Abstract
Bovine mastitis has become increasingly important issues for farmers and consumers, leading to large economic losses in the dairy industry worldwide. Because treatment of mastitis is difficult and costly, improved mastitis resistance through selective breeding would be advantageous. The toll‐like receptor 4 (TLR4) is an important player in recognising pathogens and activating immune responses. However, its roles in mastitis occurrence and the underlying molecular mechanisms are unclear. In this study, a single nucleotide polymorphism, rs8193069 (T → C) in TLR4 gene was detected in a Holstein cow resource population in southern China. Association analysis with 5‐year production traits, haematology, and biochemistry parameters revealed that individuals with genotype CC had significantly lower somatic cell counts (SCC), lower fat percentage, but higher 305‐day milk (p < 0.05) and total milk yield (p < 0.01). Both genotypes CC and CT had lower lymphocyte counts (#LYMPH) (p < 0.01) and basophil counts (#BASO) (p < 0.05) than TT. Genotype CC had a less level of triglyceride (p < 0.01) and creatine kinase (p < 0.05) than CT. Further analysis based on the production data revealed significant positive correlations between SCC and #LYMPH. Analysis of TLR4 protein structure and properties suggested that the missense mutation on the 674th amino acid from Thr to Ile reduced the flexibility and hydrophilicity of TIR domain, implying a weakened binding ability of TLR4 to its adaptors. In conclusion, allele C of rs8193069 was the major allele in Holstein cows that indicated a greater genetic potential to mastitis resistance and milk yields, probably via the LPS‐TLR4 inflammatory signalling. This study offers a marker to improve mastitis resistance in the dairy cow population in southern China.
Keywords: association analysis, mastitis, single nucleotide polymorphism, somatic cell count, toll‐like receptor 4
Short abstract
A single nucleotide polymorphism, rs8193069 (T → C) in TLR4 gene was detected in 785 Holstein cows from southern China. The rs8193069 of TLR4 is significantly associated with both performance traits and blood parameters in Holstein cows. The mutation of rs8193069 from T to C contributes the mastitis resistance with decreased SCC in milk, probably by weakening the binding ability of the TIR domain to TLR4 adaptors and LPS/TLR4‐NF‐κB signalling mediated inflammation.
1. INTRODUCTION
In dairy cattle production, the most common disease is mastitis, which is characterised by inflamed mammary tissue due to physical trauma or microorganism infection (Miles & Huson, 2020). Many factors, including herd and farm management, milking management, the milking machine and the cow itself, contribute to mastitis development (Miles & Huson, 2020), while pathogen infections, including pathogenic fungi, bacteria, mycoplasma and viruses are main causative factors. The populations of mastitis pathogens and/or risk of mastitis increase in summer season as ambient temperature and moisture increases (Hogan & Smith, 2012; Fukushima et al., 2020). In southern China, provinces such as Hunan, Hubei, Shanghai, Guangdong, the cow's environment belong to subtropical monsoon climate. Therefore, circumstances in these areas are detrimental to dairy cattle production due to the heat stress and high humidity. As a result, the treatment of mastitis is difficult and costly. Although much research has gone into its aetiology and diagnosis, there are still large economic losses every year.
White blood cells or leukocytes constitute most somatic cells in milk samples from cows with mastitis. The somatic cell count (SCC) increases sharply in response to pathogenic bacteria such as Streptococcus agalactiae and Staphylococcus aureus, which are causative agents of mastitis (Ruegg & Petersson‐Wolfe, 2018). Therefore, SCC is not only an indicator of the quality of milk but is also used for mastitis diagnosis in dairy cows (Schukken et al., 2003). Generally, the acceptable SCC is less than 100,000 cells/ml, which means that cows with a SCC over 100,000 can be judged as having a higher prevalence of mastitis pathogens (Schwarz et al., 2010).
The toll‐like receptor 4 (TLR4) is a type I transmembrane protein, a member of the toll‐like receptor (TLR) family, which is recognised as the pattern recognition receptor (PRR). It is involved in the recognition of pathogens, as well as host danger signals that are released during tissue damage (Wang et al., 2018). It is expressed by cells of the innate immune system when stimulated by structural motifs, known as pathogen‐associated molecular patterns (PAMPs), from bacteria, viruses and fungi (Lu et al., 2008). TLR4 recognises lipopolysaccharide (LPS), a PAMP present in many Gram‐negative bacteria and some Gram‐positive bacteria. A dramatic increase in TLR4 protein has been detected in the mammary gland during mastitis (Wang et al., 2018), indicating that the TLR4 may be associated with mastitis in the dairy cow. Coinciding with this, one single nucleotide polymorphism (SNP) in TLR4 gene, rs8193069, has been found to be significantly associated with SCC in commercial dairy cattle populations (Wang et al., 2008). Nevertheless, contrary results have been shown in other studies (Wang et al., 2018). In addition, roles of rs8193069 in mastitis are not underpinned merely by association analyses using production traits, and there was little information between rs8193069 and health traits. At last, effects of rs8193069 on bovine TLR4 protein properties in terms of immune crosstalk have not been discussed before. To better understand roles of TLR4 in the risk of mastitis, here our objectives were to investigate the associations of rs8193069 in TLR4 with production traits in addition to haematology and biochemistry parameters in a southern China Holstein resource population, and to investigate the potential molecular mechanisms of rs8193069 affecting mastitis. Based on statistical model construction and TLR4 protein structure and properties predictions, several associations were identified and the potential mechanism of rs8193069 affecting mastitis resistance of dairy cows through LPS‐TLR4 inflammatory signalling was proposed.
2. MATERIALS AND METHODS
2.1. Animals and production phenotypes
The resource population from a large‐scale Holstein dairy farm located in the district of Wuhan City, P.R. China comprised of 785 Holstein cows, which were fed with TMR diet, milked three times daily and implemented regular and standard performance testing by dairy herd improvement (DHI).
A total of 1255 sets of production phenotypic data were collected in different lactations from 2015 to 2020, including 777 in parity 1 (P1), 386 in P2 and 92 in P3. Each set comprised of eight production traits from a cow in a parity, including lactation days (d), 305 d milk yield (kg), total milk yield (kg), peak milk (kg), peak day (d), fat percentage (%), protein percentage (%) and somatic cell (104/ml).
2.2. Blood sample collection and DNA extraction
Blood samples were collected via coccygeal vein in EDTA tubes (Wuhan Zhiyuan Medical Treatment Technology Co., Ltd., China). Samples for haematology were refrigerated at 4°C before detections, all samples were detected within 8 h following collections. Samples for DNA extraction were stored at −80°C until used. Genomic DNA was extracted from blood samples with a TIANamp Blood DNA kit [Tiangen Biochemical Technology (Beijing) Co. Ltd., China]. DNA concentration and purity were measured in a Nanodrop™ 2000 spectrophotometer. Serums for biochemistry were obtained by centrifuging coagulated blood at 3000 rpm for 15 min at 4°C. The samples were then equated and stored at −80°C until used.
2.3. Snapshot micro‐sequencing
The snapshot micro‐sequencing technology was used to for genotyping the rs8193069 in bovine TLR4 gene (Table 1). One pair of amplification extension primer was designed using online software Primer 3.0 (http://bioinfo.ut.ee/primer3‐0.4.0/) based on the bovine TLR4 sequence (GenBank accession number: NC_037335.1). The forward primer was 5′‐ GTGGGAGCTGAGCAAATGATG‐3′, and the reverse primer was 5′‐CGCMCCCAGTCTTCATCCTG‐3′, where M denotes the annex base (A/C). The length of the amplified product was 266 bp.
TABLE 1.
Details of rs8193069 in bovine TLR4 gene
| SNP | Amino acid | ||
|---|---|---|---|
| Location on chromosome | 8:107067611 | Location on protein | 674 |
| Location on CDS | 2021 | ||
| Alleles | C/T | Amino acid variation | T(Thr)/I(Ile) |
| Codons | ACC/ATC | ||
| Mutation type | Missense variant |
CDS: coding sequence.
The PCR amplification was performed using 20 μl of the reaction mixture containing 1 × GC‐I buffer (Takara), 3.0 mM Mg2+, 0.3 mM dNTP, 1 U HotStar Taq polymerase (Qiagen Inc.), 1 μl genomic DNA (5‐10 ng/μl), 1 μl (1 μM) pooled primers and PCR grade water. The PCR amplification conditions consisted of preformation at 95°C for 2 min; 11 cycles of denaturation at 94°C for 20 s, annealing at 65°C for 40 s with every cycle involving a decrease in temperature of 0.5°C and an extension at 72°C for 1.5 min; 24 cycles of denaturation at 94°C for 20 s, annealing at 59°C for 30 s and extension at 72°C for 1.5 min; 72°C for 2 min and storage at 4°C.
A total of 10 μl PCR products were purified with SAP (5 U) and exonuclease I (2 U) at 37°C for 1 h, followed by inactivation at 75°C for 15 min. The single base extension reaction was then conducted in 10 μl reaction system containing 5 μl SNaPshot™ Multiplex Kit (ABI), 2 μl purified PCR products, 1 μl extension primer (1.6 μM) and 2 μl PCR grade water. The cycling conditions consisted of preformation at 96°C for 1 min, 28 cycles at 96°C for 10 s, at 55°C for 5 s and at 60°C for 30 s, and storage at 4°C. The extension products were purified the same as the amplified products. Then, the purified product was denatured for 3 min and tested by sequencing machine ABI3730XL. Raw data were analysed using GeneMapper 4.1 (Applied Biosystems Co., Ltd., USA).
2.4. Bioinformatics analysis of TLR4 protein structure and properties
The conservative domain and three‐dimensional structure were performed with the sequence of bovine TLR4 protein (NC_037335.1). The InterPro server (http://www.ebi.ac.uk/interpro/scan.html) was used to predict the conservative domain (Hunter et al., 2009). Three‐dimensional structures were predicted with online server I‐TASSER (http://zhanglab.ccmb.med.umich.edu/I‐TASSER/) (Yang & Zhang, 2015). The PyMOL v1.6.x was used to visualise and edit the 3D structure (Shringi, 2005). The effect of rs8193069 on hydrophilicity of TLR4 proteins were predicted by Protscale online tools (https://web.expasy.org/protscale/) (Gasteiger et al., 2005). The effect of rs8193069 on flexibility was predicted by PredyFlexy online (https://www.dsimb.inserm.fr/dsimb_tools/predyflexy/) (Bornot et al., 2011).
2.5. Haematology and serum biochemistry assays
The haematological parameters were determined using an ADVIA® 2120i Hematology System with the standard reagents (Siemens Healthcare Diagnostics Inc., USA). A total of 24 parameters for each animal were collected in bovine mode, including total red blood cell count (RBC, 106/μl), haemoglobin concentration (HGB, g/dl), haematocrit (HCT, %), mean corpuscular volume (MCV, fL), mean corpuscular haemoglobin (MCH, pg), mean corpuscular haemoglobin concentration (MCHC, g/dl), red blood cell haemoglobin (CH, pg), red blood cell distribution width (RDW, %), haemoglobin distribution width (HDW, g/dl), platelet count (PLT, 103/μl), mean platelet volume (MPV, fL), white blood cell count (WBC, 103/μl), total neutrophil count (#NEUT, 103/μl), eosinophils (#EOS, 103/μl), basophils (#BASO, 103/μl), lymphocytes (#LYMPH, 103/μl), monocytes (#MON, 103/μl), large unstained cells (#LUC, 103/μl) and the proportion of neutrophils (%NEUT), eosinophils (%EOS), basophils (%BASO), lymphocytes (%LYM), monocytes (%MON) and large unstained cells (%LUC).
Biochemical parameters were determined using an automatic biochemistry analyser HITEC 7100 (Hitachi, Ltd., Japan) with the matching commercial kits (Wako Pure Chemical Industries, Ltd., Japan). A total of 17 biochemical parameters for each animal were collected, including total bilirubin (TB, mg/dl), total protein (TP, g/dl), Albumin (ALB, g/dl), aspartate transaminase (AST, U/L), alanine aminotransferase (ALT, U/L), alkaline phosphatase (ALP, U/L), total cholesterol (TC, mg/dl), triglyceride (TG, mg/dl), glucose (GLU, mg/dl), calcium (Ca, mg/dl), phosphorus (P, mg/dl), creatinine (CREA, mg/dl), low‐density lipoprotein (LDL, mg/dl), high‐density lipoprotein (HDL, mg/dl), urea nitrogen (BUN, mg/dl), gamma‐glutamyl transpeptidase (GGT, U/L) and creatine kinase (CK, U/L).
2.6. Statistical analysis
A total of 785 cows from the resource population were involved in association analysis. The allele and genotype frequency of rs8193069 in TLR4 gene were estimated using EXCEL. The Hardy–Weinberg equilibrium was determined using a chi‐squared (χ2) test (p < 0.05). A normality test for each trait was performed using univariate progress of SAS online program (https://welcome.oda.SAS.com/). Data were transformed by BOX–COX method, and the outliers were eliminated by ‘mean ± 3 × standard deviation (SD)’. General Linear Model (GLM) progress was used to compare variation in all traits between different genotypes. The genetic effects of rs8193069 on production traits, SCC, haematology and biochemical traits were analysed with the linear model of SAS with the following equation:
where Yijklmno is the dependent variable; μ is the overall mean; Yeari is the fixed effect of calving year (i = 2014 to 2020); Seasonj is the fixed effect of calving season (j = spring, summer, autumn or winter); Phasek is the fixed effect of lactation stage (k ≤ 100 d, 100–200 d or >200 d); Sirel is the fixed effect of the sire (l = 1, 2, 3, to 48); Paritym is the fixed effect of parity (m = 1, 2, or 3); Genotypen is the fixed effect of SNP (n = CC, CT, or TT), eijklmno is the random residual effect (Wang et al., 2018). The differences among the effects of different genotypes on each trait were compared with Duncan method. Finally, CORR progress was used to analyse the correlation among the traits.
3. RESULTS
3.1. Allele and genotype frequencies of rs8193069
The allele and genotype frequencies for rs8193069 are shown in Table 2. The genotypes included CC (n = 665), CT (n = 118) and TT (n = 2), giving frequencies of 92.2% and 7.8% for C and T, respectively. Individual frequencies of the genotypes are in Hardy–Weinberg equilibrium (p = 0.497).
TABLE 2.
Distribution of rs8193069 in the studied resource population (n = 785)
| Genotype | Number | Genotype frequency | Allele | Allele frequency | χ 2 | p Value |
|---|---|---|---|---|---|---|
| C/C | 665 | 0.847 | C | 0.922 | 1.399 | 0.497 |
| C/T | 118 | 0.150 | T | 0.078 | ||
| T/T | 2 | 0.003 |
3.2. Effect of rs8193069 on bovine TLR4 protein structure and properties
Prediction of conserved domains of TLR4 (Figure 1a) showed that the 674th amino acid corresponding to rs8193069 was located at the start of the Toll/interleukin‐1 receptor (TIR) domain. The 3D‐structure prediction showed that missense mutation from T (Thr) to I (Ile) of rs8193069 induced a slight conformational change of the nearby amino acids and added a β‐fold downstream (Figure 1b and c), but neither of them had significant effect on the whole structure of the protein.
FIGURE 1.
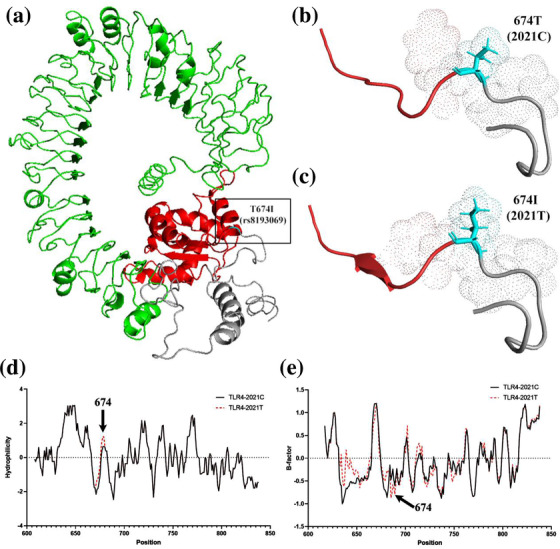
Effects of rs8193069 on TLR4 protein structure and function. (a) The location of amino acid variation corresponding to rs8193069 on theoretical three‐dimensional model of TLR4 protein. The variation is marked with cyan. The TIR domain (marked with red) is located in the membrane and contains amino acids 674–814. The extracellular part of TLR4 is indicated with green. (b), (c) The predicted three‐dimensional structure of TLR4 when the rs8193069 was C and T, respectively. (d) Effects of rs8193069 on hydrophilicity of TLR4. It shows the region at amino acid 603–841. The mutation of rs8193069 from C to T results in increased hydrophilicity values of the nearby amino acids. Higher positive values indicate stronger hydrophobicity and lower negative values indicate stronger hydrophilicity. (e) Effect of rs8193069 on flexibility of TLR4. It shows the region at amino acid 603–841. The mutation of rs8193069 from C to T results in decreased B‐factor values of the nearby amino acids, which indicate the reduced flexibility
Further protein property analysis of TLR4 showed that the mutation of rs8193069 from C to T increased the hydrophilicity values (Figure 1d), indicating an increased hydrophobicity of nearby amino acids. The mutation also decreased the B‐factor values (Figure 1e), suggesting that the flexibility of nearby amino acids was reduced because of the mutation.
3.3. Associations of rs8193069 with production traits
Associations of rs8193069 with 8 production traits are presented in Table 3. Significant effects (p < 0.05) of rs8193069 were detected on 305 d milk yield, SCC and Total milk yield. In comparison with genotype CT, individuals with genotype CC had higher 305 d milk yield and lower SCC. Total milk yield and fat percentage were extremely significantly associated with rs8193069 (p < 0.01). Individuals with genotype CC had higher total milk yield but lower fat percentage compared to genotypes CT. No significant differences were found for lactation days, peak milk, peak day and protein percentage among the three genotypes.
TABLE 3.
Association of rs8193069 with production traits
| Production traits | Genotype | Phenotypic valueMean ± SD † | F | p Value |
|---|---|---|---|---|
| Lactation day | C/C | 309.096 ± 17.489 | 2.280 | 0.103 |
| C/T | 297.408 ± 18.412 | |||
| T/T | 246.127 ± 50.837 | |||
| 305 d milk yield | C/C | 10073.005 ± 324.166b | 3.840 | 0.022* |
| C/T | 9765.951 ± 346.743a | |||
| T/T | 8310.936 ± 1035.947ab | |||
| Total milk yield | C/C | 10324.004 ± 596.00B | 5.360 | 0.005** |
| C/T | 9667.778 ± 627.447A | |||
| T/T | 7557.115 ± 1732.460 AB | |||
| Peak milk | C/C | 42.851 ± 1.368 | 2.860 | 0.058 |
| C/T | 41.704 ± 1.440 | |||
| T/T | 38.892 ± 3.977 | |||
| Peak day | C/C | 119.770 ± 10.550 | 0.230 | 0.795 |
| C/T | 118.126 ± 11.106 | |||
| T/T | 103.356 ± 30.668 | |||
| Fat percentage | C/C | 4.245 ± 0.098A | 6.940 | 0.001** |
| C/T | 4.377 ± 0.103B | |||
| T/T | 4.596 ± 0.283AB | |||
| Protein percentage | C/C | 3.235 ± 0.048 | 2.500 | 0.083 |
| C/T | 3.272 ± 0.050 | |||
| T/T | 3.372 ± 0.139 | |||
| Somatic cell count | C/C | 20.816 ± 2.718a | 4.340 | 0.013* |
| C/T | 22.001 ± 2.864a | |||
| T/T | 41.800 ± 7.900b |
All values are expressed as mean ± SD.
The upper capitals (A, B, C)/double asterisk and (a, b, c)/signal asterisk were used when the difference is highly significant (p < 0.01) and significant (p < 0.05), respectively.
3.4. Association of rs8193069 with haematology and biochemistry parameters
The estimated effects of rs8193069 on haematology and biochemistry parameters are shown in Table 4. rs8193069 had extremely significant effects on #LYMPH and TG (p < 0.01) and significant effects on MCV, MCHC, #BASO and CK (p < 0.05). Individuals with genotypes CC and CT had lower MCV, #LYMPH, #BASO and higher MCHC than genotype TT. Individuals with genotypes CC had lower TG and CK than genotype CT. Details of haematology and serum biochemistry parameters that not significantly associated with rs8193069 are shown in supplementary tables (Tables S1 and S2).
TABLE 4.
Association of rs8193069 with haematology and biochemistry parameters
| Indexes † | Genotype | Phenotypic valueMean ± SD ‡ | F | p Value |
|---|---|---|---|---|
| MCV | C/C | 48.458 ± 0.450a | 4.370 | 0.013* |
| C/T | 48.996 ± 0.641a | |||
| T/T | 60.694 ± 4.375b | |||
| MCHC | C/C | 356.378 ± 1.269b | 3.640 | 0.027* |
| C/T | 355.157 ± 1.808b | |||
| T/T | 324.450 ± 12.342a | |||
| #LYMPH | C/C | 5.917 ± 0.325A | 5.320 | 0.005** |
| C/T | 5.830 ± 0.464A | |||
| T/T | 16.157 ± 3.163B | |||
| #BASO | C/C | 0.135 ± 0.008a | 3.060 | 0.048* |
| C/T | 0.132 ± 0.011a | |||
| T/T | 0.319 ± 0.076b | |||
| TG | C/C | 12.704 ± 0.501A | 5.36 | 0.005** |
| C/T | 14.373 ± 0.696B | |||
| T/T | 18.867 ± 4.719AB | |||
| CK | C/C | 82.084 ± 0.361a | 3.03 | 0.049* |
| C/T | 84.338 ± 0.845b | |||
| T/T | 83.550 ± 6.177ab |
Meanings of all abbreviations have been indicated in Section 2.
All values are expressed as Mean ± SD.
The upper capitals (A, B, C)/double asterisk and (a, b, c)/signal asterisk were used when the difference is highly significant (p < 0.01) and significant (p < 0.05), respectively.
3.5. Correlation analysis between SCC and haematology or serum biochemistry
The relationship between SCC and haematological or biochemical parameters were further analysed. As shown in Table 5, there were extremely significant correlations between SCC and RBC, HGB, #LYMPH and TP (p < 0.01), and significant correlations between SCC and %EOS, #BASO, ALB, ALT, CA and P (p < 0.05). There were positive correlations between SCC and #LYMPH, #BASO and TP, and negative correlations between SCC and RBC, HGB, %EOS, ALB, ALT, CA and P.
TABLE 5.
Correlation between SCC and haematology and biochemistry
| Indexes † | R value | p Value | Indexes | R value | p Value |
|---|---|---|---|---|---|
| WBC | −0.016 | 0.713 | TB | −0.021 | 0.635 |
| RBC | −0.134 | 0.002** | TP | 0.163 | 0.000** |
| HGB | −0.137 | 0.001** | ALB | −0.088 | 0.050* |
| HCT | 0.035 | 0.418 | AST | −0.082 | 0.059 |
| MCV | 0.050 | 0.244 | ALT | −0.088 | 0.041* |
| MCH | −0.003 | 0.938 | ALP | −0.044 | 0.318 |
| MCHC | −0.041 | 0.342 | TC | −0.046 | 0.288 |
| CHCM | −0.037 | 0.398 | TG | −0.012 | 0.776 |
| CH | 0.048 | 0.271 | GLU | −0.079 | 0.074 |
| RDW | −0.040 | 0.352 | CA | −0.111 | 0.012* |
| HDW | −0.021 | 0.635 | P | −0.113 | 0.011* |
| PLT | 0.027 | 0.530 | CREA | 0.022 | 0.616 |
| MPV | 0.003 | 0.944 | HDL | −0.056 | 0.193 |
| %NEUT | −0.036 | 0.403 | LDL | −0.044 | 0.306 |
| %LYMPH | 0.069 | 0.108 | BUN | 0.010 | 0.823 |
| %MONO | −0.057 | 0.185 | GGT | −0.009 | 0.838 |
| %EOS | −0.097 | 0.025* | CK | 0.024 | 0.580 |
| %BASO | −0.020 | 0.645 | |||
| %LUC | −0.017 | 0.692 | |||
| #NEUT | 0.080 | 0.063 | |||
| #LYMPH | 0.126 | 0.004** | |||
| #MONO | 0.056 | 0.197 | |||
| #EOS | −0.023 | 0.593 | |||
| #BASO | 0.086 | 0.047* | |||
| #LUC | 0.034 | 0.434 |
Meanings of all abbreviations have been indicated in Section 2. The double asterisk and signal asterisk indicate the difference is highly significant (p < 0.01) and significant (p < 0.05), respectively.
4. DISCUSSION
4.1. Effects of rs8193069 on TIR domain stability
The TLR4 protein consists of three parts: an extracellular domain composed of many leucin‐rich repeats (LRRs) that mediate LPS recognition and receptor dimerisation (Ohto et al., 2012); a transmembrane domain composed of an α‐helix and the TIR domain, which is in the cytosolic face. The TIR domain, which is unique to the TLR system, is essential for downstream signal transduction (O'Neill & Bowie, 2007) and is responsible for mediating the protein‐protein interactions between the TLR4 and adaptor proteins (Mahita & Sowdhamini, 2017), including myeloid differentiation primary‐response gene 88 (MyD88), MyD88‐adaptor‐like (MAL, also known as TIRAP), TIR‐domain‐containing adaptor protein inducing IFN‐β (TRIF; also known as TICAM1), TRIF‐related adaptor molecule (TRAM; also known as TICAM2) and sterile α‐ and armadillo‐motif containing protein (SARM) (O'Neill & Bowie, 2007). The nuclear factor κB (NF‐κB) is activated after a series of signal transduction chains (Fitzgerald et al., 2001), followed by translocation into nucleus, inducing the synthesis and secretion of proinflammatory cytokines and type I interferons, initiating an inflammatory response.
The amino acid 674 corresponding to rs8193069 is the beginning of the TIR region and located at the junction between the N‐terminal and the TIR domain. Poltorak et al. (1998) found a missense mutation Pro712His in the TIR domain of TLR4 in C3H/HeJ mice, which led to the tolerance to LPS. The homology modelling of TLR4 showed that hydrophobicity played a crucial role in the binding of TLR4 protein to its adaptor proteins (Gu et al., 2002), while Pro712His changed the hydrophobicity and charge of the corresponding position, resulting in a decreased activity of LPS/TLR4 signal transduction pathway. Similarly, our analysis showed that the mutation of rs8193069 from C to T increased the hydrophobicity of the 674th amino acid and the strength of the hydrophobic interactions, which is obviously beneficial to the binding of TLR4 to its adaptors. The change of hydrophobicity also affects the stability of protein. When the hydrophilic residue mutates to the hydrophobic one, the stability of protein will increase (Gallardo et al., 2010).
The B‐factor (atomic displacement parameter) in protein crystal structures is regarded as an important indicator of the protein structure, reflecting its flexibility and dynamics (Zheng et al., 2005). A large B‐factor indicates high mobility of individual atoms and side chains, while a small B‐factor indicates high stability (Bornot et al., 2011). Our prediction results of B‐factor showed that the flexibility of area near the 674th amino acid reduced when rs8193069 mutated from C to T, implying the stability of this region was improved. The TLR4 protein with CC genotype may therefore has a weakened binding ability to adaptors, leading to reduced or even impaired activity of the LPS/TLR4 signal transduction pathway.
4.2. Effects of rs8193069 on the mastitis resistance in Holstein cows through LPS/TLR4‐NF‐κB signal transduction pathway
The rs8193069 is a functional SNP in TLR4 gene which has been widely examined in dairy cows. Previous studies have found that rs8193069 was associated with milk yields, fat percentages and protein percentages (Zhou et al., 2017; Wang et al., 2018). However, the association between this SNP and SCC is still undetermined among different populations (Wang et al., 2018). In this study, rs8193069 was found to be significantly associated with SCC in the trialled Holstein cow population, showing that individuals with genotype TT had higher SCC. As increased SCC in the milk indicates long term intra‐mammary inflammation (Roldan‐Montes et al., 2020), a lower SCC is free of, or at least, low infection (Pighetti & Elliott, 2011). Therefore, the allele C of rs8193069 is the major allele, in other words, individuals with genotype CC would have a greater genetic potential for mastitis resistance under the similar environment.
To better elucidate the underlying molecular mechanism on the contribution of rs8193069 to mastitis resistance in dairy cows, further associations of rs8193069 with haematology and biochemistry parameters were investigated. Our results showed that individuals with genotypes CC and CT had lower #LYMPH and #BASO than genotype TT, indicating mutation from C to T promoted the production of leukocytes. Lymphocytes and basophils are essential in provoking inflammation (Bartoli‐Leonard et al., 2021; Hou et al., 2021), thus individuals with genotype CC will have a healthier status with low inflammatory stress during milk production. Further analysis based on the actual production data and health data from the same resource population validated the above results, revealing that SCC was significantly positively correlated with both #LYMPH and #BASO. It is to say that the number of lymphocytes and basophils in blood may be important factors affecting SCC. Based on these results, a mechanism was proposed that the binding ability of TLR4 to its adaptors in individuals with genotype CC was weaker than genotypes TC and TT (Figure 2). Individuals with genotype CC could be stimulated by PAMPs such as LPS, but with weakened ability to activate the LPS/TLR4‐NF‐κB pathway. As a result, cytokine expressions and inflammatory response were inhibited, and subsequently, the reduced proliferations of lymphocytes and basophils in the blood and less SCC in milk, which has been used to measure the risk of mastitis (Scarsella et al., 2021). So, individuals with genotype CC manifest greater capacity of resistance to LPS‐induced mastitis or tolerance. To further verify this, additional function validation experiments will be needed in the future.
FIGURE 2.
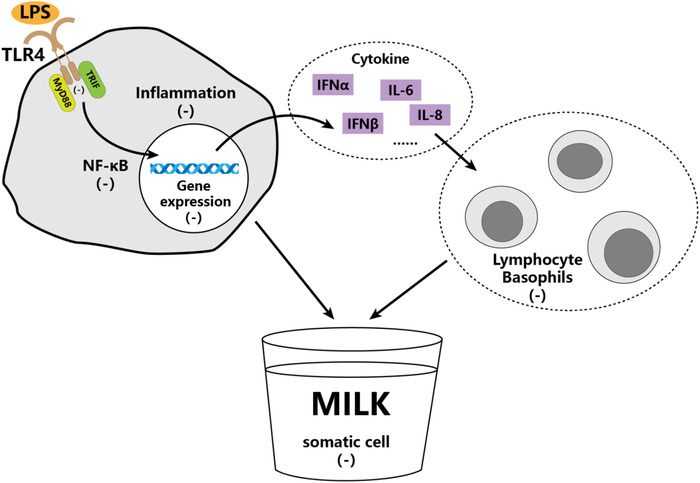
Molecular mechanism hypothesis of rs8193069 on SCC in milk or its contribution to mastitis resistance based on results in this study. The major allele C of rs8193069 may reduce the binding ability between TLR4 protein and its adaptors. This leads to the reduced activity of NF‐κB, decreased cytokines expression and lymphocyte/basophils proliferation, resulting in reduced SCC in milk. The pathway has been described in detail in the text. IFN‐α, Interferon‐α; IFN‐β, Interferon‐β; IL‐6, interleukin‐6; IL‐8, interleukin‐8
5. CONCLUSION
The rs8193069 in bovine TLR4 is significantly associated with both production traits and blood parameters in Holstein cows, where the allele C of rs8193069 is the major allele. The mutation of rs8193069 from T to C contributes the mastitis resistance with decreased SCC in milk, probably by weakening the binding ability of the TIR domain to TLR4 adaptors and LPS/TLR4‐NF‐κB signalling mediated inflammation.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
AUTHOR CONTRIBUTIONS
Hongbo Chen: conceptualisation (supporting); methodology (equal); writing – original draft (equal); writing – review and editing (equal); visualisation (lead); funding acquisition (equal). Chenhui Liu: Methodology (equal); software (lead); formal analysis (equal); writing – original draft (equal). Min Xiang: Data curation (equal); investigation (equal); resources (equal). Jie Yu: Investigation (equal); resources (equal). Yu Xia: Investigation (equal); resources (equal). Xiuzhong Hu: Data curation (equal); investigation (equal). Dingfa Wang: Resources (equal). Bifei Tao: Resources (equal). Yongjin Zhang: Data curation (equal). Lei Cheng: Conceptualisation (lead); methodology (equal); writing – review and editing (equal); visualisation (equal); funding acquisition (equal); project administration (lead).
ETHICS STATEMENT
The authors confirm that the ethical policies of the journal, as noted on the journal's author guidelines page, have been adhered to and all animal procedures were performed according to protocols approved by the Hubei Province for Biological Studies Animal Care and Use Committee. Protocols were carried out in accordance with Hubei Provincial Regulation on Administration of Laboratory Animals (10/1/2005). The US National Research Council's guidelines for the Care and Use of Laboratory Animals were followed.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1002/vms3.671
Supporting information
TABLE S1 Haematology parameters not significantly associated with rs8193069
TABLE S2 Biochemistry parameters not significantly associated with rs8193069
ACKNOWLEDGEMENTS
The authors thank Wuhan Bright Dairy Co., Ltd for providing the resource population and production data. We appreciate Dr. Liangyu Shi in Institute of Animal Science, Chinese Academy of Agricultural Sciences (CAAS) for her deep discussion with us on the corrective linear statistical model.
Chen, H. , Liu, C. , Xiang, M. , Yu, J. , Xia, Y. , Hu, X. , Wang, D. , Tao, B. , Zhang, Y. , & Cheng, L. (2022). Contribution of the mutation rs8193069 in TLR4 to mastitis resistance and performance in Holstein cows in southern China. Veterinary Medicine and Science, 8, 357–366. 10.1002/vms3.671
Present address
Yongjin Zhang, Innovation Team of Pig Genome Design and Breeding, Agricultural Genome Institute at Shenzhen, Chinese Academy of Agricultural Sciences, Shenzhen, P. R. China.
Hongbo Chen and Chenhui Liu contributed equally to the work.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Bartoli‐Leonard, F. , Zimmer, J. , & Aikawa, E. (2021). Innate and adaptative immunity: The understudied driving force of heart valve disease. Cardiovascular Research, cvab273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornot, A. , Etchebest, C. , & de Brevern, A. G. (2011). Predicting protein flexibility through the prediction of local structures. Proteins, 79(3):839–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald, K. A. , Palsson‐McDermott, E. M. , Bowie, A. G. , Jefferies, C. A. , Mansell, A. S. , Brady, G. , Brint, E. , Dunne, A. , Gray, P. , Harte, M. T. , McMurray, D. , Smith, D. E. , Sims, J. E. , Bird, T. A. , & O'Neill, L. A. (2001). Mal (MyD88‐adapter‐like) is required for Toll‐like receptor‐4 signal transduction. Nature, 413(6851):78–83. [DOI] [PubMed] [Google Scholar]
- Fukushima, Y. , Kino, E. , Furutani, A. , Minamino, T. , Mikurino, Y. , Horii, Y. , Honkawa, K. , & Sasaki, Y. (2020). Epidemiological study to investigate the incidence and prevalence of clinical mastitis, peracute mastitis, metabolic disorders and peripartum disorders, on a dairy farm in a temperate zone in Japan. BMC Veterinary Research, 16(1), 389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallardo, O. , Pastor, F. I. , Polaina, J. , Diaz, P. , Łysek, R. , Vogel, P. , Isorna, P. , González, B. , & Sanz‐Aparicio, J. (2010). Structural insights into the specificity of Xyn10B from Paenibacillus barcinonensis and its improved stability by forced protein evolution. The Journal of Biological Chemistry, 285(4), 2721–2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasteiger, E. , Hoogland, C. , Gattiker, A. , Duvaud, S. , Wilkins, M. R. , Appel, R. D. , & Bairoch A. (2005). Protein identification and analysis tools on the ExPASy server. In: John, M. Walker (Ed.), The Proteomics Protocols Handbook (pp. 571–607). Humana Press. [Google Scholar]
- Gu, C. G. , Hu, Y. D. , Li, S. , & Li, L. (2002). Homology modeling for cytoplasmic region of toll‐like receptor 4. Journal of Immunology, 18(2), 95–98. [Google Scholar]
- Hunter, S. , Apweiler, R. , Attwood, T. , Bairoch, A. , Bateman, A. , Binns, D. , Bork, P. , Das, U. , Daugherty, L. , Duquenne, L. , Finn, R. D. , Gough, J. , Haft, D. , Hulo, N. , Kahn, D. , Kelly, E. , Laugraud, A. , Letunic, I. , Lonsdale, D. , & Yeats, C. (2009). InterPro: The integrative protein signature database. Nucleic Acids Research, 37(Suppl 1), D211–D215, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan, J. , & Smith, K. L. (2012). Managing environmental mastitis. Veterinary Clinics of North America: Food Animal Practice, 28(2), 217–224. [DOI] [PubMed] [Google Scholar]
- Hou, T. , Tsang, S. M. , Kan, L. Y. , Li, P. , Chu, I. M. , Lam, C. W. , & Wong C. K. (2021). IL‐37 targets TSLP‐primed basophils to alleviate atopic dermatitis. International Journal of Molecular Sciences, 22(14), 7393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, Y. C. , Yeh, W. C. , & Ohashi, P. S. (2008). LPS/TLR4 signal transduction pathway. Cytokine, 42(2), 145–151. [DOI] [PubMed] [Google Scholar]
- Mahita, J. , & Sowdhamini, R. (2017). Integrative modelling of TIR domain‐containing adaptor molecule inducing interferon‐β (TRIF) provides insights into its autoinhibited state. Biology Direct, 12(1), 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles, A. M. , & Huson, H. J. (2020). Time‐and population‐dependent genetic patterns underlie bovine milk somatic cell count. Journal of Dairy Science, 103(9), 8292–8304. [DOI] [PubMed] [Google Scholar]
- Ohto, U. , Yamakawa, N. , Akashi‐Takamura, S. , Miyake, K. , & Shimizu, T. (2012). Structural analyses of human toll‐like receptor 4 polymorphisms D299G and T399I. The Journal of Biological Chemistry, 287(48), 40611–40617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill, L. A. , & Bowie, A. G. (2007). The family of five: TIR‐domain‐containing adaptors in Toll‐like receptor signalling. Nature Reviews Immunology, 7(5), 353–364. [DOI] [PubMed] [Google Scholar]
- Pighetti, G. M. , & Elliott, A. A. (2011). Gene polymorphisms: The keys for marker assisted selection and unraveling core regulatory pathways for mastitis resistance. Journal of Mammary Gland Biology and Neoplasia, 16(4), 421–432. [DOI] [PubMed] [Google Scholar]
- Poltorak, A. , He, X. , Smirnova, I. , Liu, M. Y. , Van Huffel, C. , Du, X. , Birdwell, D. , Alejos, E. , Silva, M. , Galanos, C. , Freudenberg, M. , Ricciardi‐Castagnoli, P. , Layton, B. , & Beutler, B. (1998). Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: Mutations in Tlr4 gene. Science, 282(5396), 2085–2088. [DOI] [PubMed] [Google Scholar]
- Roldan‐Montes, V. , Cardoso, D. F. , Hurtado‐Lugo, N. A. , do Nascimento, A. V. , de Abreu Santos, D. J. , Scalez, D. C. B. , de Freitas, A. C. , Herrera, A. C. , Albuquerque, L. G. , de Camargo, G. M. F. , & Tonhati, H. (2020). Polymorphisms in TLR4 gene associated with somatic cell score in Water buffaloes (Bubalus bubalis). Frontiers in Veterinary Science, 7, 568249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruegg, P. L. , & Petersson‐Wolfe, C. S. (2018). Mastitis in dairy cows. Veterinary Clinics of North America: Food Animal Practice, 34(3), ix–x. [DOI] [PubMed] [Google Scholar]
- Scarsella, E. , Zecconi, A. , Cintio, M. , & Stefanon, B. (2021). Characterization of microbiome on feces, blood and milk in dairy cows with different milk leucocyte pattern. Animals (Basel), 11(5), 1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schukken, Y. H. , Wilson, D. J. , Welcome, F. , Garrison‐Tikofsky, L. , & Gonzalez, R. N. (2003). Monitoring udder health and milk quality using somatic cell counts. Veterinary Research, 34(5), 579–596. [DOI] [PubMed] [Google Scholar]
- Schwarz, D. , Diesterbeck, U. S. , Failing, K. , König, S. , Brügemann, K. , Zschöck, M. , Wolter, W. , & Czerny, C. P. (2010). Somatic cell counts and bacteriological status in quarter foremilk samples of cows in Hesse, Germany—A longitudinal study. Journal of Dairy Science, 93(12), 5716–5728. [DOI] [PubMed] [Google Scholar]
- Shringi, R. P. (2005). Pymol software for 3d visualization of aligned molecules. Biomaterials, 26(1), 63–72.15193881 [Google Scholar]
- Wang, M. , Song, H. , Zhu, X. , Xing, S. , Zhang, M. , Zhang, H. , Wang, X. , Yang, Z. , Ding, X. , Karrow, N. A. , König, S. , & Mao, Y. (2018). Toll‐like receptor 4 gene polymorphisms influence milk production traits in Chinese Holstein cows. Journal of Dairy Research, 85(4), 407–411. [DOI] [PubMed] [Google Scholar]
- Wang, X. , Xu, S. , Gao, Z. , Li, J. , Ren, H. , & Luoren, Z. (2008). Cloning and SNP screening of the TLR4 gene and the association between its polymorphism and somatic cell score in dairy cattle. South African Journal of Animal Science, 38(2), 101–109. [Google Scholar]
- Yang, J. , & Zhang, Y. (2015). I‐TASSER server: New development for protein structure and function predictions. Nucleic Acids Research, 43(W1), W174–W181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng, Y. , Bailey, T. L. , & Teasdale, R. D. (2005). Prediction of protein B‐factor profiles. Proteins, 58(4), 905–912. [DOI] [PubMed] [Google Scholar]
- Zhou, H. , Cheng, L. , Gong, H. , Byun, S. O. , Edwards, G. R. , & Hickford, J. G. (2017). Variation in the Toll‐like Receptor 4 (TLR4) gene affects milk traits in dairy cows. Journal of Dairy Research, 84(4), 426–429. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
TABLE S1 Haematology parameters not significantly associated with rs8193069
TABLE S2 Biochemistry parameters not significantly associated with rs8193069
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


