Abstract
This study investigated whether the concentrations of four metals [lead (Pb), mercury (Hg), manganese (Mn), and aluminum (Al)] are correlated in cord blood and childhood blood samples from Jamaican children. Cord blood samples were obtained from 21 pregnant women enrolled in the second Jamaican Birth Cohort Study from July 1, 2011 to September 30, 2011, and blood samples were drawn from their children who participated in a follow up study when the children were 4–8 years old. Correlations were assessed by the Pearson or the Spearman’s rank correlation coefficient. The mean ages of children at the childhood visit and their mother at the child’s birth were 5.5 years and 29.8 years, respectively. About 47.6% of children were male. Statistically significant correlations between cord blood and childhood blood concentrations of Pb (rSpearman= 0.45; P = 0.04) and Mn (rPearson = 0.48; P = 0.03) were found, and these remained significant when adjusted for the child’s sex, age, or both. For Al and Hg, rSpearman= 0.29 and 0.08, respectively, but the correlations were not statistically significant (both P ≥ 0.20). A significant correlation between cord blood and childhood blood Pb concentrations for children 4–8 years old has not been previously reported.
Keywords: age-and sex adjusted, correlation, cord blood, childhood blood, lead, manganese, Jamaica
Subject classification codes: Human exposure
Introduction
Exposure to heavy metals during pregnancy, and the prenatal[1] or childhood[2–4] periods have adverse influences on neurodevelopment, including risk of attention-deficit/hyperactivity disorder (ADHD)[5] and autism spectrum disorder (ASD).[6] For example, prenatal exposure to lead (Pb) adversely affects the intelligence quotient (IQ) and cognition in children.[7] Childhood exposure to Pb is associated with ADHD and ASD as well.[8] In addition, excess prenatal manganese (Mn) exposure is also consistently reported to be associated with externalizing problems.[6] Similar adverse effects have been reported regarding childhood exposure to mercury (Hg),[9,10] Mn,[11,12] and aluminum (Al).[13,14]
Childhood exposure to heavy metals such as Pb[8] and Hg[15]may influence ASD,[16] possibly through mechanisms involving inter-individual variation in genes such as those encoding glutathione-S-transferases (GSTs) that, have a critical role in protecting cells against oxidative stress by detoxification of exogenous chemicals and heavy metals.[15,17] Some studies also reported that long-term childhood exposures to Pb and Al have an effect on the pro-oxidant/antioxidant balance in mammalian cells.[18,19] Oxidative stress is thought to be involved in the pathogenesis of ASD.[20]
Some researchers have used cohort study designs to have an opportunity to assess both prenatal and childhood exposure to certain heavy metals in order to investigate their possible effects on physical growth and neurodevelopment.[21–23] A limited number of these cohort studies investigated whether there was a significant correlation between prenatal and childhood exposures for some heavy metals.[21–23] For example, Rabinowitz et al. assessed the correlation between Pb concentrations measured in umbilical cord blood from 200 newborns in Boston, and Pb concentrations assessed in blood of the same children followed semi-annually during the first 2 years of life. The authors reported that the arithmetic mean of Pb blood concentrations did not change from birth to 2 years old, but Spearman’s rank correlation coefficients of blood Pb concentrations at different ages tended to increase with age. Specifically, Spearman’s rank correlation coefficients between blood Pb concentrations at birth and 6 months, 6 and 12 months, 12 and 18 months, and 18 and 24 months were 0.10, 0.20, 0.09 and 0.19, respectively.[23] In addition, using data from other cohort studies, a moderate Spearman’s rank correlation (Spearman’s r = 0.49) has been reported between prenatal and childhood dentine Mn levels.[22] Moreover, Sabra et al. reported a Spearman’s rank correlation of r = 0.4 between maternal and fetal serum levels for Cd, but did not find any such correlations for the other heavy metals (Hg and Pb).[24] Kot et al., on the other hand, assessed the correlations of Pb and Mn concentrations in placenta, with those measured in fetal membrane, umbilical cord, and afterbirth samples and reported that Pearson correlations between the umbilical cord and placenta concentrations for Pb and Mn were 0.09 and −0.10, respectively. Pearson correlations between the placenta and fetal membrane concentrations for these two metals were 0.11 and 0.10, respectively. The correlations between the umbilical cord and fetal membrane were −0.23, and 0.63, respectively, which were both statistically significant at the 5% level of significance,[21] although the correlation between umbilical cord and fetal membrane for Pb concentrations was negative (−0.23).
It has been recognized that the roles of prenatal and childhood exposures to environmental chemicals in complex disorders such as ASD may be challenging to assess. For example, if there is an interaction between prenatal and childhood environmental exposures to heavy metals such as Pb, Hg, Mn, and Al (i.e., a synergistic effect) in relation to ASD, studies would benefit from having exposure data for both the prenatal and childhood periods. However, only cohort studies may have an opportunity to obtain this information, which is exceedingly rare. In situations where only one of these two exposures is available, it is important to assess or know to what extent these two exposures are correlated.
Since 2009, Rahbar et al. investigated the possible association of childhood exposure to four heavy metals (Pb, Hg, Mn, and Al) with ASD in Jamaican children who were 2–8 years old.[25–27] Since assessment of both prenatal and childhood exposures to heavy metals was not possible due to limitations of the age-and sex-matched case-control study design of Epidemiological Research on Autism in Jamaica (ERAJ),[25–27] the study team thought it important to investigate whether prenatal exposures to these metals are correlated to those measured during the childhood period. Although a previous study[23] reported the correlation between Pb exposures during the prenatal and childhood periods, they only followed children until 24 months of age. However, in the ERAJ study in Jamaica, the prenatal exposure to the four metals is assessed based on cord blood, and the childhood exposure for the same metals was measured when children were in the age range of 4–8 years. Therefore, using metal concentrations in childhood blood samples from Jamaican children that participated in the ERAJ study and in a cord blood sub-study of the second Jamaican Birth Cohort Study (JA Kids 2011,[28]) allowed the concentrations of four metals (Pb, Hg, Mn, and Al) to be compared. If there is a high (e.g., r ≥ 0.70) positive correlation between prenatal and childhood exposures to these metals, then one can estimate exposures to these metals during the prenatal period from those that were available for the childhood period from the ERAJ study.
Materials and Methods
General Description
Since 2009, the ERAJ research team at the University of Texas Health Science Center at Houston (UTHealth) has collaborated with faculty in the Department of Child and Adolescent Health, at the University of the West Indies (UWI) at Mona in Jamaica. As part of this research, an age- and sex-matched case-control study was conducted to investigate the role of childhood exposure to four heavy metals (Pb, Hg, Mn, and Al) in Jamaican children 2–8 years of age in relation to ASD.[25–27] In 2011, while the ERAJ study was implementing phase I, the second Jamaican Birth Cohort Study (JA Kids study) was conducted in Jamaica.[28] The JA Kids study enrolled nearly 9600 women throughout Jamaica, and collected information regarding socioeconomic status (SES), and exposure to environmental hazards including some heavy metals by administering SES and other questionnaires to mothers during the third trimester. Of these pregnant women, 5200 were interviewed at the time of delivery from July to September of 2011, and information regarding pregnancy duration and exposure to four metals (Pb, Hg, Mn, and Al) was collected. The mothers also provided information about SES, pregnancy duration and exposure to environmental hazards in their third trimester and at the time of delivery.
Due to the overlap between the ERAJ Phase I and JA Kids studies, this study had the opportunity to conduct a cord blood sub-study by collecting 144 cord blood samples from mothers who delivered their newborns at the UWI Hospital in Kingston, Jamaica. However, after the research team completed the data quality checks, it was found that only 130 children from this subsample were born during the enrollment period of the JA Kids study. Additionally, some children were lost to follow up for various reasons including refusals, migration, or incomplete/incorrect contact information. Therefore, there were 106 children in this subsample who had the potential to be included in the follow up of the JA Kids cord blood sub-study. After removing three sets of twins, heavy metal concentration data were only available for 100 pregnant mothers and their 100 newborns, which have already been analyzed to assess the possible association between the concentrations of the four metals in cord blood and select birth outcomes such as birthweight.[29]
Study population
In 2013, UTHealth received funding from NIEHS to implement the ERAJ-Phase II (ERAJ-2) study.[30] Therefore, when the ERAJ-2 study was implemented, the JA Kids families who had participated in the cord blood sub-study were invited to participate in the follow up study of their children, which included drawing approximately 15 mL of blood that was used for the assessment of the heavy metals, polychlorinated biphenyls (PCBs) and organochlorine (OC) pesticides, and a portion of this blood was stored for future use as previously described.[31,32]
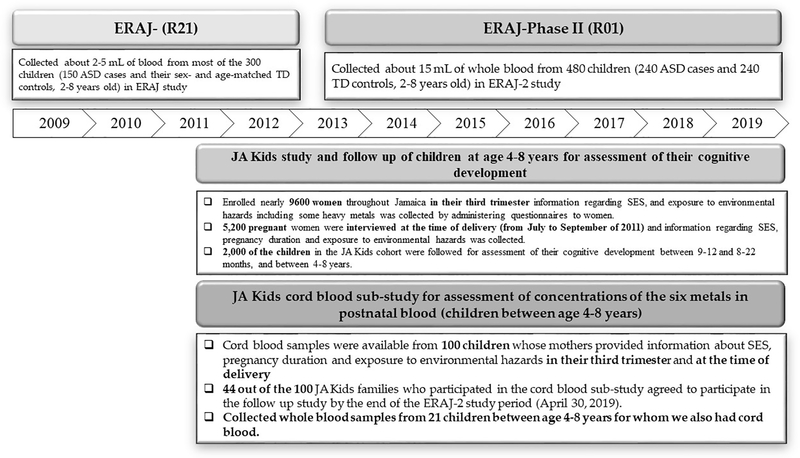
In this study, informed consent was obtained from parents/guardians and also by child’s assent (if the child was 7–8 years old). Forty-four (44) out of the 100 JA Kids families who participated in the cord blood sub-study agreed to participate in the follow-up study by the end of the ERAJ-2 study period (April 30, 2019). The UWI team was successful in obtaining blood samples from 21 of these 44 children during the follow-up period. This effort resulted in the availability of the concentrations of four heavy metals (Pb, Hg, Mn, and Al) in 21 pairs of cord blood and childhood blood samples for analysis in this study. Figure 1 shows the timeline of these studies.
Figure 1.
Timeline for the JA Kids cord blood sub-study, the JA Kids study, and two phases of the ERAJ study (ERAJ, ERAJ-2).
Assessment of heavy metals exposures
For collection of cord blood samples, a protocol was developed by the ERAJ study and UWI teams to describe the procedures necessary for collecting cord blood from infants at delivery UWI Hospital in Kingston, Jamaica. The best time to collect cord blood is immediately after delivery of the infant(s) and prior to expulsion of the placenta as the blood immediately starts to clot. As a result, after the infant was delivered and the umbilical cord was double clamped and cut, the needle was inserted for the blood draw just above the clamp that remained on the umbilical cord. Only one 10 mL lavender top EDTA tube was collected per infant. Two mL of cord blood separated in another lavender top EDTA, which were pre-screened for several trace elements or metals, including lead, mercury, manganese, and aluminum. Cord blood aliquots were allowed to clot for 30–60 minutes at ambient/room temperature, during which time they were transferred from the hospital to the Molecular Biology Lab of UWI.[29,33]
Similarly, for collection of blood from children, the study team developed and implemented standard operating procedures for the blood draw. The UWI team obtained written consent from parents/guardians and child’s assent (if the child was 7–8 years old) before drawing about 2 mL of whole blood in lavender top EDTA for assessment of exposure to heavy metals during childhood.
Samples of cord blood and blood from children that were intended for assessment of heavy metals analysis were stored at −20°C at the Molecular Biology Lab of UWI and in the UWI CARIGEN lab, respectively, without any processing and then shipped to the Michigan Department of Human Health Services (MDHHS) in Lansing, Michigan at ambient temperature on ice packs for trace metal analyses.
The 21 pairs of cord blood and childhood blood samples were analyzed for concentrations of the four metals by a Centers for Disease Control and Prevention (CDC)-certified laboratory at the MDHHS. MDHHS followed standard protocols for analyzing these samples using a PerkinElmer Elan DRC II inductively-coupled plasma mass spectrometer (PerkinElmer, Waltham, MA, USA). Specifically, MDHHS used method number ITB001A (Environmental Health Method: Blood Lead and Cadmium ICPDRCMS) that is based on the CDC guideline. (https://www.cdc.gov/nchs/data/nhanes/nhanes_05_06/pbcd_d_met_lead_cadmium.pdf) Furthermore, MDHHS is a college of American Pathologists (CAP) and Clinical Laboratory Improvement Amendments 1988 (CLIA’88) accredited laboratory. They follow the quality control (QC) plan established in these guidelines. For QCs MDHHS purchased SeronormTM Trace Elements Whole Blood which were characterized following accreditation guidelines. Once characterized ±3SD ranges were established for each analyte at each QC level. The QCs must meet this established criteria in order to accept the run. The limits of detection (LoD) for Pb, Hg, Mn, and Al were 0.25μg/dL, 0.25μg/L, 2.5μg/L, and 5μg/L, respectively. For Mn, all concentrations were above the LoD. Metal concentrations below their LoDs were identified for Pb, Hg, and Al were replaced with their respective LoD/√2.[34] The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Institutional Review Boards (IRBs) of UTHealth and UWI, Mona campus, in Kingston, Jamaica (Project identification code: HSC-SPH-09–0059).
Statistical Analysis
Descriptive analyses were performed on demographic characteristics and SES of the study participants, including sex and race of the child, maternal and paternal education levels, and assets owned by the family. The means of the metal concentrations in cord blood and the child’s blood were also examined. The concentrations of Mn were normally distributed for both cord blood and childhood blood, but the distributions of concentrations for Pb, Hg, and Al were skewed. Therefore, we log-transformed these data using the natural logarithm (ln) to produce a distribution that better approximated a normal distribution. For metals for which more than 70% of the concentrations were above their respective LoDs, median differences between cord blood and childhood blood are reported, except for Mn, for which mean differences are reported. In addition, the concentrations for the four metals in the cord blood and childhood blood were compared using the Wilcoxon signed-rank test.
Since the distribution of Mn concentrations was approximately normal, the Pearson correlation coefficient between concentration of Mn in cord blood and childhood blood was assessed. For metals with skewed distributions for concentrations, the Spearman’s rank correlation coefficient was assessed as well. However, for assessment of Pb, Hg, and Al correlations, log-transformed concentrations were used. In addition, for metals that had more than 90% of concentrations above their respective LoDs, linear regression models were fitted to predict concentrations of select metals (e.g., Pb) in childhood blood based on the concentration in cord blood. For example, the straight-line relationship between blood Pb concentrations in cord blood and childhood blood is used to estimate the equation of the fitted line based on the estimated y-intercept and slope coefficient. For nonparametric multivariable assessment of association of Pb concentrations in cord blood with childhood blood, first, both concentrations (in cord blood and childhood blood) were rank ordered within their own groups. Then the general linear model (GLM) was applied by considering the rank of childhood blood concentrations as the dependent variable. The independent variables included the rank of the cord blood Pb concentrations, which were also adjusted by sex, age, and both age and sex in different models. Since Mn concentrations were normally distributed, for multivariable assessment of association of Mn concentrations in cord blood with childhood blood, we applied the same methodology using GLM but we used the actual concentration, rather than the rank ordered concentrations. All statistical tests were performed at a 5% level of significance. All descriptive and inferential statistical analyses were conducted using SAS 9.4.[35]
Results
About 47.6% of children in this study who provided both cord blood and childhood blood samples (n = 21) were male with mean age of 5.5 years (range 3.8–6.9 years). The mean age of mothers at birth of their index child was 29.8 years (range 21.2–44.4). Demographic and socioeconomic characteristics of the children are in Table 1.
Table 1.
Demographic characteristics and socioeconomic status of children and their parents, for families who participated in the JA Kids cord blood sub-study and follow up visits (n = 44).
| Variables | Categories | n = 44 |
n = 23 |
n = 21 |
P value d |
|---|---|---|---|---|---|
| N (%) | N (%) | N (%) | |||
|
| |||||
| Sex of the child | Male | 19 (43.2) | 9 (39.1) | 10 (47.6) | 0.57 |
| Female | 25 (56.8) | 14 (60.9) | 11 (52.4) | ||
|
| |||||
|
Age of the child (months) |
Age < 60 | 9 (20.4) | 4 (17.40) | 5 (23.8) | 0.60 |
| Age ≥ 60 | 35 (79.6) | 19 (82.6) | 16 (76.2) | ||
|
| |||||
| Race of the child | Afro-Caribbean | 38 (86.4) | 18 (78.3) | 20 (95.2) | 0.13 |
| Mixed | 6 (13.6) | 5 (21.7) | 1 (4.8) | ||
|
| |||||
|
Paternal education
a (at child’s birth) |
Up to high school * | 23 (53.5) | 12 (52.2) | 12 (60.0) | 0.43 |
| Beyond high school ** | 20 (46.5) | 11 (47.8) | 8 (40.0) | ||
|
| |||||
|
Maternal education
b (at child’s birth) |
Up to high school * | 10 (25.0) | 5 (22.7) | 5 (27.8) | 0.71 |
| Beyond high school ** | 30 (75.0) | 17 (77.3) | 13 (72.2) | ||
|
| |||||
| Assets owned c | Family owns a home | 24 (60.0) | 14 (70.0) | 10 (50.0) | 0.20 |
| Family rented a home | 10 (25.0) | 4 (20.0) | 6 (30.0) | 0.47 | |
| Family home without any payments |
6 (15.0) | 2 (10.0) | 4 (20.0) | 0.38 | |
| Washing machine | 32 (80.0) | 18 (90.0) | 14 (70.0) | 0.13 | |
| Family owns a car | 27 (67.5) | 14 (70.0) | 13 (65.0) | 0.74 | |
Note: Demographic characteristics and socioeconomic status of children and their parents, for families who participated in the JA Kids cord blood sub-study follow up visit when the children were 4–8 years old (n=44), those children who did not provide childhood blood samples during the follow up visit, (n=23), and those who provided both cord- and childhood- blood samples during the follow up visit, n=21.
Up to high school education includes: attended primary, junior-secondary, and secondary/ high/technical schools
Beyond high school education includes: attended vocational, tertiary college, or university.
Paternal education was missing for 1 father.
Maternal education was missing for 4 mothers.
Assets owned by the household were missing for 4 families.
P-values are for comparison of results between 23 and 21 children.
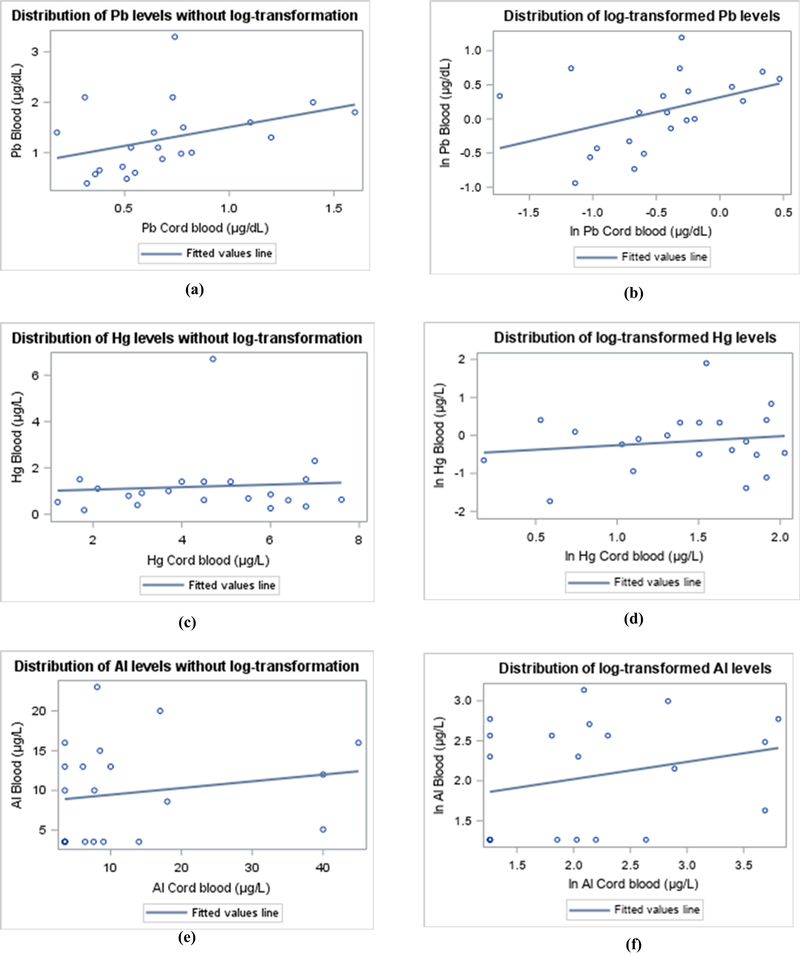
The linear association between the concentrations of Pb, Hg, and Al in cord blood and childhood blood before and after log-transformation are displayed using scatter plots in Figure 2.
Figure 2.
Linear association between untransformed lead (Pb) (a), mercury (Hg) (c), and aluminum (Al) (e) concentrations in cord blood and childhood blood, as well as the associations of ln-transformed Pb (b), Hg (d), and Al (f) concentrations in cord blood and childhood blood (n = 21).
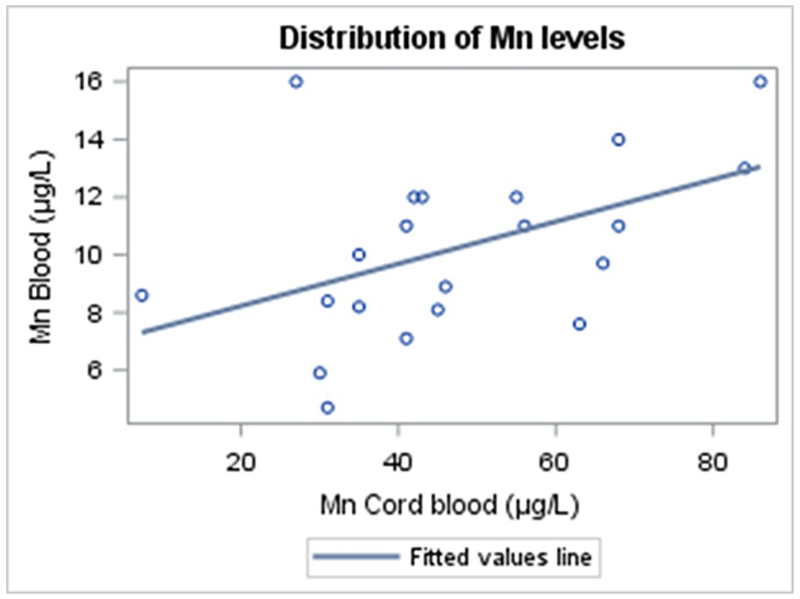
Figure 3 shows that the distribution of Mn concentrations in cord blood and childhood blood were approximately normal, hence not transformed.
Figure 3.
Linear association between untransformed manganese (Mn) concentrations in cord blood and untransformed manganese concentrations in childhood blood, n = 21.
The geometric mean (GM) concentrations of Pb and Hg in cord blood were 0.62μg/dL and 4.00μg/L, respectively. The GM blood concentrations of Pb and Cd in childhood blood were 1.12μg/dL, and 0.85μg/L, respectively. The arithmetic mean concentration of Mn in cord blood was 47.64μg/L, whereas that of childhood blood was 10.25μg/L. Arithmetic mean concentrations of other metals in cord blood and childhood blood are reported in Table 2.
Table 2.
Characteristics of distribution of the four heavy metal concentrations in cord blood and childhood blood, for different subsets of families who participated in the JA Kids cord blood sub-study and their follow up visit (n = 44).
| Heavy Metals | Limits of detection (LOD) | Cord blood (n = 44) c | Cord blood (n = 23) c | Cord blood (n = 21) | P value d | Childhood blood (n = 21) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| % Below LoD | Geometric mean | % Below LoD | Geometric mean | % Below LoD | Geometric mean | % Below LoD | Geometric mean | |||
| Lead (Pb) (μg/dL) a | 0.25μg/dL | 2.4 | 0.62 | 4.8 | 0.62 | 4.8 | 0.62 | 0.96 | 0.0 | 1.12 |
| Mercury (Hg) (μg/L) a | 0.25μg/L | 0.0 | 3.93 | 0.0 | 3.86 | 0.0 | 4.00 | 0.81 | 4.8 | 0.85 |
| Aluminum (Al) (μg/L) a | 5μg/L | 31.0 | 8.78 | 33.3 | 9.11 | 33.3 | 8.46 | 0.77 | 38.1 | 7.77 |
| Manganese (Mn) (μg/L) b | 2.5μg/L | 0.0 | 46.08 b | 0.0 | 44.52 b | 0.0 | 47.64 b | 0.58 e | 0.0 | 10.25 b |
Note: Geometric mean of four heavy metal concentrations in cord blood and childhood blood, for families who participated in the JA Kids cord blood sub-study follow up visit (n=44), those children who did not provide childhood blood samples during the follow up visit, (n=23), and those who provided both cord- and childhood- blood samples during the follow up visit, when children were 4–8 years old, n=21.
Since the distribution of blood concentrations of lead, mercury, and aluminum were not normal for cord blood and childhood blood, we reported geometric mean (Exp. [Mean (ln metal concentration)])
Since the distribution of blood manganese concentration was approximately normal for both cord blood and childhood blood, we reported arithmetic mean concentration for manganese
Two children had cord blood samples, but the amount of blood in the samples was not sufficient to be evaluated, as a result, they are missing.
P-values for comparison of geometric mean cord blood heavy metal concentrations between groups of 23 and 21 children.
P-value comparison of arithmetic mean cord blood manganese concentrations between groups of 23 and 21 children.
A comparison of median metal concentrations in cord blood and childhood blood revealed that the median difference was statistically significant for Pb = −0.27μg/dL, (P = <0.001), for Hg = 3.10μg/L, (P <0.001), and for Mn (mean difference) = 37.39μg/L, (P <0.001). Additional details are provided in Table 3.
Table 3.
Comparison of median concentrations by applying Wilcoxon signed-rank test to the difference between cord blood and childhood blood concentrations (n = 21 pairs).
| Heavy Metals | Median/Mean Cord blood | Q1 = 25th percentile | Q3 = 75th percentile | Median/Mean Childhood blood | Q1 = 25th percentile | Q3 = 75th percentile | Median/Mean difference | P value |
|---|---|---|---|---|---|---|---|---|
| Lead (Pb) (μg/dL) | 0.66 | 0.49 | 0.78 | 1.10 | 0.72 | 1.60 | −0.27 | <0.001 |
| Mercury (Hg) (μg/L) | 4.50 | 3.00 | 6.00 | 0.85 | 0.60 | 1.40 | 3.10 | <0.001 |
| Aluminium (Al) (μg/L) | 7.70 | 3.54 | 14.00 | 10.00 | 3.54 | 10.00 | 0.00 | 0.77 |
| Manganese (Mn) (μg/L) * | 47.64 | 35.00 | 63.00 | 10.25 | 8.20 | 12.00 | 37.39 | <0.001** |
Since the distribution of the difference between cord blood and childhood blood metal concentrations was not normal, we applied Wilcoxon signed-rank to test the difference.
Arithmetic means for Mn.
P-value is for the comparison of means of the blood Mn concentrations between the prenatal and childhood periods using a paired t-test.
Statistically significant correlations were found between cord blood and childhood blood concentrations of Pb (Spearman’s r = 0.45; P = 0.04) and Mn (Pearson r = 0.48; P = 0.03). The Spearman’s correlation coefficients for Al and Hg, were 0.29 (P = 0.20) and 0.08 (P = 0.72), respectively, which were not statistically significant. Furthermore, using linear regression models, a line between metal concentrations in cord blood and childhood blood was fitted and used to estimate the y-intercept. Then the significance of the y-intercept being statistically different from zero was evaluated for each heavy metal with the following results: Pb (y-intercept = −0.53μg/dL, P = 0.0001), Hg (y-intercept = 1.40μg/L, P < 0.001), Al (y-intercept = 1.48μg/L, P = 0.02), and Mn (y-intercept = 16.01μg/L, P = 0.27). Additional details are provided in Table 4.
Table 4.
Pairwise correlation coefficients between concentrations of four metals in cord blood and childhood blood as well as estimated coefficient using linear regression models (n = 21 pairs).
| Spearman correlation coefficients | Childhood blood | P value a | y-Intercept* | Slope coefficient | Intercept P-value | ||||
|---|---|---|---|---|---|---|---|---|---|
| Pb | Hg | Al | Mn | ||||||
| Cord blood | Pb | 0.45 | 0.04 | −0.53 | 0.40 | 0.0001 | |||
| Hg | 0.08 | 0.72 | 1.40 | 0.10 | < 0.001 | ||||
| Al | 0.29 | 0.20 | 1.48 | 0.32 | 0.02 | ||||
| Mn b | 0.48 | 0.03 | 16.01 | 3.09 | 0.27 | ||||
P-value for correlation coefficients.
Pearson correlation coefficient.
In linear regression analysis the equation for the model is: (Expected value of ln of cord blood metal concentrations = Intercept + β*ln of childhood blood concentrations of the same metal), For Mn the equation of the linear regression analysis model is: (Expected value of cord blood Mn concentrations = Intercept + β*childhood blood concentrations of Mn)
The findings from these GLMs indicate that the adjusted linear associations reported in the analyses of univariable associations remained significant in the multivariable models for Pb and Mn. For example, when adjusted for sex, age, or both age and sex in GLMs, the adjusted slope of the linear association between the two concentrations of Pb remained consistent and significant (i.e., when adjusted by sex (Adj. Slope coefficient = 0.52; P = 0.03); by age (Adj. Slope coefficient = 0.47; P = 0.03); and by both age and sex (Adj. Slope coefficient = 0.52; P = 0.03). The findings for Mn when adjusted for both age and sex (Adj. Slope coefficient = 0.08; P = 0.03) were also similar to those obtained after adjustment for age and sex separately as shown in Table 5.
Table 5.
Associations of Pb and Mn concentrations in cord-blood with childhood blood using general linear model (GLM) (n = 21 pairs).
| Adjusted slope of linear association | Adjusted by Sex | Adjusted by Age | Adjusted by Sex and Age | ||||
|---|---|---|---|---|---|---|---|
| Slope coefficient | P value | Slope coefficient | P value | Slope coefficient | P value | ||
| Cord blood | Pb * | 0.52 | 0.03 | 0.47 | 0.03 | 0.52 | 0.03 |
| Mn | 0.08 | 0.03 | 0.07 | 0.03 | 0.08 | 0.03 | |
Pb concentrations are based on ranks but Mn concentrations are based on actual values of concentrations
Discussion
In this study, significant correlations were found between cord blood and childhood blood Pb and Mn concentrations for children 4–8 years old in Jamaica (Spearman’s rank correlation = 0.45, P < 0.04 for Pb; Pearson correlation coefficient = 0.48, P < 0.03 for Mn). However, none of the correlations for Al and Hg were statistically significant. Rabinowitz et al. have previously reported significant Spearman’s rank correlation coefficients between cord blood Pb concentrations and Pb concentrations in childhood blood from the same children followed at birth and 6 months (r = 0.10), 6 and 12 months (r = 0.20), 12 and 18 months (r = 0.09), and 18 and 24 months (r = 0.19).[23] Therefore, findings reported here extend significant correlation between cord blood and childhood blood Pb concentrations to children 4–8 years old.
Although only cord blood concentrations of these metals were available for this study, a few other studies reported a significant correlation between concentrations of heavy metals in maternal blood and cord blood. For example, al-Saleh et al. reported a significant correlation between Pb concentrations in maternal blood and cord blood in pregnant women living in Riyadh City, Saudi Arabia, (r = 0.83, P < 0.01).[36] Srivastava et al. also reported similar findings from a study in India (r = 0.53, P < 0.01), which supports the results of al-Saleh’s study.[37] Similarly, Wang et al. conducted a study in the central part of China and reported a similar correlation between Pb concentrations in maternal blood and cord blood, (r = 0.68, P < 0.01).[38] Additionally, Arbuckle et al. showed the median Pb concentrations in maternal blood during the 1st trimester (0.60μg/dL) and 3rd trimester (0.56μg/dL) were significantly lower than those in cord blood (0.77μg/dL) in the Maternal-Infant Research on Environmental Chemicals (MIREC) study in Canada). They also showed significant Spearman’s correlations between Pb concentrations during the 1st and 3rd trimesters (r = 0.72, P < 0.01), and between maternal Pb concentrations measured in the) 3rd trimester and cord blood (r = 0.56, P < 0.01).[39] All these findings suggest potential correlation between blood Pb concentrations in the prenatal period and in children up to the age of 8 years in populations with continuous exposure to Pb from various sources such as in Jamaica, though future studies are needed to confirm this association.
Based on available literature, this study is also the first to report a significant correlation between Mn concentrations in cord blood and childhood blood (Pearson correlation coefficient = 0.48, P < 0.03) for children 4–8 years old in Jamaica. After adjustment for sex, age, and both age and sex, this association remained consistent and statistically significant. Arbuckle et al. also reported a significant Spearman’s correlations for Mn concentrations between “1st and 3rd trimesters” (r = 0.65, P < 0.01) and “3rd trimester and cord blood” (r = 0.25, P < 0.01).[39] In addition, the findings from this study suggest that the mean Mn concentration in cord blood (47.64μg/L) was significantly higher (nearly 4 times) than in childhood blood (10.25μg/L). Similarly, Arbuckle et al. reported the median Mn concentrations in maternal blood increased from the 1st trimester to the 3rd trimester (median difference = 3.3μg/L),) and the median ratio of cord blood Mn concentration to maternal blood Mn concentration was 2.5.[39] It is well-known that during pregnancy, maternal blood Mn concentrations increase due to the increased demand for micronutrients necessary for fetal growth.[39–41]
In this study, the Spearman’s correlation between Hg concentrations in cord blood and childhood blood was not statistically significant (r = 0.08; P = 0.72). Although there are no published studies that assessed the correlation of Hg concentrations in cord blood and childhood blood, some studies reported a significant correlation between Hg concentrations in maternal blood and cord blood. For example, in a study of pregnant women who lived in New York City, Lederman et al. reported a significant correlation between Hg concentrations in maternal blood and cord blood (Spearman’s r = 0.83, P < 0.01).[42]
In this study, the Spearman’s correlation between Al concentrations in cord blood and childhood blood was not statistically significant, (r = 0.29; P = 0.20). There are no published studies that assessed the correlation of the Al concentrations in cord blood and in childhood blood. Few studies have reported the levels of environmental exposure to Al in other parts of the world,[43–45] but in Jamaica due to the export of bauxite to other countries, exposure to Al could be studied further particularly among children living near bauxite mining areas.[46]
The findings in the Jamaican study of significant positive correlations between prenatal and childhood exposures to Pb and Mn have important implications for designing future studies of early life Pb and Mn exposures. Specifically, the findings could guide future investigators as to whether collection of both cord blood and childhood blood is necessary for assessment of environmental exposures to Pb and Mn. If there is a high (e.g., r ≥ 0.70) positive correlation between prenatal and childhood exposures to these metals, then one can estimate exposures to these metals during the prenatal period from those that were available for the childhood period from the ERAJ study. The findings provide greater evidence that assessment of exposure to Pb in both cord blood and childhood blood may not be necessary, though these results require replication in other populations with a larger sample size.
Limitations
There are some limitations to this study. First, although our sample size of 21 pairs of metals concentrations in cord blood and childhood blood provides at least 80% power to detect true correlations of 0.3 or higher, the sample size may not have adequate power to detect statistically significant correlations between cord blood and childhood blood metal concentrations for Al and Hg if the true correlation is less than 0.3.
Very few studies have reported any correlations between prenatal and childhood exposure to Al. Therefore, the study team has not been able to find adequate literature to discuss the findings related to Al in the context of similar studies in other settings. Hg[47] and Mn[48] are assessed more reliably from hair samples which the study team was not able to collect for this study. Therefore, findings of this study may be particularly relevant for designing future studies of continuous early life Pb[49] exposure in circumstances where there is continuous exposure to Pb in the population, and Pb concentrations can be assessed by using blood samples. More specifically, recent, serial Pb measurements may offer a better assessment of long-term and short-term exposures in the population.
Conclusions
The main findings in this study demonstrated a positive correlation between cord blood and childhood blood concentrations for Mn and Pb in Jamaican children. We also found that the median Mn concentration in cord blood was about four times that of childhood blood, possibly due to the increased demand for micronutrients necessary for fetal growth. Since blood is considered as the best biomarker for assessment of exposure to Pb, while Mn is best assessed using hair, these results may be particularly relevant for designing future studies of early life Pb exposure.
Acknowledgements
This research is co-funded by the National Institute of Environmental Health Sciences (NIEHS) by a grant (R01ES022165), as well as the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and the National Institutes of Health, Fogarty International Center (NIH-FIC) by a grant (R21HD057808) awarded to the University of Texas Health Science Center at Houston. We also acknowledge the support provided by the Child Cohort Study (JA Kids Study), supported by a grant from Inter-American Development Bank (IDB) Project Number (ATN/JF-12312-JA) awarded to the University of the West Indies, Mona Campus, in Jamaica. In addition, we acknowledge the support provided by the Biostatistics/ Epidemiology/Research Design (BERD) component of the Center for Clinical and Translational Sciences (CCTS) for this project. CCTS is mainly funded by the NIH Centers for Translational Science Award (NIH CTSA) grant (UL1 RR024148), awarded to the University of Texas Health Science Center at Houston in 2006 by National Center for Research Resources (NCRR), and its 2012 renewal (UL1 TR000371) and another 2019 grant (UL1 TR003167) by the National Center for Advancing Translational Sciences (NCATS.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NICHD, NIH-FIC, NIEHS, IDB, NCRR, or NCATS. We acknowledge that the collection and management of survey data were done using REDCap.[50] In addition, we acknowledge contributions by colleagues in the Analytical Chemistry Lab at MDHHS for analyzing and storing the cord blood and whole blood samples for the assessments of heavy metal concentrations, under a service contract.
Footnotes
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Freire C; Amaya E; Gil F; Fernandez MF; Murcia M; Llop S; Andiarena A; Aurrekoetxea J; Bustamante M; Guxens M; Ezama E; Fernandez-Tardon G; Olea N Prenatal co-exposure to neurotoxic metals and neurodevelopment in preschool children: The Environment and Childhood (INMA) Project. Sci Total Environ. 2018, 621, 340–351. [DOI] [PubMed] [Google Scholar]
- 2.Boucher O; Jacobson SW; Plusquellec P; Dewailly E; Ayotte P; Forget-Dubois N; Jacobson JL; Muckle G Prenatal methylmercury, postnatal lead exposure, and evidence of attention deficit/hyperactivity disorder among Inuit children in Arctic Quebec. Environ. Health Perspect. 2012, 120 (10), 1456–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lanphear BP The impact of toxins on the developing brain. Annu Rev Public Health 2015, 36, 211–230. [DOI] [PubMed] [Google Scholar]
- 4.Rahbar MH; Samms-Vaughan M; Lee M; Zhang J; Hessabi M; Bressler J; Bach MA; Grove ML; Shakespeare-Pellington S; Beecher C; McLaughlin W; Loveland KA Interaction between a mixture of heavy metals (lead, mercury, arsenic, cadmium, manganese, aluminum) and GSTP1, GSTT1, and GSTM1 in relation to autism spectrum disorder. Research in Autism Spectrum Disorders 2020, 79, 101681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Forns J; Fort M; Casas M; Caceres A; Guxens M; Gascon M; Garcia-Esteban R; Julvez J; Grimalt JO; Sunyer J Exposure to metals during pregnancy and neuropsychological development at the age of 4 years. Neurotoxicology 2014, 40, 16–22. [DOI] [PubMed] [Google Scholar]
- 6.Sanders AP; Claus HB; Wright RO Perinatal and Childhood Exposure to Cadmium, Manganese, and Metal Mixtures and Effects on Cognition and Behavior: A Review of Recent Literature. Curr Environ Health Rep. 2015, 2 (3), 284–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taylor CM; Kordas K; Golding J; Emond AM Effects of low-level prenatal lead exposure on child IQ at 4 and 8 years in a UK birth cohort study. Neurotoxicology 2017, 62, 162–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gorini F; Muratori F; Morales MA The Role of Heavy Metal Pollution in Neurobehavioral Disorders: a Focus on Autism. Review Journal of Autism and Developmental Disorders 2014, 1 (4), 354–372. [Google Scholar]
- 9.Li H; Li H; Li Y; Liu Y; Zhao Z Blood Mercury, Arsenic, Cadmium, and Lead in Children with Autism Spectrum Disorder. Biol. Trace Elem. Res. 2018, 181 (1), 31–37. [DOI] [PubMed] [Google Scholar]
- 10.Genuis SJ; Kelln KL Toxicant exposure and bioaccumulation: a common and potentially reversible cause of cognitive dysfunction and dementia. Behav Neurol 2015, 2015, 620143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Claus HB; Schnaas L; Ettinger AS; Schwartz J; Lamadrid-Figueroa H; Hernandez-Avila M; Amarasiriwardena C; Hu H; Bellinger DC; Wright RO; Tellez-Rojo MM Associations of early childhood manganese and lead coexposure with neurodevelopment. Environ. Health Perspect. 2012, 120 (1), 126–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rahbar MH; Samms-Vaughan M; Saroukhani S; Lee M; Zhang J; Bressler J; Hessabi M; Shakespeare-Pellington S; Grove ML; Loveland KA Interaction of Blood Manganese Concentrations with GSTT1 in Relation to Autism Spectrum Disorder in Jamaican Children. J Autism Dev. Disord. 2021, 51(6),1953–1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Exley C; Clarkson E Aluminium in human brain tissue from donors without neurodegenerative disease: A comparison with Alzheimer’s disease, multiple sclerosis and autism. Sci Rep. 2020, 10 (1), 7770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mold M; Umar D; King A; Exley C Aluminium in brain tissue in autism. J Trace Elem. Med Biol. 2018, 46, 76–82. [DOI] [PubMed] [Google Scholar]
- 15.Garrecht M; Austin DW The plausibility of a role for mercury in the etiology of autism: a cellular perspective. Toxicol. Environ. Chem. 2011, 93 (5–6), 1251–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Charman T; Jones CR; Pickles A; Simonoff E; Baird G; Happé F Defining the cognitive phenotype of autism. Brain Res 2011, 1380, 10–21. [DOI] [PubMed] [Google Scholar]
- 17.Bjørklund G; Skalny AV; Rahman MM; Dadar M; Yassa HA; Aaseth J; Chirumbolo S; Skalnaya MG; Tinkov AA Toxic metal(loid)-based pollutants and their possible role in autism spectrum disorder. Environ. Res 2018, 166, 234–250. [DOI] [PubMed] [Google Scholar]
- 18.Hsu PC; Guo YL Antioxidant nutrients and lead toxicity. Toxicology 2002, 180 (1), 33–44. [DOI] [PubMed] [Google Scholar]
- 19.Kumar V; Gill KD Oxidative stress and mitochondrial dysfunction in aluminium neurotoxicity and its amelioration: a review. Neurotoxicology 2014, 41, 154–166. [DOI] [PubMed] [Google Scholar]
- 20.Bjørklund G; Meguid NA; El-Bana MA; Tinkov AA; Saad K; Dadar M; Hemimi M; Skalny AV; Hosnedlová B; Kizek R; Osredkar J; Urbina MA; Fabjan T; El-Houfey AA; Kałużna-Czaplińska J; Gątarek P; Chirumbolo S Oxidative Stress in Autism Spectrum Disorder. Mol. Neurobiol. 2020, 57 (5), 2314–2332. [DOI] [PubMed] [Google Scholar]
- 21.Kot K; Kosik-Bogacka D; Lanocha-Arendarczyk N; Malinowski W; Szymanski S; Mularczyk M; Tomska N; Rotter I Interactions between 14 Elements in the Human Placenta, Fetal Membrane and Umbilical Cord. Int J Environ. Res Public Health 2019, 16 (9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mora AM; Arora M; Harley KG; Kogut K; Parra K; Hernandez-Bonilla D; Gunier RB; Bradman A; Smith DR; Eskenazi B Prenatal and postnatal manganese teeth levels and neurodevelopment at 7, 9, and 10.5 years in the CHAMACOS cohort. Environ. Int 2015, 84, 39–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rabinowitz M; Leviton A; Needleman H Variability of blood lead concentrations during infancy. Arch. Environ. Health 1984, 39 (2), 74–77. [DOI] [PubMed] [Google Scholar]
- 24.Sabra S; Malmqvist E; Saborit A; Gratacos E; Gomez Roig MD Heavy metals exposure levels and their correlation with different clinical forms of fetal growth restriction. PLoS One 2017, 12 (10), e0185645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rahbar MH; Samms-Vaughan M; Ardjomand-Hessabi M; Loveland KA; Dickerson AS; Chen Z; Bressler J; Shakespeare-Pellington S; Grove ML; Bloom K; Wirth J; Pearson DA; Boerwinkle E The role of drinking water sources, consumption of vegetables and seafood in relation to blood arsenic concentrations of Jamaican children with and without Autism Spectrum Disorders. Sci Total Environ. 2012, 433, 362–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rahbar MH; Samms-Vaughan M; Loveland KA; Ardjomand-Hessabi M; Chen Z; Bressler J; Shakespeare-Pellington S; Grove ML; Bloom K; Pearson DA; Lalor GC; Boerwinkle E Seafood Consumption and Blood Mercury Concentrations in Jamaican Children With and Without Autism Spectrum Disorders. Neurotoxicity Research 2013, 23 (1), 22–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rahbar MH; Samms-Vaughan M; Ma J; Bressler J; Loveland KA; Ardjomand-Hessabi M; Dickerson AS; Grove ML; Shakespeare-Pellington S; Beecher C; McLaughlin W; Boerwinkle E Role of Metabolic Genes in Blood Arsenic Concentrations of Jamaican Children with and without Autism Spectrum Disorder. Int J Environ. Res Public Health 2014, 11 (8), 7874–7895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Samms-Vaughan M Risk resilience child development-results from Jamaican birth cohort studies (JPMMS) and 2011 (JA Kids). 10–13-2014.
- 29.Rahbar MH; Samms-Vaughan M; Dickerson AS; Hessabi M; Bressler J; Coore Desai C; Shakespeare-Pellington S; Reece J-A; Morgan R; Loveland KA; Grove ML; Boerwinkle E Concentration of Lead, Mercury, Cadmium, Aluminum, Arsenic and Manganese in Umbilical Cord Blood of Jamaican Newborns. Int. J. Environ. Res. Public Health 2015, 12 (5), 4481–4501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rahbar MH; Samms-Vaughan M; Lee M; Christian MA; Bressler J; Hessabi M; Grove ML; Shakespeare-Pellington S; Coore Desai C; Reece JA; Loveland KA; Beecher C; McLaughlin W; Boerwinkle E Interaction between manganese and GSTP1 in relation to autism spectrum disorder while controlling for exposure to mixture of lead, mercury, arsenic, and cadmium. Research in Autism Spectrum Disorders 2018, 55, 50–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rahbar MH; Samms-Vaughan M; Pitcher MR; Bressler J; Hessabi M; Loveland KA; Christian MA; Grove ML; Shakespeare-Pellington S; Beecher C; McLaughlin W; Boerwinkle E Role of Metabolic Genes in Blood Aluminum Concentrations of Jamaican Children with and without Autism Spectrum Disorder. Int. J Environ Res. Public Health 2016, 13 (11), 1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bach MA; Samms-Vaughan M; Hessabi M; Bressler J; Lee M; Zhang J; Shakespeare-Pellington S; Grove ML; Loveland KA; Rahbar MH Association of polychlorinated biphenyls and organochlorine pesticides with autism spectrum disorder in Jamaican children. Research in Autism Spectrum Disorders 2020, 76, 101587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rahbar MH; Samms-Vaughan M; Hessabi M; Dickerson AS; Lee M; Bressler J; Tomechko SE; Moreno EK; Loveland KA; Desai CC; Shakespeare-Pellington S; Reece J-A; Morgan R; Geiger MJ; O’Keefe ME; Grove ML; Boerwinkle E Concentrations of Polychlorinated Biphenyls and Organochlorine Pesticides in Umbilical Cord Blood Serum of Newborns in Kingston, Jamaica. Int. J. Environ. Res. Public Health 2016, 13 (10), 1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meeker JD; Sathyanarayana S; Swan SH Phthalates and other additives in plastics: human exposure and associated health outcomes. Philos. Trans. R. Soc. Lond B Biol Sci 2009, 364 (1526), 2097–2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.SAS Institute Inc. SAS® 9.4. 2013. Cary, NC, SAS Institute Inc. [Google Scholar]
- 36.al-Saleh I; Shinwari N; Nester M; Mashhour A; Moncari L; El Din MG; Rabah A Longitudinal study of prenatal and postnatal lead exposure and early cognitive development in Al-Kharj, Saudi Arabia: a preliminary results of cord blood lead levels. J Trop. Pediatr 2008, 54 (5), 300–307. [DOI] [PubMed] [Google Scholar]
- 37.Srivastava S; Mehrotra PK; Srivastava SP; Tandon I; Siddiqui MK Blood lead and zinc in pregnant women and their offspring in intrauterine growth retardation cases. J Anal. Toxicol. 2001, 25 (6), 461–465. [DOI] [PubMed] [Google Scholar]
- 38.Wang C; Huang L; Zhou X; Xu G; Shi Q Blood lead levels of both mothers and their newborn infants in the middle part of China. Int J Hyg. Environ. Health 2004, 207 (5), 431–436. [DOI] [PubMed] [Google Scholar]
- 39.Arbuckle TE; Liang CL; Morisset AS; Fisher M; Weiler H; Cirtiu CM; Legrand M; Davis K; Ettinger AS; Fraser WD Maternal and fetal exposure to cadmium, lead, manganese and mercury: The MIREC study. Chemosphere 2016, 163, 270–282. [DOI] [PubMed] [Google Scholar]
- 40.Ashley-Martin J; Dodds L; Arbuckle TE; Ettinger AS; Shapiro GD; Fisher M; Monnier P; Morisset AS; Fraser WD; Bouchard MF Maternal and cord blood manganese (Mn) levels and birth weight: The MIREC birth cohort study. Int J Hyg. Environ. Health 2018, 221 (6), 876–882. [DOI] [PubMed] [Google Scholar]
- 41.Zota AR; Ettinger AS; Bouchard M; Amarasiriwardena CJ; Schwartz J; Hu H; Wright RO Maternal blood manganese levels and infant birth weight. Epidemiology 2009, 20 (3), 367–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lederman SA; Jones RL; Caldwell KL; Rauh V; Sheets SE; Tang D; Viswanathan S; Becker M; Stein JL; Wang RY; Perera FP Relation between cord blood mercury levels and early child development in a World Trade Center cohort. Environ. Health Perspect. 2008, 116 (8), 1085–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Agency for Toxic Substances and Disease Registry (ATSDR). Toxicological profile for Aluminum. 2008. Atlanta, GA, Agency for Toxic Substances and Disease Registry (ATSDR). 2–23-2016. [PubMed] [Google Scholar]
- 44.al-Saleh I; Shinwari N Aluminum in Saudi children. Biometals 1996, 9 (4), 385–392. [DOI] [PubMed] [Google Scholar]
- 45.Nicolescu R; Petcu C; Cordeanu A; Fabritius K; Schlumpf M; Krebs R; Kramer U; Winneke G Environmental exposure to lead, but not other neurotoxic metals, relates to core elements of ADHD in Romanian children: performance and questionnaire data. Environ Res 2010, 110 (5), 476–483. [DOI] [PubMed] [Google Scholar]
- 46.Lalor GC Geochemical mapping in Jamaica. Environmental Geochemistry and Health 1996, 18 (3), 89–97. [DOI] [PubMed] [Google Scholar]
- 47.Branco V; Caito S; Farina M; Teixeira da RJ; Aschner M; Carvalho C Biomarkers of mercury toxicity: Past, present, and future trends. J Toxicol. Environ. Health B Crit Rev 2017, 20 (3), 119–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Eastman RR; Jursa TP; Benedetti C; Lucchini RG; Smith DR Hair as a biomarker of environmental manganese exposure. Environ. Sci Technol. 2013, 47 (3), 1629–1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Barbosa F Jr.; Tanus-Santos JE; Gerlach RF; Parsons PJ A critical review of biomarkers used for monitoring human exposure to lead: advantages, limitations, and future needs. Environ. Health Perspect. 2005, 113 (12), 1669–1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Harris PA; Taylor R; Thielke R; Payne J; Gonzalez N; Conde JG Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009, 42 (2), 377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]