Abstract
Objective
Anti-Müllerian hormone (AMH) is recognized as the most important biomarker for ovarian reserve. In this cross-sectional study, we aimed to explore the potential association of AMH with central obesity or general obesity in women with polycystic ovary syndrome (PCOS).
Methods
In this cross-sectional study, 179 patients with PCOS were enrolled and underwent anthropometric measurements (BMI and waist circumference (WC)) and serum AMH level detection. Pearson’s correlation and multivariable logistic regression analyses were performed to determine the associations of AMH with central obesity and general obesity.
Results
Subjects with increasing BMI showed significantly lower values of AMH (median (interquartile range (IQR)) 8.95 (6.03–13.60) ng/mL in normal weight group, 6.57 (4.18–8.77) ng/mL in overweight group, and 6.03 (4.34–9.44) ng/mL in obesity group, P = 0.001), but higher levels of systolic blood pressure, fasting insulin, total cholesterol, triglycerides, LDL-c, obesity indices (WC, hip circumferences, waist-to-hip ratio, waist-to-height ratio (WHtR), and Chinese visceral adiposity index (CVAI)). Compared with the group of PCOS women without central obesity, the group with central obesity had significantly lower value of AMH (median (IQR) 8.56 (5.29–12.96) ng/mL vs 6.22 (4.33–8.82) ng/mL; P = 0.003). Pearson’s correlation analysis showed that AMH was significantly and negatively correlated with BMI (r = −0.280; P < 0.001), WC (r = −0.263; P < 0.001), WHtR (r = −0.273; P < 0.001), and CVAI (r = −0.211; P = 0.006). Multivariate logistic regression analysis with adjustment for potential confounding factors showed that AMH was independently and negatively associated with central obesity but was not significantly associated with general obesity.
Conclusions
AMH was independently and negatively associated with central obesity. Closely monitoring the WC and AMH should be addressed in terms of assessing ovarian reserve in women with PCOS.
Keywords: anti-Müllerian hormone, polycystic ovary syndrome, central obesity, general obesity
Introduction
Polycystic ovary syndrome (PCOS) is a highly prevalent endocrine–metabolic disorder afflicting women of reproductive age (1). It is associated with reproductive, metabolic, and psychological disorders including anovulatory infertility, obesity, type 2 diabetes mellitus (T2DM), cardiovascular diseases, and anxiety. Although not all women with PCOS have all the components of this syndrome, obesity is common in PCOS, affecting about 50–80% of patients among different studies and ethnic groups (2). Obesity can reduce the quality of life of PCOS patients by contributing to irregular menstruation, oligo/anovulation, negative effects on self-esteem and self-image (3, 4), and delayed fertility and infertility (5). Therefore, obesity has represented a major burden for women with PCOS. Weight loss has been shown to improve both the reproductive and metabolic features of PCOS (6).
Anti-Müllerian hormone (AMH) is a peptide growth factor, which belongs to the transforming growth factor β superfamily. It plays a mediator role particularly in the development of primordial follicles and the selection of follicles (7). Since serum AMH levels are proportional to the number of developing follicles in the ovaries and it differs only slightly during the menstrual cycle, serum concentration of AMH in females may represent both the quality and quantity of the ovarian follicle pool (8, 9). A large amount of data has shown that AMH levels are generally much higher in women with PCOS than in normal women. In the recent update on PCOS, the author indicated that the overall serum AMH levels are two- to three-fold higher in women with a diagnosis of PCOS than in women with normal reproductive function (2). So, there is an increasing interest in testing the level of AMH as a marker for ovarian reserve in patients with PCOS (10, 11). Various studies have explored the relationship of AMH and obesity, but reported contradictory results (6). Some of them have reported an inverse correlation between AMH levels and general (peripheral) obesity in women with PCOS (5, 12, 13), whereas others have observed a positive correlation (7) or no relationship at all (14, 15, 16). Besides, several studies have demonstrated that central (abdominal) obesity, one of the important patterns of obesity, is also closely linked to PCOS (17, 18, 19). However, regarding PCOS, studies exploring the potential association between plasma AMH and central obesity are limited.
Obesity could be generally divided into general obesity which is commonly evaluated by BMI and central obesity with waist circumference (WC) as the most common measure (20). Although imaging approaches, such as CT and MRI, are gold standards for detecting abdominal adiposity, they are relatively expensive, time-consuming, and unavailable in large populations (21). Alternatively, other anthropometric parameters such as waist-to-height ratio (WHtR) and waist-to-hip ratio (WHR) can also indicate abdominal adiposity accumulation to some degree (22). It should be noted that the Chinese visceral adiposity index (CVAI), a recently established indicator which is based on age, BMI, WC, and metabolic parameters (fasting triglycerides (TG) and HDL-c) (23), has been proven to be a reliable index for the evaluation of abdominal fat dysfunction in the Chinese population (24). The CVAI was proposed for the first time from a cross-sectional study with 485 subjects and was further validated in a study with 6495 subjects, which was strongly and positively associated with visceral fat area (r = 0.68) quantitated by CT (24). CVAI has also been considered to serve as a better predictor of metabolic syndrome (MS), T2DM, and prediabetes than BMI, WC, WHtR, and/or WHR in Chinese populations (23). To elucidate the potential association between body fat distribution and ovarian function in PCOS, we examined the relationship of AMH with central obesity and general obesity in women with PCOS.
Materials and methods
Subjects
This cross-sectional study was conducted in the Endocrinology and Diabetes Department of the First Affiliated Hospital of Xiamen University in Xiamen, China. All participants signed the informed consent forms. A total of 179 patients with PCOS were recruited. Diagnosis of PCOS was based on Rotterdam criteria with two of the following: (i) oligo- and/or anovulation, (ii) clinical and/or biochemical signs of hyperandrogenism, and (iii) polycystic ovarian morphology, defined as the presence of ≥12 follicles of 2–9 mm and/or ovarian volume of ≥10 mL in at least one ovary on transvaginal or abdominal ultrasound (25). Diagnoses were made following the exclusion of any other causes of hyperandrogenemia, such as congenital adrenal hyperplasia, androgen-secreting tumors, and Cushing’s syndrome (1, 26). The ethics committee of the First Affiliated Hospital of Xiamen Medical University approved the study protocol.
Clinical and biochemical measurements
All participants received sociodemographic questionnaires and physical examination (height, weight, blood pressure, WC, and hip circumferences (HC)) by a well-trained researcher using standardized protocols. Demographics included age, smoking, alcohol consumption, and past medical history. Height and body weight were measured by using a calibrated scale without shoes and heavy outer garments to the nearest 0.5 cm and 0.1 kg, respectively. WC was measured at the midpoint between the last palpable rib and the top of the iliac crest, and HC was measured at the level of maximal protrusion of the gluteal muscles (25, 27). Blood pressure was measured using OMRON digital sphygmomanometer, after sitting for at least 15 min. BMI was calculated as weight (kg)/ height (m2). The WHR was calculated as WC (cm) /HC (cm). WHtR was calculated as WC (cm)/ height (m). CVAI was calculated as follows: CVAI (females) = −187.32 + 1.71 × age + 4.23 × BMI + 1.12 × WC (cm) + 39.76 × Log10TG (mmol/L) −11.66 × HDL-c (mmol/L). All subjects were grouped into three BMI categories (normal weight: <24 kg/m2; overweight: 24.0–27.9 kg/m2; and obesity: ≥28 kg/m2) and two WC categories (normal weight: WC<85.0 cm and central obesity: WC≥85.0 cm) according to a Chinese Guideline (28).
Blood samples after a 12-h overnight fast were collected through antecubital vein puncture from each subject. All biochemical measurements were tested in the Central Laboratory of the First Affiliated Hospital, Xiamen University. The concentrations of total cholesterol (TC), TG, HDL-c, and LDL-c were estimated by HITACHI 7450 analyzer (Hitachi). Fasting insulin concentration was measured using electrochemiluminescence immunoassay (Roche Elecsys Insulin Test, Roche Diagnostics). Fasting blood glucose (FBG) was measured by means of the hexokinase method. Testosterone was quantified using chemiluminescent immunoassay analysis (Siemens Healthcare Diagnostics Inc, Massachusetts, USA; Siemens ADVIA centaur XP immunoassay System, Erlangen, Germany). AMH assessment was performed by electrochemiluminescence using reagents supplied by Roche Diagnostics GmbH. The theoretical sensitivity of this method is 0.01 ng/mL.
Statistical analyses
All statistical analyses were performed using SPSS version 21.0 software (IBM Corporation). Data were presented as mean ± s.d. or as median (interquartile range (IQR)) for continuous variables or as number and percentage for categorical variables. Skewness and kurtosis tests were applied for the calculation of normality. All subjects were stratified by BMI and WC. Differences among the three groups were analyzed on continuous variables using one-way ANOVA for those with normal distributions and Kruskal–Wallis test for those with skewed distributions and on categorical variables using chi-square test. Differences between two groups were analyzed on continuous variables using the Student’s t-test for those with normal distribution and Mann–Whitney U-test for those with skewed distribution and on categorical variables using chi-square test.
Pearson’s correlation analysis was performed to analyze the correlation of AMH with BMI, WC, WHtR, and CVAI. Multivariable logistic regression analysis was used to calculate the adjusted odds ratios (OR) and 95% CI of AMH for general obesity and central obesity in different models with adjustment for potential confounders. For the logistic regression analyses, model 1 was adjusted for age; systolic blood pressure (SBP) and diastolic blood pressure (DBP) were further adjusted for in model 2; FBG, TC, TG, LDL-c, HDL-c, and testosterone were further adjusted for in model 3. All P -values were two-sided, and P-value <0.05 was considered statistically significant.
Results
In the study, 179 women who met the criteria for PCOS with available AMH levels were included, of which 92 (51.39%) were diagnosed with general obesity defined as those with BMI ≥28 kg/m2 and 121 (67.59%) were diagnosed with central obesity defined as those with WC >85 cm. The median (IQR) of AMH was 6.71 (4.51–10.05) ng/mL for all subjects, the mean (±s.d.) of age was 27.9 ± 4.9-years old, and the mean (±s.d.) of BMI was 27.54 ± 4.83 kg/m2. The level of AMH decreased with the increase of BMI and WC (Tables 1 and 2).
Table 1.
Anthropometric information and biochemical characteristics in women with PCOS by BMI category.
| Normal weight, BMI <24 kg/m2 | Overweight, 24≤ BMI <28 kg/m2 | Obesity, BMI ≥28 kg/m2 | P value | |
|---|---|---|---|---|
| n | 40 | 47 | 92 | |
| Age (years) | 28.3 ± 4.0 | 26.9 ± 4.9 | 27.5 ± 5.1 | 0.438 |
| Smoking | 0 | 0 | 0 | NS |
| Occasional drinking (n, %) | 4 (10.0) | 1 (2.1) | 13 (14.1) | 0.060 |
| SBP (mmHg) | 112 ± 11 | 118 ± 12 | 122 ± 13 | <0.001b |
| DBP (mmHg) | 79 ± 9 | 80 ± 10 | 82 ± 11 | 0.205 |
| BMI (kg/m2) | 21.16 ± 2.02 | 26.07 ± 1.19 | 31.68 ± 2.95 | <0.001b |
| WC (cm) | 73.6 ± 5.9 | 87.7 ± 5.3 | 98.5 ± 8.7 | <0.001b |
| HC (cm) | 90.6 ± 7.1 | 100.7 ± 4.7 | 110.6 ± 6.0 | <0.001b |
| WHR | 0.81 ± 0.05 | 0.87 ± 0.05 | 0.89 ± 0.06 | <0.001b |
| WHtR | 0.46 ± 0.04 | 0.55 ± 0.03 | 0.61 ± 0.06 | <0.001b |
| CVAI | 27.54 ± 16.11 | 59.89 ± 15.55 | 97.86 ± 21.92 | <0.001b |
| FBG (mmol/L) | 4.81 (4.43–5.12) | 4.98 (4.67–5.45) | 4.93 (4.64–5.36) | 0.09 |
| Fasting insulin (pmol/L) | 50.36 (40.89–77.75) | 107.1 (89.45–147.28) | 145.35 (111.77–192.74) | <0.001b |
| TC (mmol/L) | 4.89 ± 1.08 | 4.94 ± 0.74 | 5.35 ± 0.85 | 0.005 |
| TG (mmol/L) | 1.12 (0.77–1.795) | 1.49 (1.02–1.96) | 1.55 (1.18–2.30) | 0.006 |
| HDL-c (mmol/L) | 1.48 (1.28–1.67) | 1.21 (1.08–1.31) | 1.2 (1.05–1.34) | <0.001b |
| LDL-c (mmol/L) | 2.37 (2.02–2.84) | 2.83 (2.31–3.36) | 2.99 (2.65–3.55) | <0.001b |
| T (ng/dL) | 42.99 (34.93–57.49) | 39.69 (31.76–46.74) | 40.63 (31.48–51.40) | 0.371 |
| AMH (ng/mL) | 8.95 (6.03–13.60) | 6.57 (4.18–8.77) | 6.03 (4.34–9.44) | 0.001a |
aP < 0.05, bP< 0.001; Values are expressed as mean ± s.d.
AMH, anti-Müllerian hormone; CVAI, Chinese visceral adiposity index; DBP, diastolic blood pressure; FBG, fasting plasma glucose; HC, hip circumference; SBP, systolic blood pressure; TC, total cholesterol; TG, triglycerides; WC, waist circumference; WHR, waist-to-hip ratio; WHtR, Waist-to-height ratio.
Table 2.
Anthropometric information and biochemical characteristics in women with PCOS by WC category.
| WC <85 cm | WC ≥85 cm | P value | |
|---|---|---|---|
| n | 58 | 121 | |
| Age (years) | 27.5 ± 4.2 | 27.6 ± 5.1 | 0.971 |
| Smoking | 0 | 0 | NS |
| Occasional drinking (n (%)) | 5 (8.6%) | 13 (10.7%) | 0.658 |
| SBP (mmHg) | 114 ± 11 | 121 ± 13 | <0.001b |
| DBP (mmHg) | 79 ± 10 | 82 ± 11 | 0.088 |
| BMI (kg/m2) | 22.82 ± 3.29 | 30.27 ± 3.56 | <0.001 |
| WC (cm) | 76.0 ± 6.2 | 96.9 ± 8.1 | <0.001b |
| HC (cm) | 93.6 ± 8.3 | 108.3 ± 6.7 | <0.001b |
| WHR | 0.81 ± 0.06 | 0.89 ± 0.05 | <0.001b |
| WHtR | 0.48 ± 0.04 | 0.60 ± 0.05 | <0.001b |
| CVAI | 36.01 ± 20.55 | 90.08 ± 24.04 | <0.001b |
| FBG (mmol/L) | 4.805 (4.44–5.1) | 4.97 (4.66–5.47) | 0.008 |
| Fasting insulin (pmol/L) | 60.53 (45.72–96.21) | 140.73 (106.61–182.87) | <0.001b |
| TC (mmol/L) | 4.87 ± 0.97 | 5.27 ± 0.84 | 0.007a |
| TG (mmol/L) | 1.15 (0.77–1.75) | 1.59 (1.21–2.23) | <0.001b |
| HDL-c (mmol/L) | 1.39 (1.15–1.61) | 1.2 (1.06–1.33) | <0.001b |
| LDL-c (mmol/L) | 2.56 (2.12–2.925) | 2.98 (2.63–3.53) | <0.001b |
| Testosterone (ng/dL) | 44.02 (35.59–57.99) | 39.31 (31.4–49.62) | 0.035a |
| AMH (ng/mL) | 8.56 (5.29–12.96) | 6.22 (4.33–8.82) | 0.003a |
aP < 0.05,bP < 0.001; Values are expressed as mean ± s.d.
AMH, anti-Müllerian hormone; CVAI, Chinese visceral adiposity index; DBP, diastolic blood pressure; FBG, fasting plasma glucose; HC, hip circumference; SBP, systolic blood pressure; TC, total cholesterol; TG, triglycerides; WC, waist circumference; WHR, waist-to-hip ratio; WHtR, Waist-to-height ratio.
Clinical characteristics of the study population
Table 1 shows the differences of anthropometric information and biochemical characteristics in PCOS women categorized by BMI. Compared with PCOS women in normal weight group, subjects in the overweight group and obesity group had significantly higher levels of SBP (112 ± 11 mmHg, 118 ± 12 mmHg, and 122 ± 13 mmHg, respectively, P < 0.001), fasting insulin (50.36 (40.89–77.75) pmol/L, 107.1 (89.45–147.28) pmol/L, and 145.35 (111.77–192.74) pmol/L, respectively, P < 0.001), TC (4.89 ± 1.08 mmol/L, 4.97 ± 0.74 mmol/L, and 5.35 ± 0.85 mmol/L, respectively, P = 0.005), TG (1.12 (0.77–1.795) mmol/L, 1.49 (1.02–1.96) mmol/L, and 1.55 (1.18–2.30) mmol/L, respectively, P = 0.006), and LDL-c (2.37 (2.02–2.84) mmol/L, 2.83 (2.31–3.36) mmol/L, and 2.99 (2.65–3.55) mmol/L, respectively, P < 0.001) and lower values for HDL-c (1.48 (1.28–1.67) mmol/L, 1.21 (1.08–1.31) mmol/L, and 1.2 (1.05–1.34) mmol/L, respectively, P < 0.001) and AMH (median (IQR) 8.95 (6.03–13.60) ng/mL, 6.57 (4.18–8.77) ng/mL, and 6.03 (4.34–9.44) ng/mL, respectively, P = 0.001). Similarly, compared to the PCOS women in the normal weight group, subjects in the overweight group and obesity group also had significantly higher levels of obesity indices such as WC (73.6 ± 5.9 cm, 87.7 ± 5.3 cm, and 98.5 ±8.7 cm, respectively, P < 0.001), HC (90.6 ± 7.1 cm, 100.7 ± 4.7 cm, and 110.6 ± 6.0 cm, respectively, P < 0.001), WHR (0.81 ± 0.05, 0.87 ± 0.05, and 0.89 ± 0.06, respectively, P < 0.001), WHtR (0.46 ± 0.04, 0.55 ± 0.03, and 0.61 ± 0.06, respectively, P < 0.001), and CVAI (27.54 ± 16.11, 59.89 ± 15.55, and 97.86 ± 21.92, respectively, P < 0.001). However, there were no statistically significant differences in age, occasional drinking, DBP, FBG, and testosterone among these three groups.
Subjects categorized by WC are presented in Table 2. There were no significant differences between the two groups in age, occasional drinking, and DBP. Compared with the group of PCOS women without central obesity, the group with central obesity had significantly greater values for SBP, FBG, fasting insulin, TC, TG, and LDL-c and lower values for HDL-c, testosterone, and AMH (median (IQR) 8.56 (5.29–12.96) ng/mL vs 6.22 (4.33–8.82) ng/mL; P = 0.003). The obesity indices such as HC (93.6 ± 8.3 cm vs 108.3 ± 6.7 cm; P < 0.001), WHR (0.81 ± 0.06 vs 0.89 ± 0.05; P < 0.001), WHtR (0.48 ± 0.04 vs 0.60 ± 0.05; P < 0.001), and CVAI (36.01 ± 20.55 vs 90.08 ± 24.04; p < 0.001) were higher in the group with central obesity than in the group without central obesity.
Correlation of AMH with obesity indices
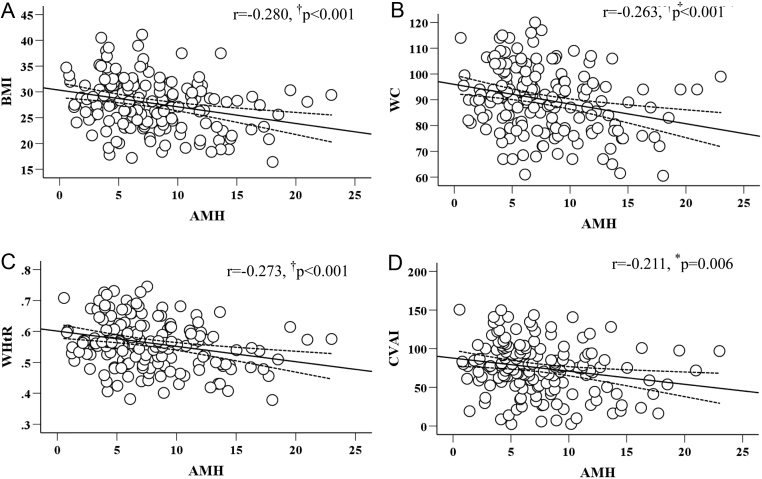
Pearson’s correlation analysis was first performed to explore the correlation between AMH and several obesity indices (BMI, WC, WHtR, and CVAI) (Fig. 1). We found that there were significant negative correlations between AMH and BMI (r = −0.280; P < 0.001), WC (r = −0.263; P < 0.001), WHtR (r = −0.273; P < 0.001), and CVAI (r = −0.211; P = 0.006).
Figure 1.
Relationship of AMH to BMI (A), WC (B), WHtR (C), and CVAI (D).
Association of AMH with central obesity and general obesity
Multivariate logistic regression analysis was performed with adjusted for different potential confounding factors to further examine the relationship of AMH with central obesity categorized by WC and general obesity defined by BMI (Table 3). In model 1 with the adjustment for age, AMH was significantly associated with central obesity, and the adjusted OR (95% CI) was 0.896 (0.831–0.966; P = 0.004). In model 2 and model 3, the association of AMH with central obesity was still statistically significant, with the adjusted OR (95% CI) of 0.883 (0.812–0.959; P = 0.003) and 0.891 (0.811–0.979; P = 0.016), respectively. However, in the three models with the same adjustments as those in multivariable logistic regression analyses, AMH was not significantly associated with general obesity.
Table 3.
Adjusted odds ratios (OR) with associated 95% CI for obesity in patients with PCOS. Model 1 was adjusted for age. Model 2 was further adjusted for SBP and DBP. Model 3 was further adjusted for TC, TG, LDL-c, HDL-c, FBG, and testosterone.
| General obesity | Central obesity | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | |
| AMH | ||||||
| Model 1 | 0.947 | 0.883–1.017 | 0.134 | 0.896 | 0.831–0.966 | 0.004a |
| Model 2 | 0.943 | 0.873–1.018 | 0.131 | 0.883 | 0.812–0.959 | 0.003a |
| Model 3 | 0.956 | 0.879–1.04 | 0.299 | 0.891 | 0.811–0.979 | 0.016a |
| Tertiles of AMH | ||||||
| Tertile 2 vs Tertile 1 | 0.596 | 0.256–1.385 | 0.229 | 0.816 | 0.296–2.25 | 0.695 |
| Tertile 3 vs Tertile 1 | 0.540 | 0.224–1.301 | 0.169 | 0.284 | 0.103–0.783 | 0.015a |
| Trend test | 0.160 | 0.015a | ||||
aP < 0.05.
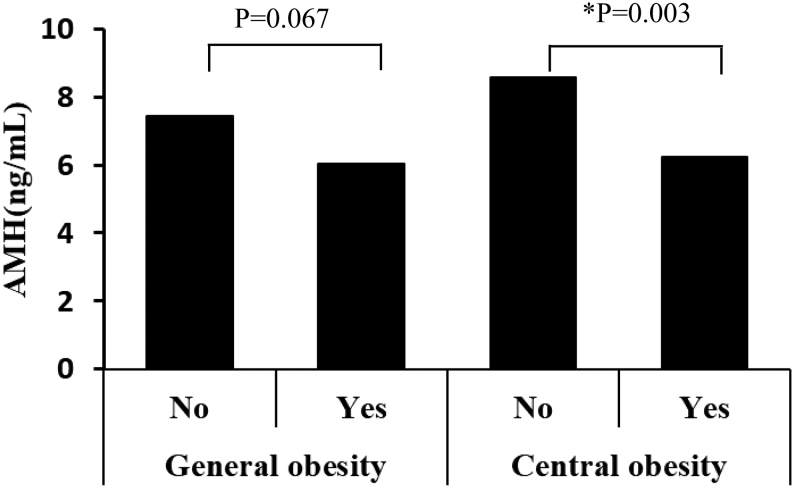
As shown in Fig. 2, AMH was significantly lower in women with central obesity than without, but showed no significant difference between general obesity and non-general obesity.
Figure 2.
Serum AMH concentrations in general obesity group and central obesity group.
Discussion
This is the first study, to the best of our knowledge, to examine the relationship of AMH with central obesity and general obesity in women with PCOS, and we found that AMH was independently and negatively associated with central obesity, but the association with general obesity was non-significant.
PCOS shares many similarities with MS in clinical features and pathophysiology. Studies showed that the prevalence of MS in PCOS population is nearly two-fold higher than age-matched control women (29). Obesity, as an essential part of MS, is a common feature in women with PCOS. Obesity has a significant impact on reproductive health, as excess body weight is the main cause for ovulatory infertility (30). Recent studies have shown serum AMH level was increasingly being recognized as the most important biomarker for ovarian reserve and was used to counsel patients regarding their reproductive outcomes (6, 9, 31). Although the relationship of AMH and obesity has been described before, findings of these studies are controversial. In one recent review, Alexis L Oldfield and colleagues (32) summarized 13 studies involving 210 obese and 550 non-obese healthy women with regular menstrual cycles, of which 5 reported decreased AMH levels with obesity, whereas 8 showed comparable AMH levels between groups. Eleni et al. (12) have observed that increasing BMI was correlated with reductions in AMH after adjusting for age, race, smoking, and site in multivariate regression models. Albu et al. (7) reported that AMH is positively correlated with BMI, especially in infertile patients younger than 35 years of normal weight and with normal ovarian reserve. Sahmay and Halawaty et al. (14, 15, 16) reported no relationship between these two markers. In the present study, we found that AMH was significantly and negatively correlated with obesity indices such as BMI, which was consistent with some previous studies (5, 13, 33). Inconsistent findings may be ascribed to the differences in the study design, populations, and sampling strategies. The mechanism underlying this inverse relationship between BMI and AMH is still unclear, but the possible explanation could be that obesity is associated with decreased ovarian reserves or that obesity is associated with follicular dysfunction (34). Therefore, the relationship of AMH level with obesity should be deemed uncertain at this time, and the biological impact of obesity on AMH production needs to be further investigated in future studies (32).
Two important patterns of obesity, which are general (peripheral) obesity and central (abdominal) obesity, are often evaluated by BMI and WC, respectively. Body fat distribution is closely related to comorbidities of obesity. But there was no difference in fat distribution by using gold standard MRI measures of visceral fat between obese and overweight women with PCOS and BMI/fat mass-matched control women (35). BMI cannot provide information on body fat distribution, while WC is an easily measurable surrogate index of abdominal fatness and a major component in diagnosing MS (18). However, there were few studies to explore the association between AMH with central obesity in patients with PCOS. In the present study, we found that AMH was associated only with central obesity but not with general obesity in women with PCOS. In parallel with our study, Sahmay et al. (14) concluded that there was no significant association between AMH and BMI-based general obesity, and the phenomenon was also seen in another study (15). The link between AMH and central obesity may be related to the following factors. First of all, central or abdominal obesity is associated with greater insulin resistance (IR) likely mediated by free fatty acids and the paracrine actions of the abdominal depot than general or peripheral obesity (30). Moreover, central obesity also worsens the insulin-related metabolic and reproductive features of PCOS (19). Mei-Jou et al. (36) speculate that IR might exert its negative effect on AMH directly or indirectly to downregulate the inhibitory effect of AMH on follicular development, thus increasing the sensitivity of granulosa cells to follicle-stimulating hormone. However, this hypothesis requires further biological research. In addition, hyperinsulinemia resulting from IR stimulates ovarian steroidogenesis and inhibits sex hormone-binding globulin production in the liver, thereby increasing the availability of free androgens. The adipose tissue also provides storage and a metabolic site for various lipid-soluble steroids, such as androgens, which contributes further to hyperandrogenism. Women with PCOS who are more overweight and obese are therefore more likely to have a worse clinical reproductive presentation (19). Accumulation of small follicles and granulosa cell proliferation due to androgen excess is the probable explanation for the association between AMH and androgen levels (37).
Our study also has some limitations. First, more accurate measurements were not performed for body composition, like CT or MRI. Secondly, since ovarian ultrasound has not been done for the participants presented with both oligo/amenorrhea and (clinical and/or biochemical) hyperandrogenism, we could not outline the accurate number of subjects in separate phenotypic subgroups of PCOS. Thirdly, the cross-sectional nature of our study disenabled us to make causal inferences. Lastly, there is no non-PCOS group in the current study, which limits the interpretation of the findings from the PCOS group. According to the recommendations from the international evidence-based guideline (38), serum AMH detection needs to improve standardization of assays and established cutoff levels for the detection of polycystic ovarian morphology (PCOM). So, it may not be necessary to measure in routine clinical practice. However, to be more accurate in the detection of PCOM, AMH measures need to be further explored based on large-scale validation in populations of different ages and ethnicities (38). Therefore, follow-up and corresponding examinations should be strengthened in future studies to obtain more accurate epidemiological data.
Conclusion
AMH was independently and negatively associated with central obesity but showed no association with general obesity. Decreased AMH production by the follicle unit may be responsible for reduced AMH with increasing WC. Closely monitoring the WC and AMH should be addressed in terms of assessing ovarian reserve in women with PCOS.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
This work was funded by Natural Science Foundation of China grant (No. 81870611), Natural Science Foundation of Fujian Province (No. 2020J011242), and Open project of State Key Laboratory of Cellular Stress Biology, Xiamen University (No. SKLCSB2019KF004).
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the Helsinki declaration and its later amendments or comparable ethical standards.
Author contribution statement
The study concept and design were framed by X Y and C L. X Z, Y H, M Z, Y C, J Y, Y H, and X Z collected data. X Z, Y H, M Z, and C L conducted the statistical data analysis and drafted the manuscript. X Y and C L contributed to discussion and revision. All authors read and approved the final manuscript.
Acknowledgement
The authors are grateful to all the subjects for their participation.
References
- 1.Escobar-Morreale HF.Polycystic ovary syndrome: definition, aetiology, diagnosis and treatment. Nature Reviews: Endocrinology 201814270–284. ( 10.1038/nrendo.2018.24) [DOI] [PubMed] [Google Scholar]
- 2.Hoeger KM, Dokras A, Piltonen T. Update on PCOS: consequences, challenges, and guiding treatment. Journal of Clinical Endocrinology and Metabolism 2021106 e1071–e1083. ( 10.1210/clinem/dgaa839) [DOI] [PubMed] [Google Scholar]
- 3.Joham AE, Palomba S, Hart R. Polycystic ovary syndrome, obesity, and pregnancy. Seminars in Reproductive Medicine 20163493–101. ( 10.1055/s-0035-1571195) [DOI] [PubMed] [Google Scholar]
- 4.Magnotti M, Futterweit W. Obesity and the polycystic ovary syndrome. Medical Clinics of North America 2007911151–116, ix–x. ( 10.1016/j.mcna.2007.06.010) [DOI] [PubMed] [Google Scholar]
- 5.Moslehi N, Shab-Bidar S, Ramezani Tehrani F, Mirmiran P, Azizi F. Is ovarian reserve associated with body mass index and obesity in reproductive aged women? A meta-analysis. Menopause 2018251046–1055. ( 10.1097/GME.0000000000001116) [DOI] [PubMed] [Google Scholar]
- 6.Chiofalo F, Ciuoli C, Formichi C, Selmi F, Forleo R, Neri O, Vuolo G, Paffetti P, Pacini F. Bariatric surgery reduces serum anti-Mullerian hormone levels in obese women with and without polycystic ovarian syndrome. Obesity Surgery 2017271750–1754. ( 10.1007/s11695-016-2528-y) [DOI] [PubMed] [Google Scholar]
- 7.Albu D, Albu A. The relationship between anti-Mullerian hormone serum level and body mass index in a large cohort of infertile patients. Endocrine 201963157–163. ( 10.1007/s12020-018-1756-4) [DOI] [PubMed] [Google Scholar]
- 8.El-Halawaty S, Rizk A, Kamal M, Aboulhassan M, Al-Sawah H, Noah O, Al-Inany H. Clinical significance of serum concentration of anti-Mullerian hormone in obese women with polycystic ovary syndrome. Reproductive Biomedicine Online 200715495–499. ( 10.1016/s1472-6483(1060379-3) [DOI] [PubMed] [Google Scholar]
- 9.Vincentelli C, Maraninchi M, Valéro R, Béliard S, Maurice F, Emungania O, Berthet B, Lombard E, Dutour A, Gaborit Bet al. One-year impact of bariatric surgery on serum anti-Mullerian-hormone levels in severely obese women. Journal of Assisted Reproduction and Genetics 2018351317–1324. ( 10.1007/s10815-018-1196-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cassar S, Teede HJ, Moran LJ, Joham AE, Harrison CL, Strauss BJ, Stepto NK. Polycystic ovary syndrome and anti-Mullerian hormone: role of insulin resistance, androgens, obesity and gonadotrophins. Clinical Endocrinology 201481899–906. ( 10.1111/cen.12557) [DOI] [PubMed] [Google Scholar]
- 11.Kim JY, Tfayli H, Michaliszyn SF, Lee S, Nasr A, Arslanian S. Anti-Mullerian hormone in obese adolescent girls with polycystic ovary syndrome. Journal of Adolescent Health 201760333–339. ( 10.1016/j.jadohealth.2016.10.015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jaswa EG, Rios JS, Cedars MI, Santoro NF, Pavone MEG, Legro RS, Huddleston HG. Increased body mass index is associated with a nondilutional reduction in antiMullerian hormone. Journal of Clinical Endocrinology and Metabolism 20201053234–3242. ( 10.1210/clinem/dgaa436) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kriseman M, Mills C, Kovanci E, Sangi-Haghpeykar H, Gibbons W. AntiMullerian hormone levels are inversely associated with body mass index (BMI) in women with polycystic ovary syndrome. Journal of Assisted Reproduction and Genetics 2015321313–1316. ( 10.1007/s10815-015-0540-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sahmay S, Usta T, Erel CT, Imamoğlu M, Küçük M, Atakul N, Seyisoğlu H. Is there any correlation between amh and obesity in premenopausal women? Archives of Gynecology and Obstetrics 2012286661–665. ( 10.1007/s00404-012-2363-x) [DOI] [PubMed] [Google Scholar]
- 15.Halawaty S, ElKattan E, Azab H, ElGhamry N, Al-Inany H. Effect of obesity on parameters of ovarian reserve in premenopausal women. Journal of Obstetrics and Gynaecology Canada 201032687–690. ( 10.1016/s1701-2163(1634573-x) [DOI] [PubMed] [Google Scholar]
- 16.Dólleman M, Verschuren WM, Eijkemans MJ, Dollé ME, Jansen EH, Broekmans FJ, van der Schouw YT. Reproductive and lifestyle determinants of anti-Mullerian hormone in a large population-based study. Journal of Clinical Endocrinology and Metabolism 2013982106–2115. ( 10.1210/jc.2012-3995) [DOI] [PubMed] [Google Scholar]
- 17.de Zegher F, López-Bermejo A, Ibáñez L. Central obesity, faster maturation, and ‘PCOS’ in girls. Trends in Endocrinology and Metabolism 201829815–818. ( 10.1016/j.tem.2018.09.005) [DOI] [PubMed] [Google Scholar]
- 18.Lim J, Han K, Kim SY, Cho YH, Yoon YS, Park HS, Yoo SJ, Kim KK. & Taskforce Team of the Obesity Fact Sheet of the Korean Society for the Study of Obesity. Effects of central obesity on maternal complications in Korean women of reproductive age. Obesity Research and Clinical Practice 201913156–163. ( 10.1016/j.orcp.2019.03.004) [DOI] [PubMed] [Google Scholar]
- 19.Lim SS, Davies MJ, Norman RJ, Moran LJ. Overweight, obesity and central obesity in women with polycystic ovary syndrome: a systematic review and meta-analysis. Human Reproduction Update 201218618–637. ( 10.1093/humupd/dms030) [DOI] [PubMed] [Google Scholar]
- 20.Zhou C, Zhan L, Yuan J, Tong X, Peng Y, Zha Y. Comparison of visceral, general and central obesity indices in the prediction of metabolic syndrome in maintenance hemodialysis patients. Eating and Weight Disorders 202025727–734. ( 10.1007/s40519-019-00678-9) [DOI] [PubMed] [Google Scholar]
- 21.Xia MF, Lin HD, Chen LY, Wu L, Ma H, Li Q, Aleteng Q, Chen Y, Sun YX, Hu Yet al. Association of visceral adiposity and its longitudinal increase with the risk of diabetes in Chinese adults: a prospective cohort study. Diabetes/Metabolism Research and Reviews 201834 e3048. ( 10.1002/dmrr.3048) [DOI] [PubMed] [Google Scholar]
- 22.Huang X, Wang Q, Liu T, Pei T, Liu D, Zhu H, Huang W. Body fat indices as effective predictors of insulin resistance in obese/non-obese polycystic ovary syndrome women in the Southwest of China. Endocrine 20196581–85. ( 10.1007/s12020-019-01912-1) [DOI] [PubMed] [Google Scholar]
- 23.Xie X, Li Q, Zhang L, Ren W. Lipid accumulation product, visceral adiposity index, and Chinese visceral adiposity index as markers of cardiometabolic risk in adult growth hormone deficiency patients: a cross-sectional study. Endocrine Practice 20182433–39. ( 10.4158/EP-2017-0007) [DOI] [PubMed] [Google Scholar]
- 24.Xia MF, Chen Y, Lin HD, Ma H, Li XM, Aleteng Q, Li Q, Wang D, Hu Y, Pan BSet al. A indicator of visceral adipose dysfunction to evaluate metabolic health in adult Chinese. Scientific Reports 20166 38214. ( 10.1038/srep38214) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tokmak A, Kokanali D, Timur H, Kuntay Kokanali M, Yilmaz N. Association between anti-Mullerian hormone and insulin resistance in non-obese adolescent females with polycystic ovary syndrome. Gynecological Endocrinology 201632926–930. ( 10.1080/09513590.2016.1193140) [DOI] [PubMed] [Google Scholar]
- 26.Lizneva D, Suturina L, Walker W, Brakta S, Gavrilova-Jordan L, Azziz R. Criteria, prevalence, and phenotypes of polycystic ovary syndrome. Fertility and Sterility 20161066–15. ( 10.1016/j.fertnstert.2016.05.003) [DOI] [PubMed] [Google Scholar]
- 27.Liang J, Wang Y, Dou L, Li H, Liu X, Qiu Q, Qi L. Neck circumference and prehypertension: the cardiometabolic risk in Chinese study. Journal of Hypertension 201533275–278. ( 10.1097/HJH.0000000000000396) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Y, Jiang Y, Wang N, Zhu M, Liu X, Wang R, Jiang F, Chen Y, Zhao Q, Zhao G. Central but not general obesity is positively associated with the risk of hyperhomocysteinemia in middle-aged women. Nutrients 201911 1614. ( 10.3390/nu11071614) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.He Y, Lu Y, Zhu Q, Wang Y, Lindheim SR, Qi J, Li X, Ding Y, Shi Y, Wei Det al. Influence of metabolic syndrome on female fertility and in vitro fertilization outcomes in PCOS women. American Journal of Obstetrics and Gynecology 2019221138.e1–138.e12. ( 10.1016/j.ajog.2019.03.011) [DOI] [PubMed] [Google Scholar]
- 30.Lim SS, Norman RJ, Davies MJ, Moran LJ. The effect of obesity on polycystic ovary syndrome: a systematic review and meta-analysis. Obesity Reviews 20131495–109. ( 10.1111/j.1467-789X.2012.01053.x) [DOI] [PubMed] [Google Scholar]
- 31.Xu H, Zhang M, Zhang H, Alpadi K, Wang L, Li R, Qiao J. Clinical applications of serum anti-Müllerian hormone measurements in both males and females: an update. Innovation 20212100091. ( 10.1016/j.xinn.2021.100091) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oldfield AL, Kazemi M, Lujan ME. Impact of obesity on anti-Mullerian hormone (AMH) levels in women of reproductive age. Journal of Clinical Medicine 202110 3192. ( 10.3390/jcm10143192) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lefebvre T, Dumont A, Pigny P, Dewailly D. Effect of obesity and its related metabolic factors on serum anti-Mullerian hormone concentrations in women with and without polycystic ovaries. Reproductive Biomedicine Online 201735325–330. ( 10.1016/j.rbmo.2017.05.013) [DOI] [PubMed] [Google Scholar]
- 34.Hwang YI, Sung NY, Koo HS, Cha SH, Park CW, Kim JY, Yang KM, Song IO, Koong MK, Kang ISet al. Can high serum anti-Mullerian hormone levels predict the phenotypes of polycystic ovary syndrome (PCOS) and metabolic disturbances in PCOS patients? Clinical and Experimental Reproductive Medicine 201340135–140. ( 10.5653/cerm.2013.40.3.135) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barber TM, Golding SJ, Alvey C, Wass JA, Karpe F, Franks S, McCarthy MI. Global adiposity rather than abnormal regional fat distribution characterizes women with polycystic ovary syndrome. Journal of Clinical Endocrinology and Metabolism 200893999–1004. ( 10.1210/jc.2007-2117) [DOI] [PubMed] [Google Scholar]
- 36.Chen MJ, Yang WS, Chen CL, Wu MY, Yang YS, Ho HN. The relationship between anti-Mullerian hormone, androgen and insulin resistance on the number of antral follicles in women with polycystic ovary syndrome. Human Reproduction 200823952–957. ( 10.1093/humrep/den015) [DOI] [PubMed] [Google Scholar]
- 37.Caglar GS, Kahyaoglu I, Pabuccu R, Demirtas S, Seker R. Anti-Mullerian hormone and insulin resistance in classic phenotype lean PCOS. Archives of Gynecology and Obstetrics 2013288905–910. ( 10.1007/s00404-013-2833-9) [DOI] [PubMed] [Google Scholar]
- 38.Teede HJ, Misso ML, Costello MF, Dokras A, Laven J, Moran L, Piltonen T, Norman RJ. & International PCOS Network. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Fertility and Sterility 2018110364–379. ( 10.1016/j.fertnstert.2018.05.004) [DOI] [PMC free article] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a