Abstract
Background
Thyroid hormone action is mediated by two forms of thyroid hormone receptors (α, β) with differential tissue distribution. Thyroid hormone receptor β (TRβ) mutations lead to resistance to thyroid hormone action in tissues predominantly expressing the β form of the receptor (pituitary, liver). This study seeks to identify the effects of mutant TRβ on pituitary size.
Methods
High-resolution 3D T1-weighted magnetic resonance images were acquired in 19 patients with RTHβ in comparison to 19 healthy matched controls. Volumetric measurements of the pituitary gland were performed independently and blinded by four different raters (two neuroradiologists, one neurologist, one neuroscientist).
Results
Patients with mutant TRβ (resistance to thyroid hormone β, RTHβ) showed elevated free tri-iodothyronine/thyroxine levels with normal thyroid-stimulating hormone levels, whereas healthy controls showed normal thyroid hormone levels. Imaging revealed smaller pituitary size in RTHβ patients in comparison to healthy controls (F(1,35) = 7.05, P = 0.012, partial η2 = 0.17).
Conclusion
RTHβ subjects have impaired sensitivity to thyroid hormones, along with decreased size of the pituitary gland.
Keywords: thyroid hormone resistance, thyroid, pituitary, thyroid hormone receptor
Introduction
Thyroid hormone receptors (TRs) mediate thyroid hormone action. They can be divided into thyroid hormone receptor alpha (TRα) and thyroid hormone receptor beta (TRβ1 and TRβ2). Mutations in encoding genes (THRA and THRB) lead to resistance to thyroid hormone action (RTH) (1, 2). Expression of these receptors is organ- and tissue-specific. TRα1 is predominantly expressed in the CNS, myocardium, skeletal muscle and gastrointestinal tract (1). TRβ1 is predominantly expressed in the liver and kidney, while TRβ2 is present in the hypothalamus, pituitary, retina and cochlea (3).
Subjects with RTHβ usually harbour heterozygous mutations in the THRB gene and may show tissue-specific symptoms reflecting local signs of hypothyroidism (i.e. growth retardation, learning difficulty, developmental delay in children, hearing impairment, nystagmus) and symptoms of hyperthyroidism (i.e. tachycardia, hyperactivity, high basal metabolic rate) (3). Subjects with homozygous mutations in the THRB gene may show developmental delay, growth and mental retardation, loss or reduced hearing ability, and colour blindness (1, 2, 3).
Diagnosis of RTHβ is difficult, since screening for thyroid malfunctioning often only includes the determination of TSH, which is generally normal, or slightly elevated in some cases. However, free thyroxine (fT4) is universally elevated (4). In RTHβ, the sensitivity of the hypothalamic–pituitary axis to thyroid hormone is reduced, due to altered signalling through TRβ2, which is predominantly expressed in the pituitary gland and hypothalamus (5).
It is known that longstanding, untreated primary hypothyroidism may lead to pituitary enlargement due to thyrotrophic hyperplasia. Thyroid-stimulating hormone (TSH)-secreting pituitary adenomas also cause hyperthyroxinaemia with non-suppressed TSH, and coexistence of TSHoma with RTHβ has been described (6, 7). The aim of the present work is to evaluate possible differences in pituitary size between 19 subjects with RTHβ and 19 healthy matched controls.
Materials and methods
Ethics statement
The institutional ethics review board of the University of Lübeck had approved all procedures prior to the study. All participants gave their written informed consent prior to their participation. The study was in accordance with the Declaration of Helsinki.
Subjects
Forty subjects were recruited for this study, 19 healthy controls and 21 patients with confirmed RTHβ due to heterozygous THRBmutations. Due to metal implants, 2 RTHβ patients had to be excluded, resulting in a group of 19 RTHβ patients, all from the UK (mean age 37.0 years, s.d. 13.7, 10 women) and 19 healthy matched controls from Lübeck, Germany (mean age 37.2 years, s.d. 13.2, 10 women). Subjects were also matched for educational school degree and after-school career, as well as height, weight and BMI (see Table 1). All procedures were conducted at the University Medical Centre Schleswig–Holstein, Campus Lübeck, Germany. The 19 RTHβ patients carried the following mutations: R320H (n = 5), R429Q (n = 3), R438H (n = 2), R383C (n = 2), M310V (n = 1), G345C (n = 1), P453S (n = 1), R243W (n = 1), T277I (n = 1), R338W (n = 1) and E460K (n = 1). Eleven patients had maternal, two paternal and six de novo inheritance. Twelve patients showed no goitre, two patients showed mild goitre, two patients had moderate goitre and one patient had large goitre secondary to high TSH and carbimazole treatment, which was stopped leading to a significantly reduced goitre size. Two patients were previously operated on elsewhere due to goitre, goitre size remained unknown. All participants underwent fasting measurements of thyroid hormones (TSH, fT4 and free tri-iodothyronine (fT3)) and lipid profiles (total, LDL and HDL cholesterol). All participants were examined by an endocrinologist. Out of the nineteen RTHβ, only one patient received propranolol, one patient received calcidol postoperatively for hypoparathyroidism, one patient received thyroxine for treatment of auto-immune hypothyroidism and one patient received atenolol for high blood pressure treatment. All other patients received no medication. The brain images were evaluated for major pathologies (e.g. stroke, tumor and signs of inflammation) by a neuroradiologist. All participants were right-handed.
Table 1.
Difference in age, height, weight, BMI between patients and controls and ratio of pituitary size to total intracranial volumes (TIV) for patients and controls. Independent t-test stated. No difference in educational degree and sex was noted, since exact matching was performed (38.1% O-level, 61.9% A-level, 10/19 (52.6%) females for both groups).
| Patients | Controls | Significance | |||
|---|---|---|---|---|---|
| Mean ratio vol/TIV (s.d.) | Mean ratio vol/TIV (s.d.) | Independent t-test | df | P | |
| Ratio of pituitary size to total intracranial volume (TIV) | 0.461 (0.085) | 0.501 (0.106) | 1.27 | 36 | 0.213 |
| Age in years, mean (s.d.) | 37.0 (13.7) | 37.2 (13.2) | 0.05 | 36 | 0.481 |
| Height in meters (m), median (s.d.) | 1.65 (0.11) | 1.69 (0.10) | 0.21 | 26 | 0.419 |
| Weight in kilograms (kg), median (s.d.) | 84.80 (19.01) | 82.50 (18.26) | −0.14 | 28 | 0.447 |
| BMI in kg/m2, median (s.d.) | 27.51 (6.58) | 27.16 (6.27) | −0.27 | 28 | 0.394 |
Blood sample analysis was performed in Cambridge, UK. For transport, the serum was separated by spinning and frozen at −80°C. TSH, fT4, fT3 and lipid profiles were measured by Advia Centaur (Siemens). The reference range for TSH was 0.35–5.5 mU/L, fT4 10–19.8 pmol/L and fT3 3.5–6.5 pmol/L.
MRI recording and analysis
MRI images were acquired using a 3.0-tesla MR scanner (Siemens Skyra; PAWP46126). High-resolution structural images were obtained with a T1-weighted 3D turbo-gradient echo sequence (192 slices, repetition time = 1900 ms, echo time = 2.44 ms, flip angle = 9°, field of view = 256 × 256 mm, voxel size 1 × 1 × 1 mm3). The volume of the pituitary gland was determined on the sagittal images, as the boundary is best defined in this orientation. The programme micron (https://www.nitrc.org/projects/mricron (8)) was used to define the region layer-by-layer with manual tracing using a mouse-guided cursor. The whole pituitary was analysed, without separating the known subdivisions of neurohypophysis and adenohypophysis within the pituitary gland, due to the imaging resolution. Fslstats from the software package fsl (9) was used to calculate the volume of the pituitary gland (in mm3) from the resulting volumes of interest (VOIs). Volumetric measurements of the pituitary gland were performed independently and blinded by four different examiners (two neuroradiologists, one neurologist, one neuroscientist). Prior to performing the volumetric analyses, the examiners agreed to the procedures during a joint session using sample brain images. For subsequent statistical analyses, the mean volume, averaged across all four raters, was calculated. Interrater agreement was calculated using the intraclass correlation coefficient (ICC) for the mean of all four raters applying a two-way mixed-effects model. According to Koo and Li (2016), the resulting kappa = 0.87 (lower to upper bound kappa = 0.8–0.92, F(37,111) = 8.0, P < 0.001) indicates a good to excellent interrater agreement. To extract the total intracranial volume (TIV) the VBM processing pipeline from the CAT toolbox (10) running under SPM 12 (v7771, https://www.fil.ion.ucl.ac.uk/spm/) and Matlab 2019b was used.
Statistics
All statistical analyses are based on R 4.0.2 using the R Project for statistical computing (https://www.R-project.org). For determining the ICC, the package psych (https://personality-project.org/r/psych/) was used, all other analyses were performed using the rstatix package (https://CRAN.R-project.org/package=rstatix). To test for the between-groups effect, a one-way ANOVA (factor levels RTHβ, control) was calculated. The potential relationship between TIV and the size of the pituitary gland per group was investigated calculating Spearman’s rho.
Results
Thyroid hormone levels
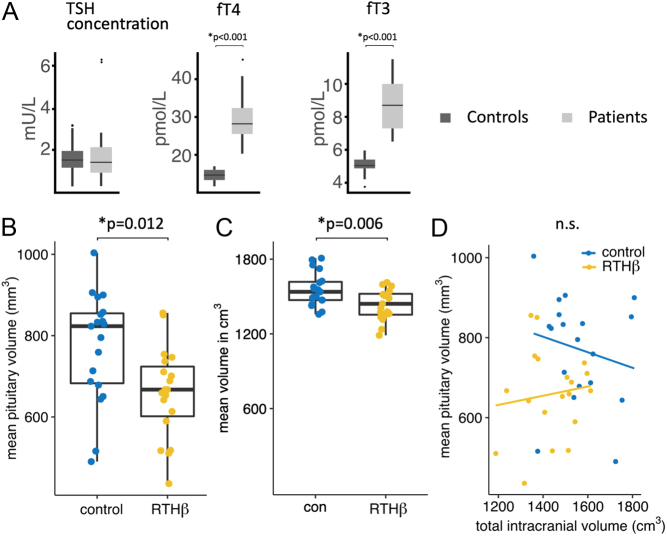
All RTHβ subjects showed increased fT3 and fT4 levels, whereas controls had normal thyroid hormone levels (see Fig. 1A). There were no significant differences for TSH for RTHβ participants vs controls, whereas significant differences were shown for fT3 (RTHβ: mean 8.6 pmol/L, s.d. 1.6 pmol/L; controls: mean: 5.1 pmol/L, s.d. 0.5 pmol/L; P< 0.001) and for fT4 (RTHβ: mean 28.4 pmol/L, s.d. 5.5 pmol/L; controls: mean: 14.6 pmol/L, s.d. 1.6 pmol/L; P< 0.001), applying a two-sample t-test.
Figure 1.
(A) Levels of TSH, fT4 and fT3 in RTHβ and healthy controls. Significant results for fT4 and fT3 are stated with no significant differences for TSH. Results are shown in box plots including mean and interquartile ranges of hormone levels. Reference range for TSH was 0.35–5.5 mU/L, fT4 10–19.8 pmol/L and fT3 3.5–6.5 pmol/L. (B) Distribution of mean pituitary volumes of controls vs patients shown by box plots including mean and interquartile range of pituitary glands (in mm3). (C) Distribution of mean total intracranial volumes of controls vs patient shown by box plots including mean and interquartile range of intracranial volumes (in cm3). (D) Scatter plot showing relationship and linear regression lines between the pituitary gland and total intracranial volumes per group. Significant results are shown, n.s., not significant.
Clinical symptoms
All subjects were examined by an endocrinologist and a neurologist with additional training in psychiatry. Out of the 19 RTHβ patients, none showed tachycardia. Other signs typical for hyperthyroidism (increased perspiration, tremors, thinning of the skin, fine brittle hair and hair loss, muscular weakness, stool frequency, weight loss, vomiting, changes in menstrual cycle) were not present. Neither did subjects experience symptoms typical for hypothyroidism (feeling cold, constipation, weight gain, shortness of breath, hoarse voice, changes of menstruation, abnormal sensation, poor hearing, dry skin, hair loss, bradycardia, swelling of the limbs, delayed relaxation of tendon reflexes, carpal tunnel syndrome). In clinical history, 9 patients reported difficulties in concentrating and 12 reported anxiety episodes. Eight patients were overweight.
Imaging results
There was a significant difference between groups in pituitary volume (F(1,36) = 7.96, P = 0.008, partial η2 = 0.18), with a smaller pituitary volume in RTHβ patients (mean volume RTHβ group: 658.8 mm3 (s.d. = 111.2), mean volume controls: 772.0 mm3 (s.d. = 134.7), see Fig. 1B). The analysis of the TIV also revealed a significant difference between groups (mean volume RTHβ group: 1435.1 cm3 (s.d.= 134.3), mean volume controls: 1556.2 cm3 (s.d.= 121.6), F(1,36) = 8.48, P = 0.006, partial η2 = 0.19, see Fig. 1C), indicating significantly reduced TIV for the RTHβ group in comparison to healthy controls. However, there was no significant linear relationship between the pituitary gland’s volume and TIV, neither for RTHβ patients nor healthy controls (RTHβ: rho = 0.12, P = 0.62; controls: rho = −0.21, P = 0.39, see Fig. 1D). We further calculated the ratio of pituitary size to TIV and tested for group differences using an independent samples t-test, revealing no significant difference between patients and controls (see Table 1). To confirm the reliability of the observation that there is no correlation between pituitary gland’s volume and TIV in healthy controls, we investigated second group of 30 healthy participants from our Centre (Center of Brain, Behavior, and Metabolism, Luebeck University), recorded with the identical imaging parameters. This sample’s mean age was 26.3, range 18–45 years, 15 men and 15 women. The analysis of this additional sample affirmed our previous observation of no significant correlation between pituitary gland size and TIV in healthy subjects (rho = −0.21, P = 0.19, data not shown). Accordingly, we did not use TIV as a covariate in the analysis of the pituitary gland’s size.
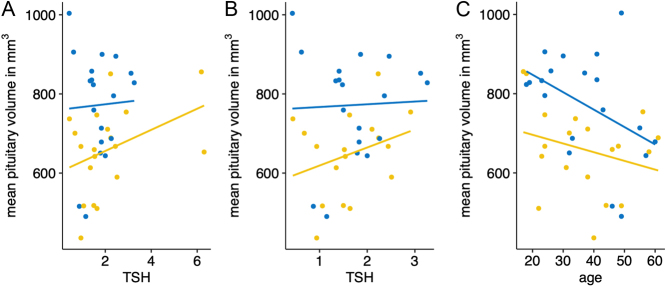
To investigate further variables influencing the pituitary gland’s volume, we tested the correlation between age or TSH level and volume per group. This revealed no association between the pituitary gland’s size and TSH level, neither for both the patient group (rho = 0.30, P = .20) nor for the controls (rho = −0.06, P = 0.81, see Fig. 2A). Even the exclusion of two patients with a TSH > 4 mU/mL does not lead to a meaningful correlation (rho = 0.26, P = 0.31, see Fig. 2B). The two subjects with increased TSH did not show significantly increased or decreased pituitary sizes in comparison to normal TSH levels (662 and 509 mm3, respectively).
Figure 2.
(A) Correlation analysis between pituitary gland size and TSH. (B) Correlation analysis after exclusion of two patients with TSH > 4 mU/mL. (C) Correlation analysis between mean pituitary gland volume and age.
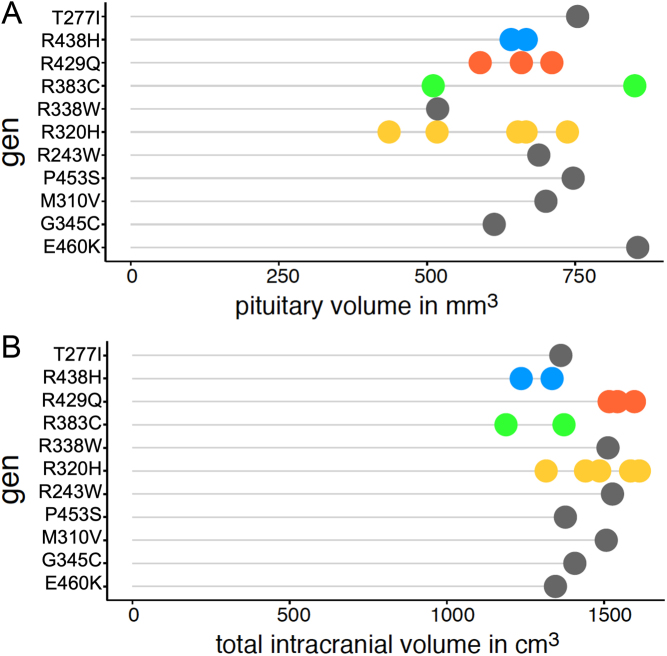
The analysis of volume and age revealed no significant correlation for the RTHß group (rho = −0.22, P = 0.37), whereas the healthy control group seems to have a faster decline of pituitary size across age and can be interpreted as a significant trend (rho = −0.44, P = 0.09) (see Fig. 2C). As can be seen from Fig. 3 the volume and TIV vary unsystematically between the individual mutations. Given the maximum number of four subjects per mutation group, a statistical test has not been performed. The mode of inheritance also does not yield a significant difference between maternal and other forms of inheritance. To test for differences, we pooled the subjects with paternal or de novo inheritance in one group and compared it to the group of subjects with maternal inheritance. Because of the low number of subjects in the paternal/de novo group, we used the parameter-free Kruskal–Wallis method to test for group differences. The test revealed no significant differences neither for pituitary volume (n = 19, H = 1.15, P = 0.283) nor for TIV (n = 19, H = 0.682, P = 0.409) (see Fig. 4).
Figure 3.
(A) Pituitary gland volume and (B) total intracranial volume (TIV) for each individual mutation. Displayed are the volumes per subject, sorted according to the respective mutation. Each genetic mutation that occurs more than once in our sample is colour- coded (see y-axis), mutations that occur only once are displayed in grey.
Figure 4.
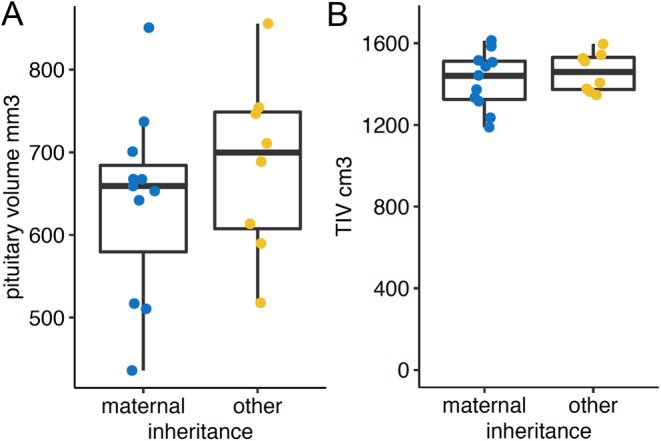
Correlation analysis between pituitary gland size (A) and total intracranial volume (TIV, B) with maternal and other (de novo, paternal) inheritance.
Discussion
Principal findings
In this study, we evaluated the pituitary sizes of RTHβ subjects in comparison to healthy controls and we found a smaller pituitary gland in RTHβ.
Findings in relation to other studies
Published data suggest a correlation between pituitary size and serum levels of TSH (11). Pituitary enlargement secondary to untreated primary hypothyroidism has been repeatedly described (12, 13, 14, 15, 16, 17, 18). Low levels of serum fT3 and fT4 cause a loss of feedback inhibition, resulting in a hypothalamic overproduction of thyrotropin-releasing hormone (TRH). This contributes to direct pituitary effects inducing hypertrophy and hyperplasia of the pituitary gland (19), which is reversible with appropriate TH replacement (20, 21).
A previous publication reported a case of RTHβ with a partially empty sella. The RTHβ subject had a proven THRB mutation, with increased fT3/4 and normal TSH. Pituitary size reduction was considered to be an incidental finding (22), but the current results suggest that pituitary size reduction is common in RTHβ. However, our study identified less a severe reduction in pituitary size, rather than an empty sella.
Literature shows that up to 15% of healthy people may have a small, clinically inapparent pituitary adenoma (6). Cases of RTHβ and incidental pituitary adenomas (23, 24) or pituitary hyperplasia (in contrast to a reduced pituitary size) (25, 26) have been reported. Regression of pituitary enlargement following thyroxine therapy in a patient with RTHbeta can occur (27). In one remarkable case, a patient with genetically confirmed RTHb (P453T mutation) underwent excision of a TSH-secreting pituitary tumor, with improvement in clinical condition and biochemistry (7, 25, 28). Many cases of RTHbeta have normal pituitary imaging (29, 30).
It is possible that defective thyroid receptor function may interfere with negative feedback of TSH leading to subsequent pituitary tumor development (6). Some cases showed pituitary enlargements in patients with slightly increased TSH (25). The reason for goitre in RTHb is likely the increase in TSH bioactivity (4). In our cohort, only two subjects showed slightly increased TSH, while all others had TSH levels within the normal range, which might explain why we did not identify any pituitary enlargement. RTHβ patients are characterized by elevated levels of thyroid hormones as well as normal or sometimes slightly elevated levels of TSH; therefore, our cohort shows fT4 and TSH levels as expected (31).
It is possible that, in our cohort with RTHβ subjects and predominantly normal TSH levels, the reduced size of the pituitary may be linked to the dysfunctional receptor, which is predominantly found in the pituitary (5). Embryonic exposure to thyroid hormone causes thyrotrope cell death (32); therefore, hereditary RTHβ could also lead to pituitary size reduction.
However, the results were surprising, since prior studies show that excessive TRH stimulation can cause pituitary enlargements due to thyrotroph and lactotroph hyperplasia (16). This is in accordance with prior studies showing RTHβ subjects with increased TSH levels and associated increase in pituitary size or adenomatous growth (7, 23, 24, 25, 28, 33). It is not known whether there is an increased frequency of pituitary adenomas in RTHbeta; reported cases may simply reflect the relatively common finding of incidentalomas seen in a healthy population. This is the first study to systematically image the pituitary gland in a group of confirmed RTHbeta patients.
Unanswered questions and future research
In the present study, we were able to observe that subjects with RTHβ have a reduced pituitary size, which has only been shown before in few case reports. Rare case reports describe an increased size of the pituitary in RTHβ. The cause for the reduced size of the pituitary in RTHβ is not clear, and more research should be applied in order to find the answer.
It remains unclear why intracranial volume is reduced in RTHβ. It may be possible that this is due to the effect of local hyperthyroidism in the brain, since TRα localized in the brain is still intact. Future research should therefore include hyperthyroid individuals as an additional control group to measure pituitary sizes.
It remains most surprising that none of the 19 individuals with RTHβ reported tachycardia. It therefore must be considered that we have studied an unusual group of individuals representing the minority of subjects with RTHβ. All of our studied subjects carried heterozygous mutations, most known heterozygous mutations are missense mutations, just like in our cohort (34). It remains unclear whether this might influence the clinical absence of tachycardia, despite high thyroid hormone concentrations. Thus, the observations and conclusions stated can possibly not be ascribed to RTHβ in general, and we therefore highly recommend that the analyses should be repeated in a more typical sample cohort.
Different results in the literature may also be explained by missing differentiation of different phenotypes of RTHβ, including generalized thyroid hormone resistance (GRTH) and pituitary thyroid hormone resistance (PRTH). GRTH indicates individuals with typical abnormal thyroid function tests and few clinical signs, whereas PRTH shows similar hormonal abnormalities with a variety of thyrotoxic features (35). Our subjects showed no significant clinical signs, therefore should be classified as GRTH. Further research will be necessary to further understand possible influences by these different phenotypes on resulting pituitary sizes.
Conclusion
This study, the first to image the pituitary gland in a cohort of confirmed RTHβ patients, identified a reduced pituitary size in patients with defective TRβ signalling in comparison to healthy controls. Given the predominant expression of TRβ in the hypothalamus and pituitary, it is possible that the change in pituitary size is mediated by reduced sensitivity to thyroid hormones in the hypothalamic–pituitary–thyroid axis. However, the results remain unexplained, since the finding of smaller pituitary size in RTH is paradoxical according to our understanding of thyroid hormone resistance.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
This work was supported by a grant of the Deutsche Forschungsgemeinschaft (MU1381 16-2) awarded to Thomas F Münte and Georg Brabant and CRC/TR 296 ‘Local control of TH action’ (LocoTact, P07) awarded to Thomas F Münte and Anna Cirkel. We acknowledge financial support by Land Schleswig-Holstein within the funding programme Open Access Publikationsfonds.
Author contribution statement
All authors met authorship criteria and participated sufficiently in the work. All authors certify that this material has not been submitted or published before. All authors confirm that all of the research meets the ethics guidelines, including adherence to the legal requirements of the country where the study was performed.
Marcus Heldmann performed the analysis and measured pituitary sizes, designed the study, discussed the results, reviewed and wrote the manuscript, revised the intellectual and technical content of the manuscript and gave final approval to the manuscript.
Krishna Chatterjee designed the study, discussed the results, reviewed and wrote the manuscript, examined and recruited the patients, revised the intellectual and technical content of the manuscript and gave final approval to the manuscript.
Carla Moran designed the study, discussed the results, reviewed and wrote the manuscript, examined and recruited the patients, revised the intellectual and technical content of the manuscript and gave final approval to the manuscript.
Berenike Rogge discussed the results, reviewed and wrote the manuscript, revised the intellectual and technical content of the manuscript and gave final approval to the manuscript. She organized the time slots of the study, drew blood, performed communication to patients in the UK, collected matched German controls, performed neurological and psychological examinations.
Julia Steinhardt discussed the results, reviewed and wrote the manuscript, revised the intellectual and technical content of the manuscript and gave final approval to the manuscript. She organized the time slots of the study, drew blood, performed communication to patients in the UK, collected matched German controls, performed neurological and psychological examinations.
Tobias Wagner-Altendorf discussed the results, reviewed and wrote the manuscript, revised the intellectual and technical content of the manuscript and gave final approval to the manuscript. He organized the time slots of the study, drew blood, performed communication to patients in the UK, collected matched German controls, performed neurological and psychological examinations.
Martin Göttlich discussed the results, reviewed and wrote the manuscript, revised the intellectual and technical content of the manuscript and gave final approval to the manuscript. He blinded the patients and controls and trained the raters to review MRIs.
Hannes Schacht performed the analysis and measured pituitary sizes, discussed the results, reviewed and wrote the manuscript, revised the intellectual and technical content of the manuscript and gave final approval to the manuscript.
Peter Schramm performed the analysis and measured pituitary sizes, discussed the results, reviewed and wrote the manuscript, revised the intellectual and technical content of the manuscript and gave final approval to the manuscript.
Georg Brabant designed and conceived the study, discussed the results, reviewed and wrote the manuscript, revised the intellectual and technical content of the manuscript and gave final approval to the manuscript.
Thomas F Münte designed and concepted the study, discussed the results, reviewed and wrote the manuscript, revised the intellectual and technical content of the manuscript and gave final approval to the manuscript.
Anna Cirkel performed the analysis and measured pituitary sizes, designed the study, wrote the first draft of the article, reviewed the manuscript, discussed the results, supervised the manuscript, revised the intellectual and technical content of the manuscript and gave final approval to the manuscript.
Acknowledgements
The authors thank the patients and their relatives for their kindness to participate and their willingness to travel and Mrs Greta Lyons, Research Nurse, University of Cambridge for her excellent support.
References
- 1.Moran C, Chatterjee K. Resistance to thyroid hormone alpha-emerging definition of a disorder of thyroid hormone action. Journal of Clinical Endocrinology and Metabolism 20161012636–2639. ( 10.1210/jc.2016-2317) [DOI] [PubMed] [Google Scholar]
- 2.Schoenmakers N, Moran C, Peeters RP, Visser T, Gurnell M, Chatterjee K. Resistance to thyroid hormone mediated by defective thyroid hormone receptor alpha. Biochimica et Biophysica Acta 201318304004–4008. ( 10.1016/j.bbagen.2013.03.018) [DOI] [PubMed] [Google Scholar]
- 3.Pappa T, Refetoff S. Human genetics of thyroid hormone receptor beta: resistance to thyroid hormone beta (RTHbeta). Methods in Molecular Biology 20181801225–240. ( 10.1007/978-1-4939-7902-8_18) [DOI] [PubMed] [Google Scholar]
- 4.Dumitrescu AM, Refetoff S. Impaired sensitivity to thyroid hormone: defects of transport, metabolism and action. In Endotext. Eds Feingold KR, Anawalt B, Boyce A, Chrousos G, de Herder WW, Dungan K, Grossmann A, Hershman JM, Hofland HJ, Kaltsas G, et al. South Dartmouth: MDText.com, 2015. (available at: https://www.ncbi.nlm.nih.gov/books/NBK285545/) [PubMed] [Google Scholar]
- 5.Abel ED, Boers ME, Pazos-Moura C, Moura E, Kaulbach H, Zakaria M, Lowell B, Radovick S, Liberman MC, Wondisford F. Divergent roles for thyroid hormone receptor beta isoforms in the endocrine axis and auditory system. Journal of Clinical Investigation 1999104291–300. ( 10.1172/JCI6397) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carvalho Cunha N, Gomes L, Bastos M. Challenging diagnosis of resistance to thyroid hormone in a patient with pituitary adenoma. BMJ Case Reports 201912 e229430. ( 10.1136/bcr-2019-229430) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Teng X, Jin T, Brent GA, Wu A, Teng W, Shan Z. A patient with a thyrotropin-secreting microadenoma and resistance to thyroid hormone (P453T). Journal of Clinical Endocrinology and Metabolism 20151002511–2514. ( 10.1210/jc.2014-3994) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rorden C, Brett M. Stereotaxic display of brain lesions. Behavioural Neurology 200012191–200. ( 10.1155/2000/421719) [DOI] [PubMed] [Google Scholar]
- 9.Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM. Fsl. Neuroimage 201262782–790. ( 10.1016/j.neuroimage.2011.09.015) [DOI] [PubMed] [Google Scholar]
- 10.Righart R, Schmidt P, Dahnke R, Biberacher V, Beer A, Buck D, Hemmer B, Kirschke JS, Zimmer C, Gaser Cet al. Volume versus surface-based cortical thickness measurements: a comparative study with healthy controls and multiple sclerosis patients. PLoS ONE 201712 e0179590. ( 10.1371/journal.pone.0179590) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamada T, Tsukui T, Ikejiri K, Yukimura Y, Kotani M. Volume of sella turcica in normal subjects and in patients with primary hypothyroidism and hyperthyroidism. Journal of Clinical Endocrinology and Metabolism 197642817–822. ( 10.1210/jcem-42-5-817) [DOI] [PubMed] [Google Scholar]
- 12.Ansari MS, Almalki MH. Primary hypothyroidism with markedly high prolactin. Frontiers in Endocrinology 20167 35. ( 10.3389/fendo.2016.00035) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Neves CP, Massolt ET, Peeters RP, Neggers SJ, de Herder WW. Pituitary hyperplasia: an uncommon presentation of a common disease. Endocrinology, Diabetes and Metabolism Case Reports 20152015150056. ( 10.1530/EDM-15-0056) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Agrawal A, Diwan SK. Pituitary hyperplasia resulting from primary hypothyroidism. Asian Journal of Neurosurgery 2011699–100. ( 10.4103/1793-5482.92171) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martinez Quintero B, Yazbeck C. Pituitary hyperplasia secondary to primary hypothyroidism. Clinical Case Reports 202081317–1318. ( 10.1002/ccr3.2863) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eiland L, Oyesiku NM, Ritchie JC, Isaacs S, Ioachimescu AG. Pathogenesis of marked pituitary enlargement and increased serum thyroid-stimulating hormone in primary hypothyroidism. Thyroid 201222101–102. ( 10.1089/thy.2011.0237) [DOI] [PubMed] [Google Scholar]
- 17.Du J, Ji H, Jin J, Gao S, Yan X, Hu S. Pituitary adenoma secondary to primary hypothyroidism: two case reports. Medicine 202099 e19222. ( 10.1097/MD.0000000000019222) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Joshi AS, Woolf PD. Pituitary hyperplasia secondary to primary hypothyroidism: a case report and review of the literature. Pituitary 2005899–103. ( 10.1007/s11102-005-3281-8) [DOI] [PubMed] [Google Scholar]
- 19.Koller KJ, Wolff RS, Warden MK, Zoeller RT. Thyroid hormones regulate levels of thyrotropin-releasing-hormone mRNA in the paraventricular nucleus. PNAS 1987847329–7333. ( 10.1073/pnas.84.20.7329) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guerrero LA, Carnovale R. Regression of pituitary tumor after thyroid replacement in primary hypothyroidism. Southern Medical Journal 198376529–531. ( 10.1097/00007611-198304000-00036) [DOI] [PubMed] [Google Scholar]
- 21.Valenta LJ, Tamkin J, Sostrin R, Elias AN, Eisenberg H. Regression of a pituitary adenoma following levothyroxine therapy of primary hypothyroidism. Fertility and Sterility 198340389–392. ( 10.1016/s0015-0282(1647307-3) [DOI] [PubMed] [Google Scholar]
- 22.Al Mohareb O, AlMalki MH, Mueller OT, Brema I. Resistance to thyroid hormone-beta co-existing with partially empty sella in a Jordanian male. Endocrinology, Diabetes and Metabolism Case Reports 2018201818-0104. ( 10.1530/EDM-18-0104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sriphrapradang C, Srichomkwun P, Refetoff S, Mamanasiri S. A novel thyroid hormone receptor beta gene mutation (G251V) in a Thai patient with resistance to thyroid hormone coexisting with pituitary incidentaloma. Thyroid 2016261804–1806. ( 10.1089/thy.2016.0450) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neamtu C, Tupea C, Paun D, Hoisescu A, Ghemigian A, Refetoff S, Sriphrapradang C. A new TRbeta mutation in resistance to thyroid hormone syndrome. Hormones 201615534–539. ( 10.14310/horm.2002.1700) [DOI] [PubMed] [Google Scholar]
- 25.Suntornlohanakul O, Sriphrapradang C. Pituitary hyperplasia mimicking thyrotropin-producing pituitary adenoma in the patient with resistance to thyroid hormone: a case report. International Journal of Neuroscience 202051–5. ( 10.1080/00207454.2020.1803304) [DOI] [PubMed] [Google Scholar]
- 26.Ando S, Sarlis NJ, Oldfield EH, Yen PM. Somatic mutation of TRbeta can cause a defect in negative regulation of TSH in a TSH-secreting pituitary tumor. Journal of Clinical Endocrinology and Metabolism 2001865572–5576. ( 10.1210/jcem.86.11.7984) [DOI] [PubMed] [Google Scholar]
- 27.Gurnell M, Rajanayagam O, Barbar I, Jones MK, Chatterjee VK. Reversible pituitary enlargement in the syndrome of resistance to thyroid hormone. Thyroid 19988679–682. ( 10.1089/thy.1998.8.679) [DOI] [PubMed] [Google Scholar]
- 28.Watanabe K, Kameya T, Yamauchi A, Yamamoto N, Kuwayama A, Takei I, Maruyama H, Saruta T. Thyrotropin-producing microadenoma associated with pituitary resistance to thyroid hormone. Journal of Clinical Endocrinology and Metabolism 1993761025–1030. ( 10.1210/jcem.76.4.8473377) [DOI] [PubMed] [Google Scholar]
- 29.Liu Z, Tsai WY, Lee CT. Resistance to thyroid hormone due to a novel THRB p.Val349Ala mutation in a Taiwanese boy. Journal of the Formosan Medical Association 20201191546–1549. ( 10.1016/j.jfma.2020.05.035) [DOI] [PubMed] [Google Scholar]
- 30.Brucker-Davis F, Skarulis MC, Grace MB, Benichou J, Hauser P, Wiggs E, Weintraub BD. Genetic and clinical features of 42 kindreds with resistance to thyroid hormone. The National Institutes of Health Prospective Study. Annals of Internal Medicine 1995123572–583. ( 10.7326/0003-4819-123-8-199510150-00002) [DOI] [PubMed] [Google Scholar]
- 31.Ortiga-Carvalho TM, Sidhaye AR, Wondisford FE. Thyroid hormone receptors and resistance to thyroid hormone disorders. Nature Reviews: Endocrinology 201410582–591. ( 10.1038/nrendo.2014.143) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tonyushkina KN, Shen MC, Ortiz-Toro T, Karlstrom RO. Embryonic exposure to excess thyroid hormone causes thyrotrope cell death. Journal of Clinical Investigation 2014124321–327. ( 10.1172/JCI70038) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Safer JD, Colan SD, Fraser LM, Wondisford FE. A pituitary tumor in a patient with thyroid hormone resistance: a diagnostic dilemma. Thyroid 200111281–291. ( 10.1089/105072501750159750) [DOI] [PubMed] [Google Scholar]
- 34.Narumi S, Cho H, Tamada I, Kozu Y, Tsuchiya T, Nagai T, Hasegawa T. One novel and two recurrent THRB mutations associated with resistance to thyroid hormone: structure-based computational mutation prediction. Clinical Pediatric Endocrinology 2010. 1991–99. ( 10.1297/cpe.19.91) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beck-Peccoz P, Chatterjee VK. The variable clinical phenotype in thyroid hormone resistance syndrome. Thyroid 19944225–232. ( 10.1089/thy.1994.4.225) [DOI] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a