Abstract
Intrauterine growth restriction combined with postnatal accelerated growth (CG-IUGR) could lead to long-term detrimental metabolic outcomes characterized by insulin resistance. As an indispensable co-receptor of Wnt signaling, LRP6 plays a critical role in the susceptibility of metabolic disorders. However, whether LRP6 is involved in the metabolic programing is still unknown. We hypothesized that CG-IUGR programed impaired insulin sensitivity through the impaired LRP6-mediated Wnt signaling in skeletal muscle. A CG-IUGR rat model was employed. The transcriptional and translational alterations of the components of the Wnt and the insulin signaling in the skeletal muscle of the male CG-IUGR rats were determined. The role of LRP6 on the insulin signaling was evaluated by shRNA knockdown or Wnt3a stimulation of LRP6. Compared with controls, the male CG-IUGR rats showed an insulin-resistant phenotype, with impaired insulin signaling and decreased expression of LRP6/β-catenin in skeletal muscle. LRP6 knockdown led to reduced expression of the IR-β/IRS-1 in C2C12 cell line, while Wnt3a-mediated LRP6 expression increased the expression of IRS-1 and IGF-1R but not IR-β in the primary muscle cells of male CG-IUGR rats. The impaired LRP6/β-catenin/IGF-1R/IRS-1 signaling is probably one of the critical mechanisms underlying the programed impaired insulin sensitivity in male CG-IUGR.
Keywords: intrauterine growth restriction, catch-up growth, insulin sensitivity, LRP6, Wnt signaling
Introduction
Accumulating evidence suggests that a disadvantageous early-life environment such as intrauterine nutritional insult is associated with adult chronic non-communicable diseases such as obesity, type 2 diabetes (T2DM), cardiovascular and cerebrovascular diseases, etc. (1, 2, 3). Intrauterine growth restriction (IUGR), caused by detrimental intrauterine environment, affects 23.8% or approximately 30 million newborns per year globally (4). In a proper environment, most of the IUGR infants will experience postnatal accelerated growth. Postnatal accelerated growth (catch-up growth), on the one hand, leads to short-term (5) and long-term health benefits, especially in cognition and academic achievement (6, 7), and on the other hand, it may exacerbate the risk of metabolic diseases in later life (7, 8, 9). A large number of studies have shown that the incidence of metabolic abnormalities including T2DM in adults with IUGR followed by postnatal accelerated growth (CG-IUGR) is much higher than that in normal people (10, 11). Insulin resistance has been considered as an early manifestation as well as a crucial mechanism for metabolic disturbance in later life of individuals with CG-IUGR (12, 13, 14, 15, 16). However, the underlying molecular pathogenesis of insulin resistance in adult CG-IUGR is still unclear.
The activation of canonical Wnt pathway begins with the binding to the frizzled receptors and its coreceptor LDL receptor-related protein 5/6 (LRP5/LRP6), resulting in the inactivation of GSK3b and the stabilization of cytoplasmic pool of β-catenin, which then enter nucleus and promote the expression of a variety of target genes including that regulate glucose homeostasis (17, 18). As a co-receptor of Wnt, LRP6 plays a critical role in the Wnt/β-catenin pathway. Genetic studies have revealed that the variations of LRP6 genes are associated with the susceptibility of a plethora of diseases, including metabolic syndrome, T2DM, cancer, etc. (19, 20, 21). Specially, several mutations of LRP6 that lead to impaired Wnt/β-catenin signaling were confirmed to be linked with impaired glucose tolerance and insulin signaling (22, 23, 24, 25). Interestingly, whether LRP6 aggravates or ameliorates insulin resistance, the key pathophysiology of T2DM, is still controversial to some extent (17, 23, 26). As a nutrient-sensitive regulator of glucose metabolism (17, 23), LRP6 possibly also plays an essential role in CG-IUGR, characterized by nutrition insults in early life. However, currently, the role of LRP6-mediated Wnt signaling in the insulin resistance of CG-IUGR is unknown.
Under normal circumstances, skeletal muscle is one of the most critical organs for maintaining glucose homeostasis (27) and is accountable for approximately 80% of insulin-mediated glucose uptake and disposal (28, 29). Thus, insulin resistance of skeletal muscle is essential for whole-body insulin sensitivity and related metabolic disorders such as T2DM (30). We previously demonstrated that rat offspring which were exposed to undernutrition in utero and postnatal overnutrition (CG-IUGR) develop aberrant growth trajectories and, as adults, a phenotype of impaired insulin sensitivity, including impaired insulin signaling in skeletal muscle (12, 13, 15, 16).
Here, using a well-established rodent model, we investigated the role of LRP6-mediated canonical Wnt signaling in the programed impaired insulin sensitivity in the skeletal muscle of male CG-IUGR rats. The transcriptional and translational alterations of certain canonical components of Wnt signaling and the insulin signaling in the skeletal muscle tissue and cells of the male CG-IUGR rats were determined. The impact of LRP6 expression, regulated by shRNA knockdown or Wnt3a stimulation, on the insulin signaling, was evaluated to further investigate the pathogenesis of metabolic programing in male CG-UGR. Our findings revealed a possible molecular mechanism linking the LRP6-mediated Wnt signaling to metabolic programming of impaired insulin sensitivity by CG-IUGR.
Materials and methods
Animal model establishment and sample collection
A CG-IUGR rat model was established by maternal nutritional restriction and litter size reduction as described in our previous studies (12, 13, 23, 26). Briefly, 15 female Sprague–Dawley rats weighing 200–250 g and 5 adult male rats were bred. Female and male rats were mated together overnight at a ratio of 2:1, and the presence of sperm in vagina was designated as day 1 of pregnancy. Pregnant rats were placed separately and randomly divided into two groups. The control group was given free access to food and water whereas the IUGR group was fed 10 g/day of the same rodent chow (about 33% of normal intake). Mothers of both groups were fed ad libitum once the rat pups were delivered. In the experiments, there were seven pregnant rats in the control group and eight in the IUGR group, and there were one pregnant rat that did not give birth of each group, one dam of the control group and two of the IUGR group that ate their babies. We excluded the dams those ate their babies, and finally included five dams in each group. The average litter size, sex ratio, birth weight and litter weight in both groups were similar. The litter size was reduced to five pups/litter at birth in the CG-IUGR group while eight pups/litter in the control group (dams = 5) to ensure postnatal accelerated growth of the rats with IUGR (dams = 5) and thus to establish the model with both IUGR and catch-up growth. BMI was calculated using the formula: weight (kg) divided by the square of height in meters. For measuring the body length of rats, the rat was put on the surface of fixed iron bars, one person held the tail of the rat, the rat's body would then be in a straight line as the rat attempted to pull away, meanwhile, another person used a ruler to measure the length from the snout to the hip of the rat. The above procedures were repeated three times for each measurement and the average value was calculated.
Considered that the programmed impairments are generally more common in male offspring than females with IUGR (31), after weaning at day 21, male rats were randomly selected as the research objects (2 pups each litter, ≥5 male rats for each group) and were placed two in one cage with free access to food and water. At the 8th week, the rats were fasted for 16 h, and then intraperitoneal glucose tolerance test (IPGTT) was performed: blood samples were collected from tail-vein before and after intraperitoneal injection of 20% glucose (2 g/kg) following standard protocols. After adaptive feeding for 1 week following IPGTT, rats were fasted overnight; thereafter, rats were killed by anesthesia before obtaining skeletal muscle (quadriceps femoris), which were immediately frozen in liquid nitrogen and then stored in −80°C freezer. Blood samples were drawn from the major abdominal vein and centrifuged at 4°C. Glucose was measured within 2 h of sample collection, and the other portion of plasma was stored at −80°C immediately after the centrifugation. All animal studies were approved by the ethical review committee of Tongji Medical College, Huazhong University of Science and Technology, and followed the regulations of the National Institutes of Health guidelines on the care and welfare of laboratory animals.
Plasma insulin and glucose measurement
Fasting plasma insulin was measured using an ELISA kit (CUSABIO, Wuhan, China) according to the manufacturers’ instructions. Glucose was measured using blood glucose test strips (Byer, Leverkusen, Germany) following the manufacturers’ instructions. AUCs of IPGTT were determined by trapezoidal approximation of glucose levels. Glucose concentrations at x min were defined as G(x), and AUC was assessed as follows: AUC (mmol/L·min) = (G (0) × 15 + G (15) × 30 + G (30) × 45 + G (60) × 90 + G(120) × 60)/2. The insulin resistance index (HOMA-IR) was calculated as fasting blood glucose level (mmol/L) × fasting insulin level (μIU/mL)/22.5.
C2C12 cell culture and shRNA lentiviral particles transduction
C2C12 cells were obtained from the American Type Culture Collection and cultured in DMEM supplemented with 10% FBS and 100 μg/mL penicillin/streptomycin following standard protocol. Cells were seeded in a 12-well plate, and after 24 h, the cells were infected at a confluence of approximately 50% by adding 1 × 106 LRP6 shRNA(m) (sc-37234-v) or control shRNA (sc-108080) with 5 μg/mL polybrene (sc-134220) in 1 mL complete optimal medium. After incubation for 24 h, the above medium was removed and replaced with 1 mL of complete medium without polybrene. Split the cells 1:5 to select stable clones expressing the shRNA through puromycin dihydrochloride (6 μg/mL, sc-108071) selection. Replaced the medium with fresh puromycin-containing medium every 2 days, until resistant colonies were identified. The puromycin-resistant colonies were expanded and assayed for stable shRNA expression. LRP6 shRNA, control shRNA, polybrene and puromycin dihydrochloride were purchased from Santa Cruz Biotechnology Inc (TX).
Primary skeletal muscle cell culture and wnt3a intervention
Skeletal muscle cells of rats were cultured using tissue explants adherent method as described in our previous study (12). Quadriceps femoris samples were sliced into 1 mm3 fragments in the cell culture medium, and the tissues were incubated and dry for 3 h at 37°C in a 5% humidified CO2-controlled atmosphere. The culture medium was gently added to prevent the tissue from floating, and the culture medium was changed every 3 days. After about 5–7 days, the primary skeletal muscle cells can be observed to climb out of the tissue and basically fill the culture bottle. Dulbecco’s modified Eagle’s medium (DMEM; Gibco), 10% heat-inactivated fetal bovine serum (Every Green, Hangzhou, China), 100 U/mL penicillin and 100 μg/mL streptomycin were added. When cells reached 70–80% confluence, cultures were digested with 2% trypsin (Gibco) and seeded in six-well plates. After 12 h of starvation with serum-free medium, cells were stimulated with recombinant Wnt3a (R&D) of 30 ng/mL for different time span as described previously (23). Subsequently, the cells were harvested for analysis.
Western blot
The proteins of skeletal muscle tissue were extracted with ice-cold radioimmunoprecipitation assay lysis buffer and the protein concentrations were measured using BCA assay kit (Beyotime, Nantong, China). Equal amounts of proteins were fractionated on a 10% SDS-polyacrylamide gel with a 5% stacking gel and electroblotted onto polyvinylidene difluoride (PVDF) membranes (Millipore). The membranes were blocked in 5% non-fat milk for 1 h at room temperature and then incubated with primary antibodies including LRP6 (1:1000 dilution; Abcam), β-catenin (1:2000 dilution; Abcam), P70S6K (1:2000 dilution; Abcam), IGF-1R (1:500 dilution; Abcam), IR (1:1000 dilution; Cell Signaling Technology), IRS-1 (1:500 dilution; Cell Signaling Technology) and mTOR (1:500 dilution; Cell Signaling Technology) overnight at 4°C. The membranes were washed for 5 min with 0.1% TBS-Tween-20 solution and repeated for three times. Thereafter, the membranes were probed with secondary horseradish-peroxidase-conjugated IgG (1:10,000 dilution; Aspen, Wuhan, China) for 2 h at room temperature. The membranes were washed with ECL solution for 5 min and repeated for five times, followed by chemiluminescence detection in the dark room.
Real-time PCR
Total RNA was isolated with TRIzol reagent (Invitrogen) according to the manufactures’ protocol. After the measurement of RNA quantity and quality on an UV arectrophotometer (Eppendorf, Hamburg, Germany), RNA was reverse-transcribed into cDNA using PrimeScriptTM RT Master Mix (TaKaRa). Relative quantitative PCR (qPCR) was performed on a CFX96 Touch PCR (Bio-Rad) using SYBR® Premix Ex TaqTM (TaKaRa) following standard protocol. Sequence-specific PCR primers were designed using Primer Premier 5.0 software (PREMIER Biosoft International, CA) and synthesized by Servicebio (Wuhan, China). The specificity of primers was checked in Primer-BLAST. Primers sequences were as follow: β-actin sense 5’-TGCTATGTTGCCCTAGACTTCG-3’, antisense 5’-GTTGGCATAGAGGTCTTTACGG-3’; LRP6 sense 5’-ATCGGATAGAAGTGACAAGGCTC-3’, antisense 5’-CTTGTCTTCCACCAGCACTCG-3’; β-catenin sense 5’-ATTACGACAGACTGCCTTCAGATC-3’, antisense 5’-GAGCAGACAGACAGCACCTTCA-3’; IR sense 5’-GTCGCTCCTATGCTCTGGTGT-3’, antisense 5’-TTCTGGTTGTCCAAGGCGTAG-3’; IRS-1 sense 5’-GGCGAGTTCTGGATGCAAGTG-3’, antisense 5’-GCGGAGGATTGTTGAGATGGT-3’. β-actin was amplified as an internal control. Relative gene expression was calculated according to the comparative threshold cycle (2−ΔΔCt) method.
Statistical analysis
Data were expressed as mean ± s.e.m. The differences between two groups were ascertained by an independent-sample t-test using IBM SPSS Statistics Version 22 (SPSS) and the GraphPad Prism version 7.0 software. The level of statistical significance was defined as *P < 0.05 (two-tailed).
Results
CG-IUGR model and systemic insulin sensitivity
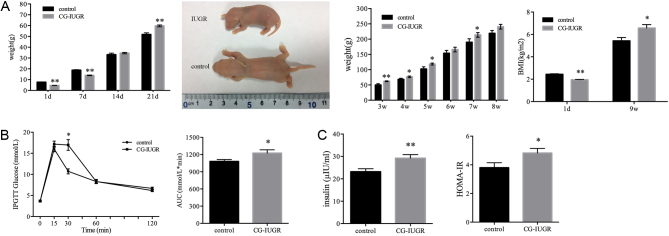
Consistent with our previous results (12, 13, 15, 16), the birth weight of the IUGR pups (n = 25) was significantly lower than the control group (n = 40) (4.62 ± 0.35 g vs 7.68 ± 0.93 g, P < 0.01, Fig. 1A). The IUGR pups showed an accelerated weight gain from postnatal day 7 and caught up with the control group on day 14, and the weight of male CG-IUGR rats was significantly higher than the control group on day 21 (59.74 ± 4.48 g vs 51.85 ± 9.08 g, P < 0.01, Fig. 1A). Thereafter, the weight of the male CG-IUGR rats kept increasing. The BMI of the male CG-IUGR group was lower at birth and higher at the 9th week than that of the control group (n = 6 for each group, Fig. 1A).
Figure 1.
CG-IUGR model and systemic insulin sensitivity. (A) Body weight and BMI between control and CGIUGR rats (n = 25 for IUGR and n = 40 for the control group before weaning, n = 6 for IUGR and the control group after weaning (two pups each litter)). (B) Blood glucose at 30 min and AUC of IPGTT were increased in the male CG-IUGR rats (n = 6 for each group). (C) Plasma insulin levels and HOMA-IR were elevated in male CG-IUGR rats (n = 6 for each group). Data are presented as mean ± s.e.m. *P < 0.05, **P < 0.01 compared with same-age controls.
IPGTT performed at the 8th week showed that the glucose at 30 min was higher in male CG-IUGR rats than the control group, and no significant differences were observed at the other time points; besides, glucose AUC of IPGTT in male CG-IUGR rats was increased than the normal control (n = 6, Fig. 1B), indicating of impaired glucose tolerance (32). Moreover, fasting plasma insulin level and HOMA-IR were elevated in male CG-IUGR rats in comparison with the control group (n = 6, Fig. 1C). These results suggested that the systemic insulin sensitivity of male CG-IUGR was decreased.
Insulin signaling was impaired in skeletal muscle of male CG-IUGR
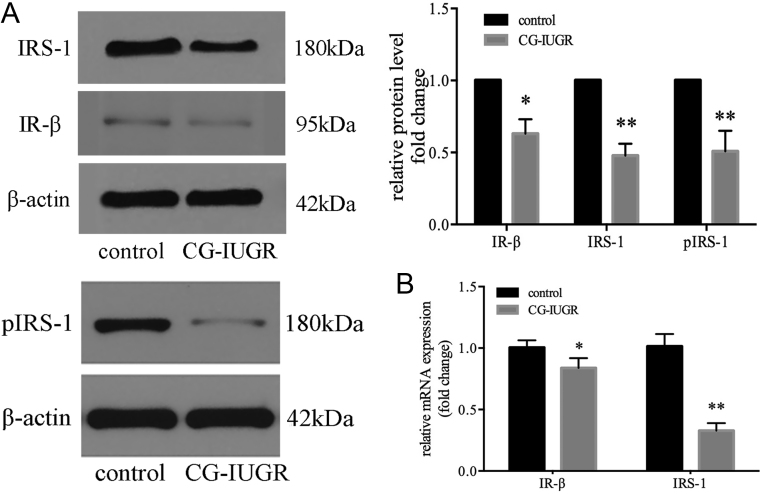
To investigate the insulin signaling in skeletal muscle of male CG-IUGR, the protein and mRNA level of insulin receptor (IR)-β and insulin receptor substrate (IRS)-1, two canonical components of insulin signal pathway, was determined. Similar to our previous studies (12), the protein levels as well as the mRNA levels of IR-β and IRS-1 were reduced in the male CG-IUGR rats (Fig. 2A and B), indicating impaired insulin signaling.
Figure 2.
Insulin signaling was impaired in the skeletal muscle of male CG-IUGR. The protein (A) and mRNA (B) levels of insulin receptor (IR)-β and insulin receptor substrate (IRS)-1 in skeletal muscle were reduced in the male CG-IUGR rats. Data are presented as mean ± s.e.m. (n = 5). *P < 0.05, **P < 0.01 compared with same-age controls.
The expression of LRP6 was decreased in skeletal muscle of male CG-IUGR
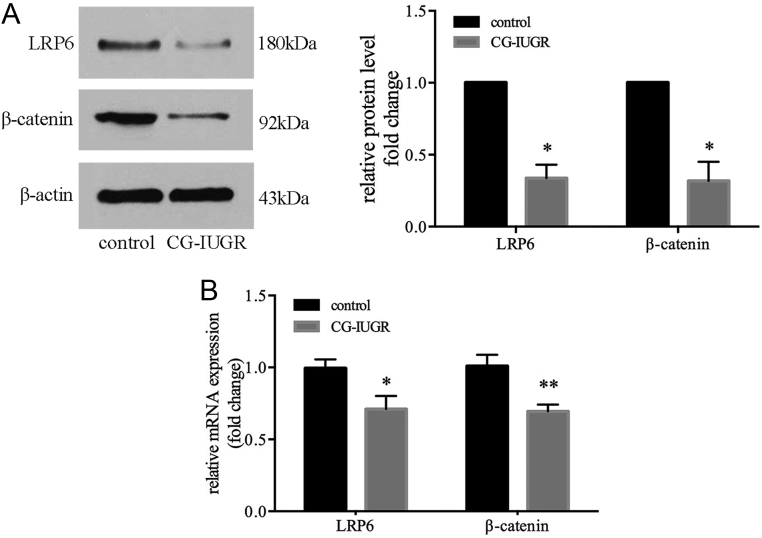
Meanwhile, the expression of LRP6 and downstream factor β-catenin in skeletal muscle was assessed. The protein and mRNA levels of LRP6 and β-catenin in male CG-IUGR were significantly lower than that of control group (Fig. 3A and B).
Figure 3.
The expression of LRP6 was decreased in the skeletal muscle of male CG-IUGR. The protein (A) and mRNA (B) levels of LRP6 and β-catenin in skeletal muscle were reduced in the male CG-IUGR rats. Data are presented as mean ± s.e.m. (n = 5). *P < 0.05, **P < 0.01 compared with same-age controls.
LRP6 is requisite for normal expression of the IR-β/IRS-1
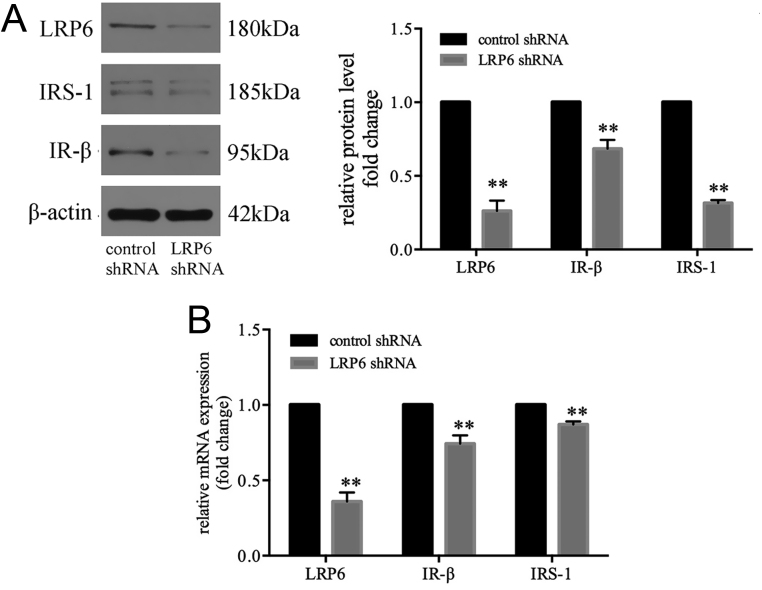
To explore the role of LRP6 in insulin signaling, LRP6 was knocked down by RNA interference in C2C12 cells. LRP6-specific shRNA markedly decreased the protein and mRNA expression of LRP6 (Fig. 4A and B). Correspondingly, the mRNA and protein levels of IR-β and IRS-1 were significantly reduced in the LRP6 knockdown group (Fig. 4A and B). These findings suggest that LRP6 is requisite for normal expression of the IR-β/IRS-1 and may play a critical role in maintaining intact insulin signaling in muscle cells.
Figure 4.
LRP6 is requisite for normal expression of the IR-β/IRS-1. LRP6 specific shRNA decreased the protein (A) and mRNA (B) expression of LRP6, IR-β and IRS-1 in C2C12 cells. Data are presented as mean ±s.e.m. (n = 3). **P < 0.01 compared with same-age controls.
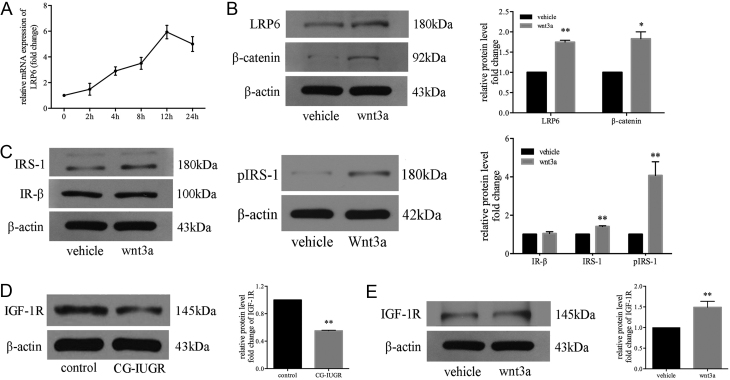
Wnt3a/LRP6 enhanced the expression of IRS-1 and IGF-1R in muscle cells of male CG-IUGR
LRP6 is the critical mediator of Wnt3a signaling (33). Since silencing of LRP6 significantly compromised the expression of IR/IRS-1 in C2C12 cells, to further examine the impact of Wnt/LRP6 signaling on the insulin signaling in the skeletal muscle of male CG-IUGR, primary muscle cells of male CG-IUGR rats were treated with Wnt3a at a concentration of 30 ng/mL for different time spans as described previously (27). We observed that the mRNA expression of LRP6 kept increasing until it reached a maximum expression at the 12th hour (Fig. 5A). Upon Wnt3a stimulation for 12 h, the protein expression levels of LRP6, β-catenin and IRS-1 were significantly increased in primary muscle cells of male CG-IUGR rats (Fig. 5B and C). Interestingly, the expression of IR-β was not altered (Fig. 5C). Considering that the insulin-like growth factor-1 receptor (IGF-1R) could also be co-activated with the IR-β by insulin (34), we then analyzed the expression of IGF-1R and found that the protein levels of IGF-1R were significantly reduced in the skeletal muscle of male CG-IUGR (Fig. 5D); moreover, the expression of IGF-1R was rescued upon Wnt-3a stimulation in the primary muscle cells of male CG-IUGR (Fig. 5E).
Figure 5.
Wnt3a/LRP6 enhanced the expression of IRS-1 and IGF-1R in muscle cells of male CG-IUGR. (A) The curve of LRP6 mRNA expression in primary muscle cells treated with Wnt3a (30 ng/mL) for different time spans in male CG-IUGR rats. (B and C) Upon Wnt3a stimulation for 12 h, the protein levels of LRP6 (B), β-catenin (B) and IRS-1 (C) were increased in the primary muscle cells of male CG-IUGR rats and the expression of IR-β (C) was not altered. (D) The expression of IGF-1R was reduced in the skeletal muscle of male CG-IUGR rats. (E) The expression of IGF-1R was increased upon Wnt-3a stimulation in the primary muscle cells of male CG-IUGR. Data are presented as mean ± s.e.m. (n = 3). **P < 0.01 compared with same age controls.
Wnt3a/LRP6 increased the expression of mTOR/S6K in muscle cells of male CG-IUGR
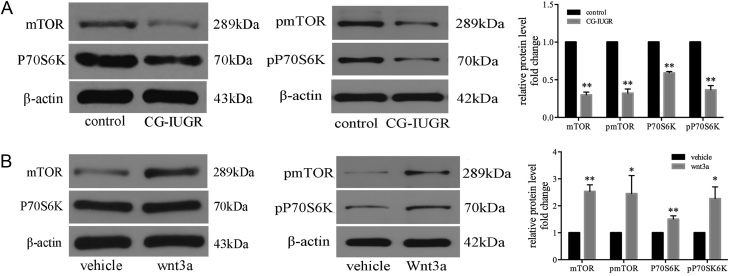
Considering that genetic as well as biochemical approaches have independently confirmed that mTOR/S6K plays a novel and essential role in the regulation of cell autonomous insulin/IGF-I sensitivity through affecting IRS-1 function (35), we also analyzed the expression of mTOR/S6K in the skeletal muscle of male CG-IUGR. We found that the protein levels of mTOR and P70S6K were significantly decreased in the skeletal muscle of male CG-IUGR (Fig. 6A). Furthermore, the expression of mTOR and P70S6K was rescued upon Wnt-3a stimulation in the primary muscle cells of male CG-IUGR (Fig. 6B).
Figure 6.
Wnt3a/LRP6 increased the expression of mTOR/S6K in skeletal muscle cells of male CG-IUGR rats. (A) The protein levels of mTOR and P70S6K were decreased in skeletal muscle of male CG-IUGR rats (n = 5). (B) The expression of mTOR and P70S6K was increased upon stimulating with Wnt3a (30 ng/mL) for 12 h in skeletal muscle cells of male CG-IUGR rats.
Discussion
The ‘developmental origins of adult disease’ hypothesis suggests that environmental insults occurring during the pivotal period of life are detrimental, leading to the adult onset of diseases (1). Moreover, nutritional insults occurring during prenatal life combined with early postnatal life may unmask or amplify the underlying defects culminating in adult diseases. Numerous studies have concluded that IUGR individuals, especially those with postnatal accelerated growth, are prone to develop insulin resistance and T2DM in adulthood (7, 8, 9, 14). Interestingly, it has been demonstrated that programmed impairments are more common in male offspring than females with IUGR. Generally, the male IUGR offspring will probably develop symptoms in later life despite living a healthy lifestyle; while the female fetuses could adapt well to the intrauterine disturbances and modify their developmental strategy in response to the altered environment, and the programmed diseases of the female IUGR could be unmasked in adult life after a ‘second hit’ such as excessive consumption of high‐fat die, living a sedentary lifestyle, etc. (31, 36). This is probably partially due to the different patterns in regulating placenta growth in the male and female fetus (36, 37, 38). In the current study, the male IUGR-CG adult rats presented an impaired insulin-sensitivite phenotype including higher BMI, fasting insulin level, HOMA-IR, glucose level of IPGTT (30 min) and AUC, indicative of impaired systemic insulin sensitivity. Furthermore, the impaired insulin sensitivity of skeletal muscle manifested as reduced expression of canonical components of insulin signaling. These findings are similar to the previous animal studies that IUGR with postnatal catch-up growth (CG-IUGR) programs impaired insulin sensitivity in adulthood (12, 13, 14, 15, 16).
The insulin receptor (IR) and insulin receptor substrate (IRS) proteins-mediated signaling is responsible for most of the metabolic actions of insulin (34), and dysregulated signaling of IRS-1 is a common underlying pathogenesis of insulin resistance (29, 39). Severe insulin resistance in skeletal muscle with a compensatory β-cell hyperplasia can be observed in IR/IRS-1+/− mice (40). And IRS-1−/− mice display peripheral insulin resistance that could be ascribed mainly to a remarkable decrease in insulin-stimulated glycogen synthesis in skeletal muscle (41). In this study, the expression of IR-β and IRS-1 in the skeletal muscle of male CG-IUGR rats was markedly reduced, indicative of impaired insulin signaling. This probably contributed to the programming of the whole-body impaired insulin sensitivity, which was consistent with human studies that the decreased expression of IRS-1 was a common underlying mechanism of insulin resistance (29, 42).
The canonical Wnt signaling pathway is highly conserved in evolution and directly controls the expression of a large number of genes related to growth and metabolism (43). LRP6 is an indispensable co-receptor of canonical Wnt signaling. Genetic studies of kindreds with severe manifestation of metabolic syndrome have led to the identification of rare pathogenic mutations in LRP6 and subsequently revealed that the Wnt/LRP6 axis was a regulator of glucose metabolism (22, 23, 24, 25). For example, Singh et al. found that a loss-of-function mutation of LRP6 impaired glucose tolerance and the IR/IRS-1-mediated insulin signaling (23). In the current study, we found that the expression of LRP6 was decreased in the skeletal muscle of the impaired insulin-sensitivite male CG-IUGR rats compared with the normal group. Moreover, consistent with the previous studies (22, 23), an RNA interference-mediated knockdown of LRP6 impaired Wnt/β-catenin signaling and strikingly reduced the expression of IR-β and IRS-1 in C2C12 cells, indicating that LRP6 mediated Wnt signaling was requisite for normal expression of the IR-β and IRS-1, as well as maintaining intact insulin signaling in skeletal muscle. Interestingly, mice heterozygous for LRP6 are shown to be less predisposed to diet-induced obesity and insulin resistance (17), and Li et al. showed that LRP6 knockdown ameliorates insulin resistance in human LO2 hepatocytes (26). These studies and our study may collectively implicate that the appropriate expression levels of LRP6 are substantial for insulin sensitivity. Furthermore, we observed that the expression of IRS-1 was rescued upon increased LRP6/β-catenin expression mediated by Wnt3a stimulation in the primary muscle cells of male CG-IUGR rats, suggesting that Wnt/LRP6 signaling could enhance the expression of IRS-1 to improved insulin sensitivity in the male CG-IUGR muscle. Intriguingly, Wnt3a stimulation did not alter the expression of IR-β in the muscle cells of male CG-IUGR, indicating that the positive effect of enhanced LRP6 expression on IRS-1 may be mediated by other factors rather than IR-β in the skeletal muscle of male CG-IUGR.
Mechanisms that regulate glucose metabolism are linked through numerous intricate molecular pathways. IR and IGF-1R, acting as identical portals in tuning-related gene expression, play a complementary biological role for each other (44, 45, 46). Besides, as IR and IGF-1R are both tyrosine kinases, the IRS-1 with a Src-homolgy 2 (SH2) domain is one of the most potent molecules attracted to them. The activation of IRS-1 by IR/IGF-R could trigger subsequent cascades of insulin signaling, leading to augmented insulin sensitivity, and ultimately increase glucose transport and glycogen synthesis (46). Additionally, skeletal muscle expresses a large amount of IGF-1R, and deletion of IGF-1R gene in skeletal muscle has been showed to impair glucose tolerance and eventually lead to T2DM at an early age (46, 47). Moreover, many studies have showed that low IGF-1 levels are associated with impaired insulin sensitivity, glucose intolerance and T2DM, possibly through IGF-IR-mediated effects on skeletal muscle (44, 45, 48). Given the above-mentioned role of IGF-1R, and that the levels of LRP6/β-catenin/IRS-1 were increased while IR-β was unaltered upon Wnt3a stimulation, we determined the expression of IGF-1R in the muscle of male CG-IUGR rats. It showed that the protein levels of IGF-1R was significantly reduced in the male CG-IUGR groups, furthermore, the expression of IGF-1R was rescued upon Wnt-3a stimulation in the primary muscle cells of male CG-IUGR rats, suggesting LRP6 mediated Wnt/β-catenin signaling probably promoted IRS-1 expression via increased the levels of IGF-1R instead of IR-β, subsequently improving insulin sensitivity in CG-IUGR.
The mammalian target of rapamycin complex 1 (mTORC1)-S6K1 signaling severs as one of the main downstream targets of IRS-1 protein, and active mTORC1-S6K1 mediates an inhibitory feedback on insulin signaling by affecting IRS-1 (35). Genetic deletion of S6K1 protects the high-fat diet-fed mice from obesity and insulin resistance by reducing IRS-1 to a similar level of that in regular chow-fed mice (49). However, although operating a negative feedback, chronic inhibition of mTORC1-S6K1 was associated with an increased risk of impaired glucose tolerance and T2DM as well as insulin resistance (50, 51), indicating the complexity of the crosstalk between mTORC1-S6K1 and insulin signaling. In our study, the level of mTORC1-S6K1 was decreased in the muscle of male CG-IUGR rats, possibly due to the impaired LRP6/β-catenin/IRS-1 signaling. Wnt3a stimulation enhanced the expression of mTORC1-S6K1 in primary muscle cells of male CG-IUGR, which could be ascribed to the Wnt3a-activated LRP6/IRS-1 signaling, consistent with the previous study which shows that LRP6 regulates glucose homeostasis by mTOR pathways (17).
Taken together, in this study, we found that the LRP6-mediated Wnt/β-catenin signaling was impaired in the skeletal muscle of impaired insulin-sensitivite male CG-IUGR rats. Furthermore, enhanced LRP6 expression induced by Wnt3a stimulation could rescue the expression of components involved in the insulin signaling pathway, where IGF-1R rather than IR-β was probably the node linking insulin signaling with Wnt/LRP6 signaling, in primary muscle cells of male CG-IUGR. These findings indicate that the impaired LRP6/β-catenin/IGF-1R/IRS-1 signaling is probably one of the critical mechanisms that underlie the programed impaired insulin sensitivity in CG-IUGR. It also provides a potential pathogenesis connecting early-life nutritional insults to later deleterious metabolic outcomes. We posit that there might be important translational implications to target modulation of LRP6 for therapeutics against insulin resistance in CG-IUGR individuals.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
This work was supported by the grant 81660268 provided by the National Natural Science Foundation of China, and by the Funding (2017280) for the Talents of High Level in the First Affiliated Hospital of Guangxi Medical University.
Author contribution statement
Wenjun Long helped to design the study, and she carried out the experiments, analyze the data and wrote the manuscript. Tuo Zhou, Xiuping Xuan and Qiuli Cao contributed to the acquisition of the data and revision of the manuscript. Zuojie Luo, Yingfen Qin and Qin Ning reviewed and revised the manuscript. Xiaoping Luo and Xuemei Xie supervised the project and revised the manuscript critically. Xuemei Xie also originated and designed the study, as well as helped to draft the manuscript. All authors were involved in the writing of the manuscript and approved the manuscript’s final version.
References
- 1.Barker DJ.The origins of the developmental origins theory. Journal of Internal Medicine 2007261412–417. ( 10.1111/j.1365-2796.2007.01809.x) [DOI] [PubMed] [Google Scholar]
- 2.Mericq V, Martinez-Aguayo A, Uauy R, Iñiguez G, Van der Steen M, Hokken-Koelega A. Long-term metabolic risk among children born premature or small for gestational age. Nature Reviews: Endocrinology 20171350–62. ( 10.1038/nrendo.2016.127) [DOI] [PubMed] [Google Scholar]
- 3.Fernandez-Twinn DS, Hjort L, Novakovic B, Ozanne SE, Saffery R. Intrauterine programming of obesity and type 2 diabetes. Diabetologia 2019621789–1801. ( 10.1007/s00125-019-4951-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sharma D, Shastri S, Farahbakhsh N, Sharma P. Intrauterine growth restriction – part 1. Journal of Maternal-Fetal and Neonatal Medicine 2016293977–3987. ( 10.3109/14767058.2016.1152249) [DOI] [PubMed] [Google Scholar]
- 5.Ehrenkranz RA, Dusick AM, Vohr BR, Wright LL, Wrage LA, Poole WK. Growth in the neonatal intensive care unit influences neurodevelopmental and growth outcomes of extremely low birth weight infants. Pediatrics 20061171253–1261. ( 10.1542/peds.2005-1368) [DOI] [PubMed] [Google Scholar]
- 6.Casey PH, Whiteside-Mansell L, Barrett K, Bradley RH, Gargus R. Impact of prenatal and/or postnatal growth problems in low birth weight preterm infants on school-age outcomes: an 8-year longitudinal evaluation. Pediatrics 20061181078–1086. ( 10.1542/peds.2006-0361) [DOI] [PubMed] [Google Scholar]
- 7.Ong KK.Catch-up growth in small for gestational age babies: good or bad? Current Opinion in Endocrinology, Diabetes, and Obesity 20071430–34. ( 10.1097/MED.0b013e328013da6c) [DOI] [PubMed] [Google Scholar]
- 8.Hermann GM, Miller RL, Erkonen GE, Dallas LM, Hsu E, Zhu V, Roghair RD. Neonatal catch up growth increases diabetes susceptibility but improves behavioral and cardiovascular outcomes of low birth weight male mice. Pediatric Research 20096653–58. ( 10.1203/PDR.0b013e3181a7c5fd) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Faienza MF, Brunetti G, Ventura A, D’Aniello M, Pepe T, Giordano P, Monteduro M, Cavallo L. Nonalcoholic fatty liver disease in prepubertal children born small for gestational age: influence of rapid weight catch-up growth. Hormone Research in Paediatrics 201379103–109. ( 10.1159/000347217) [DOI] [PubMed] [Google Scholar]
- 10.Godfrey KM, Barker DJ. Fetal nutrition and adult disease. American Journal of Clinical Nutrition 200071 (Supplement) 1344S–1352S. ( 10.1093/ajcn/71.5.1344s) [DOI] [PubMed] [Google Scholar]
- 11.Eriksson JG, Osmond C, Kajantie E, Forsén TJ, Barker DJ. Patterns of growth among children who later develop type 2 diabetes or its risk factors. Diabetologia 2006492853–2858. ( 10.1007/s00125-006-0459-1) [DOI] [PubMed] [Google Scholar]
- 12.Liao L, Zheng R, Wang C, Gao J, Ying Y, Ning Q, Luo X. The influence of down-regulation of suppressor of cellular signaling proteins by RNAi on glucose transport of intrauterine growth retardation rats. Pediatric Research 201169497–503. ( 10.1203/PDR.0b013e31821769bd) [DOI] [PubMed] [Google Scholar]
- 13.Ye J, Zheng R, Wang Q, Liao L, Ying Y, Lu H, Cianflone K, Ning Q, Luo X. Downregulating SOCS3 with siRNA ameliorates insulin signaling and glucose metabolism in hepatocytes of IUGR rats with catch-up growth. Pediatric Research 201272550–559. ( 10.1038/pr.2012.123) [DOI] [PubMed] [Google Scholar]
- 14.Berends LM, Fernandez-Twinn DS, Martin-Gronert MS, Cripps RL, Ozanne SE. Catch-up growth following intra-uterine growth-restriction programmes an insulin-resistant phenotype in adipose tissue. International Journal of Obesity 2013371051–1057. ( 10.1038/ijo.2012.196) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zheng RD, Liao LH, Ye J, Wang CB, Gao JZ, Ying YQ, Ning Q, Luo XP. Effects of SOCS 1/3 gene silencing on the expression of C/EBPα and PPARγ during differentiation and maturation of rat preadipocytes. Pediatric Research 201373263–267. ( 10.1038/pr.2012.190) [DOI] [PubMed] [Google Scholar]
- 16.Xie X, Lin T, Zhang M, Liao L, Yuan G, Gao H, Ning Q, Luo X. IUGR with infantile overnutrition programs an insulin-resistant phenotype through DNA methylation of peroxisome proliferator-activated receptor-γ coactivator-1α in rats. Pediatric Research 201577625–632. ( 10.1038/pr.2015.32) [DOI] [PubMed] [Google Scholar]
- 17.Liu W, Singh R, Choi CS, Lee HY, Keramati AR, Samuel VT, Lifton RP, Shulman GI, Mani A. Low density lipoprotein (LDL) receptor-related protein 6 (LRP6) regulates body fat and glucose homeostasis by modulating nutrient sensing pathways and mitochondrial energy expenditure. Journal of Biological Chemistry 20122877213–7223. ( 10.1074/jbc.M111.286724) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Niehrs C.The complex world of WNT receptor signalling. Nature Reviews: Molecular Cell Biology 201213767–779. ( 10.1038/nrm3470) [DOI] [PubMed] [Google Scholar]
- 19.Grant SF, Thorleifsson G, Reynisdottir I, Benediktsson R, Manolescu A, Sainz J, Helgason A, Stefansson H, Emilsson V, Helgadottir Aet al. Variant of transcription factor 7-like 2 (TCF7L2) gene confers risk of type 2 diabetes. Nature Genetics 200638320–323. ( 10.1038/ng1732) [DOI] [PubMed] [Google Scholar]
- 20.Saxena R, Gianniny L, Burtt NP, Lyssenko V, Giuducci C, Sjögren M, Florez JC, Almgren P, Isomaa B, Orho-Melander Met al. Common single nucleotide polymorphisms in TCF7L2 are reproducibly associated with type 2 diabetes and reduce the insulin response to glucose in nondiabetic individuals. Diabetes 2006552890–2895. ( 10.2337/db06-0381) [DOI] [PubMed] [Google Scholar]
- 21.Jin T.Current understanding on role of the Wnt signaling pathway effector TCF7L2 in glucose homeostasis. Endocrine Reviews 201637254–277. ( 10.1210/er.2015-1146) [DOI] [PubMed] [Google Scholar]
- 22.Mani A, Radhakrishnan J, Wang H, Mani A, Mani MA, Nelson-Williams C, Carew KS, Mane S, Najmabadi H, Wu Det al. LRP6 mutation in a family with early coronary disease and metabolic risk factors. Science 20073151278–1282. ( 10.1126/science.1136370) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Singh R, De Aguiar RB, Naik S, Mani S, Ostadsharif K, Wencker D, Sotoudeh M, Malekzadeh R, Sherwin RS, Mani A. LRP6 enhances glucose metabolism by promoting TCF7L2-dependent insulin receptor expression and IGF receptor stabilization in humans. Cell Metabolism 201317197–209. ( 10.1016/j.cmet.2013.01.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Singh R, Smith E, Fathzadeh M, Liu W, Go GW, Subrahmanyan L, Faramarzi S, McKenna W, Mani A. Rare nonconservative LRP6 mutations are associated with metabolic syndrome. Human Mutation 2013341221–1225. ( 10.1002/humu.22360) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang ZM, Luo JQ, Xu LY, Zhou HH, Zhang W. Harnessing low-density lipoprotein receptor protein 6 (LRP6) genetic variation and Wnt signaling for innovative diagnostics in complex diseases. Pharmacogenomics Journal 201818351–358. ( 10.1038/tpj.2017.28) [DOI] [PubMed] [Google Scholar]
- 26.Li L, Xue J, Wan J, Zhou Q, Wang S, Zhou Y, Zhao H, Wang X. LRP6 knockdown ameliorates insulin resistance via modulation of autophagy by regulating GSK3β signaling in human LO2 hepatocytes. Frontiers in Endocrinology 201910 73. ( 10.3389/fendo.2019.00073) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Petersen KF, Dufour S, Savage DB, Bilz S, Solomon G, Yonemitsu S, Cline GW, Befroy D, Zemany L, Kahn BBet al. The role of skeletal muscle insulin resistance in the pathogenesis of the metabolic syndrome. PNAS 200710412587–12594. ( 10.1073/pnas.0705408104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Petersen KF, Shulman GI. Pathogenesis of skeletal muscle insulin resistance in type 2 diabetes mellitus. American Journal of Cardiology 20029011G–18G. ( 10.1016/s0002-9149(0202554-7) [DOI] [PubMed] [Google Scholar]
- 29.DeFronzo RA, Tripathy D. Skeletal muscle insulin resistance is the primary defect in type 2 diabetes. Diabetes Care 200932 (Supplement 2) S157–S163. ( 10.2337/dc09-S302) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu H, Ballantyne CM. Skeletal muscle inflammation and insulin resistance in obesity. Journal of Clinical Investigation 201712743–54. ( 10.1172/JCI88880) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aiken CE, Ozanne SE. Sex differences in developmental programming models. Reproduction 2013145R1–R13. ( 10.1530/REP-11-0489) [DOI] [PubMed] [Google Scholar]
- 32.Sakaguchi K, Takeda K, Maeda M, Ogawa W, Sato T, Okada S, Ohnishi Y, Nakajima H, Kashiwagi A. Glucose area under the curve during oral glucose tolerance test as an index of glucose intolerance. Diabetology International 2016753–58. ( 10.1007/s13340-015-0212-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yamamoto H, Komekado H, Kikuchi A. Caveolin is necessary for Wnt-3a-dependent internalization of LRP6 and accumulation of beta-catenin. Developmental Cell 200611213–223. ( 10.1016/j.devcel.2006.07.003) [DOI] [PubMed] [Google Scholar]
- 34.Kido Y, Burks DJ, Withers D, Bruning JC, Kahn CR, White MF, Accili D. Tissue-specific insulin resistance in mice with mutations in the insulin receptor, IRS-1, and IRS-2. Journal of Clinical Investigation 2000105199–205. ( 10.1172/JCI7917) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yoon MS.The role of mammalian target of rapamycin (mTOR) in insulin signaling. Nutrients 201791, 176. ( 10.3390/nu9111176) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cheong JN, Wlodek ME, Moritz KM, Cuffe JS. Programming of maternal and offspring disease: impact of growth restriction, fetal sex and transmission across generations. Journal of Physiology 20165944727–4740. ( 10.1113/JP271745) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Buckberry S, Bianco-Miotto T, Bent SJ, Dekker GA, Roberts CT. Integrative transcriptome meta-analysis reveals widespread sex-biased gene expression at the human fetal-maternal interface. Molecular Human Reproduction 201420810–819. ( 10.1093/molehr/gau035) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rosenfeld CS.Sex-specific placental responses in fetal development. Endocrinology 20151563422–3434. ( 10.1210/en.2015-1227) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Taniguchi CM, Emanuelli B, Kahn CR. Critical nodes in signalling pathways: insights into insulin action. Nature Reviews: Molecular Cell Biology 2006785–96. ( 10.1038/nrm1837) [DOI] [PubMed] [Google Scholar]
- 40.Copps KD, White MF. Regulation of insulin sensitivity by serine/threonine phosphorylation of insulin receptor substrate proteins IRS1 and IRS2. Diabetologia 2012552565–2582. ( 10.1007/s00125-012-2644-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Previs SF, Withers DJ, Ren JM, White MF, Shulman GI. Contrasting effects of IRS-1 versus IRS-2 gene disruption on carbohydrate and lipid metabolism in vivo. Journal of Biological Chemistry 200027538990–38994. ( 10.1074/jbc.M006490200) [DOI] [PubMed] [Google Scholar]
- 42.Karlsson HK, Zierath JR. Insulin signaling and glucose transport in insulin resistant human skeletal muscle. Cell Biochemistry and Biophysics 200748103–113. ( 10.1007/s12013-007-0030-9) [DOI] [PubMed] [Google Scholar]
- 43.Kim W, Kim M, Jho EH. Wnt/β-catenin signalling: from plasma membrane to nucleus. Biochemical Journal 20134509–21. ( 10.1042/BJ20121284) [DOI] [PubMed] [Google Scholar]
- 44.Sandhu MS, Heald AH, Gibson JM, Cruickshank JK, Dunger DB, Wareham NJ. Circulating concentrations of insulin-like growth factor-I and development of glucose intolerance: a prospective observational study. Lancet 20023591740–1745. ( 10.1016/S0140-6736(0208655-5) [DOI] [PubMed] [Google Scholar]
- 45.Succurro E, Andreozzi F, Marini MA, Lauro R, Hribal ML, Perticone F, Sesti G. Low plasma insulin-like growth factor-1 levels are associated with reduced insulin sensitivity and increased insulin secretion in nondiabetic subjects. Nutrition, Metabolism, and Cardiovascular Diseases 200919713–719. ( 10.1016/j.numecd.2008.12.011) [DOI] [PubMed] [Google Scholar]
- 46.Aguirre GA, De Ita JR, de la Garza RG, Castilla-Cortazar I. Insulin-like growth factor-1 deficiency and metabolic syndrome. Journal of Translational Medicine 201614 3. ( 10.1186/s12967-015-0762-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fernández AM, Kim JK, Yakar S, Dupont J, Hernandez-Sanchez C, Castle AL, Filmore J, Shulman GI, Le Roith D. Functional inactivation of the IGF-I and insulin receptors in skeletal muscle causes type 2 diabetes. Genes and Development 2001151926–1934. ( 10.1101/gad.908001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yakar S, Liu JL, Fernandez AM, Wu Y, Schally AV, Frystyk J, Chernausek SD, Mejia W, Le Roith D. Liver-specific igf-1 gene deletion leads to muscle insulin insensitivity. Diabetes 2001501110–1118. ( 10.2337/diabetes.50.5.1110) [DOI] [PubMed] [Google Scholar]
- 49.Um SH, Frigerio F, Watanabe M, Picard F, Joaquin M, Sticker M, Fumagalli S, Allegrini PR, Kozma SC, Auwerx Jet al. Absence of S6K1 protects against age- and diet-induced obesity while enhancing insulin sensitivity. Nature 2004431200–205. ( 10.1038/nature02866) [DOI] [PubMed] [Google Scholar]
- 50.Veilleux A, Houde VP, Bellmann K, Marette A. Chronic inhibition of the mTORC1/S6K1 pathway increases insulin-induced PI3K activity but inhibits Akt2 and glucose transport stimulation in 3T3-L1 adipocytes. Molecular Endocrinology 201024766–778. ( 10.1210/me.2009-0328) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kezic A, Popovic L, Lalic K. mTOR inhibitor therapy and metabolic consequences: where do we stand? Oxidative Medicine and Cellular Longevity 201820182640342. ( 10.1155/2018/2640342) [DOI] [PMC free article] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a