Abstract
Carbapenems are the last resort antimicrobials for the treatment of extended spectrum β-lactamases (ESBLs) producing Enterobacteriaceae. Emergence of carbapenems resistant group B2 uropathogenic E. coli (UPEC) is a major concern because of their high virulence. Prevalence of these enzymes and multidrug resistance (MDR) among B2 UPEC isolates from Iraqi outpatients with acute urinary tract infection (UTI) was evaluated in this research. Urine cultures were performed and the isolates were identified biochemically. Escherichia coli isolates were tested for phylogroup reference by quadraplex PCR, then B2 isolates were detected for antimicrobial resistance by disc diffusion test and carbapenemase genes by PCR. Escherichia coli was the most prevalent among Gram-negative isolates (66.6%) and B2 was the most detected phylogroup among E. coli isolates (33.9%). Most of B2 isolates showed high resistance rates to tested antimicrobials, especially β-lactams with MDR revealed in 100% of them. Whereas, low resistance rates were noted against carbapenems, aminoglycosides and nitrofurantoin. Carbapenemase genes were detected in 76.3% of B2 isolates. Of which, blaOXA-48 was the most frequent (57.8%), followed by blaPER (47.3%), blaKPC (15.7%), blaVEB and blaVIM (10.5%, for each). Whereas, blaGES and blaIMP genes were not found. Coproduction of these genes occurred among 17 isolates. The combination of blaOXA-48 and blaPER was the most frequent (41.1%). All carbapenemase producing isolates were MDR. These results revealed high prevalence of carbapenemase genes and MDR among B2 UPEC recovered in this study. In the study area. it is strongly advised to use aminoglycosides and nitrofurantoin for empirical treatment of UPEC.
Introduction
Escherichia coli (E. coli) is the most versatile known microorganism [1]. It is a common commensal of gastrointestinal tract of human and animal. Also, it comprises pathogenic strains divided into intestinal or diarrhoeagenic E. coli (DEC) and extraintestinal pathogenic E. coli (ExPEC). The main infections caused by ExPEC are urinary tract infections (UTIs), sepsis, meningitis, and wound infections [2–4]. A large proportion of humans are affected by UTIs, with annual prevalence of about 150 million cases. Yearly, $5 billion are spent for treatment of UTIs in the USA with estimated cases of 11 million per year [5]. Uropathogenic E. coli is the most prevalent ExPEC and it is the primary cause of UTIs all over the world [3]. Uropathogenic E. coli strains have a wide variety of virulence factors which include: immune suppressors, adhesins (fimbrial and afimbrial adhesins), siderophore systems, the serum resistance, the capsular polysaccharide K antigen, and toxins [3, 5].
Carbapenems emerged as bactericidal β-lactam antimicrobials with confirmed activity in severe infections caused by extended spectrum β-lactamases (ESBLs) producers [6]. These antimicrobials have broad spectrum antibacterial activity and have a unique structure that is composed of a carbapenem linked to a β-lactam ring which provides protection against most β lactamases such as metallo-β-lactamases (MBLs) and ESBLs [7]. Therefore; carbapenems are considered one of the most reliable drugs for treating bacterial infections and the occurrence and distribution of resistance to them constitute a major public health problem [8]. There are three possible mechanisms for carbapenems’ resistance in the family Enterobacteriaceae. These are: efflux pump overactivity, porin loss or mutation, and carbapenemase production, which is the main mechanism of resistance to these antimicrobials [9–11]. Carbapenemases are enzymes (β-lactamases) that are encoded by both chromosomal and plasmid-mediated genes and structurally belonging to different Ambler classes (A, B, and D). These enzymes can hydrolyze a broad range of β-lactams, including carbapenems, cephalosporins, penicillin, and aztreonam. Also, bacterial strains possessing carbapenemases are often resistant to multiple drugs (MDR) [11]. Most of these enzymes have been mainly found in Enterobacteriaceae, Pseudomonas aeruginosa, and Acinetobacter baumannii. For the Enterobacteriaceae family, class A carbapenemases (KPC enzymes) appeared in North Carolina (USA) in 1996 and then spread to Europe; class B discovered as VIM-1 in E. coli in Greece, but rapidly spread in Klebsiella pneumoniae (K. pneumoniae), becoming endemic in that country as well as in other European countries; and OXA-48 (class D) which was first reported in Turkey in K. pneumoniae, and later occurred in other Mediterranean countries [8, 11]. Nowadays, carbapenemases in Enterobacteriaceae are mainly found in K. pneumoniae, and to a much lesser extent in Escherichia coli (E. coli) and other enterobacterial species, with a higher prevalence in southern Europe and Asia than in other parts of the world. It has recently been concluded that global spread of carbapenems-resistant enterobacterial isolates (CRE) in the future will be dominated in the hospital environment by K. pneumoniae producing all types of carbapenemases, mainly KPC, VIM, NDM, and OXA-48, and in the community by E. coli having NDM or OXA-type (OXA-48 and OXA- 181) enzymes [11].
Multidrug resistance has been increased all over the world that is considered a public health threat. Several recent investigations reported the emergence of multidrug-resistant bacterial pathogens from different origins including humans, birds, cattle, and fish that increase the need for routine application of the antimicrobial susceptibility testing to detect the antibiotic of choice as well as the screening of the emerging MDR strains [12–15]. Worldwide, there is a major concern regarding the high prevalence of antimicrobial resistance and MDR among UPEC. By 2050, it has been estimated that more than 3 million people may lose their lives each year as a result of increase in MDR. A major concern in this respect is the spread of carbapenem-resistant strains all over the world [3].
Strains of E. coli are divided into eight phylogroups (A, B1, B2, C, D, E, F and clade I) of which group B2 strains are the most virulent and the most common causative agents of UTI [16, 17]. Emergence of carbapenem resistant group B2 UPEC is a major concern because of their high virulence. Strains with high antimicrobial resistance accompanied by high virulence may emerge and this may lead to treatment failure and loss of effective treatment [10]. So that, awareness should be taken to prevent such strains from reaching our patients especially those who are immunocompromised. One of the effective measurements to achieve this aim is to do periodic surveillance of antimicrobial resistance of UPEC by both phenotypic and genotypic procedures. Selection of appropriate prevention and containment options requires good knowledge of the prevalence and incidence of carbapenemases. Strains-producing these enzymes are not limited to hospital environment, but also occur among hospitals, long-term care facilities, community, animals, and the environment [11]. As it is difficult to develop novel antimicrobial agents, efforts should be concentrated on the prevention of the spread of carbapenemase producers by early detection and reinforced hygiene measures [18] and careful monitoring of use of antimicrobials for UTI treatment is necessary [19]. In addition, predominance of carbapenemases among B2 E. coli isolates was reported from different parts of the world [20–23]. Hence, this research was designed to evaluate the prevalence of carbapenemase genes and MDR among B2 UPEC isolates from Iraqi outpatients in Wasit Province with acute UTI by multiplex PCR protocols.
Materials and methods
Specimen collection and processing
Midstream urine samples were collected from outpatients attending AlKarama hospital and Al-Kut hospital for Gynecology and Obstetrics and Pediatrics in Al-Kut/Wasit Province/Iraq, during the period from July, 2018 to January, 2019. The urine samples were collected into sterile screw capped test tubes and streaked immediately on MacConkey agar (HIMEDIA, India) and Blood agar (HIMEDIA, India) plates for bacterial isolation [24].
Isolation, identification and phylogenetic grouping of E. coli
Collected urine samples were cultured immediately after collection on MacConkey agar and Blood agar plates and incubated aerobically at 37°C for 24 h. Positive urine cultures were defined by a growth of single morphotype of colony with counts >105CFU/ml [24]. From each plate single colony (with the appropriate color and morphology, that is, characteristics of E. coli) was selected and subcultured onto MacConkey agar plate again, incubated, a single colony was selected and subcultured onto tryptic soy agar (TSA) (HIMEDIA, India) plate, and then kept in refrigerator for further work.
The isolates were identified biochemically by API20E rapid test system depending on the manufacturer’s instructions (BioMerieux, France).
Thereafter, E. coli isolates were classified into eight phylogenetic groups (A, B1, B2, C, D, E, F, and clade I) according to the presence of chuA, yjaA, and arpA genes and TspE4.C2 DNA fragment, depending on methods provided by Clermont et al. [17] At first the DNA was extracted from the isolates by boiling method described by Yamamoto et al. [25] with modification which included suspending 24 hr. old bacterial culture (3 loopfuls) on TSA in 1 ml of sterile 1X TE buffer (pH 8.0) (Bio Basic, Canada) instead of sterile D.W. The cell suspension was boiled in water bath at 95˚C for 10 minutes. The suspension was centrifuged at 10,000 rpm for 5 minutes. The supernatant which contains purified DNA was dispensed in 100 μl aliquots and stored at -20˚C till use. The phylogroups were determined by quadraplex PCR (Tables 1 and 2).
Table 1. Primer’s sequence for detection of E. coli phylogroups.
| Gene | Primer name | Primer sequence (5′- 3′) | Product size (bp) | Source of primer |
|---|---|---|---|---|
| chuA | chuA. 1b | ATGGTACCGGACGAACCAAC | 288 | Clermont et al. [17]; Clermont et al. [26] |
| chuA. 2b | TGCCGCCAGTACCAAAGACA | |||
| yjaA | yjaA. 1b | CAAACGTGAAGTGTCAGGAG | 211 | Clermont et al. [17] |
| yjaA. 2b | AATGCGTTCCTCAACCTGTG | |||
| TspE4.C2 | TspE4.C2.1b | CACTATTCGTAAGGTCATCC | 152 | Clermont et al. [17] |
| TspE4C2. 2b | AGTTTATCGCTGCGGGTCGC | |||
| arpA | AceK.f | AACGCTATTCGCCAGCTTGC | 400 | Clermont et al. [17]; Clermont et al. [27] |
| ArpA1.r | TCTCCCCATACCGTACGCTA | |||
| arpA | ArpAgpE.f | GATTCCATCTTGTCAAAATATGCC | 301 | Lescat et al. [28] |
| ArpAgpE.r | GAAAAGAAAAAGAATTCCCAAGAG | |||
| trpA | trpAgpC.1 | AGTTTTATGCCCAGTGCGAG | 219 | Lescat et al. [28] |
| trpAgpC.2 | TCTGCGCCGGTCACGCCC | |||
| trpA | trpBA.f | CGGCGATAAAGACATCTTCAC | 489 | Clermont et al. [29] |
| trpBA.r | GCAACGCGGCCTGGCGGAAG |
Table 2. Components of 50μl PCR master mix and amplification conditions for detection of UPEC phylogroups [17].
| PCR reaction | SterileD.W. | Primers | DNA | Amplification conditions |
|---|---|---|---|---|
| Quadruplex | 38 μl | 8 μl: 1 μl each of: | 5 μl | 1. Initial denaturation at 94°C for 4 min. 2. 30 cycles of: • Denaturation at 94°C for 5 s. • Annealing at 57°C (group E) or 59°C (quadruplex and group C) for 20 s. • Extension at 72°C for 1 min. |
| • chuA-1b, chuA-2b | ||||
| • yiaA.1b, yiaA.2b | ||||
| • TspE4C2.1b, | ||||
| TspE4C2.2b • Acek.f, ArpA.r | ||||
| Group E | 41 μl | 4 μl: 1 μl each of: | 5 μl | 3. Final extension at 72°C for 5 min. |
| • ArpAgpE.f, ArpAgpE.r | ||||
| • trpBA.f, trpBA.r | ||||
| Group C | 41 μl | 4 μl: 1 μl each of: | 5 μl | |
| • trpAgpC.1, trpAgpC.2 | ||||
| • trpBA.f, trpBA.r |
This work was approved by the Scientific Committee of the College of Science/ Wasit University/ Wasit Province/ Iraq (Scientific project discussing committee) and also it was performed after obtaining permission from Health Administration of Wasit, Wasit Province/Iraq. Oral consent was obtained from each patient for collecting specimens and publication of this report. The reason for just obtaining oral consent without the need for written consent is that collection of urine specimens is part of routine clinical laboratory work for diagnosis of these infections. All patients’ data were anonymous.
Antimicrobial susceptibility of the isolates
Antimicrobial susceptibility of the isolates was performed by the disk diffusion method according to the instructions of CLSI [30] using Mueller-Hinton agar (HIMEDIA, India). The used antimicrobials were obtained from Bioanalyse/ Turkey and included: ampicillin (AMP: 10 μg); amoxicillin-clavulanic acid (AMC: 20/10 μg); cefoxitin (FOX: 30 μg); cefotaxime (CTX: 30 μg); ceftazidime (CAZ: 30 μg); ceftriaxone (CRO: 30 μg); cefepime (FEP: 30 μg); aztreonam (ATM: 30 μg); imipenem (IPM: 10 μg); meropenem (MEM: 10 μg); gentamicin (CN: 10 μg); amikacin (AK: 30 μg); tetracycline (TE: 30 μg); nalidixic acid (NA: 30 μg); ciprofloxacin (CIP: 5 μg); trimethoprim-sulfamethoxazole (SXT: 1.25/23.75 μg); and nitrofurantoin (F: 300 μg).
Molecular detection of carbapenemase genes
Protocols for multiplex PCR that were developed by Dallenne et al. [31] were followed for amplification of carbapenemase genes. For each primer (Table 3), 100 μl of working solution was prepared by diluting the stock solution (100 pmol/μl) by 1X TE buffer ((Bio Basic, Canada)) (pH 8.0) depending on the general dilution equation: C1V1 = C2V2. So that 1μl of forward primer and 1μl of reverse primer of each gene should contain the appropriate concentration to be added to the PCR master mix (final volume 50 μl). Primer’s concentrations ranged from 10 pmole/μl to 25 pmole/μl (Table 4).
Table 3. Primers’ sequence of carbapenemase genes [31].
| Multiplex PCR pool | Primer name | Sequence (5’-3’) | Amplicon size (bp) |
|---|---|---|---|
| Multiplex-I: blaVEB, blaPER and blaGES | MultiGES-F | AGTCGGCTAGACCGGAAAG | 399 |
| MultiGES-R | TTTGTCCGTGCTCAGGAT | ||
| MultiPER-F | GCTCCGATAATGAAAGCGT | 520 | |
| MultiPER-R | TTCGGCTTGACTCGGCTGA | ||
| MultiVEB-F | CATTTCCCGATGCAAAGCGT | 648 | |
| MultiVEB-R | CGAAGTTTCTTTGGACTCTG | ||
| Multiplex-II: blaGES and blaOXA-48-like | MultiGES-F | AGTCGGCTAGACCGGAAAG | 399 |
| MultiGES-R | TTTGTCCGTGCTCAGGAT | ||
| MultiOXA-48-F | GCTTGATCGCCCTCGATT | 281 | |
| MultiOXA-48-R | GATTTGCTCCGTGGCCGAAA | ||
| Multiplex III: blaIMP, blaVIM, and blaKPC | MultiIMP-F | TTGACACTCCATTTACDGb | 139 |
| MultiIMP-R | GATYGAGAATTAAGCCACYCTb | ||
| MultiVIM-F | GATGGTGTTTGGTCGCATA | 390 | |
| MultiVIM- R | CGAATGCGCAGCACCAG | ||
| MultiKPC-F | CATTCAAGGGCTTTCTTGCTGC | 538 | |
| MultiKPC-R | ACGACGGCATAGTCATTTGC |
bY = T or C; R = A or G; S = G or C; D = A or G or T.
Table 4. Components of multiplex PCR master mix for the detection of carbapenemase genes (final volume 50 μl) and amplification conditions [31].
| Multiplex PCR pool | Primer name | Required primer’s concentration (pmol/50μl) | Added quantities of PCR master mix components (μl) | Amplification conditions | ||
| Primer | DNA | Sterile D.W. | 1. Initial denaturation at 94°C for 10 min. 2. 30 cycles of: • denaturation at 94°C for 40s. • annealing at 55°C for amplification of blaVIM, blaIMP, blaKPC genes, and 57°C for amplification of blaGES, and blaOXA-48 genes. • extension at 72°C for 1min; 3. Final elongation step at 72°C for 7 min. |
|||
| PCR pool-I: blaVEB, blaPER and blaGES | MultiGES-F & R | 15 for each F and R primer | 1 for each F and R primer | 5 | 39 | |
| MultiPER-F & R | ||||||
| MultiVEB-F & R | ||||||
| PCR pool-II: blaGES and blaOXA-48-like | MultiGES-F & R | 20 for each F and R primer | 41 | |||
| MultiOXA-48-F & R | ||||||
| PCR pool-III: blaIMP, blaVIM, and blaKPC | MultiIMP-F & R | 25 for each F and R primer | 39 | |||
| MultiVIM-F & R | ||||||
| MultiKPC-F | 25 | |||||
| MultiKPC-R | 10 | |||||
Statistical analysis
Differences in the distributions of the studied determinants were tested by Chi square (SPSS software, version 2.1, IBM, NC, USA). A P value of ≤ 0.05 was considered to indicate statistical significance.
Results and discussion
Prevalence of E. coli among uropathogens
Of 1003 urine samples (one sample per patient) only 359 (35.7%) were positive for bacterial culture, where 168 (46.7%) of them were positive for Gram-negative bacteria. All of these Gram-negative isolates were identified biochemically by API20E rapid test according to index provided by the manufacturer (BioMerieux, France).
Among all positive bacterial cultures, E. coli was one of the commonly isolated bacteria (112/359: 31.9%). Escherichia coli also was the most commonly isolated species among Gram-negative bacteria (112/168: 66.6%), followed by K. pneumoniae (28: 16.6%), Proteus mirabilis (P. mirabilis) and Pseudomonas spp. (11: 6.5%, for each) and Enterobacter spp. (6: 3.5%). These results were similar to those obtained by other Iraqi researchers such as that which was carried out in Kirkuk city by Alsamarai and Ali [32] where they showed 41.6% of urine samples were culture positive and E. coli was the predominantly isolated bacteria (57.7%), followed by K. pneumoniae (14.5%), and Proteus spp. (10.3%). In Baghdad, Ghaima et al. [33] reported that 57.9% specimens were positive for bacterial culture and E. coli was the most common bacteria (34.0%) followed by Klebsiella spp. (14.6%); Proteus spp. (4.5%); Pseudomonas spp. (3.7%)and Enterobacter sp. (1.4%). Also, similar results were reported from other countries as those achieved by Rafalskiy et al. [34] in the Russian Federation where they found that 64.2% of the isolated uropathogens were Gram-negative and E. coli was the most prevalent (49.1%), followed by K. pneumoniae (9.5%), P. mirabilis (2.9%), P. aeruginosa (1.7%) and Enterobacter spp. (1.0%). In Grenada, Sharma et al. [35] showed that 65.4% of the isolates were Gram-negative bacteria with E. coli being the frequently isolated species (51.5%), followed by K. pneumoniae (20.0%) and P. mirabilis (10.0%). The high predominance of E. coli in patients with UTI is expected and it is well known by physicians and researchers all over the world as this bacterium represents a normal component of the intestinal microbiota of humans and animals and has strains with the potential of causing UTI and other extraintestinal infections. These UPEC strains have virulence traits that allow their successful colonization. So that, these virulence traits are considered the most important features differentiating the ExPEC from commensal and enteric E. coli [36]. Uropathogenic E. coli exhibit various virulence-associated factors (VFs) that assist them in attaching to, invading, and injuring the host. Among these virulence factors are adhesins (e.g. fmbriae), siderophores, iron-acquisition systems, capsules, toxins (e.g. haemolysin), invasins, and serum resistance associated proteins [36, 37].
Phylogroups of E. coli isolates
In the present study, a quadraplex PCR assay developed by Clermont et al. [17] was applied to detect phylogroups’ reference of E. coli isolates (n = 112). The highest frequency of this study’s isolates was in group B2 (33.9%), followed by group A (24.1%), group D (17.8%), group B1 (8.0%), group C (4.4%) and group F (3.5%), whereas group E was not detected in any isolate. In addition, 9 isolates (8.0%) were non-typeable (Table 5). This predominance of phylogroup B2 among UPEC isolates is consistent with other studies conducted by Iraqi researchers including Merza and Jubrael [38] in Duhok city and Ahmed et al. [39] in Baghdad, who demonstrated that group B2 was the most frequently recovered phylogroup (56.7% and 36.0%, respectively). Furthermore, in ThiQar city, Hussain and Saleh [40] showed that 74% of UPEC belonged to phylogenetic group B2. Also, studies from different countries indicated that this group was the most prevalent among UPEC isolates. Among these studies, Iranpour et al. [41] in Iran, Massot et al. [42] in the Paris area, Miranda-Estrada et al. [43] in Mexico, Katongole et al. [44] in Uganda and Zhong et al. [45] in China, reported that group B2 had the highest frequency (39.3%, 34.0%, 42%, 40% and 16.7%, respectively) among UPEC isolates. This predominance of group B2 among UPEC isolates may be attributed to the fact that most virulence factors and antibiotic resistance genes existed jointly within this group and this could enhance survival fitness in urinary tract as recognized by many researchers such as Johnson et al. [46]; Lee et al. [47]; Najafi et al. [48]; Lara et al. [49] and Ali et al. [50].
Table 5. Distribution of E. coli phylogroups among 112 UPEC isolates from outpatients with acute UTI.
| Phylogroup | E. coli isolates (n = 112) | |
|---|---|---|
| No. | % | |
| B2 | 38 | 33.9 |
| B1 | 9 | 8.0 |
| A | 27 | 24.1 |
| D | 20 | 17.8 |
| F | 4 | 3.5 |
| C | 5 | 4.4 |
| E | 0 | 0 |
| Nontypable (NT) | 9 | 8.0 |
Resistance of the B2 E. coli isolates to antimicrobials
Susceptibility to 17 antimicrobials was performed for all of the B2 UPEC isolates (n = 38). Resistance to β-lactams was the most common except carbapenems (imipenem and meropenem) to which all of the isolates were sensitive. High resistance rates were also found against tetracycline, trimethoprim-sulfamethoxazole, and nalidixic acid. Whereas, the isolates showed high sensitivity to ciprofloxacin, gentamicin, nitrofurantoin and amikacin. (Table 6). Recently, similar resistance patterns were published by Iraqi researchers [23].
Table 6. Antimicrobial resistance of group B2 E. coli isolates from outpatients with UTI.
| Antimicrobial category | Antimicrobial agent | No. (%) of isolates* (n = 38) |
|---|---|---|
| Penicillins | Ampicillin (AMP) | 38 (100) |
| Penicillins + β-lactamase inhibitors | Amoxicillin-clavulanic acid (AMC) | 34 (89.4) |
| 3rd and 4th generation cephalosporins | Cefepime (FEP) | 32 (84.2) |
| Ceftazidime (CAZ) | 30 (78.9) | |
| Cefotaxime (CTX) | 28 (73.6) | |
| Ceftriaxone (CRO) | 25 (65.7) | |
| Monobactams | Aztreonam (ATM) | 25 (65.7) |
| Cephamycins | Cefoxitin (FOX) | 15 (39.4) |
| Carbapenems | Imipenem (IPM) | 0 |
| Meropenem (MEM) | 0 | |
| Tetracyclines | Tetracycline (TE) | 28 (73.6) |
| Trimethoprim | trimethoprim-sulfamethoxazole (SXT) | 21 (55.2) |
| Quinolones | Nalidixic acid (NA) | 17 (44.7) |
| Ciprofloxacin (CIP) | 10 (26.3) | |
| Aminoglycosides | Gentamicin (CN) | 7 (18.4) |
| Amikacin (AK) | 1 (2.6) | |
| Nitrofurans | Nitrofurantoin (F) | 3 (7.8) |
*Resistant and intermediate.
Antimicrobial resistance in UPEC is a major concern in both humans and animals at a worldwide scale due to its increased resistance to several antibiotics [51], and expanding resistance to different classes of antimicrobial agents generally [52]. Indiscriminate and widespread use of antibiotics in addition to the practice of prescribing antibiotics to treat UTI without bacterial characterization led to increased resistance among uropathogens and to decreased effectiveness of oral therapies [5, 53], which gave an alarming level of antimicrobial resistance developing in UTI pathogens. Thus, rapid initiation of appropriate empirical treatment requires a good knowledge of epidemiological data concerning the sensitivity of uropathogens to antibacterial agents [54]. Furthermore, plasmids harboring resistance determinants can be transferred between bacteria, even between species, leading to the acquisition of resistance to new antibiotics via the emergence of mutant strains [55]. Also, some bacteria produce multiple β-lactamases, which may reduce the efficiency of β-lactam/β-lactamases inhibitor combination [5]. The rapid development of resistance to β-lactam antibiotics attributed to the emergence of ESBLs in the enteric bacteria [56]. This may be due to the excessive use of expanded spectrum cephalosporins (ESC) during clinical practice, where several studies have found a relationship between third-generation cephalosporins use and acquisition of ESBL-producing strains [57]. Therefore, the limited use of these antibiotics might be helpful to inhibit/avoid the emerging or spreading of multidrug-resistant Gram-negative bacteria [58]. One hundred percent sensitivity to imipenem and meropenem was observed among all B2 UPEC isolates investigated in this work. For bacterial infections, these antibacterials are the most reliable last-resort treatment [59]. Furthermore, the low resistance rates against aminoglycosides (gentamicin: 18.4% and amikacin: 2.6%) reported in the present study may be attributed to the rare use of these antibiotics in AL-Kut hospitals which may be due to their high costs in comparison with β-lactams [57]. Nitrofurantoin resistance was also noted in 7.8% of E. coli clinical isolates, and this may be due to the lower frequency use of this drug.
In addition, 100% of this study included isolates were MDR, while none of them were XDR or PDR. This may be due to the fact that E. coli pathogens have developed resistance to every class of antibiotics introduced to treat human and animal infections [60], and these infections are particularly challenging to treat [50]. This high prevalence of MDR among the isolates of the present study is alarming and necessitate the need for the clinicians to ensure the use of appropriate antibiotics for recommended periods in adequate doses in order to prevent emergence of multidrug resistant organisms. Many factors may have contributed to such high rates of resistance including misuse of antibiotics by health care professionals or non-skilled practitioners, misuse of antibiotics by the general public and inadequate surveillance due to lack of information arising from routine antimicrobial susceptibility testing, like reports from other developing countries. Careless usage of antibiotics is the most important factor that facilitates the development of MDR, which triggers the selection and distribution of antibiotic-resistant pathogens in clinical practice [56, 61]. Iraq is one of the developing countries where antibiotics are sold over the counter, an attitude that encourages self-medication. On the other hand, it is remarked that during period, a group of antibiotics become more used than others without susceptibility tests, which may lead to variability in their resistance [62]. Other factors known to influence the evolution and transfer of MDR among microorganisms are incomplete doses, ease of access, over prescribing antibiotics without laboratory results and indiscriminate use of antimicrobials in agriculture and livestock sectors [61]. Antibiotics resistance arises quickly and spreads rapidly, especially when resistance genes are horizontally transferred via plasmids and integrons among individuals, among species, and even among bacterial kingdom [63]. Much of the problem of antimicrobial resistance has been shown to be due to the presence of transferable plasmids encoding MDR and their dissemination among different enterobacterial species and it is common for a single plasmid to simultaneously mediate resistance to multiple antimicrobials and to be shared among different bacterial genera [64].
These MDR isolates showed resistance to most antimicrobials tested in this study except carbapenems (all isolates were sensitive), amikacin (2.6%) and nitrofurantoin (7.8%). These results ensure that continuous and inappropriate use of antimicrobials is a major risk factor for development of resistance. It was realized that the inappropriate use of antimicrobials has been shown to play a pivotal role in the emergence of MDR organisms [65, 66]. Also, MDR is largely associated with ESBLs’ production as mentioned by several researchers [64, 65, 67–69].
Distribution of carbapenemase genes among the isolates
All of this study included B2 E. coli isolates were ESBLs producers with predominance of CTX-M-1 (unpublished data). In this work, investigated carbapenemase genes included: blaOXA-48, blaPER, blaKPC, blaVEB, blaVIM, blaGES and blaIMP (Figs 1 and 2). Out of 38 B2 UPEC isolates, 29 (76.3%) were carbapenemase producers. Of these carbapenemases, blaOXA-48 gene was the most frequent (57.8%), followed by blaPER (47.3%), blaKPC (15.7%), blaVEB and blaVIM (10.5%, for each). Whereas, no PCR-amplification products were noticed with blaGES and blaIMP genes (Table 7).
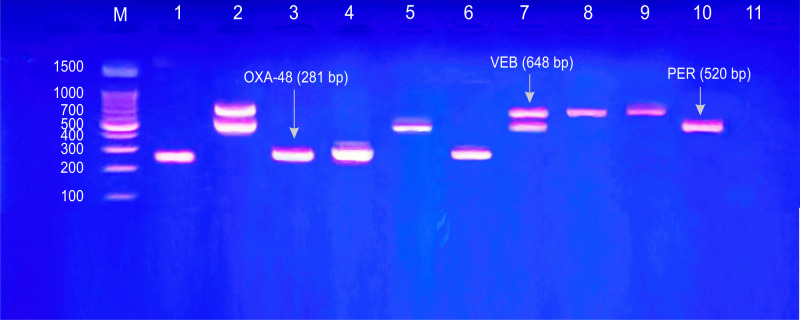
Fig 1. Gel electrophoresis of PCR amplified products for detection of carbapenemases genes: Multiplex I & II.
Multiplex I & II: blaGES (399 bp), blaPER (520 bp), blaVEB (648 bp) and blaOXA-48 (281 bp). Lane m: DNA Ladder (100 pb); Lanes 1, 3, 4 and 6: positive results for blaOXA-48; Lanes 2 and 7: positive results for blaVEB and blaPER; Lanes 5 and 10: positive results for blaPER; Lanes 8 and 9: positive results for blaVEB; Lane 11: negative results for all genes.
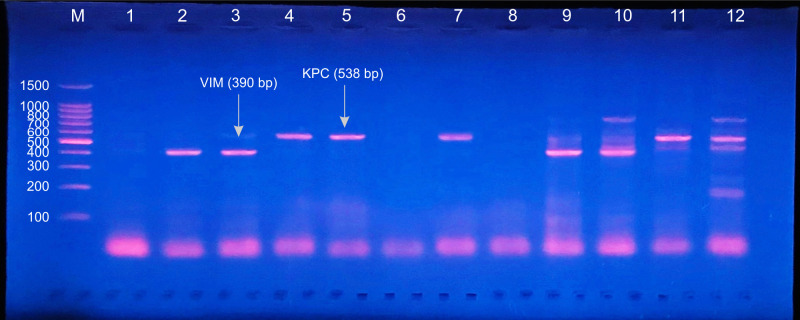
Fig 2. Gel electrophoresis of PCR amplified products for detection of carbapenemases genes: Multiplex III.
Multiplex III: blaIMP (139 bp), blaVIM (390 bp) and blaKPC (538 bp). Lane m: DNA Ladder (100 pb); Lanes 1, 6 and 8: negative results for blaIMP, blaVIM and blaKPC; Lanes 2, 3, 9 and 10: positive results for blaVIM; Lanes 4, 5, 7, 11, and 12: positive results for blaKPC.
Table 7. Distribution of carbapenemase genes among B2 E. coli isolates from outpatients with acute UTI.
| Carbapenemase gene | No. (%) of isolates (n = 38) |
|---|---|
| bla OXA-48 | 22 (57.8) |
| bla PER | 18 (47.3) |
| bla KPC | 6 (15.7) |
| bla VEB | 4 (10.5) |
| bla VIM | 4 (10.5) |
| bla GES | 0 |
| bla IMP | 0 |
Also, coproduction of these enzymes occurred among 17 isolates. The combination of blaOXA-48 and blaPER was the most frequent (41.1%) (Table 8). Antimicrobial susceptibility of these carbapenemase producers was depicted in Table 9.
Table 8. Coproduction of carbapenemase genes by B2 E. coli isolates from outpatients with acute UTI.
| Combination of Carbapenemase genes | No. (%) of isolates (n = 17) |
|---|---|
| blaOXA-48 + blaPER | 7 (41.1) |
| blaOXA-48 +blaVIM | 1 (5.8) |
| blaOXA-48 +blaKPC | 1 (5.8) |
| blaVEB +blaPER | 1 (5.8) |
| blaOXA-48 + blaPER + blaKPC | 3 (17.6) |
| blaOXA-48 + blaVIM + blaKPC | 1 (5.8) |
| blaOXA-48 + blaPER + blaVEB | 1 (5.8) |
| blaOXA-48 + blaPER + blaKPC + blaVEB | 1 (5.8) |
| blaOXA-48 + blaPER +blaVIM +blaVEB | 1 (5.8) |
Table 9. Antimicrobial resistance of carbapenemase producing B2 UPEC isolates.
| Antimicrobial category | Antimicrobial agent | No. (%) of isolates* (n = 29) |
|---|---|---|
| Penicillins | Ampicillin (AMP) | 29 (100) |
| Penicillins + β-lactamase inhibitors | Amoxicillin-clavulanic acid (AMC) | 27 (93.1) |
| 3rd and 4th generation cephalosporins | Cefepime (FEP) | 24 (82.7) |
| Ceftazidime (CAZ) | 24 (82.7) | |
| Cefotaxime (CTX) | 23 (79.3) | |
| Ceftriaxone (CRO) | 21 (72.4) | |
| Monobactams | Aztreonam (ATM) | 21 (72.4) |
| Cephamycins | Cefoxitin (FOX) | 10 (34.4) |
| Carbapenems | Imipenem (IPM) | 0 |
| Meropenem (MEM) | 0 | |
| Tetracyclines | Tetracycline (TE) | 21 (72.4) |
| Trimethoprim | trimethoprim-sulfamethoxazole (SXT) | 19 (65.5) |
| Quinolones | Nalidixic acid (NA) | 16 (55.1) |
| Ciprofloxacin (CIP) | 9 (31) | |
| Aminoglycosides | Gentamicin (CN) | 7 (24.1) |
| Amikacin (AK) | 1 (3.4) | |
| Nitrofurans | Nitrofurantoin (F) | 3 (10.3) |
*Resistant and intermediate.
From these results, it seems likely that the situation is more complicated than it is expected as the isolates: (1) belonged to the highly virulent phylogroup B2 and (2) carried not only one gene but combination of genes in addition to ESBLs’ production. This study results revealed that all carbapenemase producers (100%) were MDR. Patterns of antimicrobial resistance and carbapenemase genes of these isolates were presented in Table 10. Multidrug resistance is not attributed to a certain set of genes but in fact it is a result of possession of a combination of different genes especially ESBLs. Nikaido [70] concluded that MDR is due to accumulation of genes, each coding for resistance to a single antimicrobial agent, on R plasmids. This accumulation of these genes is carried out by transposons, integrons, and insertion sequences (ISCR). In UPEC, the mechanisms of antimicrobial resistance are diverse and comprise: production of inactivating enzymes either hydrolytic enzymes such as β-lactamases or no hydrolytic enzymes such as aminoglycoside acetyl transferase enzymes. Other mechanisms include: active efflux pumps, alteration of target site, horizontal gene transfer by insertion sequences, gene cassettes, integrons, and transposons [3, 71]. Eshetie et al. [72] reported that carbapenemase producing Enterobacteriaceae had significant drug resistance rates compared to other MDR Enterobacteriaceae and that production of these enzymes is one of the main mechanisms in the occurrence of drug resistance in this family. So that, Jain et al. [73] concluded that antimicrobial resistance due to β-lactamases is emerging as a major problem in UTI; routine surveillance of these β -lactamases will help in control of treatment failures. In fact, one of the critical priorities of the World Health Organization is carbapenem-resistant ESBL-producing bacterial pathogens for which new antibiotics should be designed [4].
Table 10. Phenotypic and genotypic resistance patterns of 29 MDR carbapenemase producing B2 UPEC isolates.
| No. (%) of isolates | Phenotypic resistance* | carbapenemase genes |
|---|---|---|
| 6 (20.6) | CTX, CRO, CAZ, FEP, ATM, AMP, AMC, SXT, TE, CIP, NA | blaOXA-48, blaVIM, blaKPC, blaPER, blaVEB |
| 3(10.3) | CTX, CRO, CAZ, FEP, ATM, AMP, AMC, TE | blaPER, blaOXA-48, blaKPC |
| 2 (6.8) | CTX, CRO, CAZ, FEP, ATM, AMP, AMC, CN, SXT, TE, F, CIP, NA | blaPER, blaOXA-48, blaKPC |
| 2 (6.8) | CAZ, FEP, FOX, AMP, AMC, TE | blaVIM, blaPER, blaOXA-48, blaKPC |
| 1 (3.4) | CTX, CRO, CAZ, FEP, FOX, ATM, AMP, AMC, CN, SXT, NA | blaPER, blaOXA-48 |
| 1(3.4) | CTX, CRO, CAZ, FEP, ATM, AMP, AMC, CN, SXT, TE, CIP, NA | blaPER, blaOXA-48 |
| 1(3.4) | CTX, CRO, CAZ, FEP, FOX, ATM, AMP, AMC, SXT, TE, CIP, NA | blaPER, blaVEB, blaOXA-48 |
| 1 (3.4) | CTX, CRO, CAZ, FEP, FOX, ATM, AMP, AMC, CN, CIP, NA | bla PER |
| 1 (3.4) | CTX, CAZ, FEP, FOX, AMP, AMC, AK, SXT, TE | bla PER |
| 1 (3.4) | CTX, CRO, CAZ, FEP, FOX, ATM, AMP, AMC, SXT, TE | blaPER, blaOXA-48 |
| 1 (3.4) | CTX, CRO, CAZ, FEP, FOX, AMP, AMC, SXT, F, CIP, NA | bla OXA-48 |
| 1 (3.4) | CTX, CRO, CAZ, FEP, ATM, AMP, AMC, CN, SXT, TE | blaPER, blaOXA-48, blaKPC |
| 1(3.4) | CTX, CRO, CAZ, FEP, FOX, ATM, AMP, AMC, TE | blaPER, blaVEB |
| Continued | ||
| 1 (3.4) | CTX, CRO, CAZ, FEP, ATM, AMP, AMC, SXT, TE | blaPER, blaVEB, blaOXA-48, blaVIM |
| 1 (3.4) | AMP, AMC, SXT, TE, NA | bla OXA-48 |
| 1 (3.4) | AMP, AMC, CN, SXT, NA | blaPER, blaOXA-48 |
| 1 (3.4) | CAZ, AMP, AMC, SXT, TE | bla PER |
| 1 (3.4) | (CTX, CRO, CAZ, FEP, ATM, AMP, AMC | bla PER |
| 1 (3.4) | CTX, ATM, AMP, NA | bla OXA-48 |
| 1 (3.4) | FEP, FOX, AMP | bla PER |
* Cephalosporins (CTX: cefotaxime, CRO: ceftriaxone, CAZ: ceftazidime, FEP: cefepime); Monobactams (ATM: aztreonam); Penicillins (AMP: ampicillin); Penicillins + β-lactamase inhibitors (AMC: amoxicillin-clavulanic acid); Trimethoprim (SXT: trimethoprim-sulfamethoxazole); Tetracyclines (TE: tetracycline), Quinolones (CIP: ciprofloxacin, NA: nalidixic acid). Aminoglycosides (CN: gentamicin, AK; amikacin; Nitrofurans (F: nitrofurantoin), Cephamycins (FOX: cefoxitin).
In a recent study [23] that was carried out in Iraq, it was found that out of 300 isolates, 11 (3.66%) of them were phenotypically resistant to carbapenems, whereas only 3 (1%) isolates were genotypically positive for carbapenemases of which blaOXA-48 and blaIMP genes were co-existed in these 3 isolates, while blaKPC, blaNDM and blaVIM were not found. Furthermore, nine of these 11 isolates belonged to B2 phylogroup and two were from B1 group. Most other studies [20–22, 74] showed the predominance of OXA-48 among E. coli isolates, especially B2 isolates. Other studies in Iraq [75] and around the world [44, 76–82] also revealed the occurrence of different carbapenemase genes among E. coli isolates.
Although, all of the isolates in this study were phenotypically susceptible to imipenem and meropenem by disc diffusion method, genotypically, carbapenemase production was obvious among them ranging from 10.5% for both VEB and VIM to 57.8% for OXA-48. This discrepancy was noticed by other researchers [20, 74]. It was explained that detection of carbapenemase-producing E. coli (CP- E. coli) isolates is often difficult due to low carbapenem MICs that may remain within the susceptibility range [83]. Also, OXA-48 enzymes are known to exhibit only low hydrolytic activity toward carbapenems [84]. Moreover, it seems better to use ertapenem disc diffusion for phenotypic detection of those especially producing OXA-48 rather than imipenem or meropenem [20]. The same researchers concluded that laboratory detection of CP-E. coli may be more difficult in comparison with CP-K. pneumoniae, particularly in the case of OXA-48, because: (i) the isolates may appear susceptible to imipenem and meropenem; and (ii) there is a high frequency of OXA-48-producing isolates without ESBL co-production.
The results of the present investigation showed high occurrence of carbapenemase genes among B2 UPEC isolates. This may be due to increased use of carbapenems by physicians for treatment of serious and even non-serious cases in the study area (personal communication). Also, in this work only B2 isolates were investigated for carbapememases among which these genes are concentrated. In spain [20], it was revealed that the CP-E. coli occurred after a continuous increase in resistance to third-generation cephalosporins and fluoroquinolones.
The production of all kinds of carbapenemases by E. coli isolates represent a major issue with further problem in UTI treatment [60]. From a therapeutic perspective, CRE represent a threat as only a few antibiotics retain activity against them. This is due to the ability of carbapenemases to hydrolyze most other β-lactam antibiotics, and to frequent coexistence in CRE isolates of additional mechanisms of resistance against other antibiotics such as fluoroquinolones and aminoglycosides [85]. Furthermore, the indiscriminate use of carbapenems can select resistance to these main drugs and sow seeds for significant therapeutic problems that may occur in the future. Efficient infection-control methods for outbreak management are also needed; and prevention approaches, e.g., antibiotic rotation, are needed to reduce the selection and spread of these highly resistant pathogens [86]. Today, the extensive international movement and exchange has helped OXA-48 producing Enterobacteriaceae to spread from many Middle-Eastern countries into other parts of the world [11].
Conclusions
This study revealed high occurrence of carbapenemase genes and MDR among phylogroup B2 UPEC isolates from outpatients with acute UTI in Wasit Province, Iraq. These findings may indicate the concentration of these genes among E. coli phylogroup B2 members. Also, this study included isolates showed high resistance rates to most antimicrobials used in this work except aminoglycosides and nitrofurantoin, so that, it is strongly recommended to use aminoglycosides and nitrofurantoin for empirical treatment of UTIs in the study area.
Supporting information
(PDF)
Acknowledgments
The authors are grateful to the crew of Microbiology laboratory, Department of Biology, College of Science, Wasit University for providing the isolates included in this work.
Data Availability
All relevant data are within the paper.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Sousa CP. The versatile strategies of Escherichia coli pathotypes: A mini review. J Venom Anim Toxins incl Trop Dis. 2006; 12 (3): 363–373. [Google Scholar]
- 2.Carroll KC, Mietzner TA, Hobden JA, Detrick B, Miller S, Mitchell TG, et al. Jawetz, Melnick & Adelberg’s Medical Microbiology. 27th. ed. New York: McGraw-Hill Education; 2016. [Google Scholar]
- 3.Bunduki GK, Heinz E, Phiri VS, Noah P, Feasey N, Musaya J. Virulence factors and antimicrobial resistance of uropathogenic Escherichia coli (UPEC) isolated from urinary tract infections: a systematic review and meta-analysis. BMC Infect Dis. 2021; 21:753. Available from: doi: 10.1186/s12879-021-06435-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martinez-Medina M. Special Issue: Pathogenic Escherichia coli: Infections and Therapies. Antibiotics. 2021; 10: 112. Available from: doi: 10.3390/antibiotics10020112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Terlizzi ME, Gribaudo G, Maffei ME. Uropathogenic Escherichia coli (UPEC) Infections: Virulence Factors, Bladder Responses, Antibiotic, and Non-antibiotic Antimicrobial Strategies. Front. Microbiol. 8:1566. Available from: doi: 10.3389/fmicb.2017.01566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hawkey PM, Livermore DM. Carbapenem antibiotics for serious infections. BMJ 2012; 344: e3236. Available from: doi: 10.1136/bmj.e3236 [DOI] [PubMed] [Google Scholar]
- 7.Knapp KM, English BK. Carbapenems. Semin. Pediatr Infect Dis. 2001; 12:175–185. [Google Scholar]
- 8.Codjoe FS, Donkor ES. Carbapenem Resistance: A Review. Med Sci. 2018; 6 (1): 2–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cuzon G, Naas T, Guibert M, Nordmann P. In vivo selection of imipenem resistant Klebsiella pneumoniae producing extended-spectrum beta-lactamase CTX-M-15 and plasmid-encoded DHA-1 cephalosporinase. Int J Antimicrob Ag. 2010a; 35: 265–8. [DOI] [PubMed] [Google Scholar]
- 10.Nordmann P, Naas T, Poirel L. Global spread of Carbapenemase producing Enterobacteriaceae. Emerg Infect Dis. 2011; 17:1791–8. doi: 10.3201/eid1710.110655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Halat DH, Moubareck CA. The Current Burden of Carbapenemases: Review of Significant Properties and Dissemination among Gram-Negative Bacteria. Antibiotics. 2020; 9 (186): 1–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Makharita RR, El-Kholy I, Hetta HF, Abdelaziz MH, Hagagy FI, Ahmed AA, Algammal AM. Antibiogram and genetic characterization of carbapenem-resistant gram-negative pathogens incriminated in healthcare-associated infections. Infect Drug Resist. 2020; 13: 3991–4002. doi: 10.2147/IDR.S276975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Algammal AM, Hetta HF, Batiha GE, Hozzein WN, El Kazzaz WM, Hashem HR, et al. Virulence-determinants and antibiotic-resistance genes of MDR-E. coli isolated from secondary infections following FMD-outbreak in cattle. Sci Rep. 2020; 10(1): 1–13. doi: 10.1038/s41598-019-56847-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Algammal AM, Hashem HR, Al-Otaibi AS, Alfifi KJ, El-Dawody EM, Mahrous E. et al. Emerging MDR-Mycobacterium avium subsp. avium in house-reared domestic birds as the first report in Egypt. BMC Microbiol. 2021a; 21(1): 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Algammal AM, Hashem HR, Alfifi KJ, Hetta HF, Sheraba NS, Ramadan H. et al. atp D gene sequencing, multidrug resistance traits, virulence-determinants, and antimicrobial resistance genes of emerging XDR and MDR-Proteus mirabilis. Sci Rep. 2021b; 11(1): 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson JR, Delavari P, Kuskowski M, Stell AL. Phylogenetic distribution of extraintestinal virulence-associated traits in Escherichia coli. J Infect Dis. 2010; 183 (1): 78–88. [DOI] [PubMed] [Google Scholar]
- 17.Clermont O, Christenson JK, Denamur E, Gordon DM. The Clermont Escherichia coli phylo-typing method revisited: improvement of specificity and detection of new phylo-groups. Environ Microbiol Rep. 2013; 5(1): 58–65. doi: 10.1111/1758-2229.12019 [DOI] [PubMed] [Google Scholar]
- 18.Delory T, Seringe E, Antoniotti G, Novakova I, Goulenok C, Paysant I. et al. Prolonged delay for controlling KPC-2-producing Klebsiella pneumoniae outbreak: The role of clinical management. Am J Infect Control. 2015; 43:1070–5. doi: 10.1016/j.ajic.2015.05.021 [DOI] [PubMed] [Google Scholar]
- 19.Kot B. Antibiotic resistance among uropathogenic Escherichia coli: A mini review. Pol J Microbiol. 2019; 68(4): 403–415. doi: 10.33073/pjm-2019-048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ortega A, Sa´ez D, Bautista V, Ferna´ndez-Romero S, Lara N, Aracil B. et al. Carbapenemase-producing Escherichia coli is becoming more prevalent in Spain mainly because of the polyclonal dissemination of OXA-48. J Antimicrob Chemother. 2016; 71: 2131–8 doi: 10.1093/jac/dkw148 [DOI] [PubMed] [Google Scholar]
- 21.El-Shaer S, Abdel-Rhman SH, Barwa R, Hassan R. Genetic characterization of extended-spectrum β-Lactamase- and carbapenemase producing Escherichia coli isolated from Egyptian hospitals and environments. PLOS ONE. 2021; 16 (7): e0255219. Available from: doi: 10.1371/journal.pone.0255219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ssekatawa K, Byarugaba DK, Nakavuma JL, Kato CD, Ejobi F, Tweyongyere R, et al. Carbapenem resistance profiles of pathogenic Escherichia coli in Uganda. Ejbio. 2021; 2(2): 63–73. [Google Scholar]
- 23.Mhawesh AA, Hamid DM, Ghasemian A. Phylogrouping, serogrouping, virulence factors and carbapenemase genes among carbapenemase-producing Escherichia coli from urinary tract infections. Indian J Forensic Med Toxicol. 2021; 15 (2): 2979–3003. [Google Scholar]
- 24.Gupte S. The Short Textbook of Medical Microbiology (including parasitology). 10th. ed. New Delhi: Jaypee Brothers Medical Publishers (P) Ltd.; 2010. [Google Scholar]
- 25.Yamamoto S, Terai A, Yuri K, Kurazono H, Takeda Y, Yoshida O. Detection of urovirulence factors in Escherichia coli by multiplex polymerase chain reaction. FEMS Immuno Med Microbiol. 1995; 12: 85–90. doi: 10.1111/j.1574-695X.1995.tb00179.x [DOI] [PubMed] [Google Scholar]
- 26.Clermont O, Bonacorsi S, Bingen E. Rapid and simple determination of the Escherichia coli phylogenetic group. App Environ Microbio. 2000; 66(10): 4555–4558. doi: 10.1128/AEM.66.10.4555-4558.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clermont O, Bonacorsi S, Bingen E. Characterization of an anonymous molecular marker strongly linked to Escherichia coli strains causing neonatal meningitis. J Clin Microbiol. 2004; 42: 1770–1772. doi: 10.1128/JCM.42.4.1770-1772.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lescat M, Clermont O, Woerther PL, Glodt J, Dion S, Skurnik D., et al. Commensal Escherichia coli strains in Guiana reveal a high genetic diversity with host‐dependent population structure. Environ Microbiol Reports. 2013; 5(1): 49–57. [DOI] [PubMed] [Google Scholar]
- 29.Clermont O, Lavollay M, Vimont S, Deschamps C, Forestier C, Branger C, et al. The CTX-M-15-producing Escherichia coli diffusing clone belongs to a highly virulent B2 phylogenetic subgroup. J Antimicrob Chemother. 2008; 61(5): 1024–1028. doi: 10.1093/jac/dkn084 [DOI] [PubMed] [Google Scholar]
- 30.CLSI. Clinical and Laboratory Standard Institute. Performance standards for antimicrobial susceptibility. Twenty-fourth information supplement. CLSI Document M100-S26. Clinical and Laboratory Standard Institute, Wayne, PA: 2016. [Google Scholar]
- 31.Dallenne C, Da Costa A, Decre D, Favier C, Arlet G. Development of a set of multiplex PCR assays for the detection of genes encoding important β-lactamases in Enterobacteriaceae. J Antimicrob Chemother. 2010; 65(3): 490–495. doi: 10.1093/jac/dkp498 [DOI] [PubMed] [Google Scholar]
- 32.Alsamarai AM, Ali S. Urinary tract infection in female in Kirkuk City, Iraq: Causative agents and antibiogram. WJPPS. 2016; 5: 261–273. [Google Scholar]
- 33.Ghaima KK, Khalaf ZS, Abdulhassan AA, Salman NY. Prevalence and antibiotic resistance of bacteria isolated from urinary tract infections of pregnant women in Baghdad hospitals. Biomed Pharmacol J. 2018; 11(4): 1989–1994. [Google Scholar]
- 34.Rafalskiy V, Pushkar D, Yakovlev S, Epstein O, Putilovskiy M, Tarasov S. et al. Distribution and antibiotic resistance profile of key Gram-negative bacteria that cause community-onset urinary tract infections in the Russian Federation: RESOURCE multicentre surveillance 2017 study. J Glob Antimicrob Resist. 2019; 21:188–194. doi: 10.1016/j.jgar.2019.09.008 [DOI] [PubMed] [Google Scholar]
- 35.Sharma D, Preston SE, Hage R. Emerging antibiotic resistance to bacterial isolates from human urinary tract infections in Grenada. Cureus. 2019; 11(9): e5752. Available from: doi: 10.7759/cureus.5752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wasiński B. Extra-intestinal pathogenic Escherichia coli-threat connected with food-borne infections. Ann Agric Environ Med. 2019; 26(4): 532–537. doi: 10.26444/aaem/111724 [DOI] [PubMed] [Google Scholar]
- 37.Kudinha T. The pathogenesis of Escherichia coli urinary tract infection. In: Samie A, editor. Escherichia coli: Recent Advances on Physiology, Pathogenesis and Biotechnological Applications. BoD–Books on Demand. In Tech; 2017. pp. 45–69. [Google Scholar]
- 38.Merza NS, Jubrael JMS. Phylogenetic grouping of uropathogenic Escherichia coli using different molecular typing methods in Kurdistan Region-Iraq. Int J Chem Bio Sci. 2015; 1(4): 284–291. [Google Scholar]
- 39.Ahmed SS, Shariq A, Alsalloom AA, Babikir IH, Alhomoud BN. Uropathogens and their antimicrobial resistance patterns: Relationship with urinary tract infections. Int J Health Sci. 2019; 13(2): 48–55. [PMC free article] [PubMed] [Google Scholar]
- 40.Hussain AR, Saleh MB. Determination of phylogenetic groups and antimicrobial susceptibility patterns for Escherichia coli isolated from patients with urinary tract infection. Journal of Education For Pure Science—University Of Thi-Qar. 2019; 9(1): 71–81. [Google Scholar]
- 41.Iranpour D, Hassanpour M, Ansari H, Tajbakhsh S, Khamisipour G, Najafi A. Phylogenetic groups of Escherichia coli strains from patients with urinary tract infection in Iran based on the new Clermont phylotyping method. BioMed Res Int. 2015; 2015:846219. Available from: doi: 10.1155/2015/846219 Epub 2015 Jan 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Massot M, Daubié AS, Clermont O, Jaureguy F, Couffignal C, Dahbi G. et al. Phylogenetic, virulence and antibiotic resistance characteristics of commensal strain populations of Escherichia coli from community subjects in the Paris area in 2010 and evolution over 30 years. Microbiol. 2016; 162(4): 642–650. doi: 10.1099/mic.0.000242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miranda-Estrada LI, Ruíz-Rosas M, López J, Parra-Rojas I, González-Villalobos E, Castro-Alarcón N. Relationship between virulence factors, resistance to antibiotics and phylogenetic groups of uropathogenic Escherichia coli in two locations in Mexico. Enferm Infecc Microbiol Clin. 2017; 35(7): 426–433. doi: 10.1016/j.eimc.2016.02.021 [DOI] [PubMed] [Google Scholar]
- 44.Katongole P, Kisawuzi DB, Bbosa HK, Kateete DP, Najjuka CF. Phylogenetic groups and antimicrobial susceptibility patterns of uropathogenic Escherichia coli clinical isolates from patients at Mulago National Referral Hospital, Kampala, Uganda. F1000Research. 2019; 8: 1828. Available from: 10.12688/f1000research.20930.1. [DOI] [Google Scholar]
- 45.Zhong YM, Liu WE, Meng Q, Li Y. Escherichia coli O25b-ST131 and O16-ST131 causing urinary tract infection in women in Changsha, China: molecular epidemiology and clinical characteristics. Infec Drug Resist. 2019; 12: 2693–2702. doi: 10.2147/IDR.S212658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Johnson JR, Kuskowski MA, Gajewski A, Soto S, Horcajada JP, De Anta MTJ, Vila J. Extended virulence genotypes and phylogenetic background of Escherichia coli isolates from patients with cystitis, pyelonephritis, or prostatitis. J Infec Dis. 2005; 191(1): 46–50. doi: 10.1086/426450 [DOI] [PubMed] [Google Scholar]
- 47.Lee JH, Subhadra B, Son YJ, Kim DH, Park HS, Kim JM, et al. Phylogenetic group distributions, virulence factors and antimicrobial resistance properties of uropathogenic Escherichia coli strains isolated from patients with urinary tract infections in South Korea. Lett Appl Microbiol. 2015; 62: 84–90. doi: 10.1111/lam.12517 [DOI] [PubMed] [Google Scholar]
- 48.Najafi A, Hasanpour M, Askary A, Aziemzadeh M, Hashemi N. Distribution of pathogenicity island markers and virulence factors in new phylogenetic groups of uropathogenic Escherichia coli isolates. Folia Microbiologica. 2017; 63(3): 335–343. doi: 10.1007/s12223-017-0570-3 [DOI] [PubMed] [Google Scholar]
- 49.Lara FBM, Nery DR, Oliveira PMd, Araujo ML, Carvalho FRQ, Messias-Silva LCF. Virulence markers and phylogenetic analysis of Escherichia coli strains with hybrid EAEC/UPEC genotypes recovered from sporadic cases of extraintestinal infections. Front Microbiol. 8:146. Available from: doi: 10.3389/fmicb.2017.00146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ali I, Rafaque Z, Ahmed I, Tariq F, Graham SE, Salzman E. et al. Phylogeny, sequence-typing and virulence profile of uropathogenic Escherichia coli (UPEC) strains from Pakistan. BMC Infect Dis. 2019; 19(1): 620. Available from: doi: 10.1186/s12879-019-4258-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Poirel L, Madec J.-Y, Lupo A, Schink A.-K, Kieffer N, Nordmann P, et al. Antimicrobial resistance in Escherichia coli. Microbiol Spectr. 2018; 6(4). Available from: doi: 10.1128/microbiolspec.ARBA-0026-2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Polse RF, Yousif SY, Assafi MS. Prevalence and antimicrobial susceptibility patterns of uropathogenic E. coli among people in Zakho, Iraq. Int J Res Med Sci. 2016; 4(4): 1219–1223. [Google Scholar]
- 53.Mazzariol A, Bazaj A, Cornaglia G. Multi-drug-resistant Gram-negative bacteria causing urinary tract infections _ a review. J Chemother. 2017; 29 suppl 1: 2–9. doi: 10.1080/1120009X.2017.1380395 [DOI] [PubMed] [Google Scholar]
- 54.Miotla P, Romanek-Piva K, Bogusiewicz M, Markut-Miotla E, Adamiak A, Wróbel A. et al. Antimicrobial resistance patterns in women with positive urine culture: Does menopausal status make a significant difference? BioMed Res Int. 2017; vol. 2017. Available from: doi: 10.1155/2017/4192908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee DS, Lee SJ, Choe HS. Community-acquired urinary tract infection by Escherichia coli in the era of antibiotic resistance. Biomed Res Int. 2018; Vol. 2018. Available from: doi: 10.1155/2018/7656752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Al-Hilali S. Genetic affinities of multiple drug resistant uropathogenic Escherichia coli isolated from patients with urinary tract infection in Najaf. Ph.D. Thesis, University of Kufa, Department of Microbiology, College of Medicine, Iraq. 2015.
- 57.Hussein AA. Distribution of ESBLs and multidrug resistance among uropathogenic Escherichia coli isolates from patients with first time and recurrent urinary tract infection: A comparison study. M.Sc. Thesis, Wasit University, College of Science, Iraq. 2019.
- 58.Lin Z, Chen T, Fu X. Correlational study between discontinuation of the fourth-generation cephalosporin and the dosage of broad-spectrum antibacterial agents as well as resistance rates of Pseudomonas aeruginosa against antimicrobials. Int J Clin Exper Med. 2018; 11(3), 2256–2263. [Google Scholar]
- 59.Meletis G. Carbapenem resistance: overview of the problem and future perspectives. Ther Adv Infec Dis. 2016; 3(1): 15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shaik S, Ranjan A, Tiwari SK, Hussain A, Nandanwar N, Kumar N. et al. Comparative genomic analysis of globally dominant ST131 clone with other epidemiologically successful extraintestinal pathogenic Escherichia coli (ExPEC) lineages. mBio. 2017; 8: e01596–17. Available from: doi: 10.1128/mBio.01596-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sanjee SA, Karim ME, Akter T, Parvez MAK, Hossain M, Jannat B, et al. Prevalence and antibiogram of bacterial uropathogens of urinary tract infections from a Tertiary Care Hospital of Bangladesh. J Sci Res. 2017; 9(3): 317–328. [Google Scholar]
- 62.Abd Al-Mayahi FS, Almohana AM. Antibiotic susceptibility patterns of Escherichia coli isolates from patients with significant bacteriuria. Al-Qadisiyha Journal for Science. 2015; 20 (1): 29–49. [Google Scholar]
- 63.Al-Hamadani AH, Al-Rikabi AM, Al-Fatlawi A.F. Detection of TEM and SHV genes in Escherichia coli and Klebsiella species isolated from cancer patients in Al-Diwaniya Governorate. Q M J. 2013; 9 (16): 22–39. [Google Scholar]
- 64.Elsayed TI, Ismail HA, Ahmed HA, Gad SAE. The occurrence of multidrug resistant E. coli which produce ESBL and cause urinary tract infections. J Appl Microbiol Biochem. 2017; 1 (2): 8.; 1(2): 8. Available from: http://www.imedpub.com/applied-microbiology-and-biochemistry. [Google Scholar]
- 65.Fernando MMPSC, Luke WANV, Mithinda JKND, Wickramasinghe RDSS, Sebastiampillai BS, Gunathilake MPML. et al. Extended spectrum beta lactamase producing organisms causing urinary tract infections in Sri Lanka and their antibiotic susceptibility pattern–A hospital based cross sectional study. BMC Infect Dis. 2017; 17 (1): 1–6. doi: 10.1186/s12879-016-2122-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Goyal D, Dean N, Neill S, Jones P, Dascomb K. Risk factors for community-acquired extended-spectrum Beta-lactamase producing Enterobacteriaceae infections–a retrospective study of symptomatic urinary tract infections. OFID. 2019; 6 (2), ofy357. Available from: doi: 10.1093/ofid/ofy357 eCollection 2019 Feb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Murray TS, Peaper DR. The contribution of extended-spectrum β-lactamases to multidrug-resistant infections in children. Curr Opin Pediatr. 2015; 27(1), 124–131. doi: 10.1097/MOP.0000000000000182 [DOI] [PubMed] [Google Scholar]
- 68.Ali FA. Distribution of CTX-M gene among Escherichia coli strains isolated from different clinical samples in Erbil City. IJB. 2018; 17 (1): 87–90. [Google Scholar]
- 69.Alqasim A, Abu Jaffal A, Alyousef AA. Prevalence of multidrug resistance and extended-spectrum β-lactamase carriage of clinical uropathogenic Escherichia coli isolates in Riyadh, Saudi Arabia. Int J Microbiol. 2018; 1–9. Available from: doi: 10.1155/2018/3026851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nikaido H. Multidrug resistance in bacteria. Annu Rev Biochem. 2009; 78: 119–146. doi: 10.1146/annurev.biochem.78.082907.145923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Peleg AY, Hooper DC. Hospital-acquired infections due to Gram-negative bacteria. N Engl J Med. 2010; 362(19):1804–1813. doi: 10.1056/NEJMra0904124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Eshetie S, Unakal C, Gelaw A, Ayelign B, Endris M, Moges F. Multidrug resistant and carbapenemase producing Enterobacteriaceae among patients with urinary tract infection at referral hospital, Northwest Ethiopia. Antimicrob Resist and Infect Control. 2015; 4(1): 1–8. doi: 10.1186/s13756-015-0054-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jain R, Pal N, Hooja S. Prevalence of β-lactamase production and multi-drug resistance among uropathogenic Escherichia coli isolates at a tertiary care hospital of North-western India. Asian J Med Sci. 2021; 12(7): 27–32. [Google Scholar]
- 74.Gauthier L, Dortet L, Cotellon G, Creton E, Cuzon G, Ponties V. et al. Diversity of carbapenemase-producing Escherichia coli isolates in France in 2012–2013. Antimicrob Agents Chemother. 2018; 62 (8): e00266–18. Available from: doi: 10.1128/AAC.00266-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Al-Sa’ady A, Mohammad G, Hussen B. Genetic relation and virulence factors of carbapenemase-producing uropathogenic Escherichia coli from urinary tract infections in Iraq. Gene Rep. 2020; 21: 100911. Available from: 10.1016/j.genrep.2020.100911. [DOI] [Google Scholar]
- 76.Dimou V, Dhanji H, Pike R, Livermore DM, Woodford N. Characterization of Enterobacteriaceae producing OXA-48-like carbapenemases in the UK. J Antimicrob Chemother. 2012; 67(7): 1660–1665. doi: 10.1093/jac/dks124 [DOI] [PubMed] [Google Scholar]
- 77.Accogli M, Giani T, Monaco M, Giufrè M, García-Fernández A, Conte V. et al. Emergence of Escherichia coli ST131 sub-clone H30 producing VIM-1 and KPC-3 carbapenemases, Italy. J Antimicrob Chemother. 2014; 69(8): 2293–2296. doi: 10.1093/jac/dku132 [DOI] [PubMed] [Google Scholar]
- 78.O’Hara JA, Hu F, Ahn C, Nelson J, Rivera JI, Pasculle AW, Doi Y. Molecular epidemiology of KPC-producing Escherichia coli: occurrence of ST131-fimH30 subclone harboring pKpQIL-like IncFIIk plasmid. Antimicrob Agents Chemother. 2014; 58(7): 4234–4237. doi: 10.1128/AAC.02182-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mathers AJ, Peirano G, Pitout JD. The role of epidemic resistance plasmids and international high-risk clones in the spread of multidrug-resistant Enterobacteriaceae. Clin Microbiol Rev. 2015; 28(3): 565–591. doi: 10.1128/CMR.00116-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.AbouZeid A, Allam A, Mahmoud S. Prevalence of extended spectrum β-lactamase producing E. coli isolated from inpatients and outpatients in Zagazig University hospitals. BFSZU. 2017; 39: 31–48. [Google Scholar]
- 81.Hojabri Z, Mirmohammadkhani M, Darabi N, Arab M, Pajand O. Characterization of antibiotic-susceptibility patterns and virulence genes of five major sequence types of Escherichia coli isolates cultured from extraintestinal specimens: a 1-year surveillance study from Iran. Infect Drug Resist. 2019. a; 12: 893–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hojabri Z, Darabi N, Arab M, Saffari F, Pajand O. Clonal diversity, virulence genes content and subclone status of Escherichia coli sequence type 131: comparative analysis of E. coli ST131 and non-ST131 isolates from Iran. BMC Microbiol. 2019. b; 19(1): 117. Available from: 10.1186/s12866-019-1493-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vila J, Saez-L´opez E, Johnson JR, Romling U, Dobrindt U, Canton R. et al. Escherichia coli: an old friend with new tidings. FEMS Microbiol Rev. 2016; 40: 437–463. doi: 10.1093/femsre/fuw005 [DOI] [PubMed] [Google Scholar]
- 84.Oueslati S, Nordmann P, Poirel L. Heterogeneous hydrolytic features for OXA-48-like β-lactamases. J Antimicrob Chemother. 2015; 70: 1059–1063. doi: 10.1093/jac/dku524 [DOI] [PubMed] [Google Scholar]
- 85.Moxon CA, Paulus S. Beta-lactamases in Enterobacteriaceae infections in children. J. Infect. 2016; 72, S41–S49. doi: 10.1016/j.jinf.2016.04.021 [DOI] [PubMed] [Google Scholar]
- 86.Rawat D, Nair D. Extended-spectrum β-lactamases in Gram-negative bacteria. J Glob Infect Dis. 2010; 2(3): 263–274. doi: 10.4103/0974-777X.68531 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
Data Availability Statement
All relevant data are within the paper.