INTRODUCTION:
Higher doses of external beam radiation therapy (EBRT) for adenocarcinoma of the prostate have been extensively examined for impact on physician reported clinical endpoints including biochemical failure, physician reported toxicity, local progression, prostate cancer specific mortality, distant metastatic disease, and overall survival.1–6 Higher doses of EBRT have shown a consistent improvement in biochemical failure and also a consistent worsening of physician reported toxicity. An improvement in overall survival with dose escalated EBRT has not been demonstrated thus far, although longer follow up of existing randomized trials will be necessary to confirm this. Dose escalated EBRT has higher rates of physician reported toxicity and likely has higher costs.1,4,7 In the absence of an overall survival improvement, or definitive prostate cancer specific mortality improvement, the impact on patient reported outcomes (PROs) associated with using dose escalated EBRT is critically important. Despite multiple prospective trials, our current understanding of the impact of dose escalated EBRT, delivered using intensity modulated radiation therapy (IMRT), on PROs needs continued assessment.8,9
The NRG/RTOG 0126 trial randomized patients to either 70.2 Gy in 39 fractions or 79.2 Gy in 44 fractions.1 Eligible patients were asked to participate in collection of detailed PROs at multiple time points. This trial represents the largest, and most complete prospective PRO assessment to have evaluated the impact of higher EBRT doses to date. The trial was designed over twenty years ago, and since this time the common type of PRO’s commonly used has evolved. This data presents an opportunity to improve our understanding of the clinical impact of dose escalated EBRT as reported by patients. Prospectively collecting PROs also enables an improved evaluation of the clinical impact of EBRT dose to critical normal structures such as the rectum, penile bulb, and bladder.
METHODS:
In the NRG/RTOG 0126 clinical trial, men were enrolled with histologically confirmed adenocarcinoma of the prostate, with a performance status of 0 to 1, and a clinical stage of T1b to T2b with either a Gleason Score of 2–6 and a PSA of at least 10 and less than 20 ng/mL, or Gleason score of 7 and a PSA of less than 15 ng/mL. Patients with any evidence of metastatic disease, or prior treatment including radiation therapy, chemotherapy, or hormonal therapy were excluded from participation. This was a multicenter, phase III clinical trial, sponsored by the National Cancer Institute (NCT01434290). Patients were randomly assigned to either standard dose EBRT, 70.2 Gy given over 39 fractions, or to dose escalated EBRT 79.2 Gy given over 44 fractions, which was considered an experimental dose. The primary endpoint was overall survival.1
Measures:
Three PRO evaluations prospectively collected in the NRG/RTOG 0126 clinical trial, included: 1. The international index of erectile function questionnaire (IIEF)10, 2. Functional alterations due to changes in eliminations (FACE) and 3. The Spitzer quality of life index (SQLI).11
The IIEF is a fifteen-item questionnaire with a total score calculated from the sum of all items. Higher scores are associated with better erectile function. The IIEF is intended to evaluate several subscales such as erectile function, orgasmic function, sexual desire, intercourse satisfaction, and overall satisfaction. Internal consistency of the IIEF demonstrates Cronbach’s alphas for the five domains ranging from 0.73 to 0.92 with an overall alpha of 0.91. The IIEF has test-retest correlation coefficients, ranging from r = 0.64 to r = 0.84 depending on the domain. Sensitivity and specificity for the IIEF have been demonstrated with those patients responding to ED treatment over time showing significant change, while patients who did not respond to treatment showed no change in IIEF.10
The FACE score is a 15-item Likert-type self-rating scale designed to measure the construct of intrusion on daily functioning caused by changes in elimination as measured by two different subscales, bowel and urinary. Dimensions of the FACE include control, fear, anxiety, and interference with activities. A lower FACE score, calculated as the sum of all items, is better than a high score. Each item on the questionnaire is scored 0–4, the maximum score (representing the worst possible function) is 56 and the lowest possible score is 0. The questions focus on items related to bowel habits, urinary, constipation, gas pain, pain with urination, and urinary control. The FACE scale is rather comparable to the more widely used EPIC questionnaire, which provides similar scores of difficult with elimination (bowel and urinary). The FACE questionnaire is provided in the supplemental materials.
The SQLI is a five item categorical questionnaire with three item response options scored from 0–2 and summed in a Likert format with total scores ranging from 0–10.11 The SQLI was used as a global measure of quality of life.
Doses to multiple local normal structures including the penile bulb, rectum, and bladder were collected and correlated with PROs. Detailed measurements of the volume of each organ receiving 65 Gy (V65), 70 Gy (V70), and 75 Gy (V75) were collected and compared between the treatment arms. These metrics were correlated with each of the corresponding PRO domains. The use of intensity modulated radiation therapy IMRT was also recorded and outcomes were compared amongst the IMRT patients and those patients being treated with 3D conformal radiation therapy (3D-CRT).
With 688 patients per arm, the primary endpoint of the quality of life portion of the study had a 90% statistical power to detect a 19% reduction in erectile dysfunction at 12 months using a test of proportions with a two-sided type I error of 0.05. Erectile dysfunction was defined as answering the first question on the IIEF (How often were you able to get an erection during sexual activity?) with a score of 0–3 indicating none/almost never to ≤ half the time. Ordinal variables, such as IIEF question 1, and non-normally distributed continuous variables, such as change scores, were compared between arms using a Wilcoxon-rank sum test. Normally distributed continuous variables were compared using a t-test and categorical variables using a chi-square test. Since many analyses may be overpowered due to the large sample size, effect sizes were computed to assess clinical meaningfulness. Improvement in IIEF was defined using the minimally important difference. Minimal clinically important differences for the IIEF total score vary according to baseline severity of erectile dysfunction ranging from mild of 2, to moderate of 5, and to severe of 7.12 In order to adjust for multiplicity, change score analyses for FACE and SQLI across all post-baseline time points were adjusted using Hochberg’s method (Hochberg 1988).13
A complete case and imputed analysis were performed for IIEF and FACE total scores. In the complete case analysis, only patients who completed all items for the IIEF and FACE were included in all analyses including subscale analyses. For the imputed data analysis, patients who completed at least 13 out of 15 items for the IIEF and FACE were included. The missing items were imputed by replacing the missing item response with the average item response (correcting for reverse scoring in the FACE) for that patient. Multi-variable analyses (MVA) using mixed effects models with maximum likelihood estimation, considered exploratory as they were not specified in the protocol but are considered appropriate in the presence of data missing at random, were conducted to determine the effect of covariates, including age (≤ 70 vs > 70), race (white vs. non-white), RT method (3D-CRT vs. IMRT) and treatment arm (standard RT vs dose escalated RT), on IIEF and FACE total scores over time. An area under the curve (AUC) analysis was performed to compare the FACE urinary and bowel symptoms across all timepoints and compared between arms using a t-test. Doses to penile bulb, rectum, and bladder, were correlated with IIEF and FACE total scores using Pearson correlation coefficients.
Correlation coefficients can be interpreted as weak if ≤0.35, moderate for 0.36–0.69, and ≥0.70 is considered a strong correlation.14,15 Percent volume of bladder, rectum, and penile bulb irradiated was compared between arms using a Wilcoxon-rank sum test. Due to the exploratory nature of these comparisons, no multiplicity adjustment was made.
RESULTS:
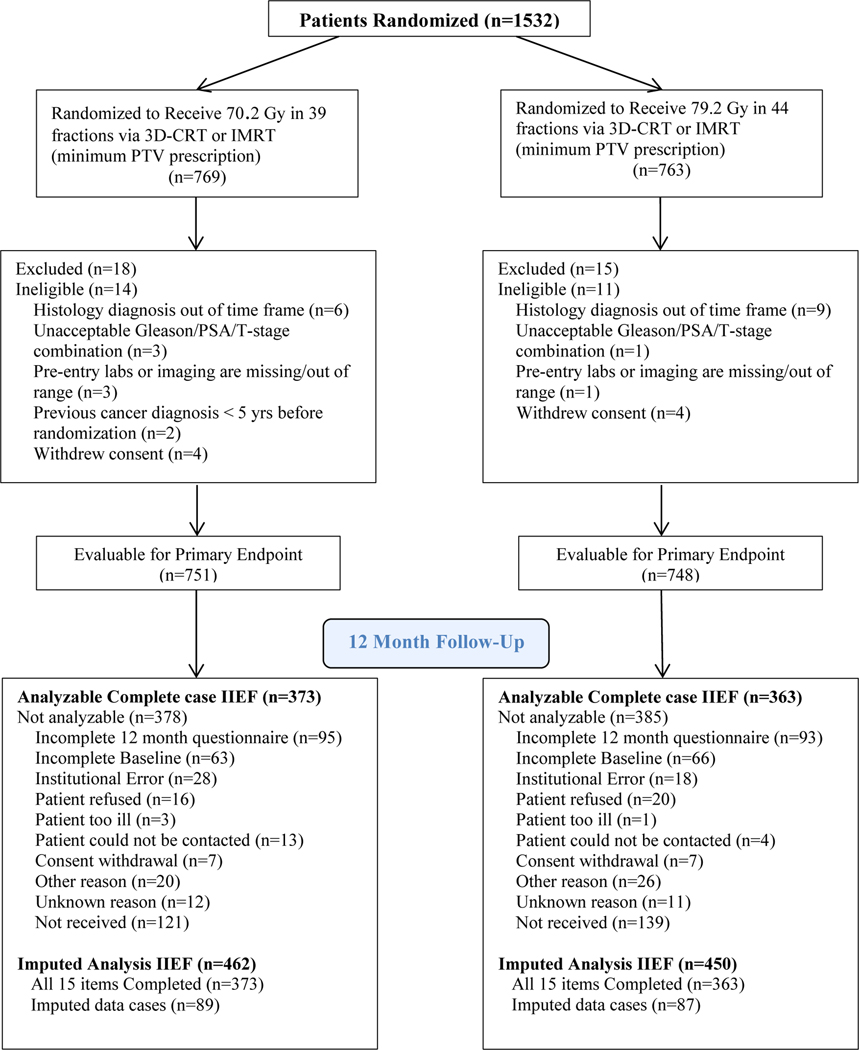
Of the 1,532 patients randomized in NRG/RTOG 0126 trial a total of 736 completed the PRO assessment with analyzable complete case data. This included 373 patients on the standard dose arm and 363 patients on the dose escalated RT arm who were analyzable for the change from baseline to 12 months in IIEF, which was the primary endpoint (Figure 1). Pre-treatment characteristics of patients participating in both the IIEF and FACE PRO were compared across treatment arms for any statistically significant differences. No statistically significant differences in patient characteristics were found when comparing across the standard and high dose treatment arms for both the IIEF and the FACE metrics.(Table 1)
Figure 1 –
CONSORT diagram.
Table 1:
Pretreatment Characteristics (Answered all 15 questions on IIEF and FACE at baseline)
| IIEF | p-value | FACE | p-value | |||
|---|---|---|---|---|---|---|
| 3D-CRT/IMRT 70.2 Gy (n=572) | 3D-CRT/IMRT 79.2 Gy (n=572) | 3D-CRT/IMRT 79.2 Gy (n=564) | 3D-CRT/IMRT 79.2 Gy (n=559) | |||
| Age(years) | ||||||
| Median | 70 | 70.5 | 71 | 71 | ||
| Min - Max | 33 – 86 | 49 – 87 | 43 – 86 | 50 – 87 | ||
| Q1 - Q3 | 64 – 74 | 65 – 74 | 0.44 | 64.5 – 74 | 64 – 74 | 0.97 |
| Race | ||||||
| White | 493 ( 86.2%) | 477 ( 83.4%) | 482 ( 85.5%) | 457 ( 81.8%) | ||
| Black | 61 ( 10.7%) | 70 ( 12.2%) | 61 ( 10.8%) | 78 ( 14.0%) | ||
| Other | 18 ( 3.1%) | 25 ( 4.4%) | 0.36 | 21 ( 3.7%) | 24 ( 4.3%) | 0.23 |
| Ethnicity | ||||||
| Hispanic or Latino | 17 ( 3.0%) | 16 ( 2.8%) | 19 ( 3.4%) | 14 ( 2.5%) | ||
| Not Hispanic or Latino | 521 ( 91.1%) | 531 ( 92.8%) | 515 ( 91.3%) | 524 ( 93.7%) | ||
| Unknown | 34 ( 5.9%) | 25 ( 4.4%) | 0.47 | 30 ( 5.3%) | 21 ( 3.8%) | 0.30 |
| Zubrod Performance Status | ||||||
| 0 | 523 ( 91.4%) | 535 ( 93.5%) | 514 ( 91.1%) | 515 ( 92.1%) | ||
| 1 | 49 ( 8.6%) | 37 ( 6.5%) | 0.18 | 50 ( 8.9%) | 44 ( 7.9%) | 0.55 |
| PSA(Study Entry) | ||||||
| <10 ng/ml | 397 ( 69.4%) | 406 ( 71.0%) | 403 ( 71.5%) | 400 ( 71.6%) | ||
| 10-<15 ng/ml | 154 ( 26.9%) | 134 ( 23.4%) | 140 ( 24.8%) | 126 ( 22.5%) | ||
| 15–20 ng/ml | 21 ( 3.7%) | 32 ( 5.6%) | 0.15 | 21 ( 3.7%) | 33 ( 5.9%) | 0.18 |
| Gleason | ||||||
| 2–6 | 91 ( 15.9%) | 89 ( 15.6%) | 78 ( 13.8%) | 84 ( 15.0%) | ||
| 7 | 481 ( 84.1%) | 483 ( 84.4%) | 0.87 | 486 ( 86.2%) | 475 ( 85.0%) | 0.57 |
| T Stage | ||||||
| T1 | 331 ( 57.9%) | 315 ( 55.1%) | 323 ( 57.3%) | 310 ( 55.5%) | ||
| T2 | 241 ( 42.1%) | 257 ( 44.9%) | 0.34 | 241 ( 42.7%) | 249 ( 44.5%) | 0.54 |
| Urinary incontinence at study entry(severity, physician reported ) | ||||||
| GRADE 0 | 544 ( 95.1%) | 537 ( 93.9%) | 540 ( 95.7%) | 522 ( 93.4%) | ||
| GRADE 1 | 23 ( 4.0%) | 29 ( 5.1%) | 19 ( 3.4%) | 29 ( 5.2%) | ||
| GRADE 2 | 5 ( 0.9%) | 4 ( 0.7%) | 5 ( 0.9%) | 5 ( 0.9%) | ||
| GRADE 3 | 0 ( 0.0%) | 1 ( 0.2%) | 0 ( 0.0%) | 1 ( 0.2%) | ||
| Unknown | 0 ( 0.0%) | 1 ( 0.2%) | 0.43* | 0 ( 0.0%) | 2 ( 0.4%) | 0.13* |
| Urinary frequency/urgency at study entry(severity, physician reported) | ||||||
| GRADE 0 | 359 ( 62.8%) | 382 ( 66.8%) | 354 ( 62.8%) | 368 ( 65.8%) | ||
| GRADE 1 | 175 ( 30.6%) | 162 ( 28.3%) | 170 ( 30.1%) | 162 ( 29.0%) | ||
| GRADE 2 | 36 ( 6.3%) | 27 ( 4.7%) | 37 ( 6.6%) | 27 ( 4.8%) | ||
| GRADE 3 | 1 ( 0.2%) | 1 ( 0.2%) | 2 ( 0.4%) | 1 ( 0.2%) | ||
| Unknown | 1 ( 0.2%) | 0 ( 0.0%) | 0.17* | 1 ( 0.2%) | 1 ( 0.2%) | 0.28* |
| RT modality | ||||||
| 3D-CRT | 378 ( 66.1%) | 386 ( 67.5%) | 353 ( 62.6%) | 344 ( 61.5%) | ||
| IMRT | 194 ( 33.9%) | 186 ( 32.5%) | 0.62 | 211 ( 37.4%) | 215 ( 38.5%) | 0.72 |
Q1 = first quartile; Q3 = third quartile.
Chi-square for urinary incontinence and urinary frequency/urgency at study entry is Grade 0 vs. Non Grade 0.
IIEF:
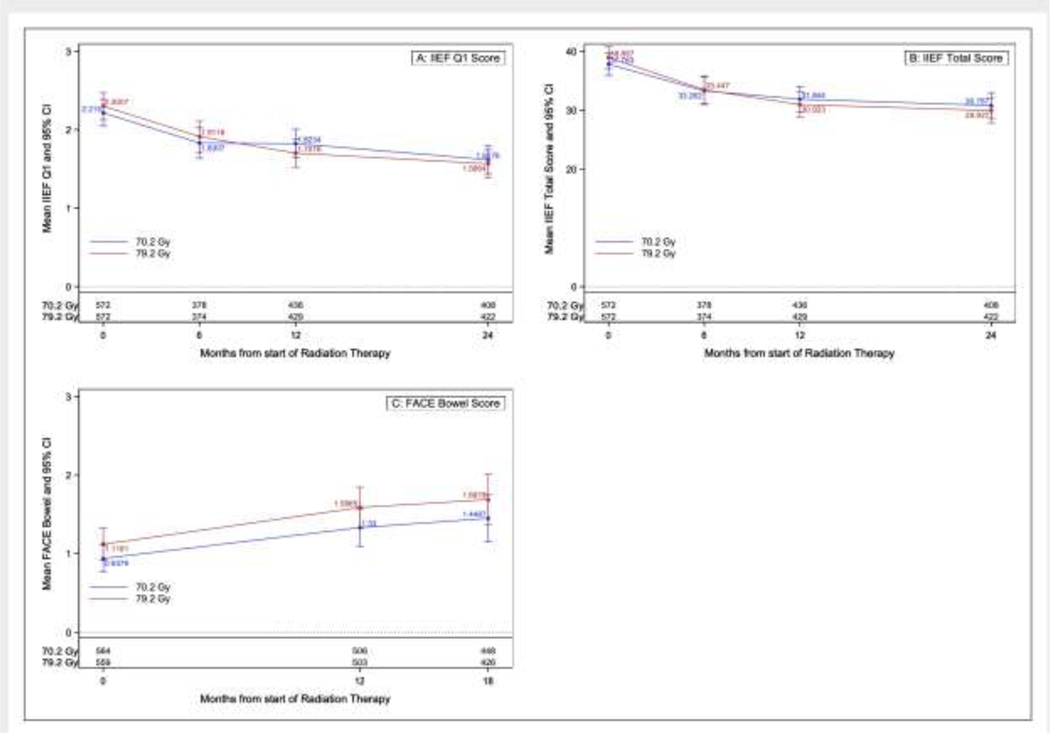
There were a total of 1144 patients that completed baseline IIEF forms and of these, 55.7%, 64.3%, and 60.8% completed the entire IIEF at 6, 12, and 24 months respectively. The presence of missing data was relatively similar in both arms. For the imputed analysis, 1304 patients completed at least 13 of the 15 questions on the IIEF at baseline and of these, 60.9%, 69.9%, and 68.0% completed at least 13 questions at 6, 12, and 24 months respectively. For the single item on erectile dysfunction (which was the planned primary endpoint) patients who completed at least 13 questions on the IIEF at 12 months also completed this question, so this question was not imputed. Erectile dysfunction at 12 months based on the single question was not significantly different between arms (38.1% for the standard dose RT arm vs. 49.7% for the dose escalated RT arm, p=0.051). There was no difference in percentage of patients with improved erectile function as defined using the minimally important difference at 6, 12, or 24 months either (Table 2). At 6 and 24 months, this score was imputed for only 2 and 3 patients, respectively (Supplemental Table 1a). There was no significant treatment arm difference found at any of these time points (Supplemental Table 2 a–b). On complete case repeated measures MVA for total IIEF score, younger age was highly significantly associated with higher IIEF total score (between group difference=4.69, standard error [SE]=0.94, p<0.001) while RT method was not (between group difference=1.09, SE=0.96, p=0.25; Table 3). A repeated measures MVA revealed that younger patients (measured as a continuous variable) had higher scores for the single question on erectile dysfunction, indicating less dysfunction (between group difference=0.41, SE=0.08, p<0.001, respectively; Supplemental Table 2b). There was no difference between treatment arms. Results for the imputed analysis were similar and showed no differences in the IIEF scores for patients treated with dose escalation versus standard dose (results not shown). Similarly RT method use was not associated with differences in PRO metrics on this imputed analysis. Figure 2 shows the mean IIEF Q1 and mean IIEF total score respectively.
Table 2.
Proportion of Patients with Improved IIEF Q1 (Erectile Dysfunction)
| 3D-CRT/IMRT 70.2 Gy | 3D-CRT/IMRT 79.2 Gy | p-value | |
|---|---|---|---|
|
| |||
| Month 6 | (n=322) | (n=315) | |
| Not improved | 301 (93.5%) | 303 (96.2%) | 0.12 |
| Improved | 21 (6.5%) | 12 (3.8%) | |
| Month 12 | (n=373) | (n=363) | |
| Not improved | 343 (92.0%) | 336 (92.6%) | 0.76 |
| Improved | 30 (8.0%) | 27 (7.4%) | |
| Month 24 | (n=342) | (n=354) | |
| Not improved | 323 (94.4%) | 336 (94.9%) | 0.78 |
| Improved | 19 (5.6%) | 18 (5.1%) | |
IIEF = International Index of Erectile Dysfunction; 3D-CRT = 3-dimensional – conventional radiation therapy; IMRT=intensity modulated radiation therapy; Gy=Gray.
Hochberg multiplicity adjustment employed based off of an overall significance level of 0.05.
Table 3.
Multivariable analysis for IIEF score
| Variables | Variable categories | Estimate | Standard deviation | P value |
|---|---|---|---|---|
|
| ||||
| IIEF score at 6 months | ||||
| RT method | 3D-CRT vs IMRT* | 2.79 | 1.75 | .11 |
| Age | <70 vs ≥70 y* | 15.04 | 1.63 | <.001 |
| Race | White* vs non-White | 2.07 | 2.30 | .37 |
| Time | Baseline vs 6 mo* | 5.45 | 0.66 | <.001 |
| Treatment arm | 3D-CRT/IMRT 70.2Gy vs 3D-CRT/IMRT 79.2Gy* | −2.06 | 1.62 | .20 |
| IIEF score at 12 months | ||||
| RT method | 3D-CRT vs IMRT* | 2.29 | 1.55 | .14 |
| Age | <70 vs ≥70 y* | 15.00 | 1.47 | <.001 |
| Race | White* vs non-White | 4.19 | 2.22 | .06 |
| Time | Baseline vs 12 mo* | 6.50 | 0.65 | <.001 |
| Treatment arm | 3D-CRT/IMRT 70.2Gy vs 3D-CRT/IMRT 79.2Gy* | −1.20 | 1.46 | .41 |
| IIEF score at 24 months | ||||
| RT method | 3D-CRT vs IMRT* | −0.33 | 1.59 | .83 |
| Age | <70 vs ≥70 y* | 14.38 | 1.47 | <.001 |
| Race | White* vs non-White | 5.74 | 2.18 | .009 |
| Salvage hormones | Yes* vs No | 7.19 | 3.13 | .022 |
| Treatment arm | 3D-CRT/IMRT 70.2Gy vs 3D-CRT/IMRT 79.2Gy* | 1.59 | 3.74 | .67 |
| Treatment arm*Salvage hormones | −2.78 | 4.08 | .49 | |
| Time | Baseline vs 24 months* | 8.59 | 0.71 | <.001 |
| IIEF score at 6, 12, and 24 months | ||||
| Baseline score | 0.65 | 0.02 | <.001 | |
| RT method | 3D-CRT vs IMRT * | 1.09 | 0.96 | .25 |
| Age | <70 vs ≥70 y* | 4.69 | 0.94 | <.001 |
| Race | White* vs non-White | 0.23 | 1.29 | .86 |
| Salvage hormones | Yes* vs No | 2.26 | 2.04 | .27 |
| Treatment arm | 3D-CRT/IMRT 70.2Gy vs 3D-CRT/IMRT 79.2Gy* | 2.42 | 2.43 | .32 |
| Treatment arm*Salvage hormones | −0.38 | 2.62 | .89 | |
| Time | −1.91 | 0.35 | <.001 | |
| IIEF single-item erectile dysfunction score at 6, 12, and 24 months† | ||||
| Baseline score | 0.56 | 0.02 | <.001 | |
| RT method | 3D-CRT vs IMRT* | 0.02 | 0.09 | .86 |
| Age | <70 vs ≥70 y* | 0.41 | 0.08 | <.001 |
| Race | White* vs non-White | 0.07 | 0.11 | .53 |
| Time | −0.17 | 0.032 | <.001 | |
| Treatment arm | 3D-CRT/IMRT 70.2Gy vs 3D-CRT/IMRT 79.2Gy* | 0.13 | 0.080 | .093 |
Abbreviations: 3D-CRT = 3-dimensional conventional radiation therapy; IIEF = International I= intensity lated radiation therapy.
Indicates the reference level.
Single item: “How often were you able to get an erection during sexual activity?”
Figure 2a –
Trends over time by treatment arm for the IIEF total score, IIEF single item erectile dysfunction, and FACE bowel.
FACE:
A total of 1123 patients completed the FACE score at baseline, 49.9%, 60.6%, 72.5%, 60.9%, and 64.6% completed all 15 items for the FACE metric at time points of 3, 6, 12, 18, and 24 months (Supplemental Table 1b). There was no difference in baseline scores between treatment arms for either subscale (Supplemental Table 3). Specifically, the results do not show any significant differences in mean change scores between treatment arms for the total score or the urinary subscale (Supplemental Tables 3a–b). The repeated measures MVA did not show any significant treatment arm differences but did show a significant difference in RT method in favor of 3D-CRT (between group difference=−0.33, SE=0.13, p=0.009; Supplemental Table 4). Imputed analyses showed similar results to the complete case analysis. Figure 2c shows the comparison of the mean FACE scores. The AUC analysis compared the FACE urinary and bowel symptoms across the IMRT and 3D-CRT cohorts and found no statistically significant differences(Supplemental Table 5).
SQLI:
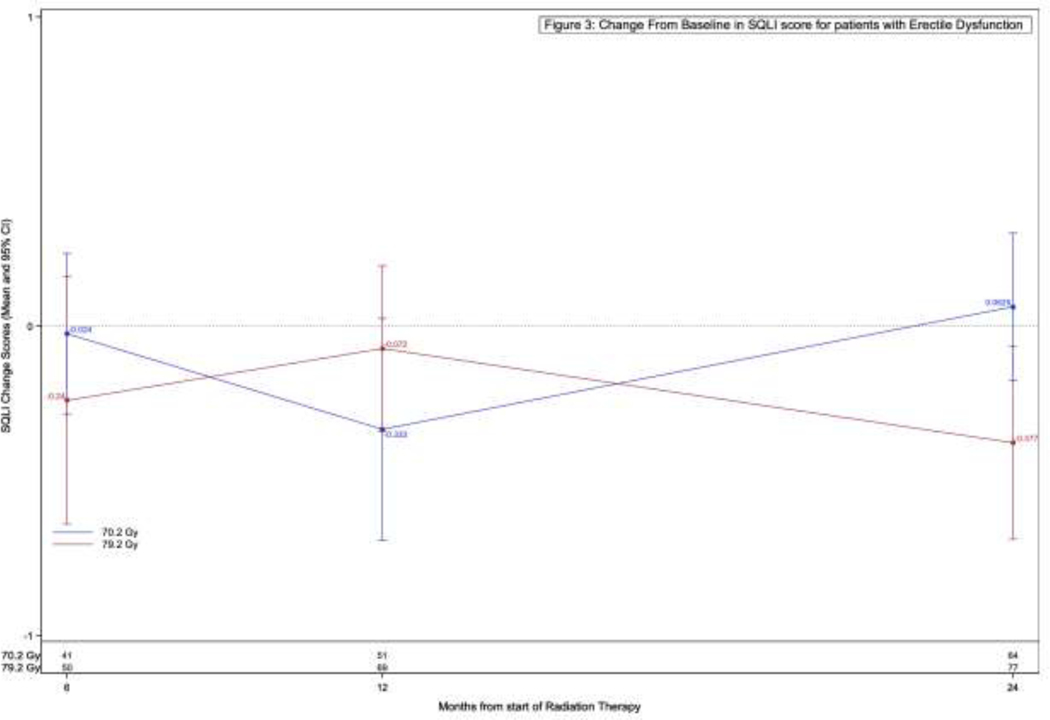
A total of 1366 (91.1%) of patients completed the SQLI at baseline, followed by 53.2%, 64.2%, 75.7%, 64.8%, and 72.2% at 3, 6, 12, 18, and 24 months respectively. With regard to the originally planned primary comparison, there was no difference in SQLI between the arms amongst all included patients. (Supplemental Table 6). When limiting the analysis to only patients with erectile dysfunction (ED) at 6, 12, and 24 months, respectively, there were again no significant differences in change from baseline to 6, 12, and 24 months in SQLI score (Figure 2b).
Figure 2b –
Change from baseline in SQLI score for patients with erectile dysfunction.
Dosimetric Analysis:
Penile bulb, rectal, and bladder doses were found to be significantly higher in the 79.2 Gy arm as compared to the 70.2 Gy arm (p<0.001; Supplemental Table 7 a–e). The only differences were seen in the higher volumetric comparisons, specifically above 65 Gy. There were no significant between arm differences in V40, V50, and V60. Volumetric doses were correlated with multiple domains of sexual function, including erectile function, orgasmic function, sexual desire, intercourse satisfaction, and overall satisfaction. No correlation between higher doses to the penile bulb and any of the metrics of sexual function at baseline, 6, 12, and 24 months were observed (results not shown). Similarly, no correlation between dose to the bladder and rectum with FACE total, urinary, or bowel scores existed at baseline, 3, 6, 12, 18, and 24 months (results not shown). All of the Spearman correlation coefficients had an absolute value <0.11 indicating weak correlations (results not shown).
DISCUSSION:
Dose escalation using EBRT is a widely applied treatment intensification strategy for patients with prostate cancer. This amplification of therapy for prostate cancer has been tested in multiple prospective clinical trials.1–6,16 Escalated EBRT dose has consistently shown an improvement in biochemical failure, spurring hypotheses that it may improve prostate cancer specific mortality.6 However, it has not been prospectively shown to improve overall survival, despite two trials, including this trial, being powered for this endpoint.1,5 Secondary to the demonstrated clinical benefits, other than overall survival, of dose escalated EBRT this treatment strategy has been adopted by radiation oncologists.17 There have been prior studies to have examined the impact of higher doses of RT on PROs.8,9 The largest of these, the MRC RT01 trial, compared 64 Gy in 32 fractions to 74 Gy in 37 fractions given with 3D-conformal RT. Both physician and patient reported GI toxicity was higher in the dose escalated RT arm, however differences were smaller (or absent) for the Los Angeles Prostate Cancer Index (UCLA PCI). Interesting, physician reported urinary toxicity rates were felt to be slightly higher for the dose escalated arm, however the patient reported toxicities showed no differences in late urinary function.8 The PROs, prospectively collected in NRG/RTOG 0126, further expanded our understanding of bowel, bladder, sexual and overall quality of life implications of increasing EBRT dose. These data also include novel methods of RT planning, such as IMRT. This data represents an enhanced assessment of the impact of EBRT dose escalation on patient outcomes in a modern era. It is well known that PROs present significant added value when understanding toxicities associated with an intervention, especially when compared with physician reported toxicity.18 Further expanding our understanding of these toxicity differences is critical to characterize the risks associatd with these interventions.
This analysis, limitations not withstanding, demonstrates no detriment to the use of dose escalated EBRT across multiple PRO metrics. This contrasts with other techniques that have shown significant detriments using other methods of radiation dose escalation, such as low dose rate (LDR) brachytherapy.19 It also contrasts with physician reported toxicity which was higher for the dose escalated EBRT arm on NRG/RTOG 0126.1 This also contrasts modestly with the MRC RT01 PRO data, which did show a small detriment in PROs associated with dose escalated EBRT using 3D-CRT. Precise etiologies for this difference between physician and PRO’s are uncertain, but very important to consider. It is moderately reassuring that across the hundreds of patients in this randomized study, for whom prospective PRO data was collected, that no clear differences are seen. This was true for both the aggregate response data, along with the imputed analysis. However, the certainty by which this conclusion can be drawn should be balanced with limitations of the data collected. Limitations not withstanding, these results favor the therapeutic ratio to further support the RT dose escalation strategy, as there was not a clear detrimental signal demonstrated across these findings. This is further supported by the significant clinical benefits demonstrated with dose escalation including improved biochemical/local/ and potentially distant control. These data also demonstrate that the use of salvage hormonal therapy, which was less frequent in the dose escalated EBRT arm, is associated with significantly worse IIEF scores at 24 months. These PRO data provide a novel metric, useful for counseling patients on the risks and benefits associated with dose escalated EBRT.
There are additional specific points that emerge from this set that are important to consider. The influence of penile bulb dose on patient reported erectile function, bladder dose on patient reported urinary function, and rectal dose on patient reported bowel function, is robustly evaluated in this prospective data set. With regard to penile bulb dose and correlations with erectile function, existing publications are conflicting. Prior publications suggest that EBRT penile bulb dose is correlated with erectile function.20–26 Several publications suggest an absence of association.27–29 Others recognize the penile bulb may be a surrogate for dose to structures more well known to contribute to erectile function.30 Despite significantly higher penile bulb doses in the experimental treatment arm, there was no difference seen in erectile function. Furthermore, volumetric assessments of penile bulb dose showed no correlations across numerous sexual function domains. This could be secondary to the relatively high dose to the penile bulb in both arms. Other analyses have demonstrated the significance of lower EBRT doses to the penile bulb.21 Alternatively, other regional structures impacting sexual function, such as the corpus cavernosum or internal pudendal artery may be responsible for radiation related erectile dysfunction.31
Similar to the analysis on the penile bulb, the correlations with FACE metrics, and measured DVH parameters, somewhat surprisingly, lacked any correlation with PROs. This is certainly an intriguing finding and highlights the importance of radiation oncologists robustly studying the influence of EBRT dose to regional organs on PROs. This also contrasts sharply with physician reported toxicities which frequently correlated with DVH metrics.32 This could be secondary to data limitations, as detailed above. However, given the absence of association between DVH data and PROs seen in this data set, novel DVH metrics that are significantly associated with PROs represent a critical and unmet clinical need.
The use of IMRT has been increasing amongst radiation oncologists significantly over the past fifteen years.17 In this clinical trial, the early experience of IMRT use was examined in a post-hoc, exploratory fashion as compared with 3D-CRT. It was shown that the use of IMRT in this trial seemed to modestly worsen the FACE bowel scores at 18 months. There were no other improvements, or decrements, in PROs associated with the use of IMRT, as it was applied in this trial. This conflicted with smaller prior analyses that have conducted similar post-hoc comparisons.9 Such a finding is important to consider, yet perhaps not surprising, considering the time frame examined and the novelty of IMRT during this trial’s years of accrual. These results are interesting, especially in that they conflict with physician reported toxicity.33 The reason for this difference is uncertain. It may be attributed to the repeated measures present in this study, or the novelty of IMRT (and limited understanding of constraints) during the time period over which this data was collected. It also highlights the previously mentioned need for novel DVH constraints that correlate with PROs.
Limitations:
Like many studies collecting PROs, some data is missing, especially with the longer follow up time points. This study specifically had lower completion rates than other recently randomized trials, such as both the ProtecT and CHHiP trials.34,35 The difficult of data collection, and potential strategies for mitigation, has been the subject prior publication and is an important learning opportunity from this trial.36 This is one of the most significant limitations of this dataset, and introduces the possibility of response bias. To account for this, additional analysis were performed including imputed analysis, specifically including partially completed PRO forms. There were no differences in any of the results between the complete case and imputed analyses. Furthermore, its important to consider the limitations of the FACE instrument. In this setting FACE is less robustly validated and studied than other PRO tools (such as EPIC), and minimally important differences of FACE are not as well understood. The FACE tool does not ask explicitly about rectal bleeding, an important symptom that should be considered. This too may have contributed to the absence of differences across the treatment arms; important differences may have been missed. Finally, we were not able to complete the originally planned quality adjusted survival analysis secondary to an absence of published methodologies to complete this analysis using the SQLI data.
Conclusions:
To our knowledge, this is the largest existing prospective randomized examination of the quality of life and PRO implications of EBRT dose in localized prostate cancer. Notwithstanding limitations, this study provides additional data to patients and providers that higher doses of EBRT do not appear to impact most PRO metrics. Most importantly, it characterizes expected PROs with current EBRT dose constraints along with their clinical relevance and significance. In summary, these data support the currently common practice of dose escalated EBRT for patients with prostate cancer and generate important points for future clinical research involving PRO data collection.
Supplementary Material
Acknowledgments
Source of Financial Support:
RTOG U10 CA21661, CCOP U10 CA37422, and ATC U24 CA 81647 grants from the National Cancer Institute
Disclosure Statement: Dr. Souhami reports other from Varian Medical Systems, other from Abbvie, other from Janssen, outside the submitted work; Dr. Hall reports institutional Research and Travel support, Elekta AB, outside the submitted work;. Dr. Lau reports other from AstraZeneca, other from Eisai, outside the submitted work; . Dr. Lock reports personal fees from Ferring, outside the submitted work; . Dr. Sandler reports personal fees from Janssen, other from Radiogel, personal fees from Caribou Publishing, outside the submitted work; and Member, ASTRO Board of Directors.. Dr. Movsas reports grants from Varian Inc, grants from Philips Inc, grants from ViewRay Inc, outside the submitted work; . Dr. Bosch reports grants from National Cancer Institute, during the conduct of the study;
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES:
- 1.Michalski JM, Moughan J, Purdy J, et al. Effect of Standard vs Dose-Escalated Radiation Therapy for Patients With Intermediate-Risk Prostate Cancer: The NRG Oncology RTOG 0126 Randomized Clinical Trial. JAMA Oncol. 2018;4(6):e180039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zietman AL, Bae K, Slater JD, et al. Randomized trial comparing conventional-dose with high-dose conformal radiation therapy in early-stage adenocarcinoma of the prostate: long-term results from proton radiation oncology group/american college of radiology 95–09. J Clin Oncol. 2010;28(7):1106–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heemsbergen WD, Al-Mamgani A, Slot A, Dielwart MF, Lebesque JV. Long-term results of the Dutch randomized prostate cancer trial: impact of dose-escalation on local, biochemical, clinical failure, and survival. Radiother Oncol. 2014;110(1):104–109. [DOI] [PubMed] [Google Scholar]
- 4.Beckendorf V, Guerif S, Le Prisé E, et al. 70 Gy versus 80 Gy in localized prostate cancer: 5-year results of GETUG 06 randomized trial. Int J Radiat Oncol Biol Phys. 2011;80(4):1056–1063. [DOI] [PubMed] [Google Scholar]
- 5.Dearnaley DP, Jovic G, Syndikus I, et al. Escalated-dose versus control-dose conformal radiotherapy for prostate cancer: long-term results from the MRC RT01 randomised controlled trial. Lancet Oncol. 2014;15(4):464–473. [DOI] [PubMed] [Google Scholar]
- 6.Pasalic D, Kuban DA, Allen PK, et al. Dose Escalation for Prostate Adenocarcinoma: A Long-Term Update on the Outcomes of a Phase 3, Single Institution Randomized Clinical Trial. Int J Radiat Oncol Biol Phys. 2019;104(4):790–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zaorsky NG, Keith SW, Shaikh T, et al. Impact of Radiation Therapy Dose Escalation on Prostate Cancer Outcomes and Toxicities. Am J Clin Oncol. 2018;41(4):409–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dearnaley DP, Sydes MR, Graham JD, et al. Escalated-dose versus standard-dose conformal radiotherapy in prostate cancer: first results from the MRC RT01 randomised controlled trial. Lancet Oncol. 2007;8(6):475–487. [DOI] [PubMed] [Google Scholar]
- 9.Al-Mamgani A, Heemsbergen WD, Peeters ST, Lebesque JV. Role of intensity-modulated radiotherapy in reducing toxicity in dose escalation for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2009;73(3):685–691. [DOI] [PubMed] [Google Scholar]
- 10.Rosen RC, Riley A, Wagner G, Osterloh IH, Kirkpatrick J, Mishra A. The international index of erectile function (IIEF): a multidimensional scale for assessment of erectile dysfunction. Urology. 1997;49(6):822–830. [DOI] [PubMed] [Google Scholar]
- 11.Spitzer WO, Dobson AJ, Hall J, et al. Measuring the quality of life of cancer patients: a concise QL-index for use by physicians. J Chronic Dis. 1981;34(12):585–597. [DOI] [PubMed] [Google Scholar]
- 12.Rosen RC, Allen KR, Ni X, Araujo AB. Minimal clinically important differences in the erectile function domain of the International Index of Erectile Function scale. Eur Urol. 2011;60(5):1010–1016. [DOI] [PubMed] [Google Scholar]
- 13.HOCHBERG Y. A sharper Bonferroni procedure for multiple tests of significance. Biometrika. 1988;75(4):800–802. [Google Scholar]
- 14.R T. Interpretation of the correlation coefficient: A basic review. JDMS; 1:35–39. 1990;1:35–39. [Google Scholar]
- 15.Akoglu H. User’s guide to correlation coefficients. Turk J Emerg Med. 2018;18(3):91–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kalbasi A, Li J, Berman A, et al. Dose-Escalated Irradiation and Overall Survival in Men With Nonmetastatic Prostate Cancer. JAMA Oncol. 2015;1(7):897–906. [DOI] [PubMed] [Google Scholar]
- 17.Malouff T, Mathy NW, Marsh S, Walters RW, Silberstein PT. Trends in the use of radiation therapy for stage IIA prostate cancer from 2004 to 2013: a retrospective analysis using the National Cancer Database. Prostate Cancer Prostatic Dis. 2017;20(3):334–338. [DOI] [PubMed] [Google Scholar]
- 18.Doward LC, Gnanasakthy A, Baker MG. Patient reported outcomes: looking beyond the label claim. Health Qual Life Outcomes. 2010;8:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rodda S, Morris WJ, Hamm J, Duncan G. ASCENDE-RT: An Analysis of Health-Related Quality of Life for a Randomized Trial Comparing Low-Dose-Rate Brachytherapy Boost With Dose-Escalated External Beam Boost for High- and Intermediate-Risk Prostate Cancer. Int J Radiat Oncol Biol Phys. 2017;98(3):581–589. [DOI] [PubMed] [Google Scholar]
- 20.Roach M, 3rd, Nam J, Gagliardi G, El Naqa I, Deasy JO, Marks LB. Radiation dose-volume effects and the penile bulb. Int J Radiat Oncol Biol Phys. 2010;76(3 Suppl):S130–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murray J, Gulliford S, Griffin C, et al. Evaluation of erectile potency and radiation dose to the penile bulb using image guided radiotherapy in the CHHiP trial. Clin Transl Radiat Oncol. 2020;21:77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fisch BM, Pickett B, Weinberg V, Roach M. Dose of radiation received by the bulb of the penis correlates with risk of impotence after three-dimensional conformal radiotherapy for prostate cancer. Urology. 2001;57(5):955–959. [DOI] [PubMed] [Google Scholar]
- 23.Roach M, Winter K, Michalski JM, et al. Penile bulb dose and impotence after three-dimensional conformal radiotherapy for prostate cancer on RTOG 9406: findings from a prospective, multi-institutional, phase I/II dose-escalation study. Int J Radiat Oncol Biol Phys. 2004;60(5):1351–1356. [DOI] [PubMed] [Google Scholar]
- 24.Wernicke AG, Valicenti R, Dieva K, Houser C, Pequignot E. Radiation dose delivered to the proximal penis as a predictor of the risk of erectile dysfunction after three-dimensional conformal radiotherapy for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2004;60(5):1357–1363. [DOI] [PubMed] [Google Scholar]
- 25.Mangar SA, Sydes MR, Tucker HL, et al. Evaluating the relationship between erectile dysfunction and dose received by the penile bulb: using data from a randomised controlled trial of conformal radiotherapy in prostate cancer (MRC RT01, ISRCTN47772397). Radiother Oncol. 2006;80(3):355–362. [DOI] [PubMed] [Google Scholar]
- 26.Magli A, Giangreco M, Crespi M, et al. Erectile dysfunction after prostate three-dimensional conformal radiation therapy. Correlation with the dose to the penile bulb. Strahlenther Onkol. 2012;188(11):997–1002. [DOI] [PubMed] [Google Scholar]
- 27.Selek U, Cheung R, Lii M, et al. Erectile dysfunction and radiation dose to penile base structures: a lack of correlation. Int J Radiat Oncol Biol Phys. 2004;59(4):1039–1046. [DOI] [PubMed] [Google Scholar]
- 28.Brown MW, Brooks JP, Albert PS, Poggi MM. An analysis of erectile function after intensity modulated radiation therapy for localized prostate carcinoma. Prostate Cancer Prostatic Dis. 2007;10(2):189–193. [DOI] [PubMed] [Google Scholar]
- 29.van der Wielen GJ, Hoogeman MS, Dohle GR, van Putten WL, Incrocci L. Dose-volume parameters of the corpora cavernosa do not correlate with erectile dysfunction after external beam radiotherapy for prostate cancer: results from a dose-escalation trial. Int J Radiat Oncol Biol Phys. 2008;71(3):795–800. [DOI] [PubMed] [Google Scholar]
- 30.Rivin del Campo E, Thomas K, Weinberg V, Roach M, 3rd. Erectile dysfunction after radiotherapy for prostate cancer: a model assessing the conflicting literature on dose-volume effects. Int J Impot Res. 2013;25(5):161–165. [DOI] [PubMed] [Google Scholar]
- 31.Spratt DE, Lee JY, Dess RT, et al. Vessel-sparing Radiotherapy for Localized Prostate Cancer to Preserve Erectile Function: A Single-arm Phase 2 Trial. Eur Urol. 2017;72(4):617–624. [DOI] [PubMed] [Google Scholar]
- 32.Michalski JM, Gay H, Jackson A, Tucker SL, Deasy JO. Radiation dose-volume effects in radiation-induced rectal injury. Int J Radiat Oncol Biol Phys. 2010;76(3 Suppl):S123–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Michalski JM, Yan Y, Watkins-Bruner D, et al. Preliminary toxicity analysis of 3-dimensional conformal radiation therapy versus intensity modulated radiation therapy on the high-dose arm of the Radiation Therapy Oncology Group 0126 prostate cancer trial. Int J Radiat Oncol Biol Phys. 2013;87(5):932–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Donovan JL, Hamdy FC, Lane JA, et al. Patient-Reported Outcomes after Monitoring, Surgery, or Radiotherapy for Prostate Cancer. N Engl J Med. 2016;375(15):1425–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dearnaley D, Syndikus I, Mossop H, et al. Conventional versus hypofractionated high-dose intensity-modulated radiotherapy for prostate cancer: 5-year outcomes of the randomised, non-inferiority, phase 3 CHHiP trial. Lancet Oncol. 2016;17(8):1047–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pugh SL, Rodgers JP, Moughan J, et al. Do reminder emails and past due notifications improve patient completion and institutional data submission for patient-reported outcome measures? Qual Life Res. 2021;30(1):81–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.