Abstract
Cyanobacterial mutants defective in acyl-acyl carrier protein synthetase (Aas) produce free fatty acids (FFAs) because the FFAs generated by deacylation of membrane lipids cannot be recycled. An engineered Aas-deficient mutant of Synechocystis sp. PCC 6803 grew normally under low-light (LL) conditions (50 µmol photons m−2 s−1) but was unable to sustain growth under high-light (HL) conditions (400 µmol photons m−2 s−1), revealing a crucial role of Aas in survival under the HL conditions. Several-times larger amounts of FFAs were produced by HL-exposed cultures than LL-grown cultures. Palmitic acid accounted for ∼85% of total FFAs in HL-exposed cultures, while C18 fatty acids (FAs) constituted ∼80% of the FFAs in LL-grown cultures. Since C16 FAs are esterified to the sn-2 position of lipids in the Synechocystis species, it was deduced that HL irradiation activated deacylation of lipids at the sn-2 position. Heterologous expression of FarB, the FFA exporter protein of Neisseria lactamica, prevented intracellular FFA accumulation and rescued the growth defect of the mutant under HL, indicating that intracellular FFA was the cause of growth inhibition. FarB expression also decreased the ‘per-cell’ yield of FFA under HL by 90% and decreased the proportion of palmitic acid to ∼15% of total FFA. These results indicated that the HL-induced lipid deacylation is triggered not by strong light per se but by HL-induced damage to the cells. It was deduced that there is a positive feedback loop between HL-induced damage and lipid deacylation, which is lethal unless FFA accumulation is prevented by Aas.
Keywords: Acyl-ACP synthetase, Lipid deacylation, Photoinhibition, Toxicity to FFAs
Introduction
Free fatty acids (FFAs) are naturally produced by cyanobacteria via deacylation of membrane lipids, but the cells do not normally accumulate FFAs or secrete them out of the cells since the FFAs are readily esterified to acyl carrier protein (ACP) by acyl-ACP synthetase (Aas) and recycled to lipids (Kaczmarzyk and Fulda 2010). Initial physiological characterization of the Aas-null mutants of model cyanobacterial species Synechococcus elongatus PCC 7942 and Synechocystis sp. PCC 6803 revealed secretion of FFAs out of the cells but there was no noticeable growth phenotype under illumination at 30–60 µmol photons m−2 s−1 (Kaczmarzyk and Fulda 2010, Ruffing 2014). Takatani et al. (2015) later showed that an Aas-deficient S. elongatus PCC 7942 grows slightly slower than the wild-type (WT) under low-light (LL) conditions (50 µmol photons m−2 s−1), and unlike WT, it cannot increase the growth rate in response to a shift to high-light (HL) conditions (400 µmol photons m−2 s−1). It was shown that lipid deacylation is enhanced under the HL condition and that the resulting FFAs destabilize photosystem II (PSII), making the cells more susceptible to photodamage (Takatani et al. 2015). These results showed the importance of Aas in acclimation of S. elongatus to the HL conditions. In this study, we characterized an Aas-deficient mutant (dAS11) constructed from Synechocystis sp. PCC 6803. HL-responsive lipid deacylation is shown to take place also in Synechocystis sp. PCC 6803, causing severe growth inhibition of the mutant. Involvement of an sn-2 specific lipase is deduced from the FFA composition of the HL-exposed cells. We show that heterologous expression of a bacterial FFA exporter prevents the accumulation of intracellular FFAs, largely rescues the growth phenotype of the mutant, and at the same time attenuates the lipase activation under HL. Based on these findings, we propose that there is a positive feedback between photodamage to the cells, which is enhanced by intracellular FFA, and upregulation of an sn-2 specific lipase. Possible roles of lipid deacylation and FFA recycling in HL acclimation of cyanobacteria are discussed.
Results
Sensitivity of an aas-deficient mutant of Synechocystis sp. PCC 6803 to high-light conditions
Fig. 1 represents the appearance of the cultures of the WT strain and an Aas-deficient mutant (dAS11) of Synechocystis sp. PCC 6803 during cultivation under LL (50 µmol photons m−2 s−1) and HL (400 µmol photons m−2 s−1) conditions. The dAS11 culture became bluish after 3–4 days of cultivation under the HL conditions and completely lost pigmentation in 7 days, whereas no decrease in pigmentation was observed under the LL conditions (Fig. 1). In a previous study in S. elongatus PCC 7942, the aas-deficient mutant dAS1 was shown to suffer from enhanced photodamage to PSII under the HL conditions, but the cells could sustain growth under HL albeit at a lower rate as compared to the WT strain (Takatani et al. 2015). These results indicated that Aas plays a more crucial role in Synechocystis sp. PCC 6803 than in S. elongatus PCC 7942 in the acclimation of the cells to the HL conditions.
Fig. 1.
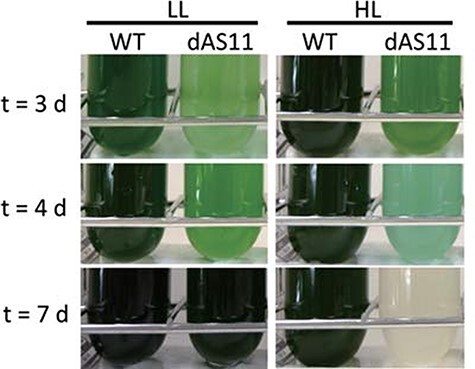
Appearance of the WT and dAS11 cultures during growth under the HL and LL conditions. Cells grown under LL conditions (50 µmol photons m−2 s−1) were inoculated into new medium to give an OD730 value of 0.01 and incubated under the LL or HL (400 µmol photons m−2 s−1) conditions for the indicated period.
Functional expression of a Neisseria lactamica FFA exporter in Escherichia coli
Aas-deficient cyanobacterial mutants produce FFAs, which can be lethal if accumulated at high levels in the cell (Kato et al. 2015, 2016, 2017). To examine whether an increase in the FFA export activity could rescue the HL-sensitive phenotype of the dAS11 mutant, we planned expression in dAS11 of the tripartite FFA efflux pump conserved among the bacteria of the Neisseria genus (Lee and Shafer 1999, see also Supplementary Fig. S1), which is composed of FarB (an MFS family transporter), MtrE (an outer membrane channel protein, OMP) and FarA (a membrane fusion protein, MFP). Since heterologous expression of the Neisseria FFA efflux pump had not been reported, we first tried it in Escherichia coli. Construction of a plasmid carrying the transcriptional fusion of the farAB operon and the mtrE gene under the control of the Ptrc promoter was unsuccessful in E. coli even in the absence of isopropyl β-D-1-thiogalactopyranoside (IPTG) (data not shown), suggesting that expression of the whole set of the proteins comprising the FFA pump might be toxic to the E. coli host. We hence examined the effects of plasmid-based expression of the Ptrc-farAB, Ptrc-farA or Ptrc-farB genes in an E. coli K12 ∆acrAB ∆emrB mutant, which lacks two (AcrAB-TolC and EmrAB-TolC) of the three efflux pumps known to mediate FFA efflux (Lennen et al. 2013). While the WT E. coli cells were resistant to lauric acid (12:0) and palmitic acid (16:0) added to the medium to a concentration of 1 mM, the ∆acrAB ∆emrB mutant was sensitive to 12:0 (Fig. 2). Plasmid-based expression of farA or farAB did not confer the cells the tolerance to exogenously added 12:0, but surprisingly, that of farB rendered the cells tolerant to 12:0 (Fig. 2C). These results indicated that expression of FarB supported export of 12:0 out of the cell, but co-expression of FarA interfered with it.
Fig. 2.
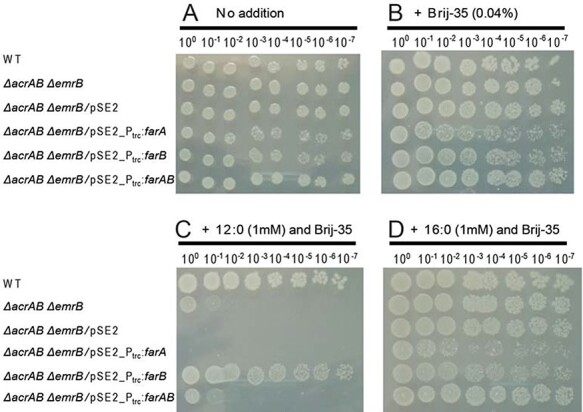
Effects of expression of either or both of the N. lactamica farA and farB genes on growth of the E. coli K12 ∆acrAB∆emrB mutant in the presence of exogenously added FFAs. Three microliters of the E. coli cell suspensions (OD600 = 1.0) and their 10-fold serial dilutions were spotted onto solid media and incubated for 1 day at 37°C. (A, B) Control experiments performed without (A) and with (B) 0.04% Brij-35. (C, D) Viability test performed in the presence of 0.04% Brij-35 and 1 mM 12:0 (C) and 16:0 (D). Numbers on the top indicate the dilution factor. The results from one of the three experiments, which yielded essentially the same results, are shown.
Negative effect of FarA on the tolerance of Synechocystis cells to extrinsic linolenic acid
Fig. 3 shows the effects of exogenously added linolenic acid (18:3) on growth of the WT Synechocystis sp. PCC 6803 strain, the dAS11 mutant and the dAS11 derivatives expressing either farA and farB or both farA and farB. Aas has been shown to facilitate passive entrance of extrinsic FFAs into the cell by consuming intracellular FFAs (Kaczmarzyk and Fulda 2010). Exogenously added 18:3 is generally toxic to cyanobacteria (Sakamoto et al. 1998, Maeda et al. 2005), but as reported previously (von Berlepsch et al. 2012, Kojima et al. 2016), the Aas-deficient mutant was tolerant to the fatty acid (FA) added to the medium to 100 µM. Expression of farA in dAS11 completely abolished the ability of the cells to grow in the presence of the FA, while that of farB did not affect it. Interestingly, the cells co-expressing farA and farB were tolerant to 18:3, showing that FarB could partially suppress the negative effect of FarA.
Fig. 3.
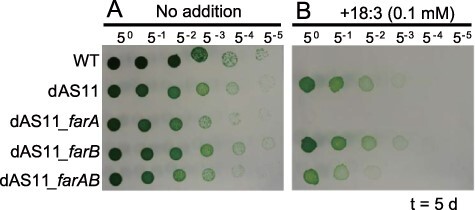
Effects of expression of either or both of the N. lactamica farA and farB genes on growth of the Aas-deficient Synechocystis sp. PCC 6803 mutant in the presence of 18:3. Three microliters of the Synechocystis cell suspensions (OD730 = 1.0) and their 5-fold serial dilutions were spotted onto solid media and incubated for 5 d at 30°C under illumination at 25 µmol photons m−2 s−1. Growth of the cells in the absence (A) and the presence (B) of 0.1 mM 18:3 is compared. Numbers on the top indicate the dilution factor. A set of data from one of the three experiments, which yielded essentially the same results, is shown.
Positive effect of FarB on the tolerance of Synechocystis cells to extrinsic FAs
Fig. 4 shows the growth curves of WT, dAS11 and the dAS11_farB strains in liquid media supplemented with various FAs under the LL conditions. Growth of the WT strain was severely inhibited by 100 µM of 12:0 and 18:3 (Fig. 4B, E, black circles), but the inhibitory effects of these FAs were partially attenuated by the deficiency of Aas as previously reported (Fig. 4B, E, white circles; von Berlepsch et al. 2012, Kojima et al. 2016; see also Fig. 3). Expression of farB was found to further improve the growth of the aas-deficient strain in the presence of these FAs (Fig. 4B, E, gray circles), suggesting the involvement of FarB in the export of the toxic FAs. Unlike 12:0 and 18:3, 16:0 is normally non-toxic to cyanobacteria (Ruffing and Trahan 2014), but at a concentration of 500 µM, it significantly inhibited the growth of dAS11 (Fig. 4C, white circles). Similar effects of exogenously added 16:0 were previously reported for a FFA-producing mutant of S. elongatus PCC 7942 and ascribed to an interference of the secretion of the endogenously produced FFA out of the cells (Kato et al. 2017). The inhibitory effect of 16:0 on dAS11 was rescued by expression of farB (Fig. 4C, gray circles), showing that FarB could mediate export of 16:0 as well as of 12:0 and 18:3.
Fig. 4.
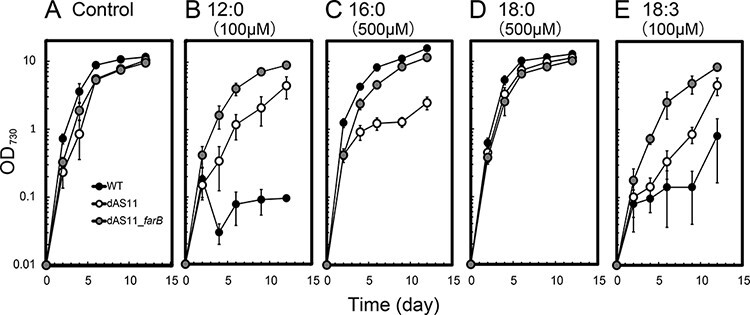
Effects of Aas deficiency and farB expression on Synechocystis growth in the presence of various FAs. Growth curves of the WT (black circles), dAS11 (white circles) and dAS11_farB (gray circles) strains were compared in the presence or absence of FAs (labeled on the top of the panel). Data shown are mean ± SE from biological triplicates.
Effects of farB expression on growth and photosynthetic activity of the aas-deficient Synechocystis mutant under high-light conditions
Fig. 5 shows the growth curves of the WT, dAS11 and dAS11_farB strains under the LL and HL conditions. Under the LL conditions, the final optical density of the dAS11 culture was comparable to that of WT, although the growth of the mutant was slower than that of WT (Fig. 5A). Cultivation under the HL conditions increased the growth rates of both WT and dAS11 as compared to those under the LL conditions, but in the case of the mutant, cell growth ceased after 3 days of exponential growth (Fig. 5B). The cells thereafter showed a decrease in pigmentation as mentioned above (Fig. 1). Interestingly, growth of the mutant resumed several days after the growth arrest, showing a recovery of pigmentation. Growth of dAS11_farB was essentially the same as that of dAS11 under the LL conditions (Fig. 5A). Even under the HL conditions, the farB expressing strain did not show the growth arrest or the bleaching phenotype (Fig. 5B). Thus, expression of FarB largely rescued the growth defect caused by the deficiency of Aas, although the final cell density of the dAS11_farB strain was somewhat lower than that attained by WT (Fig. 5B).
Fig. 5.
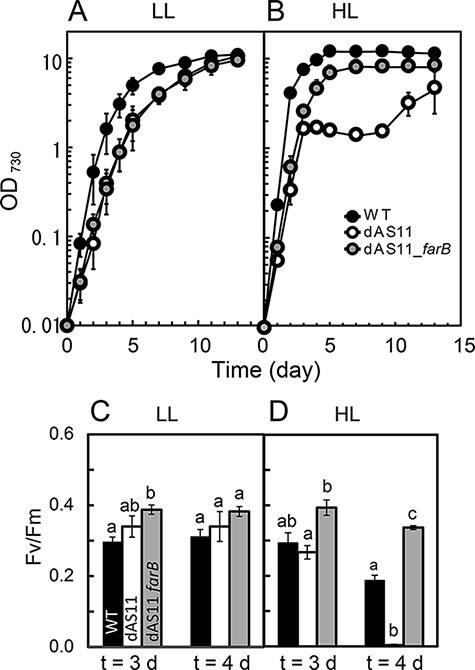
Rescue by farB expression of the HL-sensitive phenotype of the Synechocystis aas mutant. Upper panels compare the growth curves of the WT (black circles), dAS11 (white circles) and dAS11_farB (gray circles) strains under the LL (A) and HL (B) conditions. Lower panels show the photosynthetic yield of the three strains after 3 and 4 d of incubation under the LL (C) and HL (D) conditions. Data shown are mean ± SE from biological triplicates. The letters denote significant differences (P < 0.05, Tukey’s test).
The PSII activity, as determined by measuring the Fv Fm−1 ratio, showed a sharp decline between the third and fourth days of incubation of the dAS11 cells under HL (Fig. 5C, D), suggesting that the decrease of PSII activity was the cause of the growth arrest. In contrast, no such decline was observed in the PSII yield of WT or the dAS11_farB strain. In the aas-deficient cells of S. elongatus PCC 7942, intracellular FFA accumulation was shown to render PSII unstable and to make the cells sensitive to HL (Takatani et al. 2015). The tolerance of PSII of the dAS11_farB strain to the HL conditions therefore suggested that FarB prevented FFA accumulation in the cells and thereby alleviated photoinhibition.
Effects of farB expression on the PSII activity and photodamage to PSII in the aas-deficient Synechocystis mutant
Fig. 6A compares the PSII activity of LL-grown cells of WT, dAS11 and dAS11_farB. The PSII activity of dAS11 was lower than that of WT by about 40%, whereas that of the dAS11_farB strain was essentially the same as that of WT. Expression of farB thus restored the activity of PSII of the aas-deficient strain to a level comparable to that observed in WT.
Fig. 6.
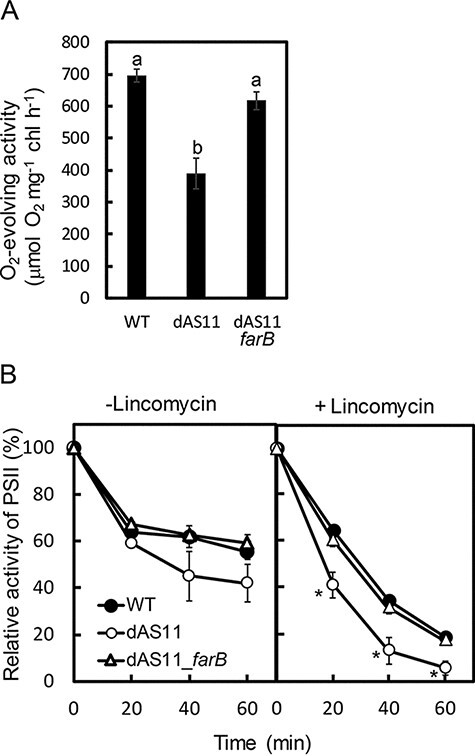
Effects of aas deficiency and farB expression on the PSII activity (A) and the sensitivity of PSII to photoinhibition (B) in Synechocystis sp. PCC 6803. (A) PSII activity of the WT, dAS11 and dAS11_farB cells grown under LL conditions (50 µmol photons m−2 s−1) for 24 h. The letters denote significant differences (P < 0.01, Tukey’s test). (B) Susceptibility of the WT (filled circles), dAS11 (open circles) and dAS11_farB (open triangle) cells to photoinhibition. The LL-grown cells were incubated under 1500 µmol photons m−2 s−1 light conditions for 60 min in the absence or presence of 200 µg ml−1 lincomycin and the PSII activity was measured at the indicated time points. One hundred percent activity for each of the strains was in the range shown in (A). Data shown are mean ± SE (bars) from three independent experiments. Asterisks indicate significant differences compared with WT (P < 0.01, t-test).
To further investigate the effects of aas deficiency and farB expression on the process of photoinhibition of PSII, the time courses of photoinhibition of WT and the mutant strains were compared in the absence and presence of lincomycin, which inhibits the repair of PSII (Fig. 6B). In the absence of lincomycin (Fig. 6B), PSII activity of the LL-grown cells was decreased by about 40% after 20 min of exposure to illumination of 1,500 µmol photons m−2 s−1 in all the strains. In WT and dAS11_farB, there was only a small decrease in the PSII activity thereafter, whereas dAS11 appeared to suffer from further decrease in the PSII activity, which was decreased to ∼40% of the initial level in 60 min of HL irradiation. However, the difference in PSII activity between dAS11 and the other strains was not statistically significant. When lincomycin was added to inhibit the repair of photodamaged PSII, on the other hand, the HL-induced decrease of PSII activity was significantly faster in dAS11 than in WT or dAS11_farB (Fig. 6B), showing that PSII was more sensitive to photodamage in dAS11 than in the other two strains. Expression of farB thus stabilized the activity of PSII in the aas-deficient mutant, suggesting that intracellular accumulation of FFA was responsible for the hypersensitivity of PSII to photodamage in dAS11.
Effects of light intensity and farB expression on the intracellular and extracellular FFA levels
Fig. 7A compares the cellular FFA content of the WT and the aas-deficient mutants after 7 days of cultivation. The intracellular FFAs were determined by LC-MS analysis of the extracts of the cells collected by centrifugation. Under the HL conditions, dAS11 cells accumulated FFAs to a level as high as 250 µg per 1 × 108 cells, which corresponded to 0.3 g of FFA ml−1 of cell volume. The cells of the farB-expressing aas-deficient mutant also accumulated larger amounts of FFAs under the HL conditions than under the LL conditions, but the FFA level in the HL-grown cells was only ∼20 µg per 1 × 108 cells (Fig. 7A). The amounts of total intracellular FFA were calculated for each of the cyanobacterial cultures (Fig. 7C), using the data in Fig. 7A and the cell density of each culture (Fig. 7B). In spite of the lower cellular FFA content of dAS11_farB cells as compared to that of dAS11 (Fig. 7A), the total intracellular FFA in the dAS11_farB culture was comparable to that in the dAS11 culture because of the high cell density of the dAS11_farB culture (Fig. 7B, E).
Fig. 7.
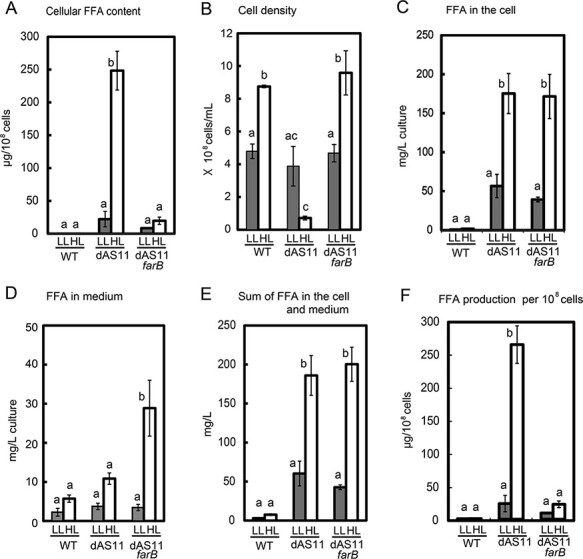
Effects of light intensity and farB expression on extracellular and intracellular FFA levels of the Synechocystis aas mutant cultures. Cells of the WT, dAS11 and dAS11_farB strains were grown in the liquid medium under the LL or HL conditions as in Fig. 5. Intracellular FFA content (A), the cell density of the culture (B) and the FFA concentration in the medium (D) were measured for each culture after 7 d of cultivation and used to calculate total intracellular FFA l−1 of culture (C), total FFA in the culture (E) and FFA production per cell (F). Data shown are mean ± SE from biological triplicates. Different letters denote significant differences (P < 0.05, Tukey’s multiple comparison test).
The amounts of FFAs secreted from the cells, as determined enzymatically after removal of the cells from the cultures by centrifugation, are shown in Fig. 7D. Low but significant amounts of extracellular FFAs (2–4 µg ml−1 of culture) were detected in LL-grown cultures of all the strains including WT. When grown under the HL conditions, the extracellular FFA level was increased in all the strains, but the highest level was attained by dAS11_farB; The HL-grown dAS11_farB cultures had 3 and 5 times more extracellular FFAs than the dAS11 and the WT cultures, respectively. The higher extracellular FFA concentration and the lower cellular FFA content in HL-grown cultures of dAS11_farB than in those of dAS11 (Fig. 7A, D) verified the contribution of FarB to FFA secretion out of the cells.
Comparison of the data in Fig. 7C, D indicated that in the HL-grown cultures of dAS11 and dAS11_farB, over 80% of FFA was retained in the cells (Fig. 7C, D). The total amount of the FFA produced by the cells under HL, as calculated from the data in Fig. 7C, D, was essentially the same in the two strains, being about 190 mg l−1 (Fig. 7E), from which the average rate of FFA production was calculated to be ∼1.1 mg l−1 h−1. Due to the much higher cell density in the dAS11_farB cultures (Fig. 7B), dAS11_farB produced only 8.6% of FFA as compared to dAS11 on a ‘per-cell’ basis (Fig. 7F).
Effects of light intensity and farB expression on the composition of the FFAs
Table 1 shows the effects of light conditions on the composition of the FFA pool in the Aas-deficient mutants. In the LL-grown dAS11 cells, the C18 FFAs including stearic acid (18:0), oleic acid (18:1) and linoleic acid (18:2) and linolenic acid (18:3) constituted 86% of the FFAs. Since the C18 FAs are esterified to the sn-1 position of the membrane lipids in Synechocystis sp. PCC 6803 (Wada and Murata 1989), the results indicated preferential deacylation of the lipids at the sn-1 position under these conditions. In the HL-grown cultures, by contrast, 16:0 was the major constituent of the FFAs accounting for 70% of the total. Given that 16:0 is esterified to the sn-2 position of the membrane lipids of Synechocystis sp. PCC 6803 except in SQDG where some 16:0 is esterified also to the sn-1 position (Wada and Murata 1989), it was deduced that HL irradiation activated deacylation of the lipids at the sn-2 position. In the case of the dAS11_farB strain, on the other hand, the major constituents of the FFAs were the C18 FAs irrespective of the light conditions. The results indicated that the strong light per se was not responsible for the activation of lipid deacylation at the sn-2 position; it was deduced that HL-induced stresses triggered the lipid deacylation at sn-2.
Table 1.
Relative amounts of the intra- and extracellular FFAs in HL- and LL-grown Synechocystis mutants
| Strain | Light conditions | FFA (mol%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 14:0 | 16:0 | 16:1 | 18:0 | 18:1 | 18:2 | 18:3 | Total C16 | Total C18 | ||
| Intracellular FFA | ||||||||||
| dAS11 | LL | 2 (1) | 20 (12) | <0.5 | 60 (11) | 5 (0) | 7 (1) | 5 (2) | 20 (12) | 77 (13) |
| HL | 3 (0) | 80 (1) | 1 (0) | 10 (0) | 1 (0) | 5 (0) | 1 (0) | 80 (1) | 17 (1) | |
| dAS11_farB | LL | <0.5 | 7 (1) | <0.5 | 68 (2) | 6 (0) | 8 (0) | 9 (1) | 7 (1) | 91 (1) |
| HL | 1 (1) | 20 (5) | 1 (0) | 50 (10) | 1 (0) | 15 (3) | 11 (2) | 21 (5) | 77 (6) | |
| Extracellular FFA | ||||||||||
| dAS11 | LL | 2 (2) | 5 (3) | 2 (0) | 14 (1) | 3 (0) | 17 (2) | 52 (5) | 7 (3) | 87 (7) |
| HL | 3 (0) | 62 (3) | 1 (0) | 16 (1) | 1 (0) | 10 (1) | 5 (1) | 63 (3) | 31 (2) | |
| dAS11_farB | LL | <0.5 | 1 (0) | 2 (0) | 23 (10) | 2 (0) | 15 (2) | 55 (9) | 2 (1) | 96 (1) |
| HL | 1 (0) | 9 (2) | 3 (1) | 21 (8) | 1 (0) | 21 (3) | 37 (4) | 12 (3) | 80 (5) | |
| Total FFA | ||||||||||
| dAS11 | LL | 2 (2) | 8 (3) | 1 (0) | 36 (11) | 4 (1) | 13 (1) | 33 (4) | 10 (3) | 85 (7) |
| HL | 3 (0) | 69 (2) | 1 (0) | 13 (1) | 1 (0) | 8 (1) | 3 (1) | 70 (1) | 25 (1) | |
| dAS11_farB | LL | <0.5 | 5 (1) | 1 (0) | 53 (6) | 5 (1) | 10 (1) | 25 (7) | 6 (1) | 93 (1) |
| HL | 1 (0) | 13 (3) | 2 (1) | 32 (8) | 1 (0) | 19 (2) | 27 (3) | 16 (4) | 79 (5) | |
Discussion
FFA production using cyanobacteria is considered to be one of the promising approaches to sustainable production of biofuels (Wang et al. 2020). Targeted inactivation of the aas gene is an essential step in the engineering of FFA-producing cyanobacterial mutants. In addition, a heterologous thioesterase gene(s) (e.g. the ‘tesA gene of E. coli), which cleaves FFA from acyl-ACP, is introduced to increase FFA production. Although cyanobacteria have endogenous lipase activity (Kaczmarzyk and Fulda 2010), contribution of the lipase(s) in FFA production has not attracted much attention. The present results show that the cultures of the Synechocystis ∆aas mutants (dAS11 and dAS11_farB) produce large amounts of total FFA (i.e. the sum of intra- and extracellular FFA) under the HL conditions (Fig. 7E), with an average rate of 1.1 mg l−1 h−1 during 7 days of cultivation. Since the corresponding rates reported for the thioesterase-expressing ∆aas mutant of S. elongatus PCC 7942 range from 2 to 3 mg of total FFAs h−1 l−1 (Kato et al. 2016, 2017), we conclude that the endogenous lipase(s) of cyanobacteria can make a significant contribution to FFA production under certain conditions.
Takatani et al. (2015) previously showed in S. elongatus PCC 7942 that PSII is more readily photodamaged in an aas-deficient mutant than in WT and speculated that FFA, which accumulated in the absence of Aas, may have destabilized PSII. The present results show that the deficiency of Aas results in destabilization of PSII also in Synechocystis sp. PCC 6803 (Fig. 6B). The rescue by the FFA exporter FarB of the adverse effect of Aas deficiency (Fig. 6B) verifies that FFA is the cause of PSII destabilization, excluding the possible involvement of lysolipid, the other product of lipid deacylation. However, the molecular mechanism of the PSII destabilization remains elusive, as FFA can act as a surfactant and have direct or indirect biochemical or biophysical effects on membranes and proteins.
In the case of S. elongatus PCC 7942, ∆aas mutant dAS1 can sustain growth under illumination of 400 µmol photons m−2 s−1 (Takatani et al. 2015), but the ∆aas mutants expressing ‘tesA suffer from severe growth inhibition caused by intracellular accumulation of FFA even at 180 µmol photons m−2 s−1 (Kato et al. 2015, 2016). Overexpression of the endogenous RND-type efflux pump (Kato et al. 2015) and removal of FFA from the culture medium (Kato et al. 2016) have been shown to help growth and FFA production of the Synechococcus mutants by decreasing intracellular FFA. The successful functional expression of FarB, an MFS family FFA exporter (Figs. 6, 7A, D), adds a new tool for further improving FFA production using engineered cyanobacterial mutants.
Functional expression of FarB not only rescued the growth deficiency and photodamage of dAS11 under HL (Figs. 5B, 6) but also drastically decreased FFA production ‘per cell’ (Fig. 7F) and the proportion of C16 FA species in the FFA fraction (Table 1). It is thus clear that strong light itself is not the signal for induction of the sn-2 specific lipase activity. From the tradeoff between robust growth and lipase upregulation, we presume that HL-induced damage acts as a signal for activation of the lipase(s). For efficient production of biofuels, it is important to increase the ‘per cell’ production of FFA. To fully exploit the activity of endogenous lipase of cyanobacteria for production of FFA, it is important to prevent inactivation (or deactivation) of the lipase(s). Currently, there is little information about cyanobacterial lipases. The Synechocystis sp. PCC 6803 genome database shows two ORFs, sll0482 and sll1969, as putative lipase genes, but with no genetic or biochemical evidence. Identification of the gene responsible for the HL-induced, sn-2-specific lipase activity and elucidation of the molecular mechanism of the regulation of the lipase protein are needed.
Although Aas has been thought to play a physiological role in uptake and utilization of exogenous FFA (Kaczmarzyk and Fulda 2010), our results show that it plays a more important role in the survival of Synechocystis sp. PCC 6803 under HL (Fig. 1); Aas prevents the formation of a harmful, positive feedback loop between photodamage and lipase upregulation by lowering intracellular FFA levels. Importance of FFA recycling has thus become apparent, but the physiological significance of lipid deacylation remains enigmatic. In this respect, a recent report suggesting involvement of FFA in the repair of photodamaged PSII is interesting (Jimbo et al. 2020). Under illumination, PSII is photodamaged, and the damaged PSII is repaired by the PSII repair mechanism. The degree of photoinhibition is therefore determined by the balance between damage and repair (Nishiyama and Murata 2014, Jimbo et al. 2019). By comparing the effects of exogenously added FFAs on PSII repair by Synechocystis sp. PCC 6803 cells, it was shown that saturated FFAs, i.e. palmitic and stearic acids, enhance the repair of PSII by accelerating de novo synthesis of the D1 protein, while linolenic acid is inhibitory to the repair process (Jimbo et al. 2020). In Synechocystis sp. PCC 6803, C18 and C16 FAs are esterified to the sn-1 and sn-2 positions of membrane lipids, respectively (Wada and Murata 1989), and the fatty acyl moiety at the sn-1 position is unsaturated by the action of desaturases (Wada and Murata 1989, 1990). Since FFAs are not always available from the natural environment of cyanobacteria, the sn-2 specific HL-inducible lipase may provide the saturated C16 FA to facilitate the repair of PSII.
FarB is an MFS-family transporter identified in Neisseria gonorrhoeae as a component of a tripartite efflux pump FarAB-MtrE, where MtrE is an OMP and FarA is a MFP (Lee and Shafer 1999). It was therefore unexpected that introduction of FarB alone could enhance FFA export of the E. coli K12 ∆acrAB∆emrB mutant (Fig. 2) and the Synechocystis sp. PCC 6803 dAS11 mutant (Fig. 4). It should be noted that gram-negative bacteria have several efflux pumps associated with multidrug resistance, among which some are capable of FFA export. Most of the multidrug resistance-associated pumps are RND-type pumps consisting of an Rnd family transporter, an inner membrane protein and an MFP. E. coli has several of this type of exporters, at least two of which (i.e. AcrAB-TolC and MdtEF-TolC) have FFA export activity (Lennen et al. 2013). There also are tripartite efflux pumps involving an MFS family protein as a transporter component, which are exemplified by FarAB-MtrE of Neisseria lactamica. Since E. coli has a FarAB-MtrE homolog (i.e. EmrAB-TolC), there are at least three efflux pumps for FFA. The E. coli mutant used in this study is deficient in AcrAB-TolC and EmrAB-TolC, but retains the MdtEF-TolC pump. The cyanobacterium S. elongatus PCC 7942, on the other hand, has two sets of genes encoding MFP and Rnd proteins, each forming an operon, and one of these (i.e. rndA1B1) was shown to be involved in FFA export (Kato et al. 2015). The expression level of rndA1B1 genes is low in the WT cells but inactivation of the operon results in loss of resistance to various exogenously added FFAs (Kato et al. 2015). Synchocystis sp. PCC 6803 have several genes for MFP and Rnd proteins, including the homologs of rndA1 and rndB1, and shows similar levels of FFA resistance as observed in the WT S. elongatus PCC 7942 cells (Kojima et al. 2016). It is therefore reasonable to assume that at least one RND-type FFA pump is functioning in Synechocystis sp. PCC 6803. It is, however, unlikely that FarB formed a heterologous tripartite complex with the proteins of E. coli or Synechocystis sp. PCC 6803 to secrete FFA out of the cell. Given that RND-type pumps secrete the substrates out of the cell not only from the cytoplasm but also from the periplasm (Alvarez-Ortega et al. 2013), we presume that FarB enhanced FFA secretion by increasing the rate of FFA transfer from the cytoplasm to the periplasm. In contrast to that of FarB, expression of FarA reduced resistance of E. coli and Synechocystis sp. PCC 6803 cells to exogenously added FFAs (Figs. 2, 3). This is likely to be due to interference by the heterologous MFP on the function of the endogenous efflux pumps.
Increased FFA production under HL, which is presumably due to activation of lipase activity, has also been reported in an aas-deficient mutant of S. elongatus PCC 7942 (Takatani et al. 2015), but as mentioned above, this mutant can sustain growth under the HL conditions (Takatani et al. 2015) and hence is more tolerant to high light conditions than the Synechocystis sp. PCC 6803 ∆aas mutant. The molecular basis of different light sensitivities of the two mutants is currently unclear. In the case of the S. elongatus PCC 7942 aas mutant, growth impairment due to accumulation of large amounts of intracellular FFA has been reported only when a foreign thioesterase is expressed to enhance FFA production (Kato et al. 2016, 2017). It is thus likely that the activity of the endogenous lipase is lower in S. elongatus PCC 7942 than in Synechocystis sp. PCC 6803. Another possible cause of the difference between the aas-deficient mutants of the two cyanobacterial strains may be the difference in FA compositions of membrane lipids. One of the notable differences between the two strains is that S. elongatus PCC 7942 does not contain polyunsaturated FAs, whereas Synechocystis sp. PCC 6803 contain linoleic acid (18:2) and α-linolenic acid (18:3) (Wada and Murata 1989, 1990). Although palmitic acid is by far the major FFA in the dAS11 cells exposed to HL, the sum of 18:2 and 18:3 constitutes about 6% of total intracellular FFA (Table 1). Toxicity of extrinsically added 18:2 and 18:3 on cyanobacteria is well documented (Sakamoto et al. 1998, Maeda et al. 2005, von Berlepsch et al. 2012, Kojima et al. 2016). Given the high ‘per-cell’ FFA content of HL-grown dAS11 cells, the endogenously produced polyunsaturated FAs may exert photooxidative damage to the cells under HL conditions. Further studies are needed to elucidate the hypersensitivity of the Synechocystis sp. PCC 6803 aas mutant to strong light.
Materials and Methods
Organisms and culture conditions
Synechocystis sp. PCC 6803 cells were grown at 30°C under continuous illumination using nitrate as the nitrogen source. HL illumination of the cultures was performed using white LED light at 400 µmol photons m−2 s−1. For LL illumination of the cultures, the light intensity was 50 µmol photons m−2 s−1 unless otherwise stated. The liquid medium used was a modification of BG-11 (Stanier et al. 1971) described previously (Suzuki et al. 1995). The solid medium was prepared by addition of 1.5% (w v−1) agar and 0.3% (w v−1) sodium thiosulfate to the liquid medium. Liquid cultures were bubbled with air supplemented with 2% (v v−1) CO2. Petri dishes containing the cells on the solid medium were incubated under the CO2-enriched air. When appropriate, kanamycin and chloramphenicol were added to the medium at 15 μg ml−1 and 5 μg ml−1, respectively.
Construction of the Neisseria farB-expressing dAS11 mutant
The engineered aas insertion mutant dAS11 (Kojima et al. 2016) was the aas-deficient Synchocystis sp. PCC 6803 mutant used in this study. N. lactamica cells were obtained from ATCC and the genomic DNA was used for polymerase chain reaction (PCR) amplification of the DNA fragments carrying the farAB operon, the farA gene and the farB gene (Lee and Shafer 1999: GenBank accession numbers AF132909 and AF132910), using the KOD plus DNA polymerase (Toyobo, Osaka, Japan) with the primer pairs a1/b3, a1/a2 and b1/b3, respectively (Supplementary Table S1). The amplified DNA fragments were cloned into the pGEM-T easy vector (Promega, Madison, WI, USA) and after verification of the nucleotide sequences excised from the plasmids by digestion with EcoRI and ligated into the EcoRI site of the E. coli-S. elongatus PCC 7942 shuttle vector pSE2 (Maeda et al. 1998) so that the far genes are transcriptionally fused to the Ptrc promoter on pSE2. The cat chloramphenicol resistance gene was amplified by PCR from the plasmid pHSG399 (Takeshita et al. 1987), using the primer pair d1/d2 (Supplementary Table S1) and ligated into the XbaI site of the pSE2 derivatives carrying the Ptrc-far fusions. Using the resultant plasmids as the template, DNA fragments carrying the Ptrc-far transcriptional fusions and the cat gene were amplified by PCR using the primer pair e1/e2 (Supplementary Table S1) and ligated into the EcoRV site of the plasmid pUM1, a cloning vector for gene introduction into the neutral site of the Synechocystis sp. PCC 6803 genome (Keta et al. unpublished results). The resultant plasmid DNAs, which were referred to as pUM1_Ptrc-farAB_cat, pUM1_Ptrc-farA_cat and pUM1_Ptrc-farB_cat, respectively, were used to transform the dAS11 cells through homologous recombination, generating kanamycin- and chloramphenicol-resistant transformants. After three rounds of streak purification of single colonies, selected colonies were analyzed by PCR to confirm the insertion of the Ptrc-far constructs into the neutral site of the chromosome with complete genome segregation (Supplementary Fig. S1).
FFA analysis
For the analysis of the intracellular and extracellular FFAs, 10-ml aliquots of the cultures were centrifuged at 1,700× g for 15 min to separate the cells and the medium. The supernatant was transferred to a 15-ml plastic tube and the cells were resuspended in 1 ml of methanol. The samples were stored at −80°C until use. For determination of the total concentration of FFAs in the medium, the supernatant was analyzed using the Free Fatty Acid Quantification Kit (Biovision, California, USA) according to the manufacturer’s instructions. For analysis of the extracellular and intracellular FFA profiles and the total FFA content in the cells, samples were extracted with a modified Folch method (Folch et al. 1957, Ikeda 2015) and analyzed by liquid chromatography-mass spectrometry (LC-MS) as described (Ikeda 2015, Takatani et al. 2015).
Effects of FFAs on E. coli growth
Construction of the E. coli K-12 ∆acrAB∆emrB strain was performed according to previously reported methods (Baba et al., Nakahigashi et al. 2009) using BW25113 as a background strain. For expression of the Neisseria farAB, farA or farB genes, pSE2_Ptrc-farAB_cat, pSE2_Ptrc-farA_cat or pSE2_Ptrc-farB_cat was introduced into the ∆acrAB∆emrB strain, respectively. A control strain was obtained by transformation of the ∆acrAB∆emrB strain with the pSE2 plasmid. For viability assays on the agar plates, cultures of E. coli in the late logarithmic phase of growth were incubated in the presence of 0.1 mM IPTG for 2 h and diluted to give an optical density (OD) of 1.0 at 600 nm, followed by serial dilution into fresh Luria–Bertani (LB) medium. About 3-μl aliquot from each dilution was spotted onto LB plates containing 1 mM lauric or palmitic acid and 0.04% (v v−1) polyoxyethyleneglycol dodecyl ether (Brij-35). The plates were incubated for 1 day at 37°C in the dark.
Effects of FFAs on cyanobacteria growth
For viability assays on the agar plates, liquid cultures in the late logarithmic phase of growth were diluted to an optical density of 1.0 at 730 nm, followed by serial dilution into fresh liquid medium. About 3-μl aliquot from each dilution was spotted onto solid media containing 0.1 mM 18:3. The plates were incubated for 5 days at 30°C under illumination at a light intensity of 25 µmol photons m−2 s−1. For growth assays in liquid media, cells were inoculated into the media supplemented with 0.1 mM or 0.5 mM of various FAs to give an OD730 value of 0.01 and incubated under illumination at 50 µmol photons m−2 s−1.
Other methods
The cell number and volume were determined by using a particle counter analyzer (CDA-1000, Sysmex). The photosynthetic yield of PSII was determined by measuring the FvFm−1 ratio using an AquaPen-C fluorometer (AP-C100, Photon Systems Instruments). The samples were dark-adapted for 5 min before measurement. For determination of the PSII activity of Synechocystis cells, the rate of oxygen evolution was measured by using an oxygen electrode (Oxygraph, Hansatech Instruments Ltd) in the presence of 1 mM p-benzoquinone.
Supplementary Material
Contributor Information
Kouji Kojima, Department of Biological Chemistry, Chubu University, 1200 Matsumoto-cho, Kasugai, 487-8501 Japan; Japan Science and Technology Agency, CREST, 4-1-8 Honmachi, Kwaguchi, Saitama 332-0012, Japan; Department of Applied Microbial Technology, Faculty of Biotechnology and Life Science, Sojo University, 4-22-1 Ikeda, Nishi-ku, Kumamoto, 860-0082 Japan.
Ui Matsumoto, Department of Biological Chemistry, Chubu University, 1200 Matsumoto-cho, Kasugai, 487-8501 Japan; Japan Science and Technology Agency, CREST, 4-1-8 Honmachi, Kwaguchi, Saitama 332-0012, Japan.
Sumie Keta, Department of Biological Chemistry, Chubu University, 1200 Matsumoto-cho, Kasugai, 487-8501 Japan; Japan Science and Technology Agency, CREST, 4-1-8 Honmachi, Kwaguchi, Saitama 332-0012, Japan.
Kenji Nakahigashi, Japan Science and Technology Agency, CREST, 4-1-8 Honmachi, Kwaguchi, Saitama 332-0012, Japan; Institute for Advanced Biosciences, Keio University, 246-2 Mizukami, Yamagata, 997-0052 Japan; Spiber Inc., Yamagata, 997-0052 Japan.
Kazutaka Ikeda, Japan Science and Technology Agency, CREST, 4-1-8 Honmachi, Kwaguchi, Saitama 332-0012, Japan; Laboratory for Metabolomics, RIKEN Center for Integrative Medical Sciences, 1-7-22 Suehiro-cho, Tsurumi-ku, Yokohama, 230-0045 Japan; Laboratory of Biomolecule Analysis, Department of Applied Genomics, Kazusa DNA Research Institute, 2-6-7 Kazusa-Kamatari, Kisarazu Chiba, 292-0818 Japan.
Nobuyuki Takatani, Japan Science and Technology Agency, CREST, 4-1-8 Honmachi, Kwaguchi, Saitama 332-0012, Japan; Graduate School of Bioagricultural Sciences, Nagoya University, Furo-cho, Chikusa-ku, Nagoya, 464-8601 Japan.
Tatsuo Omata, Japan Science and Technology Agency, CREST, 4-1-8 Honmachi, Kwaguchi, Saitama 332-0012, Japan; Graduate School of Bioagricultural Sciences, Nagoya University, Furo-cho, Chikusa-ku, Nagoya, 464-8601 Japan.
Makiko Aichi, Department of Biological Chemistry, Chubu University, 1200 Matsumoto-cho, Kasugai, 487-8501 Japan; Japan Science and Technology Agency, CREST, 4-1-8 Honmachi, Kwaguchi, Saitama 332-0012, Japan.
Supplementary Data
Supplementary data are available at PCP online.
Data Availability
The data underlying this article are available in the article and in its online supplementary material.
Funding
Japan Science and Technology Agency (JST)-Core Research for Evolutionary Science and Technology (CREST) program (JPMJCR11V5); JST-Mirai program (JPMJMI17EE) from JST.
Disclosures
The authors have no conflicts of interest to declare.
References
- Alvarez-Ortega C., Olivares J. and Martinez J. (2013) RND multidrug efflux pumps: what are they good for? Front Microbiol 5: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba T., Ara T., Hasegawa M., Takai Y., Okumura Y., Baba M., et al. (2006) Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol. Syst. Biol. 2: 2006.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folch J., Lees M. and Sloane Stanley G.H. (1957) A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 226: 497–509. [PubMed] [Google Scholar]
- Ikeda K. (2015) Mass-spectrometric analysis of phospholipids by target discovery approach. InBioactive Lipid Mediators: Current Reviews and Protocols. Edited by Yokomizo, T. and Murakami, M. Part IV 25, pp. 349–356. Springer, Tokyo. [Google Scholar]
- Jimbo H., Izuhara T., Hihara Y., Hisabori T. and Nishiyama Y. (2019) Light-inducible expression of translation factor EF-Tu during acclimation to strong light enhances the repair of photosystem II. Proc. Natl. Acad. Sci. U. S. A. 116: 21268–21273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimbo H., Takagi K., Hirashima T., Nishiyama Y. and Wada H. (2020) Long-chain saturated fatty acids, palmitic and stearic acids, enhance the repair of photosystem II. Int. J. Mol. Sci. 21: 7509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczmarzyk D. and Fulda M. (2010) Fatty acid activation in cyanobacteria mediated by acyl-acyl carrier protein synthetase enables fatty acid recycling. Plant Physiol. 152: 1598–1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato A., Takatani N., Ikeda K., Maeda S.I. and Omata T. (2017) Removal of the product from the culture medium strongly enhances free fatty acid production by genetically engineered Synechococcus elongatus. Biotechnol. Biofuels 10: 141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato A., Takatani N., Use K., Uesaka K., Ikeda K., Chang Y., et al. (2015) Identification of a cyanobacterial RND-type efflux system involved in export of free fatty acids. Plant Cell Physiol. 56: 2467–2477. [DOI] [PubMed] [Google Scholar]
- Kato A., Use K., Takatani N., Ikeda K., Matsuura M., Kojima K., et al. (2016) Modulation of the balance of fatty acid production and secretion is crucial for enhancement of growth and productivity of the engineered mutant of the cyanobacterium Synechococcus elongatus. Biotechnol. Biofuels 9: 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima K., Keta S., Uesaka K., Kato A., Takatani N., Ihara K., et al. (2016) A simple method for isolation and construction of markerless cyanobacterial mutants defective in acyl-acyl carrier protein synthetase. Appl. Microbiol. Biotechnol. 100: 10107–10113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee E.H. and Shafer W.M. (1999) The farAB-encoded efflux pump mediates resistance of gonococci to long-chained antibacterial fatty acids. Mol. Microbiol. 33: 839–845. [DOI] [PubMed] [Google Scholar]
- Lennen R.M., Politz M.G., Kruziki M.A. and Pfleger B.F. (2013) Identification of transport proteins involved in free fatty acid efflux in Escherichia coli. J. Bacteriol. 195: 135–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda H., Sakuragi Y., Bryant D.A. and DellaPenna D. (2005) Tocopherols protect Synechocystis sp. strain PCC 6803 from lipid peroxidation. Plant Physiol. 138: 1422–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda S., Kawaguchi Y., Ohe T. and Omata T. (1998) cis-acting sequences required for NtcB-dependent, nitrite-responsive positive regulation of the nitrate assimilation operon in the cyanobacterium Synechococcus sp. strain PCC 7942. J. Bacteriol. 180: 4080–4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakahigashi K., Toya Y., Ishii N., Soga T., Hasegawa M., Watanabe H., et al. (2009) Systematic phenome analysis of Escherichia coli multiple-knockout mutants reveals hidden reactions in central carbon metabolism. Mol. Syst. Biol. 5: 306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama Y. and Murata N. (2014) Revised scheme for the mechanism of photoinhibition and its application to enhance the abiotic stress tolerance of the photosynthetic machinery. Appl. Microbiol. Biotechnol. 98: 8777–8796. [DOI] [PubMed] [Google Scholar]
- Ruffing A.M. (2014) Improved free fatty acid production in cyanobacteria with Synechococcus sp. PCC 7002 as host. Front. Bioeng. Biotechnol. 2: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruffing A.M. and Trahan C.A. (2014) Biofuel toxicity and mechanisms of biofuel tolerance in three model cyanobacteria. Algal Res. 5: 121–132. [Google Scholar]
- Sakamoto T., Delgaizo V.B. and Bryant D.A. (1998) Growth on urea can trigger death and peroxidation of the cyanobacterium Synechococcus sp. strain PCC 7002. Appl. Environ. Microbiol. 64: 2361–2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanier R.Y., Kunisawa R., Mandel M. and Cohen-Bazire G. (1971) Purification and properties of unicellular blue-green algae (order Chroococcales). Bacteriol. Rev. 35: 171–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki I., Horie N., Sugiyama T. and Omata T. (1995) Identification and characterization of two nitrogen-regulated genes of the cyanobacterium Synechococcus sp. strain PCC7942 required for maximum efficiency of nitrogen assimilation. J. Bacteriol. 177: 290–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takatani N., Use K., Kato A., Ikeda K., Kojima K., Aichi M., et al. (2015) Essential role of acyl-ACP synthetase in acclimation of the cyanobacterium Synechococcus elongatus strain PCC 7942 to high-light conditions. Plant Cell Physiol. 56: 1608–1615. [DOI] [PubMed] [Google Scholar]
- Takeshita S., Sato M., Toba M., Masahashi W. and Hashimoto-Gotoh T. (1987) High-copy-number and low-copy-number plasmid vectors for lacZ alpha-complementation and chloramphenicol- or kanamycin-resistance selection. Gene 61: 63–74. [DOI] [PubMed] [Google Scholar]
- von Berlepsch S., Kunz H.-H., Brodesser S., Fink P., Marin K., Flügge U.-I., et al. (2012) The acyl-acyl carrier protein synthetase from Synechocystis sp. PCC 6803 mediates fatty acid import. Plant Physiol. 159: 606–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada H. and Murata N. (1989) Synechocystis PCC6803 mutants defective in desaturation of fatty acids. Plant Cell Physiol. 30: 971–978. [Google Scholar]
- Wada H. and Murata N. (1990) Temperature-induced changes in the fatty acid composition of the cyanobacterium, Synechocystis PCC6803. Plant Physiol. 92: 1062–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Chen L., Yang S. and Tan X. (2020) Photosynthetic conversion of carbon dioxide to oleochemicals by cyanobacteria: recent advances and future perspectives. Front. Microbiol. 11: 634. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in the article and in its online supplementary material.


