Abstract
The amygdala plays an important role in the interpretation of emotionally significant stimuli and has strong projections to brainstem regions regulating muscle tone and sleep. Cataplexy, a symptom of narcolepsy, is a loss of muscle tone usually triggered by sudden, strong emotions. Extracellular single-unit recordings were carried out in the amygdala of narcoleptic dogs to test the hypothesis that abnormal activity of a subpopulation of amygdala neurons is linked to cataplexy.
Of the 218 cells recorded, 31 were sleep active, 78 were active in both waking and rapid-eye-movement sleep, 88 were maximally active during waking, and 21 were state independent. Two populations of cells showed a significant change in activity with cataplexy. A population of sleep active cells localized to central and basal nucleus increased discharges prior to and during cataplexy. A population of wake active cells localized to the cortical nucleus decreased activity prior to and during cataplexy. We hypothesize that these cell populations have a role in mediation or modulation of cataplexy through interactions with meso-pontine regions controlling atonia. The anticholinesterase physostigmine, at doses which increased cataplexy, did not alter the activity of the cataplexy-related cells or of other amygdala cells, suggesting that its effect on cataplexy is mediated ‘downstream’ of the amygdala. The α-1 blocker prazosin, at doses which increased cataplexy, increased discharge in a subgroup of the cataplexy active cells and in a number of other amygdala cells, indicating that prazosin may modulate cataplexy by its action on amygdala cells or their afferents. Published by Elsevier Science Ltd on behalf of IBRO.
Keywords: amygdala, narcolepsy, cataplexy, neuronal activity, sleep, dog
Narcolepsy is an incurable sleep disorder characterized by excessive daytime sleepiness, sudden attacks of muscle atonia called cataplexy, fragmented night-time sleep, sleep paralysis and hypnagogic hallucinations (Guilleminault, 1994). Narcoleptics frequently have rapid-eye-movement (REM) sleep at sleep onset, while normals typically have non-REM (NREM) sleep at sleep onset (Rechtschaffen et al., 1963). Cataplexy and sleep paralysis are thought to represent abnormal triggering in waking of the REM-sleep muscle tone suppression mechanism (Siegel et al., 1991). Laughter, anger or the sudden onset of other strong emotions can trigger cataplexy (Aldrich, 1990; Rechtschaffen et al., 1963). In the 1970s, narcolepsy was described in dogs (Knecht et al., 1973; Mitler et al., 1974). Canine cataplexy is triggered by vigorous play, the eating of favored foods or other forms of emotional excitement and is linked to a mutation of the hypocretin-2 receptor gene (Lin et al., 1999).
Several lines of evidence suggest that the amygdala is involved in sleep regulation. The amygdala is strongly activated in REM sleep (Maquet et al., 1996; Nofzinger et al., 1997). Stimulation of the central nucleus of the amygdala in normal cats increases REM-sleep duration (Calvo et al., 1996). Electrical stimulation of the central nucleus of the amygdala during REM sleep increases ponto-geniculo-occipital wave amplitude (Deboer et al., 1998). Stimulation of the ventral amygdala produces electroencephalographic (EEG) synchrony (Kreindler and Steriade, 1964). Noradrenergic denervation of amygdala reduces the REM-sleep rebound after sleep deprivation (Charifi et al., 2000). Lesions of the amygdala in restrained rhesus monkeys resulted in more sleep and a higher proportion of REM sleep than their control animals (Benca et al., 2000).
Recent findings have demonstrated that there is a substantially decreased number of neurons producing hypocretin in the hypothalamus of human narcoleptics (Peyron et al., 2000b; Thannickal et al., 2000). Hypocretin axons have been found in many brain regions including the amygdala (Peyron et al., 2000a). Earlier data from our laboratory reported neuronal degeneration in the forebrain of narcoleptic dogs, peaking just before symptom onset (Siegel et al., 1999). The highest levels of axonal degeneration were found in the amygdala. There are strong projections from the central nucleus of the amygdala to the dorsolateral pontine cholinergic and noradrenergic cell groups involved in the generation of REM sleep (Wallace et al., 1989). The amygdala has been shown to make a major contribution to the regulation of emotional processes, particularly to the detection of emotionally significant events and the production of appropriate responses (Llamas et al., 1977; Rolls, 1992; Swanson and Petrovich, 1998). We hypothesize that the neuronal degeneration seen in canine narcolepsy (Siegel et al., 1999) disinhibits amygdala cells and that these disinhibited cells are activated during sudden, strong emotions. These cells then activate the brainstem motor inhibitory system, mono- or polysynaptically, resulting in cataplexy. Alternatively or simultaneously a population of amygdala cells that decrease activity during cataplexy may cause the disfacilitation of key groups of neurons required for the maintenance of muscle tone. In the current study, we have recorded the amygdala neurons during normal sleep states and during cataplexy in narcoleptic dogs to determine if the amygdala has cells that significantly alter their activity in relation to cataplexy.
EXPERIMENTAL PROCEDURES
Cells were recorded from the amygdala of narcoleptic Doberman pinschers using microwire recording techniques as described previously (Siegel et al., 1992; Wu et al., 1999). Doberman pinschers were bred in our animal facility (VAGLAHS). All experiments were conducted in accordance with the National Research Council Guide for the Care and Use of Animals and all procedures were reviewed and approved by the Internal Animal Care and Use Committee of the V.A. Greater Los Angeles Healthcare system. Under isoflurane anesthesia, microdrives containing 32- and 64-μm stainless steel microwires (California Fine Wire, Grover Beach, CA, USA) aimed at the nucleus centralis of the amygdala were implanted, with the tip of the electrodes 1 mm above the target area. Stainless steel screw electrodes were implanted in the frontal sinus for the recording of the EEG and electrooculogram (EOG).
For the recording of hippocampal theta activity, tripolar twisted electrodes, made of three 256-μm formvar insulated stainless steel wires (California Fine Wire), with 1 mm vertical separation were placed in the dorsal hippocampus. Electromyogram (EMG) activity was recorded from the dorsal neck muscles with teflon-coated multistranded (0.035 cm) stainless steel wires (Cooner Wire, Chatsworth, CA, USA).
Unit and physiological recording in sleep and cataplexy
The dogs were free to move around the chamber (1.2 m3 in size) during the recordings. Electrodes were moved in 80-μm increments until a cell or cells with signal-to-noise ratio of at least 3-to-1 was isolated. The activity of each cell was then characterized throughout sleep–waking states and during cataplexy. Unit pulses from the window discriminator and the digitized action potentials, cortical and hippocampal EEG, EOG and EMG were amplified by a Grass model 78 E polygraph and recorded continuously on a PC with Spike2 software (Cambridge Electronic Design Ltd., Cambridge, UK) and on the polygraph. Microwire signals were filtered, with roll off of signals below 300 Hz and above 10 kHz, and digitized at a frequency of 25 kHz. Cortical and hippocampal EEG, and EOG signals were filtered with 1 Hz low-frequency and 100 Hz high-frequency cut-off and sampled at 200 Hz. EMG was filtered between 30 and 3 kHz and sampled at 1 kHz (Wu et al., 1999). The baseline sleep–wake discharge profile of each cell was established across at least one complete sleep–wake cycle. Cataplexy was then induced either by the introduction of soft food (Pedigree, by Kalkan) or by giving novel toys or play objects to the dog. Cataplexy was defined as an abrupt loss of muscle tone in the alert waking state triggered during play or food intake and was accompanied by theta activity similar to that seen in REM sleep (Wu et al., 1999). Eyes typically remained open and tracked moving objects. Sometimes cataplectic attacks were followed by the onset of REM sleep. Cataplexy normally terminated with the dog lifting his neck above the floor and then rising to its feet. A minimum of five cataplexies were observed during the recording of each cell. In some cases, the anticholinesterase physostigmine salicylate (50–100 μg/kg, i.v.; Forest Pharmaceutical, St. Louis, MO, USA) or the α-1 antagonist prazosin hydrochloride (0.5 mg/kg, p.o.; UDL Laboratories, Rockford, IL, USA) was administered to increase the frequency and the intensity of cataplexy. Physostigmine administered i.v. produces increased cataplexy within 10 min of administration. The effect of physostigmine was assessed during waking, 10 min after injection. Oral prazosin administration has its initial effect after 90 min. The effect of prazosin on cell activity was assessed between 90 and 120 min after drug administration during sleep and waking states. Cells were held for recording for as long as needed to complete the experimental protocol (6–72 h).
Narcoleptic dogs display partial cataplexies in which only the hind limbs collapse and complete cataplexies in which all four limbs collapse and the whole body contacts the floor. Partial cataplexies typically last less than 5 s while complete cataplexies may last from 10 s to a few minutes. Because of the shortness of the partial cataplexy episodes, only complete cataplexies were used for quantification of the changes in unit discharge rate during cataplexy.
To characterize the temporal relationship between unit and EMG activity, both were averaged with respect to the onset and offset of cataplexy. The time of onset and offset was determined from the digitized EMG. The onset of cataplexy was defined as the initial point of a period in which EMG became completely atonic for at least 1 s. The offset of cataplexy was defined as the point at which EMG activity resumed. Unit activity and EMG were averaged for 10 s before and 10 s after the onset/offset points. Average spike rate of first 5 s was (from −10 to −5 s) analyzed and mean and standard deviation values calculated. This mean value was compared with every bin rate (bin size=400 ms) from −5 to 10 s. When the firing rate across three consecutive bins deviated more than ±2 S.D. from the mean of first 5 s, it was scored as a change in firing rate. Comparison of unit discharge rate in different behavioral states was done using analysis of variance. Significant differences between paired states were determined by post-hoc Bonferroni analyses.
Histology
At the completion of recording, iron was deposited by passing a 15-μA, 20-s anodal current through the electrodes from which cells had been recorded. Dogs were killed by first deeply anesthetizing them with sodium pentobarbitol (50 mg/kg, i.v.), then perfusing with buffered saline and paraformaldehyde. Four series of 60-μm brain sections were cut across the whole length from anterior to posterior amygdala. One series was stained with Cresyl Violet for nuclei identification and an adjacent series was stained first for the Prussian Blue reaction for iron to locate the tip of electrode and then with Cresyl Violet. Recording sites were reconstructed.
RESULTS
A total of 218 cells were recorded from the amygdaloid complex of four dogs and observed long enough to see the activity across at least one complete sleep–wake cycle. The mean discharge rates during wakefulness and sleep stages were calculated from 40–300-s blocks (mean sample duration 74 s during waking and 70 s during sleep) of stable wakefulness and sleep (NREM or REM). Wakefulness was classified as either active waking (AW) in which dog was moving freely in the chamber or quietwaking (QW) in which the dog was awake but sitting quietly. The neurons were defined as sleep active if their discharge rate was at least 30% higher during NREM and/or REM sleep as compared to either AW or QW (using whichever had the higher firing rate). Wake (W)/REM active cells were those that had discharge rate at least 30% higher during AW waking and REM sleep as compared to NREM. Wake active cells were defined as those that were at least 30% more active during both AW and QW as compared to both NREM and REM sleep. Neurons not meeting any of the above criteria were defined as state independent. Six, 30-s epochs were taken from each state and compared with each other for significance.
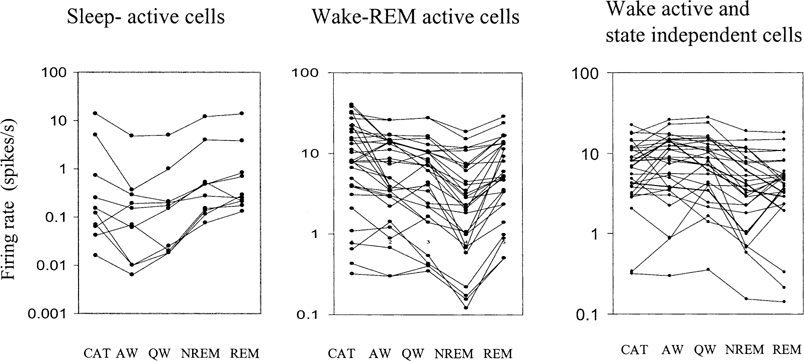
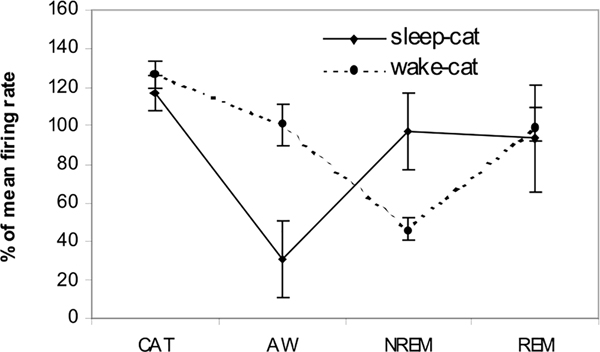
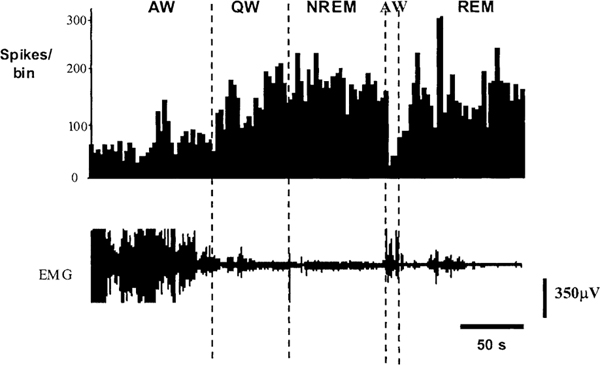
Four types of cells were seen (Table 1). These were sleep active, W/REM active, wake active and state independent. Figure 1 shows the behavior of sleep active and REM active cells during different sleep–wake states and cataplexy. The anatomical distribution of all recorded cells is shown in Figs. 2 and 3. One hundred thirty two cells were examined during cataplexy. Two types of cataplexy active cells were observed, sleep active–cataplexy active and W/REM/cataplexy active (Fig. 4). Sleep active–cataplexy active cells had at least 30% higher discharge (as compared to wake state) during sleep and cataplexy. W/REM/cataplexy active cells had at least 30% higher discharge (as compared to NREM) during W/REM and cataplexy.
Table 1.
Cells recorded from the amygdala of the narcoleptic dog
| Cell type | Overall totals | Central/basal nucleus |
Anterior amygdala/cortical nucleus |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| recorded | tested for cataplexy | CAT+ | CAT− | inconsistent rate change | recorded | tested for cataplexy | CAT+ | CAT− | inconsistent rate change | ||
|
| |||||||||||
| Sleep active | 31 | 30 | 17 | 6 | 3 | 8 | 1 | 1 | 1 | ||
| W/REM active | 78 | 24 | 14 | 10 | 2 | 2 | 54 | 48 | 37 | 6 | 5 |
| Wake active | 88 | 20 | 11 | 4 | 5 | 2 | 68 | 42 | 20 | 17 | 5 |
| State indifferent | 21 | 12 | 9 | ||||||||
| Total cells recorded | 218 | 86 | 132 | ||||||||
CAT+: Cells activated during cataplexy; CAT−: cells inactivated during cataplexy.
Fig. 1.
Discharge rate profile of sleep active and W/REM active cells recorded across all sleep/waking states and during cataplexy. For sleep active cells, the discharge rate is minimal during waking states. For W/REM active cells the discharge rate is minimal during NREM sleep. CAT – Cataplexy.
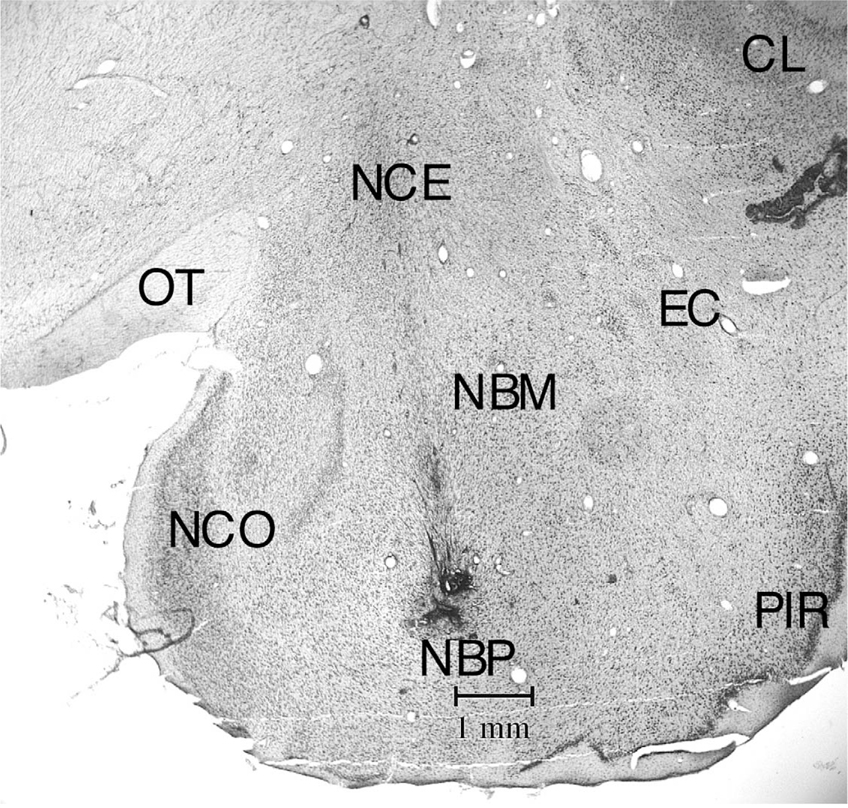
Fig. 2.
Coronal section (Cresyl Violet stained) at R3 level showing electrode track passing through the regions of nucleus centralis and basalis. EC – External capsule, NBM – nucleus basalis magnocellularis, NBP – nucleus basalis parvocellularis, NCE – nucleus centralis, NCO – nucleus cortical amygdala; OT – optic tract, PIR – piriform cortex, CL – claustrum.
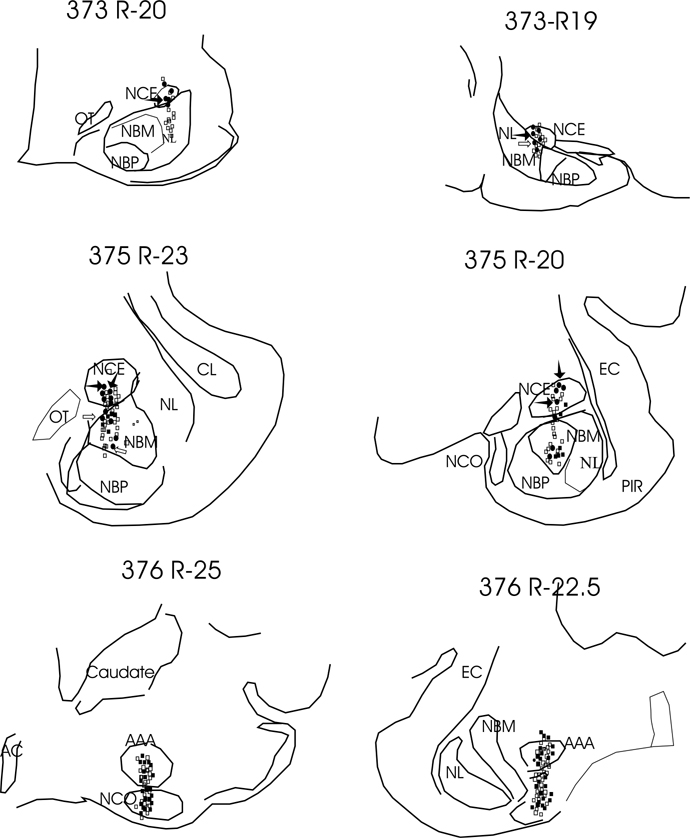
Fig. 3.
Anatomical distribution of cell types recorded in the amygdala. R19, R20, R22.5, R23, and R25 posterior to anterior levels from atlas of (Lim et al., 1960). Filled circles, sleep active cells; filled squares, AW cells; open squares, W/REM active cells. Filled arrows, sleep active–cataplexy active cells, blank arrows, sleep active–cataplexy inactive cells. AAA – Anterior amygdala nuclei; AC – anterior commissure, EC – external capsule, NBM – nucleus basalis magnocellularis, NBP – nucleus basalis parvocellularis, NCE – nucleus centralis, NCO – nucleus cortical amygdala, NL – nucleus lateralis, OT – optic tract, PIR – piriform cortex. 373, 375 and 376 are dog identification numbers.
Fig. 4.
Percent change (±S.E.M.) in firing rate of two types of cataplexy active (sleep active–cataplexy active and AW–cataplexy active) cells. Mean across all the states taken as 100%. CAT –Cataplexy.
Characteristics of sleep active cells in cataplexy
A total of 31 sleep active cells were recorded. The firing pattern of a representative sleep active cell is shown in Fig. 5. These cells were recorded from nucleus centralis (16 of 31), basalis (14 of 31) and cortical nucleus (1 of 31). These cells were very slow or silent during waking and increased firing during NREM and REM (Fig. 6) (average firing rate in mean Hz±S.E.M.: AW, 0.2±0.1; QW, 0.3±0.1; NREM, 0.9±0.2; REM, 1.1±0.2. Firing rate during NREM and REM was not significantly different (Fig. 4).
Fig. 5.
Histogram showing increase in firing rate of sleep active neuron during transition from AW to NREM and REM sleep. Bin size is 30 s.
Fig. 6.
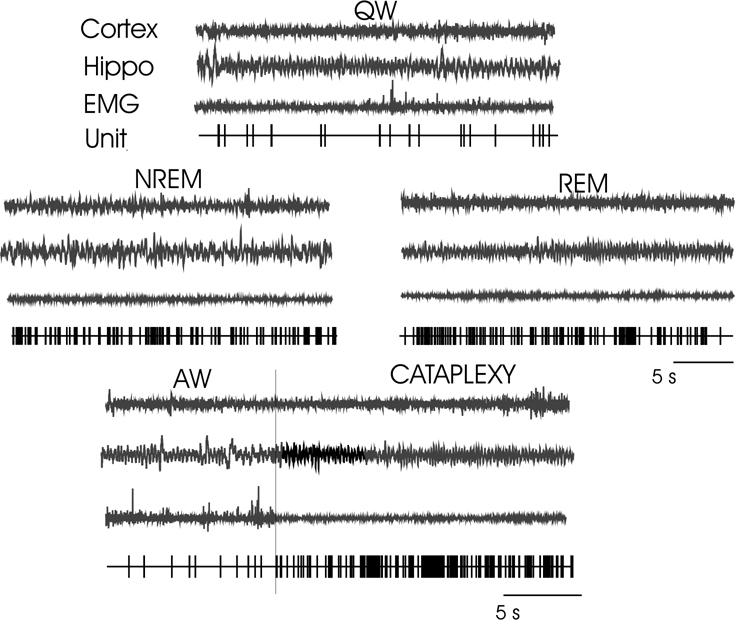
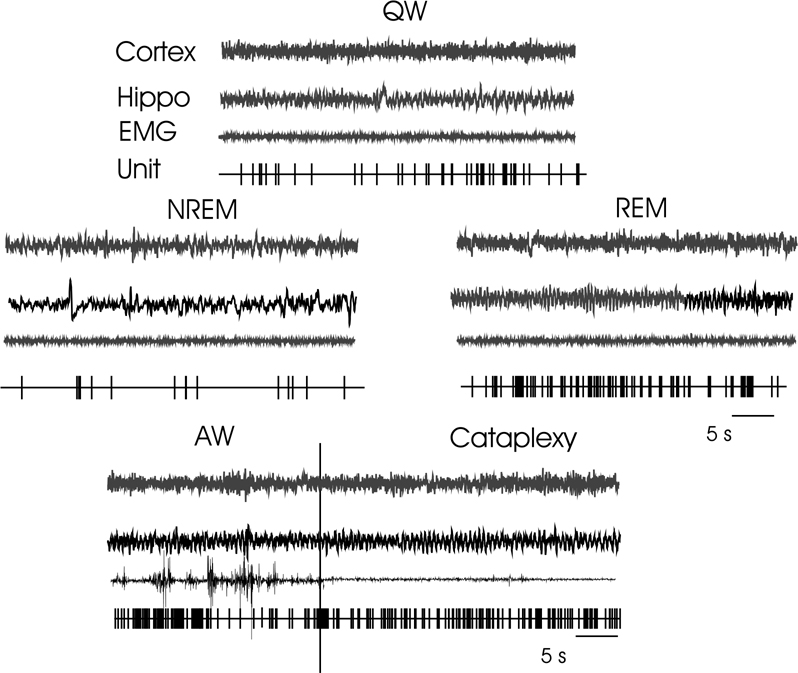
Discharge pattern of a sleep active–cataplexy active cell during QW, Cortex (EEG from sensorimotor cortex), Hippo (EEG from hippocampus), EMG (from dorsal neck muscle), Unit (computer triggered spike display), NREM sleep, REM sleep, and transition from AW to cataplexy.
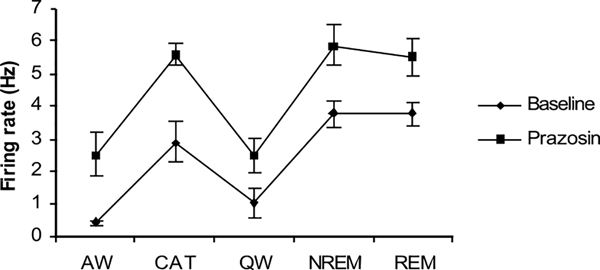
Of these 31 cells, 17 were observed during cataplexy. Of the nine cells with the same pre- and post-drug behavior, six were sleep active-cataplexy active and 3 were sleep active–cataplexy-inactive (Fig. 1). The firing pattern of a representative sleep active–cataplexy active cell is shown in Fig. 6. Their firing was negatively correlated with muscle tone with higher discharge rates in QW than in AW and maximal discharge during sleep and cataplexy (Fig. 7). The firing pattern of these cells during cataplexy was opposite to the pattern we have previously reported in the locus coeruleus (LC)(Fig. 8) (Wu et al., 1999). In the LC, cells reduce their firing during NREM and cease discharge in REM sleep. LC cells are tonically active in waking but completely cease discharge in cataplexy. The average firing rate of the amygdala sleep active–cataplexy active cells during cataplexy was not significantly different from their rate in REM and NREM sleep but significantly greater than that in the AW state out of which cataplexy arose and than the rate in QW (F3,16 = 3.62, P<0.05, Bonferroni’s paired analysis; NREM vs. AW, P<0.05; NREM vs. QW, P<0.05). For three sleep active cells (which were each observed during at least 15 cataplexy episodes) unit activity and EMG were averaged for 10 s before and 10 s after the onset/offset points. Unit firing increased by at least 2 S.D. above the baseline firing rate (the average combined baseline rate was 1.34±0.31 whereas average rate during cataplexy was 3.83±0.21) at the onset point of cataplexy episodes and unit firing fell (1.47±0.38) by at least 2 S.D. below cataplexy firing rate) at the offset of cataplexy (Fig. 8). Prazosin increased the wake (QW) firing rate of three of these sleep active–cataplexy active cells by 72.4±8.9%, whereas the activity of the remaining three cells was not changed (Fig. 9).
Fig. 7.
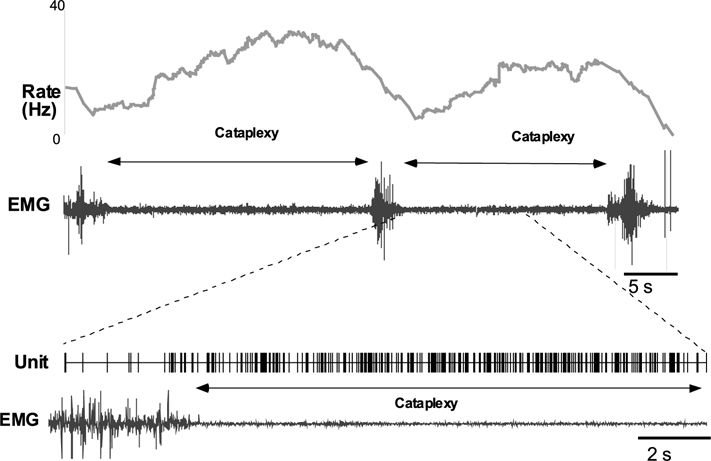
Instantaneous rate plot showing increase in discharge with cataplexy in sleep active–cataplexy active cell. Pair of traces in the lower part of the figure is an enlargement of the interval around the onset of cataplexy showing an increase of unit firing with decrease in the muscle tone.
Fig. 8.
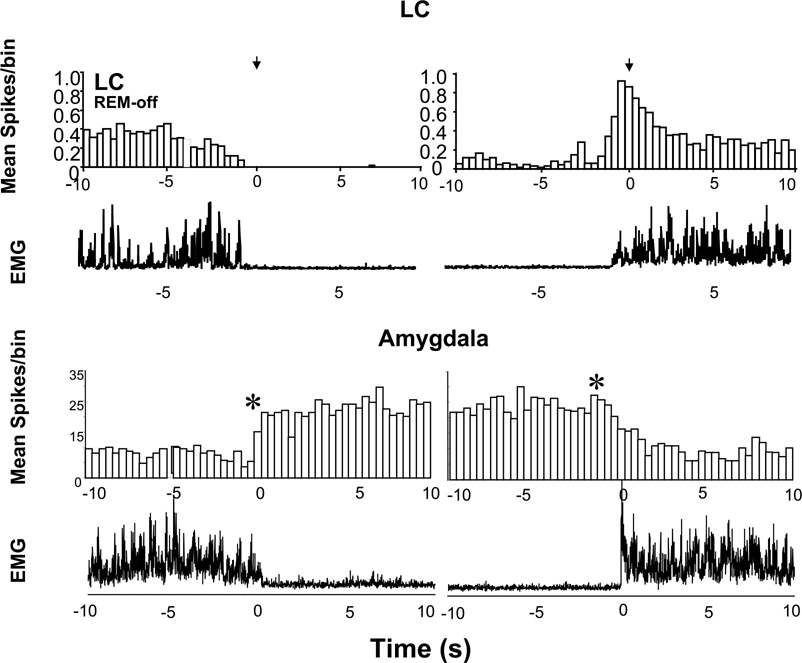
Average spike histogram before and after the cataplexy onset and offset in LC (REM-off cell) and amygdala (sleep active–cataplexy active cell). Twenty cataplexy episodes (mean duration 23.7±3.7 s) were averaged across four LC cells (Wu et al., 1999). Fifteen cataplexy episodes (mean duration 40.4±5.45 s) were averaged across three amygdala cells. The bin size of the histogram was 400 ms for both cells. Note the increased discharge in the amygdala cataplexy-on cell with reduction in muscle tone and the inverse relation between LC and amygdala cataplexy-on cells at onset and offset of cataplexy. Asterisks denote significant difference (mean±2 S.D.) in firing rate before and after the onset and offset of cataplexy episodes. Note that changes in discharge rate of amygdala cell accompany or precede changes in simultaneously recorded EMG (lowest trace).
Fig. 9.
Effect of prazosin on firing rate of sleep active–cataplexy active cells (n = 3). Prazosin increased the firing rate of these cells. CAT – Cataplexy.
Of the 17 sleep active cells tested for cataplexy, nine showed the same activity profile across sleep and waking states in pre- and post-drug conditions, whereas the remaining eight cells did not show the same behavior in pre- and post-drug conditions during the 6-h period of post-drug observation. The above-mentioned eight cells whose sleep–wake discharge profile was altered by prazosin were no longer ‘sleep active’ in post-drug conditions. Instead, their waking firing rates were increased to levels that were not significantly less than their post-prazosin sleep rates. Firing rate during cataplexy was not significantly different from either wake or sleep rates.
The effect of physostigmine was observed on 11 sleep active cells including five sleep active–cataplexy active cells. Although physostigmine greatly increased the propensity for cataplexy, it did not have any effect on the wake firing rate of these cells.
Characteristics of W/REM active cells in cataplexy
A total of 78 W/REM active cells were recorded. Their discharge pattern is seen in Fig. 10. Their mean firing rate was AW 6.2±1.6; QW 4.3±1.1; NREM 1.8±0.4; REM 6.6±1.3. Sixty-two of these cells were tested during cataplexy. The average firing rate during cataplexy was 10.4±2.3 Hz, not significantly different from REM and AW but significantly greater than NREM) (F4,100 = 3.94, P<0.005, pairwise Bonferroni; cataplexy vs. NREM, P<0.005). Cataplexy onset was not linked to a significant change in the discharge rate of these cells, since cataplexy arises out of AW. The behavior of W/ REM active cells in the amygdala during cataplexy can be contrasted with that of pontine W/REM active cells. Most pontine W/REM active cells greatly reduce discharge rate in cataplexy (Siegel et al., 1992). W/REM active cells were scattered in nucleus centralis, basalis and cortical amygdala.
Fig. 10.
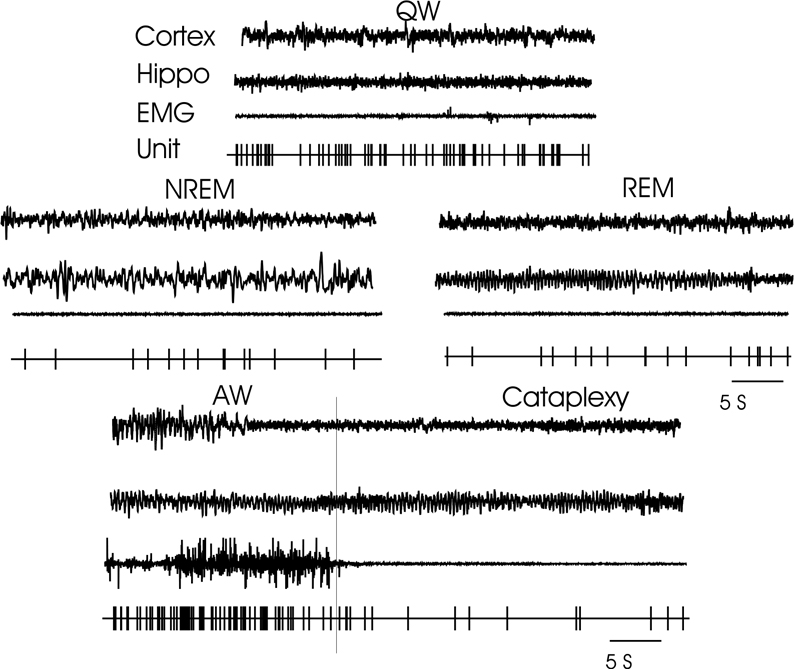
Discharge pattern of wake/REM/cataplexy active cell during AW, QW, NREM, REM sleep, and cataplexy. For abbreviations, see legend of Fig. 6.
Characteristics of wake active cells in cataplexy
A total of 88 wake active cells were recorded. These cells were mainly seen in the cortical amygdala nucleus. Their mean firing rate was AW 17.3±4.5; QW 16.9±4.1; NREM 6.7±1.5; REM 8.5±1.9. Firing rate during AW was significantly greater than that in REM (F4,398 = 3.62 P<0.05,). Fifty three of the wake active cells were observed during cataplexy. Two distinct cataplexy response cell types were seen. In the first (n = 24), firing rate during cataplexy was 19.7±3.2 significantly greater than NREM and REM sleep (F4,90 = 3.25, P<0.05, pairwise Bonferroni; cataplexy vs. NREM P<0.005; cataplexy vs. REM P<0.05) but not significantly different from AW and QW stages. Firing rate during cataplexy of the other cell type was very different (Fig. 11). In this type, the ‘wake active–cataplexy inactive’ cell, the mean firing rate (n = 23) during cataplexy was 7.3±2.2, significantly decreased from QW (F4,111 = 3.01, P<0.05) but was not significantly different from that of NREM and REM. The discharge pattern of these cells was in contrast to sleep active–cataplexy active cells (Fig. 8) whose firing rate during cataplexy was higher than in AW. Neither physostigmine nor prazosin produced any consistent effect on firing rate of wake active–cataplexy active or wake active–cataplexy-inactive cells.
Fig. 11.
Discharge pattern of wake active–cataplexy inactive cell during AW, QW, NREM, REM sleep, and cataplexy. For abbreviations, see legend of Fig. 6.
Characteristics of state indifferent cells in cataplexy
A total of 21 state indifferent cells were recorded. Their mean firing rate was AW 6.7±3.1; QW 5.9±2.9; NREM 5.4±3.4; REM 4.9±3.1. Firing rate during cataplexy was not significantly different from that of any of the sleep–wake states. Neither prazosin nor physostigmine produced any significant effect on the discharge rate of these cells.
DISCUSSION
We found that cataplexy active cells were present in the amygdala. They were primarily localized to nucleus centralis, basalis and cortical amygdala. All the sleep active–cataplexy active cells were localized in nucleus centralis and nucleus basalis. We also found a population of wake active–cataplexy inactive cells. These were localized to the cortical nucleus of the amygdala.
Our observations on the sleep cycle discharge of amygdala cells in the narcoleptic dog agree with earlier studies of the amygdala in normal cats (Jacobs and McGinty, 1971; Reich et al., 1983; Szymusiak and McGinty, 1986; White and Jacobs, 1975). These studies noted the presence of a population of cells localized to nucleus basalis and centralis of amygdala that fired slowly in waking and increased discharge in NREM or/and REM sleep.
In the current study we identified a subset of cells in amygdala which discharged at high rates only in cataplexy and sleep. This kind of cell has been previously been seen only in the medial medulla (Siegel et al., 1991) and dorsolateral pons (Siegel et al., 1992; Wu et al., 1994) where they are believed to be output cells for the suppression of muscle tone (Siegel et al., 1991). Amygdala sleep active–cataplexy active cells fired maximally during NREM, REM sleep and cataplexy and reduced firing rate significantly during QW with a further reduction during AW. Therefore, like the medullary and pontine cataplexy active cells, their discharge rate was inversely correlated with muscle tone. In contrast to the discharge pattern shown by cataplexy active cells, most brainstem and amygdala cells in both normal and narcoleptic animals maintain higher discharge rates in AW than in QW. In both normal and narcoleptic animals, a high percentage of the cells that have higher discharge rates in AW than in QW are maximally active in REM sleep (Siegel et al., 1991).
Cataplexy active cells were located in the nucleus centralis and basalis. These are the major output and input nuclei of amygdala. Nucleus centralis projects heavily to the mesopontine reticular formation and magnocellular basal forebrain nuclei (the basal nucleus of Meynert and the nucleus of horizontal limb of diagonal band) (Mesulam et al., 1983; Price and Amaral, 1981; Russchen et al., 1985). The fibers which innervate the cholinergic magnocellular basal forebrain nuclei sweep through the basal forebrain and continue on to the thalamus, hypothalamus and brain stem (Russchen et al., 1985). While they do not, therefore terminate within the basal forebrain, the fibers form en passant synapses with cells of the magnocellular nuclei. Fibers from the central nucleus are concentrated around the large cell bodies of the cholinergic cell groups. The diagonal band of Broca and magnocellular preoptic region are the major components of the basal forebrain hypnogenic region. Sleep and wake active neurons are found throughout this area (Szymusiak and McGinty, 1986).
The central and medial nucleus of the amygdala sends major projections to the bed nucleus of the stria terminalis and the full rostrocaudal extent of the lateral hypothalamus (Holstege et al., 1985; Krettek and Price, 1978; Price and Amaral, 1981). These projections originate mainly in the central nucleus, with minor additional contributions from the medial and anterior cortical nuclei (Amaral et al., 1992). The bed nucleus of stria terminalis and lateral hypothalamus project to the brainstem nuclei (retrorubral nucleus, periaqueductal gray, ventral paralemniscal tegmental field, pedunculopontine nucleus, LC complex and nucleus magnocellularis) (Amaral et al., 1992; Hopkins and Holstege, 1978; Krettek and Price, 1978; Wallace et al., 1989, 1992). Neurons from these brainstem nuclei in turn project to the pontine and medullary regions involved in the suppression of muscle tone (Lai et al., 1999). Direct projections from the limbic system to spinal cord including projections from hypocretin (orexin) neurons in the lateral hypothalamus have also been found (Kuypers and Maisky, 1977; van den Pol, 1999). Thus, abnormal activation of cataplexy active amygdala neurons during waking may activate brainstem motor inhibitory systems such as the pontine inhibitory area and nucleus magnocellularis.
Recent studies found a mutation in the hypocretin (orexin) receptor-2 gene in narcoleptic dogs (Lin et al., 1999). Chemelli et al. (1999) reported that hypocretin knockout mice exhibit a phenotype with episodes of ‘behavioral arrest’ and sleep onset REM sleep, abnormalities resembling those of narcolepsy. The mutated receptor gene may alter the discharge rate of hypocretin target neurons (Lin et al., 1999; Sakurai et al., 1998; Siegel, 1999) and cause the degeneration of a subgroup of these neurons (Siegel et al., 1999). The central nucleus of amygdala has dense projections to and from the hypocretin-rich area (Krettek and Price, 1978; Price and Amaral, 1981). Thus, altered interactions between the hypocretin system and the amygdala may explain the emotional triggering of cataplexy as well as the sleepiness that is characteristic of narcolepsy.
The cessation of LC neuronal activity is linked to the initiation of cataplexy (Wu et al., 1999). Norepinephrine released from LC axons facilitates motor neuronal activity (Fung and Barnes, 1981; Lai and Barnes, 1985). The amygdala has a direct projection to the LC (Wallace et al., 1992). The amygdala may modulate motor activity linked to cataplexy through its projections to LC.
Narcolepsy is characterized by sleep attacks and loss of muscle tone. The participation of sleep active cells in cataplexy suggests a role for these amygdala cells in the genesis of both of these symptoms of narcolepsy. Narcoleptic dogs show degenerative changes in the forebrain at the time of symptom onset (Siegel et al., 1999). The amygdala, particularly the basal, central and anterior nuclei, showed the highest level of labelling for degenerated cells and axons. Abnormal levels of cataecholamines and their metabolites (Faull et al., 1986), increased concentration of dopamine and its metabolite (DOPAC), (Nishino et al., 1994), an increase in D2 receptors (Bowersox et al., 1987; Nishino et al., 1994), and an increase in the number of α-1 adrenergic receptors (Mignot et al., 1988a; Mignot et al., 1988b) are found in the amygdala of narcoleptic dogs and may result from the degenerative changes that we have seen.
We have hypothesized that these degenerative changes may be linked to an alteration in immune function (Ichinose et al., 1998; Siegel, 1999) resulting from the mutation of the hypocretin receptor gene that has recently been described (Lin et al., 1999). Our current findings suggest that degeneration leads to the disinhibition or disfacilitation of the cataplexy-on and cataplexy-off cells. Disinhibition of amygdala sleep active neurons may contribute to the sleepiness that characterizes narcolepsy.
We find that α-1 blocker prazosin, at doses that increase cataplexy, increased activity in many amygdala neurons, including a subgroup of cataplexy active neurons. Thus, prazosin effects on cataplexy may be mediated by direct or indirect effects on the amygdala. In contrast, the cholinesterase inhibitor physostigmine, at doses that increased cataplexy, did not alter the activity of any amygdala neurons. This finding suggest that physostigmine affects cataplexy ‘downstream’ of the amygdala perhaps by altering the activity of brainstem cholinoceptive neurons.
To summarize, the amygdala has a major role in the integration of emotions. It strongly projects to the LC and medullary brainstem regions linked to muscle tone control and to basal forebrain hypnogenic regions. It contains a population of sleep active neurons. In the present work we show that amygdala contains a population of cataplexy-related cells. These data suggest that the amygdala is involved in the modulation or mediation of cataplexy and in the sleep abnormalities of narcolepsy.
Acknowledgements
This research was supported by the Medical Research Service of the Department of Veterans Affairs and USPHS Grant NS 14610.
Abbreviations:
- AW
active waking
- EEG
electroencephalographic/electroencephalogram
- EMG
electromyogram
- EOG
electrooculogram
- NREM
non-rapid eye movement
- LC
locus coeruleus
- QW
quietwaking
- REM
rapid eye movement
- W
wake
REFERENCES
- Aldrich MS, 1990. Narcolepsy N. Engl. J. Med. 323, 389–394. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Price JL, Pitkanen A, Carmichael ST, 1992. Anatomical organization of the primate amygdaloid complex. In: Aggleton JP (Ed.), The Amygdala. Wiley-Liss, New York, pp. 1–67. [Google Scholar]
- Benca RM, Obermeyer WH, Shelton SE, Droster J, Kalin NH, 2000. Effects of amygdala lesion on sleep in rhesus monkeys. Brain Res 879, 130–138. [DOI] [PubMed] [Google Scholar]
- Bowersox SS, Kilduff TS, Faull KF, Zeller-DeAmicis L, Dement WC, Ciaranello RD, 1987. Brain dopamine receptor levels elevated in canine narcolepsy. Brain Res 402, 44–48. [DOI] [PubMed] [Google Scholar]
- Calvo JM, Simon-Arceo K, Fernandez-Mas R, 1996. Prolonged enhancement of REM sleep produced by carbachol microinjection into the amygdala. NeuroReport 7, 577–580. [DOI] [PubMed] [Google Scholar]
- Charifi C, Paut-Pagno L, Debilly G, Cespuglio R, Jouvet M, Valtax JL, 2000. Effect of noradrenergic denervation of the amygdala upon recovery after sleep deprivation in the rat. Neurosci. Lett. 287, 41–44. [DOI] [PubMed] [Google Scholar]
- Chemelli RM, Willie JT, Sinton CM, Elmquist JK, Scammell T, Lee C, Richardson JA, Williams SC, Xiong Y, Kisanuki Y, Fitch TE, Nakazato M, Hammer RE, Saper CB, 1999. Narcolepsy in orexin knockout mice: Molecular genetics of sleep regulation. Cell 98, 437–451. [DOI] [PubMed] [Google Scholar]
- Deboer T, Sanford LD, Ross RJ, Morrison AR, 1998. Effects of electrical stimulation in the amygdala on ponto-geniculo-occipital waves in rats. Brain Res 793, 305–310. [DOI] [PubMed] [Google Scholar]
- Faull KF, Zeller-DeAmicis LC, Radde L, Bowersox SS, Baker TL, Kilduff TS, Dement WC, 1986. Biogenic amine concentrations in the brains of normal and narcoleptic canines: current status. Sleep 9, 107–110. [DOI] [PubMed] [Google Scholar]
- Fung SJ, Barnes CD, 1981. Evidence of facilitatory coerulospinal action in lumbar motoneurons of cats. Brain Res 216, 299–311. [DOI] [PubMed] [Google Scholar]
- Guilleminault C, 1994. Narcolepsy syndrome. In: Kryger MH, Roth T, Dement WC (Eds.), Principles and Practice of Sleep Medicine. W.B. Saunders, PA, pp. 549–561. [Google Scholar]
- Holstege G, Meiners L, Tan K, 1985. Projections of the bed nucleus of the stria terminalis to the mesencephalon, pons, and medulla oblongata in the cat. Exp. Brain Res. 58, 379–391. [DOI] [PubMed] [Google Scholar]
- Hopkins DA, Holstege G, 1978. Amygdaloid projections to the mesencephalon, pons and medulla oblongata in the cat. Exp. Brain Res 32, 529–547. [DOI] [PubMed] [Google Scholar]
- Ichinose M, Asaim M, Sawadam M, Sasaki K, Ouyang S, 1998. Induction of output current by orexin-B in mouse peritoneal macrophages. FEBS Lett 440, 51–54. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, McGinty DJ, 1971. Amygdala unit activity during sleep and waking. Exp. Neurol 33, 1–15. [DOI] [PubMed] [Google Scholar]
- Knecht CD, Oliver JE, Redding R, Selcer R, Johnson G, 1973. Narcolepsy in a dog and a cat. J. Am. Vet. Med. Assoc 162, 1052–1053. [PubMed] [Google Scholar]
- Kreindler A, Steriade M, 1964. EEG patterns of arousal and sleep induced by stimulating various amygdaloid levels in the cat. Arch. Ital. Biol 102, 576–586. [PubMed] [Google Scholar]
- Krettek JE, Price JL, 1978. Amygdaloid projections to subcortical structures within the basal forebrain and brainstem in the rat and cat. J. Comp. Neurol 178, 225–254. [DOI] [PubMed] [Google Scholar]
- Kuypers HG, Maisky VA, 1977. Funicular trajectories of descending brain stem pathways in cat. Brain Res 136, 159–165. [DOI] [PubMed] [Google Scholar]
- Lai YY, Barnes CD, 1985. A spinal projection of serotonergic neurons of the locus coeruleus in the cat. Neurosci. Lett 58, 159–164. [DOI] [PubMed] [Google Scholar]
- Lai YY, Clements JR, Wu XY, Shalita T, Wu JP, Kuo JS, Siegel JM, 1999. Brainstem projections to the ventromedial medulla in cat: retrograde transport horseradish peroxidase and immunohistochemical studies. J. Comp. Neurol 408, 419–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim RKS, Liu C, Moffit RC, 1960. A Stereotaxic Atlas of the Dog’s Brain. Thomas, Springfield, IL. [Google Scholar]
- Lin L, Faraco J, Li R, Kadotani H, Rogers W, Lin X, Qiu X, de Jong PJ, Nishino S, Mignot E, 1999. The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell 98, 365–376. [DOI] [PubMed] [Google Scholar]
- Llamas L, Avendano C, Reinoso-Suarez F, 1977. Amygdaloid projections to prefrontal and motor cortex. Science 195, 794–796. [DOI] [PubMed] [Google Scholar]
- Maquet P, Peters J, Aerts J, Delfiore G, Degueldre C, Luxen A, Franck G, 1996. Functional neuroanatomy of human rapid-eye-movement sleep and dreaming. Nature 383, 163–166. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ, Levey AI, Wainer BH, 1983. Cholinergic innervation of cortex by the basal forebrain: cytochemistry and cortical connections of the septal area, diagonal band nuclei, nucleus basalis (substantia innominata), and hypothalamus in the rhesus monkey. J. Comp. Neurol 214, 170–197. [DOI] [PubMed] [Google Scholar]
- Mignot E, Guilleminault C, Bowersox S, Rappaport A, Dement WC, 1988a. Effect of alpha1-adrenoceptors blockade with prazosin in canine narcolepsy. Brain Res 444, 184–188. [DOI] [PubMed] [Google Scholar]
- Mignot E, Guilleminault C, Bowersox S, Rappaport A, Dement WC, 1988b. Role of central alpha-1 adrenoceptors in canine narcolepsy. J. Clin. Invest 82, 885–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitler MM, Boysen BG, Campbell L, Dement WC, 1974. Narcolepsy-cataplexy in a female dog. Exp. Neurol 45, 332–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishino S, Reid MS, Dement WC, Mignot E, 1994. Neuropharmacology and neurochemistry of canine narcolepsy. Sleep 17, S84–S92. [DOI] [PubMed] [Google Scholar]
- Nofzinger EA, Mintun MA, Wiseman M, Kupfer DJ, Moore RY, 1997. Forebrain activation in REM sleep: an FDG PET study. Brain Res 770, 192–201. [DOI] [PubMed] [Google Scholar]
- Peyron C, Faraco J, Rogers W, Ripley B, Overeem S, Charnay Y, Nevsimalova S, Aldrich MS, Reynolds D, Albin R, Li R, Hungs M, Pedrazzoli M, Padigaru M, Kucherlapati M, Fan J, Maki R, Lammers GJ, Bouras C, Kucherlapati R, Nishini S, Mignot E, 2000b. A mutation in a case of early onset narcolepsy and a generalized absence of hypocretin peptides in human narcoleptic brains. Nat. Med 6, 991–997. [DOI] [PubMed] [Google Scholar]
- Peyron C, Tighe DK, van den Pol AN, De Lecca L, Heller HC, Sutcliffe JG, Kilduff TS, 2000a. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J. Neurosci 18, 9996–10015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price JL, Amaral DG, 1981. An autoradiographic study of the projections of the central nucleus of the monkey amygdala. J. Neurosci 1, 1242–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rechtschaffen A, Wolpert EA, Dement WC, Mitchel SA, Fisher C, 1963. Nocturnal sleep of narcoleptics. Electroencephalogr. Clin. Neurophysiol 15, 599–609. [DOI] [PubMed] [Google Scholar]
- Reich H, Rupprecht U, Stumpf H, Stock G, 1983. Modulation of unit activity in the amygdala of unrestrained cats during the sleep-waking cycle. Neurosci. Lett 35, 209–214. [DOI] [PubMed] [Google Scholar]
- Rolls E, 1992. Neurophysiology and functions of the primate amygdala. In: Aggleton JP (Ed.), The Amygdala. Wiley-Liss, New York, pp. 143–165. [Google Scholar]
- Russchen FT, Amaral DG, Price JL, 1985. The afferent connections of the substantia innominata in the monkey, Macaca fascicularis. J. Comp. Neurol 242, 1–27. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richardson JA, Kozlowski GP, Wilson S, Arch JR, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M, 1998. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell 92, 573–585. [DOI] [PubMed] [Google Scholar]
- Siegel JM, 1999. Narcolepsy: A key role for hypocretins (orexins). Cell 98, 409–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel JM, Nienhuis R, Fahringer HM, Chiu C, Dement WC, Mignot E, Lufkin R, 1992. Activity of medial mesopontine units during cataplexy and sleep-waking states in the narcoleptic dog. J. Neurosci 12, 1640–1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel JM, Nienhuis R, Fahringer HM, Paul R, Shiromani P, Dement WC, Mignot E, Chiu C, 1991. Neuronal activity in narcolepsy: identification of cataplexy-related cells in the medial medulla. Science 252, 1315–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel JM, Nienhuis R, Gulyani S, Ouyang S, Wu MF, Mignot E, Switzer RC, McMurry G, Cornford M, 1999. Neuronal degeneration in canine narcolepsy. J. Neurosci 19, 248–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson LW, Petrovich GD, 1998. What is the amygdala? Trends Neurosci 21, 323–331. [DOI] [PubMed] [Google Scholar]
- Szymusiak R, McGinty D, 1986. Sleep-related neuronal discharge in the basal forebrain of cats. Brain Res 370, 82–92. [DOI] [PubMed] [Google Scholar]
- Thannickal TC, Moore RY, Nienhuis R, Ramanathan L, Gulyani S, Aldrich MS, Cornford M, Siegel JM, 2000. Reduced number of hypocretin neurons in human narcolepsy. Neuron 27, 469–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Pol AN, 1999. Hypothalamic hypocretin (orexin): robust innervation of the spinal cord. J. Neurosci 19, 3171–3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace DM, Magnuson DJ, Gray TS, 1989. The amygdalo-brainstem pathway: selective innervation of dopaminergic, noradrenergic and adrenergic cells in the rat. Neurosci. Lett 97, 252–258. [DOI] [PubMed] [Google Scholar]
- Wallace DM, Magnuson DJ, Gray TS, 1992. Organization of amygdaloid projections to brainstem dopaminergic, noradrenergic, and adrenergic cell groups in the rat. Brain Res. Bull 28, 447–454. [DOI] [PubMed] [Google Scholar]
- White TJ, Jacobs BL, 1975. Single unit activity in the lateral amygdala of the cat during sleep and waking. Electroencephalogr. Clin. Neurophysiol 38, 331–333. [DOI] [PubMed] [Google Scholar]
- Wu M, Gulyani S, Yao E, Mignot E, Phan B, Siegel JM, 1999. Locus coeruleus neurons:cessation of activity during cataplexy. Neuroscience 91, 1389–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu MF, Nienhuis R, Mignot E, Dement WC, Siegel JM, 1994. Pontine cataplexy-related cells in the narcoleptic dog. Soc. Neurosci. Abstr 20, 793. [Google Scholar]