Abstract
Objective(s):
We investigated the association of metabolic syndrome (MetS) and its components [abdominal obesity, elevated triglycerides (TG), low HDL cholesterol, elevated blood pressure (BP), and impaired fasting glycemia (IFG)] with neurocognitive impairment in youth with perinatally-acquired HIV (YPHIV) or who are perinatally HIV-exposed uninfected (YPHEU).
Design:
Observational study with a comparison group of 350 YPHIV and 68 YPHEU ages 10–19 years.
Methods:
Youth with MetS components measured between 1 year before and 3 months after a baseline neurocognitive assessment (Wechsler Intelligence Scale) were selected from the Pediatric HIV/AIDS Cohort Study (PHACS). A sub-group completed another assessment 3 years later. We assessed the association of each baseline MetS component with five standardized neurocognitive indices at baseline and changes in indices over time.
Results:
At baseline, 15% of YPHIV and 18% of YPHEU met criteria for ≥2 MetS components. Among YPHIV, there was no association between MetS components and neurocognitive indices at baseline; however, over time, elevated baseline BP was associated with a greater decrease in mean Perceptual Reasoning scores (−4.3;95%CI: −8.8,0.3) and ≥2 MetS components with a greater decrease in mean Processing Speed scores (−5.1;95%CI: −9.4,−0.8). Among YPHEU, elevated TG was associated with lower mean Verbal Comprehension, Perceptual Reasoning, and Full-scale IQ scores at baseline, and IFG with lower mean Verbal Comprehension scores.
Conclusions:
Components of MetS in YPHIV (elevated BP) and YPHEU (elevated TG and IFG) were associated with lower neurocognitive performance index scores. Studies to elucidate how modifying metabolic risk factors early in life may improve neurocognitive outcomes in this population are warranted.
Keywords: neurocognitive function, perinatally-acquired HIV, HIV-exposure, children, metabolic syndrome
Introduction
The increased effectiveness of combination antiretroviral therapy (ART) has led to substantial declines in morbidity and mortality in adults living with HIV (ALWH); however, neurocognitive complications of HIV remain prevalent (Eggers et al., 2017; Heaton et al., 2010; Michael et al., 2020). Neurodevelopmental deficits have also been reported in youth with HIV, even among those initiating ART early in life (Hoare et al., 2018; Laughton et al., 2013, 2018; Ruel et al., 2012; Strehlau et al., 2016; Yadav et al., 2017). In addition, metabolic complications of HIV infection and ART, including dyslipidemia (Tassiopoulos et al., 2008), fat redistribution (Dzwonek et al., 2006; Jacobson et al., 2011), and insulin resistance (Frigati et al., 2019; Geffner et al., 2018; Gojanovich et al., 2020), have been documented extensively and may be the result of complex interactions between HIV infection, ART, age, race/ethnicity, socioeconomics, diet, and other factors including inflammation (Wohl et al., 2006).
The term metabolic syndrome (MetS) was popularized in 1977 to identify individuals at increased risk for cardiovascular disease (Alberti et al., 2009). Definitions of MetS in adults have been developed by, among others, the National Cholesterol Education Program (NCEP) and the International Diabetes Federation (IDF) (Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults, 2001). Components include (1) abdominal obesity, (2) elevated triglycerides (TG), (3) low high-density lipoprotein (HDL) cholesterol, (4) elevated blood pressure (BP), and (5) impaired fasting glycemia (IFG), with only slight differences among the various definitions. The IDF definition has also been adapted for children (Zimmet et al., 2007).
MetS is commonly present in ALWH (Jacobson et al., 2006). In the general HIV-uninfected adult population, cardiovascular risk factors, including components of MetS, have been linked to lower cognitive performance and brain abnormalities (Anstey et al., 2008; Debette et al., 2010; Levin et al., 2014; Siervo et al., 2014; Vieira et al., 2011). Studies in ALWH on ART have reported associations between cardiovascular risk factors and cognitive impairment (Fabbiani et al., 2013; Macaluso et al., 2020; McCutchan et al., 2012; Sattler et al., 2015; Valcour et al., 2005, 2006). Recent studies also suggest MetS is associated with global neurocognitive deficits, and that these associations may differ between adults with and without HIV (Yu et al., 2019).
It is unclear whether these associations extend to youth living with perinatally-acquired HIV (YPHIV) or who are perinatally HIV-exposed uninfected (YPHEU), as the intersection of metabolic and neurocognitive health has not been well-studied in these groups. YPHIV have lifelong exposure to HIV and ART which may drive persistent inflammation and immune activation, and these processes, in turn, may underlie metabolic and cognitive problems (Benki-Nugent et al., 2019; Wilkinson et al., 2018). While some studies report stable neurocognitive performance among YPHIV on a group level, unfavorable changes in neurocognitive performance over time in some domains has also been reported for YPHIV; it is possible this could be due to changing HIV disease or metabolic dysfunction (Kerr et al., 2019; Malee et al., 2017; Robbins et al., 2020; Van den Hof et al., 2020). As such, YPHIV are a critical population to investigate, particularly since earlier identification of the association between MetS and poor neurocognition may provide a window for screening and intervention that is lost in older adult populations. In addition, understanding whether perinatal HIV modifies this association even at earlier ages is important. The aim of our study was to examine the association of individual MetS components with neurocognitive outcomes at baseline in YPHIV and YPHEU, and the association between individual MetS components at baseline and change in neurocognitive outcomes over time in YPHIV.
Methods
Study Population
Participants for this study were selected from the Adolescent Master Protocol (AMP) of the Pediatric HIV/AIDS Cohort Study (PHACS) network, a prospective cohort study designed to define the impact of HIV infection and antiretroviral therapy on pre-adolescents and adolescents with perinatal HIV infection with a group of HIV uninfected children with perinatal exposure to HIV as a comparison group. PHACS enrolled 451 YPHIV and 227 YPHEU from March 2007 through November 2009 at 15 clinical sites in the United States (US) including Puerto Rico, with annual follow-up visits. The analytic dataset included youth with a neurocognitive assessment at age ≥10 years (with the first assessment defined as “baseline”) and all five MetS components [based on International Diabetes Federation (IDF) criteria] measured between 1 year before and 3 months after that baseline assessment (Alberti et al., 2006, 2009; Zimmet et al., 2007). Participants with a second neurocognitive examination approximately 3 years after baseline (hereafter called “year 3”) were included in longitudinal analyses. Participants who were pregnant at baseline were excluded. Participating sites and the Harvard T.H. Chan School of Public Health obtained Institutional Review Board (IRB) approvals. Written informed consent was obtained from the parent/legal guardian and assent was obtained from participants according to local IRB guidelines.
Outcomes of Interest: Neurocognitive Function
The primary outcomes of interest were neurocognitive function at baseline and change in neurocognitive function between the baseline and year 3 evaluation, for each neurocognitive measure separately. Neurocognitive function was measured using either the Wechsler Intelligence Scale for Children, Fourth Edition (WISC-IV) if the participants were 10-<16 years of age, or the Wechsler Adult Intelligence Scale, Fourth Edition (WAIS-IV) if the participants were ≥16 years at the assessment (Wechsler, 2003, 2008). Specific indices included Full-scale IQ (FSIQ) which represents overall cognitive ability, as well as standardized index scores (mean=100, SD=15) for the Verbal Comprehension, Perceptual Reasoning, Working Memory, and Processing Speed domains. Change over time in each neurocognitive index score was defined as the year 3 score minus baseline score. All tests were administered by a psychologist/psychometrist under direct supervision by a psychologist. Protocol neuropsychologists reviewed any test results suggesting unusual assessment circumstances, and only scores determined to be valid were included in the analysis.
Exposures of Interest: MetS Components
The primary exposures of interest were the individual components of MetS at baseline as binary variables based on the IDF criteria: abdominal obesity (waist circumference ≥90th percentile for 10-<16 years and ≥94 cm or ≥80 cm for males and females ≥16 years, respectively), elevated TG (≥150 mg/dL), low HDL cholesterol (<40 mg/dL for 10-<16 years for both sexes and males ≥16 years, and <50 mg/dL for females ≥16 years), high BP (systolic BP ≥130 mm Hg or diastolic BP ≥85 mm Hg), and IFG (≥100 mg/dL) (Supplementary Table 1) (Alberti et al., 2006, 2009; Zimmet et al., 2007). Blood samples were collected after a >8-hour fast to assay lipid sub-fractions, including TG and HDL cholesterol, and glucose levels according to standardized protocols (Geffner et al., 2018; Miller et al., 2012). Anthropometric and BP measurements were measured at the same visit. For this analysis, we used two definitions for MetS – the first meeting ≥3 and the second ≥2 of the five individual components.
Covariates
Covariate information was obtained from clinical charts, questionnaires, or physical examinations. For both groups, the following covariates were considered as potential confounders: sex, age, self-reported race/ethnicity (non-Hispanic Black vs. not non-Hispanic Black), primary language (English vs. Bilingual/Other), household income at baseline (≤$20,000 vs. >$20,000), and Tanner stage at baseline (1–2 vs. 3–4 vs. 5). For YPHIV, in addition to the above, we considered age at ART initiation, nadir CD4 cell count, peak HIV RNA level, and antiretroviral (ARV) use at baseline in categories [≥3 ARV classes, protease inhibitor (PI)-based combination ART, integrase strand transfer inhibitor (INSTI)-based combination ART, non-nucleoside reverse-transcriptase inhibitor (NNRTI)-based combination ART, 3 nucleoside reverse transcriptase inhibitors (NRTIs), other, and not on ARV].
Statistical Analysis
Baseline sociodemographic and clinical characteristics, as well as the distribution of exposure and outcome measures, were compared between YPHIV and YPHEU using t-, Wilcoxon, and Chi-square tests as appropriate.
Baseline:
Within YPHIV and YPHEU separately, we assessed the association between each binary MetS component and each neurocognitive index at baseline by fitting linear regression models using generalized estimating equations (GEE) with robust variance, unadjusted and adjusted for a priori confounders (age, sex, race/ethnicity, primary language, household income, and Tanner stage, plus age at ART initiation, nadir CD4 cell count, peak HIV RNA level, and ARV use for YPHIV). Given limited power, we did not conduct a formal test for effect modification by HIV status. We presented stratified findings and qualitatively assessed effect modification of associations between each MetS component and each neurocognitive index by HIV status.
Longitudinal:
To assess the representativeness of the subset with longitudinal neurocognitive assessments, distributions of baseline binary exposures and continuous outcomes were compared between participants with and without longitudinal measurements, separately within YPHIV and YPHEU, using t-, Wilcoxon, and Chi-square tests. Given the small sample size of YPHEU with available longitudinal measurements and apparent lack of change in neurocognitive indices from baseline to year 3 in YPHEU, the longitudinal association between each binary MetS component at baseline and change in each neurocognitive index over time was only assessed in YPHIV. This was done by fitting linear regression models using GEE with robust variance, specifying the distribution as normal and the identity link, unadjusted and adjusted for a priori confounders (age, sex, and race/ethnicity) and other potential confounders that had associations of p<0.10 with at least one of the neurocognitive indices. Additional analyses considering each of the MetS components on a continuous scale were conducted to determine if findings were consistent with those where we considered dichotomous MetS components.
Statistical analyses were performed using SAS® 9.4 (SAS Institute, Cary, NC). All statistical tests were two-sided; emphasis was placed on consistency of results across analyses under various assumptions.
Results
Study Population
Of the 678 AMP participants, 599 (398 YPHIV, 201 YPHEU) completed either a WISC-IV or WAIS-IV (Figure 1). Seventy-nine participants were excluded because they did not have a neurocognitive assessment completed ≥10 years of age, were missing scores for all outcomes of interest, or were pregnant at the time of the assessment. A total of 418 participants (350 YPHIV and 68 YPHEU) had all five MetS components measured between 1 year before and up to 3 months after the neurocognitive assessment and included in baseline analyses. Among YPHIV, median time between the MetS measurement and neurocognitive assessment was 0 days [IQR (interquartile range): −19, 0]. Among YPHEU, median time between the MetS measurement and neurocognitive assessment was −1 day (IQR: −19, 0). In addition, 183 YPHIV and 58 YPHEU completed a neurocognitive assessment at both baseline and year 3.
Figure 1:
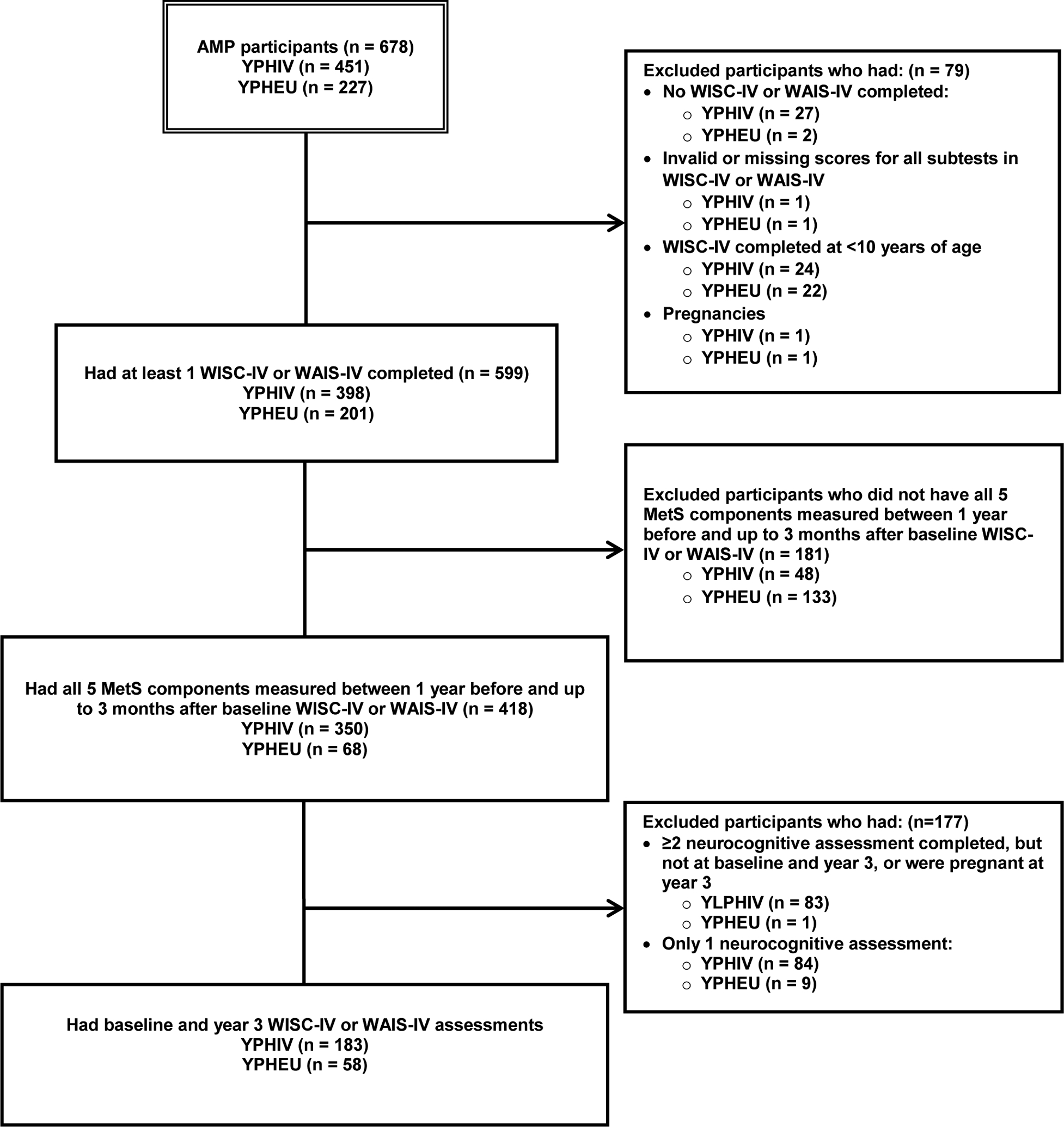
Study population derivation for current analysis of youth with perinatally-acquired HIV (YPHIV) and youth who are HIV-exposed uninfected (YPHEU) from the Adolescent Master Protocol (AMP) of the Pediatric HIV/AIDS Cohort Study (PHACS) network
Abbreviations: WISC-IV – Wechsler Intelligence Scale for Children, Fourth Edition; WAIS-IV – Wechsler Adult Intelligence Scale, Fourth Edition; MetS – metabolic syndrome
Participant Characteristics
Participant characteristics are shown in Table 1. YPHIV were slightly older (median age 12.8 vs 11.6 years) and more often Non-Hispanic Black, living in a household with income >$20,000, and reporting English as a primary language compared to YPHEU. Approximately one-third (37%) of YPHIV reported that their biological mother was one of their primary caregivers compared to 68% of YPHEU. There were no differences in the distribution of sex, Tanner stage, caregiver education, and physical activity between groups. At baseline, 89% of YPHIV had a CD4 count >350 cells/μL, 69% had a viral load ≤400 copies/mL, and 58% were on PI-based combination ART. Median age at ART initiation was 2.96 years; 26% had a nadir CD4 T-cell >500 cells/μL, and 73% had a peak viral load >100,000 copies/mL.
Table 1:
Demographic characteristics of youth with perinatally-acquired HIV (YPHIV) and youth who are HIV-exposed uninfected (YPHEU) in the baseline sample
| Characteristic | YPHIV (N=350) | YPHEU (N=68) | P-value | |
|---|---|---|---|---|
| Age at WISC-IV or WAIS-IV1, years | Median (IQR) | 12.8 (11.5, 14.5) | 11.6 (10.7, 13.4) | <0.001* |
| Min, Max | 10.0, 18.6 | 10.0, 15.8 | ||
| Sex | M | 160 (46%) | 32 (47%) | 0.84** |
| F | 190 (54%) | 36 (53%) | ||
| Weight Z-score2 | Mean (s.d.) | 0.10 (1.27) | 0.78 (1.33) | <0.001*** |
| Min, Max | −4.15, 2.93 | −2.23, 3.53 | ||
| Height Z-score2 | Mean (s.d.) | −0.33 (1.17) | 0.36 (1.21) | <0.001*** |
| Min, Max | −4.57, 3.09 | −2.22, 4.41 | ||
| BMI Z-score2 | Mean (s.d.) | 0.31 (1.15) | 0.76 (1.21) | 0.006*** |
| Min, Max | −3.28, 2.93 | −2.45, 2.71 | ||
| Tanner stage2 | 1–2 | 122 (35%) | 31 (46%) | 0.24** |
| 3–4 | 165 (47%) | 27 (40%) | ||
| 5 | 63 (18%) | 10 (15%) | ||
| Race/ethnicity | Not non-Hispanic Black | 126 (36%) | 33 (49%) | 0.054** |
| Non-Hispanic Black | 223 (64%) | 35 (51%) | ||
| Unknown | 1 | 0 | ||
| Caregiver education2 | High school or below | 195 (56%) | 41 (60%) | 0.53** |
| Greater than high school | 152 (44%) | 27 (40%) | ||
| Missing | 3 | 0 | ||
| Annual household income2 | ≤$20,000 | 151 (46%) | 44 (68%) | 0.001** |
| >$20,000 | 180 (54%) | 21 (32%) | ||
| Missing | 19 | 3 | ||
| Number of household members supported by income1 | Median (Q1, Q3) | 4 (3, 5) | 4 (3, 5) | 0.49* |
| Primary language reported in WISC-IV or WAIS-IV | English | 304 (87%) | 50 (74%) | 0.002** |
| Spanish | 27 (8%) | 15 (22%) | ||
| Bilingual/Other | 19 (5%) | 3 (4%) | ||
| One of the primary caregivers is the biological mother | Yes | 127 (37%) | 46 (68%) | <0.001** |
| No | 219 (63%) | 22 (32%) | ||
| Unknown | 4 | 0 | ||
| Physical activity (vigorous minutes of activity per day >75th percentile)3 | Yes | 54 (20%) | 16 (26%) | 0.30** |
| No | 218 (80%) | 46 (74%) | ||
| Unknown | 78 | 6 | ||
| Age at ART initiation, years | Median (IQR) | 2.96 (1.09, 5.59) | ||
| Min, Max | 0.15, 19.65 | |||
| Nadir CD4 T-cell, cells/μL | >500 | 90 (26%) | ||
| 201–500 | 177 (51%) | |||
| 51–200 | 53 (15%) | |||
| ≤50 | 30 (9%) | |||
| Peak HIV RNA, copies/mL | ≤10,000 | 14 (5%) | ||
| 10,001–100,000 | 69 (22%) | |||
| 100,001–1,000,000 | 225 (73%) | |||
| Baseline CD4 count, cells/μL | >500 | 265 (76%) | ||
| 351–500 | 44 (13%) | |||
| 200–350 | 29 (8%) | |||
| <200 | 11 (3%) | |||
| Missing | 1 | |||
| Baseline HIV RNA, copies/mL | ≤400 | 239 (69%) | ||
| 401–1000 | 20 (6%) | |||
| 1001–10,000 | 46 (13%) | |||
| >10,000 | 42 (12%) | |||
| Missing | 3 | |||
| Antiretroviral (ARV) treatment at baseline | ≥3 ARV classes | 42 (12%) | ||
| PI-based cART | 200 (58%) | |||
| INSTI-based cART | 7 (2%) | |||
| NNRTI-based cART | 53 (15%) | |||
| 3 NRTIs | 8 (2%) | |||
| Other | 14 (4%) | |||
| Not on ARV | 20 (6%) | |||
| Missing | 6 |
Wilcoxon Test
Chi-Square Test
T-Test
WISC-IV: 411/418 (98%); WAIS-IV: 7/418 = 2%
Data on weight Z-score, height Z-score, BMI Z-score, Tanner stage, caregiver education, and household income were retained if reported up to 1 year before and 3 months after WISC-IV or WAIS-IV.
Data on physical activity was retained if reported within 2 years of age at WISC-IV or WAIS-IV. This is due to the fact that we only have approximate age at which physical activity was measured.
Abbreviations: BMI – body mass index; NRTI – nucleoside reverse transcriptase inhibitor; NNRTI – non-nucleoside reverse transcriptase inhibitor; PI – protease inhibitor; INSTI – integrase strand transfer inhibitor (INSTI); IQR – interquartile range; cART – combination antiretroviral therapy
Prevalence of MetS Components
A smaller proportion of YPHIV had abdominal obesity (17% vs 34%, p=0.002) and IFG (3% vs 7%, p=0.045), and a higher proportion had elevated TG (22% vs 7%, p=0.006) compared to YPHEU (Table 2). No differences were observed in the other categorical MetS components—reduced HDL and elevated BP. Among YPHIV, no participants with IFG were diagnosed with Type 2 DM at or prior to glucose measurement, but two participants without IFG were. Among YPHEU, no participants with or without IFG were diagnosed with Type 2 DM.
Table 2:
Metabolic syndrome (MetS) components and neurocognitive indices from WISC-IV or WAIS-IV between YPHIV and YPHEU in baseline sample
| YPHIV (N=350) | YPHEU (N=68) | P-value | ||
|---|---|---|---|---|
| MetS components 1 | ||||
| Abdominal obesity | N (%) | 60 (17%) | 23 (34%) | 0.002 |
| Elevated triglycerides | N (%) | 76 (22%) | 5 (7%) | 0.006 |
| Low HDL cholesterol | N (%) | 74 (21%) | 9 (13%) | 0.13 |
| Elevated blood pressure | N (%) | 21 (6%) | 2 (3%) | 0.31 |
| Impaired fasting glycemia | N (%) | 9 (3%) | 5 (7%) | 0.045 |
| Fulfilled criteria for ≥2 MetS components | N (%) | 52 (15%) | 12 (18%) | 0.56 |
| Fulfilled criteria for ≥3 MetS components | N (%) | 12 (3%) | 5 (7%) | 0.13 |
| Neurocognitive indices | ||||
| WISC-IV or WAIS-IV | WISC-IV | 343 (98%) | 68 (100%) | 0.24* |
| WAIS-IV | 7 (2%) | 0 (0%) | ||
| Verbal Comprehension score | Median (IQR) | 89 (77, 98) | 85.00 (75.00, 99.50) | 0.56** |
| Mean (SD) | 87.37 (15.65) | 86.71 (16.96) | ||
| Min, Max | 45, 138 | 53, 130 | ||
| Perceptual Reasoning score | Median (IQR) | 92 (82, 102) | 91 (82, 103) | 0.65** |
| Mean (SD) | 90.15 (15.81) | 91.66 (14.67) | ||
| Min, Max | 45, 131 | 59, 121 | ||
| Working Memory score | Median (IQR) | 91 (80, 99) | 94 (80, 99) | 0.62** |
| Mean (SD) | 88.25 (16.27) | 89.96 (13.08) | ||
| Min, Max | 50, 135 | 65, 120 | ||
| Processing Speed score | Median (IQR) | 87 (78, 97) | 91 (83, 97) | 0.077** |
| Mean (SD) | 86.35 (15.60) | 90.00 (10.29) | ||
| Min, Max | 50, 128 | 65, 115 | ||
| Full-scale IQ score | Median (IQR) | 86 (76, 95) | 84.50 (77.00, 98.00) | 0.80** |
| Mean (SD) | 85.32 (16.11) | 86.97 (14.40) | ||
| Min, Max | 40, 130 | 60, 117 |
Chi-Square Test
Wilcoxon Test
Abbreviations: WISC-IV - Wechsler Intelligence Scale for Children, Fourth Edition; WAIS-IV - Wechsler Adult Intelligence Scale, Fourth Edition; YPHIV - youth living with perinatally-acquired HIV; YPHEU – youth who are HIV-exposed uninfected; HDL - high-density lipoprotein; SD – standard deviation; IQR – interquartile range
Refer to Supplementary Table 1 for definitions of MetS components
At baseline, 3% of YPHIV and 7% of YPHEU met criteria for ≥3 MetS components; 15% of YPHIV and 18% of YPHEU met criteria for ≥2 MetS components. Among those who met criteria for ≥2 MetS components, the most common MetS components were reduced HDL (65%) and elevated TG (63%) in YPHIV, whereas the most common were abdominal obesity (100%) and reduced HDL cholesterol (67%) in YPHEU.
Baseline Analysis of Neurocognitive Indices
Neurocognitive outcomes at baseline are shown in Table 2. Among YPHIV, no associations between baseline MetS components and baseline neurocognitive indices were observed in unadjusted models or models adjusted for age, sex, race/ethnicity, primary language, household income, Tanner stage, age at ART initiation, nadir CD4, peak HIV RNA, and ARV use (Supplementary Table 2). Among YPHEU, however, elevated TG was associated with lower mean Verbal Comprehension (−10.0; 95% CI: −17.7, −2.2), Perceptual Reasoning (−7.9; 95%CI: −13.2, −2.5), and FSIQ (−7.5; 95%CI: −14.6, −0.5) scores. In addition, IFG was associated with a lower mean Verbal Comprehension score (−12.7; 95%CI: −24.7, −0.7) in models adjusted for age, sex, race/ethnicity, primary language, household income, and Tanner stage. This was consistent with additional analyses treating TG as a continuous variable: a one-unit increase in TG (mg/dL) was associated with a 0.14 lower Verbal Comprehension score (95%CI: −0.21, −0.07), 0.07 lower Perceptual Reasoning score (95%CI: −0.11, −0.03), and 0.05 lower FSIQ score (95%CI: −0.10, 0.00), and a one-unit increase in IFG (mg/dL) was associated with a 0.33 lower Verbal Comprehension score (95%CI: −0.65, −0.00).
Longitudinal Analysis of Neurocognitive Indices
Proportions of YPHIV and YPHEU meeting each MetS criterion at baseline were similar between participants with and without longitudinal measurements (Supplementary Table 3). At year 3, 34% of YPHIV and 19% of YPHEU with neurocognitive assessments completed the WAIS-IV. Neurocognitive scores at baseline, year 3, and the change are shown in Figure 2. All neurocognitive scores except Processing Speed score declined on average, between 1.5 and 2.1 points, from baseline to year 3 for YPHIV, while there were no changes in mean scores for YPHEU. Due to the lack of change in neurocognitive scores over time and the small sample of YPHEU, we chose to focus on longitudinal models in YPHIV.
Figure 2:
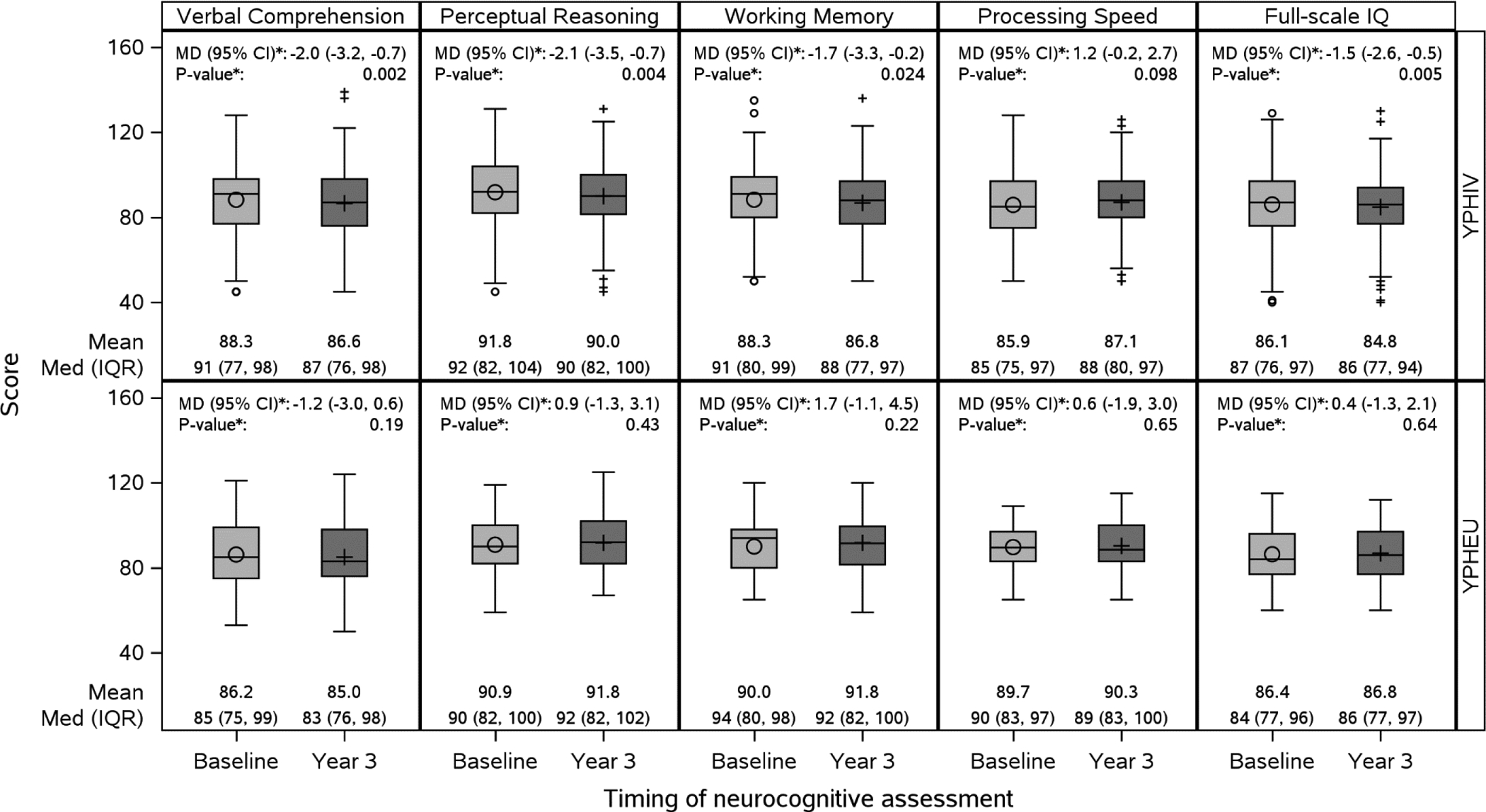
Boxplot of baseline and year 3 neurocognitive scores and mean difference between baseline and year 3 for youth with perinatally-acquired HIV (YPHIV) and youth who are HIV-exposed uninfected (YPHEU) in the longitudinal sample
Abbreviations: Med – median; IQR – interquartile range; MD – mean difference; CI – confidence interval
*From paired t-tests
Characteristics of the 183 YPHIV included in the longitudinal analyses were mostly similar to those without longitudinal measurements, with a few exceptions (data not shown). YPHIV included in the longitudinal analyses had a lower mean nadir CD4 count (320 vs. 386 cells/μL, p<0.001) and higher HIV RNA viral load (2.6 vs. 1.8 log₁₀ copies/mL, p<0.001) near their baseline neurocognitive assessment, and initiated ART slightly later (3.8 vs. 2.1 years, p<0.001) than those excluded from longitudinal analyses.
Among 183 YPHIV with longitudinal data, elevated BP at baseline was associated with a greater decrease in mean Perceptual Reasoning scores over time (−4.3; 95%CI: −8.8, 0.3) in adjusted analyses (Table 3). In addition, meeting criteria for ≥2 MetS components was associated with a greater decrease in mean Processing Speed scores over time (−5.1; 95%CI: −9.4, −0.8) in adjusted analyses.
Table 3:
Unadjusted and adjusted models assessing the association of each MetS component at baseline with change over time1 in each neurocognitive index in YPHIV over time
| Unadjusted | Adjusted2 | |||||
|---|---|---|---|---|---|---|
| Outcome | N | Estimates (95% CI) | P-value | N | Estimates (95% CI) | P-value |
| Exposure 1) Abdominal obesity | ||||||
| Change in Verbal Comprehension | 180 | −3.2 (−6.9, 0.4) | 0.080 | 166 | −2.3 (−6.3, 1.6) | 0.25 |
| Change in Perceptual Reasoning | 180 | 0.8 (−2.7, 4.3) | 0.65 | 166 | 0.7 (−2.9, 4.2) | 0.72 |
| Change in Working Memory | 177 | −1.5 (−4.7, 1.7) | 0.37 | 163 | −0.7 (−4.5, 3.0) | 0.70 |
| Change in Processing Speed | 182 | −1.0 (−4.8, 2.9) | 0.63 | 168 | 0.2 (−3.7, 4.2) | 0.90 |
| Change in Full-scale IQ | 181 | −1.2 (−3.9, 1.6) | 0.40 | 167 | −0.4 (−3.4, 2.7) | 0.81 |
| Exposure 2) Elevated triglycerides | ||||||
| Change in Verbal Comprehension | 180 | −0.2 (−3.1, 2.7) | 0.89 | 166 | −0.5 (−3.5, 2.6) | 0.76 |
| Change in Perceptual Reasoning | 180 | 0.7 (−3.0, 4.4) | 0.72 | 166 | 0.9 (−3.3, 5.1) | 0.67 |
| Change in Working Memory | 177 | 1.9 (−1.5, 5.3) | 0.27 | 163 | 2.3 (−1.4, 6.1) | 0.22 |
| Change in Processing Speed | 182 | −0.9 (−4.4, 2.6) | 0.62 | 168 | −1.2 (−4.7, 2.2) | 0.48 |
| Change in Full-scale IQ | 181 | 0.5 (−2.2, 3.3) | 0.70 | 167 | 0.3 (−2.5, 3.1) | 0.82 |
| Exposure 3) Low HDL-cholesterol | ||||||
| Change in Verbal Comprehension | 180 | −1.4 (−4.2, 1.3) | 0.30 | 166 | −2.3 (−5.1, 0.5) | 0.10 |
| Change in Perceptual Reasoning | 180 | −0.2 (−4.1, 3.6) | 0.91 | 166 | 0.7 (−3.4, 4.8) | 0.74 |
| Change in Working Memory | 177 | −1.9 (−5.5, 1.8) | 0.32 | 163 | −1.1 (−5.2, 3.0) | 0.60 |
| Change in Processing Speed | 182 | −2.5 (−6.6, 1.6) | 0.23 | 168 | −3.5 (−7.9, 0.8) | 0.11 |
| Change in Full-scale IQ | 181 | −1.8 (−4.2, 0.7) | 0.16 | 167 | −2.0 (−4.8, 0.7) | 0.15 |
| Exposure 4) Raised blood pressure | ||||||
| Change in Verbal Comprehension | 180 | 0.8 (−4.2, 5.8) | 0.76 | 166 | 0.7 (−4.1, 5.5) | 0.78 |
| Change in Perceptual Reasoning | 180 | −4.2 (−8.4, 0.0) | 0.051 | 166 | −4.3 (−8.8, 0.3) | 0.068 |
| Change in Working Memory | 177 | 2.9 (−3.2, 9.0) | 0.35 | 163 | 4.6 (−2.0, 11.3) | 0.17 |
| Change in Processing Speed | 182 | −0.1 (−5.6, 5.4) | 0.97 | 168 | −1.0 (−7.2, 5.1) | 0.74 |
| Change in Full-scale IQ | 181 | −0.8 (−4.5, 2.9) | 0.67 | 167 | −0.7 (−5.0, 3.5) | 0.74 |
| Exposure 5) Impaired fasting glycemia | ||||||
| Change in Verbal Comprehension | 180 | −1.6 (−7.3, 4.1) | 0.59 | 166 | −1.8 (−7.5, 4.0) | 0.55 |
| Change in Perceptual Reasoning | 180 | −2.7 (−9.2, 3.8) | 0.42 | 166 | −2.0 (−8.8, 4.8) | 0.56 |
| Change in Working Memory | 177 | 2.8 (−3.5, 9.2) | 0.38 | 163 | 3.3 (−1.9, 8.5) | 0.21 |
| Change in Processing Speed | 182 | −0.9 (−6.3, 4.5) | 0.74 | 168 | 0.4 (−4.5, 5.3) | 0.87 |
| Change in Full-scale IQ | 181 | −0.5 (−5.6, 4.6) | 0.85 | 167 | 0.4 (−4.2, 5.0) | 0.86 |
| Exposure 6) Fulfilled criteria for ≥2 MetS components | ||||||
| Change in Verbal Comprehension | 180 | −1.2 (−4.4, 2.1) | 0.48 | 166 | −0.6 (−4.1, 2.9) | 0.73 |
| Change in Perceptual Reasoning | 180 | 1.5 (−2.6, 5.5) | 0.48 | 166 | 1.6 (−2.9, 6.1) | 0.50 |
| Change in Working Memory | 177 | −0.7 (−4.3, 2.8) | 0.68 | 163 | 0.5 (−3.6, 4.5) | 0.82 |
| Change in Processing Speed | 182 | −4.2 (−8.2, −0.1) | 0.045 | 168 | −5.1 (−9.4, −0.8) | 0.019 |
| Change in Full-scale IQ | 181 | −1.3 (−4.2, 1.6) | 0.37 | 167 | −1.1 (−4.4, 2.1) | 0.49 |
Change over time in each neurocognitive index score was defined as year 3 score minus baseline score
Models are adjusted for age at neurocognitive assessment (years), sex, race/ethnicity (NH Black vs not NH Black), primary language reported in WISC/WAIS (English vs Bilingual/Other), household income (≤$20,000 vs >$20,000), Tanner stage (3–4 and 5 vs 1–2), age (years) first started HAART, nadir CD4, and ARV use.
Abdominal obesity was associated with a greater decrease in mean Verbal Comprehension scores over time in unadjusted models (−3.2; 95%CI: −6.9, 0.4), but this association was attenuated after adjusting for potential confounders (−2.3; 95%CI: −6.3, 1.6). On the continuous scale, for every one-unit increase in waist circumference Z-score at baseline, there was an additional mean decrease of 1.64 (95%CI: −3.00, −0.27) points in Verbal Comprehension over time (data not shown).
Discussion
To our knowledge, this is the first study to examine the association between components of MetS and neurocognitive outcomes in YPHIV and YPHEU. In our cohort of youth aged 10–19, 3% of YPHIV and 7% of YPHEU met criteria for ≥3 MetS components and 15% of YPHIV and 18% of YPHEU met criteria for ≥2 MetS components. For YPHIV, the most prevalent MetS components were reduced HDL and elevated TG and, for YPHEU, the most prevalent components were abdominal obesity and reduced HDL cholesterol. We found that some components of MetS in YPHIV (raised blood pressure) and YPHEU (elevated TG and IFG) were associated with lower neurocognitive performance in childhood/adolescence.
A systematic review of 85 studies found a median MetS prevalence (based on ≥3 MetS components) of 3.3% (range 0–19.2%) in the general pediatric population (Friend et al., 2013). MetS prevalence for YPHIV in our study was similar to this estimate, while the MetS prevalence for YPHEU was higher than in the general population. Another study, using nationally-representative data from 1999–2014 in youth ages 12–19 years in the US, found MetS prevalences ranging from 6.25% in the Northeast to 11.42% in the Midwest (DeBoer et al., 2019). MetS prevalence for YPHIV in our study fell below this range, while the MetS prevalence for YPHEU fell within this range. It is important to note that there is no consensus definition for pediatric MetS currently. For example, in the IDF criteria, the US definition of elevated fasting glucose is used for IFG, rather than the European definition of elevated fasting insulin. Use of different definitions may lead to different classifications for MetS. Thus, for youth, the focus on individual components may be more meaningful (Magge et al., 2017). There is some debate over whether incremental MetS risk (e.g., meeting one or two, but not three or more, criteria for MetS) is as important as full-fledged MetS when investigating cognitive impairment in adults in the general population. Some studies suggest that meeting one or two factors may be as detrimental as meeting three or more (Lamar et al., 2015; Vieira et al., 2011). We found 15% YPHIV and 18% YPHEU exhibited MetS by meeting criteria for ≥2 components, raising the notion that these youth may also benefit from further monitoring.
Prevalence estimates of MetS among populations with HIV are varied. A meta-analysis using various MetS definitions estimated the global pooled prevalence of MetS among people with HIV to range from 16.7 to 31.3%, with substantial heterogeneity by age and other factors (Nguyen et al., 2016). This meta-analysis included few pediatric studies. In a study of Spanish youth with HIV ages 2–18 years, MetS prevalence was 1.97% by the IDF criteria and 5.92% by the National Cholesterol Education Program-Adult Treatment Panel III (NCEP-ATP III) criteria (Espiau et al., 2016). Our estimate of 3% in YPHIV was in this range. In young adults, a recent Thai study found MetS prevalence (NCEP-ATP III criteria) to be 10.6% in YPHIV ages 15–25 years (Aurpibul et al., 2020). Another cohort of young adults ages 18–30 years in France with perinatally-acquired HIV found a MetS prevalence of 13.2% in men and 10.4% in women (Arrive et al., 2018). Additional follow-up is necessary to understand whether the prevalence of MetS observed in our study will continue to increase as these adolescents age into young adulthood.
Among YPHIV who met criteria for ≥2 MetS components, the most common components were reduced HDL (65%) and elevated TG (63%). The profile of abnormal lipids without abdominal obesity observed among the YPHIV in our study falls in line with reports of a “thin and hypercholesterolemic” pattern in other studies of YPHIV (Lindsey et al., 2012), a pattern that continues to persist at older ages. For example, in the aforementioned French study of young adults with HIV, the most common cluster of abnormalities for those with MetS was reduced HDL, elevated TG, and elevated BP (Arrive et al., 2018). In another study of MetS in ALWH ages 25–64 years, most with MetS (77%) had reduced HDL, elevated TG, and one additional abnormality (Jacobson et al., 2006). On the other hand, for YPHEU who met criteria for ≥2 MetS components in this study, the most common were abdominal obesity (100%) and reduced HDL cholesterol (67%).
Among YPHIV, we found that elevated BP was associated with a decrease in Perceptual Reasoning scores over time. Hypertension could mediate the effects of HIV and ART on cognition and has been linked to cognitive function in the general population (Asiimwe et al., 2020; Jiménez-Balado et al., 2019; Pasipanodya et al., 2019). An earlier study in the AMP cohort found an association between fibrinogen, a marker of coagulation, and Perceptual Reasoning scores (Kapetanovic et al., 2010). Together with our BP finding, this suggests a cascade of microvascular events could be associated with neurocognitive impairment in the context of pediatric HIV.
We found that, among YPHIV, meeting criteria for ≥2 MetS components was associated with lower Processing Speed scores over time. In a study of 109 ALWH, DM and elevated TG were the MetS components most strongly associated with increased global neurocognitive deficits (Yu et al., 2019). Monitoring these components of MetS as YPHIV continue to age will be increasingly important, particularly since YPHIV are less likely to exhibit frank obesity as a MetS component. Though the mechanism linking MetS and cognitive decline is not well-understood, microvascular dysfunction and inflammation from metabolic perturbations may offer an explanation. For example, YPHIV have been shown to have endothelial dysfunction, an early marker of subclinical cardiovascular disease risk compared to youth without HIV (Dirajlal-Fargo et al., 2017; Mahtab et al., 2020). Endothelial dysfunction reflects potentially reversible vascular damage (Flammer et al., 2012) and has been shown to be associated with cognitive impairment (Gorelick et al., 2011; Wright & Flores, 2015), making it an important parameter to consider in the context of cardiometabolic and neurocognitive health among YPHIV.
Of note, Perceptual Reasoning and Processing Speed were the domains associated with MetS components in our study among YPHIV. Processing Speed is a domain that has been repeatedly shown to be affected in youth with HIV (Phillips et al., 2016). In addition, there have been studies done indicating deficits in perceptual-performance domains in young children ages 6–8 years with HIV (Kandawasvika et al., 2015).
In cross-sectional analyses, YPHEU with elevated TG had lower Verbal Comprehension, Perceptual Reasoning, and FSIQ scores, and those with IFG had a lower mean Verbal Comprehension score. These associations have been reported in HIV-unexposed and uninfected adults. Among African-Americans adults in the general population, elevated TG and IFG have both been shown to be associated with poorer verbal scores (Sims et al., 2008; Skinner et al., 2015). As Verbal Comprehension can be affected by knowledge and cultural experience, there may be sociodemographic factors, such as parental education or food insecurity reflecting healthcare inequities, that contribute to decreased Verbal Comprehension scores (Wechsler, 2003, 2008).
Our study was limited by the lack of a comparison group of HIV-unexposed and uninfected youth. Eligible YPHEU for this analysis was also a small group (n=68) and may be subject to selection bias since they represent the first YPHEU enrolled in PHACS when metabolic assays were not performed routinely on this group (Geffner et al., 2018; Miller et al., 2012). This may also affect the generalizability of results to the larger YPHEU population. Lastly, as discussed above, there is the issue that there are no consensus guidelines or validated diagnostic criteria for MetS in youth (Weiss et al., 2013). We chose to use the US definition to more closely align with our study population, and our decision to evaluate each MetS component separately as a predictor of interest allowed us to more closely investigate associations with neurocognitive domains.
In conclusion, in our study, 15% of YPHIV and 18% of YPHEU met criteria for ≥2 of the individual MetS components. Components of MetS in YPHIV (elevated BP) and YPHEU (elevated TG and IFG) were associated with lower neurocognitive performance index scores in childhood and adolescence. Future studies to elucidate how modifying metabolic risk factors early in life may improve short- and long-term neurocognitive outcomes in this population are warranted.
Supplementary Material
Acknowledgments:
We thank the participants and families for their participation in PHACS, and the individuals and institutions involved in the conduct of PHACS. The study was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development with co-funding from the National Institute on Drug Abuse, the National Institute of Allergy and Infectious Diseases, the National Institute of Mental Health, the National Institute of Neurological Disorders and Stroke, the National Institute on Deafness and Other Communication Disorders, the National Institute of Dental and Craniofacial Research, the National Cancer Institute, the National Institute on Alcohol Abuse and Alcoholism, the Office of AIDS Research, and the National Heart, Lung, and Blood Institute through cooperative agreements with the Harvard T.H. Chan School of Public Health (HD052102) (Principal Investigator: George R Seage III; Program Director: Liz Salomon) and the Tulane University School of Medicine (HD052104) (Principal Investigator: Russell Van Dyke; Co-Principal Investigator: Ellen Chadwick; Project Director: Patrick Davis). Data management services were provided by Frontier Science and Technology Research Foundation (PI: Suzanne Siminski), and regulatory services and logistical support were provided by Westat, Inc (PI: Julie Davidson).
Funding:
The study was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development with co-funding from the National Institute on Drug Abuse, the National Institute of Allergy and Infectious Diseases, the National Institute of Mental Health, the National Institute of Neurological Disorders and Stroke, the National Institute on Deafness and Other Communication Disorders, the National Institute of Dental and Craniofacial Research, the National Cancer Institute, the National Institute on Alcohol Abuse and Alcoholism, the Office of AIDS Research, and the National Heart, Lung, and Blood Institute through cooperative agreements with the Harvard T.H. Chan School of Public Health (HD052102) (Principal Investigator: George R Seage III; Program Director: Liz Salomon) and the Tulane University School of Medicine (HD052104) (Principal Investigator: Russell Van Dyke; Co-Principal Investigator: Ellen Chadwick; Project Director: Patrick Davis).
Conflicts of Interest:
Shiau: none
Yu: none
Jacobson: none
Nichols: none
McFarland: none
Chen: J.S.C. receives research support paid to institution from Gilead Sciences, Inc.
Dirajlal-Fargo: none
Surowiec: none
Geffner: M.E.G. receives research support from Novo Nordisk; consultant fees from Adrenas, Daiichi Sankyo, Eton Pharmaceuticals, Neurocrine Biosciences, Novo Nordisk, Pfizer, and QED; and royalties from McGraw-Hill and UpToDate; and serves on a data safety monitoring board for Ascendis.
Jao: none
The following institutions, clinical site investigators and staff participated in conducting PHACS AMP and AMP Up in 2019, in alphabetical order: Ann & Robert H. Lurie Children’s Hospital of Chicago: Ellen Chadwick, Margaret Ann Sanders, Kathleen Malee, Yoonsun Pyun; Baylor College of Medicine:, Mary Paul, Shelley Buschur, Chivon McMullen-Jackson, Lynnette Harris; Bronx Lebanon Hospital Center: Murli Purswani, Mahboobullah Mirza Baig, Alma Villegas; Children’s Diagnostic & Treatment Center: Lisa- Gaye Robinson, Sandra Navarro, Patricia Garvie; Boston Children’s Hospital: Sandra K. Burchett, Rebecca Pinsky, Adam R. Cassidy; Jacobi Medical Center: Andrew Wiznia, Marlene Burey, Ray Shaw; Rutgers - New Jersey Medical School: Arry Dieudonne, Linda Bettica, Juliette Johnson, Karen Surowiec; St. Christopher’s Hospital for Children: Janet S. Chen, Taesha White, Mitzie Grant; St. Jude Children’s Research Hospital: Katherine Knapp, Jamie Russell-Bell, Megan Wilkins, Erick Odero; San Juan Hospital Research Unit/Department of Pediatrics, San Juan Puerto Rico: Midnela Acevedo-Flores, Heida Rios, Vivian Olivera; Tulane University School of Medicine: Margarita Silio, Medea Gabriel, Patricia Sirois; University of California, San Diego: Stephen A. Spector, Megan Loughran, Veronica Figueroa, Sharon Nichols; University of Colorado Denver Health Sciences Center: Elizabeth McFarland, Carrie Chambers, Emily Barr, Mary Glidden; University of Miami: Gwendolyn Scott, Grace Alvarez, Juan Caffroni, Anai Cuadra
Note: The conclusions and opinions expressed in this article are those of the authors and do not necessarily reflect those of the National Institutes of Health or U.S. Department of Health and Human Services.
References
- Alberti KGMM, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart J-C, James WPT, Loria CM, Smith SC, International Diabetes Federation Task Force on Epidemiology and Prevention, Hational Heart, Lung, and Blood Institute, American Heart Association, World Heart Federation, International Atherosclerosis Society, & International Association for the Study of Obesity. (2009). Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation, 120(16), 1640–1645. 10.1161/CIRCULATIONAHA.109.192644 [DOI] [PubMed] [Google Scholar]
- Alberti KGMM, Zimmet P, & Shaw J (2006). Metabolic syndrome—A new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabetic Medicine: A Journal of the British Diabetic Association, 23(5), 469–480. 10.1111/j.1464-5491.2006.01858.x [DOI] [PubMed] [Google Scholar]
- Anstey KJ, Lipnicki DM, & Low L-F (2008). Cholesterol as a risk factor for dementia and cognitive decline: A systematic review of prospective studies with meta-analysis. The American Journal of Geriatric Psychiatry: Official Journal of the American Association for Geriatric Psychiatry, 16(5), 343–354. 10.1097/JGP.0b013e31816b72d4 [DOI] [PubMed] [Google Scholar]
- Arrive E, Viard J-P, Salanave B, Dollfus C, Matheron S, Reliquet V, Arezes E, Nailler L, Vigouroux C, Warszawski J, & Groups, on behalf of the A. C. C. and E. study. (2018). Metabolic risk factors in young adults infected with HIV since childhood compared with the general population. PLOS ONE, 13(11), e0206745. 10.1371/journal.pone.0206745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asiimwe SB, Farrell M, Kobayashi LC, Manne-Goehler J, Kahn K, Tollman SM, Kabudula CW, Gómez-Olivé FX, Wagner RG, Montana L, Berkman LF, Glymour MM, & Bärnighausen T (2020). Cognitive differences associated with HIV serostatus and antiretroviral therapy use in a population-based sample of older adults in South Africa. Scientific Reports, 10(1), 16625. 10.1038/s41598-020-73689-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aurpibul L, Namwongprom S, Sudjaritruk T, & Ounjaijean S (2020). Metabolic syndrome, biochemical markers, and body composition in youth living with perinatal HIV infection on antiretroviral treatment. PloS One, 15(3), e0230707. 10.1371/journal.pone.0230707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benki-Nugent SF, Martopullo I, Laboso T, Tamasha N, Wamalwa DC, Tapia K, Langat A, Maleche-Obimbo E, Marra CM, Bangirana P, Boivin MJ, & John-Stewart GC (2019). High Plasma Soluble CD163 During Infancy Is a Marker for Neurocognitive Outcomes in Early-Treated HIV-Infected Children. Journal of Acquired Immune Deficiency Syndromes (1999), 81(1), 102–109. 10.1097/QAI.0000000000001979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debette S, Beiser A, Hoffmann U, Decarli C, O’Donnell CJ, Massaro JM, Au R, Himali JJ, Wolf PA, Fox CS, & Seshadri S (2010). Visceral fat is associated with lower brain volume in healthy middle-aged adults. Annals of Neurology, 68(2), 136–144. 10.1002/ana.22062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBoer MD, Filipp SL, & Gurka MJ (2019). Geographical variation in the prevalence of obesity and metabolic syndrome among US adolescents. Pediatric Obesity, 14(4), e12483. 10.1111/ijpo.12483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirajlal-Fargo S, Sattar A, Kulkarni M, Bowman E, Funderburg N, & McComsey GA (2017). HIV-positive youth who are perinatally infected have impaired endothelial function. AIDS (London, England), 31(14), 1917–1924. 10.1097/QAD.0000000000001556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzwonek AB, Lawson MS, Cole TJ, & Novelli V (2006). Body fat changes and lipodystrophy in HIV-infected children: Impact of highly active antiretroviral therapy. Journal of Acquired Immune Deficiency Syndromes (1999), 43(1), 121–123. 10.1097/01.qai.0000230523.94588.85 [DOI] [PubMed] [Google Scholar]
- Eggers C, Arendt G, Hahn K, Husstedt IW, Maschke M, Neuen-Jacob E, Obermann M, Rosenkranz T, Schielke E, Straube E, & German Association of Neuro-AIDS und Neuro-Infectiology (DGNANI). (2017). HIV-1-associated neurocognitive disorder: Epidemiology, pathogenesis, diagnosis, and treatment. Journal of Neurology, 264(8), 1715–1727. 10.1007/s00415-017-8503-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espiau M, Yeste D, Noguera-Julian A, González-Tomé M, Falcón-Neyra L, Gavilán C, Navarro-Gómez M, Mellado-Peña M, Gracia-Casanova M, Colino-Gil M, Méndez M, Calavia LC, Fortuny C, Carrascosa A, & Soler-Palacín P (2016). Metabolic Syndrome in Children and Adolescents Living with HIV. The Pediatric Infectious Disease Journal, 35(6). 10.1097/INF.0000000000001118 [DOI] [PubMed] [Google Scholar]
- Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. (2001). Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA, 285(19), 2486–2497. 10.1001/jama.285.19.2486 [DOI] [PubMed] [Google Scholar]
- Fabbiani M, Ciccarelli N, Tana M, Farina S, Baldonero E, Di Cristo V, Colafigli M, Tamburrini E, Cauda R, Silveri MC, Grima P, & Di Giambenedetto S (2013). Cardiovascular risk factors and carotid intima-media thickness are associated with lower cognitive performance in HIV-infected patients. HIV Medicine, 14(3), 136–144. 10.1111/j.1468-1293.2012.01044.x [DOI] [PubMed] [Google Scholar]
- Flammer AJ, Anderson T, Celermajer DS, Creager MA, Deanfield J, Ganz P, Hamburg NM, Lüscher TF, Shechter M, Taddei S, Vita JA, & Lerman A (2012). The assessment of endothelial function: From research into clinical practice. Circulation, 126(6), 753–767. 10.1161/CIRCULATIONAHA.112.093245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friend A, Craig L, & Turner S (2013). The prevalence of metabolic syndrome in children: A systematic review of the literature. Metabolic Syndrome and Related Disorders, 11(2), 71–80. 10.1089/met.2012.0122 [DOI] [PubMed] [Google Scholar]
- Frigati LJ, Jao J, Mahtab S, Asafu Agyei N-A, Cotton MF, Myer L, & Zar HJ (2019). Insulin Resistance in South African Youth Living with Perinatally Acquired HIV Receiving Antiretroviral Therapy. AIDS Research and Human Retroviruses, 35(1), 56–62. 10.1089/AID.2018.0092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geffner ME, Patel K, Jacobson DL, Wu J, Miller TL, Hazra R, Gerschenson M, Sharma T, Silio M, Jao J, Takemoto JK, Van Dyke RB, DiMeglio LA, & Pediatric HIV/AIDS Cohort Study (PHACS). (2018). Changes in insulin sensitivity over time and associated factors in HIV-infected adolescents. AIDS (London, England), 32(5), 613–622. 10.1097/QAD.0000000000001731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gojanovich GS, Jacobson DL, Jao J, Russell JS, Van Dyke RB, Libutti DE, Sharma TS, Geffner ME, & Gerschenson M (2020). Mitochondrial Dysfunction and Insulin Resistance in Pubertal Youth Living with Perinatally Acquired HIV. AIDS Research and Human Retroviruses. 10.1089/AID.2020.0067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorelick PB, Scuteri A, Black SE, Decarli C, Greenberg SM, Iadecola C, Launer LJ, Laurent S, Lopez OL, Nyenhuis D, Petersen RC, Schneider JA, Tzourio C, Arnett DK, Bennett DA, Chui HC, Higashida RT, Lindquist R, Nilsson PM, … American Heart Association Stroke Council, Council on Epidemiology and Prevention, Council on Cardiovascular Nursing, Council on Cardiovascular Radiology and Intervention, and Council on Cardiovascular Surgery and Anesthesia. (2011). Vascular contributions to cognitive impairment and dementia: A statement for healthcare professionals from the american heart association/american stroke association. Stroke, 42(9), 2672–2713. 10.1161/STR.0b013e3182299496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Clifford DB, Franklin DR, Woods SP, Ake C, Vaida F, Ellis RJ, Letendre SL, Marcotte TD, Atkinson JH, Rivera-Mindt M, Vigil OR, Taylor MJ, Collier AC, Marra CM, Gelman BB, McArthur JC, Morgello S, Simpson DM, … CHARTER Group. (2010). HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology, 75(23), 2087–2096. 10.1212/WNL.0b013e318200d727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoare J, Fouche J-P, Phillips N, Joska JA, Myer L, Zar HJ, & Stein DJ (2018). Structural brain changes in perinatally HIV-infected young adolescents in South Africa. AIDS (London, England), 32(18), 2707–2718. 10.1097/QAD.0000000000002024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson DL, Patel K, Siberry GK, Van Dyke RB, DiMeglio LA, Geffner ME, Chen JS, McFarland EJ, Borkowsky W, Silio M, Fielding RA, Siminski S, Miller TL, & Pediatric HIV/AIDS Cohort Study. (2011). Body fat distribution in perinatally HIV-infected and HIV-exposed but uninfected children in the era of highly active antiretroviral therapy: Outcomes from the Pediatric HIV/AIDS Cohort Study. The American Journal of Clinical Nutrition, 94(6), 1485–1495. 10.3945/ajcn.111.020271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson DL, Tang AM, Spiegelman D, Thomas AM, Skinner S, Gorbach SL, & Wanke C (2006). Incidence of metabolic syndrome in a cohort of HIV-infected adults and prevalence relative to the US population (National Health and Nutrition Examination Survey). Journal of Acquired Immune Deficiency Syndromes (1999), 43(4), 458–466. 10.1097/01.qai.0000243093.34652.41 [DOI] [PubMed] [Google Scholar]
- Jiménez-Balado J, Riba-Llena I, Abril O, Garde E, Penalba A, Ostos E, Maisterra O, Montaner J, Noviembre M, Mundet X, Ventura O, Pizarro J, & Delgado P (2019). Cognitive Impact of Cerebral Small Vessel Disease Changes in Patients With Hypertension. Hypertension (Dallas, Tex.: 1979), 73(2), 342–349. 10.1161/HYPERTENSIONAHA.118.12090 [DOI] [PubMed] [Google Scholar]
- Kandawasvika GQ, Kuona P, Chandiwana P, Masanganise M, Gumbo FZ, Mapingure MP, Nathoo K, & Stray-Pedersen B (2015). The burden and predictors of cognitive impairment among 6- to 8-year-old children infected and uninfected with HIV from Harare, Zimbabwe: A cross-sectional study. Child Neuropsychology: A Journal on Normal and Abnormal Development in Childhood and Adolescence, 21(1), 106–120. 10.1080/09297049.2013.876493 [DOI] [PubMed] [Google Scholar]
- Kapetanovic S, Leister E, Nichols S, Miller T, Tassiopoulos K, Hazra R, Gelbard HA, Malee KM, Kammerer B, Mendez AJ, Williams PL, & Pediatric HIV/AIDS Cohort Study Team. (2010). Relationships between markers of vascular dysfunction and neurodevelopmental outcomes in perinatally HIV-infected youth. AIDS (London, England), 24(10), 1481–1491. 10.1097/QAD.0b013e32833a241b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr SJ, Puthanakit T, Malee KM, Thongpibul K, Ly PS, Sophonphan J, Suwanlerk T, Kosalaraksa P, Ounchanum P, Aurpibul L, Kanjanavanit S, Ngampiyaskul C, Chettra K, Robbins R, Paul R, Ananworanich J, & Mellins CA (2019). Increased Risk of Executive Function and Emotional Behavioral Problems Among Virologically Well-Controlled Perinatally HIV-Infected Adolescents in Thailand and Cambodia. Journal of Acquired Immune Deficiency Syndromes (1999), 82(3), 297–304. 10.1097/QAI.0000000000002132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamar M, Rubin LH, Ajilore O, Charlton R, Zhang A, Yang S, Cohen J, & Kumar A (2015). What Metabolic Syndrome Contributes to Brain Outcomes in African American & Caucasian Cohorts. Current Alzheimer Research, 12(7), 640–647. 10.2174/1567205012666150701102325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laughton B, Cornell M, Boivin M, & Van Rie A (2013). Neurodevelopment in perinatally HIV-infected children: A concern for adolescence. Journal of the International AIDS Society, 16, 18603. 10.7448/IAS.16.1.18603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laughton B, Cornell M, Kidd M, Springer PE, Dobbels EFM-T, Rensburg AJV, Otwombe K, Babiker A, Gibb DM, Violari A, Kruger M, & Cotton MF (2018). Five year neurodevelopment outcomes of perinatally HIV-infected children on early limited or deferred continuous antiretroviral therapy. Journal of the International AIDS Society, 21(5), e25106. 10.1002/jia2.25106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin BE, Llabre MM, Dong C, Elkind MSV, Stern Y, Rundek T, Sacco RL, & Wright CB (2014). Modeling metabolic syndrome and its association with cognition: The Northern Manhattan study. Journal of the International Neuropsychological Society: JINS, 20(10), 951–960. 10.1017/S1355617714000861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsey JC, Jacobson DL, Li H, Houseman EA, Aldrovandi GM, & Mulligan K (2012). Using cluster heat maps to investigate relationships between body composition and laboratory measurements in HIV-infected and HIV-uninfected children and young adults. Journal of Acquired Immune Deficiency Syndromes (1999), 59(3), 325–328. 10.1097/QAI.0b013e31823fdbec [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macaluso F, Weber K, Dellinger E, Holman S, Minkoff H, Minkoff H, Keating S, & Gustafson D (2020). Body mass index and leptin are associated with executive function over 10 years in women with and without HIV infection. The Women’s Interagency HIV Study (WIHS) (4981). Neurology, 94(15 Supplement), 4981. [Google Scholar]
- Magge SN, Goodman E, Armstrong SC, COMMITTEE ON NUTRITION, SECTION ON ENDOCRINOLOGY, & SECTION ON OBESITY. (2017). The Metabolic Syndrome in Children and Adolescents: Shifting the Focus to Cardiometabolic Risk Factor Clustering. Pediatrics, 140(2). 10.1542/peds.2017-1603 [DOI] [PubMed] [Google Scholar]
- Mahtab S, Zar HJ, Ntusi NAB, Joubert S, Asafu-Agyei NAA, Luff NJ, Jele N, Liesl Z, Myer L, & Jao J (2020). Endothelial dysfunction in South African youth living with perinatally acquired HIV on antiretroviral therapy. Clinical Infectious Diseases: An Official Publication of the Infectious Diseases Society of America. 10.1093/cid/ciaa396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malee KM, Chernoff MC, Sirois PA, Williams PL, Garvie PA, Kammerer BL, Harris LL, Nozyce ML, Yildirim C, Nichols SL, & Memory and Executive Functioning Study of the Pediatric HIV/AIDS Cohort Study. (2017). Impact of Perinatally Acquired HIV Disease Upon Longitudinal Changes in Memory and Executive Functioning. Journal of Acquired Immune Deficiency Syndromes (1999), 75(4), 455–464. 10.1097/QAI.0000000000001441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCutchan JA, Marquie-Beck JA, Fitzsimons CA, Letendre SL, Ellis RJ, Heaton RK, Wolfson T, Rosario D, Alexander TJ, Marra C, Ances BM, Grant I, & CHARTER Group. (2012). Role of obesity, metabolic variables, and diabetes in HIV-associated neurocognitive disorder. Neurology, 78(7), 485–492. 10.1212/WNL.0b013e3182478d64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael HU, Naidoo S, Mensah KB, Ramlall S, & Oosthuizen F (2020). The Impact of Antiretroviral Therapy on Neurocognitive Outcomes Among People Living with HIV in Low- and Middle-Income Countries (LMICs): A Systematic Review. AIDS and Behavior. 10.1007/s10461-020-03008-8 [DOI] [PubMed] [Google Scholar]
- Miller TI, Borkowsky W, DiMeglio LA, Dooley L, Geffner ME, Hazra R, McFarland EJ, Mendez AJ, Patel K, Siberry GK, Van Dyke RB, Worrell CJ, Jacobson DL, Pediatric HIV/AIDS Cohort Study (PHACS), Shearer W, Cooper N, Harris L, Purswani M, Baig M, … Willen E (2012). Metabolic abnormalities and viral replication are associated with biomarkers of vascular dysfunction in HIV-infected children. HIV Medicine, 13(5), 264–275. 10.1111/j.1468-1293.2011.00970.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen KA, Peer N, Mills EJ, & Kengne AP (2016). A Meta-Analysis of the Metabolic Syndrome Prevalence in the Global HIV-Infected Population. PloS One, 11(3), e0150970. 10.1371/journal.pone.0150970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasipanodya EC, Montoya JL, Campbell LM, Hussain MA, Saloner R, Paolillo EM, Jeste DV, Letendre SL, McCutchan JA, Heaton RK, & Moore DJ (2019). Metabolic Risk Factors as Differential Predictors of Profiles of Neurocognitive Impairment Among Older HIV+ and HIV- Adults: An Observational Study. Archives of Clinical Neuropsychology: The Official Journal of the National Academy of Neuropsychologists. 10.1093/arclin/acz040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips N, Amos T, Kuo C, Hoare J, Ipser J, Thomas KGF, & Stein DJ (2016). HIV-Associated Cognitive Impairment in Perinatally Infected Children: A Meta-analysis. Pediatrics, 138(5). 10.1542/peds.2016-0893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins RN, Zimmerman R, Korich R, Raymond J, Dolezal C, Choi CJ, Leu CS, Nguyen N, Malee K, Wiznia A, Abrams EJ, & Mellins CA (2020). Longitudinal trajectories of neurocognitive test performance among individuals with perinatal HIV-infection and -exposure: Adolescence through young adulthood. AIDS Care, 32(1), 21–29. 10.1080/09540121.2019.1626343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruel TD, Boivin MJ, Boal HE, Bangirana P, Charlebois E, Havlir DV, Rosenthal PJ, Dorsey G, Achan J, Akello C, Kamya MR, & Wong JK (2012). Neurocognitive and motor deficits in HIV-infected Ugandan children with high CD4 cell counts. Clinical Infectious Diseases: An Official Publication of the Infectious Diseases Society of America, 54(7), 1001–1009. 10.1093/cid/cir1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattler FR, He J, Letendre S, Wilson C, Sanders C, Heaton R, Ellis R, Franklin D, Aldrovandi G, Marra CM, Clifford D, Morgello S, Grant I, McCutchan JA, & CHARTER Group. (2015). Abdominal obesity contributes to neurocognitive impairment in HIV-infected patients with increased inflammation and immune activation. Journal of Acquired Immune Deficiency Syndromes (1999), 68(3), 281–288. 10.1097/QAI.0000000000000458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siervo M, Harrison SL, Jagger C, Robinson L, & Stephan BCM (2014). Metabolic syndrome and longitudinal changes in cognitive function: A systematic review and meta-analysis. Journal of Alzheimer’s Disease: JAD, 41(1), 151–161. 10.3233/JAD-132279 [DOI] [PubMed] [Google Scholar]
- Sims RC, Madhere S, Gordon S, Clark E, Abayomi KA, Callender CO, & Campbell AL (2008). Relationships among Blood Pressure, Triglycerides and Verbal Learning in African Americans. Journal of the National Medical Association, 100(10), 1193–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner JS, Morgan A, Hernandez-Saucedo H, Hansen A, Corbett S, Arbuckle M, Leverenz JB, Wilkins CH, Craft S, & Baker LD (2015). Associations between Markers of Glucose and Insulin Function and Cognitive Function in Healthy African American Elders. Journal of Gerontology & Geriatric Research, 4(4). 10.4172/2167-7182.1000232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strehlau R, Kuhn L, Abrams EJ, & Coovadia A (2016). HIV-associated neurodevelopmental delay: Prevalence, predictors and persistence in relation to antiretroviral therapy initiation and viral suppression. Child: Care, Health and Development, 42(6), 881–889. 10.1111/cch.12399 [DOI] [PubMed] [Google Scholar]
- Tassiopoulos K, Williams PL, Seage GR, Crain M, Oleske J, Farley J, & International Maternal Pediatric Adolescent AIDS Clinical Trials 219C Team. (2008). Association of hypercholesterolemia incidence with antiretroviral treatment, including protease inhibitors, among perinatally HIV-infected children. Journal of Acquired Immune Deficiency Syndromes (1999), 47(5), 607–614. 10.1097/QAI.0b013e3181648e16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valcour VG, Sacktor NC, Paul RH, Watters MR, Selnes OA, Shiramizu BT, Williams AE, & Shikuma CM (2006). Insulin resistance is associated with cognition among HIV-1-infected patients: The Hawaii Aging With HIV cohort. Journal of Acquired Immune Deficiency Syndromes (1999), 43(4), 405–410. 10.1097/01.qai.0000243119.67529.f5 [DOI] [PubMed] [Google Scholar]
- Valcour VG, Shikuma CM, Shiramizu BT, Williams AE, Watters MR, Poff PW, Grove JS, Selnes OA, & Sacktor NC (2005). Diabetes, insulin resistance, and dementia among HIV-1-infected patients. Journal of Acquired Immune Deficiency Syndromes (1999), 38(1), 31–36. 10.1097/00126334-200501010-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Hof M, Ter Haar AM, Scherpbier HJ, van der Lee JH, Reiss P, Wit FWNM, Oostrom KJ, & Pajkrt D (2020). Neurocognitive Development in Perinatally Human Immunodeficiency Virus-infected Adolescents on Long-term Treatment, Compared to Healthy Matched Controls: A Longitudinal Study. Clinical Infectious Diseases: An Official Publication of the Infectious Diseases Society of America, 70(7), 1364–1371. 10.1093/cid/ciz386 [DOI] [PubMed] [Google Scholar]
- Vieira JR, Elkind MSV, Moon YP, Rundek T, Boden-Albala B, Paik MC, Sacco RL, & Wright CB (2011). The metabolic syndrome and cognitive performance: The Northern Manhattan Study. Neuroepidemiology, 37(3–4), 153–159. 10.1159/000332208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D (2003). Manual for the Wechsler Intelligence Scale for Children—Fourth Edition. Psychological Corporation. [Google Scholar]
- Wechsler D (2008). The Wechsler Adult Intelligence Scale, Fourth Edition.
- Weiss R, Bremer AA, & Lustig RH (2013). What is metabolic syndrome, and why are children getting it? Annals of the New York Academy of Sciences, 1281, 123–140. 10.1111/nyas.12030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson JD, Williams PL, Yu W, Colan SD, Mendez A, Zachariah JPV, Van Dyke RB, Shearer WT, Margossian RE, Lipshultz SE, & Pediatric HIV/AIDS Cohort Study (PHACS). (2018). Cardiac and inflammatory biomarkers in perinatally HIV-infected and HIV-exposed uninfected children. AIDS (London, England), 32(10), 1267–1277. 10.1097/QAD.0000000000001810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohl DA, McComsey G, Tebas P, Brown TT, Glesby MJ, Reeds D, Shikuma C, Mulligan K, Dube M, Wininger D, Huang J, Revuelta M, Currier J, Swindells S, Fichtenbaum C, Basar M, Tungsiripat M, Meyer W, Weihe J, & Wanke C (2006). Current concepts in the diagnosis and management of metabolic complications of HIV infection and its therapy. Clinical Infectious Diseases: An Official Publication of the Infectious Diseases Society of America, 43(5), 645–653. 10.1086/507333 [DOI] [PubMed] [Google Scholar]
- Wright CB, & Flores A (2015). Vascular contributions to cognitive impairment. Neurology: Clinical Practice, 5(3), 201–208. 10.1212/CPJ.0000000000000118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav SK, Gupta RK, Garg RK, Venkatesh V, Gupta PK, Singh AK, Hashem S, Al-Sulaiti A, Kaura D, Wang E, Marincola FM, & Haris M (2017). Altered structural brain changes and neurocognitive performance in pediatric HIV. NeuroImage. Clinical, 14, 316–322. 10.1016/j.nicl.2017.01.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu B, Pasipanodya E, Montoya JL, Moore RC, Gianella S, McCutchan A, Ellis R, Heaton RK, Jeste DV, Moore DJ, & Marquine MJ (2019). Metabolic Syndrome and Neurocognitive Deficits in HIV Infection. Journal of Acquired Immune Deficiency Syndromes, 81(1), 95–101. 10.1097/QAI.0000000000001964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmet P, Alberti KGM, Kaufman F, Tajima N, Silink M, Arslanian S, Wong G, Bennett P, Shaw J, Caprio S, & IDF Consensus Group. (2007). The metabolic syndrome in children and adolescents—An IDF consensus report. Pediatric Diabetes, 8(5), 299–306. 10.1111/j.1399-5448.2007.00271.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


