Abstract
Introduction
Multisystem inflammatory syndrome in children (MIS-C) is a rarely seen severe complication of coronavirus disease-2019 (COVID-19). Although fever is one of the indispensable symptoms, other infections should be considered in the differential diagnosis during the pandemic.
Case report
An 8-year-old and a 16-year-old female patient were admitted with fever, vomiting, headache. Both had fulfilled the criteria and were diagnosed with MIS-C. However, they both had remarkable persistent costovertebral angle tenderness, which was unexpected in MIS-C. In Case-1, urine analysis showed microscopic hematuria without pyuria, and urine culture showed no bacterial growth. Case-2 had microscopic hematuria and pyuria with Escherichia coli growth in urine culture. Contrast-enhanced computed tomography showed wedge-shaped hypodense multiple lesions in bilateral kidneys for Case-1, in the right kidney for Case-2. They diagnosed acute focal bacterial nephritis (AFBN).
Conclusions
The diagnostic criteria of MIS-C can overlap with the symptoms of other severe septic infections such as AFBN, which is a rare urinary tract infection, diagnosed by imaging of localized renal inflammatory mass-like or wedge-shaped lesion. A detailed anamnesis and careful physical examination may help differential diagnosis.
Keywords: MIS-C, fever, acute focal bacterial nephritis, costovertebral angle tenderness, flank pain
Introduction
Multisystem inflammatory syndrome in children (MIS-C) is a severe complication following the new coronavirus disease-2019 (COVID-19) occurring in childhood. The pathophysiology is not well understood, and it is suggested that it associates an abnormal immune response with certain clinical similarities to Kawasaki disease, macrophage activation syndrome, or cytokine release syndrome.1 The exact mechanisms whereby SARS-CoV-2 triggers this type of abnormal immune response are not clear. It has been hypothesized that patients with severe MIS-C present persistent immunoglobulin G (IgG) antibodies and a propensity for activation of monocytes and CD8+ T cells, coupled with persistent cytopenia, that differ from the findings regularly seen in acute COVID-19.2
Although all perceptions and perspectives have shifted to COVID-19 inevitably during the pandemic, it is imperative to keep in mind other infectious diseases in the differential diagnosis. Careful examination of the patients for the signs and symptoms of other infectious diseases is crucial to excluding other plausible diagnoses. One of these signs is costovertebral angle (CVA) tenderness. Here, two cases admitted with MIS-C-like clinical features and one of whom showed COVID-19 enzyme-linked immunosorbent assay (ELISA) test positivity for IgG are presented. They were misdiagnosed as MIS-C in the first place. However, both cases had flank pain with CVA tenderness, and on further investigation, they were both diagnosed with acute focal bacterial nephritis (AFBN), an upper urinary tract infection (UTI).
Case reports
Case-1
An 8-year-old girl presented with fever for three days, headache, flank pain, vomiting, lethargy. There was no history of cough, difficulty in breathing, diarrhea, dysuria. Before presenting, she had experienced alteration in consciousness, not recognizing her parents for an hour. She had had contact with a COVID-19 patient about three weeks previously. She was a previously healthy child without medication, except for the history of enuresis and frequent lower UTI. Her family history was unremarkable. The physical examination revealed fever (39.2°C, axillary) and tachycardia (135 beats/minute). Her blood pressure was 105/64 mmHg (below the 90th percentile according to the age, gender and height of the patient). Respiratory sounds and rate were normal (20 breaths/minute). She had normal skin turgor, moist oral mucosa. No mucocutaneous skin lesion or edema was noted. There was no rigidity, rebound tenderness, or organomegaly, but bilateral CVA tenderness was remarkable in the abdominal examination. She had no growth retardation (height of 1.40 m with standard deviation score (SDS) 1.45 and weight of 30 kg with SDS 0.26).
On laboratory analyses, there was leukocytosis, thrombocytopenia, neutrophilia with high inflammatory markers (elevated C-reactive protein, fibrinogen, procalcitonin, and D-dimer levels) – Table 1. The estimated glomerular filtration rate was calculated as 70 mL/min/1.73m2 by the Schwartz formula.3 Also, she had a high brain-natriuretic peptide (BNP) with normal troponin I levels. Urinalysis showed microscopic hematuria and (2+) proteinuria without nitrite or leukocyte esterase positivity. After obtaining urine and blood cultures, vancomycin (60 mg/kg/day in four divided doses), cefotaxime (150 mg/kg/day in three divided doses), and acyclovir (30 mg/kg/day in three divided doses) were started with intravenous route. Abdominal ultrasonography and echocardiography were found to be normal.
Table 1.
Summary of the cases
| Clinical features | Case-1 | Case-2 | |
|---|---|---|---|
| Age, sex | 8 years old, female | 16 years old, female | |
| Past medical history | Enuresis, frequent UTI | None | |
| Signs and symptoms | - Fever for three days | - Fever for four days | |
| - Headache | - Headache | ||
| - Vomiting | - Vomiting | ||
| - Altered mental status | - Right flank pain | ||
| - Bilateral flank pain | - Hypotension | ||
| - Bilateral CVA tenderness | - Right CVA tenderness | ||
| - Right lower abdominal tenderness | |||
| Laboratory features | Normal range | Case-1 | Case-2 |
| Leukocytes (×103/mm3) | 4.2-10.6 | 16 (↑) | 24 (↑) |
| Neutrophils (×103/mm3) | 2.0-6.9 | 13.3 (↑) | 21.9 (↑) |
| Lymphocytes (×103/mm3) | 0.6-3.4 | 1.1 | 0.5 (↓) |
| Thrombocytes (×103/mm3) | 150-450 | 127 (↓) | 155 |
| Triglycerides (mg/dL) | 30-200 | 109 | 181 |
| Ferritin (mcg/L) | 6-320 | 224 | 190 |
| CRP (mg/L) | 0-5 | 214 (↑) | 302 (↑) |
| PCT (mcg/L) | 0.04-0.1 | 27.1 (↑) | 1.17 (↑) |
| Urea (mg/dL) | 10-38 | 42 (↑) | 25 |
| Creatinine (mg/dL) | 0.5-1.2 | 1.1 | 1.1 |
| GFR (mL/min/1.73m2) | 70 (↓) | 82.5 (↓) | |
| AST / ALT (U/L) | 0-35 | 9 / 11 | 20 / 13 |
| Albumin (g/dL) | 3.5-5.2 | 3 (↓) | 3.6 |
| LDH (U/L) | 110-250 | 243 | 300 (↑) |
| Troponin I (ng/L) | 2.5-46 | <2.5 | <2.5 |
| BNP (ng/L) | 2-100 | 259 (↑) | 143 (↑) |
| Fibrinogen (mg/dL) | 170-420 | 454 (↑) | 645 (↑) |
| D-dimer (mcg/L) | 0-440 | 2000 (↑) | 3850 (↑) |
| aPTT / PT (sec) | 21-36 / 11-15 | 28.9 / 15.6 | 34.9 / 23.3 |
| INR | 0.8-1.2 | 1.29 (↑) | 2.17 (↑) |
| SARS-CoV-2 IgG (s/co) | 0-1.3 | 3.57 (↑) | 0.28 |
| SARS-CoV-2 IgM (s/co) | 0-0.9 | 0.16 | 0.10 |
| SARS-CoV-2 RT-PCR | Negative | Negative | |
| Urinalysis, pH/ density/ erythrocyte(E)/ leucocyte(L)/ protein(P)/ nitrite(N) | 5.5 /1019/ E(+3)/ L(-)/ P(+2)/ N(-) | 6.0/ 1021/ E(+3)/ L(+2)/ P(+2)/ N(-) | |
| Urine culture | Sterile | E. coli – 105 CFU/mL | |
| Blood culture | Contaminated | Sterile | |
| Imaging of kidneys | |||
| USG | No finding | No finding | |
| CT with contrast enhancement | Bilateral multiple hypodense lesions | A hypodense lesion in the right kidney | |
ALT – alanine aminotransferase; aPTT – activated partial thromboplastin time; AST – aspartate transaminase; BNP – brain natriuretic peptide; CFU – colony-forming unit; CVA – costovertebral angle; CRP – C-reactive protein; CT – computed tomography; E. coli – Escherichia coli; GFR – glomerular filtration rate; LDH – lactate dehydrogenase; PCT – procalcitonin; PT – prothrombin time; RT-PCR – reverse transcription-polymerase chain reaction; SARS-CoV-2 – severe acute respiratory syndrome coronavirus 2; USG – ultrasonography; UTI – urinary tract infection.
Meanwhile, the result of the SARS-CoV-2 RT-PCR test came back negative. However, the COVID-19 ELISA was positive for IgG and negative for IgM. Due to fever duration, clinically severe illness requiring hospitalization, multisystem organ involvement (cardiac, hematological, renal, gastrointestinal, neurological), she was diagnosed with MIS-C (Table 2). Intravenous immunoglobulin (IVIG) (2 g/kg/day), enoxaparin sodium (1 mg/kg/day) and prednisolone (2 mg/kg/day) were started.
Table 2.
Comparison of criteria and inconsistent findings for MIS-C
| Criteria of MIS-C | Case-1 | Case-2 |
|---|---|---|
| Age below 21 years | Yes | Yes |
| Fever higher than 38°C for more than 24 hours | Yes | Yes |
| Requiring hospitalization due to severe illness | Yes | Yes |
| Multisystem organ involvement (more than two) | Cardiac (elevated BNP) Renal (acute kidney injury with decreased GFR) Hematological (elevated D-dimer, thrombocytopenia) Gastrointestinal (abdominal pain, vomiting) Neurological (altered mental status, headache) |
Cardiac (hypotension, shock, elevated BNP) Renal (acute kidney injury with decreased GFR) Hematological (elevated D-dimer) Gastrointestinal (abdominal pain, vomiting) |
| Laboratory evidence of inflammation | Elevated levels of: | Elevated levels of: |
| C-reactive protein | C-reactive protein | |
| Procalcitonin | Procalcitonin | |
| Fibrinogen | Fibrinogen | |
| D-dimer | D-dimer | |
| Neutrophilia | LDH | |
| Hypoalbuminemia | Neutrophilia | |
| Lymphocytopenia | ||
| Current or recent SARS-CoV-2 infection, exposure within four weeks | RT-PCR (-) | RT-PCR (-) |
| ELISA IgG(+), IgM(-) | ELISA IgG(-), IgM(-) | |
| Exposure (+), 3 weeks previously | Exposure: unknown | |
| Inconsistent findings with MIS-C | Bilateral flank pain | Right flank pain |
| Bilateral CVA tenderness | Right CVA tenderness | |
| Bilateral multiple hypodense renal lesions on abdomen CT. | A hypodense lesion in the right kidney on abdomen CT |
BNP – brain natriuretic peptide; CT – computed tomography; ELISA – enzyme-linked immunosorbent assay; RT-PCR – reverse transcription-polymerase chain reaction; SARS-CoV-2 – severe acute respiratory syndrome coronavirus 2.
Case-2
A 16-year-old female patient presented to the hospital with fever for four days, right flank pain, headache, and vomiting. There was no cough, difficulty in breathing, diarrhea, dysuria. She had no known chronic disease, history of urinary tract infection. She had no known COVID-19 contact. Her family history was irrelevant. On physical examination, she had a fever (38.8°C, axillary), tachycardia (124 beats/minute), and hypotension (73/37 mmHg). Auscultation of the lungs was normal. Remarkable rebound tenderness and guarding in the right lower quadrant indicated acute abdomen. In addition, right CVA tenderness was found.
On laboratory analysis, she had leukocytosis, neutrophilia, lymphocytopenia, and high inflammatory markers (elevated C-reactive protein, procalcitonin, fibrinogen, lactate dehydrogenase, and D-dimer levels) – Table 1. The estimated glomerular filtration rate was calculated as 82.5 mL/min/1.73m2 by the Schwartz formula.3 Slightly elevated BNP with normal troponin I levels were also found. In urinalysis, there was microscopic hematuria, 2+ proteinuria, leukocyte esterase reaction. After obtaining urine and blood cultures, vancomycin (60 mg/kg/day in four divided doses), meropenem (60 mg/kg/day in three divided doses), and amikacin (15 mg/kg/day) were started. Due to hypotension, intravenous sodium chloride 0.9% bolus (20 cc/kg) was given twice. However, hypotension persisted, and noradrenalin infusion was started. Systolic functions were found as normal on echocardiography.
Since the patient had acute abdomen, oral intake was stopped entirely after pediatric surgery consultation. Abdominal ultrasonography did not reveal any pathology.
Meanwhile, SARS-CoV-2 RT-PCR and ELISA test results came back negative. Due to fever duration, clinically severe illness requiring hospitalization, multisystem organ involvement (cardiac, hematological, renal, gastrointestinal), she was diagnosed with MIS-C (Table 2).
Clinical course of both cases
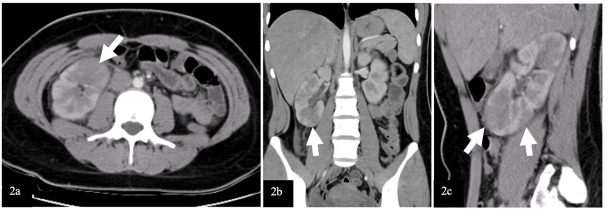
Due to the acute abdomen clinical finding in Case-2 and the significant CVA tenderness in both cases (bilateral in Case-1 and unilateral in Case-2), contrast-enhanced abdominal computed tomography (CT) was performed for differential diagnosis. In Case-1, nonhomogeneous hypodense mass-like lesions with decreased contrast enhancement in both kidneys were detected (Figure 1). In Case-2, a similar lesion was seen in the lower pole of the right kidney (Figure 2). Both cases were diagnosed with AFBN since these lesions were specific for it.
Figure 1.
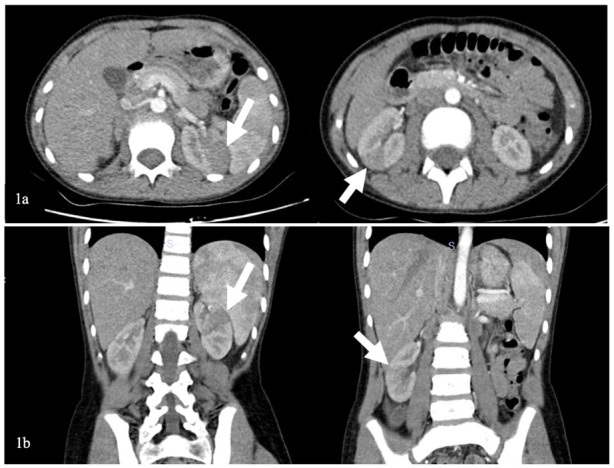
Images of contrast-enhanced abdominal CT of Case-1
(1a) Axial section, (1b) Coronal section. Bilateral wedge-shaped hypodense lesions with decreased contrast enhancement are shown with white arrows.
Figure 2.
Images of contrast-enhanced abdominal CT of Case-2
(2a) Axial sections, (2b) Coronal section, (2c) Parasagittal section. Wedge-shaped hypodense multiple lesions with decreased contrast enhancement on the right kidney are shown with white arrows.
Case-1 was admitted to the pediatric nephrology inpatient clinics. The treatment was continued with intravenous cefotaxime and acyclovir at the doses mentioned above. Her general condition improved, and her fever regressed on follow-up. The midstream urine culture on admission resulted in no bacterial growth. Neurological impairment did not repeat during follow-up. Electroencephalogram (EEG) performed on the 7th day of hospitalization was found normal. After 14 days of hospitalization, she was discharged to continue oral cefixime (8 mg/kg/day) treatment for seven more days. The urodynamic study resulted in overactive bladder with decreased functional bladder capacity. The treatment was continued with antibiotic prophylaxis and oxybutynin. Voiding cystourethrography (VCUG) revealed grade 3 vesicoureteral reflux in the right kidney. Four months after the infection, a radionuclide scan with dimercaptosuccinic acid (DMSA) was performed showing no hypoactive scar lesions on both kidneys.
Case-2 was treated in the pediatric intensive care unit for two days. After resolution of hypotension and cessation of inotrope infusion, she was transported to the pediatric nephrology inpatient clinics. A DMSA scan was performed to support the diagnosis of AFBN, and it showed hypoactive lesions on the lower pole of the left kidney and the upper and lower poles of the right kidney. The midstream urine culture on admission showed Escherichia coli growth. The treatment was continued with only intravenous meropenem at the dose mentioned above during hospitalization for 14 days and peroral cefixime (8 mg/kg/day) treatment for 7 days. Then, antibiotic prophylaxis was started. The control DMSA and VCUG are planned, and waiting for the results.
Discussion
Some of the first reports of MIS-C emerged from the UK, of children with an unexplained multisystemic inflammation which has similar characteristics with Kawasaki disease and toxic shock syndrome.4 These cases are reported across the world with the spread of the COVID-19 pandemic. Most children affected had negative RT-PCR tests with high IgG antibody levels, indicating past infection.5
The diagnostic criteria of MIS-C have been described by the Centers for Disease Control and Prevention (CDC) as age below 21 years old, fever lasting more than 24 hours, high inflammatory markers, more than two system involvement, no plausible alternate diagnosis, COVID-19 exposure in the last four weeks, or evidence of past infection.6 Gastrointestinal and cardiac involvements are reported as common presentations.7,8 Kidney involvement is reported between 10% to 46% in MIS-C patients.9,10 Both presented cases had gastrointestinal and cardiac involvement in addition to renal and hematological involvement. Case-1 also had neurological system involvement (Table 2). Therefore, Case-1 fulfilled the diagnostic criteria of MIS-C with clinical and laboratory aspects and with positive serology of SARS-CoV-2. However, Case-2 had no known COVID-19 contact, although clinical and laboratory characteristics suited MIS-C (Table 2). In countries like Turkey, where COVID-19 incidence rates are very high, anyone can be considered in a suspected position about COVID-19 contact. Therefore, Case-2 had also been misdiagnosed with MIS-C.
Since the pandemic has spread all around the world with high contagion rates, uncertain prognosis, and newly isolated virus variants, the healthcare providers may become hyperalert on the diagnosis of MIS-C. However, MIS-C is a diagnosis of exclusion, and other severe infections may present similar clinical features. Therefore, clinicians should focus on unexpected findings and the findings incompatible with MIS-C to avoid overdiagnosis and delay in correct diagnosis.11 Careful evaluation of each case is mandatory. In the presented cases, flank pain with CVA tenderness was the unexpected finding, suggesting the possibility of a plausible alternative diagnosis (Table 2).
Costovertebral angle tenderness is one of the specific indicators for kidney pathology and is often seen in acute pyelonephritis.12 However, it may present in other diseases such as nephrolithiasis, kidney abscess, vesicoureteral reflux, obstructive pathologies of the urinary tract, retrocecal appendicitis, retroperitoneal abscess.13 In a patient presenting with fever and CVA tenderness, the clinician should consider first upper UTI, among other diagnoses. In selected cases, imaging techniques can be used for differential diagnosis. Herein, due to the presence of CVA tenderness, we performed contrast-enhanced CT imaging, which showed hypodense wedge-shaped kidney lesions indicating AFBN (Figure 1 and 2).14
Acute focal bacterial nephritis (AFBN) or acute lobar nephronia is a rare bacterial UTI that causes non-liquefactive inflammatory lesions localized to the cortex of the kidneys and is considered to be located in a spectrum between acute pyelonephritis and renal abscess. It mainly occurs as ascending infection in children with urinary tract anomalies, although reported also in healthy children. The incidence is 8.6% in children with febrile UTI and 19.2% in healthy children with first febrile UTI.14,15
Persistent fever, severe flank pain/ abdominal pain, and rapid deterioration of the general condition are remarkable symptoms of AFBN. Laboratory analysis shows intense inflammation with leukocytosis, neutrophilia, and high acute phase reactant levels.16 These clinical and laboratory findings are all compatible with the criteria of MIS-C and may cause the misdiagnosis with MIS-C. However, a careful anamnesis indicating predisposing features to UTI (like enuresis and frequent UTI in Case-1) and physical examination revealing specific symptoms of upper UTI (like CVA tenderness) help the differential diagnosis.
Pyuria, bacteriuria, or bacterial isolation in urine culture may not be detected in 5-10% of AFBN patients since the infection does not involve the calyceal system.17 Therefore, these laboratory findings may not help in differential diagnosis with MIS-C, as Case-1 presented in this report.
Early diagnosis and effective treatment are essential in terms of preventing renal scars and morbidity such as hypertension, proteinuria, and renal failure.18 The diagnosis of AFBN is based on radiologic examinations. In many cases, there is no finding on kidney ultrasonography (USG), although it may show nephromegaly, focal lesions with poorly defined irregular margins. Contrast-enhanced abdomen CT is the gold standard imaging technique for the diagnosis of AFBN. Herein, both cases had no finding on kidney USG. The lesions were observed on CT as the typical wedge-shaped, poorly defined hypodense lesions after contrast-medium administration.14 The DMSA scan of the kidneys may help in the diagnosis of AFBN and the detection of renal scarring during follow-up.19 In Case-1, the diagnosis of AFBN was supported with the DMSA scan. In Case-2, the DMSA scan in follow-up shows no scars indicating the effective treatment.
In the treatment of AFBN it is recommended to continue with intravenous antibiotics until the fever response is seen. The duration of antibiotic therapy can be planned as two or three weeks, depending on the presence of complicated lesions (microabscess formation, liquefaction areas) on CT imaging.14,19 Renal abscess formation develops in approximately 25% of patients who are not given appropriate and adequate antibiotic therapy.20 In the presented cases, we preferred to continue the antibiotic treatment for three weeks (intravenously for two weeks and peroral for one week). We didn’t see progression to renal abscess.
Conclusions
The diagnostic criteria of MIS-C can overlap with the symptoms of other severe septic infections. It may cause misdiagnosis with MIS-C due to the hyperalert situation of clinicians worldwide. To avoid misdiagnosis and delay of correct diagnosis, MIS-C should be diagnosed after exclusion of other possible diagnoses. Acute focal bacterial nephritis is a severe upper urinary tract infection that can mimic MIS-C and is easily misdiagnosed with MIS-C. A detailed anamnesis and careful physical examination may help differential diagnosis.
Footnotes
Consent: Written informed consents were obtained from the parents for publication of these case reports and accompanying images.
Authors’ contributions statement: DA, BKD and AKA contributed to manuscript conception and design. GE, ÖÖŞ and GÜ contributed to data collection and writing. SAÇ, FM, DYÇ and DA contributed to reviewing and providing criticism for the draft version of the manuscript. All authors read and approved the final version of the manuscript.
Conflicts of interest: All authors – none to declare.
Funding: None to declare.
References
- 1.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bartsch YC, Wang C, Zohar T, et al. Humoral signatures of protective and pathological SARS-CoV-2 infection in children. Nat Med. 2021;27:454–62. doi: 10.1038/s41591-021-01263-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schwartz GJ, Brion LP, Spitzer A. The use of plasma creatinine concentration for estimating glomerular filtration rate in infants, children, and adolescents. Pediatr Clin North Am. 1987;34:571–90. doi: 10.1016/S0031-3955(16)36251-4. [DOI] [PubMed] [Google Scholar]
- 4.Riphagen S, Gomez X, Gonzalez-Martinez C, Wilkinson N, Theocharis P. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet. 2020;395:1607–8. doi: 10.1016/S0140-6736(20)31094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Radia T, Williams N, Agrawal P, et al. Multisystem inflammatory syndrome in children & adolescents (MIS-C): a systematic review of clinical features and presentation. Paediatr Respir Rev. 2021;38:51–7. doi: 10.1016/j.prrv.2020.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention Health Alert Network (HAN) Multisystem inflammatory syndrome in children (MIS-C) associated with coronavirus disease 2019 (COVID-19) [Accessed on: 15 April 2021]. Available at: https://emergency.cdc.gov/han/2020/han00432.asp .
- 7.Jiang L, Tang K, Levin M, et al. COVID-19 and multisystem inflammatory syndrome in children and adolescents. Lancet Infect Dis. 2020;11:e276–88. doi: 10.1016/S1473-3099(20)30651-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harahsheh AS, Krishnan A, DeBiasi RL, et al. Cardiac echocardiogram findings of severe acute respiratory syndrome coronavirus-2-associated multi-system inflammatory syndrome in children Cardiol Young. 2021 Aug 5:1–9. doi: 10.1017/S1047951121003024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sethi SK, Rana A, Adnani H, et al. Kidney involvement in multisystem inflammatory syndrome in children: a pediatric nephrologist’s perspective. Clin Kidney J. 2021;14:2000–11. doi: 10.1093/ckj/sfab073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deep A, Upadhyay G, du Pré P, et al. Acute kidney injury in pediatric inflammatory multisystem syndrome temporally associated with severe acute respiratory syndrome coronavirus-2 pandemic: experience from PICUs across United Kingdom. Crit Care Med. 2020;48:1809–18. doi: 10.1097/CCM.0000000000004662. [DOI] [PubMed] [Google Scholar]
- 11.Harahsheh AS, Dahdah N, Newburger JW, et al. Missed or delayed diagnosis of Kawasaki disease during the 2019 novel coronavirus disease (COVID-19) pandemic. J Pediatr. 2020;222:261–2. doi: 10.1016/j.jpeds.2020.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bent S, Nallamothu BK, Simel DL, Fihn SD, Saint S. Does this woman have an acute uncomplicated urinary tract infection? JAMA. 2002;287:2701–10. doi: 10.1001/jama.287.20.2701. [DOI] [PubMed] [Google Scholar]
- 13.Belyayeva M, Jeong JM. StatPearls. Treasure Island (FL): StatPearls Publishing; 2021. Acute pyelonephritis. [PubMed] [Google Scholar]
- 14.Cheng CH, Tsau YK, Chen SY, Lin TY. Clinical courses of children with acute lobar nephronia correlated with computed tomographic patterns. Pediatr Infect Dis J. 2009;28:300–3. doi: 10.1097/INF.0b013e31818ffe7d. [DOI] [PubMed] [Google Scholar]
- 15.Yang CC, Shao PL, Lu CY, et al. Comparison of acute lobar nephronia and uncomplicated urinary tract infection in children. J Microbiol Immunol Infect. 2010;43:207–14. doi: 10.1016/S1684-1182(10)60033-3. [DOI] [PubMed] [Google Scholar]
- 16.Erfidan G, Alaygut D, Soyaltın E, et al. Urinary tract infection that a pediatric nephrologist must keep in mind: answers. Pediatr Nephrol. 2020;35:795–7. doi: 10.1007/s00467-019-04438-w. [DOI] [PubMed] [Google Scholar]
- 17.Sekine H, Kawasaki Y, Ohara S, Suyama K, Hosoya M. Focal bacterial nephritis without pyuria in a boy presenting with high urinary β2-MG and NAG levels. Fukushima J Med Sci. 2014;60:91–4. doi: 10.5387/fms.2014-3. [DOI] [PubMed] [Google Scholar]
- 18.Cheng CH, Tsau YK, Hsu SY, Lee TL. Effective ultrasonographic predictor for the diagnosis of acute lobar nephronia. Pediatr Infect Dis J. 2004;23:11–4. doi: 10.1097/01.inf.0000105202.57991.3e. [DOI] [PubMed] [Google Scholar]
- 19.Cheng CH, Tsau YK, Lin TY. Effective duration of antimicrobial therapy for the treatment of acute lobar nephronia. Pediatrics. 2006;117:e84–9. doi: 10.1542/peds.2005-0917. [DOI] [PubMed] [Google Scholar]
- 20.Vijayakumar M, Prahlad N, Nandhini G, Prasad N, Muralinath S. Child with acute lobar nephronia. Indian J Nephrol. 2010;20:162–5. doi: 10.4103/0971-4065.70847. [DOI] [PMC free article] [PubMed] [Google Scholar]