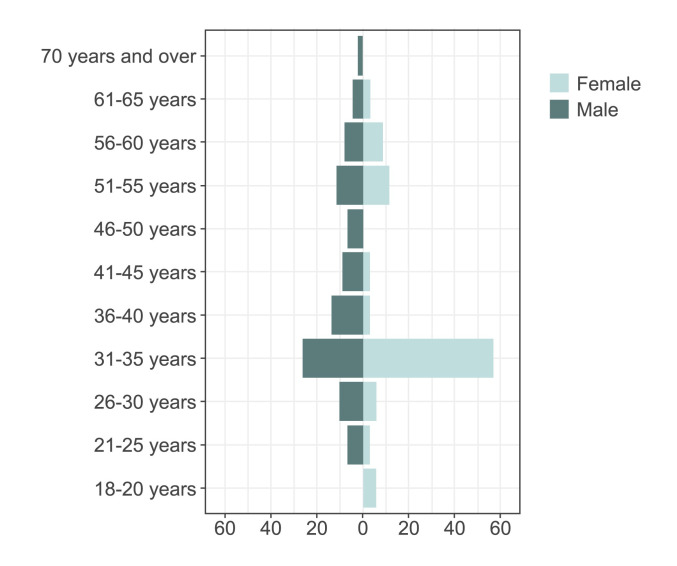Abstract
Introduction
Development of highly active antiretroviral therapy marked an important step forward in the management of people living with HIV and fixed dose combinations are now available to be used as modern antiretroviral regimens. The single-tablet regimen bictegravir/emtricitabine/tenofovir alafenamide (BIC/FTC/TAF) was recently approved in Europe and included in international guidelines and recommendations. It became available in Romania in early 2021. We present the real-world results from a retrospective analysis of patients initiating BIC/FTC/TAF in two HIV centers in Romania.
Methods
This retrospective analysis included patients treated with BIC/FTC/TAF (first-line or switch) in two HIV centers in Romania, one in Bucharest and one in Iași. We collected data on baseline patient characteristics, reasons for initiation of BIC/FTC/TAF and preliminary clinical and laboratory efficacy, safety and tolerability data. All assessments had been performed according to local practice. Statistical analyses were mostly descriptive and association analysis was performed to assess changes in laboratory parameters from baseline to data cut-off (October 2021).
Results
In total, 122 patients were initiated on BIC/FTC/TAF in routine clinical practice from February to October 2021 in the two HIV centers, either as first-line or switch. The majority of patients were male (71%). The median age at baseline was 35.0 years (IQR 32.0-50.8 years). Overall, 91 patients (75%) were treatment-experienced and the most frequent reason for switch was treatment simplification (79%). The mean ± standard deviation follow-up duration on treatment with BIC/FTC/TAF was 101.6 ± 64.2 days until the cut-off date for this analysis. We found no significant changes in lipid values, blood glucose or liver enzymes, coupled with a significant decrease in viral load (p=0.001). A low number of adverse events occurred during the treatment period (n=4): two cases of fatigue and two gastrointestinal reactions. No patient discontinued BIC/FTC/TAF and the overall tolerability was good.
Conclusions
The insights of the first report on BIC/FTC/TAF use in routine clinical practice in Romania provide an overview of effectiveness and safety to local clinicians treating this patient population.
Keywords: PLWH, BIC/FTC/TAF, real-world, Romania
Introduction
The development of highly active antiretroviral therapy (HAART) marked an important step forward in the management of people living with HIV (PLWH). Initial treatment regimens incurred a high pill burden, both in antiretroviral (ARV)-naïve and experienced patients, thus increasing the risk of therapeutic fatigue, non-adherence and consequent lack of efficacy.1 An important improvement in the quality of life of PLWH was noticed after the introduction into clinical practice of single-tablet regimens with fixed dose combinations (FDCs).2
One of the most recent classes of ARVs, integrase inhibitors (INSTIs), have shown strong efficacy data and good safety and tolerability, supplemented with high barrier to resistance,3-8 thus leading to the inclusion in European, North American and World Health Organization (WHO) guidelines as top treatment choices in PLWH.9-11 Accumulated evidence support INSTIs as first-line and switch options,9,12 allowing a versatile individualization of treatment. Currently, five INSTI-based FDCs (three single-tablet three-drug regimens (BIC/FTC/TAF, DTG/3TC/ABC and EVG/c/FTC/TAF) and two two-drug regimens (DTG/3TC and DTG/ RPV) are used in Europe, including Romania.9
BIC/FTC/TAF was approved in Europe in 201813 and became available in Romania at the beginning of 2021, being indicated for the treatment of adults infected with HIV-1 without history of resistance to integrase inhibitors, emtricitabine or tenofovir.14 Clinical trials and meta-analyses showed high efficacy.6-8,15-18 Moreover, switch studies revealed a lower rate of drug-drug interactions and a favorable safety profile compared to other active regimens.16 Coupled with the comfort in administration, these attributes place this combination high among the current treatment choices, considering the need to re-think HIV management as that for a lifelong, chronic infection.19 Real-world evidence data need to be generated to ensure the results from clinical trials may be generalized in broader populations and to assess to which extent they can be replicated in daily clinical practice.
After 40 years of HIV and over 20 years of experience with ARV therapy (ART),20,21 infectious disease specialists in Romania see an advancement in the role of ART, from lifesaver to quality-of-life keeper for PLWH. In this context, selection of the most appropriate treatment option takes into consideration long-term goals such as efficacy and tolerability, as well as the ease of administration which comes with a lower pill burden.
The current retrospective analysis aimed to describe the baseline characteristics of PLWH being switched to or initiated on BIC/FTC/TAF in two clinical centers in Romania, and to analyze the real-life efficacy and tolerability data.
Methods
This retrospective analysis included all patients with HIV infection who were initiated on BIC/FTC/TAF (either as first-line therapy or as switch from a previous regimen) between February and October 2021 in two tertiary infectious disease clinics in Romania (the National Institute for Infectious Diseases “Prof. Dr. Matei Balș” (Center 1) in Bucharest and the Clinical Hospital of Infectious Diseases in Iași (Center 2).
The analysis was approved by the Bioethics Committee of the National Institute for Infectious Diseases “Prof. Dr. Matei Balș” in Bucharest and the Clinical Infectious Diseases Hospital “Sf Parascheva” in Iași, with a waiver for informed consent due to the retrospective nature of the analysis, based on data collected in routine clinical practice. All clinical procedures were performed according to the local practice, no additional tests or examinations were requested for this analysis. Data were collected by the consultation of medical patient charts.
This retrospective analysis was not designed to confirm or reject any pre-defined hypothesis. Statistical analyses were of explorative and descriptive nature. Descriptive statistics included mean ± standard deviation (SD) for parametric variables and median (interquartile range – IQR) for non-parametric variables, as well as frequency and percentages for categorical variables. All statistical analyses were performed for the full analysis set, which included all patients with data available at the time of the analysis.
Changes in laboratory parameters from baseline to data cut-off were assessed with the Wilcoxon signed-rank test for continuous non-parametrical data and the paired two-sample t-test for continuous parametrical data.
All statistical analyses were performed using R programming language version 3.6.2 (https://www-rproject.org/). Missing data were not replaced.
Results
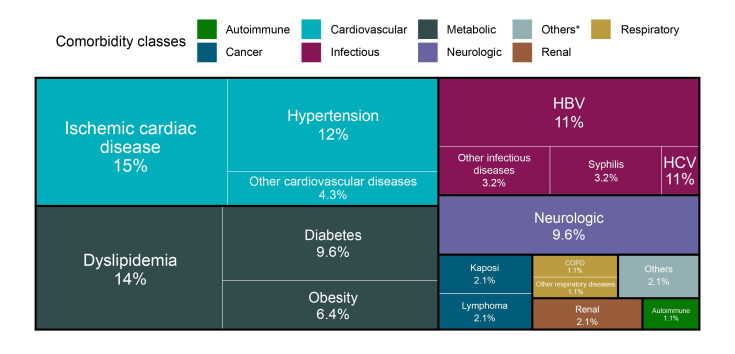
This analysis included a total of 122 patients started on BIC/FTC/TAF in routine clinical practice from February to October 2021, of which 89 in Center 1 and 33 in Center 2. Their baseline demographic and clinical characteristics are presented in Table 1. The majority of patients (73.8%) were less than 50 years of age (Figure 1), 43 (35%) of them belonging to the Romanian pediatric HIV cohort. Information about comorbidities were available for 92 patients. The most common associated diseases were ischemic cardiac disease (15%), hypertension (12%), dyslipidemia (14%) and co-infection with HBV (11%) (Figure 2).
Table 1.
Baseline patient characteristics (prior to initiation of BIC/FTC/TAF)
| Variable | N | Baseline value |
|---|---|---|
| Male, n (%) | 122 | 87 (71) |
| Age, years, median (IQR) | 122 | 35.0 (32.0-50.8) |
| Caucasian, n (%) | 122 | 122 (100) |
| Comorbidities | ||
| None, n (%) | 92 | 45 (49) |
| 1-2, n (%) | 92 | 34 (37) |
| ≥3, n (%) | 92 | 13 (14) |
| BMI, kg/m2, mean ± SD | 70 | 25.5 ± 4.4 |
| Random blood glucose, mg/dL, mean ± SD | 122 | 104.2 ± 26.3 |
| Total cholesterol, mg/dL, mean ± SD | 119 | 192.2 ± 54.1 |
| LDL-cholesterol, mg/dL, mean ± SD | 106 | 118.6 ± 48.2 |
| HDL-cholesterol, mg/dL, mean ± SD | 109 | 43.4 ± 17.7 |
| Triglycerides, mg/dL, mean ± SD | 119 | 172.0 ± 119.1 |
| Creatinine clearance (Cockcroft-Gault), mL/min, mean ± SD | 75 | 114.9 ± 31.3 |
| eGFR (MDRD), mL/min/1.73 m2, mean ± SD | 121 | 93.1 ± 26.7 |
| Urea, mg/dL, mean ± SD | 122 | 34.5 ± 11.0 |
| Alanine aminotransferase, U/L, mean ± SD | 122 | 35.6 ± 33.1 |
| Alkaline phosphatase, U/L, mean ± SD | 101 | 99.2 ± 80.1 |
| Gamma-glutamyl transferase, U/L, mean ± SD | 119 | 57.3 ± 177.4 |
| Current CD4 count, cells/mm3, median (IQR) | 121 | 435 (196-644) |
| Nadir CD4, cells/mm3, median (IQR) | 122 | 215 (100-367) |
| Undetectable HIV-1 RNA, n (%) | 115 | 41 (36) |
| >1000 copies/mL HIV-1 RNA, n (%) | 115 | 43 (37) |
| ≤1000 copies/mL HIV-1 RNA, n (%) | 115 | 31 (27) |
| HIV-1 RNA, log10 copies/mL, median (IQR) in patients with detectable viremia | 74 | 9.7 (4.4-12.4) |
BIC/FTC/TAF – bictegravir/emtricitabine/tenofovir alafenamide; eGFR – estimated glomerular filtration rate; HDL – high-density lipoprotein; HIV-1 RNA – human immunodeficiency virus-1 ribonucleic acid; LDL – low-density lipoprotein; MDRD – modification of diet in renal disease; SD – standard deviation.
Figure 1.
Age pyramid in the overall cohort (n=122)
Figure 2.
Distribution of comorbidities by disease group
*Others include psychiatry and urological diseases.
COPD – chronic obstructive pulmonary disease; HBV – hepatitis B virus; HCV – hepatitis C virus.
A total of 30 patients (25%) were treatment-naïve, and among them 20 were classified as late presenters based on a CD4 cell count below 350 cells/mm3 at the time of HIV diagnosis. Treatment-naïve patients had a median age of 35 years old (IQR 25-41 years), and associated the following comorbidities in decreasing order of their frequency: neurologic conditions (n=3), HBV coinfection (n=2), arterial hypertension (n=2), Kaposi sarcoma (n=2), other lymphomas (n=1), ischemic heart disease (n=1), diabetes mellitus (n=1). Treatment-naïve patients were initiated on BIC/FTC/TAF at a median CD4 cell count of 199 (IQR 117-339) cells/mm3 and a median HIV viral load of 533000 (IQR 134000-890000) copies/mL, and displayed rapid immunological and virological response, with an increase to a median CD4 cell count of 320 (IQR 224-431) cells/mm3 and a decrease in HIV viral load to a median of 156 (98-308) copies/mL over the course of follow-up in this analysis, with 25 of the patients achieving a viral load below 1000 copies/mL at their first on-treatment evaluation. Treatment initiation was generally well tolerated, with only 2 patients reporting an adverse event, i.e., fatigue, which was self-limited in all cases.
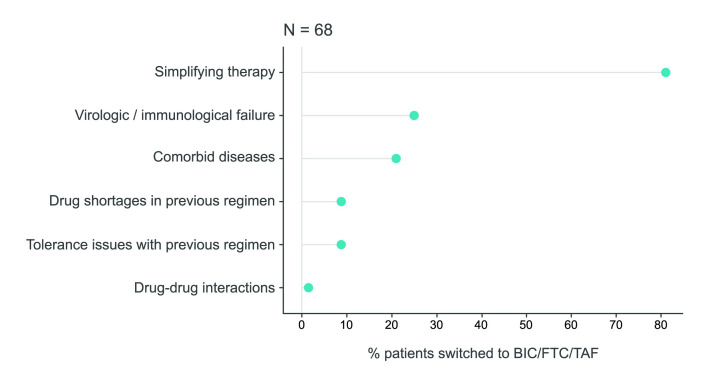
In total, 91 patients (75%) were treatment-experienced, with a median of 6 (IQR 3-11) previous ARV regimens and a median pill burden of 3 (IQR 1-4 and a range of 1 to 7) pills/day prior to the switch. Among treatment-experienced patients, 48 (52.7%) had detectable HIV-RNA and 20 (22.0%) had HIV viral loads >1000 copies/mL at the time of the switch, while after the switch 8 patients had detectable HIV-RNA and only 2 patients still had an HIV viral load >1000 copies/mL (data available for 12 patients). Patients who underwent a switch to BIC/FTC/TAF had had various different ART regimens, most frequently 2 nucleos(t)ide reverse transcriptase inhibitors (NRTIs) + INSTI (46.2%) (Table 2). Simplification of treatment was the most frequent reason for switch (79%), followed by immunological/virological failure (25%), and the presence of comorbid diseases (21%) (Figure 3). Change of treatment due to tolerability issues was recorded as the reason for switch in 8.8% of the patients. For an equal proportion (48.5%) of patients undergoing a switch to BIC/FTC/TAF, the physician reported one or two reasons for this change. Treatment switch was generally well tolerated, with 2 patients reporting an adverse event, i.e., self-limited diarrhea (n=1) and nausea (n=1).
Table 2.
Distribution of ARV regimens prior to the switch to BIC/FTC/TAF
| ARV regimen | N (%) |
|---|---|
| 2NRTIs + INSTI | 42 (46.2%) |
| 2NRTIs + PI/c or PI/r | 26 (28.6%) |
| 2NRTIs + PI/c or PI/r + INSTI | 6 (6.6%) |
| 2NRTIs + NNRTI | 2 (2.2%) |
| NRTI + PI/r + INSTI | 2 (2.2%) |
| NNRTI + PI/r | 2 (2.2%) |
| NNRTI + PI/r + INSTI | 2 (2.2%) |
| Other | 7 (7.7%) |
Missing: n=2; % are calculated from the total number of treatment-experienced patients, n=91.
Other included: CCR5 inhibitor + INSTI, CCR5 inhibitor + NRTI + PI/r, 2NRTIs + NNRTI + INSTI, NNRTI + INSTI, PI/r + 3 NRTIs.
CCR5 – C-C chemokine receptor type 5; BIC/FTC/TAF – bictegravir/emtricitabine/tenofovir alafenamide; INSTI – integrase strand transfer inhibitor; NRTI – nucleos(t)ide reverse transcriptase inhibitor; NNRTI – non-nucleoside reverse transcriptase inhibitor; PI – protease inhibitor; /c – pharmacologically boosted with cobicistat; /r – pharmacologically boosted with ritonavir.
Figure 3.
Reasons for switching to BIC/FTC/TAF
It was possible to provide more than one answer.
BIC/FTC/TAF - bictegravir/emtricitabine/tenofovir alafenamide.
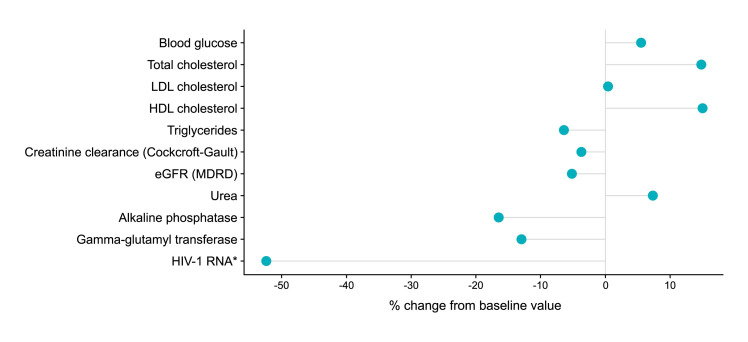
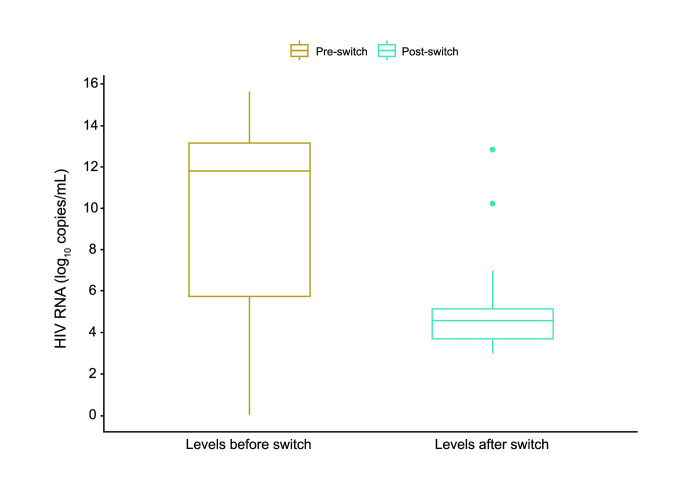
After initiation of BIC/FTC/TAF (first-line or switch), the mean ± SD follow-up duration until data cut-off for this analysis was 101.6 ± 64.2 days for the entire study group. We identified no statistically significant changes in liver function, renal function, lipid values, blood glucose, other biochemical parameters during follow-up compared to baseline values (Figure 4 and Table 3). HIV viral load assessment was repeated during the available follow-up period in 21 of the 74 patients with detectable viral load at baseline, and a statistically significant decrease was noticed (difference between median values of 7.2 log10 copies/mL), with a large effect size (r=0.57, V=23, p=0.001) (Figure 5).
Figure 4.
Difference between pre- and post- BIC/FTC/TAF initiation, expressed as percentage of baseline median value
*In a separate Wilcoxon signed-rank test that included 21 patients with complete data in both pre- and post- setting, the change from baseline was statistically significant (V=23, p=0.001) with Δ median value (% from baseline median value) = 7.2 log10 copies/mL (61%) and a large effect size, r=0.57.
BIC/FTC/TAF – bictegravir/emtricitabine/tenofovir alafenamide; eGFR – estimated glomerular filtration rate; HDL – high-density lipoprotein; HIV-1 RNA – human immunodeficiency virus-1 ribonucleic acid; LDL – low-density lipoprotein; MDRD – modification of diet in renal disease.
Table 3.
Changes in laboratory values from baseline (prior to BIC/FTC/TAF initiation) to last follow-up prior to data cut-off
| Parameter | Baseline | Current | Change (Δ median) | Statistical analysis | Effect size | |||
|---|---|---|---|---|---|---|---|---|
| N | Median | N | Median | Statistic test | P value | |||
| Random blood glucose, mg/dL | 122 | 99.0 | 26 | 104.5 | 5.5 | 167.5* | 0.904 | Small (r=0.08) |
| Total cholesterol, mg/dL | 119 | 192.0 | 22 | 220.5 | 28.5 | 1.39(20)** | 0.180 | Small (Cohen’s d=0.30) |
| LDL-cholesterol, mg/dL | 106 | 120.5 | 19 | 121.0 | 0.5 | -0.12(17)** | 0.906 | Small (Cohen’s d=0.03) |
| HDL-cholesterol, mg/dL | 109 | 40.0 | 17 | 46.0 | 6.0 | 103* | 0.218 | Small (r=0.17) |
| Triglycerides, mg/dL | 119 | 141.0 | 21 | 132.0 | -9.0 | 86* | 0.490 | Small (r=0.05) |
| Creatinine clearance (Cockcroft-Gault), mL/min | 75 | 113.1 | 18 | 109.0 | -4.2 | 0.77(16)** | 0.451 | Small (Cohen’s d=0.19) |
| eGFR (MDRD), mL/min/1.73 m2 | 121 | 89.1 | 27 | 84.5 | -4.6 | -0.6(25)** | 0.552 | Small (Cohen’s d=0.12) |
| Urea, mg/dL | 122 | 34.0 | 28 | 36.5 | 2.5 | 229.5* | 0.073 | Small (r=0.09) |
| Alkaline phosphatase, U/L | 101 | 73.0 | 13 | 61.0 | -12.0 | 34* | 0.442 | Small (r=0.12) |
| Gamma-glutamyl transferase, U/L | 119 | 27.0 | 24 | 23.5 | -3.5 | 101* | 0.626 | Small (r=0.01) |
| HIV-1 RNA, log10 copies/mL | 21 | 5.1 | 21 | 1.99 | -3.1 | 23* | 0.001 | Large (r=0.57) |
Wilcoxon signed rank test statistic: V-statistic
Paired t-test statistic: t-statistic(df)
eGFR – estimated glomerular filtration rate; HDL – high-density lipoprotein; LDL – low-density lipoprotein; MDRD – modification of diet in renal disease.
Figure 5.
Changes in viral load (log10 copies/mL) from initiation of BIC/FTC/TAF to data cut-off in patients with data recorded pre- and post-switch
BIC/FTC/TAF – bictegravir/emtricitabine/tenofovir alafenamide; HIV-1 RNA – human immunodeficiency virus-1 ribonucleic acid.
During the duration of the available follow-up, we identified no events of special interest such as liver-related or kidney-related adverse events, significant increases in serum lipid or glucose parameters, immune reconstitution inflammatory syndrome (IRIS), autoimmune reactions, or osteonecrosis. We noted a low occurrence rate of adverse events labelled as frequent in the summary of product characteristics, such as: headache (0%), diarrhea (1.1%), nausea (1.1%), dizziness (0%), fatigue (2.2%), depression (0%), abnormal dreams (0%). None of the patients discontinued BIC/FTC/ TAF and the overall tolerability was good.
Discussion
With the current access to highly active antiretroviral therapy, the majority of PLWH may achieve and maintain virological suppression and immune control. Timely diagnosis and ART initiation, coupled with the efficacy and safety of modern ART regimens, have led to improvement of life expectancy and an overall shift of treatment objectives from simple virological suppression to virological suppression with improved quality of life. PLWH now live longer and develop age-related comorbidities, with specific therapeutic and monitoring needs. However, late presenters still represent an important share of newly-diagnosed cases (two-thirds of the treatment-naïve patients in our analysis), and may pose important therapeutic management issues. Optimization of treatment algorithms in this patient population is possible provided that novel ARV combinations are available, with good efficacy and safety profiles and good tolerability.
This is the first analysis of data for PLWH receiving BIC/FTC/TAF in real world settings in two large HIV centers in Romania, following the recent introduction of this FDC into clinical practice in Romania. Most of the patients treated with this FDC were ARV-experienced (75%), and less than half of them were fully virologically suppressed at baseline while almost one quarter (22%) had high pre-switch HIV viral loads suggestive of virological failure. Simplification of the treatment regimen was the top reason mentioned by clinicians initiating the switch, but virological or immunological failure was also an important contributing reason in 25% of cases.
Our results reinforce the effectiveness of BIC/FTC/TAF in our cohort of PLWH in general and particularly in the subgroup of PLWH who had highly replicating HIV infection at baseline. A large share of the patients from our analysis (35%) belonged to the Romanian pediatric HIV cohort. These patients are, by definition, multi-experienced to ART, as they have lived with HIV infection since early childhood,22 and have experienced multiple episodes of therapeutic success and failure, as well as multiple challenges in terms of adherence to older ART with a high pill burden.19
In this real word data analysis, we found a low frequency of adverse events in patients receiving BIC/FTC/TAF, and specifically no cases of adverse events of special interest, as defined in the summary of product characteristics. These data are consistent with results from clinical trials and the BICStar observational cohort reporting high suppression rates in patients treated with BIC/FTC/TAF FDC.23
Although the sample size for this analysis was relatively low, and the duration of follow-up was not uniform, the results may already inform clinicians on the expected real-world outcomes. As in other therapeutic areas, the COVID-19 pandemic had reduced to a certain extent the number of medical visits and the frequency of viro-immunological monitoring was consequently impacted. Limitations are inherently related to the retrospective nature of the analysis, the inability to measure relevant laboratory parameters at standardized time points and in all patients initiating BIC/FTC/TAF, and the fact that the data come from two infectious disease clinics in Romania which might have different protocols in place for periodic monitoring and for assessing the occurrence of adverse events. We also acknowledge that the number of reported adverse events is low, a difference that probably stems from the patient’s or the clinician’s interpretation of whether or not a reported sign or symptom should be classified as an adverse event, interpretation which is not present in clinical trials, where any untoward medical occurrence is recorded and reported. The current analysis is further limited by the fact that data on resistance testing were not routinely collected for treatment-experienced patients who had high viral loads suggestive of virological failure at the time of the switch and that we do not have information on the baseline rate of INSTI resistance.
The treatment landscape in Romania is complex and sometimes challenging for infectious disease specialists. This report on one of the most recent single tablet regimens introduced in clinical practice provides the first highlights of its efficiency and tolerability in a local patient population and supports BIC/FTC/TAF as a valid treatment option in PLWH with different ART history, comorbidity burden, or viral suppression levels.
Conclusions
This retrospective analysis supports the effectiveness and safety of treatment with BIC/FTC/TAF in PLWH irrespective of their disease continuum and can inform clinical practice regarding real-world expected outcomes of treatment with BIC/FTC/TAF.
Footnotes
Authors’ contributions statement: All authors had equal contributions. All authors read and approved the final version of the manuscript.
Conflicts of interest: All authors – none to declare.
Funding: Medical writing support was provided by Raluca Voicu, MD of MedInteractiv Plus and was funded by Gilead Sciences Europe Ltd and Neola Pharma Romania, which had no involvement in the data collection, drafting of the manuscript, analysis and interpretation of the data, and the decision to submit for publication. They had only administrative roles in providing financial support for the statistical analysis and medical writing activities.
References
- 1.Chen IW, Sun HY, Hung CC. Meta-analysis of efficacy and safety of coformulated bictegravir, emtricitabine, and tenofovir alafenamide among people living with HIV. Infect Dis Ther. 2021;10:1331–46. doi: 10.1007/s40121-021-00449-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shin YH, Park CM, Yoon CH. An overview of human immunodeficiency virus-1 antiretroviral drugs: general principles and current status. Infect Chemother. 2021;53:29–45. doi: 10.3947/ic.2020.0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clotet B, Feinberg J, van Lunzen J, et al. Once-daily dolutegravir versus darunavir plus ritonavir in antiretroviral-naive adults with HIV-1 infection (FLAMINGO): 48 week results from the randomised open-label phase 3b study. Lancet. 2014;383:2222–31. doi: 10.1016/S0140-6736(14)60084-2. [DOI] [PubMed] [Google Scholar]
- 4.Molina JM, Lamarca A, Andrade-Villanueva J, et al. Efficacy and safety of once daily elvitegravir versus twice daily raltegravir in treatment-experienced patients with HIV-1 receiving a ritonavir-boosted protease inhibitor: randomised, double-blind, phase 3, non-inferiority study. Lancet Infect Dis. 2012;12:27–35. doi: 10.1016/S1473-3099(11)70249-3. [DOI] [PubMed] [Google Scholar]
- 5.Sax PE, DeJesus E, Mills A, et al. Co-formulated elvitegravir, cobicistat, emtricitabine, and tenofovir versus co-formulated efavirenz, emtricitabine, and tenofovir for initial treatment of HIV-1 infection: a randomised, double-blind, phase 3 trial, analysis of results after 48 weeks. Lancet. 2012;379:2439–48. doi: 10.1016/S0140-6736(12)60917-9. [DOI] [PubMed] [Google Scholar]
- 6.Gallant J, Lazzarin A, Mills A, et al. Bictegravir, emtricitabine, and tenofovir alafenamide versus dolutegravir, abacavir, and lamivudine for initial treatment of HIV-1 infection (GS-US-380-1489): a double-blind, multicentre, phase 3, randomised controlled non-inferiority trial. Lancet. 2017;390:2063–72. doi: 10.1016/S0140-6736(17)32299-7. [DOI] [PubMed] [Google Scholar]
- 7.Sax PE, Pozniak A, Montes ML, et al. Coformulated bictegravir, emtricitabine, and tenofovir alafenamide versus dolutegravir with emtricitabine and tenofovir alafenamide, for initial treatment of HIV-1 infection (GS-US-380-1490): a randomised, double-blind, multicentre, phase 3, non-inferiority trial. Lancet. 2017;390:2073–82. doi: 10.1016/S0140-6736(17)32340-1. [DOI] [PubMed] [Google Scholar]
- 8.Daar ES, DeJesus E, Ruane P, et al. Efficacy and safety of switching to fixed-dose bictegravir, emtricitabine, and tenofovir alafenamide from boosted protease inhibitor-based regimens in virologically suppressed adults with HIV-1: 48 week results of a randomised, open-label, multicentre, phase 3, non-inferiority trial. Lancet HIV. 2018;5:e347–56. doi: 10.1016/S2352-3018(18)30091-2. [DOI] [PubMed] [Google Scholar]
- 9.European AIDS Clinical Society. EACS Guidelines 2021 (version 11.0) [Accessed on: 10 November 2021]. Available at: https://www.eacsociety.org/media/final2021eacsguidelinesv11.0_oct2021.pdf.
- 10.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents Living with HIV. Department of Health and Human Services, 2021. [Accessed on: 10 November 2021]. Available at: https://clinicalinfo.hiv.gov/sites/default/files/guidelines/documents/AdultandAdolescentGL.pdf.
- 11.World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection, 2nd edition. 2016. [Accessed on: 10 November 2021]. Available at: https://www.who.int/hiv/pub/arv/arv-2016/en/
- 12.Raffi F, Esser S, Nunnari G, Pérez-Valero I, Waters L. Switching regimens in virologically suppressed HIV-1-infected patients: evidence base and rationale for integrase strand transfer inhibitor (INSTI)-containing regimens. HIV Med. 2016;17(Suppl 5):3–16. doi: 10.1111/hiv.12440. [DOI] [PubMed] [Google Scholar]
- 13.Biktarvy authorization details in Europe. [Accessed on: 25 November 2021]. Available at: https://www.ema.europa.eu/en/medicines/human/EPAR/biktarvy.
- 14.Biktarvy Summary of Product Characteristics, 2021. [Accessed on: 25 November 2021]. Available at: https://www.ema.europa.eu/en/medicines/human/EPAR/biktarvy#product-information-section.
- 15.Molina JM, Ward D, Brar I, et al. Switching to fixed-dose bictegravir, emtricitabine, and tenofovir alafenamide from dolutegravir plus abacavir and lamivudine in virologically suppressed adults with HIV-1: 48 week results of a randomised, double-blind, multicentre, active-controlled, phase 3, non-inferiority trial. Lancet HIV. 2018;5:e357–65. doi: 10.1016/S2352-3018(18)30092-4. [DOI] [PubMed] [Google Scholar]
- 16.Maggiolo F, Rizzardini G, Molina JM, et al. Bictegravir/emtricitabine/tenofovir alafenamide in virologically suppressed people with HIV aged ≥65 years: week 48 results of a phase 3b, open-label trial. Infect Dis Ther. 2021;10:775–88. doi: 10.1007/s40121-021-00419-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Workowski K, Orkin C, Sax P, et al. Four-year outcomes of B/F/TAF in treatment-naïve adults. Poster presented at virtual CROI 2021, March 6-10, 2021, P# 2268
- 18.Rockstroh JK, Molina JM, Post F, et al. Long-term follow-up after a switch to bictegravir, emtricitabine, tenofovir alafenamide (B/F/TAF) from a boosted protease inhibitor-based regimen. Poster presented at Glasgow 2020 virtual meeting, 5-8 October 2021, P# 036. [Accessed on: 10 November 2021]. Available at: https://www.natap.org/2020/GLASGOW/GLASGOW_62.htm.
- 19.Streinu-Cercel A, Săndulescu O, Poiană C, et al. Consensus statement on the assessment of comorbidities in people living with HIV in Romania. Germs. 2019;9:198–210. doi: 10.18683/germs.2019.1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Streinu-Cercel A. Forty years of HIV. Germs. 2021;11:146. doi: 10.18683/germs.2021.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gokengin D, Oprea C, Begovac J, et al. HIV care in Central and Eastern Europe: How close are we to the target? Int J Infect Dis. 2018;70:121–30. doi: 10.1016/j.ijid.2018.03.007. [DOI] [PubMed] [Google Scholar]
- 22.Streinu-Cercel A, Săndulescu O, Ceapraga G, et al. Prevalence of osteo-renal impairment in the Romanian HIV cohort. BMC Infect Dis. 2016;16(Suppl 1):93. doi: 10.1186/s12879-016-1397-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spinner CD, Stoehr A, Wong A, et al. Starting or switching to bictegravir/emtricitabine/ tenofovir alafenamide (B/F/TAF) in clinical practice: Pooled 12-month results from the global BICSTaR study. Poster presented at Glasgow 2020 virtual meeting, 5-8 October 2021, P# 046. [Accessed on: 10 November 2021]. Available at: https://www.natap.org/2020/GLASGOW/GLASGOW_62.htm.