Abstract
Purpose
To report 3 otherwise healthy patients with Herpes zoster reactivation shortly after administration of a mRNA vaccine against the novel COVID-19 virus.
Observations
Patient 1 is a 54 year old who presented with Herpes zoster meningitis complicated by enhancing nodular leptomeningeal lesions of the spinal cord. The subsequent two patients had Herpes zoster ophthalmicus of the cornea (Case 2) and eyelid (Case 3). All three presented within 2 weeks of receiving the Pfizer/BioNTech COVID-19 vaccine.
Conclusions
Herpes zoster may be a side effect of m RNA vaccination against the Sars-CoV2 vaccine and requires further investigation.
Keywords: Herpes zoster, COVID-19 mRNA vaccine, Vaccination, Adverse reactions
1. Introduction
In December 2019, a number of lower respiratory infections were first detected in Wuhan, China. Initially characterized as a “pneumonia of unknown etiology”, the disease was later attributed to a novel form of the coronavirus (2019-nCoV, “COVID-19”). Since then, the virus has rapidly spread on a global scale causing illnesses ranging from common cold symptoms to severe respiratory distress requiring mechanical ventilation to death. To date, around the world, there have been over 243 million confirmed cases of COVID-19 including 4.9 million deaths.1
With the rapidly rising number of worldwide cases caused by the novel coronavirus, there was great impetus to quickly develop a vaccine. Companies from around the world began working on a vaccine in early 2020. Given relatively high rates of morbidity and mortality from Covid-19, vaccine development needed to be accelerated including the time to conceptualize an approach, formulate the vaccine, and then perform large placebo controlled clinical trials-a process that has typically taken 10–15 years. Yet, by December 2020, a vaccine against the Sars-CoV-2 virus was developed by Pfizer and BioNTech and became the first fully-tested immunization to be approved for emergency use. The rapidity of the novel vaccine was in part due to years of previous research developing new mRNA vaccines against related coronaviruses such as SARS and MERS, multinational cooperation, large influxes of funding from both public and private sources, and expedited approvals through emergency-use regulations. Since the initial Pfizer/BionTech vaccine, there has been 1 other mRNA vaccine (Moderna) and 1 adenovirus vector vaccine (Johnson & Johnson/Janssen) that have undergone Phase 3 clinical trial testing and approved for emergency use in the United States. To date, over 109 million, 92 million, and 7 million doses of Pfizer, Moderna, and J&J vaccines have been administered in the United States respectively.2
Under the watchful eye of federal establishments such as the Centers For Disease Control (CDC) and the Food and Drug Administration (FDA), over 414 million doses of COVID-19 vaccine have been administered in the United States alone. From the onset, there were a number of well-known side effects associated with the vaccines including injection site pain, fatigue, myalgias, nausea, fever, chills, all of which are generally self-limited and associated with a number of other vaccines. However, anaphylaxis and venous thrombosis have also been documented to be potentially more serious side effects associated with vaccination.3 On April 15,2021, the J&J vaccine was temporarily placed on ‘pause’ by the CDC and FDA in order to further investigate reports of associated thromboses. Similar clotting issues have not been reported with relation to the 2 mRNA vaccines.
To our knowledge, there has only been one published report of ocular side effects from the Covid-19 vaccine.4 A report from Israel by Furer et al. documented six patients with herpes zoster following the vaccination with the Pfizer/BioNTech mRNA vaccine.4 In that case series, patients with autoimmune inflammatory rheumatic diseases (AIIRD) such as rheumatoid arthritis, undifferentiated connective tissue disorder, vasculitis, and myositis were noted to develop Herpes zoster infection shortly after vaccination. One patient in their case series developed Herpes zoster ophthalmicus, without corneal involvement, and there were otherwise no cases of disseminated herpes zoster disease.
Herein we report three cases of patients not on immunosuppressive therapy who presented with Herpes zoster after Covid-19 vaccination.
2. Case 1
A 54 year-old healthy male patient received the first dose of the Pfizer/BioNTech mRNA vaccine and started to experience severe headache, fever and chills the next day. Three days later, after severe worsening of symptoms, the patient presented to the emergency room and was later discharged with a presumed diagnosis of a viral illness. The patient subsequently collapsed the next day at home and was readmitted to the hospital for further workup which included CT, MRI and MRA of the brain in order to exclude a brain bleed. Images were all negative. Two days later, the patient underwent a spinal tap due to progressively worsening symptoms and the CSF analysis revealed 750 white blood cells per mm3 with lymphocytic predominance. Herpes zoster PCR was positive in the CSF, with an otherwise negative meningitis-encephalitis panel. High dose intravenous antivirals were immediately initiated for three days followed by 10 days of oral acyclovir (2000 mg twice a day).
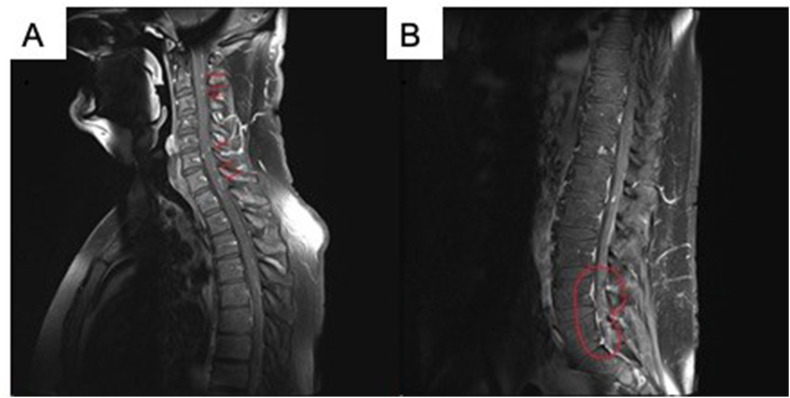
The patient followed up at our institution soon after hospital discharge and completion of the oral antiviral regimen. Follow-up testing included an MRI of the brain and total spine imaging nine days later, which revealed multiple small enhancing leptomeningeal nodules throughout the dorsal cervical and thoracic spinal cord, along with abnormal enhancement involving the cauda equina nerve roots (see Fig. 1). The differential diagnosis of neuro-sarcoid, disseminated leptomeningeal metastasis, or infection was raised by the scan. Evaluation with CT of chest, abdomen, and pelvis were negative for lymphadenopathy or neoplasm. A follow-up CSF study showed 200 WBC's with 89% lymphs, and negative herpes zoster PCR. A follow up MRI of the spine 25 days later showed dramatic resolution of the leptomeningeal nodules. The patient has since returned back to his baseline 6 weeks after initial diagnosis of VZV meningitis.
Fig. 1.
Case 1-Herpes zoster Meningitis. MRI of the spine taken after diagnosis of VZV Meningitis showing enhancing lepto-meningeal nodules.
3. Case 2
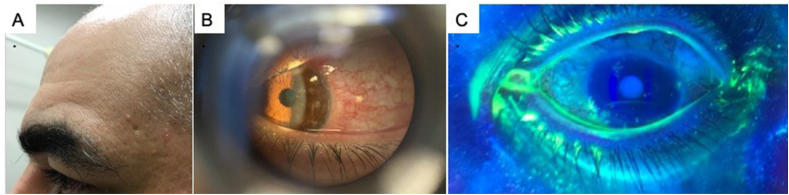
A 46 year-old patient with controlled hypertension and asthma was referred for a left corneal abrasion by an outpatient facility. On examination the patient reported discomfort of the left eye 1 day after the Pfizer/BioNTech vaccine. Discomfort was associated with redness, discharge, and tearing. Over the next 4 days, the left eye symptoms progressively worsened necessitating a visit to an emergency walk-in facility where he was diagnosed with a corneal abrasion, started on fluoroquinolone eye drops, and referred to our practice. In the office, no abrasion was seen. Rather, there were several discrete small corneal infiltrates seen, that mildly stained for fluorescein. The patient was not a contact lens wearer, reported no trauma or recent swimming pool exposure. The patient was prescribed topical antibiotics and discharged from the office. The patient presented to the emergency room the very next day with worsened left eye redness, photophobia, blurred vision along with left sided head pain, scalp tenderness, and a new vesicular rash in the V1 dermatomal distribution (Fig. 2a). The corneal infiltrates were again seen with evidence of pseudodendritic fluorescein staining (Fig. 2b and c). The diagnosis of Herpes zoster ophthalmicus (HZO) was made and the patient was discharged on oral valacyclovir and topical gancyclovir gel. At the most recent follow up visit, 2 weeks after initial diagnosis, the patient had improved subjective symptoms but with persistent corneal infiltrates.
Fig. 2.
Case 2-Herpes zoster ophthalmicus. A. Small mildly erythematous vesicular lesions seen on the scalp in V1 dermatomal distribution. B. Slit lamp showing white corneal infiltrates. C. Fluorescein staining reveals mild uptake in areas of corneal opacity along with possible inferior pseudo-dendrite.
4. Case 3
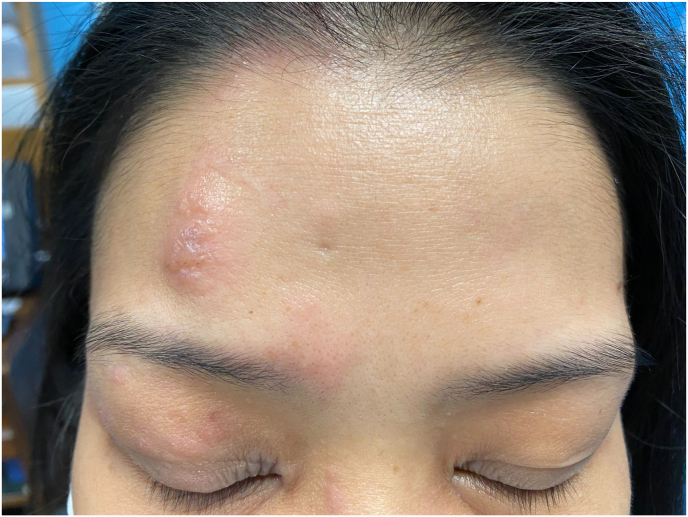
A 34 year-old female nurse with past medical history of eczema, not on immunosuppressives, referred herself to our practice for a possible conjunctivitis of the right eye two weeks after receiving the first dose of her Pfizer/BioNTech COVID-19 vaccination. She first noted a right sided skin rash above her eyelid five days prior to presentation. At the time she covered the lesion with a band-aid thinking that it was related to her history of eczema. However, when the band-aid was removed during her clinical exam, she was noted to have a typical V1 rash of Herpes zoster with a marginal upper lid lesion as well (Fig. 3). Her cornea was clear without evidence of dendritic/pseudodendritic lesions. The diagnosis of HZO was made and the patient was started on antivirals.
Fig. 3.
Case 3-Herpes zoster ophthalmicus. Raised erythematous vesicular lesions in a V1 dermatomal distribution.
5. Discussion
The Covid-19 pandemic has caused worldwide morbidity and mortality. The race to fight the pandemic has been a global one with collaboration among many laboratories to create an effective vaccine. To that end, over 6 vaccines have been developed worldwide that have been highly effective in reducing disease burden, hospitalizations and death.
In this case series, we report 3 patients with Herpes zoster after administration of the first dose of the Pfizer/BioNTech COVID-19 vaccine. The onset of Herpes zoster was between a few days to two weeks after vaccination. In the first case, tissue diagnosis by PCR in a very severe case of meningeal zoster was made. This case was notable for the rare finding of enhancing nodular lesions involving the spinal cord leptomeninges. The subsequent two patients had Herpes zoster ophthalmicus with clinical manifestations mainly involving the cornea (Case 2) and eyelid (Case 3). While the third case was a young woman with eczema, an immune mediated condition, none of the three patients were on immunosuppressive therapy nor had otherwise contributory medical history.
This case series is the third such published report of Herpes zoster reactivation following the vaccination with the Pfizer/BioNTech mRNA vaccine. In contrast to our case series, all six patients in the series published by Furer et al. were on immunosuppressive therapy for some form of autoimmune inflammatory rheumatic disease (AIIRD). The patients all developed typical Herpes zoster skin rashes 2 days to 2 weeks after receiving the vaccine. Only one patient in that series developed Herpes zoster ophthalmicus, and the overall prevalence of Herpes zoster corresponded to 1.2% in patients with AIIRD compared to none in controls.4 Mechanistically they propose that activation of the virus is a result of both an altered CD4+ and CD8+ cellular response, as well as a dysregulated innate immune response through toll-like receptors.4 The association between immunosuppression and ocular morbidity still needs to be better understood, as while immunosuppression on the one hand increases viral loads and drives inflammation, as the eye is a region of immunoprivilege, immunosuppression may also work to dampen any hyperinflammatory response.5
There was a recent publication noting Herpes zoster ophthalmicus in four patients with Covid-19 infection.6 There was also a recent report of Herpes zoster reported after vaccination (one patient),7 and an additional one of two patients where one of the two was on immunosuppressants.8
Although the causal link between the Covid vaccine and Herpes zoster cannot be considered definitive based on the cases presented here or in conjunction with the series published by Furer et al. given the small sample size of this study and relatively rapid onset of symptoms in Cases 1 and 2 following vaccine administration, further investigation is certainly warranted. In the United States, vaccine-related adverse events are reported through the Vaccine Adverse Event Reporting System (VAERS) (https://vaers.hhs.gov/). It should be noted that even though the current repository of vaccine side effects may not include cases such as those presented here, since most patients and doctors have not recognized this as a possible complication or side effect of the vaccine, the VAERS system accepts reports even if a causative link has not been proven.
While we acknowledge that Herpes zoster is a common infection worldwide and cannot exclude the possibility that its co-occurrence is purely coincidental, manifestations of the Herpes virus can be devastating. In 2018, the American Academy of Ophthalmology updated its guidelines6 to recommend the recombinant zoster vaccine (RZV; Shingrix, Glaxo-SmithKline, Philadelphia, PA) to all eligible immunocompetent adults over the age of 50, such as Case 1 presented here.9 Therefore, it is not only important for providers to encourage all patients over the age of 50 to receive the Shingles vaccine, but also to remain vigilant for this condition in those who have recently received the COVID-19 vaccine.
6. Conclusions
This is the second known case series of Herpes zoster shortly after COVID-19 vaccine administration. While the few number of cases presented here is not enough to prove a definite causality between the two events, there is need for monitoring and reporting of potential side effects of the novel mRNA vaccines to ensure the safety of all our patients.
Patient consent
Written informed consent was obtained from patients for publication of these case reports and any accompanying images.
Funding
No funding or grant support.
Acknowledgements and Disclosures
The following authors have no financial disclosures: DRL, RR, EC, SLG.
References
- 1.WHO Coronavirus (COVID-19) Dashboard. https://covid19.who.int. Accessed 25 Oct. 2021.
- 2.CDC COVID data tracker.” Centers for disease Control and prevention. 28 Mar. 2020. https://covid.cdc.gov/covid-data-tracker
- 3.Interim clinical Considerations for Use of COVID-19 vaccines | CDC. 16 apr. 2021. https://www.cdc.gov/vaccines/covid-19/info-by-product/clinical-considerations.html
- 4.Furer Victoria, et al. Herpes zoster following BNT162b2 MRNA covid-19 vaccination in patients with autoimmune inflammatory rheumatic diseases: a case series.” rheumatology, no. keab345. Silverchair. Apr. 2021 doi: 10.1093/rheumatology/keab345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chau Charlene Y.C., et al. Ophthalmological considerations for COVID-19 vaccination in patients with inflammatory eye diseases and autoimmune disorders.” Ophthalmology and therapy, mar. Springer Link. 2021 doi: 10.1007/s40123-021-00338-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nofal, A, Fawzy, MM. HErpes zoster ophthalmicus in Covid-19 patients. Inter Jnl Derm, Vol 59, Issue 12, 1545-1546. [DOI] [PMC free article] [PubMed]
- 7.Bostan, E, Yalici-Armagan B. HErpes zoster following inactivated Covid-19 vaccine: a coexistence or coincidence? Jnl Cosmetic Derm. Vol 20, Issue 6, 1566-1567. [DOI] [PubMed]
- 8.Van Dam C.S., Lede I., Schaar J., et al. Herpes zoster after COVID vaccination. Inter Soc Infec Diseases. Aug 2021 doi: 10.1016/j.ijid.2021.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Policy Statement Recommendations for herpes zoster vaccine for patients 50 years of age and older. Ophthalmology. 2018;125:1813–1816. [Google Scholar]