Abstract
Oxidative stress is a main cause of tissue damage and highly associated with incidence of human chronic diseases. Among the major target organs attacked by reactive oxygen species (ROS) is the liver. Protocatechuic acid (PCA) is a phenolic compound found in green tea, acai oil and some mushroom species that possesses strong antioxidative and anti-inflammatory activity and may have benefits as a natural phytochemical for prevention of human diseases. However, the protective effect of PCA on hydrogen peroxide (H2O2)-induced oxidative stress specifically in the liver has not yet been investigated. The current study aims to observe if PCA possesses protective activity against H2O2-induced oxidative stress in HepG2 human liver cancer cells. Relative to untreated control cells, treatment of HepG2 cells with PCA reduced H2O2-induced cell death and mitigated H2O2-induced production of ROS; furthermore, it mitigated the H2O2-induced increase of caspase-3/7 enzyme activity, expression of cleaved poly(ADP-ribose) polymerase (PARP), expression of endoplasmic reticulum (ER) stress genes including activating transcription factor 4 (ATF4), serine/threonine-protein kinase/endoribonuclease inositol-requiring enzyme 1 α (IRE1α) and phosphorylation of p38 mitogen-activated protein kinases (MAPK). These findings indicate that PCA effectively protects hepatic cells from H2O2-induced oxidative stress and cell death.
Keywords: Protocatechuic acid, Hepatocytes, HepG2, Oxidative stress, Apoptosis
Graphical abstract
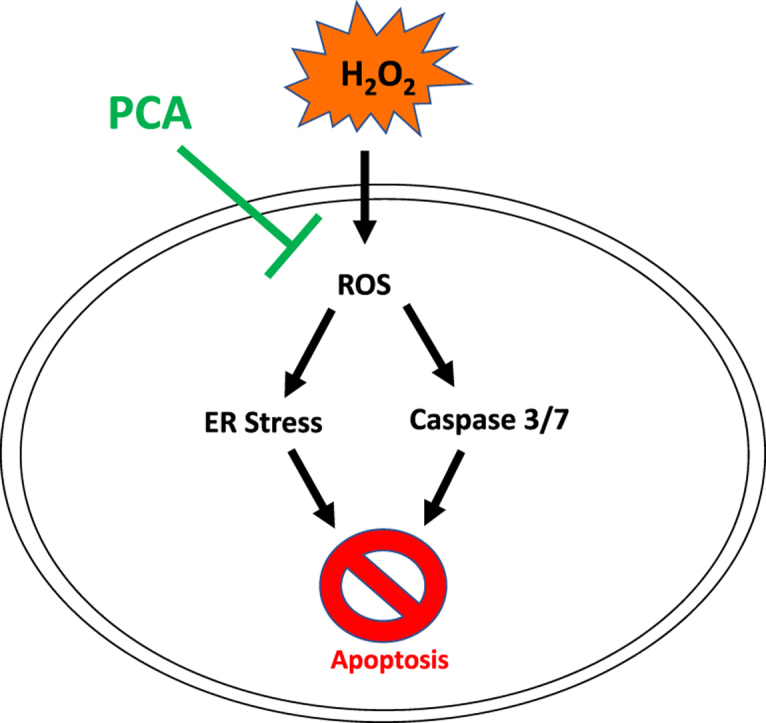
Highlights
-
•
Protocatechuic acid restored hydrogen peroxide-induced suppression of cell viability.
-
•
Protocatechuic acid mitigated hydrogen peroxide-induced generation of reactive oxygen species.
-
•
Protocatechuic acid mitigated hydrogen peroxide-induced apoptosis and endoplasmic reticulum stress.
1. Introduction
Oxidative stress is known to damage human tissues and is considered connected to many diseases including cancer (Halliwell, 2007), cardiovascular disease (Csanyi and Miller, 2014), and Alzheimer’s disease (Valko et al., 2007). While oxygen itself is essential for mitochondrial oxidative phosphorylation and cellular respiration, some of the oxygen derivatives produced during metabolism can negatively affect cells and tissues. For example, excessive levels of reactive oxygen species (ROS) such as hydrogen peroxide (H2O2) and superoxide can harm many tissues, inducing oxidative tissue damage that in turn causes pathological events. In particular, excessive oxidative stress can readily damage liver tissue and is linked to hepatic diseases such as fatty liver, liver fibro-proliferative diseases (Cichoz-Lach and Michalak, 2014), and hepatoma and hepatocarcinoma (Wang et al., 2016). To reduce oxidative stress and prevent associated diseases, food sources containing bioactive compounds and nutraceuticals with high antioxidant capacity are widely used. Phenols are well-known phytochemicals having strong antioxidative activity; in particular, phenolic compounds have been reported to contribute to liver protection by lowering oxidative stress and therefore reducing the risks of hepatic diseases (Saha et al., 2019).
Protocatechuic acid (3,4-dihydroxybenzoic acid, PCA) is a phenolic compound found in green tea; it is also abundant in acai oil (Pacheco-Palencia et al., 2008) and some mushroom species (Lee et al., 2008). PCA has been proven to have significant anti-oxidative and anti-inflammatory response in murine hepatocytes and to prevent hepatic toxicity (Liu et al., 2002) and liver cancer (Lin et al., 2007). And since many liver diseases are linked to excessive oxidative stress (Cichoz-Lach and Michalak, 2014), PCA potentially can be used in preventing and treating liver diseases as food ingredients and pharmacological sources. Especially, we expect that the diseases due to compromised H2O2 reduction in human body such as decreased expression of peroxisome can easily be treated with PCA. In addition, PCA’s antioxidant capacity can be linked to anti-inflammatory activity and the phenolic compound can be used to treat inflammation-related diseases such as neurodegenerative diseases (Kaewmool et al., 2020), coronary artery disease (Li et al., 2020a), and allergic asthma (Li et al., 2020b).
In this study, we investigated whether PCA possesses protective properties against H2O2-induced oxidative stress in HepG2 human hepatoblastoma cells.
2. Materials and methods
2.1. Materials
Human liver cancer cells (HepG2) were purchased from the American Type Culture Collection (Manassas, VA, USA). Antibodies for PARP (#9542), p-p38 (#9211), p-38 (#9212), ATF4 (#11815), and β-actin (#5125) were purchased from Cell Signaling Technology (Danvers, MA, USA). The Caspase-3/7 assay kit was purchased from Cayman Chemicals (Ann Arbor, MI, USA). All other chemical compounds and instruments were obtained from Fisher Scientific (Waltham, MA, USA).
2.2. Cell culture and treatment
HepG2 cells were incubated at 37 °C under a humid atmosphere with 5% CO2. The cells were cultured in Dulbecco’s modified Eagle’s media (DMEM) containing 10% fetal bovine serum (FBS), 1% penicillin, and 1% streptomycin. PCA was dissolved in dimethyl sulfoxide (DMSO) and diluted with culture media. The final concentration of DMSO in the media was limited to 0.1% (v/v).
2.3. Cell viability
HepG2 cells were plated in 96-well culture plates at a density of 5 × 103 cells/well. After one day of incubation, the cells were pre-treated with 0, 25, or 50 μM of PCA in culture media under 37 °C for 2 hours and then co-treated with 600, 800, or 1000 μM H2O2 in culture media containing 0, 25, or 50 μM of PCA for 24 hours at 37 °C. Afterwards, MTT solution (dye was dissolved in DMSO) was added to each well and the cells incubated for 2 hours at 37 °C. Finally, the optical density was measured at 540 nm using an ELISA microplate reader (Bio-Tek Instruments Inc., Winooski, VT, USA).
2.4. Western blot
HepG2 cells were plated in 60 mm culture plates at a density of 3 × 105 cells/plate and incubated at 37 °C for one day. Then, the cells were pre-treated with different concentrations (0, 25, and 50 μM) of PCA in the growth media for 2 h and subsequently co-treated with 800 μM H2O2 in growth media containing the same concentrations of PCA for a 24-h incubation at 37 °C. After incubation, the cells were washed twice with pre-chilled phosphate buffered saline (PBS) and lysed in in radio-immunoprecipitation assay (RIPA) buffer containing combined protease inhibitor and phosphatase inhibitor. To quantify protein in the lysates, bicinchoninic acid (BCA) assays were conducted according to the manufacturer’s manual. Next, the protein samples were mixed with 5X loading buffer, heat denatured, and separated by means of 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The separated proteins were then transferred to nitrocellulose transfer membranes and the membranes blocked with 5% skim milk in Tris-buffered saline with 0.1% Tween 20 (TBST). After washing, the membranes were saturated with primary antibody at 4 °C overnight, followed by saturation with secondary antibodies. Finally, chemiluminescent signal was generated using Pierce ECL Western blotting substrate and images developed with a ChemiDoc MP Image System (Bio-Rad, Hercules, CA, USA). Stripping buffer was used to rip antibodies from the membranes in order to bind different ones.
2.5. Analysis of ROS production
HepG2 cells were seeded at 1 × 105 cells/well in a 24-well culture plate. After one-day incubation at 37 °C, the cells were treated with 0, 25, or 50 μM of PCA in serum-free DMEM containing 25 μM of 2ʹ,7ʹ-dichlorofluorescin diacetate (DCFH-DA) for 1 h at 37 °C. Then, 800 μM H2O2 in Hanks’ Balanced Salt solution (HBSS) was added to the cells, followed by a 1-h incubation at 37 °C. The cells were washed with HBSS buffer, and fluorescence was detected with excitation at 485/20 and emission at 528/20 using an ELISA multiplate reader (Bio-Tek Instruments Inc). For visible images, fluorescent microscopy (ECLIPS Ti; Nikon, Melville, NY, USA) was used to detect green-colored cells.
2.6. Caspase activity assay
HepG2 cells were plated in a 24-well culture plate at a density of 5 × 104 cells/well and incubated at 37 °C for one day. Then, the cells were pre-treated with different concentrations (0, 25, or 50 μM) of PCA in growth media for 2 h, and subsequently treated with 800 μM H2O2 in growth media containing the same concentrations of PCA. After one-day incubation at 37 °C, the Caspase-3/7 assay kit was utilized according to the manufacturer’s instructions. First, bicinchoninic acid assays were conducted to evaluate the amount of protein in each lysate sample. Then, the lysates were placed in 96-well plates and the Caspase-Glo 3/7 reagent was added. After a 30-min incubation at 37 °C, sample luminescence was measured with a microplate reader. The measured activity was calibrated with reference to the total protein amount to identify activity per unit of protein.
2.7. Statistical analysis
The results were statistically analyzed using one-way analysis of variance (ANOVA). Differences between groups were assessed by Duncan’s multiple test, and p-values less than 0.05 were considered significantly different. Three independent replicates were analyzed, and the data are presented as means ± standard deviations.
3. Results
3.1. Effect of PCA on viability of HepG2
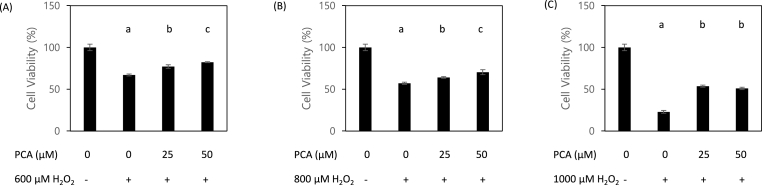
H2O2 is a well-known and broadly-used inducer of oxidative damage. We investigated the effect of PCA treatment on viability of HepG2 cells co-treated with H2O2. To determine the optimal degree of H2O2-induced oxidative stress, we first tested three concentrations of H2O2 (600, 800, and 1000 μM) and determined significant reduction of cell viability to occur in a dose-dependent manner. Co-treatment with PCA at doses of 25 and 50 μM significantly mitigated H2O2-induced reduction of cell viability for all three tested concentrations of H2O2 (Fig. 1A–C), with a dose-dependent response to PCA being observed in cells treated with 600 or 800 μM of H2O2. These findings indicate that PCA can protect liver cells from H2O2-induced repression of cell viability in a dose-dependent manner.
Fig. 1.
Protocatechuic acid (PCA) restored hydrogen peroxide (H2O2)-induced suppression of cell viability. A protective effect of PCA against oxidative stress was observed in HepG2 cells co-treated with H2O2 (600, 800, and 1000 μM) and PCA (0, 25, and 50 μM). Different letters indicate significant difference at p < 0.05.
3.2. Effect of PCA on ROS generation
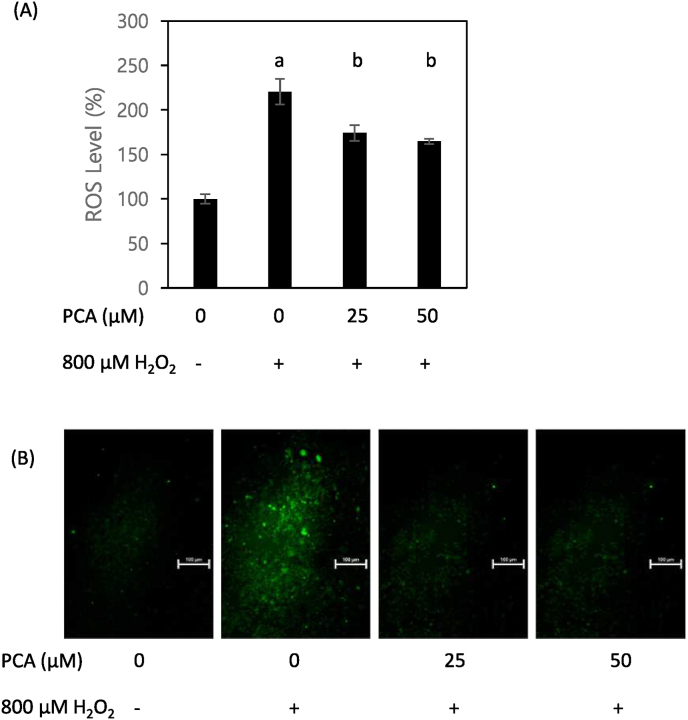
H2O2 is a well-known and broadly-used inducer of oxidative damage, which damage is mediated by the production of ROS. To examine if the H2O2-induced reduction of cell viability and its restoration under PCA co-treatment is associated with ROS, we measured the production and release of ROS by means of a fluorescence assay in HepG2 cells treated with H2O2 and various concentrations of PCA. The results indicate that PCA possesses effective antioxidant capacity, as ROS emission was significantly reduced in PCA-treated cells relative to the non-PCA (vehicle)-treated group (Fig. 2A). In addition, the greatest density of green fluorescent light was achieved in cells with no PCA treatment; that density was reduced when PCA was provided to the cells (Fig. 2B). These data suggest that the antioxidant capacity of PCA can effectively eliminate ROS produced by H2O2.
Fig. 2.
PCA mitigated H2O2-induced generation of reactive oxygen species (ROS). Emission from ROS (A) and intensity of fluorescent green color (B) were measured in HepG2 cells co-treated with H2O2 (800 μM) and PCA (0, 25, and 50 μM). Different letters indicate significant difference at p < 0.05. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
3.3. Effect of PCA on apoptosis
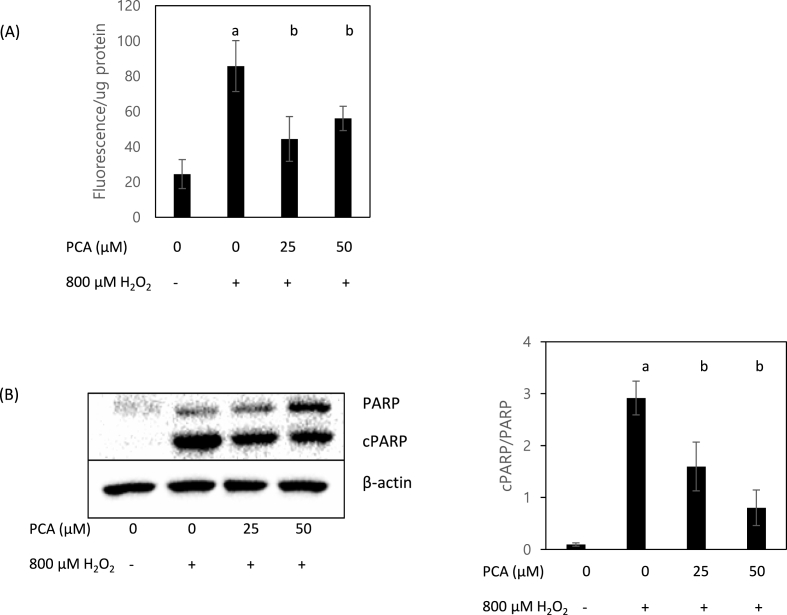
ROS play a central role in the regulation of cell signaling involved in apoptosis, which is a highly regulated cellular process usually held in tissue homeostasis (Redza-Dutordoir and Averill-Bates, 2016). Among the most critical enzymes involved in apoptosis are caspases; during apoptosis, caspases are activated and degrade various cellular compartments via reacting with cysteines in target proteins. Notably, oxidative stress induces apoptosis by activating caspase-3-dependent pathways (Carvour et al., 2008). Accordingly, we measured caspase-3/7 enzyme activity as a biomarker of apoptosis. As shown in Fig. 3A, when under H2O2-induced oxidative stress, caspase-3/7 activity per unit protein was significantly decreased in cells treated with PCA compared to those not so treated. In addition, the ratio of cPARP to PARP (a molecular marker of caspase-dependent apoptosis) decreased significantly in cells treated with PCA (Fig. 3B).
Fig. 3.
PCA mitigated H2O2-induced increase of caspase 3/7 enzyme activity. Activity of caspase-3/7 enzyme per unit protein (A) and expression of cleaved PARP (cPARP) (B) were measured in HepG2 cells co-treated with H2O2 (800 μM) and PCA (0, 25, and 50 μM). Different letters indicate significant difference at p < 0.05.
3.4. Effect of PCA on ER stress and other signal pathways
ER stress, also called the unfolded protein response (USR), is a response activated by the accumulation of improperly folded, unfolded, or misfolded proteins in the ER lumen. As such, an inflow of proteins that are not acceptable negatively affects ER function and ultimately causes apoptosis in living cells. ER stress is notable as being a cause of several hepatic diseases including hepatic steatosis, non-alcoholic fatty liver disease (NAFLD), and liver cancer (Liu and Green, 2019).
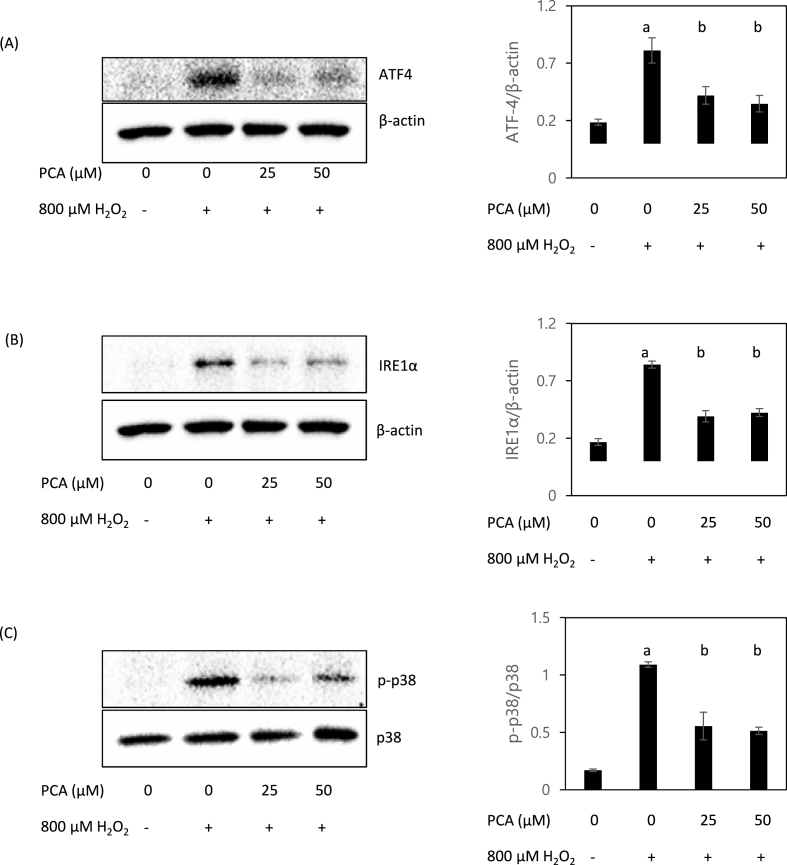
We measured ATF4 expression as a molecular marker of ER stress, and found ATF4 to be both highly induced by H2O2 treatment and significantly inhibited by PCA under the oxidative stress condition (Fig. 4A). Moreover, expression of IRE1α was also activated by H2O2 and the expression was significantly inhibited by PCA treatment (Fig. 4B). Since ER stress is also known to induce apoptosis via p38 MAPK in hepatic cells (Ren et al., 2021), we also measured the phosphorylation of p38. The ratio of phosphorylated p38 (activated form) to p38 was increased by H2O2 but decreased by co-treatment with PCA (Fig. 4C). This data indicates that PCA can effectively reduce ER stress and therefore suppress apoptosis and the associated tissue damage caused by ROS-induced oxidative stress.
Fig. 4.
PCA mitigated H2O2-induced expression of endoplasmic reticulum (ER) stress genes. Western blotting was performed to measure expression of ATF4 (A), IRE1α (B) and phosphorylation of p38 (C) in HepG2 cells co-treated with H2O2 (800 μM) and PCA (0, 25, and 50 μM). Different letters indicate significant difference at p < 0.05.
4. Discussion
Green tea is widely known to contain various kinds of bioactive compounds effective in preventing several diseases including cancer (Yang and Wang, 2010) and cardiovascular disease (Sumpio et al., 2006). In particular, green tea contains many phenolic compounds that are known to be strong antioxidants and have been used to treat diseases including hepatotoxicity. Recently, several studies have indicated the potential protective effect of PCA against many diseases (Kakkar and Bais, 2014), a property that can be associated with its antioxidant capacity. However, not much research has yet investigated the protective activity of PCA against H2O2-induced oxidative damage in hepatic cells specifically. It was evaluated that a plant extract containing PCA has been shown to be effective in relieving H2O2-induced oxidative in hepatic tissue by antioxidant enzymes (CAT and SOD-1) and HO-1/Nrf2 activation (Je and Lee, 2015). Also, PCA suppressed H2O2-induced oxidative damage in mice livers by enhancing Nrf2 expression (Ibitoye and Ajiboye, 2020). Therefore, we evaluated here the effects of PCA on H2O2-induced oxidative stress in HepG2 cells for the purpose of exploring the potential use of PCA in liver tissue protection.
Our results indicate that PCA significantly suppressed H2O2-induced apoptosis; furthermore, cell viability was preserved when cells under oxidative stress were pre- and post-treated with PCA. These findings indicate that the protective effect of PCA against apoptosis is a main mechanism of protection against cell death from oxidative stress. Although the inhibitory activity of 50 μM of PCA on apoptosis seemed to be greater than treatment with 25 μM in cells subjected to 600–800 μM H2O2, no dose-dependency was observed under 1000 μM H2O2-induced oxidative stress. It is possible that there was PCA’s anti-cancer activity in liver cancer cells, as proved in previous research (Yin et al., 2009). Therefore, we can conclude that PCA effectively inhibits H2O2-induced liver cell apoptosis, but research with non-cancer liver cells should be conducted to provide further evidence that PCA’s protective effect on liver is dose-dependent.
Caspases are enzymes that have key roles in cell apoptosis in many living organisms. Among the available therapies for diseases featuring abnormal apoptosis, such as Alzheimer’s disease, is the inhibition of caspase activity in a target organ so that abnormal cell death can be reduced. In our research, the expression of caspase-3/7 in cells under oxidative stress was significantly inhibited by PCA, although the effect was not dose-dependent. Therefore, we speculate that PCA can be an efficient material for treating hepatic diseases or hepatotoxicity caused by abnormal ROS.
PARP constitutes a family of proteins that catalyze DNA repair and stabilize and protect cells from external cytotoxic stimuli. Cleaved PARP is an inactive form that cannot repair damaged DNA; as such, the level of cPARP relative to PARP is under specific circumstances a good indication of failure of DNA repair capacity and severity of apoptosis and so can be used as a molecular marker of apoptosis. Our data suggests that the ratio of cPARP to PARP significantly decreased in cells co-treated with H2O2 and PCA. Consequently, it can be concluded that H2O2-induced oxidative stress makes DNA in hepatic tissue vulnerable to damage, and PCA promotes the preservation of PARP proteins. Since cleavage of PARP is connected to and activated by caspase-3 (Boulares et al., 1999), our data indicates that PCA’s inhibitive activity on H2O2-induced caspase-3 activity is also linked to preservation of PARP proteins.
Hydrogen peroxide is generated during the biochemical chain reaction of metabolism in mitochondria and degraded by the peroxisome in cells. However, excess H2O2 produces highly interactive chemicals called ROS, such as free radicals, that can damage DNA, proteins, and lipids (Kirkland, 1991) (Zhu et al., 2005). Therefore, eliminating ROS is critical for maintaining biological homeostasis; notably, dietary antioxidants have a significant role in the removal of free radicals. In cells exposed to H2O2-induced oxidative stress, the generated ROS interact with DCFH-DA and produce a specific fluorescent emission under the appropriate excitation. Here, this emission in Hep2G cells was significantly reduced when the cells were treated with PCA. ROS also produced green fluorescent light in H2O2-treated cells, and the intensity of the green color was reduced when PCA was provided to the cells. Therefore, it can be concluded that the antioxidant capacity of PCA efficiently scavenges ROS in hepatic cells.
The main functions of the ER are transporting proteins in cells and stabilizing proteins for their proper function. When unfolded or misfolded proteins are present in the ER, these functions are perturbed; accumulation of such proteins can thus lead to impaired ER function and ultimately cell death (Adams et al., 2019). Cellular ER stress caused by oxidative stress can be identified by quantifying ATF4 protein. In this study, ATF4 expression was highest in cells treated with H2O2 alone, while PCA significantly suppressed its expression and therefore contributed to cell protection. IRE1α is a sensor protein indicating the level of ER stress and a good indicator of predicting cellular fate under specific circumstances (Riaz et al., 2020). Therefore, investigating the activation of IRE1α is a critical factor in predicting cell apoptosis in tissues and organs. Moreover, IRE1α also protects hepatic tissues from hepatotoxicity and thus activation of the protein is a significant factor for measuring the level of hepatotoxicity in several hepatic diseases (Hur et al., 2012). In this research, we investigated the inhibitory activity of PCA on IRE1α activation. The activation level was highest in the cells treated with H2O2 only, and PCA treatment significantly suppressed the protein activation caused by the oxidative stress.
p38 is a mitogen-activated protein kinase that responds to stress stimuli. Proteins in the MAPK family are activated by phosphorylation, which is highly regulated; abnormally increased activation of p38 can be linked to apoptosis (Deng et al., 2008) (Bao et al., 2016). In Hep2G cells, we investigated the effect of PCA on the p38 MAPK pathway as reflected in the ratio of phosphorylated p38 to parent p38. The results indicate that expression of phosphorylated p38 in HepG2 cells is increased with H2O2 treatment, but that oxidation-induced expression is significantly suppressed by PCA. However, no difference was observed from phosphorylated ERK and JNK (data not shown). We speculate that p38 MAPK is significantly associated with apoptosis rather than others (Ji et al., 2010) (Yin et al., 2019) (Wu et al., 2006).
5. Conclusion
PCA protects hepatocytes from ROS-mediated apoptosis under oxidative stress. Our results propose potential use of PCA as a functional dietary ingredient for the prevention and treatment of hepatic diseases. Further research is needed to verify the benefits of PCA in diverse oxidative stress and inflammation related diseases such as cardiovascular diseases and allergies.
CRediT authorship contribution statement
Wu-Joo Lee: Methodology, Visualization, Formal analysis, Writing – original draft. Seong-Ho Lee: Conceptualization, Project administration, Supervision, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We thank Dr. Hee-Seop Lee for his technical support.
References
- Adams C.J., Kopp M.C., Larburu N., Nowak P.R., Ali M.M.U. Structure and molecular mechanism of ER stress signaling by the unfolded protein response signal activator IRE1. Front. Mol. Biosci. 2019;6:11. doi: 10.3389/fmolb.2019.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao H., Liu P., Jiang K., Zhang X., Xie L., Wang Z., Gong P. Huaier polysaccharide induces apoptosis in hepatocellular carcinoma cells through p38 MAPK. Oncol. Lett. 2016;12(2):1058–1066. doi: 10.3892/ol.2016.4686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulares A.H., Yakovlev A.G., Ivanova V., Stoica B.A., Wang G., Iyer S., Smulson M. Role of poly(ADP-ribose) polymerase (PARP) cleavage in apoptosis. Caspase 3-resistant PARP mutant increases rates of apoptosis in transfected cells. J. Biol. Chem. 1999;274:22932–22940. doi: 10.1074/jbc.274.33.22932. [DOI] [PubMed] [Google Scholar]
- Carvour M., Song C., Kaul S., Anantharam V., Kanthasamy A. Chronic low-dose oxidative stress induces caspase-3-dependent PKCdelta proteolytic activation and apoptosis in a cell culture model of dopaminergic neurodegeneration. Ann. N. Y. Acad. Sci. 2008;1139:197–205. doi: 10.1196/annals.1432.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cichoz-Lach H., Michalak A. Oxidative stress as a crucial factor in liver diseases. World J. Gastroenterol. 2014;20:8082–8091. doi: 10.3748/wjg.v20.i25.8082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csanyi G., Miller F.J., Jr. Oxidative stress in cardiovascular disease. Int. J. Mol. Sci. 2014;15:6002–6008. doi: 10.3390/ijms15046002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng J., Qian Y., Geng L., Chen J., Wang X., Xie H., Zheng S. Involvement of p38 mitogen‐activated protein kinase pathway in honokiol‐induced apoptosis in a human hepatoma cell line (hepG2) Liver Int. 2008;28(10):1458–1464. doi: 10.1111/j.1478-3231.2008.01767.x. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Oxidative stress and cancer: have we moved forward? Biochem. J. 2007;401:1–11. doi: 10.1042/BJ20061131. [DOI] [PubMed] [Google Scholar]
- Hur K.Y., So J.S.., Ruda V., Frank-Kamenetsky M., Fitzgerald K., Koteliansky V., Iwawaki T., Glimcher L.H., Lee A.H. IRE1α activation protects mice against acetaminophen-induced hepatotoxicity. J. Exp. Med. 2012;209(2):307–318. doi: 10.1084/jem.20111298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibitoye O.B., Ajiboye T.O. Protocatechuic acid protects against menadione-induced liver damage by up-regulating nuclear erythroid-related factor 2. Drug. Chem. Toxicol. 2020;43(6):567–573. doi: 10.1080/01480545.2018.1523187. [DOI] [PubMed] [Google Scholar]
- Je J.Y., Lee D.B. Nelumbo nucifera leaves protect hydrogen peroxide-induced hepatic damage via antioxidant enzymes and HO-1/Nrf2 activation. Food Funct. 2015;6(6):1911–1918. doi: 10.1039/c5fo00201j. [DOI] [PubMed] [Google Scholar]
- Ji C., Ren F., Ma H., Xu M. The roles of p38MAPK and caspase-3 in DADS-induced apoptosis in human HepG2 cells. J. Exp. Clin. Cancer Res. : CR (Clim. Res.) 2010;29:50. doi: 10.1186/1756-9966-29-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaewmool C., Kongtawelert P., Phitak T., Pothacharoen P., Udomruk S. Protocatechuic acid inhibits inflammatory responses in LPS-activated BV2 microglia via regulating SIRT1/NF-kappaB pathway contributed to the suppression of microglial activation-induced PC12 cell apoptosis. J. Neuroimmunol. 2020;341:577164. doi: 10.1016/j.jneuroim.2020.577164. [DOI] [PubMed] [Google Scholar]
- Kakkar S., Bais S. A review on protocatechuic Acid and its pharmacological potential. ISRN pharmacol. 2014:952943. doi: 10.1155/2014/952943. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkland J.B. Lipid peroxidation, protein thiol oxidation and DNA damage in hydrogen peroxide-induced injury to endothelial cells: role of activation of poly(ADP-ribose)polymerase. Biochim. Biophys. Acta. 1991;1092:319–325. doi: 10.1016/s0167-4889(97)90007-0. [DOI] [PubMed] [Google Scholar]
- Lee Y.S., Kang Y.H., Jung J.Y., Lee S., Ohuchi K., Shin K.H., Kang I.J., Park J.H., Shin H.K., Lim S.S. Protein glycation inhibitors from the fruiting body of Phellinus linteus. Biol. Pharmaceut. Bull. 2008;31:1968–1972. doi: 10.1248/bpb.31.1968. [DOI] [PubMed] [Google Scholar]
- Li L., Liu S., Tang H., Song S., Lu L., Zhang P., Li X. Effects of protocatechuic acid on ameliorating lipid profiles and cardio-protection against coronary artery disease in high fat and fructose diet fed in rats. J. Vet. Med. Sci. 2020;82:1387–1394. doi: 10.1292/jvms.20-0245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Wu Y., Guo X., Wang R., Mao Y., Zhao Y., Zou J., Li C., Chen Y., Yang Y. Protocatechuic acid supplement alleviates allergic airway inflammation by inhibiting the IL-4Ralpha-STAT6 and Jagged 1/Jagged2-Notch1/Notch2 pathways in allergic asthmatic mice. Inflamm. Res. : off. J. Eur. Histamine Res. Soc. 2020;69:1027–1037. doi: 10.1007/s00011-020-01379-1. [et al] [DOI] [PubMed] [Google Scholar]
- Lin H.H., Chen J.H., Huang C.C., Wang C.J. Apoptotic effect of 3,4-dihydroxybenzoic acid on human gastric carcinoma cells involving JNK/p38 MAPK signaling activation. Int. J. Cancer J. Int. du cancer. 2007;120:2306–2316. doi: 10.1002/ijc.22571. [DOI] [PubMed] [Google Scholar]
- Liu C.L., Wang J.M., Chu C.Y., Cheng M.T., Tseng T.H. In vivo protective effect of protocatechuic acid on tert-butyl hydroperoxide-induced rat hepatotoxicity. Food Chem. Toxicol. : Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2002;40:635–641. doi: 10.1016/s0278-6915(02)00002-9. [DOI] [PubMed] [Google Scholar]
- Liu X., Green R.M. Endoplasmic reticulum stress and liver diseases. Liver Res. 2019;3:55–64. doi: 10.1016/j.livres.2019.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacheco-Palencia L.A., Mertens-Talcott S., Talcott S.T. Chemical composition, antioxidant properties, and thermal stability of a phytochemical enriched oil from Acai (Euterpe oleracea Mart.) J. Agric. Food Chem. 2008;56:4631–4636. doi: 10.1021/jf800161u. [DOI] [PubMed] [Google Scholar]
- Redza-Dutordoir M., Averill-Bates D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta. 2016;1863:2977–2992. doi: 10.1016/j.bbamcr.2016.09.012. [DOI] [PubMed] [Google Scholar]
- Ren Z., Chen S., Pak S., Guo L. A mechanism of perhexiline's cytotoxicity in hepatic cells involves endoplasmic reticulum stress and p38 signaling pathway. Chem. Biol. Interact. 2021;334:109353. doi: 10.1016/j.cbi.2020.109353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riaz T.A., Junjappa R.P, Handigund M., Ferdous J., Kim H.R., Chae H.J. Role of endoplasmic reticulum stress sensor IRE1α in cellular physiology, calcium, ROS signaling, and metaflammation. Cells. 2020;9(5):1160. doi: 10.3390/cells9051160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha P., Talukdar A.D., Nath R., Sarker S.D., Nahar L., Sahu J., Choudhury M.D. Role of natural phenolics in hepatoprotection: a mechanistic review and analysis of regulatory network of associated genes. Front. Pharmacol. 2019;10:509. doi: 10.3389/fphar.2019.00509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumpio B.E., Cordova A.C., Berke-Schlessel D.W., Qin F., Chen Q.H. Green tea, the "Asian paradox," and cardiovascular disease. J. Am. Coll. Surg. 2006;202:813–825. doi: 10.1016/j.jamcollsurg.2006.01.018. [DOI] [PubMed] [Google Scholar]
- Valko M., Leibfritz D., Moncol J., Cronin M.T., Mazur M., Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Wang Z., Li Z., Ye Y., Xie L., Li W. Oxidative stress and liver cancer: etiology and therapeutic targets. Oxid. Med. Cell. Longev. 2016:7891574. doi: 10.1155/2016/7891574. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J.H., Hong L.C., Tsai Y.Y., Chen H.W., Chen W.X., Wu T.S. Mitogen-activated protein kinase (MAPK) signalling pathways in HepG2 cells infected with a virulent strain of Klebsiella pneumoniae. Cell Microbiol. 2006;8:1467–1474. doi: 10.1111/j.1462-5822.2006.00725.x. [DOI] [PubMed] [Google Scholar]
- Yang C.S., Wang X. Green tea and cancer prevention. Nutr. Cancer. 2010;62:931–937. doi: 10.1080/01635581.2010.509536. [DOI] [PubMed] [Google Scholar]
- Yin M.C., Lin C.C., Wu H.C., Tsao S.M., Hsu C.K. Apoptotic effects of protocatechuic acid in human breast, lung, liver, cervix, and prostate cancer cells: potential mechanisms of action. J. Agric. Food Chem. 2009;57:6468–6473. doi: 10.1021/jf9004466. [DOI] [PubMed] [Google Scholar]
- Yin P., Zheng N., Dong J., Xu C., Zhang X., Ding G. Alsterpaullone induces apoptosis of HepG2 cells via a p38 mitogen-activated protein kinase signaling pathway. Oncol. Lett. 2019;17:1177–1183. doi: 10.3892/ol.2018.9700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu D., Tan K.S., Zhang X., Sun A.Y., Sun G.Y., Lee J.C. Hydrogen peroxide alters membrane and cytoskeleton properties and increases intercellular connections in astrocytes. J. Cell Sci. 2005;118:3695–3703. doi: 10.1242/jcs.02507. [DOI] [PubMed] [Google Scholar]