Abstract
A survey of drug combinations employed by the poultry industry indicates that they have played an important role in the control of coccidiosis in chickens. The mode of action of their component drugs is described. Advantages that accrue from their use may include a reduction in potential toxicity, a broadening of their spectrum of activity against different species of Eimeria, activity against different stages of the life cycle, and improved efficacy due to synergism between component drugs. Integration of management procedures involving rotation of drug combinations with vaccination is desirable because this has been shown to result in a restoration of drug sensitivity where drug resistance is present and could contribute to the sustainable control of coccidiosis. Threats to the future use of the most widely used combinations, those that include ionophores, stem from the recent desire to eliminate antibiotics from poultry feeds.
Keywords: Drug combinations, Anticoccidials, Eimeria, Coccidiosis, Chicken
1. Introduction
For many years the poultry industry has been almost entirely dependent upon the use of anticoccidial drugs for the control coccidiosis, a major enteric disease of the chicken caused by apicomplexan parasites of the genus Eimeria (McDougald, 2008). From the 1950s onwards, intensification of poultry production has involved raising large numbers of birds on deep litter in enclosed houses at ever increasing stocking densities. Rearing birds in this manner provides ideal conditions for the transmission of parasites that have an orofecal life cycle. Intensification was accompanied with a change of emphasis from the treatment of frank disease, often by including drugs in the drinking water, to prophylactic medication in which drugs are incorporated continuously in the feed to combat coccidiosis (Chapman, 2009). A succession of drugs, with improved efficacy, safety, and greater species spectrum of activity have been introduced, but one by one these were rendered ineffective due to the acquisition of drug resistance (Chapman, 1997; Joyner, 1970; Ryley, 1980). One method believed to prolong the useful life of anticoccidial drugs has been the introduction of combinations of anticoccidials comprising drugs with different modes of action, and such combinations are widely employed by the poultry industry. Another approach has been the introduction of control programs in which the use of drugs, including combinations, is alternated with the use of live vaccines in order to restore drug sensitivity (Chapman and Jeffers, 2014). Various reasons have been put forward to justify the use of drug combinations, such as a reduction in potential toxicity, improved efficacy resulting from the synergism between component drugs, efficacy against different stages of the Eimeria life cycle, a greater range of species spectrum of activity, and reduced probability of the development of drug resistance. In this review, we describe combinations of anticoccidial drugs that have been used to control coccidiosis including those that inhibit the folic acid pathway, co-factor uptake, electron transport, and ion transport across the parasite cell membrane. We consider the important role drug combinations have played in the past to control coccidiosis and their current utility for prevention of this disease.
2. Life cycle
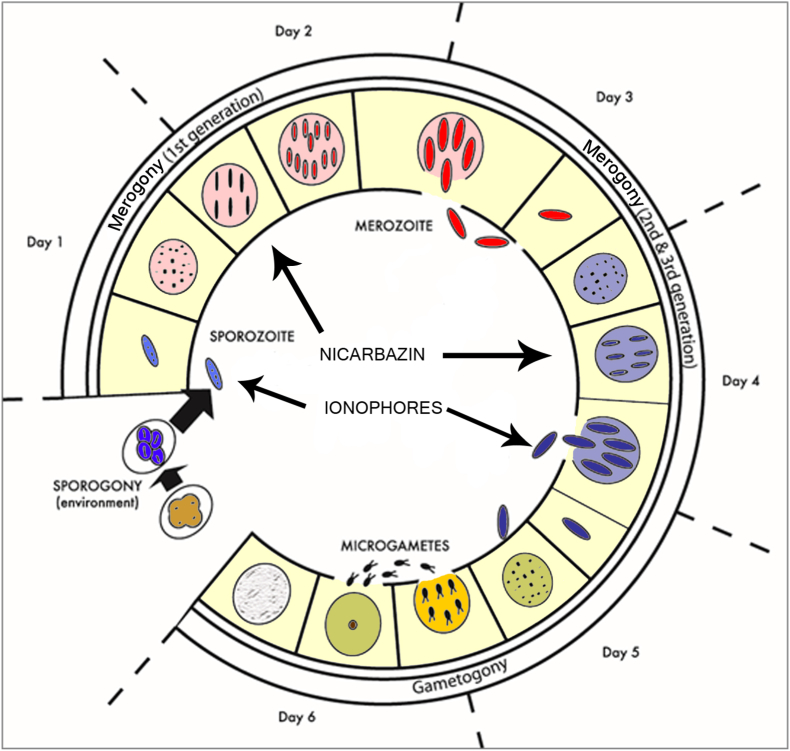
Knowledge of the complex life cycle of Eimeria species is helpful in understanding the sites of action of anticoccidial drugs. The life cycle involves three successive phases, merogony, gametogony, and sporogony, the first two of which occur in the intestine of the bird and the third in the environment (Fig. 1). The infective transmission stage is the oocyst, which when sporulated contains four sporocysts each containing two sporozoites. Following ingestion of the sporulated oocyst, the sporozoites are released from the sporocysts and penetrate epithelial cells in the intestine. This is followed by the merogonic phase of multiplication that results in the release of further motile stages, the merozoites, that invade other epithelial cells. This process is repeated several times and is followed by gametogony, the sexual phase of the life cycle, that results in the formation of new oocysts that are passed in the feces. The third phase of the life cycle (sporogony or sporulation), in which sporocysts and sporozoites are formed, occurs in the environment of the poultry house. A key feature of the Eimeria life cycle, with implications for the selection of drug resistant mutants, is the haploid state of the genome as all stages of the post-meiotic phase of the life cycle (from sporoplasm formation within the sporulating oocyst to gametes) are haploid and consequently subject to the selective pressure of drugs.
Fig. 1.
Life cycle of Eimeria illustrating the three phases (sporogony, merogony, gametogony) and stages affected by nicarbazin (asexual meronts) and ionophores (sporozoites and merozoites). Diagram based upon an illustration by Reid (1972).
Most anticoccidial drugs inhibit intracellular meront stages of the life cycle whereas ionophores target the motile sporozoites and merozoites that are present in the gut lumen (Chapman, 1997). Sites of development in the intestine vary for different species of Eimeria. Thus E. acervulina, E. mitis, E. maxima, and E. praecox develop in the duodenum and jejunum, E. brunetti in the lower intestine and rectum, E. necatrix in the lower intestine and ceca, and E. tenella in the ceca (Long et al., 1976). Although some older drugs are more effective against species that develop in the duodenum and jejunum (e.g. sulfonamides), and others are more effective against species that develop in the ceca (e.g. amprolium), more recent drugs have a broad species spectrum of activity.
3. Definitions and isobologram analysis
Several terms have been employed to describe the interactions of anticoccidial drugs although often insufficient data has been published to justify their use. Examples include “additive responses” where the effect of two drugs given in combination equals the mathematical summation of their effects when given alone, and “synergistic responses” where the combined effect of two drugs is greater than the sum of their effects when given separately. The term “potentiation” applies to cases when an ineffective drug enhances the response to another drug and “double blockade” where two drugs act against separate components of the same biochemical pathway.
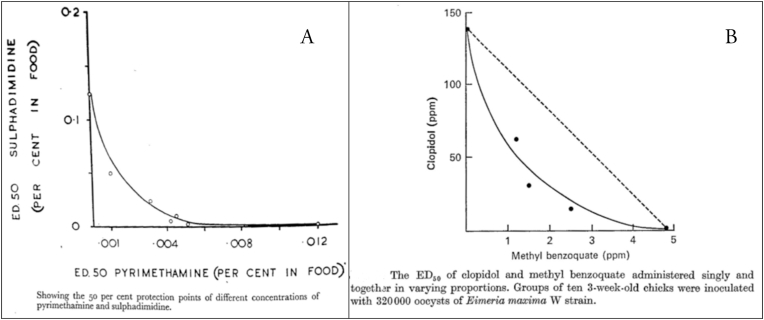
The classic method to investigate interaction between two drugs and to identify synergistic responses requires a dose-response study involving an isobologram analysis. Typically, this involves calculation of an ED50 (50% effective dose) based upon the component drugs used alone and in combination at various concentrations (see Fig. 2). Concentrations selected for use in drug combinations are often influenced as much by economic as well as scientific considerations (Ryley, 1975). Isobolograms have been constructed in the case of sulfadimidine and pyrimethamine (Kendall, 1956; Kendall and Joyner, 1956), sulfaquinoxaline and an experimental drug (Ball, 1964), and methyl benzoquate and clopidol (Joyner and Norton, 1978). The effect of one drug in combination with another may result from molecular interactions with the target cell. Of the several drug interactions reported for Eimeria none have been fully explained biochemically.
Fig. 2.
Isobolograms illustrating the synergism between (A) sulphadimidine and pyrimethamine (Kendall and Joyner, 1956; ©Elsevier) and (B) clopidol and methyl benzoquate (Joyner and Norton, 1978; ©Cambridge University Press). Data for E. tenella and E. maxima respectively. Reproduced with permission.
In this article, the chemical components of drug combinations are described but subsequently, to avoid repetition, the commercial names, listed in Table 1, are used.
Table 1.
Drug combinations that have been used in some countries to control coccidiosis in poultry1.
| Combination | Concentrations (ppm) | Trade names | Application |
|---|---|---|---|
| Sulfaquinoxaline + pyrimethamine | 83.3 + 8.5 | Whitsyn®* | Feed |
| Sulfaquinoxaline + diaveridine | 80.6 + 20 | Solquin® | Water |
| Sulfadimethoxine + ormetoprim | 125 + 75 | Rofenaid® | Feed |
| Sulfanitran + roxarsone + aklomide | 200 + 50 + 250 | Novastat®* | Feed |
| Sulfanitran + roxarsone + nitromide | 300 + 50 + 250 | Unistat®* | Feed |
| Sulfaquinoxaline + sulfamezathine + sulfamerazine | 0.04–0.025% | PoultrySulfa®* | Water |
| Amprolium + ethopabate | 125 + 8 | Amprolmix® | Feed |
| Amprolium + sulfaquinoxaline + ethopabate | 100 + 60 + 5 | Pancoxin®* | Feed |
| Amprolium + sulfaquinoxaline + ethopabate + pyrimethamine | 100 + 60 + 5 + 5 | Supacox® | Feed |
| Methyl benzoquate + clopidol | 8.35 + 100 | Lerbek® | Feed |
| Narasin + nicarbazin | 50 + 50 | Maxiban® | Feed |
| Maduramicin + nicarbazin | 7.5 + 80 | Gromax® | Feed |
| Maduramicin + diclazuril | 5 + 2.5 | Atozuril® | Feed |
| Monensin + nicarbazin | 40 + 40 | Monimax® | Feed |
Partly based upon Noack et al. (2019) and Ryley and Betts (1973). Combinations with an asterisk are currently unavailable or no longer used commercially.
4. Anticoccidial drugs used in combinations
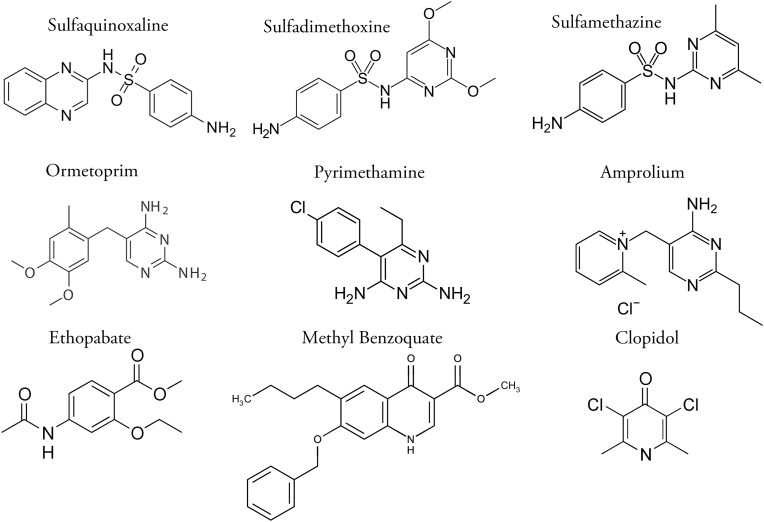
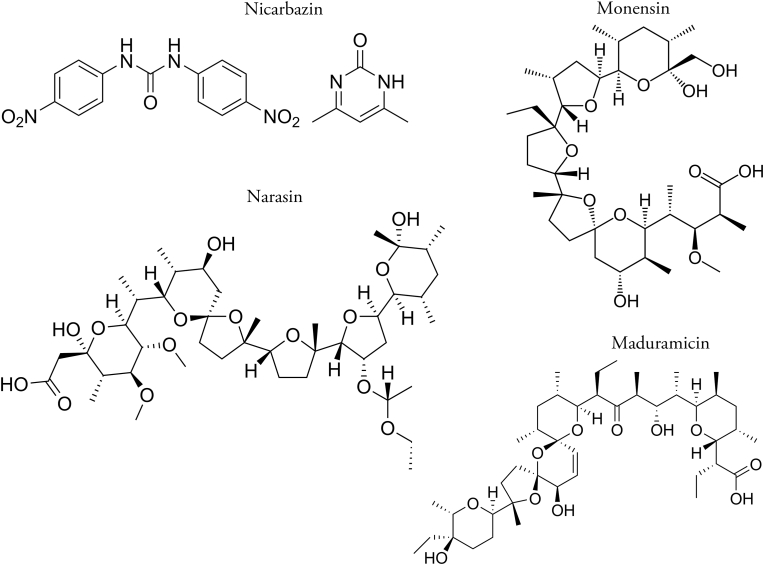
Anticoccidial drugs used to control coccidiosis fall into two categories, synthetic drugs (produced by chemical synthesis and often referred to as “chemicals”) and ionophorous polyether antimicrobials (ionophores) produced by fermentation. The chemical structures of some of the drugs used in combinations are illustrated in Fig. 3, Fig. 4. Synthetic drugs are sometimes employed, mainly in the first feeds given to broiler chickens. Since the early 1970's, however, ionophores have been the principal drugs used for the control of coccidiosis in this class of stock.
Fig. 3.
Chemical structures of synthetic anticoccidials used in drug combinations.
Fig. 4.
Chemical structures of ionophore anticoccidials and nicarbazin used in drug combinations.
4.1. Synthetic drug combinations
The first anticoccidial drug combinations to be investigated, introduced in the 1950s, were sulfonamides and various diaminopyrimidines such as pyrimethamine, diaveridine, and ormetropim. Pyrimethamine was found to potentiate the activity of sulfanilamide, sulfamerazine, sulfadimidine, and sulfaquinoxaline (Horton-Smith et al., 1960; Joyner and Kendall, 1955; Lux, 1954). Subsequently, the interaction of sulfadimidine and pyrimethamine was investigated and an isobologram constructed based upon the dose-response of these compounds (Fig. 2A). This showed that their combined effect was greater than their separate effects indicating a synergistic interaction (Kendall, 1956; Kendall and Joyner, 1956). Other combinations involving sulfonamides and compounds such as roxarsone, nitromide and aklomide were investigated but are no longer available having been superseded by more effective drugs. The interaction of these drugs was considered to be additive rather than synergistic (Ryley and Betts, 1973). In the United States a mixture of sulfadimethoxine and ormetoprim (Rofenaid), and a mixture of three sulfonamides, sulfamerazine, sulfamezathine and sulfaquinoxaline (PoultrySulfa), are approved for the treatment of coccidiosis, and in Australia a mixture of sulfaquinoxaline and diaveridine (Solquin). In some EU countries, sulfonamides and their combinations have been withdrawn because of concerns regarding potential for residues in edible tissues and transferable drug resistance (Noack et al., 2019).
For many years, the arsenical roxarsone was included with ionophores in poultry feeds in the United States as the combination, in some experiments, showed an additive effect (Bafundo et al., 1989; McDougald et al., 1981). Roxarsone has some anticoccidial activity against E. tenella (Ryley and Betts, 1973), and is said to improve growth rate and feed utilization, and enhance pigmentation in broilers (Jones, 2007). Concerns that organic arsenic present in roxarsone could transform into inorganic arsenic in poultry litter led in 2013 in the USA to its voluntary withdrawal from use (Federal Register, 2013, 2014 78 FR 69992, 79 FR 10976).
In the 1960s, amprolium was introduced in combinations with ethopabate, sulfaquinoxaline, and pyrimethamine (Amprol Plus, Pancoxin, and Supacox respectively) primarily to obtain a broad species spectrum of activity but also to combat the development of resistance (Chapman, 1980; Ryley, 1980). Amprol Plus and sulfonamide combinations are still used today in some countries for the treatment of birds showing clinical signs of coccidiosis, but the 3 and 4 drug combinations (Pancoxin and Supacox) are no longer available. Treatment is usually undertaken by inclusion of drugs in the drinking water and is often a salvage operation because by the time the disease is clinically apparent significant mortality may have occurred.
A combination of the quinolone methyl benzoquate and clopidol (Lerbek) was introduced in the 1970s for the control of coccidiosis to “fortify” the clopidol component (Ryley, 1975, 1980). It was demonstrated that the interaction was synergistic because the combination gave far better control than could be accounted for by a simple additive effect (Joyner and Norton, 1978) (Fig. 2B). Synergism between other quinolones (amquinolate, buquinolate, and decoquinate), and clopidol has also been demonstrated (Challey and Jeffers, 1973). A curious feature of Lerbek is that repeated propagation of E. maxima in the presence of the drug resulted in the formation of abnormal bisporocystic oocysts similar in appearance to those produced by members of the related apicomplexan genus Isospora (Norton and Joyner, 1978).
Nicarbazin was introduced in 1955 for the control of coccidiosis (Chapman, 1994a). This drug is a molecular complex of 4,4′-dinitrocarbanilide (DNC) and 2-hydroxy-4,6-dimethylpyrimidine (HDP) present in a 1:1 M ratio (Cuckler et al., 1955). It is thought that the complex results from hydrogen bonding between the complexing agent HDP and the urea portion of the substituted carbanilide. While DNC has anticoccidial activity when used alone HDP lacks activity against Eimeria. However, the potency of DNC is enhanced ten-fold when this molecule is complexed with HDP but no increase in anticoccidial efficacy is observed with a simple mixture of the two compounds.
4.2. Nicarbazin - ionophore combinations
The most recent combinations introduced are those with nicarbazin and the ionophores maduramicin, monensin, narasin, and semduramicin (Gromax, Monimax, Maxiban, Aviax Plus, respectively) (Callender and Jeffers, 1980; Vereecken et al., 2020). The concentrations employed in the components are well below those that would show efficacy when given alone and therefore it was concluded that their action is synergistic. Thus, in the case of nicarbazin and narasin, concentrations ranging from 0 to 108 g/ton of nicarbazin and narasin when used alone and in combination were examined and a 1:1 ratio gave the best response (Tonkinson et al., 1987). The strains of E. tenella and E. acervulina used in these experiments were sensitive to both drugs, resistant to one drug but sensitive to the other, or resistant to both. Interestingly, the synergism demonstrated was significant regardless of the sensitivity of the parasites to the two drugs. In a recent study, the effect of Monimax was compared with the component drugs used separately (Vereecken et al., 2020). Monensin and nicarbazin, normally included in the feed at 100 and 125 ppm respectively, showed only partial efficacy at 40 ppm evaluated by weight gain and no efficacy evaluated by feed conversion. By contrast, the combination of 40 ppm nicarbazin +40 ppm monensin provided complete control of infection judged by greater daily weight gain, feed intake, and lower feed conversion. Currently, in major poultry producing countries, ionophore combinations with nicarbazin are among the most widely employed drugs for the control of coccidiosis in broiler chickens. Combinations of monensin and lasalocid, and monensin and clopidol have been reported, and claimed to be synergistic, but have not been utilized commercially (McDougald, 1977, 1978).
4.3. Plant combinations
In recent years there has been interest in the role that plant products, some of which contain compounds with anticoccidial activity, may play in the control of coccidiosis (Muthamilselvan et al., 2016). An example is a combination of quillaja and yucca that contain saponins and are often used to promote intestinal health and immunity in poultry (Bafundo et al., 2020). In a series of studies they were shown, in addition to improving gut health, to have beneficial effects against infections with Eimeria species, necrotic enteritis, and improve the effectiveness of live coccidiosis vaccines (Bafundo et al., 2021a,b). Synergism has been claimed for a 1:1 mixture of the natural alkaloid berberine and amprolium (Malik et al., 2016).
5. Mode of action of anticoccidial drugs used in combinations
5.1. Drugs that affect the folic acid pathway
Whereas host cells are able to take up and utilize folic acid, it is believed that coccidia, like other apicomplexan parasites, are unable to do so and therefore must synthesize this co-factor. Sulfonamides act against Eimeria species by inhibiting this pathway, specifically the enzyme dihydropteroate synthetase, thus preventing the synthesis of dihydrofolate (Wang, 1982). A subsequent step in the pathway involves the reduction of dihydrofolate to tetrahydrofolate by the enzyme dihydrofolate reductase (DHFR), this enzyme is inhibited by drugs such as pyrimethamine, trimethoprim, and ormetoprim. Pyrimethamine is a potent inhibitor of DHFR extracted from oocysts, but a less effective inhibitor of this enzyme obtained from chicken liver (Wang et al., 1975). Ethopabate, a substituted benzoic acid, was also shown to interfere with the folic acid pathway and shows a synergistic interaction with sulfaquinoxaline and pyrimethamine indicating a different mode of action from the latter. Thus, in experiments with E. brunetti, when pyrimethamine was combined with ethopabate or sulfaquinoxaline, there was a 10-fold enhancement of efficacy of the latter two drugs (McManus et al., 1967).
5.2. Drug that affects thiamine uptake
Amprolium is a thiamine antagonist and competitively inhibits carrier mediated uptake of thiamine by meronts of E. tenella. It has also been reported to cause abnormal oocyst wall formation and inhibit oocyst sporulation (Ball et al., 1987). Thiamine transport in the parasite was 50 times more susceptible to inhibition than in the chicken (James, 1980). Because the drug lacks the hydroxymethyl group of thiamine it cannot be pyrophosphorylated and thereby participate in biochemical pathways of thiamine metabolism (Rogers, 1962).
5.3. Drugs that affect electron transport
Quinolones, such as methyl benzoquate, buquinolate, amquinolate, and decoquinate inhibit respiration of E. tenella by blocking electron transport in the parasite mitochondrion (Wang, 1975, 1976). The site of action in the electron transport chain was identified as a point beyond co-enzyme Q and near cytochrome b. The pyridine clopidol also affects the electron transport chain but at a different point from the quinolones. According to Fry and Williams (1984), analysis of cytochrome spectra and the effects of inhibitors such as azide and cyanide upon electron transport indicated the presence of two biochemical pathways with different terminal oxidases, a proposal first made by Wang (1978). One of these pathways, possibly an o-type cytochrome oxidase, is believed to contain a binding site with a high affinity for decoquinate but low affinity for clopidol. The other pathway, terminating in a cytochrome aa3 oxidase, is more sensitive to clopidol than decoquinate. They propose a branched or parallel electron transport chain in coccidia and found that low concentrations of both drugs, when used in combination, caused a greater inhibition of electron transport than expected from a summation of their separate effects. The interaction of these drugs was therefore considered to be synergistic and data to support this contention was provided by Joyner and Norton (1978).
The active component of nicarbazin (4,4′- dinitrocarbanilide or DNC) inhibits succinate-linked NAD reduction in beef heart mitochondria (Dougherty, 1974) and also inhibits an energy dependent transhydrogenase and the accumulation of Ca2+ ions by rat liver mitochondria. Whether this is relevant to its mode of action is not known but Wang (1978) believes it could cause the uncoupling of mitochondrial function in coccidia.
5.4. Drugs that affect ion transport across cell membranes
Ionophores such as monensin, narasin, and maduramicin are able to transport cations across cell membranes and affect a diverse range of processes dependent upon ion transport (Chapman et al., 2010). They are accumulated in the cell membrane of sporozoites (and merozoites) before penetrating epithelial cells. They cause an influx of sodium ions across the parasite cell membrane that results in a concomitant stimulation of the sodium pump (Na+/K+ ATPase) that is responsible for pumping excess sodium out of the cell (Smith and Galloway, 1983). This is an energy dependent process that results in enhanced utilization of ATP. Lactate production is increased indicating a stimulation of glycolysis and resulting in depletion of amylopectin, the carbohydrate energy reserve. Eventually, accumulation of sodium ions in the cell results in water entering the parasite which swells and ruptures (Smith et al., 1981; Smith and Strout, 1979). Another explanation for the mechanism of action is that the drug is able to interrupt host cell invasion by sporozoites (del Cacho et al., 2007). The outer membrane of the sporozoite contains lipid rafts and a protein, flotillin-1, was identified in sporozoites of E. tenella at the apex of the cell, a region that mediates cell invasion. Monensin was found to disrupt the localization of flotillin-1 within raft structures, resulting in the loss of ability to invade host cells. Support for this mode of action was that the effect was reduced in a monensin-resistant line of E. tenella.
6. Rationales for using drug combinations
6.1. Reduction in toxicity
Most anticoccidial drugs show some toxicity if used above their approved use concentrations (Chapman, 2018). Overdosing usually results in non-specific signs such as reduced feed intake, depression, incoordination, and poor growth and therefore diagnosis of toxicity may be difficult (Reece, 1988). A disadvantage of the practical use of sulfonamides was that unduly high concentrations were required to control the cecal species E. tenella and this resulted in toxicity exemplified by a hemorrhagic syndrome (Ryley and Betts, 1973). However, in combination with DHFR inhibitors such as pyrimethamine it was possible to lower the concentrations of either compound thereby reducing toxic effects of the drugs. Pyrimethamine is potentially toxic (Ryley and Betts, 1973) whereas an alternative folic acid antagonist, diaveridine is much less so, furthermore it was effective against the cecal as well as intestinal species of Eimeria (Clarke, 1962, 1964). Another drug combination, sulfaquinoxaline plus trimethoprim, was also investigated and efficacy against bacterial as well as coccidial infections demonstrated (White and Williams, 1983). An advantage of trimethoprim and ormetoprim as sulfonamide synergists is that they are rapidly eliminated from avian species whereas pyrimethamine persists in tissues for a prolonged period (Goetting et al., 2011).
Ionophores have generally been found to be safe in target animals receiving an approved dosage, but a long list of clinical signs has been reported from misuse (Dowling, 1992; Keshavarz and McDougald, 1982). Nicarbazin also has side effects in broilers under some environmental conditions (Beers et al., 1989; McDougald and McQuistion, 1980; Wiernusz and Teeter, 1995) and cannot be used in layers at its approved concentrations because of affects upon egg production (Chapman, 2017). A comparison of nicarbazin at 125 ppm and Maxiban at the combined approved use level of 80 ppm was undertaken in controlled environmental chambers under conditions designed to induce heat stress (Harris and Macy, 1988). Birds fed nicarbazin had significantly higher mortality, gained less weight, and had a poorer feed conversion ratio than those given the combination. Long et al. (1988) suggested that Maxiban may reduce heat distress induced mortality, as chicks subjected to a 42 °C ambient temperature had a higher survivability than birds fed nicarbazin alone. An advantage of combinations involving ionophores and nicarbazin is that the concentrations employed for each drug are well below those that could result in toxicity. Thus in the EU, there is a zero-day withdrawal period specified for their inclusion in poultry feeds. As a consequence of the lack of potential toxicity, Monimax is approved in the EU for control of coccidiosis in chickens reared for laying (Bampidis et al., 2018). Furthermore, any concerns regarding tissue residues should be alleviated, as the depletion time and concentration level for the active component of nicarbazin (DNC) in birds given Maxiban is significantly reduced (Lima et al., 2017).
6.2. Broadening the species spectrum of activity
One attribute of the sulfonamides and drugs such as ethopabate is their superior activity against the intestinal species of Eimeria (E. acervulina, E. maxima, E. brunetti) as opposed to cecal species E. tenella and E. necatrix (Ryley and Betts, 1973). Amprolium, however is more effective against E. tenella and E. necatrix than the intestinal species. The combination of amprolium with ethopabate and subsequently sulfaquinoxaline was therefore intended to enhance the species spectrum of activity (Davies and Joyner, 1963; Long, 1963). In the UK, the inclusion of pyrimethamine even provided a 4-drug mixture (Supacox). Such multiple combinations of 3 and 4 four drugs are unlikely to be introduced today because of the costs and difficulty of obtaining product approval from registration authorities.
6.3. Efficacy against different stages of the Eimeria life cycle
Synthetic anticoccidial drugs inhibit the development of the asexual phases of the life cycle, principally intracellular meronts of the first and second generation. Sulfonamides have little activity against the first generation but are most effective against the second asexual cycle, a fact that favors their use for the treatment of birds that are already infected (Davies and Kendall, 1954; Kendall, 1956). Amprolium causes retardation in the development of first-generation meronts and inhibition of oocyst sporulation whereas quinolones and clopidol prevent further development of sporozoites once they have penetrated intestinal cells (Ryley and Wilson, 1976). Nicarbazin is reported to have some effect against first generation meronts and suppresses development of second generation meronts (Chapman, 1994a; Cuckler and Malanga, 1956).
The ionophores have been shown to kill the extracellular stages, the motile sporozoites and merozoites, as the drugs are taken up by the parasite before they penetrate intestinal epithelial cells of the host. An advantage of combinations that comprise ionophores and nicarbazin is that any motile parasites that escape the action of the ionophore are likely to be subsequently killed by nicarbazin due to its effects upon the meront generations.
6.4. Improvement in efficacy
A possible advantage of drug combinations is that their use may result in improved efficacy in the control of coccidiosis. Thus, in the case of the early combinations, drugs with indifferent activity were combined in order to give acceptable efficacy (Ryley, 1980). Ideally, in order to demonstrate improved efficacy it would be necessary to conduct growth trials, preferably conducted in floor-pens where treatments can be replicated, in which a combination and component drugs are employed at their commercially approved concentrations and current field isolates utilized as an infection source. Thus, in a floor-pen study, Maxiban gave a better final weight when included in the starter feed of broilers than birds given nicarbazin (Bafundo et al., 1990). It appears that no other such trials have been reported in the literature.
6.5. Reduced probability of developing drug resistance
The practice of prophylactic control of coccidiosis, in which large numbers of broiler chickens are given anticoccidial drugs continuously in the feed, has resulted in the inevitable development of drug resistance. The parasite is constantly exposed throughout its life cycle to agents designed to promote its demise (Chapman, 1982). One objective for the use of drug combinations is that this may reduce or delay the development of resistance (reviewed by Chapman, 1982). For example, Lerbek was introduced to take advantage of the synergistic effects noted between its two components and hopefully to delay the appearance of drug resistance (Ryley, 1975). Anticoccidial combinations involving an ionophore and nicarbazin were introduced as an effective means of controlling ionophore-resistant coccidia (Bafundo and Jeffers, 1990). The principal method to develop resistance has been repeated propagation of parasites in birds given gradually increasing concentrations of drug and by this means it was shown that resistance to some develops rapidly (e.g. quinolones) whereas resistance to others (such as amprolium) develops slowly (Chapman, 1982). It has been suggested that in the case of quinolones resistance results from a single mutation whereas for amprolium several successive mutations may be involved (Chapman, 1978). In the case of nicarbazin resistance was difficult to achieve (McLoughlin and Gardiner, 1967) and initial attempts to develop resistance to ionophores were unsuccessful (Chapman, 1976; Weppelman et al., 1977) although subsequently decreases in susceptibility were observed (Chapman et al., 2010). A slight increase in reproductive ability of E. acervulina was found in birds given monensin after 60 generations of selection, whereas E. tenella did not increase its reproductive capacity in the presence of this drug (Bafundo and Jeffers, 1990).
An attempt to calculate mutation rates to anticoccidial drugs in E. tenella indicated that for the quinolone amquinolate, the frequency of resistant parasites was 5.8 × 10−8 per wild type oocyst (Weppelman et al., 1977). These authors believed that it should be possible to select resistance to optimal concentrations of other drugs providing the chickens are inoculated with sufficiently large numbers of oocysts. They were unsuccessful with amprolium, nicarbazin, and monensin but argued that the numbers of oocysts utilized in their experiments may have been insufficient and that single-step mutants might be present at frequencies lower than 5 × 10−9. No evidence to support this was provided. Chapman (1978) believes that initial selection of mutants resistant to sub-optimal concentrations of drugs may be essential for the subsequent selection of mutants resistant to higher concentrations. The nature of the Eimeria life cycle, involving repeated asexual multiplication and production of haploid sporozoites and merozoites, will ensure that genes for any resistant mutant will be immediately expressed, and have a selective advantage in medicated chickens compared with drug -sensitive counterparts. The existence of a sexual process provides opportunities for recombination that may permit innovative ways for coccidia to evade control by anticoccidials. Sexual reproduction may result in the generation of novel genes and the haploid endogenous development provides strong selection pressure to ensure that novel, adaptive changes are quickly fixed in the population. There have been several experimental studies demonstrating that new resistance phenotypes may arise by genetic recombination, a phenomenon first reported by Jeffers (1974a) for amprolium and decoquinate. This indicated a capacity for cross-fertilization, independent segregation, and the production of hybrid progeny (Chapman et al., 2013). However, such phenotypes require that strains employed be already resistant to the drugs studied (Chapman, 1984; Joyner and Norton, 1975, 1977).
An advantage of drug combinations may be that the chance of selecting resistant mutants when component compounds are used simultaneously is much less than if those compounds are used alone (Bryson and Szybalski, 1955). Experimental evidence to support this was found by Norton and Joyner (1975, 1978) who obtained resistance to 10 ppm methyl benzoquate and 125 ppm of clopidol after 3 propagations of E. maxima in medicated birds but were unable to develop resistance to a combination of the drugs. Furthermore, Joyner and Norton (1978) have argued that if there is a synergistic interaction when drugs are given simultaneously then a drug resistant recombinant might be eliminated even though it carried factors for both drugs involved. They also considered, that in the case of these two drugs, close physical linkage of contributing genetic loci may be an explanation for incompatible resistance combinations. An interesting phenomenon described for the quinolone decoquinate and clopidol (meticlorpindol) was collateral drug sensitivity in which strains sensitive to one compound were found to be sensitive to the other (Jeffers and Challey, 1973). Furthermore, this inverse relationship between the sensitivity to these drugs extended to a large number of isolates obtained from the field. This could have provided an additional rationale for the use of such combinations. However, Joyner and Norton (1978) were unable to demonstrate collateral sensitivity between methyl benzoquate and clopidol in a drug sensitive strain of E. maxima. Although they were unable to develop resistance to both these drugs when administered simultaneously, they found it relatively easy to do so by selecting resistance to methyl benzoquate in a strain already resistant to clopidol (Joyner and Norton, 1978). This is likely the mechanism by which resistance to Lerbek appeared in the field.
There have been a few other attempts to select drug resistance to combinations. Thus, a decrease in sensitivity of E. tenella to Novastat was found after five serial propagations of a sensitive strain in the presence of suboptimal levels of the drug (McLoughlin and Chute, 1973) and resistance to Maxiban could be developed after 11 serial passages of E. tenella in medicated birds (Tamas and Wilks, 1989). However, Bafundo and Jeffers (1990) found that sixty generations of selection were required to develop resistance to Maxiban with E. acervulina, but only partial resistance developed after 52 generations with E. tenella.
6.6. Permit the acquisition of immunity
A consequence of limited control of Eimeria infections is that sufficient parasites may escape the effects of a drug to allow the development of a protective immune response (Chapman, 1999). This could be desirable for coccidiosis control programs where extended withdrawal periods are employed (removal of drugs from the feed prior to slaughter) that may expose birds to infection later in life. Thus, with mixtures of sulfaquinoxaline and amprolium it was considered important to select concentrations that would not interfere with immunity development (Davies and Joyner, 1963). In the case of Amprol Plus, no suppression of the development of immunity to E. tenella was observed (Karlsson and Reid, 1978) and nicarbazin did not prevent acquisition of immunity to E. acervulina, E. tenella or E. necatrix (Cuckler and Malanga, 1956). It has been shown that ionophores do not prevent the acquisition of immunity (Chapman et al., 2010) and Jeffers (1989) believes that immunity may account for the effectiveness of ionophores in the field. The presence of low-level lesions in birds given Monimax indicates that some parasite recycling is occurring in the presence of the combination (Vereecken et al., 2020). Similarly, in a study of the drug-sensitivity of field isolates of E. tenella, the development of lesions was not prevented by Maxiban (Chapman, 1989). In view of the reduced concentrations of ionophores and nicarbazin in these drug combinations it seems likely that they may not interfere with immunity development. This, however, remains to be investigated.
7. Sensitivity of strains isolated from the field to drug combinations
7.1. Synthetic drugs
Many studies have been conducted on the sensitivity of field strains to anticoccidial drugs but few for drug combinations (reviewed by Chapman, 1982; Cuckler et al., 1969; Ryley and Betts, 1973). Thus, of the early combinations, Novastat was unable to control field strains of E. tenella resistant to nicarbazin and other drugs (McLoughlin and Chute, 1973). However, the combination has limited efficacy and therefore this was not considered to be acquired resistance (Ryley and Betts, 1973). Similarly, a decrease in sensitivity was observed in E. brunetti to a mixture of sulfaquinoxaline and diaveridine (Darvisul) from 1964 to 1966 (Hodgson et al., 1969; Warren et al., 1966). Sulfaquinoxaline plus pyrimethamine (Whitsyn) was found effective against E. acervulina when evaluated by lesion scores in the upper intestine and administered in the drinking water, but as anticipated had no activity against the cecal species E. tenella (Mathis et al., 1984). The apparent lack of correlation with previous reports may have been attributed to a higher drug intake with drinking water administration, and better absorption of the drug via water than the feed.
In surveys conducted in the United States, resistance to amprolium combinations has been demonstrated (Jeffers, 1974b,c; McDougald et al., 1986). In the UK, a survey carried out by Hodgson et al. (1969) over a three-year period from 1964 to 1966 revealed a significant increase in strains of E. acervulina and E. maxima resistant to Pancoxin. None of the strains of E. maxima investigated by Chapman (1980) were sensitive to Pancoxin likely reflecting the widespread use of this combination. Resistance to this drug has also been reported for a strain of E. tenella (Ryley and Betts, 1973). Chapman (1980) also investigated the sensitivity of field strains of E. maxima to the four-drug mixture Supacox. Most isolates were sensitive suggesting that the potentiation achieved by the inclusion of pyrimethamine improved the efficacy of the combination. The finding of 1 resistant strain and 2 that were partially resistant suggested that resistance might have become more widespread. However, Supacox was withdrawn from commercial use before this could be realized.
As already discussed, resistance could not be developed to Lerbek experimentally (Joyner and Norton, 1978), however field isolates of E. acervulina and E. maxima resistant to this combination have been described (Chapman, 1980; Kawazoe et al., 1991). Thus of 9 isolates of E. maxima obtained from broiler farms, 6 were considered resistant, 1 partially resistant, and 2 sensitive to Lerbek (Chapman, 1980). By contrast, of 6 isolates from breeder farms all were sensitive. The results correlated with the use of the component compounds because neither methyl benzoquate nor clopidol had been used at breeder farms but had been employed extensively in broilers. Most broiler isolates had acquired resistance to methyl benzoquate and clopidol prior to the introduction of Lerbek hence the failure of the combination to control these strains. Lerbek would have been effective if resistance to its components had not emerged prior to its introduction, but unfortunately this was not the case (Williams, 1998). However, an investigation of the sensitivity of Eimeria species from Belgian broiler farms indicated a 50% improvement in weight gain, feed conversion and oocyst production compared with infected controls in birds given Lerbek (Peeters et al., 1994). Similarly, a limited study of field isolates from Dutch poultry farms conducted in 2001 indicated that 7 isolates of E. acervulina, 1 of E. maxima, and 4 of E. tenella were sensitive to Lerbek with none resistant to the drug (Peek and Landman, 2003). It was not stated whether Lerbek had been previously used at these farms. More recently, in a survey of the occurrence of Eimeria species in Colombia, no indication of resistance to the methyl benzoquate/clopidol combination was observed (Mesa et al., 2021). The observations suggest that there could be a role for older drugs and combinations in the control of coccidiosis if local strains could be shown to be sensitive to them.
7.2. Ionophore combinations
Chapman (1989) reported that of 15 isolates of E. tenella from broiler farms, 3 were resistant, 7 partially resistant, and 5 sensitive to Maxiban. More recently, 26 isolates of Eimeria were collected from 2003 to 2006 in the USA and their sensitivity to nicarbazin and Maxiban investigated (Bafundo et al., 2008). Most isolates were sensitive to nicarbazin but only 22% overall were sensitive to Maxiban. Of the species present in these isolates, 88% of E. acervulina, 75% of E. maxima, and 29% of E. tenella were considered either resistant or partially resistant to this drug. Evidently, a considerable decline in sensitivity in the ionophore component of the combination had occurred which they attributed to the over-usage of such drugs for many years by the poultry industry. Strains of 3 species (E. acervulina, E. maxima, and E. tenella) were obtained from 13 countries throughout Europe over a period of 7 years and their sensitivity to Monimax, utilizing standard sensitivity tests, was performed (Anon, 2019a). Monimax significantly decreased lesion scores of all 3 species, significantly improved daily weight gain and feed intake, and decreased feed conversion compared with infected unmedicated controls. Recently, the sensitivity of Brazilian field isolates of E. acervulina and E. maxima to various drugs, including Maxiban and nicarbazin plus semduramicin (Aviax Plus), indicated that strains were resistant to these combinations (Kraieski et al., 2021).
Resistance to more than one drug (multiple resistance), whether arising through successive exposure to different drugs or genetic recombination between pre-existing resistant strains is widespread in the field and this, no doubt, is one reason why some of the older combinations are no longer used (Chapman, 1984).
8. Shuttle and rotation programs
Ionophore combinations are typically employed to control coccidiosis during the rearing of broiler chickens in so-called “shuttle programs”, in which different drugs are used in successive feeds given during the life of a single flock (Chapman, 2001). Combinations may also be employed in “rotation” programs that involve the use of different drugs in successive flocks. The use of combination products in the first feed provided (starter ration) and sometimes in subsequent grower feeds has become a common practice. As an example, in the EU Monimax is recommended for use in the feed of broilers to 21 days of age followed by an ionophore, salinomycin or monensin. Shuttle programs were thought to reduce the probability of selecting drug resistant strains and are widely employed by the broiler industry (McDougald, 2008). Ideally, such alternation should be between drugs with different modes of action, but this is not often the case. The poultry industry is fortunate that, unlike many other areas of veterinary medicine, a variety of agents are approved for the control of coccidiosis, however, the occurrence of resistance to synthetic drugs and ionophores is so widespread only limited opportunities are available. In the only study attempting to simulate a rotation program, E. tenella was propagated in birds given alternately nicarbazin, followed by zoalene, amprolium and Unistat (McLoughlin and Chute, 1975). After 10 propagations for each drug, the strain was resistant to all except nicarbazin. They concluded that changing drugs does not prevent the acquisition of resistance. Resistance is generally thought to be stable, nevertheless, relaxation of drug selection pressure may be advantageous in that given time sensitive strains might recolonize broiler houses. Anecdotal evidence suggests that this may be the case for some of the older synthetic drugs, however, experimental evidence to support this contention is lacking.
9. Restoration of drug sensitivity
Ionophore combinations are also employed in rotation programs in which drugs and vaccines are alternated in successive flocks. Improvements in delivery systems for live vaccines, especially the use of spray cabinets in the hatchery, has allowed their economic deployment for the vaccination of broiler chickens. Furthermore, some vaccines comprise strains of Eimeria, attenuated or non-attenuated, that have never been exposed to drugs, and it is believed that use of such vaccines may repopulate broiler houses with drug-sensitive strains, thus resulting in a restoration of drug sensitivity. Restoration of sensitivity to monensin, salinomycin, and diclazuril following use of the live non-attenuated vaccine Coccivac has been reported (Chapman, 1994b; Chapman and Jeffers, 2015; Mathis and Broussard, 2006) and to monensin and diclazuril following use of the attenuated vaccine Paracox-5 (Peek and Landman, 2006). More recently, restoration of drug sensitivity to a range of drugs, including Maxiban and Monimax following use of the attenuated vaccine ADVENT has been demonstrated (Vereecken et al., 2021). Rotation programs involving anticoccidial drugs and vaccines are widely utilized in the USA and many of these employ drug combinations as the chemotherapy component. Similar programs involving drug combinations have been tailored for local conditions and husbandry practices in other countries.
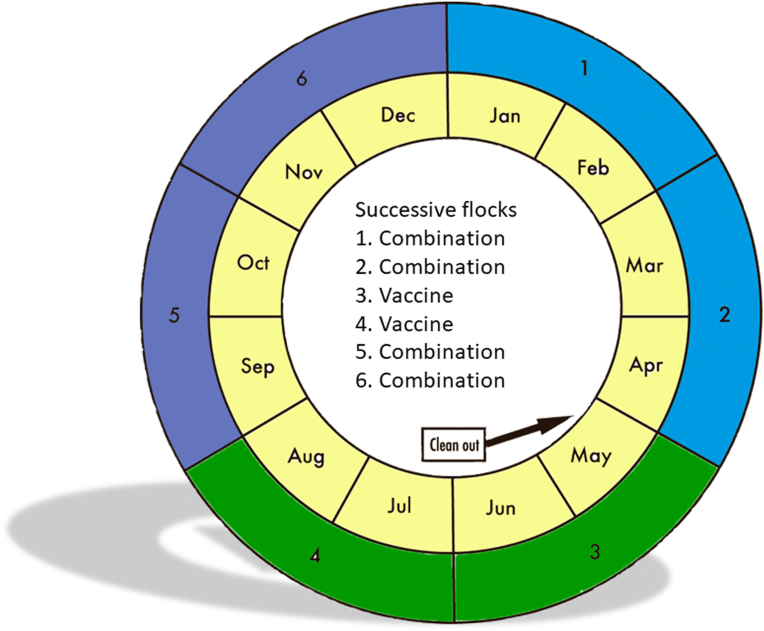
A proposal for a rotation program involving drug combinations and vaccines, based upon a diagram by Chapman and Jeffers (2015) is provided in Fig. 5. An annual program involving 6 successive flocks is illustrated. The combination may be used in both starter and grower feeds, in the starter feed followed by a different ionophore, or in the grower feed following use of a synthetic drug. Ideally, in those situations where an annual clean-out is conducted, removal of litter should be undertaken prior to vaccination in order to remove as many drug-resistant parasites present as possible.
Fig. 5.
Rotation program comprising a yearly chicken production cycle involving six flocks given drug combinations and vaccines.
10. Future for drug combinations
Control of coccidiosis by inclusion of drugs in poultry feeds has been achieved by use of synthetic compounds and ionophores. Indeed, control of coccidiosis by drugs and their combinations since the 1950s may be considered the golden age of chemotherapy. The widespread development of drug resistance, however, has led to a decline in use of some older synthetic compounds, and many are no longer available. In recent years there has been renewed interest in such drugs and several are now employed in programs as alternatives to ionophores. There may in the future, therefore, still be a role for drug combinations comprising synthetic drugs in the control of coccidiosis. The principal drug combinations employed today are those comprising ionophores and nicarbazin. Threats to their future use, however, are that some poultry companies, in order to satisfy perceived customer demands, are marketing chickens as “raised without antibiotics”. This has led to compromised production including reduced livability, increased feed costs, poor feed conversion, increased incidence of various disease conditions, and negative effects upon welfare (Cervantes, 2015; Karavolias et al., 2018). In some countries, the poultry meat sector has come under pressure to reduce or even remove the use of ionophores for the control of coccidiosis because they have activity against gram positive bacteria and are classified as antibiotics (Parker et al., 2021). It should be noted, however, that ionophores are not currently approved for use in human medicine (Anon, 2019b). It is unclear, how the poultry industry will accommodate the likely deterioration in the health of chickens if ionophores are no longer available for the control of coccidiosis. As already described, several advantages accrue from the use of ionophore combinations, including a reduced probability of toxicity, efficacy against several stages of the life cycle, and broad species spectrum of activity against Eimeria. Furthermore, one drug combination (Monimax) is approved for use in the EU for controlling coccidiosis in other classes of stock, that includes fattening turkeys, and for the rearing phase of layer birds. Nevertheless, the principal use of ionophores will be in broiler chickens and, despite evidence of resistant strains having developed, they are widely employed by the broiler industry and likely to remain so in the immediate future. A contributing factor maybe that the usage of ionophores has expanded because of their antibacterial properties, such as control of clostridial enteritis (Bafundo et al., 2008). Thus, monensin and narasin are able to protect birds from developing lesions of Clostridium perfringens but the combination of narasin and nicarbazin was unable to do so (Lanckriet et al., 2010). Although drug resistance has proved to be a major factor contributing to the failure of anticoccidial agents, rotation programs involving drug combinations and vaccination may be an effective means of controlling coccidiosis. It is concluded that several advantages may accrue from the use of drug combinations and that, for the present, they are likely to remain among the principal means to control this disease. Further research is desirable, including the influence of combinations upon the acquisition of immunity, effects upon the performance of birds raised under different environmental conditions, and their integration in various management regimes including vaccination.
Declaration of competing interest
The authors declare there is no conflict of interest. The sponsors had no role in the design, execution, interpretation, or writing of the study.
Acknowledgments
The authors acknowledge Elsevier for permission to reproduce Fig. 2A (Kendall, S.B. and Joyner, L.P., 1956. The synergism between pyrimethamine and sulphadimethylpyrimidine in the control of Eimeria tenella. J. Comp. Path. 66, 145–150) and Cambridge University Press for permission to reproduce Fig. 2B (Joyner, L.P. and Norton, C.C., 1978. The activity of methyl benzoquate and clopidol against Eimeria maxima: Synergy and drug resistance. Parasitology 76, 369–377). We thank Huvepharma N.V. for providing funds to support publication of this manuscript.
References
- Anon . 2019. Meta-analysis on Results of Anticoccidial Testing Performed over a Period of Seven Years. Technical Bulletin No. 34. Huvepharma NV, Uitbreidingstraat 80, 2600 Antwerp, Belgium. [Google Scholar]
- Anon . World Health Organization; Geneva: 2019. Critically Important Antimicrobials for Human Medicine, 6th Revision. 2019. Licence: CC BY-NC-SA 3.0 IGO. [Google Scholar]
- Bafundo K.W., Jeffers T.K. Selection for resistance to monensin, nicarbazin, and the monensin plus nicarbazin combination. Poultry Sci. 1990;69:1485–1490. doi: 10.3382/ps.0691485. [DOI] [PubMed] [Google Scholar]
- Bafundo K.W., Schlegel B.F., Tonkinson L.V., Donovan D.J. Research Note: effect of narasin and roxarsone combinations on Eimeria tenella infections in floor-pen raised broilers. Poultry Sci. 1989;68:1011–1014. doi: 10.3382/ps.0681011. [DOI] [PubMed] [Google Scholar]
- Bafundo K.W., Duerr I., McNaughton J.L., Johnson A.B. The effects of a quillaja and yucca combination on performance and carcass traits of coccidia-vaccinated broilers exposed to an enteric disease challenge. Poultry Sci. 2021;100 doi: 10.1016/j.psj.2021.101391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bafundo K.W., Gard D.I., Schlegel B.F., Tonkinson L.V. Shuttle programs in broilers: effective use of maxiban, nicarbazin, monensin, and narasin. Poultry Sci. 1990;69(Suppl):111. [Google Scholar]
- Bafundo K.W., Cervantes H.M., Mathis G.F. Sensitivity of Eimeria field isolates in the United States: responses of nicarbazin-containing anticoccidials. Poultry Sci. 2008;87:1760–1767. doi: 10.3382/ps.2008-00129. [DOI] [PubMed] [Google Scholar]
- Bafundo K.W., Johnson A.B., Mathis G.F. The effects of a combination of Quillaja saponaria and Yucca schidigera on Eimeria spp. in broiler chickens. Avian Dis. 2020;64:300–304. doi: 10.1637/aviandiseases-D-20-00016. [DOI] [PubMed] [Google Scholar]
- Bafundo K.W., Gomez L., Lumpkins B., Mathis G.F., McNaughton J., Duerr I. Concurrent use of saponins and live coccidiosis vaccines: the influence of a quillaja/yucca combination on anticoccidial effects and performance results of vaccinated broilers. Poultry Sci. 2021;100:100905. doi: 10.1016/j.psj.2020.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball S.J. Synergistic action of sulphaquinoxaline and 2-amino-4-dimethylamino-5-(4-chlorophenyl)-6-ethylpyrimidine in caecal coccidiosis in chickens. J. Comp. Pathol. 1964;74:487–499. doi: 10.1016/s0368-1742(64)80055-2. [DOI] [PubMed] [Google Scholar]
- Ball S.J., Pittilo R.M., Norton C.C., Joyner L.P. Ultrastructural studies of the effects of amprolium and dinitolmide on Eimeria acervulina macrogametes. Parasitol. Res. 1987;73:293–297. doi: 10.1007/BF00531080. [DOI] [PubMed] [Google Scholar]
- Bampidis V., Azimonti G., et al. EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP) Safety and efficacy of Monimax® (monensin sodium and nicarbazin) for chickens for fattening and chickens reared for laying. EFSA J. 2018;16(11) doi: 10.2903/j.efsa.2018.5459. Published 2018 Nov 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beers K.W., Raup T.J., Bottje W.G., Odom T.W. Physiological responses of heat-stressed broilers fed nicarbazin. Poultry Sci. 1989;68:428–434. doi: 10.3382/ps.0680428. [DOI] [PubMed] [Google Scholar]
- Bryson V., Szybalski W. Microbial drug resistance. Adv. Genet. 1955;7:1–46. [PubMed] [Google Scholar]
- Callender M.E., Jeffers T.K. Eli Lilly and Co.; 1980. Anticoccidial Combinations Comprising Nicarbazin and the Polyether Antibiotics. assignee. US Pat. No. 4,218,438. [Google Scholar]
- Cervantes H.M. Antibiotic-free poultry production: is it sustainable? J. Appl. Poultry Res. 2015;24:91–97. [Google Scholar]
- Challey J.R., Jeffers T.K. Synergism between 4-hydroxyquinoline and pyridone coccidiostats. J. Parasitol. 1973;59:502–504. [PubMed] [Google Scholar]
- Chapman H.D. Eimeria tenella in chickens: studies on resistance to the anticoccidial drugs monensin and lasalocid. Vet. Parasitol. 1976;2:187–196. [Google Scholar]
- Chapman H.D. In: Avian Coccidiosis. Long P.L., Boorman K.N., Freeman B.M., editors. Brit. Poult Sci. Ltd; Edinburgh: 1978. Drug resistance in coccidia; pp. 387–412. [Google Scholar]
- Chapman H.D. Studies on the sensitivity of field isolates of Eimeria maxima to combinations of anticoccidial drugs. Avian Pathol. 1980;9:67–76. doi: 10.1080/03079458008418387. [DOI] [PubMed] [Google Scholar]
- Chapman H.D. In: The Biology of the Coccidia. Long P.L., editor. University Park Press; Baltimore: 1982. Anticoccidial drug resistance; pp. 429–452. [Google Scholar]
- Chapman H.D. Development by genetic recombination of a line of Eimeria tenella resistant to robenidine, decoquinate, and amprolium. Z. Parasitenkd. 1984;70:437–441. doi: 10.1007/BF00926683. [DOI] [PubMed] [Google Scholar]
- Chapman H.D. In: Coccidia and Intestinal Coccidiomorphs. Yvoré P., editor. INRA Publications; 1989. Field isolates of Eimeria tenella: sensitivity to diclazuril, maduramicin, narasin, salinomycin and a mixture of nicarbazin/narasin; pp. 323–326. (Vth International Coccidiosis Conference, Tours, France). [Google Scholar]
- Chapman H.D. A review of the biological activity of the anticoccidial drug nicarbazin and its application for the control of coccidiosis in poultry. Poultry Sci. Rev. 1994;5:231–243. [Google Scholar]
- Chapman H.D. Sensitivity of field isolates of Eimeria to monensin following the use of a coccidiosis vaccine in broiler chickens. Poultry Sci. 1994;73:476–478. doi: 10.3382/ps.0730476. [DOI] [PubMed] [Google Scholar]
- Chapman H.D. Biochemical, genetic, and applied aspects of drug resistance in Eimeria parasites of the fowl. Avian Pathol. 1997;26:221–244. doi: 10.1080/03079459708419208. [DOI] [PubMed] [Google Scholar]
- Chapman H.D. Anticoccidial drugs and their effects upon the development of immunity to Eimeria infections in poultry. Avian Pathol. 1999;28:521–535. doi: 10.1080/03079459994317. [DOI] [PubMed] [Google Scholar]
- Chapman H.D. Use of anticoccidial drugs in broiler chickens in the USA: analysis for the years 1995-1999. Poultry Sci. 2001;80:572–580. doi: 10.1093/ps/80.5.572. [DOI] [PubMed] [Google Scholar]
- Chapman H.D. A landmark contribution to poultry science – prophylactic control of coccidiosis in poultry. Poultry Sci. 2009;88:813–815. doi: 10.3382/ps.2008-00316. [DOI] [PubMed] [Google Scholar]
- Chapman H.D. In: Egg Innovations and Strategies for Improvements. Hester P.Y., editor. Academic Press; 2017. Coccidiosis in egg laying poultry; pp. 571–577. [Google Scholar]
- Chapman H.D. Applied strategies for the control of coccidiosis in poultry. CAB Rev. 2018;13(No.26):1–11. doi: 10.1079/PAVSNNR201813026. [DOI] [Google Scholar]
- Chapman H.D., Jeffers T.K. Vaccination of chickens against coccidiosis ameliorates drug resistance in commercial poultry production. Int. J. Parasitol. Drugs Drug Resist. 2014;4:214–217. doi: 10.1016/j.ijpddr.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman H.D., Jeffers T.K. Restoration of sensitivity to salinomycin in Eimeria following 5 flocks of broiler chickens reared in floor-pens using drug programs and vaccination to control coccidiosis. Poultry Sci. 2015;94:943–946. doi: 10.3382/ps/pev077. [DOI] [PubMed] [Google Scholar]
- Chapman H.D., Jeffers T.K., Williams R.B. Forty years of monensin for the control of coccidiosis in poultry. Poultry Sci. 2010;89:1788–1801. doi: 10.3382/ps.2010-00931. [DOI] [PubMed] [Google Scholar]
- Chapman H.D., Barta J.R., Blake D., Gruber A., Jenkins M., Smith N.C., Suo X., Tomley F.M. In: Rollinson D., editor. vol. 83. Academic Press; Amsterdam, The Netherlands: 2013. A selective review of advances in coccidiosis research; pp. 93–171. (Adv. Parasitol). [DOI] [PubMed] [Google Scholar]
- Clarke M.L. A mixture of diaveridine and sulphaquinoxaline as a coccidiostat for poultry- I. Preliminary studies on efficiency against Eimeria tenella and E. necatrix infections, and on toxicity in poultry. Vet. Rec. 1962;74:845–848. [Google Scholar]
- Clarke M.L. A mixture of diaveridine and sulphaquinoxaline as a coccidiostat for poultry II. Efficiency against Eimeria acervulina, E. brunetti, and E. maxima infections together with a field survey of coccidiosis in S.E. England. Vet. Rec. 1964;76:818–822. [Google Scholar]
- Cuckler A.C., Malanga C.M. The effect of nicarbazin on the development of immunity to avian coccidia. J. Parasitol. 1956;42:593–607. [PubMed] [Google Scholar]
- Cuckler A.C., Malanga C.M., Basso A.J., O'Neill R.C. Antiparasitic activity of substituted carbanilide complexes. Science. 1955;122:244–245. doi: 10.1126/science.122.3162.244-a. [DOI] [PubMed] [Google Scholar]
- Cuckler A.C., McManus E.C., Campbell W.C. Development of resistance in coccidia. Acta Vet. 1969;38:87–99. [Google Scholar]
- Davies S.F.M., Joyner L.P. Design of therapy for the control of species of Eimeria in the domestic fowl. J. Comp. Pathol. 1963;73:379–390. doi: 10.1016/s0368-1742(63)80040-5. [DOI] [PubMed] [Google Scholar]
- Davies S.F.M., Kendall S.B. The practical application of sulphamezathine therapy for caecal coccidiosis. Vet. Rec. 1954;66:19–21. [Google Scholar]
- del Cacho E., Gallego M., Sánchez-Acedo C., Lillehoj H.S. Expression of flotillin-1 on Eimeria tenella sporozoites and its role in host cell invasion. J. Parasitol. 2007;93:328–332. doi: 10.1645/GE-992R.1. [DOI] [PubMed] [Google Scholar]
- Dougherty H.M. Inhibition of mitochondrial energy transduction by carbanilides. Fed. Proc. 1974;33:1657. [Google Scholar]
- Dowling L. Ionophore toxicity in chickens: a review of pathology and diagnosis. Avian Pathol. 1992;21:355–368. doi: 10.1080/03079459208418854. [DOI] [PubMed] [Google Scholar]
- Federal Register Final rule; withdrawal of approval of new animal drug applications. Carbarsone; Roxarsone. 2013 November 22 78 FR 69992. [Google Scholar]
- Fry M., Williams R.B. Effects of decoquinate and clopidol on electron transport in mitochondria of Eimeria tenella (Apicomplexa: coccidia) Biochem. Pharmacol. 1984;33:229–240. doi: 10.1016/0006-2952(84)90480-5. [DOI] [PubMed] [Google Scholar]
- Goetting V., Lee K.A., Tell L.A. Pharmacokinetics of veterinary drugs in laying hens and residues in eggs: a review of the literature. J. Vet. Pharmacol. Therapeut. 2011;34:521–556. doi: 10.1111/j.1365-2885.2011.01287.x. [DOI] [PubMed] [Google Scholar]
- Harris G.C., Macy L.B. Effect of nicarbazin and MaxibanTM on broiler performance when fed to different age broiler males subjected to environmental stress. Poultry Sci. 1988;67(Suppl 1):16. [Google Scholar]
- Hodgson J.N., Ball S.J., Ryan K.C., Warren E.W. The incidence of drug resistant strains of Eimeria in chickens in Great Britain, 1966. Br. Vet. J. 1969;125:31–35. doi: 10.1016/s0007-1935(17)49161-5. [DOI] [PubMed] [Google Scholar]
- Horton-Smith C., Long P.L., Collier H.O.J. Potentiation of sulfadimidine by 2,4-diamino-6,7-di-isopropylpteridine and other 6,7-disubstituted 2,4-diaminopteridines against Eimeria infections of chicks. Br. J. Pharmacol. 1960;15:298–303. doi: 10.1111/j.1476-5381.1960.tb01248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James S. Thiamine uptake in isolated schizonts of Eimeria tenella and the inhibitory effects of amprolium. Parasitology. 1980;80:313–322. doi: 10.1017/s0031182000000779. [DOI] [PubMed] [Google Scholar]
- Jeffers T.K. Genetic transfer of anticoccidial drug resistance in Eimeria tenella. J. Parasitol. 1974;60:900–904. [PubMed] [Google Scholar]
- Jeffers T.K. Eimeria tenella: incidence, distribution, and anticoccidial drug resistance of isolants in major broiler-producing areas. Avian Dis. 1974;18:74–84. [PubMed] [Google Scholar]
- Jeffers T.K. Eimeria acervulina and E. maxima: incidence, distribution, and anticoccidial drug resistance of isolants in major broiler-producing areas. Avian Dis. 1974;18:331–342. [PubMed] [Google Scholar]
- Jeffers T.K. In: Coccidia and Intestinal Coccidiomorphs. Yvoré P., editor. INRA Publications; Tours, France: 1989. Anticoccidial drug resistance: a review with emphasis on the polyether ionophores; pp. 295–308. (Proc. Vth International Coccidiosis Conference). [Google Scholar]
- Jeffers T.K., Challey J.R. Collateral sensitivity to 4-hydroxyquinolines in Eimeria acervulina strains resistant to meticlorpindol. J. Parasitol. 1973;59:624–630. [PubMed] [Google Scholar]
- Jones F.T. A broad view of arsenic. Poultry Sci. 2007;86:2–14. doi: 10.1093/ps/86.1.2. [DOI] [PubMed] [Google Scholar]
- Joyner L.P. Coccidiosis: problems arising from the development of anticoccidial drug resistance. Exp. Parasitol. 1970;28:122–128. doi: 10.1016/0014-4894(70)90077-9. [DOI] [PubMed] [Google Scholar]
- Joyner L.P., Kendall S.B. Synergism in the chemotherapy of Eimeria tenella. Nature. 1955;176:975. doi: 10.1038/176975a0. [DOI] [PubMed] [Google Scholar]
- Joyner L.P., Norton C.C. Transferred drug resistance in Eimeria maxima. Parasitology. 1975;71:385–392. doi: 10.1017/s0031182000047168. [DOI] [PubMed] [Google Scholar]
- Joyner L.P., Norton C.C. Further observations on the genetic transfer of drug resistance in Eimeria maxima. Parasitology. 1977;74:205–213. [Google Scholar]
- Joyner L.P., Norton C.C. The activity of methyl benzoquate and clopidol against Eimeria maxima: synergy and drug resistance. Parasitology. 1978;76:369–377. doi: 10.1017/s003118200004823x. [DOI] [PubMed] [Google Scholar]
- Karavolias J., Salois M.J., Baker K.T., Watkins K. Raised without antibiotics: impact on animal welfare and implications for food policy. Transl. Anim. Sci. 2018;2:337–348. doi: 10.1093/tas/txy016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson T., Reid W.M. Development of immunity to coccidiosis in chickens administered anticoccidials in the feed. Avian Dis. 1978;22:487–495. [PubMed] [Google Scholar]
- Kawazoe U., Chapman H.D., Shaw M. Sensitivity of field isolates of E. acervulina to salinomycin, maduramicin, and a mixture of clopidol and methyl benzoquate in the chicken. Avian Pathol. 1991;20:439–446. doi: 10.1080/03079459108418782. [DOI] [PubMed] [Google Scholar]
- Kendall S.B. Synergy between pyrimethamine and sulphonamides used in the control of Eimeria tenella. Proc. Roy. Soc. Med. 1956;49:874–877. [PubMed] [Google Scholar]
- Kendall S.B., Joyner L.P. The synergism between pyrimethamine and sulphadimethylpyrimidine in the control of Eimeria tenella. J. Comp. Pathol. 1956;66:145–150. doi: 10.1016/s0368-1742(56)80015-5. [DOI] [PubMed] [Google Scholar]
- Keshavarz K., McDougald L.R. Anticoccidial drugs: growth and performance depressing effects in young chickens. Poultry Sci. 1982;61:699–705. doi: 10.3382/ps.0610699. [DOI] [PubMed] [Google Scholar]
- Kraieski A.L., Salles G.B.C., Muniz E.C., Nascimento D.V.J., Lima Neto A.J., Santos I.L., Madeira A.M.B.N. Sensitivity of field isolates of Eimeria acervulina and E. maxima from three regions in Brazil to eight anticoccidial drugs. Poultry Sci. 2021;100 doi: 10.1016/j.psj.2021.101233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanckriet A., Timbermont L., De Gussem M., Marien M., Vancraeynest D., Haesebrouck F., Ducatelle R., Van Immerseel F. The effect of commonly used anticoccidials and antibiotics in a subclinical necrotic enteritis model. Avian Pathol. 2010;39:63–68. doi: 10.1080/03079450903505771. [DOI] [PubMed] [Google Scholar]
- Lima A.L., Barreto F., Rau R.B., Silva G.R.D., Lara L.J.C., Figueiredo T.C., Assis D.C.S., Cançado S.D.V. Determination of the residue levels of nicarbazin and combination nicarbazin-narasin in broiler chickens after oral administration. PLoS One. 2017;12(7) doi: 10.1371/journal.pone.0181755. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long P.L. The effect of a combination of sulphaquinoxaline and amprolium against different species of Eimeria in chickens. Vet. Rec. 1963;75:645–650. [Google Scholar]
- Long P.L., Joyner L.P., Millard B.J., Norton C.C. A guide to the laboratory techniques used in the study and diagnosis of avian coccidiosis. Folia Vet. Lat. 1976;6:201–217. [PubMed] [Google Scholar]
- Long P.L., Johnson J., McKenzie M.E. Anticoccidial activity of combinations of narasin and nicarbazin. Poultry Sci. 1988;67:248–252. doi: 10.3382/ps.0670248. [DOI] [PubMed] [Google Scholar]
- Lux R.E. The chemotherapy of Eimeria tenella I. Dihydropyrimidines and dihydrotriazenes. Antibiot. Chemother. (Wash. D C) 1954;4:971–977. [PubMed] [Google Scholar]
- Malik T.A., Kamili A.N., Chishti M.Z., Tanveer S., Ahad S., Johri R.K. Synergistic approach for treatment of chicken coccidiosis using berberine – a plant natural product. Microb. Pathog. 2016;93:56–62. doi: 10.1016/j.micpath.2016.01.012. [DOI] [PubMed] [Google Scholar]
- Mathis G.F., Broussard C. Increased level of Eimeria sensitivity to diclazuril after using a live coccidiosis vaccine. Avian Dis. 2006;50:321–324. doi: 10.1637/7455-101305R1.1. [DOI] [PubMed] [Google Scholar]
- Mathis G.F., McDougald L.R., McMurray B. Effectiveness of therapeutic anticoccidial drugs against recently isolated coccidia. Poultry Sci. 1984;63:1149–1153. doi: 10.3382/ps.0631149. [DOI] [PubMed] [Google Scholar]
- McDougald L.R. Eli Lilly and Co.; 1977. Coccidiocidal Combination of Monensin and Metichlorpindol. assignee. US Pat. No. 4,061,755. [Google Scholar]
- McDougald L.R. Eli Lilly and Co.; 1978. Coccidiocidal Combinations. assignee. US Pat. No. 4,083,962. [Google Scholar]
- McDougald L.R. In: Diseases of Poultry. twelfth ed. Saif Y.M., Fadly A.M., Glisson J.R., McDougald L.R., Nolan L.K., Swayne D.E., editors. Blackwell Publishing; 2008. Protozoal infections; pp. 1067–1085. [Google Scholar]
- McDougald L.R., McQuistion T.E. Mortality from heat stress in broiler chickens influenced by anticoccidial drugs. Poultry Sci. 1980;59:2421–2423. [PubMed] [Google Scholar]
- McDougald L.R., Keshavarz K., Rosenstein M. Anticoccidial activity of salinomycin (AHR-3096C) and compatibility with roxarsone in floor-pen experiments with broilers. Poultry Sci. 1981;60:2416–2422. doi: 10.3382/ps.0602416. [DOI] [PubMed] [Google Scholar]
- McDougald L.R., Fuller L., Solis J. Drug-sensitivity of 99 isolates of coccidia from broiler farms. Avian Dis. 1986;30:690–694. [PubMed] [Google Scholar]
- McLoughlin D.K., Chute M.B. Efficacy of Novastat against twelve strains of Eimeria tenella and the development of a Novastat-resistant strain. Avian Dis. 1973;17:582–585. [PubMed] [Google Scholar]
- McLoughlin D.K., Chute M.B. Sequential use of coccidiostats: effect on development by Eimeria tenella of resistance to amprolium, nicarbazin, Unistat and zoalene. Avian Dis. 1975;19:424–428. [PubMed] [Google Scholar]
- McLoughlin D.K., Gardiner J.L. Drug resistance in Eimeria tenella: V. the experimental development of a nicarbazin-resistant strain. J. Parasitol. 1967;53:930–932. [PubMed] [Google Scholar]
- McManus E.C., Oberdick M.T., Cuckler A.C. Response of six strains of Eimeria brunetti to two antagonists of para-aminobenzoic acid. J. Protozool. 1967;14:379–381. doi: 10.1111/j.1550-7408.1967.tb02013.x. [DOI] [PubMed] [Google Scholar]
- Mesa C., Gómez-Osorio L.M., López-Osorio S., Williams S.M., Chaparro-Gutiérrez J.J. Survey of coccidia on commercial broiler farms in Colombia: frequency of Eimeria species, anticoccidial sensitivity, and histopathology. Poultry Sci. 2021;100 doi: 10.1016/j.psj.2021.101239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthamilselvan T., Kuo T., Wu Y., Yang W. Herbal remedies for coccidiosis control: a review of plants, compounds, and anticoccidial actions. Evid. Based Complement. Alternat. Med. 2016;2016 doi: 10.1155/2016/2657981. Epub 2016 Jun 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noack S., Chapman H.D., Selzer P.M. Anticoccidial drugs of the livestock industry. Parasitol. Res. 2019;118:2009–2026. doi: 10.1007/s00436-019-06343-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton C.C., Joyner L.P. The development of drug-resistant strains of Eimeria maxima in the laboratory. Parasitology. 1975;71:153–165. doi: 10.1017/s0031182000053233. [DOI] [PubMed] [Google Scholar]
- Norton C.C., Joyner L.P. The appearance of bisporocystic oocysts of Eimeria maxima in drug treated chicks. Parasitology. 1978;77:243–248. doi: 10.1017/s0031182000050216. [DOI] [PubMed] [Google Scholar]
- Parker C.D., Lister S.A., Gittins J. Impact assessment of the reduction or removal of ionophores used for controlling coccidiosis in the UK broiler industry. Vet. Rec. 2021;189:e513. doi: 10.1002/vetr.513. [DOI] [PubMed] [Google Scholar]
- Peek H.W., Landman W.J.M. Resistance to anticoccidial drugs of Dutch avian Eimeria spp. field isolates originating from 1996, 1999 and 2001. Avian Pathol. 2003;32:391–401. doi: 10.1080/0307945031000121149. [DOI] [PubMed] [Google Scholar]
- Peek H.W., Landman W.J.M. Higher incidence of Eimeria spp. field isolates sensitive for diclazuril and monensin associated with the use of live coccidiosis vaccination with ParacoxTM-5 in broiler farms. Avian Dis. 2006;50:434–439. doi: 10.1637/7486-121205R.1. [DOI] [PubMed] [Google Scholar]
- Peeters J.E., Derijcke J., Verlinden M., Wyffels R. Sensitivity of avian Eimeria spp. to seven chemical and five ionophore anticoccidials in five Belgian integrated broiler operations. Avian Dis. 1994;38:483–493. [PubMed] [Google Scholar]
- Reece R.L. Review of adverse effects of chemotherapeutic agents in poultry. World’s Poult. Sci. J. 1988;44:193–216. [Google Scholar]
- Register Federal. 2014. Final Rule; Technical Amendment; Zoetis Inc.; Withdrawal of Approval of New Animal Drug Applications for Combination Drug Medicated Feeds Containing an Arsenical Drug, February 27, 2014; p. 79. FR 10976. [Google Scholar]
- Reid W.M. Anticoccidials used in the poultry industry: time of action against the coccidial life cycle. Folia Vet. Lat. 1972;2:641–667. [Google Scholar]
- Rogers E.F. Thiamine antagonists. Ann. N. Y. Acad. Sci. 1962;98:412–429. doi: 10.1111/j.1749-6632.1962.tb30563.x. [DOI] [PubMed] [Google Scholar]
- Ryley J.F. Lerbek, a synergistic mixture of methyl benzoquate and clopidol for the prevention of chicken coccidiosis. Parasitology. 1975;70:377–384. doi: 10.1017/s0031182000052148. [DOI] [PubMed] [Google Scholar]
- Ryley J.F. Drug resistance in coccidia. Adv. Vet. Sci. Comp. Med. 1980;24:99–120. [PubMed] [Google Scholar]
- Ryley J.F., Betts M.J. Chemotherapy of chicken coccidiosis. Adv. Pharmacol. Chemother. 1973;11:221–293. doi: 10.1016/s1054-3589(08)60459-7. [DOI] [PubMed] [Google Scholar]
- Ryley J.F., Wilson R.G. Laboratory studies with some older anticoccidials. Parasitology. 1976;73:287–309. doi: 10.1017/s0031182000046989. [DOI] [PubMed] [Google Scholar]
- Smith II C.K., Galloway R.B. Influence of monensin on the cation influx and glycolysis of Eimeria tenella sporozoites in vitro. J. Parasitol. 1983;69:666–670. [PubMed] [Google Scholar]
- Smith II C.K., Strout R.G. Eimeria tenella: accumulation and retention of anticoccidial ionophores by extracellular sporozoites. Exp. Parasitol. 1979;48:325–330. doi: 10.1016/0014-4894(79)90115-2. [DOI] [PubMed] [Google Scholar]
- Smith II C.K., Galloway R.B., White S.L. Effect of ionophores on survival, penetration, and development of Eimeria tenella sporozoites in vitro. J. Parasitol. 1981;67:511–516. [PubMed] [Google Scholar]
- Tamas T., Wilks G. Development of resistance against narasin and narasin+nicarbazin. Poultry Sci. 1989;68(Suppl. 1):146. [Google Scholar]
- Tonkinson L.V., Jeffers T.K., Callender M.E. Anticoccidial efficacy of various ratios of narasin and nicarbazin. Poultry Sci. 1987;66(Suppl. 1):187. [Google Scholar]
- Vereecken M., Dehaeck B., Berge A.C., Marien M., Geerinckx M., De Gussem K. Synergistic effect of a combination of nicarbazin and monensin against coccidiosis in the chicken caused by Eimeria spp. Avian Pathol. 2020;49:389–393. doi: 10.1080/03079457.2020.1756226. [DOI] [PubMed] [Google Scholar]
- Vereecken M., Dehaeck B., Rathinam T., Schelstraete W., De Gussem K., Chapman H.D. Restoration of the sensitivity of Eimeria acervulina to anticoccidial drugs in the chicken following use of a live coccidiosis vaccine. Vet. Parasitol. 2021;292 doi: 10.1016/j.vetpar.2021.109416. [DOI] [PubMed] [Google Scholar]
- Wang C.C. Studies of the mitochondria from Eimeria tenella and inhibition of the electron transport by quinolone coccidiostats. Biochim. Biophys. Acta. 1975;396:210–219. doi: 10.1016/0005-2728(75)90035-3. [DOI] [PubMed] [Google Scholar]
- Wang C.C. Inhibition of the respiration of Eimeria tenella by quinolone coccidiostats. Biochem. Pharmacol. 1976;25:343–349. doi: 10.1016/0006-2952(76)90225-2. [DOI] [PubMed] [Google Scholar]
- Wang C.C. In: Avian Coccidiosis. Proc. 13th Poultry Science Symposium. Long P.L., Boorman K.N., Freeman B.M., editors. British Poultry Science Ltd; Edinburgh, UK: 1978. Biochemical and nutritional aspects of coccidia; pp. 135–184. [Google Scholar]
- Wang C.C. In: The Biology of the Coccidia. Long P.L., editor. Baltimore University Park Press; Baltimore: 1982. Biochemistry and physiology of coccidia; pp. 167–228. [Google Scholar]
- Wang C.C., Stotish R.L., Poe M. Dihydrofolate reductase from Eimeria tenella: rationalization of chemotherapeutic efficacy of pyrimethamine. J. Protozool. 1975;22:564–568. doi: 10.1111/j.1550-7408.1975.tb05234.x. [DOI] [PubMed] [Google Scholar]
- Warren E.W., Ball S.J., Mackenzie D.R. The incidence of drug-resistant strains of Eimeria species in chickens in Great Britain, 1964/65. Br. Vet. J. 1966;122:534–543. doi: 10.1016/s0007-1935(17)49161-5. [DOI] [PubMed] [Google Scholar]
- Weppelman R.M., Battaglia J.A., Wang C.C. Eimeria tenella: the selection and frequency of drug-resistant mutants. Exp. Parasitol. 1977;42:56–66. doi: 10.1016/0014-4894(77)90061-3. [DOI] [PubMed] [Google Scholar]
- White G., Williams R.B. Evaluation of a mixture of trimethoprim and sulphaquinoxaline for the treatment of bacterial and coccidial diseases of poultry. Vet. Rec. 1983;113:608–612. [PubMed] [Google Scholar]
- Wiernusz C.J., Teeter R.G. Nicarbazin effects on broiler thermobalance during high ambient temperature stress. Poultry Sci. 1995;74:577–580. doi: 10.3382/ps.0740577. [DOI] [PubMed] [Google Scholar]
- Williams R.B. Analysis of the phenotypes in field populations of E. acervulina expressing dual drug-resistance to decoquinate and clopidol. Avian Pathol. 1998;27:67–73. doi: 10.1080/03079459808419276. [DOI] [PubMed] [Google Scholar]