RATIONALE
Resuscitative Endovascular Balloon Occlusion of the AORTA (REBOA) has continued to evolve as a viable tool of modern trauma resuscitation.(1-10) Developed from the convergence of trauma and endovascular surgery (1-3), REBOA has increasingly been employed at select centers as a resuscitative adjunct for trauma patients with life threatening non-compressible truncal hemorrhage.(4-11) Even as prospective registry data seeks to capture and analyse outcomes of early REBOA utilization, on-going device innovation and technique refinement seek to mitigate potential risks associated with aortic occlusion.
It is hypothesized that early utilization of REBOA preserves cerebral perfusion and coronary filling in the setting of life-threatening hypotension and hypovolemia secondary to hemorrhage. Laboratory data has demonstrated that the use of REBOA in the setting of hemorrhagic shock results in increased central aortic pressure, carotid flow, and brain oxygenation(1-4) and early reports suggest REBOA may improve outcomes in select patient populations.(6,7) These benefits of REBOA must be weighed against the consequence of sustained complete aortic occlusion, primarily profound distal ischemia and associated reperfusion injury. (1,12-15) Continued refinement of aortic occlusion techniques are required to minimize these adverse effects, improve outcomes, and expand the populations of patients who will experience benefits from the resuscitative capabilities of REBOA.
One method to decrease distal ischemia and reperfusion injury is to allow titrated controlled low-volume aortic flow distal to the site of occlusion, a technique termed partial-REBOA (P-REBOA).(16) P-REBOA has the potential to maintain the benefits of preserved perfusion above the level of occlusion, while creating permissive regional hypoperfusion to areas of uncontrolled hemorrhage distal to the balloon. This strategy aims to strike a balance between minimizing ongoing hemorrhage and lessening distal ischemia, a balance driven by the rate of aortic blood flow past the balloon. Additionally, P-REBOA may serve to mitigate the potentially detrimental effects of supraphysiologic proximal pressure and afterload associated with complete aortic occlusion. Regardless of the aortic zone of deployment, P-REBOA as an adjunct to complete occlusion may decrease the degree of distal ischemia and minimize the risk for significant acute reperfusion injury after complete balloon deflation. We describe below the key steps required to effectively employ P-REBOA using existing technologies.
DESCRIPTION OF TECHNIQUE FOR PARTIAL REBOA
STEP 1: ESTABLISHING REBOA
Establishing arterial access and the introduction of wire and REBOA catheters has been previously described. [2] Briefly, current technologies for REBOA require arterial access through the common femoral artery. Arterial access is accomplished via percutaneous ultrasound-guided access, surgical cut-down, or by upsizing existing femoral access to accommodate the large sheaths required for some types of REBOA catheters. Selection and positioning of the initial sheath is predicated on the type of catheter used for REBOA (Table 1). Recent advances in balloon development, specifically the newly available FDA approved REBOA-ER®, allow for percutaneous access through a 7 French sheath. The ER-REBOA catheter should then be secured in place using the stabilization device that comes with most central venous access kits or by directly suturing it in place. It is imperative that a reliable method of stabilization be used, as transitioning from complete occlusion to partial causes loss of friction between the aortic wall and balloon can result in rapid distal migration of the balloon.
Table 1:
Balloon Catheters for P-REBOA
| Size | Minimal Sheath Requirement |
Balloon Volume (mL) |
Maximum Diameter (mm) |
Advantages | |
|---|---|---|---|---|---|
| Coda | 12 Fr | 12 Fr | 60 mL | 46 | Common, low cost, majority of REBOA cases to date |
| Coda-LP | 9 Fr | 9 Fr | 30 mL | 32 | Common, low cost, lower profile than Coda |
| REBOA-ER | 7 Fr | 7 Fr | 24 mL | 32 | Low profile, integrated proximal pressure port |
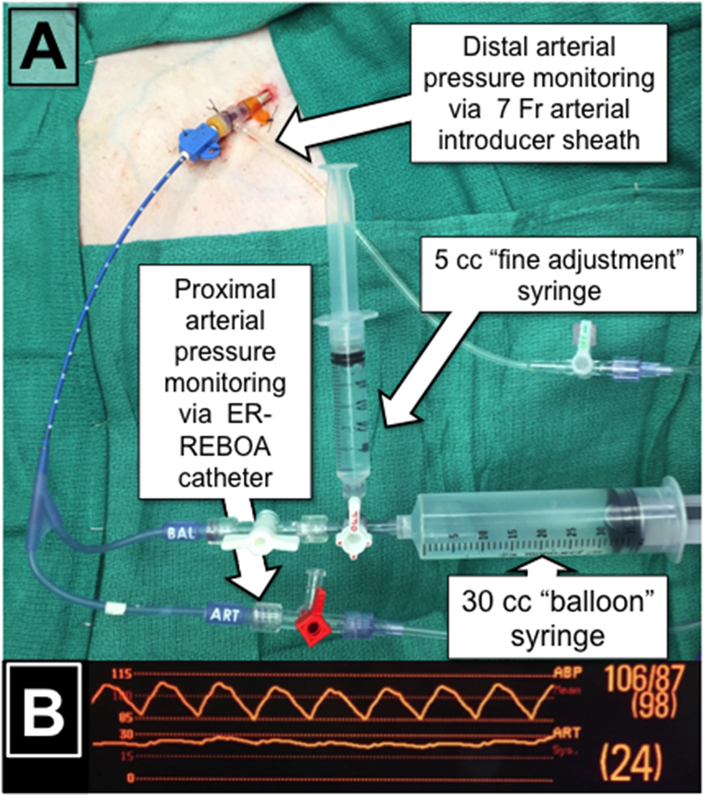
Time and resources permitting, a 3-way stopcock may be fitted onto the balloon port of the catheter with a large syringe filled with saline, for initial balloon inflation (20-30cc depending on chosen catheter), as well as an empty 3-5cc syringe (Figure 1A). Setup of the catheter with both syringes prior to insertion allows for greater fidelity when regulating balloon volumes during P-REBOA as described below. If complete assembly of a two-syringe system is not feasible, including a 3-way stopcock between the large insufflation syringe and the REBOA catheter will allow for a smaller syringe to be added once P-REBOA is initiated.
Figure 1:
(A) Typical syringe setup with ER-REBOA catheter for P-REBOA (B) Proximal and distal blood pressures from a single ER-REBOA catheter placed through a 7 French sheath.
STEP 2: PROXIMAL AND DISTAL PRESSURE MONITORING
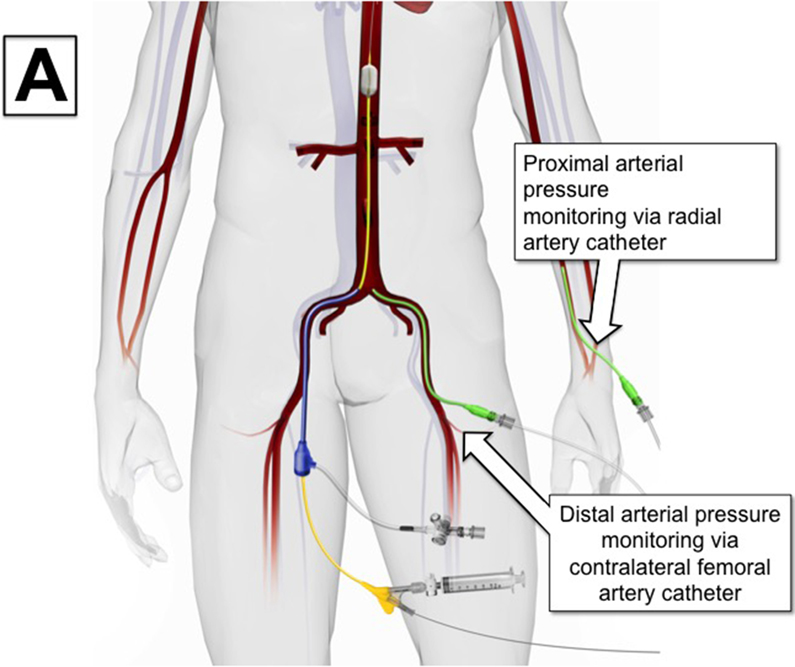
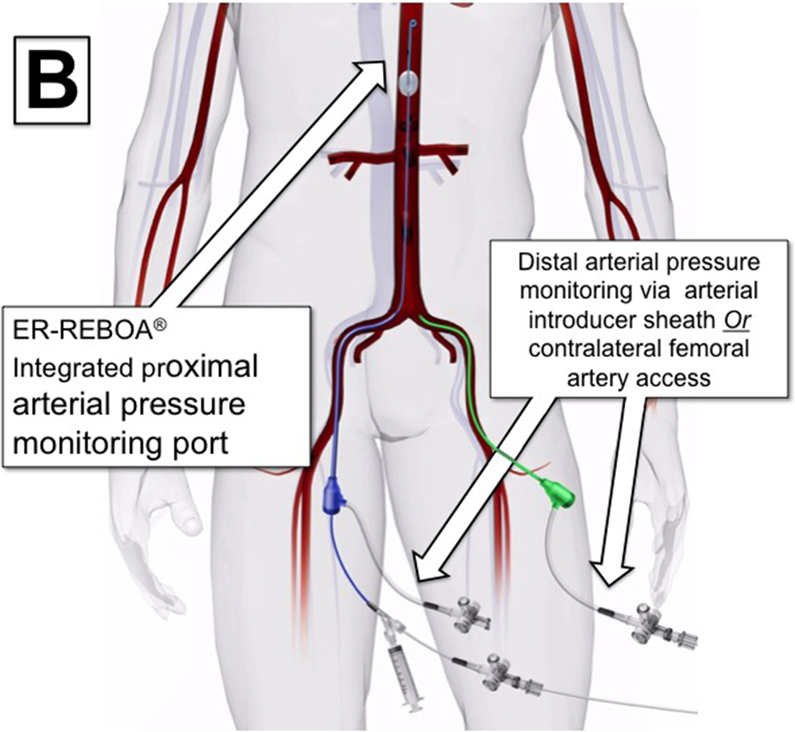
Partial REBOA can be considered when proximal hemodynamics suggest resuscitation reserves are adequate to support titrated distal perfusion. The first step to transition to P-REBOA is to obtain arterial blood pressure monitoring proximal and distal to the site of aortic occlusion. This can be accomplished by three methods. When using catheters that do not have proximal blood pressure monitoring capabilities, proximal blood pressure should be obtained using a radial or brachial arterial line. Distal arterial monitoring can then be accomplished either by placement of an arterial line in the contralateral femoral artery from the REBOA catheter or by direct transduction of arterial pressure from the flush-port of the arterial sheath (Figure 2A). In circumstances when a REBOA-ER® catheter is used, proximal pressure can be transduced directly off the catheter’s integrated proximal pressure port (Figure 2B, Figure 1A). It should be noted that contralateral groin access may be required for angiographic procedures, limiting the ability to maintain distal monitoring in some situations.
Figure 2:
Variations on Proximal and Distal Arterial Pressure Monitoring
Current bedside monitors capable of transducing two pressure tracings simultaneously are required for optimization of P-REBOA (Figure 1B). The tracing corresponding to the distal pressure should be scaled to detect low pressure, typically in the range of 0-30 mmHg. A small amount of distal pressure should be detected even in the presence of complete REBOA due to collateral circulation and the inherent pressurization of distal vascular beds. A faintly pulsatile waveform may be encountered, but is not typical in settings of severe volume depletion and shock. Once proximal and distal arterial pressures have been transduced, the patient’s physiologic ability to tolerate partial distal perfusion should be assessed by taking into account ongoing resuscitation, physiologic response to REBOA, and injury characteristics.
STEP 3: INTRODUCTION OF DISTAL FLOW
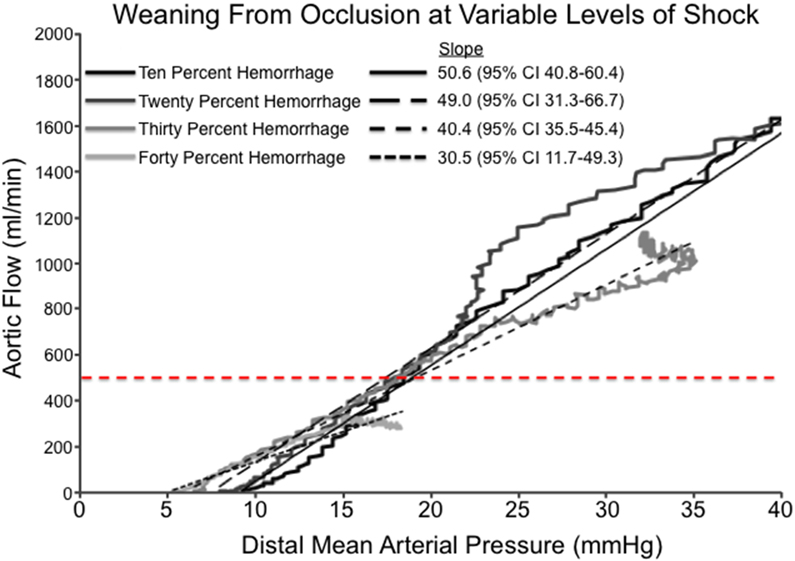
In the authors’ translational and clinical experience with P-REBOA, an initial period of complete occlusion is advisable to allow for assessment of hemodynamics and time for clot stabilization prior to transitioning to P-REBOA. Provided appropriate resuscitation efforts and sufficient proximal mean arterial pressure are present, the provider may consider the reintroduction of distal flow. Titration of distal flow is based off careful observation of the distal arterial pressure waveform. Experience in swine models of partial REBOA suggests that titration from complete occlusion to full aortic flow occurs over an extremely narrow range of balloon volume. Initial titration of balloon volume should commence by removing 0.5cc of saline at a time using a large volume syringe until a pulsatile waveform is observed in the distal arterial pressure reading (Figure 1B). At this time a period of observation of the proximal hemodynamics should be allowed to determine if any further distal perfusion will be tolerated by the current physiologic state. If proximal hemodynamics allow, further deflation of the REBOA balloon from initial pulsatile waveforms to goal distal systolic pressures should be carried out using a 3-5cc syringe with small incremental changes every 30 seconds to allow for hemodynamic equilibrium at each step. An initial target distal mean arterial pressure should not exceed an increase of 10 mmHg over the baseline pressure. Swine experiments have demonstrated that this 10mmHg increase in distal MAP corresponds with an aortic flow of 250-500 ml/min dependent upon the severity of shock (Figure 3A). From the author’s experience, initial flow rates greater than 500 ml/min may lead to hemodynamic destabilization, thus further balloon deflation to increase distal pressure and flow should only be attempted after hemodynamics stabilize.
Figure 3:
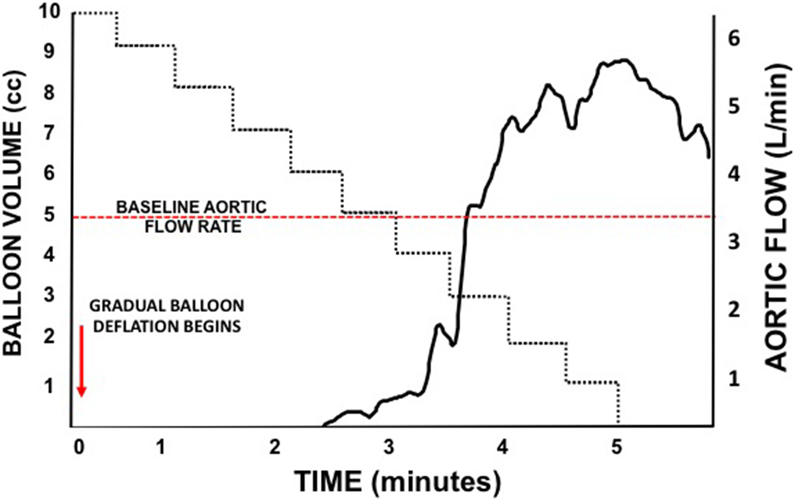
(A) Relationship between distal mean arterial blood pressure and aortic flow during computer controlled reinstitution of aortic flow after complete occlusion in a swine model of progressive iterative hemorrhage. (B) Representative Aortic Flow During Iterative Coda Balloon Deflation over a 5-minute period. Aortic flow resumes after half the balloon volume is removed and within a small range of continued deflation, rebounds to nearly twice baseline aortic flow rates.
The provider must realize that the range of balloon deflation to achieve stable distal flow is very narrow. Animal models have demonstrated that small changes in balloon size by even 0.5cc of saline result in exponential changes in flow across the balloon (Figure 3B). Furthermore, the distal vasodilation that occurs rapidly during even transient periods of occlusion combined with a high proximal distal pressure gradients can result in rapid increases in aortic flow rates. These large changes in distal hemodynamics are hypothesized to lead to clot destabilization and ongoing hemorrhage. Aortic diameter, the product of volume status, catecholamine response, and individual patient physiology will also result in differences in the relationship between aortic flow rates and the level of balloon deflation. The response of individual patients to ongoing resuscitation will likely make this relationship dynamic and a dedicated person to continuously titrate balloon volume, when available, is recommended.
STEP 4: MAINTENANCE OF PARTIAL REBOA
While not directly measured, the main determinate of distal perfusion is the aortic flow that results from the complex interplay between the proximal to distal pressure gradient, intravascular volume status, cardiac performance, vascular tone, aortic diameter, and the degree of balloon inflation. The dynamic nature of these interrelated factors demands constant attention to the absolute proximal and distal pressure and associated waveforms. Although the physiologic mechanisms driving response to P-REBOA are complex, balloon volume and the extent of resuscitation are the only variables that can be manipulated until hemorrhagic control is obtained. In order for P-REBOA to be successful, careful attention to maintain a blunted distal systolic pressure wave and ensuring no more than a 10 mmHg increase in distal mean arterial pressure while ensuring adequacy of proximal physiology is required. An initial favorable hemodynamic response to P-REBOA may not be sustained due to redistribution of blood volume, ongoing hemorrhage, and the systemic effects of anaerobic metabolites. Attention to detail with rapid responses to decreased proximal pressures by reverting back to a complete occlusive state may be necessary. After stabilization and in the appropriate clinical context, more liberal weaning, as described below, can occur.
STEP 5: WEANING FROM PARTIAL OR COMPLETE REBOA
Large animal data suggests that weaning from REBOA is better tolerated if a period of P-REBOA has been achieved, but the following protocol for weaning can be used to wean from either partial or complete occlusion. An initial goal is to increase the distal systolic arterial pressure by 50% from the current baseline in sequential steps every 5 minutes. Suggestions of 5-minute increments are based upon the known dynamic physiologic changes that occur as distal ischemic metabolites are washed out into the proximal circulation where they can have effects on vascular tone and cardiac output. As noted when establishing P-REBOA during STEP 3, small changes in balloon volume will result in large changes in aortic flow. Consideration of the duration of complete and partial aortic occlusion is necessary to estimate the extent of metabolic burden while weaning to zero occlusion. Short periods of complete REBOA may allow for relatively rapid balloon deflation without subsequent hemodynamic decompensation. If however the complete occlusive phase has been prolonged or larger metabolic burdens are suspected, a balance must be achieved between expedited reintroduction of flow to ischemic distal tissue beds and the known physiologic instability that results from ischemia-reperfusion. In these instances, balloon deflation should occur in a more gradual fashion.
STEP 6: REMOVAL OF THE CATHETER AND SHEATH
Catheter and sheath removal have been previously described. [2] Removal of common femoral sheath access must be undertaken once stabilization and determination that femoral access is no longer required. The choice of technique for sheath removal is dependent on the access size, potentially requiring direct repair for access >7.5F. A thorough assessment of lower extremity perfusion ipsilateral to the site of access is important following femoral access removal, particularly if a sheath is to be left in place. Advances in REBOA catheters now on the market have made it possible for smaller femoral sheaths to be used that do not necessitate surgical repair at the time of removal and minimize the potential from sheath induced distal limb ischemia. Removal of a percutaneously placed introducer sheath ≤7 French does not require routine surgical exposure or fluoroscopic visualization during removal, and can be removed utilizing a variety of closure devices or with direct manual pressure held for 20 minutes. Sound judgment by a practitioner experienced with arterial sheath removal must be utilized, as even small sheaths pose a risk for access site complications including free extravasation, pseudoaneurysm formation, thrombosis, and distal embolization. The potential for distal thrombosis may be more pronounced in the setting of vasospasm in younger trauma patients or in the context of modern damage control resuscitation practices that emphasize early transexamic acid and fresh frozen plasma. Any concern for potential limb ischemia or when challenges are encountered during sheath insertion should guide the provider towards further interrogation of the access site and distal limb perfusion utilizing angiography or open surgical exploration.
CONCLUSION
The utilization of P-REBOA as described here requires only simple modifications of previously described REBOA techniques. [2,8,9] This approach combines the benefits of preserved critical organ perfusion above the level of occlusion with regionalized hypotensive resuscitation distal to the balloon. P-REBOA may prove to mitigate the ischemia-reperfusion injury associated with total aortic occlusion, while potentially extending the duration of intervention beyond the “golden hour”.
Funding Sources:
Dr. Johnson is the recipient of a training grant from the National Heart, Lung and Blood Institute: K12HL108964. This project was partly supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through grant number UL1 TR000002.
Abbreviations:
- AIS
abbreviated injury score
- ED
Emergency Department
- GCS
Glasgow Coma Scale
- ICU
intensive care unit
- ISS
Injury Severity Score
- LOS
length of stay
- mmHg
millimeters of mercury
- SBP
systolic blood pressure
- TBI
traumatic brain injury
Footnotes
Financial Disclosures: None
Conflicts of Interest: None
The Endovascular Variable Aortic Control (EVAC) Study Group is:
Ian Brown, MD, PhD2; Jeremy Cannon, MD1; Anders Davidson, MD2; Joseph DuBose, MD2,4,5; Sarah-Ashley Ferencz, MD2; Joseph Galante, MD2; Travis Gerlach, MD2,3,4; Kevin Grayson, DVM, PhD8;Rachel Hight, MD2,3,4; M. Austin Johnson, MD6, PhD; Lucas P. Neff, MD2,3,4,8; Todd Rasmussen, MD7; Rachel Russo, MD2; James Sampson MD2,4,5,8; Timothy K. Williams, MD2,5,8
Affiliations:
1. Department of Surgery, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, Pennsylvania, 19104
2. Department of Surgery, UC Davis Medical Center, 2315 Stockton Blvd. Sacramento, California 94535
3. Department of General Surgery, David Grant USAF Medical Center, Travis Air Force Base, California 94533
4. Department of Surgery, Uniformed Services University of the Health Sciences, Bethesda, Maryland 20814
5. Heart, Lung and Vascular Center, David Grant USAF Medical Center, Travis Air Force Base, California 94533
6. Department of Emergency Medicine, University of California Davis Medical Center, Sacramento, CA 95917
7. US Combat Casualty Care Research Program, US Army Medical Research and Material Command, Fort Detrick, MD
8. Clinical Investigation Facility, David Grant USAF Medical Center, Travis Air Force Base, CA, 94535
Bibliography
- 1.Markov NP, Percival TJ, Morrison JJ, Ross JD, Scott DJ, Spencer JR, Rasmussen TE. Physiologic tolerance of descending thoracic aortic balloon occlusion in a swine model of hemorrhagic shock. Surgery. 2013. Jun;153(6):848–56. [DOI] [PubMed] [Google Scholar]
- 2.Stannard A, Eliason JL, Rasmussen TE. Resuscitative Endovascular Balloon Occlusion of the Aorta (REBOA) as an Adjunct for Hemorrhagic Shock. The Journal of Trauma: Injury, Infection, and Critical Care. 2011. Dec;71(6):1869–72. [DOI] [PubMed] [Google Scholar]
- 3.Morrison JJ, Percival TJ, Markov NP, Villamaria C, Scott DJ, Saches KA, Spencer RA, Rasmussen TE. Aortic balloon occlusion is effective in controlling pelvic hemorrhage. Journal of Surgical Research. Elsevier Ltd; 2012. Oct 1;177(2):341–7. [DOI] [PubMed] [Google Scholar]
- 4.Biffl WL, Fox CJ, Moore EE. The role of REBOA in the control of exsanguinating torso hemorrhage. Journal of Trauma and Acute Care Surgery. 2015. May;78(5):1054–8. [DOI] [PubMed] [Google Scholar]
- 5.Saito N, Matsumoto H, Yagi T, Hara Y, Hayashida K, Motomura T, Mashiko K, Iida H, Yokota H. Evaluation of the safety and feasibility of resuscitative endovascular balloon occlusion of the aorta. Journal of Trauma and Acute Care Surgery. 2015. May;78(5):897–904. [DOI] [PubMed] [Google Scholar]
- 6.Brenner ML, Moore LJ, DuBose JJ, Tyson GH, McNutt MK, Albarado RP, Holcomb JB, Scalea TM, Rasmussen TE. A clinical series of resuscitative endovascular balloon occlusion of the aorta for hemorrhage control and resuscitation. Journal of Trauma and Acute Care Surgery. 2013. Sep;75(3):506–11. [DOI] [PubMed] [Google Scholar]
- 7.Moore LJ, Brenner M, Kozar RA, Pasley J, Wade CE, Baraniuk MS, Scalea T, Holcomb JB. Implementation of resuscitative endovascular balloon occlusion of the aorta as an alternative to resuscitative thoracotomy for noncompressible truncal hemorrhage. J Trauma Acute Care Surg. 2015. Oct;79(4):523–30–discussion530–2. [DOI] [PubMed] [Google Scholar]
- 8.Tsurukiri J, Akamine I, Sato T, Sakurai M, Okumura E, Moriya M, Yamanaka H, Ohta S. Resuscitative endovascular balloon occlusion of the aorta for uncontrolled haemorrahgic shock as an adjunct to haemostatic procedures in the acute care setting. Scand J Trauma Resusc Emerg Med. 2016;24(1):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brenner M, Hoehn M, Teeter W, Stein D, Scalea T. Trading scalpels for sheaths: Catheter-based treatment of vascular injury can be effectively performed by acute care surgeons trained in endovascular techniques. J Trauma Acute Care Surg. 2016. Feb 17;:1. [DOI] [PubMed] [Google Scholar]
- 10.Morrison JJ, Galgon RE, Jansen JO, Cannon JW, Rasmussen TE, Eliason JL. A systematic review of the use of resuscitative endovascular balloon occlusion of the aorta in the management of hemorrhagic shock. J Trauma Acute Care Surg. 2016. Feb;80(2):324–34. [DOI] [PubMed] [Google Scholar]
- 11.Qaism Z, Brenner M, Menaker J, Scalea T. Resuscitative endovascular balloon occlusion of the aorta. Resuscitation. 2015. Nov;96:275–9. [DOI] [PubMed] [Google Scholar]
- 12.Juel IS, Solligard E, Lyng O, Stomholm T, Tvedt KE, Johnsen H, Jynge P, Saether OD, Aadhl P, Gronbech JE. Intestinal injury after thoracic aortic cross-clamping in the pig. J Surg Res. 2004. Apr;117(2):283–95. [DOI] [PubMed] [Google Scholar]
- 13.Bown MJ, Nicholson ML, Bell PR, Sayers RD. Cytokines and inflammatory pathways in the pathogenesis of multiple organ failure following abdominal aortic aneurysm repair. Eur J Vasc Endovasc Surg. 2001. Dec;22(6):485–95. [DOI] [PubMed] [Google Scholar]
- 14.Miller CC 3rd, Villa MA, Sutton J, Lau D, Keyhani K, Estrera AL, Azizzadeh A, Coogan SM, Safi HJ. Serum myoglobin and renal morbidity and mortality following thoracic and thoraco-abdominal aortic repair: does rhabdomyolysis play a role? Eur J Vasc Endovasc Surg. 2009. Apr;37(4):388–94. [DOI] [PubMed] [Google Scholar]
- 15.Morrison JJ, Ross JD, Markov NP, Scott DJ, Spencer JR, Rasmussen TE. The inflammatory sequelae of aortic balloon occlusion in hemorrhagic shock. J Surg Res. 2014. Oct;191(2):423–31. [DOI] [PubMed] [Google Scholar]
- 16.Russo RM, Williams TK, Grayson JK, Lamb CM, Cannon JW, Clement NF, Galante JM, Neff LP. Extending the golden hour: Partial resuscitative endovascular balloon occlusion of the aorta (P-REBOA) in a highly lethal swine liver injury model. J Trauma Acute Care Surg. 2015. Dec 14;:1. [DOI] [PubMed] [Google Scholar]