Abstract
Whilst COVID-19 vaccination strategies continue to receive considerable emphasis worldwide, the extent to which routine immunisation (RI) has been impacted during the first year of the pandemic remains unclear. Understanding the existence, extent, and variations in RI disruptions globally may help inform policy and resource prioritisation as the pandemic continues.
We modelled historical, country-specific RI trends using publicly available vaccination coverage data for diphtheria, tetanus and pertussis-containing vaccine first-dose (DTP1) and third-dose (DTP3) from 2000 to 2019.
We report a 2·9% (95 %CI: [2·2%; 3·6%]) global decline in DTP3 coverage from an expected 89·2% to a reported 86·3%; and a 2·2% decline in DTP1 coverage (95 %CI: [1·6%; 2·8%]). These declines translate to levels of coverage last observed in 2005, thus suggesting a potential 15-years setback in RI improvements. Further research is required to understand which factors – e.g., health seeking behaviours or non-pharmaceutical interventions – linked to the COVID-19 crisis impacted vaccination coverage.
Keywords: Routine immunisation, Pandemic, Coverage, Modelling, Global health
1. Introduction
The COVID-19 pandemic has impacted society and public health infrastructures worldwide, influencing mobility [1], access to health services [2], livelihoods and poverty [3]. While COVID-19 vaccination strategies continue to receive considerable emphasis [4], [5], the extent to which routine immunisation (RI) has been impacted during the first year of the pandemic remains unclear. Indeed, the World Health Organisation (WHO) pulse surveys reported disruptions in the first half of 2020 [2], and while some later studies suggested a potential recovery [6], recent observations again hinted at global coverage declines [7], [8].
RI is estimated to prevent four to five million (M) deaths worldwide every year [9]. As such, there is an urgent need for assessing potential changes in RI coverage, as declines may result in considerable added morbidity and mortality.
2. Materials and methods
2.1. Data collection
We investigated changes in RI coverage using two key indicators: diphtheria-tetanus-pertussis first-dose (DTP1) and third-dose (DTP3) vaccine coverage. DTP3 serves as a general marker for immunisation system performance, used by national and global immunisation stakeholders [10]. DTP1 is used as a proxy for inequity – quantifying Zero Dose (ZD) children, those that receive no childhood vaccinations [11]. We compiled vaccination coverage data from the WHO and United Nations Children’s Fund (UNICEF) Estimates of National Immunisation Coverage (WUENIC) [12], [13] for the last 20 years, using the latest (October 2021) WUENIC data release. Countries were excluded if (a) they did not have complete time series coverage estimates for 2000–2019 inclusive to enable expected coverage modelling (three countries); or (b) they had not yet reported 2020 coverage through WUENIC (16 countries).
2.2. Statistical analysis
We used AutoRegressive Integrated Moving Average (ARIMA) modelling [14] to capture temporal trends in coverage for each country from 2000 to 2019, and predicted expected coverage levels in 2020 for each country and vaccine dose. Prior to investigating differences between expected and observed coverage, historic and predicted time series were assessed and countries were removed from analyses if they met one of three criteria – (1) large volatility in coverage estimates (over 10 percentage points) in the last decade since this may indicate high uncertainty in point estimates, (2) strong influence of most recent coverage estimates (i.e., 2018 or and 2019) contributing to model fitting, corroborated by WUENIC documentation indicating potential anomalous or rare events, and (3) ARIMA-models predicted coverage improvement greater than or equal to five percentage points from WUENIC-reported 2020 levels, since this may not be programmatically feasible. See Supplementary Text for details on removed countries and contexts by dose. We additionally conducted analyses with no exclusions as a sensitivity study.
After removing countries for which reliable temporal trends could not be assessed, changes in coverage were measured as the difference between the reported and expected coverage for 2020, expressed as percentage values, for the remaining 167 countries per vaccine dose. The significance of global changes was assessed using a t-test against the null hypothesis of the absence of change. Heterogeneities between groups of countries (UN regions or income groups) were tested using linear models with coverage change as a response variable and the corresponding ANOVA. Differences between individual countries were assessed by comparing the 95% confidence intervals derived from the linear models.
For additional validation we conducted the same analysis for measles-containing vaccine first-dose (MCV1) to compare whether similar trends were seen across other vaccine doses and immunisation touchpoints (MCV1 is typically administered at age 9-months compared to six-weeks for DTP1 and 10-weeks for DPT3).
We calculated missed immunisations by combining the estimated changes in coverage with surviving infant population estimates (medium variant births minus infant deaths) of the United Nations World Population Prospects (UNWPP) for 2020 [15].
All analyses were conducted using R [16] and can be reproduced using a publicly available reportfactory including all required data and scripts [17].
3. Results
3.1. Global trends
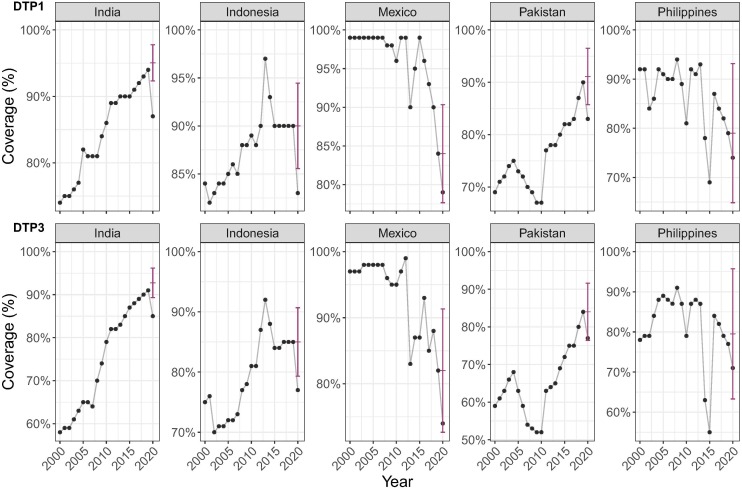
After excluding countries for which reliable coverage predictions could not be obtained (see Supplementary Text for details), we were able to estimate differences between expected and observed coverage in 2020 for 167 countries for DTP1 and DTP3 – examples shown in Fig. 1 .
Fig. 1.
Expected and reported 2020 vaccine coverage for DTP1 and DTP3: example of five countries with most additional missed DTP3 immunisations in 2020. These graphs show WUENIC-reported coverage data (black dots) and the corresponding ARIMA predictions and the associated 95% confidence intervals (red bars). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
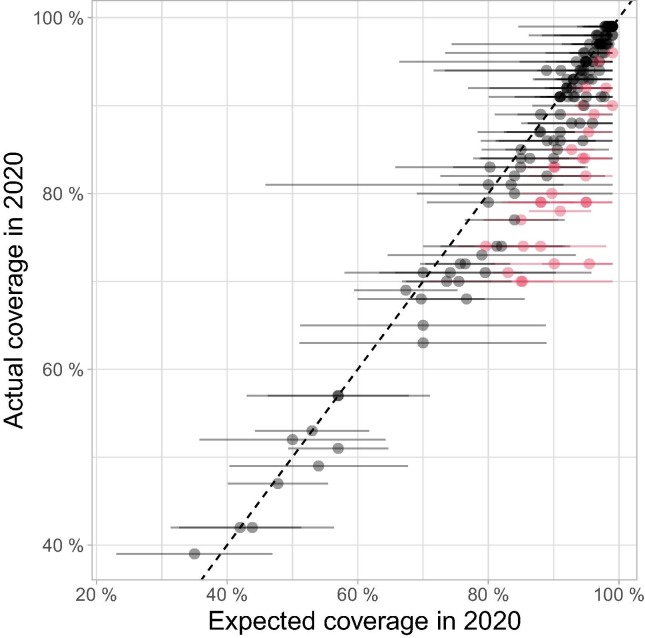
The exact magnitude of coverage decline was often hard to assess for individual countries due to uncertainties in model predictions (Fig. 2 , Supplementary Table 1 and 2), but general trends remained clearly apparent.
Fig. 2.
Comparison between 2020 WUENIC-reported DTP3 coverage and expectations derived from historical trends. This scatterplot shows country coverage (WUENIC-reported actual values and ARIMA-predicted expectations) as dots. Lines around individual points illustrate the 95% confidence intervals (CI) of ARIMA predictions. Countries showing significant departure from expected values, i.e., for which actual coverage is outside the 95% CI of predictions, are indicated in red; countries without such significant departure from expected results are shown in black. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Results suggest an average global decline in DTP3 coverage of 2·9% (95 %CI: [2·2%; 3·6%]), from an expected 89·2% to a reported 86·3% across 167 reporting countries, translating to a point estimate of 4·5 M additional unimmunised children for DTP3 in 2020 in these countries.
Similar trends were seen for DTP1 - an average global coverage decline of 2·2% (95 %CI: [1·6%; 2·8%]) from an expected 92·9% to a reported 90·7% across the 167 countries analysed here, equivalent to a point estimate of 4·1 M additional Zero Dose children.
We note that results hold for both DTP1 and DTP3 when no countries are excluded from analysis; and that similar trends were seen for MCV1 (an average global coverage decline of 2·7%, 95 %CI: [2.0%; 3.4%], from an expected 88.3% to a reported 85.6% across 167 countries). See Supplementary Materials for more details on these sensitivity analyses.
3.2. Heterogeneities
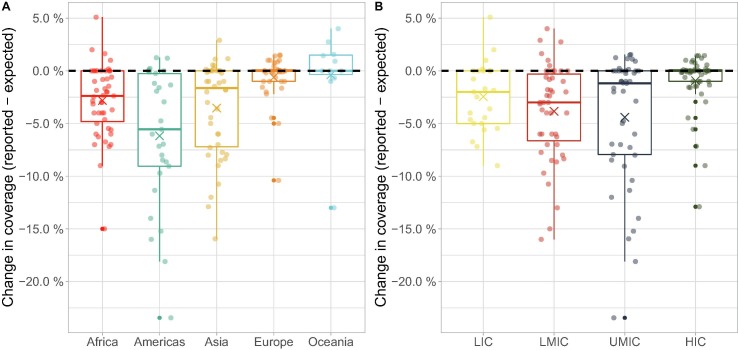
Patterns of RI coverage significantly varied across United Nations regions (Fig. 3 A; ANOVA: F = 22·4, df = 162, p < 2.2x10-16), with the strongest decline observed in the Americas (6·2% decline, 95 %CI: [4·6%; 7·7%]), Asia (3.5% decline, 95 %CI: [2·2%; 4·8%]) and Africa (2·8% decline, 95 %CI: [1·6%; 4·0%]), while Europe (mean change = −0·6%; 95 %CI: [−2·0%; +0·7%]) and Oceania (mean change = −0·4%; 95 %CI: [−2·9%; +2·2%]) did not show any significant change.
Fig. 3.
Differences between expected and reported DTP3 vaccine coverage in 2020 across (A) UN regions and (B) income groups. Points represent individual countries, grouped, and coloured according to (A) UN region classification and (B) World Bank income groups. Country coordinates on the X-axis were jittered for visibility. Values on the y-axis are indicated as absolute differences between reported and expected vaccine coverage, in percentages. Boxes show the median (50%), upper (75%) and lower (25%) quartile changes in coverage for each group, with whiskers extending to either the minimum/ maximum changes or the quartile value plus 1.5 times the interquartile range, and crosses indicating the average. The black dashed horizontal lines indicate no change in coverage. LIC: Low-income Country. LMIC: Lower-middle-income Country. UMIC: Upper-middle-income Country. HIC: High-income Country.
Similar heterogeneities were observed when considering income groups (Fig. 3 B; ANOVA: F = 22·6, df = 163, p < 7·1-15), with stronger declines in coverage observed in lower-middle-income countries (LMICs; mean decline: 3·8%; 95 %CI: [2·6%; 5·1%]) and in upper-middle-income countries (UMICs; mean decline: 4·4%; 95 %CI: [3·1%; 5·7%]), than in low-income countries (LICs; mean decline: 2·4%; 95 %CI: [0·7%; 4·2%]), while high-income countries (HICs; mean change: −0·9%; 95 %CI: [-2·2%; 0·3%]) did not show any significant change.
As UN regions and income groups are highly correlated (non-parametric Chi-square test: X 2 = 115·4, p < 10−5), we also tested whether heterogeneities due to one variable (regions or income groups) remained after accounting for the effect of the other one. Interestingly, regional differences remained after accounting for differences in income groups (ANOVA: F = 5·67, df = 159, p < 2·7 × 10-4), but evidence for the converse was weak (ANOVA: F = 2·67, df = 159, p = 0·05).
3.3. Country-level missed immunisations
These results on 167 countries represent ∼94% of the global surviving infant population. Our results suggest a strong impact, with large additional missed immunisations versus expected, in some countries. For DTP3 29 countries (17%) and for DTP1 32 countries (19%), reported coverage significantly (p < 0.05) lower than expected in 2020. For example, in India an estimated 3·5 M children did not receive their DTP3 vaccine in 2020, of which 52% (95 %CI: [29%; 75%]), i.e. 1·8 M, were associated with coverage declines during the first year of the pandemic; and in Indonesia an estimated 1·1 M children missed DTP3 vaccinations, of which 35% (95 %CI: [10%; 60%]), i.e., 400 thousand (k), were associated with coverage declines in 2020. Table 1 details results for the 10 countries with point estimate greatest additional missed DTP3 immunisations in 2020 (see also Fig. 1). Similar trends are seen for ZD children using DTP1 results. Detailed results for all analysed countries can be found in the Supplementary Tables S1 (DTP1), S2 (DTP3) and S3 (MCV1).
Table 1.
Estimated DTP3 coverage declines and missed immunisations for 10 countries with most additional missed immunisations.
| Country | ISO code | UN region | Income group | ARIMA modelled DTP3 expected 2020 coverage [95% CI] | WUENIC reported 2020 DTP3 coverage | Change in DTP3 coverage (mean) | Total missed immunisations 2020 | Additional missed immunisations 2020 (mean) |
|---|---|---|---|---|---|---|---|---|
| India | IND | Asia | LMIC | 92·7% [89·3%; 96·2%] | 85·0% | −7·7% | 3,505,350 | 1,808,022 |
| Pakistan | PAK | Asia | LMIC | 84·0% [76·4%; 91·6%] | 77·0% | −7·0% | 1,309,160 | 398,439 |
| Indonesia | IDN | Asia | LMIC | 85·0% [79·3%; 90·7%] | 77·0% | −8·0% | 1,078,470 | 375,119 |
| Philippines | PHL | Asia | LMIC | 79·5% [63·3%; 95·7%] | 71·0% | −8·5% | 621,470 | 182,408 |
| Mexico | MEX | Americas | UMIC | 82·0% [72·7%; 91·3%] | 74·0% | −8·0% | 562,380 | 173,039 |
| Uganda | UGA | Africa | LIC | 96·2% [91·4%; 99·0%] | 89·0% | −7·2% | 176,000 | 114,949 |
| Peru | PER | Americas | UMIC | 90·1% [80·4%; 99·0%] | 72·0% | −18·1% | 159,040 | 102,807 |
| Mozambique | MOZ | Africa | LIC | 88·0% [82·0%; 94·0%] | 79·0% | −9·0% | 230,160 | 98,639 |
| Argentina | ARG | Americas | UMIC | 85·3% [78·9%; 91·7%] | 74·0% | −11·3% | 193,700 | 84,529 |
| Iraq | IRQ | Asia | UMIC | 81·3% [70·1%; 92·5%] | 74·0% | −7·3% | 288,600 | 80,770 |
Numbers displayed in bold font indicate significant differences (p < 0.05) between expected and observed coverage. LIC: Low-income Country. LMIC: Lower-middle-income Country. UMIC: Upper-middle-income Country.
4. Discussion
While the estimated decline in coverage (DTP3: 2·9% (95 %CI: [2·2%; 3·6%]) may seem small, this reduced level of coverage was last observed in the analysed countries in 2005, thus suggesting a potential 15-years setback in RI improvements. Note that global vaccination coverage has remained relatively stagnant over the last decade in many countries, so that the average decline in coverage observed here may often reflect changes between 2019 and 2020. The RI disruption observed in this study suggests there may be greater risk of vaccine-preventable disease outbreaks in the coming years, in the absence of multi-faceted catch-up and adaptation strategies, such as Supplementary Immunisation Activities (SIAs) to reach missed children or periodic intensification of routine immunisation [18].
The estimated changes in RI coverage reported in this study suggest a smaller global decline (approximately 1/3rd the magnitude) than previously found using alternative methodology and data [19]. We believe our findings may be more robust owing to a more comprehensive dataset including data from more countries (167 here vs. 94), plus increased data from the end of 2020 (annual here vs. majority of data from January-September 2020), and the use of WUENIC-reported data (less prone to data quality and completeness issues than administrative data). The observed discrepancies are compatible with a rebound of global RI coverage in late 2020 [6].
An important contribution of this work, beyond the global trends reported, is that it offers a replicable rationale for estimating and comparing the impact of COVID-19 on RI across countries and vaccine programmes, facilitating prioritisation of interventions and resources to those most-affected. Declines in DTP1 coverage indicate increases in the quantity of ZD children in some countries – suggesting that the most vulnerable populations have been strongly impacted by the reductions in RI observed in the first year of the pandemic and reinforced existing inequities in access to healthcare. ZD populations in key ZD “hotspots” (e.g., India, Pakistan, and Indonesia) are estimated to have increased significantly in 2020, posing a genuine public health threat in the coming years. To alleviate such risks and reduce immunisation inequities, SIAs targeted specifically at these populations should be considered. Additional research is needed to investigate heterogeneities in RI decline at finer scales and identify subpopulations which may have experienced even greater losses to RI coverage.
RI disruption may be worsened by the acceleration of COVID-19 vaccination campaigns, particularly in low- and middle-income countries where absorption capacity may be challenged [20], potentially competing with RI services. Careful monitoring of the interaction, trade-offs and synergies between RI and COVID-19 vaccinations is essential. Further studies are needed to understand which factors linked to the COVID-19 crisis impacted vaccination coverage, such as changes in health-seeking behaviours or non-pharmaceutical intervention policies, in order to successfully and efficiently address pandemic-associated losses to coverage.
5. Conclusions
As the COVID-19 pandemic continues to affect healthcare systems globally, maintaining the appropriate balance between access to routine immunisation and pandemic response will be essential to reduce both the direct and indirect mortality and morbidity associated with COVID-19. This research provides a transparent and replicable rationale for estimating gaps in RI coverage across countries, producing an objective measure for missed immunisations and coverage disruptions. As such, it can form a basis for identifying countries most affected by declines in RI coverage and prioritising efforts to alleviate the indirect impact of COVID-19.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: It is noted that BE has been employed by the Clinton Health Access Initiative in the Global Vaccines team in the last three years; and is currently employed by Gavi. All research contained in this manuscript was conducted during a postgraduate qualification, outside and independent of employment. Neither facilities, data, nor any other forms of input from the Clinton Health Access Initiative or Gavi, were used in this study. The research and manuscript are independent of the Clinton Health Access Initiative and Gavi, and the findings have not been discussed, reviewed, or endorsed by the Clinton Health Access Initiative, the Gavi Secretariat, or any Alliance members.
Acknowledgments
Acknowledgements
TJ receives funding from the Global Challenges Research Fund (GCRF) project ‘RECAP’ managed through RCUK and ESRC (ES/P010873/1), from the National Institute for Health Research - Health Protection Research Unit for Modelling Methodology, and from the Medical Research Council (grant number MC_PC_19065). These funders had no role in the design and conduct of this study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
BE is an MSc student at the London School of Hygiene and Tropical Medicine and received no funding.
Funding
Funders had no role in the design and conduct of this study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Data sharing
All analyses were conducted using free software R [16], and can be reproduced using a publicly available reportfactory including all required data and scripts [17] used to produce the results presented in this publication, and available on GitHub at: https://github.com/bevans249/modelling_covid_impact_RI.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.vaccine.2022.01.044.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Barbieri D.M., Lou B., Passavanti M., Hui C., Hoff I., Lessa D.A., et al. Impact of COVID-19 pandemic on mobility in ten countries and associated perceived risk for all transport modes. PLoS One. 2021;16:e0245886. doi: 10.1371/journal.pone.0245886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. Pulse survey on continuity of essential health services during the COVID-19 pandemic: interim report, 27 August 2020. World Health Organization; 27 Aug 2020 [cited 21 Nov 2021]. Available: https://www.who.int/publications/i/item/WHO-2019-nCoV-EHS_continuity-survey-2020.1.
- 3.Impact of COVID-19 on people’s livelihoods, their health and our food systems. [cited 21 Nov 2021]. Available: https://www.who.int/news/item/13-10-2020-impact-of-covid-19-on-people’s-livelihoods-their-health-and-our-food-systems.
- 4.National Academies of Sciences, Engineering, and Medicine, Health and Medicine Division, Board on Population Health and Public Health Practice, Board on Health Sciences Policy, Committee on Equitable Allocation of Vaccine for the Novel Coronavirus. Framework for Equitable Allocation of COVID-19 Vaccine. National Academies Press; 2020. [PubMed]
- 5.Eccleston-Turner M., Upton H. International collaboration to ensure equitable access to vaccines for COVID-19: The ACT-accelerator and the COVAX facility. Milbank Q. 2021;99:426–449. doi: 10.1111/1468-0009.12503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shet A, Carr K, Danovaro-Holliday CM, Sodha SV, Prosperi C, Wunderlich J, et al. Impact of the SARS-CoV-2 pandemic on routine immunization services: evidence of disruption and recovery from 169 countries and territories. 2021. doi:10.2139/ssrn.3850009. [DOI] [PMC free article] [PubMed]
- 7.World Health Organization. Weekly Epidemiological Record. 2021 Nov. Report No.: No 44, 2021, 96, 537–548. Available: https://apps.who.int/iris/bitstream/handle/10665/348056/WER9644-eng-fre.pdf?sequence=1.
- 8.Muhoza P., Danovaro-Holliday M.C., Diallo M.S., Murphy P., Sodha S.V., Requejo J.H., et al. Routine vaccination coverage - worldwide, 2020. MMWR Morb Mortal Wkly Rep. 2021;70(43):1495–1500. doi: 10.15585/mmwr.mm7043a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Immunization. [cited 21 Nov 2021]. Available: https://www.who.int/news-room/facts-in-pictures/detail/immunization.
- 10.Reported Diphtheria Tetanus toxoid and Pertussis (DTP3) immunization coverage among 1-year-olds (%). [cited 21 Nov 2021]. Available: https://www.who.int/data/gho/indicator-metadata-registry/imr-details/3092.
- 11.Zero-dose children and missed communities. [cited 21 Nov 2021]. Available: https://www.gavi.org/our-alliance/strategy/phase-5-2021-2025/equity-goal/zero-dose-children-missed-communities.
- 12.Burton A., Monasch R., Lautenbach B., Gacic-Dobo M., Neill M., Karimov R., et al. WHO and UNICEF estimates of national infant immunization coverage: methods and processes. Bull World Health Organ. 2009;87:535–541. doi: 10.2471/BLT.08.053819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burton A., Kowalski R., Gacic-Dobo M., Karimov R., Brown D. A formal representation of the WHO and UNICEF estimates of national immunization coverage: a computational logic approach. PLoS ONE. 2012:e47806. doi: 10.1371/journal.pone.0047806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hyndman R.J., Khandakar Y. Automatic time series forecasting: the forecast package for R. J Stat Softw. 2008;27:1–22. [Google Scholar]
- 15.World Population Prospects - Population Division - United Nations. [cited 21 Nov 2021]. Available: https://population.un.org/wpp/.
- 16.R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2021. Available: https://www.R-project.org/.
- 17.Evans B, Jombart T. Analysis of changes in worldwide routine immunisation coverage in 2020. 2021. doi:10.5281/zenodo.5750111.
- 18.Catch-up vaccination. [cited 19 Jan 2022]. Available: https://www.who.int/teams/immunization-vaccines-and-biologicals/essential-programme-on-immunization/implementation/catch-up-vaccination.
- 19.Causey K., Fullman N., Sorensen R.J.D., Galles N.C., Zheng P., Aravkin A., et al. Estimating global and regional disruptions to routine childhood vaccine coverage during the COVID-19 pandemic in 2020: a modelling study. Lancet. 2021;398(10299):522–534. doi: 10.1016/S0140-6736(21)01337-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.What needs to change to enhance covid-19 vaccine access. [cited 21 Nov 2021]. Available: https://www.who.int/news/item/24-09-2021-what-needs-to-change-to-enhance-covid-19-vaccine-access.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.