Abstract
Introduction
Coronavirus disease 2019 (COVID-19) has greatly impacted medical care practices. Although the effects on infectious disease treatment and infection control, such as antimicrobial resistance, have been specified, very few reports exist on the specific effects of COVID-19.
Methods
We investigated the effects of COVID-19 on daily medical practices at a tertiary hospital in Japan by comparing the use of hand sanitizers, the detection of bacteria from blood cultures, and the amount dose of antibacterial drugs used for one year before (April 2019 to March 2020, fiscal year 2019.) and after COVID-19 admissions began (April 2020 to March 2021, fiscal year 2020).
Results
The use of hand sanitizers increased by 1.4–3 times during the year after COVID-19 admissions began; the incidence of methicillin-susceptible Staphylococcus aureus and all S. aureus detected in blood cultures reduced in all departments. No decrease was observed in the usage of all antibacterial drugs; rather, the usage of all antibacterial drugs tended to increase in all departments. Therefore, no significant change was observed in the detection of drug-resistant bacteria and the trends of antibacterial drug use based on the acceptance of COVID-19 patients.
Conclusions
The prevalence of drug-resistant bacteria and trends of antibacterial drug use remained unchanged despite the increased use of hand sanitizers due to the admission of patients with COVID-19.
Keywords: Antimicrobial resistance, COVID-19, Hand sanitizers, Infection control
Abbreviations
- ABPC/SBT
ampicillin/sulbactam
- AMR
antimicrobial resistance
- AUD
antimicrobial use density
- CFPM
cefepime
- CMZ
cefmetazole
- COVID-19
coronavirus disease 2019
- CRE
carbapenem-resistant Enterobacteriaceae
- CTRX
ceftriaxone
- CVC
central venous catheter
- DDD
defined daily doses
- DOT
days of therapy
- MEPM
meropenem
- MIC
minimum inhibitory concentration
- MRSA
methicillin-resistant Staphylococcus aureus
- MSSA
methicillin-sensitive Staphylococcus aureus
- TEIC
teicoplanin
- VCM
vancomycin
1. Introduction
The world's first case of coronavirus disease 2019 (COVID-19) was confirmed in China in December 2019 [1], and the first case in Japan was reported in January 2020 [2,3]. Since then, the number of patients with COVID-19 has continued to increase, and the emergency department of our hospital began accepting COVID-19 patients with severe illness in April 2020. Our hospital is equipped to provide advanced medical care, including extracorporeal membrane oxygenation; therefore, the majority of patients have serious illnesses, and only a few patients have mild to moderate illnesses. The COVID-19 pandemic has had a major impact on our daily hospital practices, partly because of the distribution of medical resources and staff to patients with COVID-19. We speculated that it may also have affected antimicrobial resistance (AMR), infectious disease treatment, and infection control. An increased focus on hand hygiene during the COVID-19 pandemic may reduce the development of AMR within healthcare settings. Conversely, increased use of empirical antimicrobial therapy among COVID-19 patients may promote AMR. Several AMR outbreaks have been reported since the start of the COVID-19 pandemic [4,5]. The COVID-19 pandemic may change doctors' prescribing habits. For example, the use of antimicrobial therapy among patients with respiratory symptoms may increase [4], and increased AMR may lead to an increase in the prescriptions of broad-spectrum antibiotics. Similarly, hospital-wide antibiotic usage may change due to the COVID-19 pandemic because approximately 70% of hospitalized COVID-19 patients receive antibiotics [[5], [6], [7]]. Healthcare workers may focus on self-protection rather than preventing cross-transmission between patients, and the use of hand sanitizers may increase [5]. In summary, the COVID-19 pandemic may affect behavior regarding infection control, antibiotic prescription, and the detection of AMR. However, there is limited information on the specific effects of COVID-19, including its effects on AMR, infectious disease treatment, and infection control. Therefore, we conducted a study to describe our hospital's approach to admitting COVID-19 patients and the effect of the COVID-19 pandemic on infectious disease treatment and infection control.
2. Methods
2.1. Setting and study design
This study was conducted at the Osaka City University Hospital, a 965-bed tertiary-care hospital in Osaka, Japan. We considered two periods: the year before the admission of COVID-19 patients began (April 2019 to March 2020, fiscal year 2019), and the year after the admission of COVID-19 patients began (April 2020 to March 2021, fiscal year 2020). We compared the following factors between the emergency department and clinical departments other than the emergency department: number of inpatients, amount of hand sanitizer used, bacteria detected in blood cultures, drug-resistance of Pseudomonas spp., and the amount of antibacterial agents used (expressed as antimicrobial use density [AUD] and days of therapy [DOT]). All bacteria detected by blood culture were included in the results, and the possibility of contamination could not be excluded. Defined daily doses (DDD), set by the World Health Organization, were used for calculating the AUD. The AUD was calculated as the total antimicrobial use in DDD per 1000 patient-days. The DOT was calculated as the number of antimicrobial therapy days per 1000 patient-days. Due to the instability of the antibacterial drug supply [8], the AUD and DOT in the fiscal year 2020 were compared to those of fiscal years 2015–2019 combined.
2.2. Variables and definitions
Each patient was included in the study only once during the observation period for each sample. The number of hand sanitizer units used per patient daily was calculated by dividing the total number of hand sanitizer units used in the hospital (or ward) by the total number of inpatients in the hospital (or ward). Because our hospital uses several different types of hand sanitizer, we obtained the total volume of each type of hand sanitizer used (mL) in the hospital (or ward) from the department that manages the purchase of hand sanitizers in our hospital. We calculated the total number of units of hand sanitizer used for each hand sanitizer by dividing the total volume of each sanitizer used (mL) by the recommended single-use amount (mL). The total number of units of hand sanitizer used was calculated by adding the number of units of all the different types of hand sanitizer used. To compare the amount of antibacterial drugs used, the AUD and DOT for each month of each period were calculated and compared between the two periods.
2.3. Microbiological analysis
All bacterial isolates were identified using colony morphologic analysis and Gram staining. Isolate identification and antimicrobial susceptibility were confirmed using the MicroScan WalkAway-96 SI system (Beckman Coulter, Inc., Brea, CA, USA). Results were interpreted according to the 2018 Clinical and Laboratory Standards Institute guidelines [9]. After screening for drug susceptibility, the presence of extended-spectrum β-lactamase (ESBL)-producing bacteria was confirmed by the disk diffusion method using clavulanate [9]; in contrast, the presence of AmpC-producing bacteria was confirmed by the disk diffusion method using boronic acid [10]. Carbapenem-resistant Enterobacteriaceae (CRE) were determined according to the standards of the Ministry of Health, Labour, and Welfare of Japan [11] for meropenem (minimum inhibitory concentration [MIC] of meropenem: ≥2 μg/mL), imipenem (MIC of imipenem: ≥2 μg/mL), and cefmetazole (MIC of cefmetazole: ≥64 μg/mL) susceptibility.
2.4. Statistical analysis
Fisher's exact test was used for univariate comparisons of categorical data, and the Mann–Whitney U test was used to compare continuous variables using EZR software (Saitama Medical Center, Jichi Medical University, Saitama, Japan) [12], which is a modified version of the R commander (version 2.4) that includes statistical functions frequently used in biostatistics. Statistical significance was set at p < 0.05.
2.5. Ethics statement
The Ethics Committee of the Osaka City University Graduate School of Medicine approved this study (No. 2021-093). The need for written informed consent was waived owing to the retrospective nature of the study.
3. Results
The number of inpatients in the fiscal year 2020 was lower than that in the fiscal year 2019 in the emergency department and other departments. While 81 patients with severe COVID-19 were admitted to the emergency department in the fiscal year 2020, the other departments admitted only 18 patients during this period. Nevertheless, it was confirmed that the amount of hand sanitizer used increased in the emergency department, the intensive care unit, and all wards in our hospital, with consumption in the emergency department increasing by approximately three-fold (Table 1 ).
Table 1.
Number of inpatients and central venous catheter and hand sanitizer usage.
| Fiscal year 2019 | Fiscal year 2020 | |
|---|---|---|
| Number of inpatients | ||
| Number of inpatients in the emergency department (person) | 556 | 473 |
| Number of inpatients in departments other than the emergency department (person) | 21,725 | 18,974 |
| Total number of inpatients (36 clinical departments) (person) |
22,281 |
19,447 |
| Hand sanitizer usage | ||
| Emergency departmenta | 48.8 | 149.9 |
| Intensive care unita | 59.7 | 76.8 |
| Other than emergency department, ICU, and NICUa | 11.6 | 17 |
| All wardsa | 14.1 | 19.8 |
Number of units of hand sanitizer per patient daily = Total number of hand sanitizer units used in our hospital (or ward)/Total number of inpatients in the hospital (or ward).
During the 2015–2019 fiscal years, the baseline detection of the proportion of methicillin-resistant S. aureus (MRSA) among S. aureus detected in the blood culture was 22.2%, and the proportion of ESBL among Escherichia coli and Klebsiella pneumoniae was 25.5% and 12.1%, respectively. Although CRE were detected in 22 cases in the fiscal year 2015–2019, none of them was carbapenemase-producing Enterobacteriaceae. The numbers of patients with methicillin-susceptible S. aureus (MSSA) and overall S. aureus bacteremia significantly reduced in the fiscal year 2020 compared with the fiscal year 2019 in all departments. In clinical departments other than the emergency department, there was an increase in the number of patients with Pseudomonas spp. bacteremia detected. There were no changes in the number of patients with other bacteria and fungi detected in blood cultures between the two periods (Table 2 ).
Table 2.
Changes in the number of isolates of bacteria and fungi detected in blood culture.
| Emergency department | Fiscal year 2019 |
Fiscal year 2020 |
p-valuea | |
|---|---|---|---|---|
| n = 86 | n = 95 | |||
| GPC | 58 (67.4%) | 61 (64.2) | 0.75 | |
| Staphylococcus aureus | 15 (17.4%) | 7 (7.4%) | 0.04 | |
| MSSA | 10 (11.6%) | 3 (3.2%) | 0.04 | |
| MRSA | 5 (5.8%) | 4 (4.2%) | 0.74 | |
| CNS | 22 (25.6%) | 32 (33.7%) | 0.26 | |
| MRCNS | 16 (18.6%) | 26 (27.4%) | 0.22 | |
| GPR | 19 (22.1%) | 20 (21.1%) | >0.99 | |
| GNC | 0 (0%) | 0 (0%) | >0.99 | |
| GNR | 23 (26.7%) | 19 (20.0%) | 0.3 | |
| ESBL (+) | 3 (3.5%) | 2 (2.1%) | 0.67 | |
| AmpC (+) | 2 (2.3%) | 2 (2.1%) | >0.99 | |
| CRE | 0 (0%) | 1 (1.1%) | >0.99 | |
| Pseudomonas spp. | 2 (2.3%) | 2 (2.1%) | >0.99 | |
| Fungi |
1 (1.2%) |
2 (2.1%) |
>0.99 |
|
| Clinical departments other than the emergency department |
n = 343 |
n = 287 |
p-valuea |
|
| GPC | 197 (57.4%) | 149 (51.9%) | 0.17 | |
| Staphylococcus aureus | 48 (14.0%) | 23 (8.0%) | 0.02 | |
| MSSA | 35 (10.2%) | 12 (4.2%) | <0.01 | |
| MRSA | 13 (3.8%) | 11 (3.8%) | >0.99 | |
| CNS | 31 (9.0%) | 27 (9.4%) | 0.89 | |
| MRCNS | 68 (19.8%) | 55 (19.2%) | 0.84 | |
| GPR | 45 (13.1%) | 30 (10.5%) | 0.33 | |
| GNC | 2 (0.6%) | 2 (0.7%) | >0.99 | |
| GNR | 128 (37.3%) | 123 (42.9%) | 0.17 | |
| ESBL (+) | 16 (4.7%) | 10 (3.5%) | 0.55 | |
| AmpC (+) | 17 (5.0%) | 9 (3.1%) | 0.32 | |
| CRE | 3 (0.9%) | 3 (1.0%) | >0.99 | |
| Pseudomonas spp. | 9 (2.6%) | 21 (7.3%) | <0.01 | |
| Fungi | 2 (0.6%) | 6 (2.1%) | 0.15 | |
CNS, coagulase-negative staphylococci; CRE, carbapenem-resistant Enterobacteriaceae; ESBL, extended spectrum beta-lactamase; GNC, gram negative cocci; GNR, gram negative rod; GPC, gram positive cocci; GPR, gram positive rod; MRCNS, methicillin-resistant coagulase-negative staphylococci; MRSA, methicillin-resistant Staphylococcus aureus; MSSA, methicillin-sensitive Staphylococcus aureus..
Fisher's exact test.
Further, the changes in susceptibility of Pseudomonas spp. in blood culture, sputum, urine, and skin to antibacterial agents were investigated because there was a rise in the detection of Pseudomonas spp. in blood culture samples obtained from clinical departments other than the emergency department. However, no significant change was observed (Supplementary material).
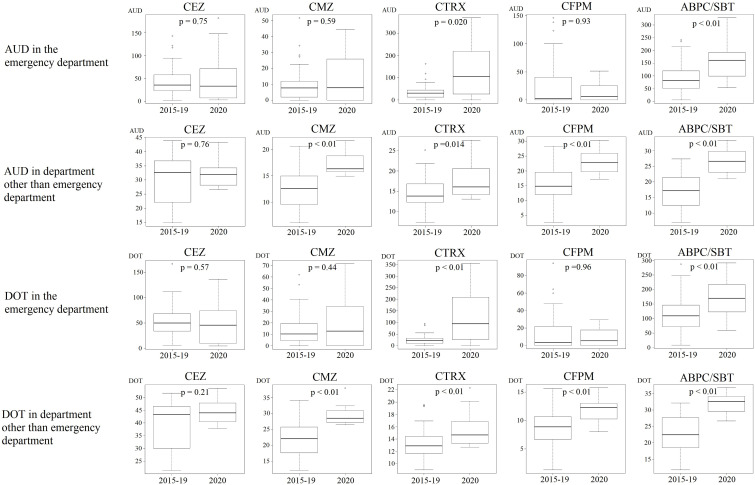
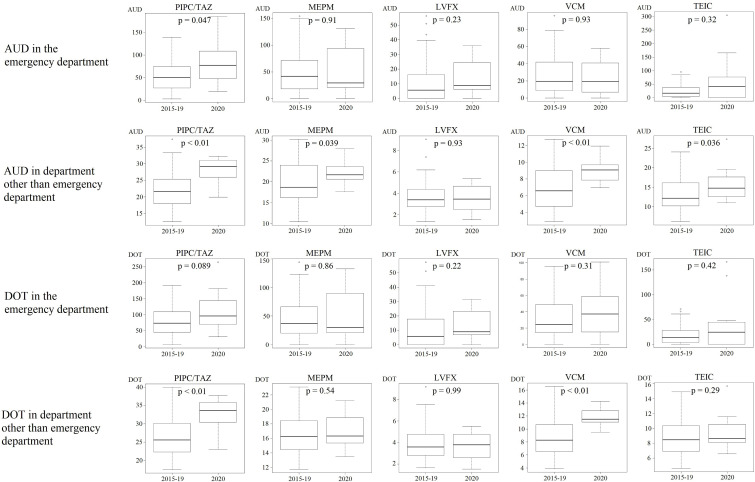
Additionally, we investigated the changes in the amount of antibacterial drugs used by calculating the AUD and DOT. In the emergency department, the AUD/DOT of ceftriaxone (CTRX) and ampicillin/sulbactam (ABPC/SBT) increased, and the AUD of piperacillin/tazobactam (PIPC/TAZ) similarly increased (Fig. 1 (a), (b)). The AUD/DOT of cefmetazole (CMZ), CTRX, cefepime (CFPM), ABPC/SBT, PIPC/TAZ, and vancomycin (VCM) increased in the clinical departments other than the emergency department, and the AUD of meropenem (MEPM) and teicoplanin (TEIC) similarly increased (Fig. 1 (a), (b)).
Fig. 1.
Box-and-whisker plots showing antimicrobial use density (AUD) and days of therapy (DOT) in fiscal years 2015–2019 and 2020. (a) Comparison of AUD/DOT between fiscal years 2015–2019 and 2020 in the emergency department and departments other than the emergency department; CEZ, CMZ, CTRX, CFPM, and ABPC/SBT. (b) Comparison of AUD/DOT between fiscal years 2015–2019 and 2020 in the emergency department and departments other than the emergency department; PIPC/TAZ, MEPM, LVFX, VCM, and TEIC. ABPC/SBT, ampicillin/sulbactam; AUD, antimicrobial use density; CAZ, ceftazidime; CEZ, cefazolin; CFPM, cefepime; CMZ, cefmetazole; CTRX, ceftriaxone; LVFX, levofloxacin; MEPM, meropenem; PIPC/TAZ, piperacillin/tazobactam; TEIC, teicoplanin; VCM, vancomycin.
4. Discussion
COVID-19 has affected various factors influencing daily medical practice and can potentially affect AMR; thus, it influences infectious disease treatment and control. One of the effects is an increased incidence of infections caused by multidrug-resistant bacteria [5,[13], [14], [15]]. This may be due to poor adherence to infection control measures because of staff shortages [13] and improper use of hand sanitizers and personal protective equipment [5,16]. Moreover, the number of antibacterial agents used in the empiric treatment of patients with COVID-19 and the incidence of infection due to drug-resistant bacteria may increase [17]. However, previous reports showed that the COVID-19 pandemic did not affect the incidence of infection due to multidrug-resistant bacteria [5]. This difference in study findings may be attributed to the differences in the nature of the COVID-19 pandemic among countries and the use of infection control measures. The incidence of COVID-19 in Japan has been lower than that of many other countries, with 1,687,422 cases among a total population of over 100 million as of September 24, 2021 [18]. In contrast, the epidemic has been more extensive in North America and Europe, with 43,734,666 cases in the United States of America, 6,994,319 cases in France, 7,664,230 cases in the United Kingdom, 4,660,314 cases in Italy, and 4,203,411 cases in Germany reported as of September 24, 2021 [18].
To the best of our knowledge, this is the first study to investigate the impact of COVID-19 on infection control and infectious disease treatment in Japan. We observed that the use of hand sanitizers increased despite a reduced number of inpatients; however, there were no obvious changes in the detection of drug-resistant bacteria or the amount of antibacterial drugs used.
A previous study showed that the COVID-19 pandemic may increase the use of hand sanitizers [4], and we observed an increase in the use of hand sanitizers in the clinical departments where COVID-19 is directly treated and in those where it is not. This may be related to the rising awareness regarding infection control when admitting COVID-19 patients. Reportedly, proper hand sanitization reduces the incidence of infection caused by resistant bacteria, such as MRSA and ESBL-producing bacteria [19,20]. However, we detected no reduction in the incidence of drug-resistant bacterial infections during the study period. This could be because of the relatively short observation period, and there could be a decrease in the incidence of drug-resistant bacterial infection because of increased hand sanitizer use if a longer observation period is considered. However, factors other than the observation period may be involved, such as inappropriate timing and method of hand sanitization. Other studies with an observation period of less than one year found a decreased incidence of MRSA infection due to the appropriate use of hand sanitizers [21,22]. Therefore, monitoring by the infection control team should focus on increased hand sanitizer use and the method and timing of hand sanitizer use, and appropriate changes should be made to the timing and method of hand sanitizer use by medical staff.
The incidence of MSSA bacteremia was similar in the samples obtained from the clinical departments that admitted many COVID-19 patients and in those that did not; therefore, the decreased incidence of MSSA bacteremia may not be an effect of COVID-19. The amount of antibacterial drugs used in the fiscal year 2020 was compared to that in fiscal years 2015–2019. This is because of the instability in the supply of some antibacterial drugs from the year 2017 [8], which is likely to have a greater effect on the results of the yearly comparisons (fiscal years 2019 and 2020). Increased CTRX and ABPC/SBT usage (based on both AUD and DOT) was confirmed in the emergency department and all other departments, and this may have contributed to the reduced incidence of MSSA bacteremia. Overall, no decrease was observed in the usage of any antibacterial drugs, and the usage of all antibacterial drugs tended to increase in the fiscal year 2020. Additionally, CTRX and ABPC/SBT usage increased based on the AUD and DOT, while PIPC/TAZ usage in the emergency department increased based on the AUD. In contrast, there was increased usage of CTRX, ABPC/SBT, and PIPC/TAZ in clinical departments other than the emergency department. Therefore, it is unlikely that COVID-19 affected the usage of these drugs. Further, the usage of all antibacterial drugs tended to increase in all departments other than the emergency department. As no reduction in usage was observed for all antibacterial drugs, even in the emergency department, it is likely that the changes in the amount of antibacterial agents used (other than CTRX and ABPC/SBT) were similar. Notably, a significant increase was observed only in the amount of antibiotics used in clinical departments other than the emergency department. This was because there was less antibiotic usage in the emergency department, making it difficult to detect a significant difference. The incidence of Pseudomonas spp. infections increased only in clinical departments other than the emergency department. This may have been caused by breakthrough infections due to increased antibiotic usage, especially CMZ, CTRX, and ABPC/SBT, which are ineffective against Pseudomonas spp. The difference in the incidence of Pseudomonas spp. bacteremia may be a possible effect of COVID-19 because the incidence of bacteremia differed between the COVID-19 ward (emergency department) and the other wards; however, the incidence in the emergency department was too low to perform statistical analysis. Studies that have shown an increased incidence of drug-resistant bacterial infections since the onset of the COVID-19 pandemic have been conducted in Germany [13], the United States of America [14], and Italy [15], where the incidence of COVID-19 is several times higher than that in Japan, and, the influence of COVID-19 may, therefore, be more marked. Additionally, our hospital mainly accepts patients with severe COVID-19, and only a few patients have mild to moderate symptoms. Thus, most of the COVID-19 patients were intubated and received mechanical ventilation, which may have aided in infection control.
Our study has several limitations. First, it was a single-center study conducted only in Japan. The COVID-19 situation varies greatly not only between countries but also between different regions within the same country. Furthermore, the process of admission of COVID-19 patients with mild, moderate, or severe disease varies between hospitals. Studies conducted in different hospitals could yield diverse results; therefore, it is necessary to investigate the effect of COVID-19 in different settings. Second, our observation period was relatively short. The relationship between the use of hand sanitizers and detection of resistant bacteria varies according to the observation period, and these findings vary between different reports [[19], [20], [21], [22]]. Furthermore, the use of antibacterial drugs can change significantly in a single year, depending on the supply. The most significant limitation of this study is that we could not investigate the method and timing of hand sanitizer use. Therefore, this needs to be clarified in a future prospective study.
In conclusion, admission of patients with COVID-19 may not affect the detection of drug-resistant bacteria and the use of antimicrobial agents. Nevertheless, increasing the awareness of healthcare staff toward the use of hand sanitizers and sufficient infection control education is needed for the increased use of hand sanitizers to have a positive effect, despite the COVID-19 pandemic.
Declarations of interest
None.
Funding
This research was supported by the Research Program on Emerging and Re-emerging Infectious Diseases from the Japan Agency Development, AMED [Grant number JP21fk0108094 and JP21fk0108133] and JSPS KAKENHI [Grant number 18K16185, 19K08958].
Authorship statement
All authors meet the ICMJE authorship criteria.
Author contributions
Waki Imoto: Conceptualization, Investigation, Resources, Writing – Original Draft; Koichi Yamada: Investigation, Resources, Writing – Review & Editing; Gaku Kuwabara: Resources, Writing – Review & Editing; Wataru Shibata: Resources, Writing – Review & Editing; Norihiro Sakurai: Resources, Writing – Review & Editing; Yuka Nonose: Resources, Writing – Review & Editing; Yasuyo Okada: Resources, Writing – Review & Editing; Kiyotaka Nakie: Resources, Writing – Review & Editing; Akiko Fujita: Resources, Writing – Review & Editing; Hiroshi Kakeya: Supervision, Project administration. All authors contributed to the writing of the final manuscript.
Acknowledgments
We would like to thank Editage (www.editage.jp) for English language editing.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jiac.2022.01.008.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., et al. A novel coronavirus from patients with pneumonia in China. N Engl J Med. 2019;(382):727–733. doi: 10.1056/NEJMoa2001017. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Watanabe M. The COVID-19 pandemic in Japan. Surg Today. 2020;50:787–793. doi: 10.1007/s00595-020-02033-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amengual O., Atsumi T. COVID-19 pandemic in Japan. Rheumatol Int. 2021;41:1–5. doi: 10.1007/s00296-020-04744-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rawson T.M., Moore L.S.P., Castro-Sanchez E., Charani E., Davies F., Satta G., et al. COVID-19 and the potential long-term impact on antimicrobial resistance. J Antimicrob Chemother. 2020;75:1681–1684. doi: 10.1093/jac/dkaa194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Monnet D.L., Harbarth S. Will coronavirus disease (COVID-19) have an impact on antimicrobial resistance? Euro Surveill. 2020;25:2001886. doi: 10.2807/1560-7917.ES.2020.25.45.2001886. 33183403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Langford B.J., So M., Raybardhan S., Leung V., Westwood D., MacFadden D.R., et al. Bacterial co-infection and secondary infection in patients with COVID-19: a living rapid review and meta-analysis. Clin Microbiol Infect. 2020;26:1622–1629. doi: 10.1016/j.cmi.2020.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rawson T.M., Moore L.S.P., Zhu N., Ranganathan N., Skolimowska K., Gilchrist M., et al. Bacterial and fungal coinfection in individuals with coronavirus: a rapid review to support COVID-19 antimicrobial prescribing. Clin Infect Dis. 2020;71:2459–2468. doi: 10.1093/cid/ciaa530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nakahama C. Toward a stable supply of antibacterial agents. Jpn J Chemother. 2020;68:510–517. [Google Scholar]
- 9.Wayne P.A. 27th Informational Supplement. M100–S28. Clinical and Laboratory Standards Institute; 2018. Performance standards for antimicrobial susceptibility testing. [Google Scholar]
- 10.Yagi T., Wachino J., Kurokawa H., Suzuki S., Yamane K., Doi Y., et al. Practical methods using boronic acid compounds for identification of class C beta-lactamase-producing Klebsiella pneumoniae and Escherichia coli. J Clin Microbiol. 2005;43:2551–2558. doi: 10.1128/JCM.43.6.2551-2558.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ministry of Health, Labour and Welfare Carbapenem-resistant Enterobacteriaceae infection. 2021. https://www.mhlw.go.jp/bunya/kenkou/kekkaku-kansenshou11/01-05-140912-1.html
- 12.Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48:452–458. doi: 10.1038/bmt.2012.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kampmeier S., Tönnies H., Correa-Martinez C.L., Mellmann A., Schwierzeck v. AA. A nosocomial cluster of vancomycin resistant enterococci among COVID-19 patients in an intensive care unit. Antimicrob Resist Infect Control. 2020;154:9. doi: 10.1186/s13756-020-00820-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nori P., Szymczak W., Puius Y., Sharma A., Cowman K., Gialanella P., et al. Emerging co-pathogens: New Delhi metallo-beta-lactamase producing Enterobacterales infections in New York City COVID-19 patients. Int J Antimicrob Agents. 2020;106179:56. doi: 10.1016/j.ijantimicag.2020.106179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Porretta A.D., Baggiani A., Arzilli G., Casigliani V., Mariotti T., Mariottini F., et al. Increased risk of acquisition of New Delhi metallo-beta-lactamase-producing carbapenem-resistant Enterobacterales (NDM-CRE) among a cohort of COVID-19 patients in a teaching hospital in Tuscany, Italy. Pathogens. 2020;635:9. doi: 10.3390/pathogens9080635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meda M., Gentry V., Reidy P., Garner D. Unintended consequences of long-sleeved gowns in a critical care setting during the COVID-19 pandemic. J Hosp Infect. 2020;106:605–609. doi: 10.1016/j.jhin.2020.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cantón R., Gijón D., Ruiz-Garbajosa P. Antimicrobial resistance in ICUs: an update in the light of the COVID-19 pandemic. Curr Opin Crit Care. 2020;26:433–441. doi: 10.1097/MCC.0000000000000755. [DOI] [PubMed] [Google Scholar]
- 18.World Health Organization WHO coronavirus (COVID-19) dashboard. https://covid19.who.int/table
- 19.Haverstick S., Goodrich C., Freeman R., James S., Kullar R., Ahrens M. Patients' hand washing and reducing hospital-acquired infection. Crit Care Nurse. 2017;37:e1–8. doi: 10.4037/ccn2017694. [DOI] [PubMed] [Google Scholar]
- 20.Johnson P.D.R., Martin R., Burrell L.J., Grabsch E.A., Kirsa S.W., O'Keeffe J., et al. Efficacy of an alcohol/chlorhexidine hand hygiene program in a hospital with high rates of nosocomial methicillin-resistant Staphylococcus aureus (MRSA) infection. Med J Aust. 2005;183:509–514. doi: 10.5694/j.1326-5377.2005.tb07151.x. [DOI] [PubMed] [Google Scholar]
- 21.MacDonald A., Dinah F., MacKenzie D., Wilson A. Performance feedback of hand hygiene, using alcohol gel as the skin decontaminant, reduces the number of inpatients newly affected by MRSA and antibiotic costs. J Hosp Infect. 2004;56:56–63. doi: 10.1016/s0195-6701(03)00293-7. [DOI] [PubMed] [Google Scholar]
- 22.Grayson M.L., Jarvie L.J., Martin R., Johnson P.D.R., Jodoin M.E., McMullan C., et al. Significant reductions in methicillin-resistant Staphylococcus aureus bacteraemia and clinical isolates associated with a multisite, hand hygiene culture-change program and subsequent successful statewide roll-out. Med J Aust. 2008;188:633–640. doi: 10.5694/j.1326-5377.2008.tb01820.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.