Highlights
-
•
Colorectal cancer patients present increased level of all MDSCs populations in peripheral blood.
-
•
Eosinophilic MDSCs can be distinguished among PMN-MDSCs in the blood of colorectal cancer patients.
-
•
In patients with colorectal cancer Mo-MDSCs correlate negatively with tumor antigen associated CD8+ T cells.
-
•
In colorectal cancer patients an increase of circulating Mo-MDSCs after surgery may be associated with a higher risk of disease recurrence during a 5-year period.
Keywords: Myeloid-derived suppressor cells (MDSCs), Colorectal cancer (CRC), Immunosuppression, T cell subsets, Tumor recurrence
Abbreviations: ARG-1, arginase-1; CRC, colorectal cancer; iNOS, inducible NO synthase; FMO, fluorescence minus one; mAbs, monoclonal antibodies; MDSC, myeloid derived suppressor cells; PBMC, peripheral blood mononuclear cells; TAA, tumor-associated antigen; TAM, tumor associated macrophages; TAN, tumor associated neutrophils; Treg, T regulatory cells
Abstract
Colorectal cancer (CRC) is the third most common malignancy. Its development and progression is associated with natural immunosuppression related, among others, to myeloid derived suppressor cells (MDSCs).
Overall, 54 patients in different stage of CRC, before any treatment were recruited into the study. The analysis included flow cytometry evaluation of blood MDSCs subsets, correlation their level with the tumor stage and T cell subsets. In the case of 11 patients, MDSCs level was evaluated before and 3 days after surgery, and these patients were monitored for cancer recurrence over 5 years.
The results showed that frequency of circulating MDSCs subsets is increased significantly in CRC patients, with highest level detected in most advanced tumor stages. Moreover, only monocytic MDSCs (Mo-MDSCs) positively correlate with regulatory Treg, and negatively with tumor Her2/neu specific CD8+ T cells. Circulating MDSCs, in contrast to tumor resident (mostly Mo-MDSCs), are negative for PD-L1 expression. Additionally, after surgery the blood level of Mo-MDSCs increases significantly, and this is associated with tumor recurrence during a 5-year follow-up.
In conclusion, Mo-MDSCs are pivotal players in CRC-related immunosuppression and may be associated with the risk of tumor recurrence after surgery.
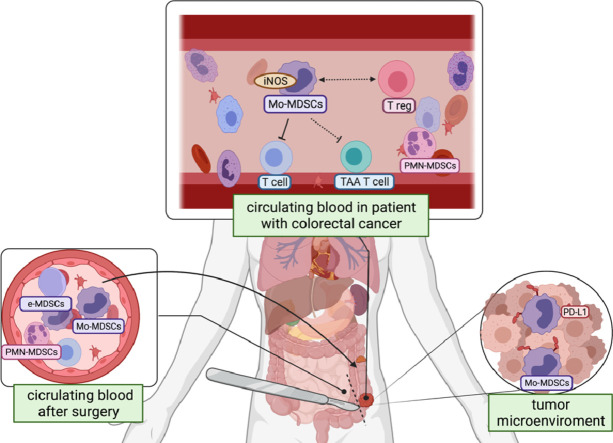
Graphical abstract
Introduction
Colorectal cancer (CRC) is the third most common malignancy diagnosed worldwide and recent data documents a shift in the mean age of CRC onset from 72 to 66 years of age [1,2]. Surgery, followed by adjuvant chemotherapy, remains the main form of CRC treatment, however for patients with selected genetic profiles, immunotherapy with bevacizumab, cetuximab, and panitumumab or molecular-targeted personalized therapy might also be included [3]. Yet approximately 50% of treated patients will develop metastases during the rest of their life [4]. Cancer development is accompanied by tumor infiltration with different immune cell subsets, having both pro- and anti-tumor activities and overall affecting the tumor growth and patients’ survival [5]. It was noticed that a low rate of blood lymphocyte-to-monocyte ratio in CRC may be associated with poor survival [6]. Moreover, the number of T lymphocytes infiltrating the tumor tissue may have a prognostic value in CRC, as CD4+ T cells were shown to negatively correlate with cancer stage [7]. On the other side, patients with a bigger population of cytotoxic lymphocytes tend to have more favorable outcomes relative to those with a higher proportion of suppressive Tregs, who succumb to earlier disease recurrence [8]. A significant component of the CRC microenvironment also constitutes the myeloid cells. This population is highly heterogenic and contains tumor associated macrophages (TAM), tumor associated neutrophils (TAN) and myeloid-derived suppressor cells (MDSCs) - all by the means of various mechanisms may contribute to tumor escape from the immune surveillance and can enhance cancer progression [9]. Among them, MDSCs currently turn attention in the context of their potential targeting in cancer therapy.
MDSCs are composed of three different subpopulations, defined depending on their origin: granulocytic (PMN-MDSCs), monocytic (Mo-MDSCs) and early-stage (e-MDSCs), which are most likely progenitors of the other two [10]. Their immunosuppressive function is mainly directed on effector T cells, being driven by a complicated signal network, where inducible NO synthase (iNOS) and arginase-1 (ARG-1) play a major role [11]. While PMN-MDSCs preferentially settle the peripheral lymphoid organs and are mainly responsible for ROS production and the release of granule-derived myeloperoxidase (MPO), Mo-MDSCs persist rather in the tumor bed and show a higher expression of iNOS, being the main producers of NO [12].
Here we aimed at characterization of MDSCs role in CRC patients, particularly in respect to the level of their subsets and its correlation with the tumor recurrence after surgery during a 5-year follow-up. In CRC, several antigens could be associated with the induction of tumor-specific T cells, including carcinoembryonic antigen (CEA), mucin-1 (MUC1), guanyl cyclase C (GUCY2C, GCC), and HER-2/neu [13,14]. In this context we asked whether MDSCs correlates with HLA-A2 HER-2/neu369–377 specific CD8+ T cells and other T cell subsets, including T reg and Th17 cells in CRC patients.
Materials and methods
Patients
Patients in different stage of CRC (TNM classification according to IUCC guidelines [15]), before any treatment were recruited into the First and Second Department of General Surgery, Jagiellonian University Medical College in Krakow. Overall, 54 patients were included in the study, however due to the several aspects investigated, the varying numbers of patients were involved in the individual phases of the study. For 11 CRC patients, examination of the blood level of MDSCs was done before and 3 days after surgery and further correlated with disease recurrence in a 5-year follow-up. In some cases, the tumor biopsy was taken in parallel. Simultaneously, 41 adult healthy blood donors were recruited as a control group. Characterization of the patients has been presented in Table 1, according to Zhang et al. [16]. All procedures were approved by the Jagiellonian University Bioethics Committee (approval no. 122.6120.128.2015 and 1072.6120.70.2018) and all subjects gave written informed consent to participate in the study.
Table 1.
Clinical characterization of the patients.
| CRC | Limiteda UICC (I+II) | Metastatica UICC (III+IV) | ||
| Characteristic | n = 54 | n = 27 | n = 27 | |
| Ageb (years) | 64±13 | 65±11.7 | 63.1 ± 14.3 | |
| Gander | Male | 30 | 14 | 16 |
| Female | 24 | 13 | 11 | |
| TNM stagec | I | 7 | 7 | – |
| II | 20 | 20 | – | |
| III | 16 | – | 16 | |
| IV | 11 | – | 11 | |
| Tumor sited | proximal | 27 | 13 | 14 |
| distal | 27 | 14 | 13 | |
| Histological grade | well/moderate | 36 | 19 | 17 |
| poor/undifferentiated | 13 | 6 | 7 | |
| undefined | 5 | 2 | 3 |
CRC patients with limited disease correspond to stage I and II, and those with metastatic disease correspond to stage III and IV.
Mean ± SD.
Staging based on TNM classification of CRC according to UICC.
Tumor site was classified as proximal or distal to the splenic flexure.
In each case, 5–10 ml of a whole blood was drawn to EDTA-containing tubes (BD Diagnostics, Vacutainer System, San Jose, CA) and within a maximum of 2 h, the peripheral blood mononuclear cells (PBMC) were isolated by standard Pancoll (PAN-Biotech, Aidenbach, Germany) density gradient centrifugation.
MDSCs analysis
For MDSCs analysis, PBMC (app. 1 × 106 cells) were stained (20 min at 4 °C) with the set of monoclonal antibodies (mAbs) listed in Table 1 (Supplementary data). After incubation, the cells were washed twice in PBS and suspended in 0.2 ml PBS. To determine the level of non-specific antibody staining and cell autofluorescence, the fluorescence minus one (FMO) control samples were incubated in parallel. The samples were analyzed in FACSCanto flow cytometer (BD Biosciences, Immunocytometry Systems, San Jose, CA) using FACSDiva 8.1 (BD Biosciences) and FlowJo v.10 software (BD Biosciences, Franklin Lakes, NJ). The Mo-MDSCs were characterized as LIN−HLA-DRlow/−CD33+CD11b+CD15−CD14+ cells, whereas PMN-MDSCs, as LIN−HLA-DRlow/−CD33+CD11b+CD15+CD14− cells, and e-MDSCs as LIN−HLA-DRlow/−CD33+CD11b+CD15−CD14−, all presented as a percent value of nucleated cells (NC) – cells positive for staining with SYTO™41 (Invitrogen, Eugene, OR) from PBMC.
In the case of tumor biopsy material, prior to staining with monoclonal antibodies tissue was fragmented and strained using small cell strainers with a nylon mesh having 40 μm pores (BD Biosciences) and washed twice in PBS. Thereafter, the cells were stained with monoclonal antibodies and MDSCs subsets were characterized as above within CD45+ cells and presented as a percent value of NC.
Detection of T regulatory (Treg) and Th17 cells
For Tregs and Th17 cells analysis, a whole blood was stained using Th17/Treg Human Phenotypic Kit (BD Pharmingen, San Diego, CA) with anti-CD4-Peridinin-Chlorophyll-Protein (PerCP), anti-IL-17A-Phycoerythrin (PE) and anti-Foxp3-Alexa Fluor (AF) 645 – conjugated mAbs, according to manufacturer's instructions. In parallel, the isotype controls were prepared to determine a non-specific binding of antibodies. Samples were analyzed in FACSCanto flow cytometer using FACSDiva software. The Treg and Th17 cells, were defined as CD4+Foxp3+ and CD4+IL-17A+ respectively, and their level was presented as percent value from CD4+ population.
HLA-A2 typing
PBMC (1 × 106/test) were incubated (20 min at 4 °C) with mouse anti-HLA-A2-Allophycocyanin (APC)-conjugated mAb (BD Pharmingen) or respective isotype control, then fixed (BD Fixative, BD Biosciences), washed in PBS and suspended in 200 μl PBS. Samples were analyzed using a FACSCanto flow cytometer and FACSDiva software. Patients positive for HLA-A2 expression were selected to the next stage, where CD8+ T cells specific for HER-2/neu369–377 (KIFGSLAFL) immunodominant peptide were analyzed.
Detection of CD8+ T cells specific for HER-2/neu epitope
The HER-2/neu antigen specific CD8+ T cells were identified using PE-labelled HLA-A2 pentamer complex (Pro5Pentamer; ProImmune, Oxford, UK) folded around the HER-2/neu369–377 specific epitope, as described previously [17]. As a negative control, staining with an HLA-A2 Negative Control Pentamer (ProImmune) was used. To minimize a non-specific staining, each pentamer was tittered before the final use. Isolated PBMC were resuspended in PBS and incubated with the indicated pentamer for 10 min. at 20 °C followed by washing and staining with FITC-conjugated anti-CD8 mAB (BD Biosciences) for 20 min. at 4 °C in the dark. Cells were washed twice and fixed before analysis by flow cytometry. Data from a minimum of 50.000 lymphocytes were collected.
MDSCs morphology assessment
The MDSCs populations were sorted using a FACSAria II, according to the following criteria: PMN-MDSCs as LIN−HLA-DRlow/−CD33+CD66b+CD14−, Mo-MDSCs as LIN−HLA-DRlow/−CD33+CD66b−CD14+, and e-MDSCs as LIN−HLA-DRlow/−CD33+CD66b−CD14−, followed by cytospin preparations (Cytospin, Sheldon, UK) and the Wright's staining (Merck, Darmstadt, Germany). Slides were analyzed by Olympus XC50 camera (Olympus, Tokyo, Japan).
Suppression of T cell proliferation assay
The MDSCs induced suppression of T cell proliferation was analyzed by H3-thymidine incorporation assay. In preliminary experiments, to establish the optimal ratio for the MDSCs inhibitory effect in cultures with autologous T cells, the FACS purified CD3+ cells and MDSCs subsets were cultured in RPMI-1640 medium containing 10% (v/v) FBS at different ratios (8:1; 4:1; 2:1) (Supplementary Fig. 1) in the presence of autologous monocytes (10% of the T cell number), and stimulated with phytohemagglutinin (PHA; Sigma Aldrich, Saint Louis, MO). After 3 days of culture, cells were pulsed with H3-thymidine (1 μCi/well; Hartmann Analytic, Braunschweig, Germany) and the ß-minus radiation was measured in a liquid scintillation counter (Beckman Coulter LS1801, Inc., Mississagua, Ontario, CA) as counts per minute (cpm). The results were calculated as mitotic index. For further tests, the ratio 2:1 (T cells: MDSCs) was selected.
iNOS expression and NO detection
Total RNA was isolated from Mo-MDSCs, and monocytes sorted by flow cytometry, using the Universal RNA Purification Kit (EURx, Gdansk, Poland), according to the manufacturer's specifications. 5 µg of total RNA was reverse transcribed to complementary DNA using NG dART RT-PCR kit (EURx). Quantitative real-time PCR was performed using SG qPCR Master Mix (2x) (EURx) and oligonucleotides complementary to transcripts of the analyzed genes using the Quant Studio 7 Real-Time PCR system (Applied Biosystems). The following oligonucleotides were used in this study:
iNOS: 5′- CAGCGGGATGACTTTCCAAG AGGCAAGATTTGGACCTGCA-3′
Gene expression was assessed by using the 2−∆Ct method. Expression level of the target genes was calculated by normalization to the reference gene - hypoxanthine-guanine phosphoribosyltransferase (HPRT). To detect NO production by MDSCs, a 4.5-diaminofluorescein-2/diacetate (DAF-2/DA, Abcam, Cambridge, UK) was used according to Strijdom et al. [18] and the cells were analyzed by flow cytometry (FACSCanto, BD Biosciences).
Statistical analysis
Statistical analysis was performed using the PRISM GraphPad 5 package (GraphPad Software Inc., San Diego, CA). Obtained data were analyzed using a T-test or one-way analysis of variance (ANOVA) with Tuckey test as a post hoc. The magnitude of the relationship between two quantitative features was evaluated using Pearson's correlation coefficient. The Kaplan–Meier curves with Log-rank analysis were used to determine the disease recurrence in two arbitrary selected patients’ groups. All data are expressed as median ± interquartile range. The pvalue < 0.05 was considered statistically significant.
Results
Peripheral blood of patients with CRC contains high level of circulating MDSCs
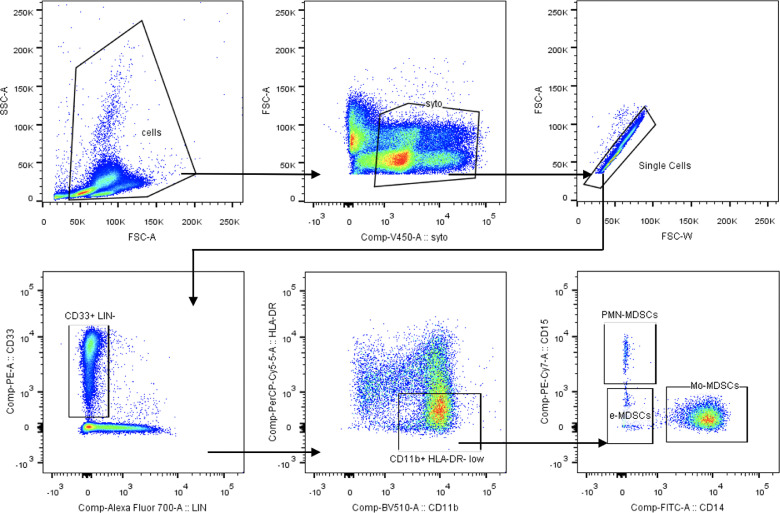
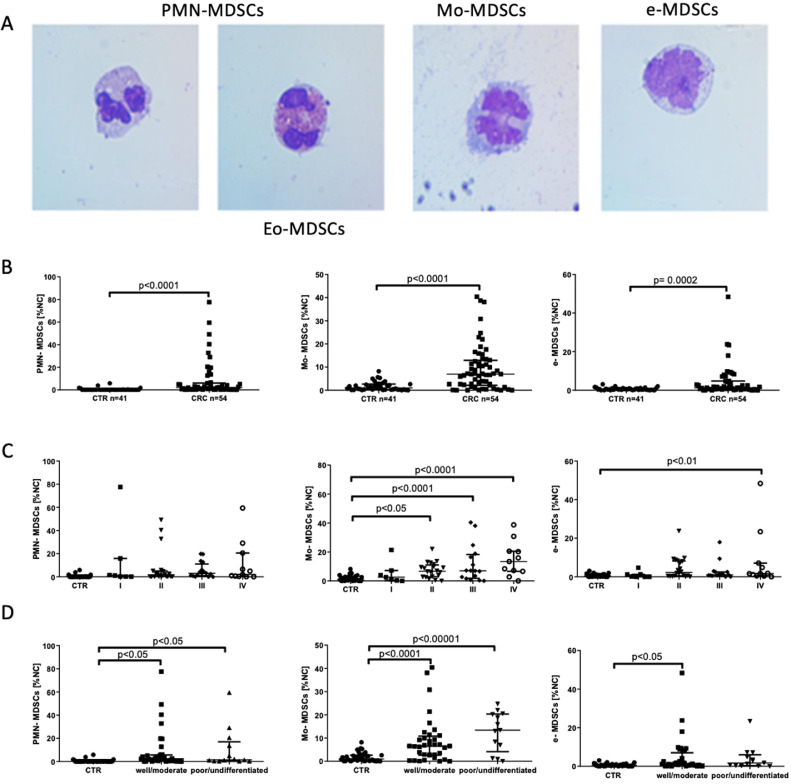
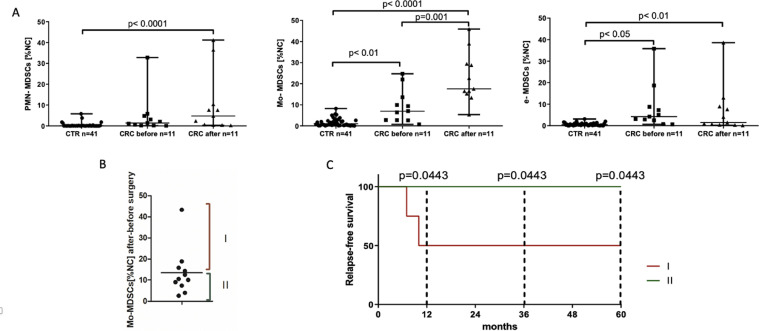
Populations of PMN-MDSCs, Mo-MDSCs and e-MDSCs were determined in peripheral blood of CRC patients according to the expression of previously defined cell surface markers [9]. The gating strategy for identification of MDSCs subsets by flow cytometry is presented in Fig. 1. The obtained data show that in CRC patients, the level (% value of PBMC) of circulating PMN-MDSCs, Mo-MDSCs and e-MDSCs was significantly higher than in healthy donors (Fig. 2B). These cells were further isolated by FACS and their morphology was analyzed microscopically after the Wright's staining (Fig. 2A). This analysis revealed that population of PMN-MDSCs is composed of two morphologically different subsets, including a recently described population of eosinophilic origin (Eo-MDSCs) [19]. Considering diversity in the clinical stage of the patients, we further analyzed how MDSCs subpopulations are distributed among the groups of patients with different CRC stages. In this context, higher levels of Mo-MDSCs and e-MDSCs in patients with most advanced cancer stage (IV) were observed. In the case of Mo-MDSCs, their level was also higher in stage II and III, while there was no difference in case of PMN-MDSCs (Fig. 2C).
Fig. 1.
Gating strategy for MDSCs population analysis.
The myeloid cells were gated as LIN−CD33+ from nucleated cells (SYTO41-positive) and single cells, then HLA-DR−/low CD11b+ cells were selected and PMN-MDSCs were identified as CD15+, Mo-MDSCs as CD14+, whereas e-MDSCs as CD15−CD14−.
Fig. 2.
MDSCs subset distribution in patients with CRC.
(A) The Wright's staining of isolated PMN-MDSCs, Mo-MDSCs and e-MDSCs. MDSCs populations were sorted out according to the following criteria: PMN-MDSCs as LIN−CD33HLA-DR−/lowCD66b+CD14−, Mo-MDSCs as LIN−CD33+HLA-DR−/lowCD66b−CD14+, and e-MDSCs as LIN−HLA-DR−/lowCD33+ CD66b−CD14− followed by cytospin preparation and the Wright's staining. Note, that within PMN-MDSCs, cells with eosinophil morphology (Eo-MDSC) were detected.
(B) Flow cytometry analysis of PMN-MDSCs, Mo-MDSCs and e-MDSCs level in CTR (n = 41), and patients with CRC (n = 54). MDSCs populations were identified by flow cytometry after gating according to cell surface marker expression, as described in A, and their levels are presented as percent values of PBMC.
(C) Frequency of PMN-MDSCs, Mo-MDSCs and e-MDSCs in relation to the stage of CRC (TNM classification, I n = 7; II n = 20; III n = 16; IV n = 11).
(D) Frequency of PMN-MDSCs, Mo-MDSCs and e-MDSCs in relation to the histological grading of CRC (well/moderate n = 36; poor/undifferentiated n = 13).
CTR - healthy donors, CRC - colorectal cancer patients, NC-nucleated cells.
In respect to tumor grading, the level of Mo-MDSCs and PMN-MDSCs was higher in the blood of patients, regardless the histological tumor classification (Fig. 2D). On the other hand, the level of e-MDSCs was elevated only in the blood of patients with the tumor differentiation status “well/moderate” (Fig. 2D). There was no difference in the level of MDSCs between patients with or without local lymph node metastases (Supplementary Fig. 2A). However, a trend for increased Mo-MDSCs and decreased of e-MDSCs frequency in patients with large tumor was observed (Supplementary Fig. 2B).
Mo-MDSCs positively correlate with the level of Treg cells and negatively with TAA CD8+ T cells in CRC patients
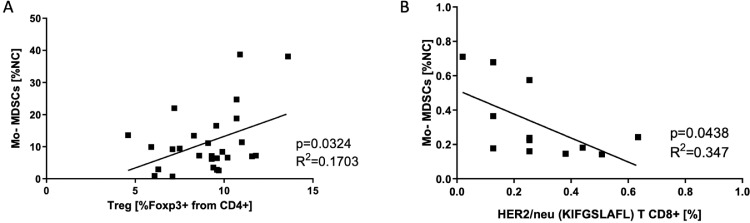
Although the interactions between MDSCs and T cells in cancer are well-documented, the relationship between MDSCs subpopulations and T cell subsets, including Treg in CRC is a matter of debate [20]. To shed more light on this aspect, we compared the level of MDSCs subsets with Treg, Th17 and CD8+ T cells specific for HLA-A2 HER-2/neu immunodominant epitope, considered as a tumor-associated antigen (TAA) in CRC [21]. We found that only Mo-MDSCs positively correlated with the level of Treg in this group of patients (Fig. 3A and Supplementary Fig. 3B). Furthermore, only these cells affected the level of CD8+ T cells specific for HLA-A2 HER-2/neu369–377 (KIFGSLAFL) immunodominant epitope in CRC patients (Fig. 3B and Supplementary Fig. 3C).
Fig. 3.
The relationship of Mo-MDSCs with T cell subpopulations.
(A) Correlation of the blood level of Mo-MDSCs with Treg cells. Mo-MDSCs were identified in peripheral blood by flow cytometry, as described in “Materials and methods”, and Treg cells as CD4+ Foxp3+. Mo-MDSCs’ levels are presented as percent values of nucleated cells (NC) from PBMC while Treg levels are presented as percent of CD4+Foxp3+ lymphocytes (n = 27).
(B) Correlation of the blood level of Mo-MDSCs with CD8+ T cells specific for Her2/neu, identified by flow cytometry. Blood level of Mo-MDSCs and HLA-A2 HER-2/neu369–377 specific CD8+ T cells is presented as percent of PBMCs and lymphocytes, respectively (n = 12).
MDSCs isolated from peripheral blood of CRC patients are immunosuppressive
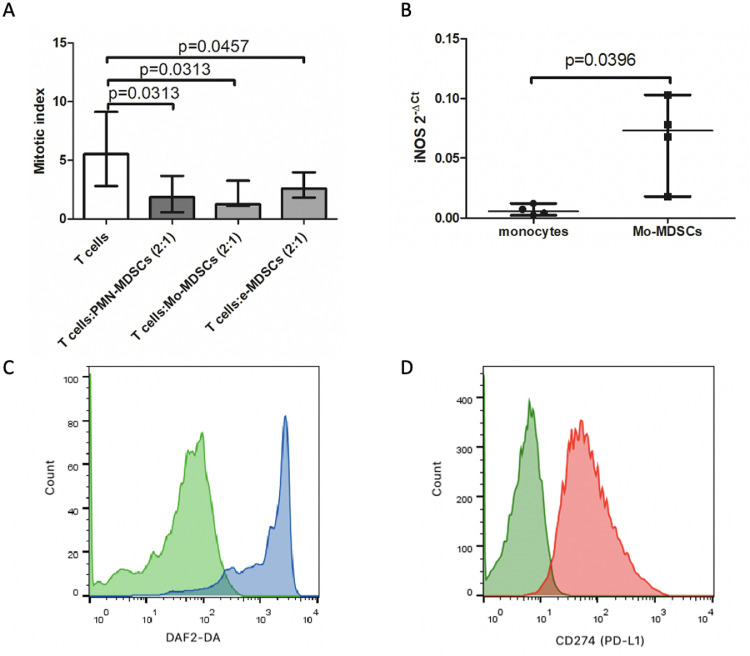
In the next set of experiments, we examined if identified cellular subsets indeed possess suppressive activity, typical for MDSCs. This was analyzed in vitro by H3-thymidine incorporation assay, evaluating the effect of MDSCs specific subsets on T-cell proliferation induced by PHA. MDSCs subsets were isolated by flow cytometry cell sorting and co-cultured for 3 days with autologous T cells, containing 10% of monocytes as accessory cells, and after pulse with H3-thymidyne, the proliferation of lymphocyte was measured. Initially, in a co-culture setting we have established a most effective T cells to MDSCs ratio as 2:1, which was used in further experiments. By this approach we show that PMN-MDSCs, Mo-MDSCs and e-MDSCs isolated from CRC patients’ blood were able to significantly inhibit T cell proliferation induced by PHA (Fig. 4A), confirming their suppressive activity.
Fig. 4.
Suppressive activity of MDSCs subsets. iNOS expression and NO production by Mo-MDSCs from CRC patients and expression of PD-L1 on Mo-DSCs depending on their localization
(A) MDSCs inhibit proliferation of autologous T-cells in CRC patients (n = 6). T cells were stimulated with PHA for 3 days in the presence of MDSCs. MDSCs subsets were sorted out by FACS as Lin−HLA-DRlow/−CD33+CD66b+CD14−; LIN−CD33+HLA-DRlow/−CD66b+CD14+; LIN−CD33+HLA-DRlow/−CD14−CD15− cells from PBMCs. MDSCs subsets were added to the culture of FACS purified autologous CD3+ T cells with 10% of autologous monocytes (sorted as CD14+HLA-DR+ cells). After 3 days of co-culture, T-cells were pulsed with H3-thymidine for additional 6 h and β− radiation was measured as cpm in a liquid scintillation counter. The index of proliferation was calculated as a ratio of PHA stimulated test culture [cpm] to non-stimulated culture [cpm].
(B) iNOS expression in monocytes and Mo-MDSCs from CRC patients detected by qRT-PCR (n = 4). The cells were sorted out by flow cytometry as Lin−CD33+HLA-DRlow/−CD66b−CD14+ and CD14+HLA-DR+ from PBMCs, respectively.
(C) Histogram overlay shows the fluorescence intensity of DAF2-DA corresponding to NO production by Mo-MDSCs (blue) in comparison to negative control (green). Data from one representative analysis out of four performed are presented.
(D) Histogram overlay shows the fluorescence intensity of PD-L1 (CD274) on Mo-MDSCs from blood (green) in comparison to Mo-MDSCs isolated from the tumor mass (red). Data from one representative flow cytometry analysis out of four performed are presented. Mo-MDSCs were gated as Lin−CD33+HLA-DRlow/−CD15−CD14+in PBMC and CD45+LIN−HLA-DRlow/−CD33+CD15−CD14+ in the tumor tissue, respectively.
Mo-MDSCs possess iNOS activity and differ in PD-L1 expression from the tumor-originated counterparts
Regulatory role of Mo-MDSCs is mainly associated with iNOS activity and NO production. To confirm this mechanism as operating in CRC patients, in the next step we analyzed the expression of iNOS-mRNA and NO production by Mo-MDSCs isolated from the patients’ blood. The data obtained by RT-PCR have shown significantly higher iNOS-mRNA expression in Mo-MDSCs than in normal blood monocytes (Fig. 4B). In parallel, using a fluorogenic dye - DAF-2 and flow cytometry analysis we have documented a significant production of NO by Mo-MDSCs isolated from the CRC-patients’ blood (Fig. 4C). In this context, we have also analyzed the expression of PD-L1 on Mo-MDSCs, as relevant in direct, cell-to-cell mediated immune suppression. The data presented in Fig. 4D show that blood Mo-MDSCs are negative, while their counterparts isolated directly from the tumor mass are highly positive for PD-L1 expression, supporting the role of Mo-MDSCs locally in the CRC tumor (Fig. 4D).
Surgical removal of colorectal cancer increases the level of circulating Mo-MDSCs which may correlate with tumor recurrence
Surgery is still a first-line therapy for CRC patients. In this context we asked, if surgical removal of the tumor may affect the level of MDSCs in patients’ blood. To this end we analyzed the level of MDSCs subsets in peripheral blood of CRC patients before and 3 days after surgery. The obtained results showed that after the treatment, frequency of circulating Mo-MDSCs increases significantly (Fig. 5A) – with an average increase of 13.5 ± 11.01%. Referring to these data, we have arbitrary divided our patients into two groups – first, composed of patients whose increase in Mo-MDSCs level was above an average value (group I, n = 4) and the second one, with the level of increase below the average (group II, n = 7) (Fig. 5B). During a five-year observation period it turned out that between these two groups there is a difference in the tumor recurrence, and patients with significant increase in the level of Mo-MDSCs after surgery present much more frequent relapse of the disease (characterization of the patients in these groups is presented in Supplementary Table 2).
Fig. 5.
MDSCs level before and after surgery treatment in relation to the CRC relapse-free time.
(A) Frequency of PMN-MDSCs, Mo-MDSCs and e-MSCSs in CRC patients’ blood before and 3 days after surgical removal of the tumor. MDSCs populations were identified by flow cytometry, as described in “Materials and methods” and presented as percent of PBMC (n = 11).
(B) Index of the increase of Mo-MDSCs level in CRC patients’ blood after surgery, calculated as the ratio of the Mo-MDSCs level after and before the treatment. Patients were divided into two groups according to the index of increase in the Mo-MDSCs level - first group (I), composed of patients whose increase in Mo-MDSCs level was above an average value (n = 4), and the second group (II), with the level of increase below the average value (n = 7).
(C) Kaplan-Meier analysis of relapse-free survival of CRC patients from group I and II during a 5-year follow-up (I n = 4; II n = 7).
CTR - healthy donors, CRC - colorectal cancer patients.
Discussion
Our study documents a significant increase in the level of PMN-MDSCs, Mo-MDSCs and e-MDSCs in the blood of CRC patients. In respect to PMN-MDSCs and Mo-MDSCs, this observation corroborates the previous reports [5,22], although the studies showing such a pattern solely for PMN-MDSCs also exist [12]. A correlation between CRC and e-MDSCs level has been already shown [10] but without reaching a statistical significance. Therefore, this paper is the first reporting an increased blood level of all identified MDSCs subsets in this group of patients. Moreover, we noticed that PMN-MDSCs contain, in majority, cells of neutrophil origin but eosinophilic MDSCs (Eo-MDSCs) can also be detected. This population was described for chronic bacterial infections [19], and to the best of our knowledge, this is the first study documenting the occurrence of such cells in cancer, and specifically in CRC.
It is widely accepted that the level of circulating MDSCs increases in the late stage of cancer [23]. In our study we have confirmed this observation only for Mo-MDSCs and e-MDSCs population frequency in blood was highest in stadium IV of CRC. Considering the level of MDSCs subsets, we did not detect any significant difference neither between the groups of patients with or without local lymph node metastases, nor between the patients with large and small tumors, however a tendency suggesting that particularly Mo-MDSCs may contribute to these aspects of CRC progression has been observed.
In our work, for phenotype identification of MDSCs subsets we followed the recommendations by Bronte et al. [9], however, recently new tips for characterization of these cells were proposed [24]. In the case of PMN-MDSCs and Mo-MDSCs, our panel of markers did not differ from the latest recommendations, but in respect to e-MDSCs the use of anti-CD123 mAb to exclude basophils from analysis has been suggested [25]. In this context, our data, although did not include CD123 staining, clearly show that e-MDSCs do not resemble basophils by morphology, and possess strong suppressive activity, a key feature for MDSCs identification [25]. Regarding phenotype, it has been further indicated that PD-L1 expression on MDSCs is increased in CRC patients, suggesting that it may be a potent mediator of immunosuppression [26]. In our study MDSCs subsets isolated from the patients’ blood were negative for PD-L1 expression, nevertheless they all efficiently inhibited proliferation of autologous T cells, confirming that MDSCs-mediated T cell suppression does not require the expression of PD-L1 [27], and suggesting that suppressive features of MDSCs represent a functional state of these cells. Contrary to peripheral blood, in the tumor MDSCs become more suppressive and are positive for PD-L1, specifically Mo-MDSCs, which are more prominent in this location, comparing to PMN-MDSCs [12]. This observation is in favor for PD-L1 upregulation by hypoxia, occurring in the tumor mass [28], and suggests that in the tumor, suppression is more dependent on direct cell-to cell contacts, although iNOS expression is also relevant for the suppressive nature of Mo-MDSCs [29]. In keeping, in our study Mo-MDSCs from CRC patients showed higher expression of iNOS mRNA than monocytes, and were able to produce NO, further documenting such an activity. In this context, we cannot exclude direct regulation of PD-L1 expression by iNOS activity in the tumor mass, as tumor-located MDSCs were shown to have a much higher expression of iNOS and NO production than their counterparts in peripheral lymphoid organs [30]. This would further clarify why tumor MDSCs are much more suppressive than their counterparts in the spleen or blood.
Immunosuppression in cancer is also associated with Treg [31], and increase in the Treg/Th17 ratio in cancer patients usually correlates with the disease progression [32]. Also, in our group of patients we observed a slightly increased blood Treg/Th17 ratio (Supplementary Fig. 2A), indicating a shift into the immune suppressive endotype. Considering the relationships between different populations of cells with regulatory functions, we further analyzed a potential correlation between the blood level of Treg and MDSCs. Although known for other types of cancer [33], in our study this was confirmed only in relation to Mo-MDSCs. Interestingly, there is no previous studies in the literature showing such a correlation in CRC patients. The regulatory functions of MDSCs can also be directed against tumor-antigen specific T cells [34]. In this context, we examined a correlation between Mo-MDSCs and HER-2/neu specific CD8+ T cells in CRC patients. While the role of HER-2/neu overexpression/amplification in CRC is less clear than in breast or gastric cancer, and controversial expression rates, ranging from 2.7 [35] to 47.7% [36] have been published, the HER2 protein levels are correlated with clinical outcomes in CRC [37]. Our data showed an inverse correlation between the blood levels of these two cell subsets. To the best of our knowledge, this is a first such an observation in CRC patients.
Although immunotherapy or molecular-targeted personalized therapy are currently available for patients with the selected genetic profiles, surgery is still a basic form of CRC treatment. However, there is little data on the effects of surgery on the MDSCs in CRC, while the available observations are largely contradictory [14,19,38,39]. In our study, we have shown that 3 days after surgery the significant increase in the frequency of blood Mo-MDSCs occurs, and its value above the average may be relevant as a prognostic factor, correlating with the recurrence of CRC during a 5-year observation period. This was not observed for other MDSCs subsets, indicating that blood level of Mo-MDSCs after surgery should be taken into consideration for assessing the risk of CRC recurrence. Similarly, the increased level of MDSCs was noticed in patient with rectal cancer 7 days after surgery [40]. These results seem to be consistent with our data in prostate cancer patients, showing that surgery was not effective in reducing the level of circulating Mo-MDSCs, or could even induce its increase [41].
Our study has potential clinical relevance but must be interpreted with caution. Its main limitation is a small size of the group of patients with a 5-year follow-up after surgery. In this context it must be stressed that patients were qualified to this group randomly and originally the group contained 18 patients, but 7 patients had to be excluded because of a highly advanced CRC (IV stage). This aspect must be taken into consideration in future studies to obtain a more potent data, however it is worth mentioning that similarly to our results, an increased frequency of postoperative CD14+HLA-DR−/low MDSCs was already correlated with an early recurrence of hepatocellular carcinoma [42].
In summary, our report indicates that in CRC patients, Mo-MDSCs play important role in cancer-related immunosuppression, and correlate with tumor size (prognostic marker in CRC) [43]. Moreover, Mo-MDSCs may be associated with tumor recurrence after surgery. Despite some limitations, our study suggests that MDSCs, and Mo-MDSCs particularly, seem potential targets for immunotherapy in CRC. In support of this view, first reports on successful therapy causing selective depletion of MDSCs in patients with advanced cancers, including CRC, have been already released [44].
CRediT authorship contribution statement
Izabela Siemińska: Conceptualization, Methodology, Formal analysis, Investigation, Writing – original draft. Kazimierz Węglarczyk: Methodology. Marta Walczak: Methodology. Agata Czerwińska: Methodology. Radosław Pach: Methodology. Mateusz Rubinkiewicz: Investigation, Methodology. Antoni Szczepanik: Investigation, Methodology. Maciej Siedlar: Supervision. Jarek Baran: Conceptualization, Formal analysis, Investigation, Writing – original draft, Supervision, Project administration, Funding acquisition.
Declaration of Competing Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Funding
The authors declare and acknowledge financial support from the Jagiellonian University Medical College internal grant (K/ZDS/005492) and EU H2020-MSCA-RISE-2017 program - grant “CANCER” (GA 777682).
Acknowledgments
Graphical abstract created with BioRender.com.
Data statement
Upon reasonable request, the raw data supporting the results of this article will be made available by the authors without undue reservation.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.tranon.2022.101346.
Appendix. Supplementary materials
References
- 1.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Charles M.M., Kahi J. Colorectal cancer 2020 epidemiological update. NEJM J. Watch. 2020;2020 doi: 10.1056/NEJM-JW.NA51140. [DOI] [Google Scholar]
- 3.Fakih M.G. Metastatic colorectal cancer: current state and future directions. J. Clin. Oncol. 2015;33:1809–1824. doi: 10.1200/JCO.2014.59.7633. [DOI] [PubMed] [Google Scholar]
- 4.Vatandoust S., Price T.J., Karapetis C.S. Colorectal cancer: metastases to a single organ. World J. Gastroenterol. 2015;21:11767–11776. doi: 10.3748/wjg.v21.i41.11767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang B., Wang Z., Wu L., Zhang M., Li W., Ding J., Zhu J., Wei H., Zhao K. Circulating and tumor-infiltrating myeloid-derived suppressor cells in patients with colorectal carcinoma. PLoS One. 2013;8 doi: 10.1371/journal.pone.0057114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu Q., Hu T., Zheng E., Deng X., Wang Z. Prognostic role of the lymphocyte-to-monocyte ratio in colorectal cancer. Medicine. 2017;96 doi: 10.1097/MD.0000000000007051. (United States) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zadka Ł., Chabowski M., Grybowski D., Piotrowska A., Dzięgiel P. Interplay of stromal tumor-infiltrating lymphocytes, normal colonic mucosa, cancer-associated fibroblasts, clinicopathological data and the immunoregulatory molecules of patients diagnosed with colorectal cancer. Cancer Immunol. Immunother. 2021:1–20. doi: 10.1007/s00262-021-02863-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Katz S.C., Bamboat Z.M., Maker A.V., Shia J., Pillarisetty V.G., Yopp A.C., Hedvat C.V., Gonen M., Jarnagin W.R., Fong Y., D'Angelica M.I., DeMatteo R.P. Regulatory T cell infiltration predicts outcome following resection of colorectal cancer liver metastases. Ann. Surg. Oncol. 2012;3(20):946–955. doi: 10.1245/S10434-012-2668-9. 2012 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bronte V., Brandau S., Chen S., Colombo M.P., Frey A.B., Greten T.F., Mandruzzato S., Murray P.J., Ochoa A., Ostrand-rosenberg S., Rodriguez P.C. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat. Commun. 2016;7:1–10. doi: 10.1038/ncomms12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ma P., Beatty P.L., McKolanis J., Brand R., Schoen R.E., Finn O.J. Circulating myeloid derived suppressor cells (MDSC) that accumulate in premalignancy share phenotypic and functional characteristics with MDSC in cancer. Front. Immunol. 2019;10 doi: 10.3389/fimmu.2019.01401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rodríguez P.C., Ochoa A.C. Arginine regulation by myeloid derived suppressor cells and tolerance in cancer: mechanisms and therapeutic perspectives. Immunol. Rev. 2008;222:180–191. doi: 10.1111/j.1600-065X.2008.00608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kumar V., Patel S., Tcyganov E., Gabrilovich D.I. The nature of myeloid-derived suppressor cells in the tumor microenvironment. Trends Immunol. 2016:1–13. doi: 10.1016/j.it.2016.01.004. xx. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Richman S.D., Southward K., Chambers P., Cross D., Barrett J., Hemmings G., Taylor M., Wood H., Hutchins G., Foster J.M., Oumie A., Spink K.G., Brown S.R., Jones M., Kerr D., Handley K., Gray R., Seymour M., Quirke P. HER2 overexpression and amplification as a potential therapeutic target in colorectal cancer: analysis of 3256 patients enrolled in the QUASAR, FOCUS and PICCOLO colorectal cancer trials. J. Pathol. 2016;238:562–570. doi: 10.1002/path.4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu P., He H., Gu Y., Wang Y., Sun Z., Yang L., Miao C. Surgical trauma contributes to progression of colon cancer by down regulating CXCL4 and recruiting MDSCs. Exp. Cell Res. 2018;370:692–698. doi: 10.1016/j.yexcr.2018.07.035. [DOI] [PubMed] [Google Scholar]
- 15.Brierley J., Gospodarowicz M., Wittekind C. John Wiley & Sons; 2017. TNM Classification of Malignant Tumours. [Google Scholar]
- 16.Zhang B., Wang Z., Wu L., Zhang M., Li W., Ding J., Zhu J., Wei H., Zhao K. Circulating and tumor-infiltrating myeloid-derived suppressor cells in patients with colorectal carcinoma. PLoS One. 2013;8:e57114. doi: 10.1371/journal.pone.0057114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stec M., Baran J., Szatanek R., Mytar B., Lenart M., Czupryna A., Szczepanik A., Siedlar M., Zembala M. Properties of monocytes generated from haematopoietic CD34+ stem cells from bone marrow of colon cancer patients. Cancer Immunol. Immunother. 2013;62:705–713. doi: 10.1007/s00262-012-1375-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Strijdom H., Muller C., Lochner A. Direct intracellular nitric oxide detection in isolated adult cardiomyocytes: flow cytometric analysis using the fluorescent probe, diaminofluorescein. J. Mol. Cell. Cardiol. 2004;37:897–902. doi: 10.1016/j.yjmcc.2004.05.018. [DOI] [PubMed] [Google Scholar]
- 19.Goldmann O., Beineke A., Medina E. Identification of a novel subset of myeloid-derived suppressor cells during chronic staphylococcal infection that resembles immature eosinophils. J. Infect. Dis. 2017;216:1444–1451. doi: 10.1093/infdis/jix494. [DOI] [PubMed] [Google Scholar]
- 20.OuYang L.Y., Wu X.J., Ye S.B., Zhang R., Li Z.L., Liao W., Pan Z.Z., Zheng L.M., Zhang X.S., Wang Z., Li Q., Ma G., Li J. Tumor-induced myeloid-derived suppressor cells promote tumor progression through oxidative metabolism in human colorectal cancer. J. Transl. Med. 2015;13:47. doi: 10.1186/s12967-015-0410-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brossart P., Stuhler G., Fiad T., Stevanovic S., Rammensee H.-.G., Kanz L., Brugger W. Her-2/neu-derived peptides are tumor-associated antigens expressed by human renal cell and colon carcinoma lines and are recognized by in vitro induced specific cytotoxic T lymphocytes1. Cancer Res. 1998;58:732–736. [PubMed] [Google Scholar]
- 22.Mandruzzato S., Solito S., Falisi E., Francescato S., Chiarion-Sileni V., Mocellin S., Zanon A., Rossi C.R., Nitti D., Bronte V., Zanovello P. IL4Rα+ myeloid-derived suppressor cell expansion in cancer patients. J. Immunol. 2009;182 doi: 10.4049/jimmunol.0803831. http://www.jimmunol.org/content/182/10/6562.long [DOI] [PubMed] [Google Scholar]
- 23.Diaz-Montero C.M., Salem M.L., Nishimura M.I., Garrett-Mayer E., Cole D.J., Montero A.J. Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin-cyclophosphamide chemotherapy. Cancer Immunol. Immunother. 2009;58:49–59. doi: 10.1007/s00262-008-0523-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sanseviero E., Kim R., Gabrilovich D.I. Methods in Molecular Biology. Humana Press Inc.; 2021. Isolation and phenotyping of splenic myeloid-derived suppressor cells in murine cancer models; pp. 19–28. [DOI] [PubMed] [Google Scholar]
- 25.Khan A.N.H., Emmons T.R., Wong J.T., Alqassim E., Singel K.L., Mark J., Smith B.E., Tario J.D., Eng K.H., Moysich K.B., Odunsi K., Abrams S.I., Segal B.H. Quantification of early-stage myeloid-derived suppressor cells in cancer requires excluding basophils. Cancer Immunol. Res. 2020;8:819–828. doi: 10.1158/2326-6066.CIR-19-0556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lu C., Redd P.S., Lee J.R., Savage N., Liu K. The expression profiles and regulation of PD-L1 in tumor-induced myeloid-derived suppressor cells. Oncoimmunology. 2016;5 doi: 10.1080/2162402X.2016.1247135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Youn J.I., Nagaraj S., Collazo M., Gabrilovich D.I. Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J. Immunol. 2008;181:5791–5802. doi: 10.4049/jimmunol.181.8.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Noman M.Z., Desantis G., Janji B., Hasmim M., Karray S., Dessen P., Bronte V., Chouaib S. PD-L1 is a novel direct target of HIF-1α, and its blockade under hypoxia enhanced: mDSC-mediated T cell activation. J. Exp. Med. 2014;211:781–790. doi: 10.1084/jem.20131916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ostrand-Rosenberg S., Sinha P., Beury D.W., Chornoguz O., Parker K.H. Cancer Immunotherapy: Immune Suppression and Tumor Growth. 2nd ed. Elsevier Inc.; 2013. Tumor-induced myeloid-derived suppressor cells; pp. 473–496. [DOI] [Google Scholar]
- 30.Gabrilovich D.I. The dawn of myeloid-derived suppressor cells: identification of arginase I as the mechanism of immune suppression. Cancer Res. 2021;81:3953–3955. doi: 10.1158/0008-5472.CAN-21-1237. [DOI] [PubMed] [Google Scholar]
- 31.Li C., Jiang P., Wei S., Xu X., Wang J. Regulatory T cells in tumor microenvironment: new mechanisms, potential therapeutic strategies and future prospects. Mol. Cancer. 2020;19:116. doi: 10.1186/s12943-020-01234-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang J., Cai D., Ma B., Wu G., Wu J. Skewing the balance of regulatory T-cells and T-helper 17 cells in breast cancer patients. J. Int. Med. Res. 2011;39:691–701. doi: 10.1177/147323001103900301. [DOI] [PubMed] [Google Scholar]
- 33.Li F., Zhao Y., Wei L., Li S., Liu J. Tumor-infiltrating Treg, MDSC, and IDO expression associated with outcomes of neoadjuvant chemotherapy of breast cancer. Cancer Biol. Ther. 2018;19:695–705. doi: 10.1080/15384047.2018.1450116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Monu N.R., Frey A.B. Myeloid-derived suppressor cells and anti-tumor T cells: a complex relationship. Immunol. Invest. 2012;41:595–613. doi: 10.3109/08820139.2012.673191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marx A.H., Burandt E.C., Choschzick M., Simon R., Yekebas E., Kaifi J.T., Mirlacher M., Atanackovic D., Bokemeyer C., Fiedler W., Terracciano L., Sauter G., Izbicki J.R. Heterogenous high-level HER-2 amplification in a small subset of colorectal cancers. Hum. Pathol. 2010;41:1577–1585. doi: 10.1016/J.HUMPATH.2010.02.018. [DOI] [PubMed] [Google Scholar]
- 36.Park D.I., Kang M.S., Oh S.J., Kim H.J., Cho Y.K., Sohn C.I., Jeon W.K., Kim B.I., Han W.K., Kim H., Ryu S.H., Sepulveda A.R. HER-2/neu overexpression is an independent prognostic factor in colorectal cancer. Int. J. Colorectal Dis. 2007;22:491–497. doi: 10.1007/S00384-006-0192-8. [DOI] [PubMed] [Google Scholar]
- 37.Wang X.Y., Zheng Z.X., Sun Y., Bai Y.H., Shi Y.F., Zhou L.X., Yao Y.F., Wu A.W., Cao D.F. Significance of HER2 protein expression and HER2 gene amplification in colorectal adenocarcinomas. World J. Gastrointest. Oncol. 2019;11:335–347. doi: 10.4251/wjgo.v11.i4.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li W., Wu K., Zhao E., Shi L., Li R., Zhang P., Yin Y., Shuai X., Wang G., Tao K. HMGB1 recruits myeloid derived suppressor cells to promote peritoneal dissemination of colon cancer after resection. Biochem. Biophys. Res. Commun. 2013;436:156–161. doi: 10.1016/j.bbrc.2013.04.109. [DOI] [PubMed] [Google Scholar]
- 39.Tang F., Tie Y., Tu C., Wei X. Surgical trauma-induced immunosuppression in cancer: recent advances and the potential therapies. Clin. Transl. Med. 2020;10:199–223. doi: 10.1002/ctm2.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yuan L., Xu B., Fan H., Yuan P., Zhao P., Suo Z. Pre- and post-operative evaluation: percentages of circulating myeloid-derived suppressor cells in rectal cancer patients. Neoplasma. 2015;62:239–249. doi: 10.4149/NEO_2015_029. [DOI] [PubMed] [Google Scholar]
- 41.Siemińska I., Rychlicka-Buniowska E., Jaszczyński J., Palaczyński M., Bukowska-Strakova K., Ryś J., Dumański J., Siedlar M., Baran J. The level of myeloid derived-suppressor cells in peripheral blood of patients with prostate cancerafter various types of therapy. Pol. J. Pathol. 2020;71:46–54. doi: 10.5114/PJP.2020.95415. [DOI] [PubMed] [Google Scholar]
- 42.Gao X.H., Tian L., Wu J., Ma X.L., Zhang C.Y., Zhou Y., Sun Y.F., Hu B., Qiu S., Zhou J., Fan J., Guo W., Yang X.R. Circulating CD14+HLA-DR−/low myeloid-derived suppressor cells predicted early recurrence of hepatocellular carcinoma after surgery. Hepatol. Res. 2017;47:1061–1071. doi: 10.1111/HEPR.12831. [DOI] [PubMed] [Google Scholar]
- 43.Kornprat P., Pollheimer M.J., Lindtner R.A., Schlemmer A., Rehak P., Langner C. Value of tumor size as a prognostic variable in colorectal cancer: a critical reappraisal. Am. J. Clin. Oncol. Cancer Clin. Trials. 2011;34:43–49. doi: 10.1097/COC.0B013E3181CAE8DD. [DOI] [PubMed] [Google Scholar]
- 44.Dominguez G.A., Condamine T., Mony S., Hashimoto A., Wang F., Liu Q., Forero A., Bendell J., Witt R., Hockstein N., Kumar P., Gabrilovich D.I. Selective targeting of myeloid-derived suppressor cells in cancer patients using DS-8273a, an agonistic TRAIL-R2 antibody. Clin. Cancer Res. 2017;23:2942–2950. doi: 10.1158/1078-0432.CCR-16-1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.