Abstract
Background
High‐dose‐rate brachytherapy (HDR BRT) has been enjoying rapid acceptance as a treatment modality offered to selected prostate cancer patients devoid of risk group, employed either in monotherapy setting or combined with external beam radiation therapy (EBRT) and is currently one of the most active clinical research areas.
Recent findings
This review encompasses all the current evidence to support the use of HDR BRT in various clinical scenario and shines light to the HDR BRT rationale, as an ultimately conformal dose delivery method enabling safe dose escalation to the prostate.
Conclusion
Valid long‐term data, both in regard to the oncologic outcomes and toxicity profile, support the current clinical indication spectrum of HDR BRT. At the same time, this serves as solid, rigid ground for emerging therapeutic applications, allowing the technique to remain in the spotlight alongside stereotactic radiosurgery.
Keywords: combined with EBRT, high‐dose‐rate, interstitial brachytherapy, monotherapy, prostate cancer, salvage
1. INTRODUCTION
Validated therapeutic modalities considered for patients diagnosed with organ‐confined prostate cancer are radical prostatectomy, 1 , 2 external beam radiation therapy (EBRT), 3 , 4 , 5 permanent low‐dose rate (LDR) brachytherapy (BRT), 6 , 7 , 8 and temporary high‐dose‐rate (HDR) BRT. 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 However, owing to the unavailability of randomized clinical trials, the optimal management remains trivial, with proposed treatment assignment being mainly determined by physician's biased guidance and patient's preference. In this regard, choice of treatment and consecutively its impact on quality of life have gained increasing importance, with BRT being favored due to its high effectiveness and, at the same time, its relatively low morbidity. Currently, validated long‐term data endorse the efficacy of BRT in the management of locally confined prostate cancer with technological advancements fueling active research in the field of HDR BRT owed mainly to refinement of the technique, 20 employment of modern biomolecular imaging, 21 , 22 , 23 and investigation of focal and focused approaches, 24 all of which ensure high standards of implant quality and precision. The dosimetric superiority of HDR BRT translates into excellent clinical results, 25 , 26 , 27 thus backing up the notion that HDR BRT is a novel alternative to permanent LDR BRT. 28
This review presents a comprehensive analysis of the rationale, current clinical indications, and oncologic outcomes, including a representative data report.
2. BACKGROUND
2.1. Rationale for HDR brachytherapy
Dose escalation data suggest that the utilization of comparatively higher dose for definitive radiation therapy (RT) in organ‐confined prostate adenocarcinoma improves biochemical control (BC) 4 , 5 , 29 but, at the same time, results in improved metastasis‐free survival (MFS). 5 , 30 , 31 , 32 , 33 , 34 Adding to that, the rational assumption can be made that further therapeutic impact improvement could be attained through dose escalation, while simultaneously enhancing dose conformity, especially in patients devoid of regionally advanced and/or metastatic tumor load. HDR BRT fully exploits its radiobiological advantage to perfectly meet this objective, through the utilization of extreme hypofractionation 35 , 36 , 37 and, at the same time, its incomparably superior three‐dimensional (3D) dosimetry. 38 HDR treatment planning enables dose optimization through multiparametric modulation, for example, catheter geometry, precalculated dwell positions, and times. 39 , 40 This allows for optimal dose modulation, with higher dose delivery to target volume and/or selectively dose reduction to organs at risk (OARs). 25
In relation, HDR BRT employs “high‐density” dosimetry, owed to the roughly twofold dwell positions number when compared to seeds in a typical LDR implant. Again in comparison to LDR, anatomic and, thus, dosimetric changes are kept to a minimum, since issues associated with LDR BRT such as migration of seed/source and deformation of tissue do not occur. 41 , 42 , 43
On the other hand, intrafractional anatomic alteration caused by organ motion during EBRT delivery, 44 , 45 , 46 as well as setup inaccuracies, is overcomed with HDR due to rectification of theses error during the implantation procedure with interactive online dosimetry or modified prior to dose delivery with real‐time anatomy‐based treatment planning. 25
This minimization of errors allows for a decrease in the therapeutic margins required beyond the intended target, thus exposing less healthy tissue in unnecessary radiation, transforming HDR BRT to the optimal intraprostatic dose‐escalation technique, where needed, especially when combined with EBRT. This proved especially important in patients whose treatment volume includes the regional lymphatic drainage, being treated to a moderate dose, yet offering an escalated intraprostatic escalated dose.
2.2. Radiobiological considerations
Radiobiological data suggest that there is variability between normal and malignant tissue and the probability of acute and late radiation sequelae development, variation which is also being noted in‐between different fractionation schedules. Adhering to the linear‐quadratic model, 47 the sensitivity of a particular tissue to altered fraction size is expressed by the α/β ratio, allowing comparison between various treatment schedules and, at the same time, estimates the impact of each given fractionation schedule on tumor control and toxicity. Recent radiobiological reports suggest an α/β ratio for prostate cancer ranging between 1.2 and 3.0 Gy, which is relatively lower than the α/β ratio of acutely and late‐reacting normal tissues. 36 , 48 , 49 Having this in mind, hypofractionated dose schemes are favored and seem to result in superior tumor control with remarkable reduction in late side effects. In this background, HDR BRT represents the ideal method for conformal dose escalation. 50
2.3. Patient selection for HDR brachytherapy
Based on the hypothesis that failure of local control in organ‐confined prostate cancer may lead to regional and distant metastasis development, histologically confirmed localized disease is the fundamental indication for HDR BRT in patients, who are considered suitable candidates for definite treatment. 51 , 52
In line with the National Comprehensive Cancer Network (NCCN) guidelines, 53 patients with low‐ and intermediate‐risk are stratified as optimal candidates for local radical treatment, considering they bear the highest probability for organ‐confined prostatic disease. Concomitantly, reports from mature retrospective series encourage the use of HDR BRT monotherapy in a selection of high‐risk patients, based on the notion that the therapeutic margin provided is superior to RP, with OARs' dose (urinary bladder and rectum) remaining significantly lower in comparison with definitive dose‐escalated EBRT plans.
On the other hand, in patients stratified as intermediate and high risk, 1 , 53 , 54 the utilization of combined HDR BRT as a boost modality with EBRT is a well‐established treatment supported by valid data. 55 , 56 , 57 , 58 Again, HDR BRT may find implementation in the regional lymphadenopathy setting, with or without the presence of distant metastatic spread, as a combination with EBRT as part of an individualized treatment concept, aiming at minimizing toxicity, with the goal of maximizing local disease control.
In the local recurrence setting after definitive RT, as proposed by international guidelines, 51 , 52 , 59 any patient presenting histological and/or radiological (also biomolecular imaging) proved prostate‐confined disease is a potential candidate for local radical treatment, therefore prostate salvage HDR BRT (sHDR BRT) should be considered.
Prior to HDR BRT, complete clinical staging should be attempted following the European Association of Urology, 60 European Society for Radiotherapy and Oncology (ESTRO), 52 and American Brachytherapy Society (ABS) 51 guidelines. Patient's precise group stratification and further on choice of therapeutic modality should be based on thorough clinical work‐up, consisting of histological confirmation of the prostatic malignancy, and clinical investigations for evaluation of possible disease spread, including digital rectal examination, transrectal ultrasound (TRUS), computed tomography (CT), bone scintigraphy, and/or magnetic resonance imaging (MRI). In uncertain cases of regional lymphadenopathy, laparoscopic pelvic lymphadenectomy or positron emission tomography may be considered for optimal staging.
Although the baseline urinary function can be predictive for functional outcome following HDR BRT, 61 neither larger glandular size nor previous transurethral resection of the prostate (TURP) (given a sufficient amount of time has surpassed [>3 months] and residual gland volume remains for image‐based 3D treatment planning), 62 , 63 , 64 should be considered as absolute contraindications.
When comparing HDR to LDR or EBRT, the exacerbation of lower urinary tract symptoms appears to be less prolonged, based on the fact that even patients with high International Prostate Symptom Score (IPSS; ≥20) tend to have a rather rapid recovery to pretreatment baseline urinary function. 65 Selection criteria for HDR BRT as monotherapy, combined with EBRT and in the salvage setting, are presented in Table 1.
TABLE 1.
Patient selection criteria for HDR BRT in the treatment of prostate cancer
| Inclusion criteria |
| Stages cT1‐T3b a |
| Any Gleason score |
| Any PSA level |
| Exclusion criteria |
| TURP within 3 months |
| IPSS >20 |
| Pubic arch interference |
| Lithotomy position not possible b |
| Anaesthesia not possible |
| Rectal fistula |
Abbreviations: IPSS, International Prostate Symptom Score; TURP, transurethral resection of the prostate.
Selected T4 tumors included with curative intent in the protocols of selected centers.
Relevant for TRUS‐guided technique, does not apply for MRI‐guided implantation.
In contrary to permanent LDR implants, HDR BRT after loading catheters can be implanted accordingly, in order to cover areas of extracapsular or the seminal vesicles' infiltration or even the bladder pouch, extending its indication to coverage of even T4 tumors, as part of individualized curative treatment concepts. 14 , 66 , 67 Previous pelvic EBRT, prior pelvic surgery, and inflammatory bowel disease are not considered absolute contraindications for HDR prostate BRT but always a very thorough evaluation of the potential risks and benefits should take place, based on anatomy‐based dosimetry including carefully defined OARs dose constraints. 25
2.4. Implantation techniques
Anaesthesia, spinal or general, is required for interstitial catheter implantation. It should be stated that catheter implantation can be carried out using TRUS‐guided technique, 13 , 68 , 69 where extensive experience exists or by MRI‐assistance. 52 , 53 Table 2 describes key features of the technique.
TABLE 2.
Key features in the HDR BRT of prostate cancer
| Important steps for high‐dose‐rate brachytherapy are: | |
|---|---|
| A. | Catheter placement under image guidance (usually TRUS) |
| B. | Imaging with catheters in place: TRUS, CT, or MRI |
| C. |
Definition/contouring of CTV, OARs, and catheter reconstruction on planning system Current step might include image co‐registration aiding at gross disease delineation: TRUS, MRI, PET |
| D. | Dwell position and time optimisation |
| E. | Quality assurance tests |
| F. | Treatment delivery |
Abbreviations: CT = computed tomography; CTV = clinical target volume; MRI = magnetic resonance imaging; OARs = organs‐at‐risk; PET = positron emission tomography; TRUS = transrectal ultrasound.
In the TRUS‐based technique, implantation is carried out transperineally with the patient placed in high lithotomy position, using a template to aid catheter placement and a continuous probe movement technique. The clinical workflow includes image acquisition of the prostate, urethra, and anterior rectal wall and the creation of virtual volumes prior to implantation for inverse treatment preplanning. 40 Three‐dimensional (3D) volume reconstruction follows based on a 0.1 cm image distance. Contouring commences based on the GEC/ESTRO guidelines. 52 Abiding on the acquired 3D anatomy, precalculated virtual catheter positions are generated, activating catheter source dwell positions located within the PTV, while radioactive source dwell times are calculated using an intraoperative treatment planning system (Figure 1). Using a dose–volume histogram (DVH) of the PTV and the OARs (ie, intraprostatic urethra, anterior rectal wall, and urinary bladder), the final evaluation of the anatomy‐oriented dose optimization 39 is performed. Once the dosimetric protocol parameters are met, TRUS‐guided implantation is carried out at the predefined catheter positions (Figure 1).
FIGURE 1.
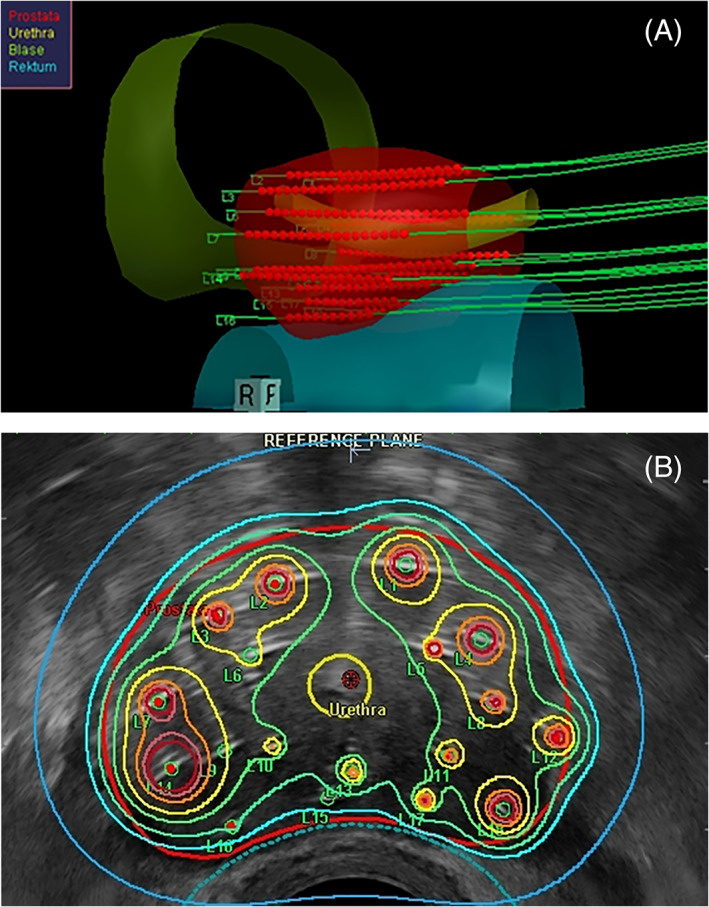
(A) Three‐dimensional reconstruction of the prostate, urethra, rectum, and bladder with template catheter trajectories for TRUS‐guided implantation as calculated for preplanning by the real‐time treatment planning system SWIFT/Oncentra Prostate (Nucletron – an Elekta Company, B.V., Veenendaal, The Netherlands). (B) Intraoperative real‐time TRUS‐based treatment planning presenting isodose distribution after anatomy‐based dose optimization. The isodose color code convention is dark red = 300 %, orange = 200 %, yellow = 150 %, green = 125 %, turquoise = 100 %, and dark blue = 50 %
In the MRI‐based implantation procedure, transperineal catheter placement is achieved by placing the patient in left lateral decubitus position, again employing a template device. The MRI‐based procedure parallels the workflow of TRUS‐guided implantation, since it involves a preplanning step based on 3D image reconstruction from the acquired preinterventional MRI sequences (of at least 0.3 cm slice thickness). The number, distribution, as well as distance between the catheters are predetermined by the preplanning which calculates the peripheral catheter arrangement with arbitrary optimization for target coverage. The maximum insertion depth and positional verification of the implanted catheters is performed by interactive MRI scanning following catheter implantation. An attempt to obtain the optimum from both worlds has already been made. In our department, a T2‐MRI sequence, with a placed urinary catheter, is obtained just before the TRUS‐guided transperineal implantation procedure begins. Based on clearly visible landmarks, such as the urinary catheter balloon, the vesicourethral junction can be easily identified, both on MRI and US images, aiding in optimal fusion of the two modalities and thereby precise prostate capsule definition, especially of the prostatic apex and base (Figure 2).
FIGURE 2.
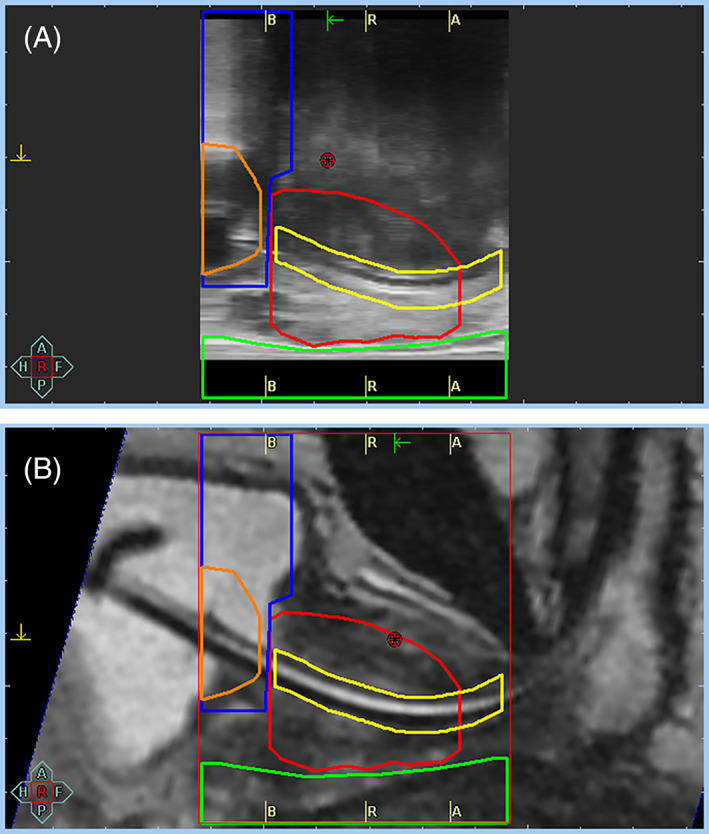
Image fusion with (A) ultrasound (US) image acquisition prior to interstitial catheter implantation and (B) magnetic resonance imaging (MRI) acquired on day of brachytherapy session with urethral catheter in place. Urethral catheter's balloon serves as a mark indicating the vesicourethral junction, a point easily identifiable both on US‐ and MRI‐images. MRI images assist in visibility of the prostatic base and apex. *blue contour = urinary bladder, red = prostate, yellow = urethra, green = rectum, orange = catheter balloon
3. CLINICAL DATA
3.1. HDR brachytherapy in combination with EBRT
Dose‐escalation trials, in reference to the management of intermediate‐ and high‐risk prostate cancer, identified a marked improvement, observed both in BC as well as MFS. 4 , 5 , 29 , 30 , 32 , 33 , 34 , 70 , 71 , 72 , 73 It is evident that the combination of EBRT with hypofractionated HDR BRT as a boost enables for safe delivery of high biologically equivalent doses to the prostate, which currently cannot be matched by any form of image‐guided EBRT. 29 , 74 , 75 , 76 Of particular importance is the comparison of HDR BRT with stereotactic approaches, in terms of conformality, proving its dosimetric superiority. 77 , 78
Randomized studies in confluence with mature retrospective data justify the superiority of combined modalities over EBRT alone in the primary treatment of localized high‐risk prostate adenocarcinoma. A randomized prospective study 55 allocated 220 patients to either combined HDR BRT with hypofractionated EBRT or EBRT alone. The EBRT‐only scheme (n = 111) consisted of 55 Gy administered over 20 fractions, whereas in the combined group (n = 109) of 35.75 Gy, EBRT was administered over 13 fractions followed by a 17‐Gy HDR boost applied in two fractions with a single implant. The combined arm proved superior in regard to mean biochemical failure‐free survival, 5.1 years versus 4.3 years in the EBRT‐only group (P = .03), with higher‐grade GU as well as GI toxicity not reaching statistical significance. In an earlier study, 56 104 patient were randomized to either conventional EBRT up to a total physical dose of 66 Gy in 33 fractions or to 35 Gy pulse‐dose‐rate BRT delivered over 48 h plus EBRT of 40 Gy in 20 fractions 2 weeks later. A recent update of this study, 79 with a median follow‐up of 14 years, reported an overall survival benefit for the combined technique, 67% in the EBRT arm compared to 77% in the combined modality arm, again without statistically significant differences in late GU and GI toxicity. Although BC remained improved in favor of the combined modality, unfortunately it did not manage to achieve statistical significance, owning mainly to the fact that the trial was underpowered. The recent ASCENDE RT Trial 80 put two‐dose escalation methods to the test, with patients being allocated between a standard arm (n = 200) consisting of ADT for 12 months and pelvic EBRT to 46 Gy plus a EBRT boost to 78 Gy and an experimental arm (n = 198) employing an LDR BRT boost with minimal peripheral prostatic dose of 115 Gy. Achieving a median follow‐up of 6.5 years, the 7‐year biochemical failure‐free survival was in favor of the BRT arm, 86% compared to 75% in the EBRT arm. The favorable oncologic outcomes of the study were associated with higher rates of acute and late GU toxicity in the LDR boost‐arm, attaining a 5‐year cumulative incidence of grade 3 GU of 18.4% for LDR BRT vs 5.2% for the EBRT boost (P < .001). 57
More recently, the TROG 03.04 RADAR study 81 randomized patients with intermediate‐ (33%) and high‐risk (66%) disease to either 6 or 18 months of leuprorelin with or without 18 months of zoledronic acid. Patients were either treated solely by EBRT (66 Gy, 70 Gy, or 74 Gy) or received a high‐dose‐rate (HDR) brachytherapy boost (19.5 Gy in three fractions). In a multivariate analysis adjusted for age, use of zoledronic acid, and other validated prognostic stratification variables, the HDR boost subgroup reported that longer duration of androgen suppression (18 months) was associated with reduced distant progression, prostate‐cancer‐specific mortality, and all‐cause mortality (sHR, 0.61, 0.67, 0.59, respectively). Again, interestingly, the HDR boost was associated with reduced PCSM risk and improved overall survival, reaching statistical significance (P < .001 for both).
Adding to that, one of the largest retrospective series from our group 19 included 303 high‐risk patients treated with EBRT delivering 45.0 Gy followed by an HDR BRT boost consisting of two fractions of 10.5‐Gy. The reported 7‐year biochemical relapse‐free survival and metastasis‐free survival rates were 88% and 93%, respectively. The reported incidence of late grade 3 GU adverse events was 2.2%, with no GI grade 3 being reported.
Acknowledging the methodological advantages of HDR BRT in comparison with LDR, in regard to the very steep fall‐off in dose beyond the PTV together with the versality of intratarget dose modulation, the avoidance of systematic errors, and imprecision in dose application due to anatomic deformities and source migration, it is only reasonable to state that all LDR outcomes can be reproducible, if not superior, 82 with the employment of HDR BRT.
Overall, the heterogeneity of clinically implemented treatment schemes poses a great challenge, especially if attempting to propose uniform recommendations for a standardized protocol. That set aside, the published oncological results on combined RT are both consistent and reproducible (Table 3). The majority of institutions employ total physical HDR doses of 12–21 Gy applied in two to four fractions, with BRT fractions ranging from 6 to 10.5 Gy. The supplemental EBRT doses range from 45 to 54 Gy (normofractionation), generating total BED 1.5 and EQD2 doses in the range of 171–366 Gy and 74–137 Gy, respectively. 9 , 10 , 14 , 15 , 16 , 56 , 59 , 68 , 69 , 79 , 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 , 91 , 92 , 93 , 94 , 95 , 96 , 97 , 98 , 99 , 100 , 101 , 102 , 103 , 104 , 105 , 106 , 107 The reported severe late GU and GI adverse events rates compare favorably with late toxicity rates in dose‐escalated EBRT series. 71 , 105 , 106 , 108 It must be noted that hypofractionated EBRT protocols are gaining momentum, 94 , 95 appearing equieffective in regard to clinical outcome, while demonstrating favorable toxicity profile.
TABLE 3.
Literature results of HDR BRT as boost modality to EBRT for clinically localised prostate cancer
| Treatment scheme | ||||||
|---|---|---|---|---|---|---|
| Study | n | Total EBRT dose (Gy/fx) | Total HDR dose (Gy/fx) | Total BED/EQD2 (Gy) | Follow‐up (y) | Biochemical control a |
| Galalae et al. 83 | 122 | 40/20 | 18/2 | 219/94 | Median 8.2 | 74% LR, 69% all‐risk groups at 5 years/8 years |
| Phan et al. 84 | 309 | 46/23 | 36/18–50.4/28 | 191–218/82–94 | Median 4.7 | 98% LR, 90% IR, 78% HR at 6 years |
| Pellizzon et al. 85 | 209 | 36–54/20–30 | 16–24/4 | 138–239/59–102 | Median 5.3 | 94.2% All‐risk groups at 3.3 years with 91.5% LR, 90.2% IR and 88.5% HR |
| Viani et al. 86 | 131 | 45–50/25 | 16–24/4–6 | 158–205/68–88 | Median 5.2 | 81% at 5 years with 87% IR and 71% HR |
| Morton et al. 87 | 123 | 37.5/15 | 15/1 | 265/114 | Median 1.2 | 100% All‐risk groups |
| Neviani et al.88 | 455 | 45/25 | 16.5–21/3 | 176–216/76–93 | Median 4.0 | 92% LR, 88% IR, 85% HR at 4 years |
| Noda et al. 89 | 59 | 50/25 | 15–18/2 | 207–243/89–104 | Median 5.1 | 100% LR at 5 years, 92% IR at 5 years, 72% HR at 5 years |
| Agoston et al. 59 | 100 | 40–60/20–30 | 8/1 or 10/1 | 144–217/62–93 | Median 5.1 | 85.5% for all risk groups at 5 years with IR 84.2% at 7 years and HR 81.6% at 7 years |
| Martinez et al. 90 | 472 | 46/23 | 16.5–23/2–3 | 184–306/79–131 | Mean 8.2 | 56.9% All risk groups for EQD2 < 93 Gy and 81.1% all‐risk groups for EQD2 > 93 Gy at 10 years |
| Prada et al. 16 | 313 | 46/23 | 23/2 | 306/131 | Median 5.7 | 100% LR, 88% IR, 79%–81% HR |
| Aluwini et al.91 | 264 | 45/25 | 18/3 | 189/81 | Median 6.2 | 97% LR and IR at 7 years |
| Whalley et al.92 | 101 | 46/23 | 17/2–19.5/3 | 211–220/91–95 | Median 4.7 | 95% IR, 68% HR at 4 years |
| Kotecha et al. 14 | 229 | 45/25–50.4/28 | 16.5–22.5/3 | 176–245/74–105 | Median 5.1 | 95% LR at 7 years, 90% IR at 7 years, 57% HR at 7 years (81% HR with BED>190 Gy) |
| Hoskin et al. 13 | 218 | 35.75/13 | 17/2 | 214/92 | Median 7.1 | 75%, 66% and 46% for all‐risk groups at 5‐, 7‐ and 10‐years |
| Helou et al. 93 |
123 60 |
37.5/15 45/25 |
15/1 20/2 |
265/114 252/108 |
Median 6.2 Median 8.5 |
97.4% at 5 years with IR 92.7% at 5 years with IR |
| Vigneault et al. 94 | 832 | 40–44/20 | 18/3–15/1 | 184–274/79–117 | Median 5.5 | 95% LR, 95% IR, 94% HR at 6 years |
| Ishiyama et al. 95 | 3424 | 39/13 | 18/2 | 243/104 | Median 5.5 | 91% IR, 81% HR at 10 years |
| Falk et al. 96 | 159 | 46/23 | 18/3 or 18/2 or 14/1 | 223–177/100–76 | Median 6.0 | 92% IR, 85% HR at 5 years |
| Strouthos et al. 19 | 314 | 45/25 | 21/2 | 267/114 | Median 5.9 | 86.3% for the entire cohort, 85.6% HR at 6 years |
Abbreviations: BED, biologically effective dose considering an a/β‐ratio for prostate cancer of 1.5 Gy, EQD2, equieffective dose administered in 2Gy‐fractions; HR, high‐risk group; IR, intermediate‐risk group; LR, low‐risk group; y, years.
Biochemical failure defined by the Phoenix definition unless specified otherwise.
3.2. HDR monotherapy
As already mentioned, HDR BRT was originally used in combination with EBRT, as a boost modality mainly due to concerns regarding normal tissue toxicity with the application of hypofractionated treatment regimes. The safety and efficacy range for HDR in the context of combined EBRT and BRT have been clearly established by dose escalation trials. 106 , 109 , 111 At the same time, the employment of other locally directed treatments such as RP, radical EBRT, and LDR BRT, together with the acknowledgment that image‐guided HDR with its anatomy‐based dose optimization offers high precision in prostate dose coverage, while simultaneously minimization of the total dose to adjacent OARs 25 laid the way for broad practice of HDR BRT in the monotherapy setting. An evergrowing body of literature considers HDR safe and effective radical treatment with consistent intermediate‐ and long‐term BC rates over a range of risk groups. 13 , 17 , 18 , 69 , 112 , 113 , 114 , 115 , 116 , 117 , 118 , 119 , 120 , 121 , 122 , 123 , 124 , 125 , 126 Although, moderate hypofraction enjoys the longest follow‐up in regard to clinical results (four to nine fractions), consistent data are reported for extreme hypofractionated protocols (one to three fractions). It should be noted that ultrahypofractionated attempts 115 , 127 , 128 (one fraction) to make HDR logistically comparable with LDR BRT have proven inferior in respect to clinical outcomes and require further validation. 115 , 129 , 130 , 131 , 132
Again, due to the variation of clinically implemented dose fractionation regimens, direct comparisons are proving difficult. Despite that, the oncological outcomes yielded for both single‐ and multiple‐implant schemes for extreme or moderated hypofractionated treatment protocols are uniform (Table 4).
TABLE 4.
Oncological results of HDR monotherapy for localised prostate cancer
| HDR protocol | ||||||||
|---|---|---|---|---|---|---|---|---|
| Study | n | Gy/Fraction | Fractions (implants) | Total | median f/u (y) | Biochemical control a | BED (Gy) | EQD2 (Gy) |
| Morton et al. 114 | 170 |
19 Gy 13.5 Gy |
1 (1 implant) 2 (2 implants) |
19 Gy 27 Gy |
6.0 |
73.5% LR/IR at 5 years 95% LR/IR at 5 years |
260–270 | 111–116 |
| Strouthos et al. 18 | 450 | 11.5 Gy | 3 (3 Implant) | 34.5 Gy | 4.7 | 96% LR, 96% IR, 92% HR at 5 years | 299 | 128 |
| Hoskin et al. 128 | 293 |
19–20 Gy 13 Gy 10.5 Gy |
1 (1 implant) 2 (1 implant) 3 (1 implant) |
19–20 Gy 26 Gy 31.5 Gy |
4.1 5.3 9.0 |
94% IR/HR at 4 years 77% IR/HR at 7 years 81% IR/HR at 7 years |
251–260 | 108–111 |
| Krauss et al. 127 | 58 | 19 Gy | 1 (1 implant) | 19 Gy | 2.9 | 93% LR/IR at 3 years | 260 | 111 |
| Yoshioka et al. 114 | 190 |
6.0 Gy 6.0 Gy 6.5 Gy |
8 (1 Implant) 9 (1 Implant) 7 (1 Implant) |
48 Gy 54 Gy 45.5 Gy |
7.6 | 93% IR, 81% HR at 5 years | 240–270 | 103–116 |
| Hauswald et al. 113 | 448 | 7.0–7.25 Gy | 6 (2 Implants) | 42–43.5 Gy | 6.5 | 98.9% LR, 95.2% IR at 10 years | 238–253 | 102–108 |
| Jawad et al. 112 |
494 |
9.5 Gy 12.0 Gy 13.5 Gy |
4 (1 Implant) 2 (1–2 Implants) 2 (1–2 Implants) |
38 Gy 24 Gy 27 Gy |
4.1 |
98% LR, 95% IR at 5 years 92% LR, 81% IR at 5 years 100% LR,79% IR at 5 years |
270–279 | 115–119 |
| Prada et al. 115 | 60 | 19.0 Gy | 1 (1 Implant) | 19 Gy | 6.0 | 66% LR, 63% IR at 6 years | 260 | 111 |
| Kukiełka et al 116 | 77 | 15.0 Gy | 3 (3 Implants) | 45 Gy | 4.7 | 96.7% all risk groups at 5 years | 495 | 212 |
| Komiya et al. 121 | 51 | 6.5 Gy | 7 (1 Implant) | 45.5 | 1.4 | 94% all risk groups at 17 months | 243 | 104 |
| Hoskin et al. 13 | 197 |
8.5–9.0 Gy 10.5 Gy 13.0 Gy |
4 (1 Implant) 3 (1 Implant) 2 (1 Implant) |
34–36 Gy 31.5 Gy 26 Gy |
3.1 |
95% IR, 87% HR at 4 years | 227–252 | 97–108 |
| Rogers et al. 119 | 284 | 6.5 Gy | 6 (2 Implants) | 39 Gy | 2.7 | 94% IR at 5 years | 208 | 89 |
| Zamboglou et al. 17 |
718 |
9.5 Gy 9.5 Gy 11.5 Gy |
4 (1 Implant) 4 (2 Implants) 3 (3 Implants) |
38 Gy 38 Gy 34.5 Gy |
4.4 | 95% LR, 93% IR 93% HR at 5 years | 279–299 | 119–128 |
| Barkati et al. 118 | 79 | 10–11.5 Gy | 3 (1 Implant) | 30–34.5 Gy | 3.3 | 85.1% LR/IR at 5 years | 230–299 | 99–128 |
| Demanes et al. 69 | 298 |
7.0 Gy 9.5 Gy |
6 (2 Implants) 4 (1 Implant) |
42 Gy 38 Gy |
5.2 | 97% LR/IR at 5 years | 238–279 | 102–119 |
| Mark et al. 117 | 301 | 7.5 Gy | 6 (2 Implants) | 45 Gy | 8.0 | 88% All risk groups at 8 years | 270 | 117 |
| Martinez et al 120 | 248 |
7.0 Gy 9.5 Gy |
6 (2 Implants) 4 (1 Implant) |
42 Gy 38 Gy |
4.8 |
87% LR/IR at 5 years 91% LR/IR at 5 years |
238–279 | 102–119 |
| Ghadjar et al.88 | 36 | 9.5 Gy | 4 (1 Implant) | 38 Gy | 3.0 | 100% LR/IR at 3 years | 279 | 119 |
| Grills et al. 123 | 65 | 9.5 Gy | 4 (1 Implant) | 38 Gy | 2.9 | 98% LR/IR at 3 years | 279 | 119 |
Abbreviations: BED, biologically effective dose considering an a/β‐ratio for prostate cancer of 1.5; EQD2, equieffective dose administered in 2.0 Gy‐fractions considering an a/β‐ratio for prostate cancer of 1.5 Gy; f/u, follow‐up; HR, high‐risk group; IR, intermediate‐risk group; LR, low‐risk group; y, years.
Biochemical failure defined by the Phoenix definition.
A great retrospective study 113 focusing on 448 patients with low‐/intermediate‐risk disease treated with six fractions in two implants (spaced 1 week apart) to a median of 43.5 Gy. Temporary ADT was administered in 42 patients (9%). The actuarial 6‐ and 10‐year overall BC rate was 98.6% and 97.8%, respectively, with a median follow‐up of 6.5 years, while no significant difference in respect to biochemical progression‐free survival being noted at 10 years between low‐ and intermediate‐risk group (98.9% vs. 95.2%). Late grade 3–4 GU toxicity was 4.7%, with one patient (0.2%) experiencing grade 4 toxicity, while no late grade 3–4 GI toxicity was observed. These results are in line with experience from other major institutions suggesting that HDR BRT in the monotherapy setting can be applicable both for intermediate‐ and selected high‐risk disease cases. 13 , 113 , 114 , 121 , 123 , 124 , 125 The Offenbach group 17 in Germany reported on 718 consecutive patients, considered to this day, one of the largest patient collectives, administering three different protocols (four fractions of 9.5 Gy in single implant, four fractions of 9.5 Gy in two implants, and three fractions of 11.5 in three implants). Intermediate‐ and high‐risk patients made up 44.9% of the collective, with 60% of high‐ and 27% of intermediate‐risk cases receiving temporary ADT. The 5‐year BC rate was 93% and 93% for intermediate‐ and high‐risk patients, respectively. Late grade 3 GU and GI were reported at 3.5% and 1.6%, respectively.
Erectile dysfunction following BRT monotherapy has been rarely reported, using various multidimensional or ordinal scales for assessment. However, potency preservation rates of 60%–90% have been documented in recent literature. 17 , 26 , 113 , 115 , 119 , 120 , 121 , 122 , 123 , 133 In the previously mentioned series by Hauswald et al., 113 315 (70%) patients managed to attain an erection sufficient for intercourse before treatment. A total of 225 patients provided data in regard to sexual function reaching a median of 6 years following treatment. An ability to engage in sexual intercourse, with or without the use of erectile aids, was reported by 60% of patients with median age of 69 years at time of assessment.
To date, only nonrandomized evaluations have put LDR and HDR monotherapy in comparison in regard to their toxicity profile and justified that high‐grade toxicities, both acute and late, are in favor of HDR. 120 , 123 A comparative, retrospective study 120 analyzed and compared HDR monotherapy (n = 248) and LDR seed patients (n = 206), indicating that temporary HDR is being associated with significantly less grade 1–2 GU toxicity, in the form of chronic dysuria (LDR 22% vs. HDR 15%) and urinary frequency/urgency (LDR 54% vs. HDR 43%). The incidence of urethral stricture was equal for both therapeutic modalities (LDR 2.5% vs. HDR 3%), while late Grade 3 GU sequelae was insignificant in both groups. At last, the 5‐year potency preservation rate was 80% for temporary HDR versus 70% for permanent seeds BRT.
Overall, the reproducible clinical data in favor of HDR monotherapy clearly reflect the current radiobiological notion for optimal tumor control through hypofractionation. Table 5 describes sufficiently the biologically effective dose (BED) values, ranging from 208 to 299 Gy, with a median value of 256 Gy (α/β‐ratio of 1.5 Gy). Calculation of the EQD2 doses provides values of the range from 89 to 128 Gy, tendering such dose coverage unachievable with the current EBRT techniques.
TABLE 5.
Late toxicity data of HDR monotherapy for localised prostate cancer
| HDR protocol | Toxicity | |||||||
|---|---|---|---|---|---|---|---|---|
| Study | n | Gy/Fraction | Fractions (implants) | Total | GU Grade 2 (%) | GU Grade 3 (%) | GI Grade 2 (%) | GI Grade 3 (%) |
| Morton et al. 114 | 170 |
19.0 Gy 13.5 Gy |
1 (1 Implant) 2 (2 Implants) |
19.0 Gy 27.0 Gy |
45 | 1 | 1 | 0 |
| Strouthos et al. 18 | 450 | 11.5 Gy | 3 (3 Implants) | 34.5 Gy | 14 | 0.8 | 0.4 | 0 |
| Hoskin et al. 128 | 293 |
19.0–20.0 Gy 13.0 Gy 10.5 Gy |
1 (1 Implant) 2 (1 Implant) 3 (1 Implant) |
19.0–20.0 Gy 26.0 Gy 31.5 Gy |
2.6 0 2.1 |
2.6 0 1.1 |
0 3.5 0 |
0 0 0 |
| Krauss et al. 127 | 58 | 19.0 Gy | 1 (1 Implant) | 19.0 Gy | 10.3 | 0 | 3.4 | 0 |
| Yoshioka et al. 114 | 190 |
6.0 Gy 6.0 Gy 6.5 Gy |
8 (1 Implant) 9 (1 Implant) 7 (1 Implant) |
48.0 Gy 54.0 Gy 45.5 Gy |
6 | 2 | 4 | 2 |
| Hauswald et al. 113 | 448 | 7.0–7.25 Gy | 6 (2 Implants) | 42–43.5 Gy | – | 4.7 | – | 0 |
| Jawad et al. 112 |
494 |
9.5 Gy 12.0 Gy 13.5 Gy |
4 (1 Implant) 2 (1–2 Implants) 2 (1–2 Implants) |
38.0 Gy 24.0 Gy 27.0 Gy |
20 | 1 | 2 | 0 |
| Prada et al. 115 | 60 | 19.0 Gy | 1 (1 Implant) | 19.0 Gy | 0 | 0 | 0 | 0 |
| Kukiełka et al 116 | 77 | 15.0 Gy | 3 (3 Implants) | 45.0 Gy | 25 | 0 | 0 | 0 |
| Komiya et al. 121 | 51 | 6.5 Gy | 7 (1 Implant) | 45.5 | QoL (IPSS, FACT‐P and IIEF) at baseline after 12 weeks | |||
| Hoskin et al. 13 | 197 |
8.5–9.0 Gy 10.5 Gy 13.0 Gy |
4 (1 Implant) 3 (1 Implant) 2 (1 Implant) |
34–36.0 Gy 31.5 Gy 26.0 Gy |
33–40 a |
3–16 a 3–6 strictures |
4–13 a | 0–1 a |
| Rogers et al. 119 | 284 | 6.5 Gy | 6 (2 Implants) | 39.0 Gy | 1.5 | 0.6 | 0 | 0 |
| Zamboglou et al. 17 |
718 |
9.5 Gy 9.5 Gy 11.5 Gy |
4 (1 Implant) 4 (2 Implants) 3 (3 Implants) |
38.0 Gy 38.0 Gy 34.5 Gy |
15.6 16.5 17.6 |
9.2 4.8 3.9 |
0 1.7 3.5 |
0.7 0 0 |
| Ghilezan et al 126 | 50 |
12.0 Gy 13.5 Gy |
2 (1 Implant) 2 (1 Implant) |
24.0 Gy 27.0 Gy |
16 | 1 | 1 | 1 |
| Barkati et al. 118 | 79 | 10–11.5 Gy | 3 (1 Implant) | 30–34.5 | 2–6 | 2–4 | 0–3 | 0 |
| Demanes et al. 69 | 298 |
7.0 Gy 9.5 Gy |
6 (2 Implants) 4 (1 Implant) |
42.0 Gy 38.0 Gy |
10 | 3 | 1 | 0 |
| Mark et al. 117 | 301 | 7.5 Gy | 6 (2 Implants) | 45.0 Gy | 3.2 | 0 | 1.3 | 1 |
| Martinez et al 120 | 248 |
7.0 Gy 9.5 Gy |
6 (2 Implants) 4 (1 Implant) |
42.0 Gy 38.0 Gy |
0.5–13 0.5 strictures |
0.5–3 3 strictures |
0–1 | 0–0.5 |
| Ghadjar et al.88 | 36 | 9.5 Gy | 4 (1 Implant) | 38.0 Gy | 25 | 11 | 6 | 0 |
| Grills et al. 123 | 65 | 9.5 Gy | 4 (1 Implant) | 38.0 Gy | 3–15 | 0–3 | 0 | 0 |
Abbreviations: FACT‐P, Functional Assessment of Cancer Therapy—Prostate; IIEF, International Index of Erectile Function; IPSS, International Prostate Symptom score; QoL, quality of life; RTOG, Radiation Therapy Oncology Group.
RTOG toxicity scale (all other toxicity data according to Common Terminology Criteria for Adverse Events).
Contrary to the clinical data arising from definitive EBRT, the potential advantageous roles of temporary ADT for patients treated with HDR monotherapy remain an unresolved issue, fueling debate, as no convincing evidence exists, 25 , 134 with those debating against the addition of ADT suggesting that the increased intraprostatic dose suffices while those for ADT addition claiming that EBRT data should be equally adopted in this clinical scenario.
It needs to be stated that the excellent oncologic results of HDR BRT have prompted the implementation of stereotactic body radiotherapy (SBRT) for the treatment of localized prostate cancer employing extreme hypofractionation and utilizing continuous image guidance to automatically track, detect, and correct for intrafraction prostate movement. 135 , 136 , 137 , 138 , 139 It seemingly combines “EBRT‐like” noninvasiveness with “HDR BRT‐like” biologic potency. 78 However, a dosimetric analysis 140 comparing virtual SBRT with actual HDR monotherapy plans from treated patients, indicated that HDR achieves significantly higher intraprostatic doses while, at the same time, provides similar urethral doses and comparatively lower maximum rectal doses. Notwithstanding this, SBRT, HDR, as well as LDR BRT have proven their efficacy, as safe for the management of localized prostate cancer. However, the validation of all the theoretical advantages as well as disadvantages of one modality over the other necessitate that randomized clinical trials are conducted, so that uncertainties concerning the clinical impact will be resolved. Adding to that, given the relatively restricted “surgical margin” associated with SBRT, it is clearly not recommended for more advanced disease presenting with extracapsular extension or seminal vesicle involvement. 136 , 141
In conclusion, HDR BRT as monotherapy proves to be an excellent modality for the management of low‐, intermediate‐, as well as carefully selected cases of high‐risk prostate cancer with long‐term follow‐up data justifying its safety and low side‐effect rate.
3.3. HDR monotherapy as salvage treatment
The optimal management of patients treated previously with definitive RT for clinically localized prostate cancer which are experiencing a biochemical recurrence (BCR) remains a challenging clinical issue, 142 with various therapeutic managements put to the test, such as salvage radical prostatectomy (sRP), salvage high‐intensity‐focused US, and salvage EBRT (sEBRT) being clinically practiced. 143 , 144 , 145 , 146 Clinical evidence suggests that approximately 70% of patients with an increase in their PSA value will experience solely a local failure, 147 , 148 , 149 devoid the variance in treatment‐related BCR definition. 150 , 151
Salvage HDR BRT (sHDR BRT) with or without ADT for clinically, histologically, and metabolically proven local recurrence after previous radical RT appears to be a safe, effective, and well‐tolerated therapeutic option which can be favorably compared with other nonradiotherapeutic local treatment modalities, in regard to disease control and toxicity rates. 149 , 152 , 154 Considering that reports about local salvage modalities are in general scarce, only a few studies report the long‐term oncological outcomes following sHDR BRT. Even though all data arise from retrospective reports and are unfortunately relatively restricted in regard to patient sample size, with reported BC of the order of up to 77%, some of them have reached a 5‐year follow‐up. Table 6 lists the clinical outcomes of published studies reporting on sHDR BRT after definitive RT. In comparison to the primary BRT setting, an increase in adverse events is observed, 25 although the toxicity rates are regarded as acceptable when compared to sRP and sEBRT. When compared with sRP series after previous definitive RT, symptomatic anastomotic strictures are reported in the range of 7%–41%, while GI toxicity focusing in rectal injury ranges in 0%–28%. At the same time, complete erectile dysfunction is of the order of 80%–100%, and complete urinary incontinence ranges from 21% to 90% of patients. 144 Following sEBRT, late grade 3 GU adverse events of 7% to 18% have been reported. 162 , 163 With regard to LDR, no randomized trial has compared LDR and HDR neither in the primary nor in salvage treatment setting; however, nonrandomized evaluations have confirmed that both acute and late high‐grade toxicities are less frequent after primary HDR than LDR monotherapy. 123 Similarly, late grade 3 GU and GI toxicity rates in the sLDR BRT literature range from 0% to 47% and 0%–20%, respectively. 157 , 158
TABLE 6.
Oncological outcomes and late toxicity rates of salvage HDR protocols
| Study | n | HDR protocol Gy/fraction | Fractions (implants) | Total dose (Gy) | Median f/u (months) | BC | Toxicity |
|---|---|---|---|---|---|---|---|
| Tharp et al.91 | 7 |
7.0 6.0 7.0 9.0 |
3 (1) 2 (2) 3 (2) 1 (2) |
21.0 24.0 42.0 18.0 |
58 | 71.5% |
28% Grade 3 GU No ≥ grade 3 GI |
| Lyszek et al.92 | 115 | 10.0 | 1 (3) | 30.0 | 60 | 46% for GS ≤6 |
1.7% Urethral fistulas 1.7% Urinary incontinence 3.4% Bladder outlet obstruction |
| Pellizzon et al.85 | 17 | 8.5–9.0 | 4 (1) | 34–36 | 47 | 70.5% |
5.9% Late grade 4 urethral strictures 5.9% Late grade 3 GI |
| Jo et al.155 | 11 | 11.0 | 2 (1) | 22.0 | 29 | 63% |
No grade 3 GI/GU Low grade 2 GU |
| Chen et al. 148 | 52 | 6.0 | 3 (2) | 36.0 | 59.6 | 51.0% at 5 years |
54% Late grade 2 GU 2% Late grade 3 GU 4% Late grade 2 GI 6% Late grade 3 sexual dysfunction |
| Oliai et al. 153 | 22 | 6.0 | 3 (2) | 36 | 45 | 95.5% at 2 years |
18% Hematuria 32% Urethral strictures |
| Yamada et al. 156 | 45 | 8.0 | 4 (1) | 32.0 | 36 | 68.5% at 5 years |
48% Late grade 2 GU 8.8% Late grade 3 GU 14% Late grade 2 GI |
| Kukieka et al. 157 | 25 | 10.0 | 1 (3) | 30.0 | 13 | 74% at 2 years |
9% Late grade 2 nocturia 4.5% Late grade 2 obstruction 4.5% Late grade 2 frequency no grade 3 GU |
|
Henriquez et al.158 |
19 | Med. 5.25 | 1–4 (1–3) | 17–39 | 48 | 77% at 5 years |
21% Late grade 3 GU No late grade 4 GU 2% Late grade 3 GI |
| Hanna et al.159 | 28 | Med. 6.0 | Med. 6 | Med. 36.0 | 83 | DMFS 11% at 15 years | N. R. |
| Wojcieszek et al. 160 | 83 | 10.0 | 1 (3) | 30.0 | 41 |
76% at 3 years 67% at 5 years |
39% Late grade 2 GU 13% Late grade 3 GU 6% Late grade 1 GI |
| Jiang et al.161 | 22 | 10.0 | 3 (3) | 30.0 | 66 | 45% at 5 years |
5% Late grade 2 GU 9% Late grade 3 GU 9% Late grade 2 GI |
| 139 |
6.0 8.0 |
6 (2) 4 (2) |
36.0 32.0 |
61 | 45% for T3, 65% for T1‐2 at 5 years |
11% Acute urinary obstruction 13% Urethral stricture |
Abbreviations: BC, biochemical control; DMFS, distant metastases‐free survival; f/u, follow‐up; GI, gastrointestinal; GS, Gleason Score; GU, genitourinary; HDR, high‐dose‐rate brachytherapy; m, months; med, median; N.R., not reported.
Once again, the heterogeneity of clinically implemented protocols makes uniform recommendations concerning the optimal dose‐fractionation scheme for whole gland sHDR BRT trivial. However, the oncological results arising from single‐ or multiple‐implant regimes are considered consistent and reproducible, irrespective of the exploiting extreme hypofractionated or moderately hypofractionated treatment.
At the same time, sHDR BRT has been applied in the focal setting for the reirradiation of radiologically detectable recurrent disease. 166 , 167 Although it is clear that a significant dose reduction to OARs can be achieved by the implication of focal HDR BRT, 168 further investigation is guaranteed to calculate the possible clinical impact both on morbidity and tumor control.
Currently, no consensus involving patient's eligibility for repeating a local therapy of organ‐confined recurrent prostate cancer exists, and the most suitable candidates have yet to be defined. Table 1 describes the selection criteria and contraindications. Nevertheless, the main rationale for HDR salvage treatment remains unchanged and is based solely on the presence of local disease in nonmetastatic patients, who are considered suitable candidates for radical therapy. The safe utilization of sHDR BRT either solely or as part of individualized treatment approach also for high‐risk patients is supported by an ever growing literature body. 148 , 156 , 157 , 160
4. CONCLUSION
HDR BRT is an excellent radio‐oncological modality for the management of prostate cancer granting an extraordinary low side‐effect rate. Valid mature follow‐up data support its safe and effective implementation in the treatment of prostate‐confined cancer regardless of risk group. However, further prospective and randomized studies are warranted to fully establish its role in clinically challenging prostate cancer cases.
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
AUTHOR CONTRIBUTIONS
All authors had full access to the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Conceptualization, I.S., E.K., N.Z., K.F.; Methodology, I.S., E.K., N.Z., K.F.; Investigation, I.S., E.K., N.Z., K.F.; Formal Analysis, I.S., E.K., N.Z., K.F.; Resources, I.S., E.K., N.Z., K.F.; Writing—Original Draft, I.S., E.K., N.Z., K.F.; Writing—Review & Editing, I.S., E.K., N.Z., K.F.; Visualization, I.S., E.K.; Supervision, I.S., E.K., N.Z., K.F.; Data Curation, I.S., E.K., N.Z., K.F.; Project Administration, I.S., E.K., N.Z., K.F.; Validation, I.S., E.K., N.Z., K.F.
ETHICAL STATEMENT
The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Strouthos I, Karagiannis E, Zamboglou N, Ferentinos K. High‐dose‐rate brachytherapy for prostate cancer: Rationale, current applications, and clinical outcome. Cancer Reports. 2022;5(1):e1450. 10.1002/cnr2.1450
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analysed in this study.
REFERENCES
- 1. D'Amico AV, Whittington R, Malkowicz SB, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998;280(11):969‐974. [DOI] [PubMed] [Google Scholar]
- 2. D'Amico AV, Whittington R, Malkowicz SB, et al. Biochemical outcome after radical prostatectomy or external beam radiation therapy for patients with clinically localized prostate carcinoma in the prostate specific antigen era. Cancer. 2002;95(2):281‐286. [DOI] [PubMed] [Google Scholar]
- 3. Kupelian P, Meyer JL. Image‐guided, adaptive radiotherapy of prostate cancer: toward new standards of radiotherapy practice. Front Radiat Ther Oncol. 2011;43:344‐368. [DOI] [PubMed] [Google Scholar]
- 4. Kuban DA, Tucker SL, Dong L, et al. Long‐term results of the M. D. Anderson randomized dose‐escalation trial for prostate cancer. Int J Radiat Oncol Biol Phys. 2008;70(1):67‐74. [DOI] [PubMed] [Google Scholar]
- 5. Zelefsky MJ, Yamada Y, Fuks Z, et al. Long‐term results of conformal radiotherapy for prostate cancer: impact of dose escalation on biochemical tumor control and distant metastases‐free survival outcomes. Int J Radiat Oncol Biol Phys. 2008;71(4):1028‐1033. [DOI] [PubMed] [Google Scholar]
- 6. Battermann JJ, Boon TA, Moerland MA. Results of permanent prostate brachytherapy, 13 years of experience at a single institution. Radiother Oncol. 2004;71(1):23‐28. [DOI] [PubMed] [Google Scholar]
- 7. Machtens S, Baumann R, Hagemann J, et al. Long‐term results of interstitial brachytherapy (LDR‐brachytherapy) in the treatment of patients with prostate cancer. World J Urol. 2006;24(3):289‐295. [DOI] [PubMed] [Google Scholar]
- 8. Zelefsky MJ, Kuban DA, Levy LB, et al. Multi‐institutional analysis of long‐term outcome for stages T1‐T2 prostate cancer treated with permanent seed implantation. Int J Radiat Oncol Biol Phys. 2007;67(2):327‐333. [DOI] [PubMed] [Google Scholar]
- 9. Galalae RM, Martinez A, Mate T, et al. Long‐term outcome by risk factors using conformal high‐dose‐rate brachytherapy (HDR‐BT) boost with or without neoadjuvant androgen suppression for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2004;58(4):1048‐1055. [DOI] [PubMed] [Google Scholar]
- 10. Demanes DJ, Rodriguez RR, Schour L, Brandt D, Altieri G. High‐dose‐rate intensity‐modulated brachytherapy with external beam radiotherapy for prostate cancer: California endocurietherapy's 10‐year results. Int J Radiat Oncol Biol Phys. 2005;61(5):1306‐1316. [DOI] [PubMed] [Google Scholar]
- 11. Potters L, Morgenstern C, Calugaru E, et al. 12‐year outcomes following permanent prostate brachytherapy in patients with clinically localized prostate cancer. J Urol. 2005;173(5):1562‐1566. [DOI] [PubMed] [Google Scholar]
- 12. Deutsch I, Zelefsky MJ, Zhang Z, et al. Comparison of PSA relapse‐free survival in patients treated with ultra‐high‐dose IMRT versus combination HDR brachytherapy and IMRT. Brachytherapy. 2010;9(4):313‐318. [DOI] [PubMed] [Google Scholar]
- 13. Hoskin P, Rojas A, Lowe G, et al. High‐dose‐rate brachytherapy alone for localized prostate cancer in patients at moderate or high risk of biochemical recurrence. Int J Radiat Oncol Biol Phys. 2012;82(4):1376‐1384. [DOI] [PubMed] [Google Scholar]
- 14. Kotecha R, Yamada Y, Pei X, et al. Clinical outcomes of high‐dose‐rate brachytherapy and external beam radiotherapy in the management of clinically localized prostate cancer. Brachytherapy. 2013;12(1):44‐49. [DOI] [PubMed] [Google Scholar]
- 15. Pistis F, Guedea F, Pera J, et al. External beam radiotherapy plus high‐dose‐rate brachytherapy for treatment of locally advanced prostate cancer: the initial experience of the Catalan Institute of Oncology. Brachytherapy. 2010;9(1):15‐22. [DOI] [PubMed] [Google Scholar]
- 16. Prada PJ, Mendez L, Fernández J, González H, Jiménez I, Arrojo E. Long‐term biochemical results after high‐dose‐rate intensity modulated brachytherapy with external beam radiotherapy for high risk prostate cancer. Radiat Oncol. 2012;7:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zamboglou N, Tselis N, Baltas D, et al. High‐dose‐rate interstitial brachytherapy as monotherapy for clinically localized prostate Cancer: treatment evolution and mature results. Int J Radiat Oncol Biol Phys. 2013;85(3):672‐678. [DOI] [PubMed] [Google Scholar]
- 18. Strouthos I, Tselis N, Chatzikonstantinou G, et al. High dose rate brachytherapy as monotherapy for localised prostate cancer. Radiother Oncol. 2018;126(2):270‐277. [DOI] [PubMed] [Google Scholar]
- 19. Strouthos I, Chatzikonstantinou G, Zamboglou N, et al. Combined high dose rate brachytherapy and external beam radiotherapy for clinically localised prostate cancer. Radiother Oncol. 2018;128(2):301‐307. [DOI] [PubMed] [Google Scholar]
- 20. Milickovic N, Mavroidis P, Tselis N, et al. 4D analysis of influence of patient movement and anatomy alteration on the quality of 3D U/S‐based prostate HDR brachytherapy treatment delivery. Med Phys. 2011;38(9):4982‐4993. [DOI] [PubMed] [Google Scholar]
- 21. Fendler WP, Schmidt DF, Wenter V, et al. 68Ga‐PSMA PET/CT detects the location and extent of primary prostate cancer. J Nucl Med. 2016;57(11):1720‐1725. [DOI] [PubMed] [Google Scholar]
- 22. Zamboglou C, Drendel V, Jilg CA, et al. Comparison of 68Ga‐HBED‐CC PSMA‐PET/CT and multiparametric MRI for gross tumour volume detection in patients with primary prostate cancer based on slice by slice comparison with histopathology. Theranostics. 2017;7(1):228‐237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schmidt‐Hegemann N‐S, Stief C, Kim T‐H, et al. Outcome after PSMA PET/CT based salvage radiotherapy in patients with biochemical recurrence after radical prostatectomy: a bi‐institutional retrospective analysis. J Nucl Med. 2019;60(2):227‐233. https://jnm.snmjournals.org/content/60/2/227.long. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Haworth A, Williams S. Focal therapy for prostate cancer: the technical challenges. J Contemp Brachytherapy. 2017;9(4):383‐389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Demanes DJ, Ghilezan MI. High‐dose‐rate brachytherapy as monotherapy for prostate cancer. Brachytherapy. 2014;13(6):529‐541. [DOI] [PubMed] [Google Scholar]
- 26. Morton GC, Hoskin PJ. Brachytherapy: current status and future strategies—can high dose rate replace low dose rate and external beam radiotherapy? Clin Oncol (R Coll Radiol). 2013;25(8):474‐482. [DOI] [PubMed] [Google Scholar]
- 27. Yoshioka Y, Yoshida K, Yamazaki H, Nonomura N, Ogawa K. The emerging role of high‐dose‐rate (HDR) brachytherapy as monotherapy for prostate cancer. J Radiat Res. 2013;54(5):781‐788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Challapalli A, Jones E, Harvey C, Hellawell GO, Mangar SA. High dose rate prostate brachytherapy: an overview of the rationale, experience and emerging applications in the treatment of prostate cancer. Br J Radiol. 2012;85(1):S18‐S27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kupelian PA, Ciezki J, Reddy CA, Klein EA, Mahadevan A. Effect of increasing radiation doses on local and distant failures in patients with localized prostate cancer. Int J Radiat Oncol Biol Phys. 2008;71(1):16‐22. [DOI] [PubMed] [Google Scholar]
- 30. Kim MM, Hoffman KE, Levy LB, et al. Prostate cancer‐specific mortality after definitive radiation therapy: who dies of disease? Eur J Cancer. 2012;48(11):1664‐1671. [DOI] [PubMed] [Google Scholar]
- 31. Zelefsky MJ, Reuter VE, Fuks Z, Scardino P, Shippy A. Influence of local tumor control on distant metastases and cancer related mortality after external beam radiotherapy for prostate cancer. J Urol. 2008;179(4):1368‐1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zelefsky MJ, Pei X, Chou JF, et al. Dose escalation for prostate cancer radiotherapy: predictors of long‐term biochemical tumor control and distant metastases‐free survival outcomes. Eur Urol. 2011;60(6):1133‐1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pahlajani N, Ruth KJ, Buyyounouski MK, et al. Radiotherapy doses of 80 Gy and higher are associated with lower mortality in men with Gleason score 8 to 10 prostate cancer. Int J Radiat Oncol Biol Phys. 2012;82(5):1949‐1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nguyen Q‐N, Levy LB, Lee AK, et al. Long‐term outcomes for men with high‐risk prostate cancer treated definitively with external beam radiotherapy with or without androgen deprivation. Cancer. 2013;119(18):3265‐3271. [DOI] [PubMed] [Google Scholar]
- 35. Brenner DJ, Martinez AA, Edmundson GK, Mitchell C, Thames HD, Armour EP. Direct evidence that prostate tumors show high sensitivity to fractionation (low alpha/beta ratio), similar to late‐responding normal tissue. Int J Radiat Oncol Biol Phys. 2002;52(1):6‐13. [DOI] [PubMed] [Google Scholar]
- 36. Nath R, Bice WS, Butler WM, et al. AAPM recommendations on dose prescription and reporting methods for permanent interstitial brachytherapy for prostate cancer: report of task group 137. Med Phys. 2009;36(11):5310‐5322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ritter M, Forman J, Kupelian P, Lawton C, Petereit D. Hypofractionation for prostate cancer. Cancer J. 2009;15(1):1‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. White EC, Kamrava MR, Demarco J, et al. High‐dose‐rate prostate brachytherapy consistently results in high quality dosimetry. Int J Radiat Oncol Biol Phys. 2013;85(2):543‐548. [DOI] [PubMed] [Google Scholar]
- 39. Mavroidis P, Katsilieri Z, Kefala V, et al. Radiobiological evaluation of the influence of dwell time modulation restriction in HIPO optimized HDR prostate brachytherapy implants. J Contemp Brachytherapy. 2010;2(3):117‐128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Karabis A, Giannouli S, Baltas D. 40 HIPO: a hybrid inverse treatment planning optimization algorithm in HDR brachytherapy. Radiother Oncol. 2005;76:S29. [Google Scholar]
- 41. Kono Y, Kubota K, Aruga T, et al. Swelling of the prostate gland by permanent brachytherapy may affect seed migration. Jpn J Clin Oncol. 2010;40(12):1159‐1165. [DOI] [PubMed] [Google Scholar]
- 42. Nakano M, Yorozu A, Saito S, et al. Seed migration after transperineal interstitial prostate brachytherapy by using loose seeds: Japanese prostate cancer outcome study of permanent iodine‐125 seed implantation (J‐POPS) multi‐institutional cohort study. Radiat Oncol. 2015;10:228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Knaup C, Mavroidis P, Esquivel C, et al. Investigating the dosimetric and tumor control consequences of prostate seed loss and migration. Med Phys. 2012;39(6):3291‐3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Shah AP, Kupelian PA, Willoughby TR, Langen KM, Meeks SL. An evaluation of intrafraction motion of the prostate in the prone and supine positions using electromagnetic tracking. Radiother Oncol. 2011;99(1):37‐43. [DOI] [PubMed] [Google Scholar]
- 45. Algan O, Jamgade A, Ali I, et al. The dosimetric impact of daily setup error on target volumes and surrounding normal tissue in the treatment of prostate cancer with intensity‐modulated radiation therapy. Med Dosim. 2012;37(4):406‐411. [DOI] [PubMed] [Google Scholar]
- 46. Mutanga TF, de Boer HCJ, Rajan V, Dirkx MLP, Incrocci L, Heijmen BJM. Day‐to‐day reproducibility of prostate intrafraction motion assessed by multiple kV and MV imaging of implanted markers during treatment. Int J Radiat Oncol Biol Phys. 2012;83(1):400‐407. [DOI] [PubMed] [Google Scholar]
- 47. Fowler JF. The linear‐quadratic formula and progress in fractionated radiotherapy. Br J Radiol. 1989;62(740):679‐694. [DOI] [PubMed] [Google Scholar]
- 48. Tucker SL, Thames HD, Michalski JM, et al. Estimation of α/β for late rectal toxicity based on RTOG 94‐06. Int J Radiat Oncol Biol Phys. 2011;81(2):600‐605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Miralbell R, Roberts SA, Zubizarreta E, Hendry JH. Dose‐fractionation sensitivity of prostate cancer deduced from radiotherapy outcomes of 5,969 patients in seven international institutional datasets: α/β = 1.4 (0.9‐2.2) Gy. Int J Radiat Oncol Biol Phys. 2012;82(1):e17‐e24. [DOI] [PubMed] [Google Scholar]
- 50. Lee WR. Extreme hypofractionation for prostate cancer. Expert Rev Anticancer Ther. 2009;9(1):61‐65. [DOI] [PubMed] [Google Scholar]
- 51. Yamada Y, Rogers L, Demanes DJ, et al. American brachytherapy society consensus guidelines for high‐dose‐rate prostate brachytherapy. Brachytherapy. 2012;11(1):20‐32. [DOI] [PubMed] [Google Scholar]
- 52. Hoskin PJ, Colombo A, Henry A, et al. GEC/ESTRO recommendations on high dose rate afterloading brachytherapy for localised prostate cancer: an update. Radiother Oncol. 2013;107(3):325‐332. [DOI] [PubMed] [Google Scholar]
- 53. Mohler JL, Antonarakis ES. NCCN guidelines updates: Management of prostate cancer. J Natl Compr Canc Netw. 2019;17(5.5):583‐586. [DOI] [PubMed] [Google Scholar]
- 54. Zelefsky MJ, Leibel SA, Gaudin PB, et al. Dose escalation with three‐dimensional conformal radiation therapy affects the outcome in prostate cancer. Int J Radiat Oncol Biol Phys. 1998;41(3):491‐500. [DOI] [PubMed] [Google Scholar]
- 55. Hoskin PJ, Rojas AM, Bownes PJ, Lowe GJ, Ostler PJ, Bryant L. Randomised trial of external beam radiotherapy alone or combined with high‐dose‐rate brachytherapy boost for localised prostate cancer. Radiother Oncol. 2012;103(2):217‐222. [DOI] [PubMed] [Google Scholar]
- 56. Sathya JR, Davis IR, Julian JA, et al. Randomized trial comparing iridium implant plus external‐beam radiation therapy with external‐beam radiation therapy alone in node‐negative locally advanced cancer of the prostate. J Clin Oncol. 2005;23(6):1192‐1199. [DOI] [PubMed] [Google Scholar]
- 57. Rodda S, Tyldesley S, Morris WJ, et al. ASCENDE‐RT: an analysis of treatment‐related morbidity for a randomized trial comparing a low‐dose‐rate brachytherapy boost with a dose‐escalated external beam boost for high‐ and intermediate‐risk prostate cancer. Int J Radiat Oncol Biol Phys. 2017;98(2):286‐295. [DOI] [PubMed] [Google Scholar]
- 58. Kishan AU, Shaikh T, Wang P‐C, et al. Clinical outcomes for patients with Gleason score 9‐10 prostate adenocarcinoma treated with radiotherapy or radical prostatectomy: a multi‐institutional comparative analysis. Eur Urol. 2017;71(5):766‐773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Agoston P, Major T, Fröhlich G, et al. Moderate dose escalation with single‐fraction high‐dose‐rate brachytherapy boost for clinically localized intermediate‐ and high‐risk prostate cancer: 5‐year outcome of the first 100 consecutively treated patients. Brachytherapy. 2011;10(5):376‐384. [DOI] [PubMed] [Google Scholar]
- 60. Heidenreich A, Bastian PJ, Bellmunt J, et al. EAU guidelines on prostate cancer. Part 1: screening, diagnosis, and local treatment with curative intent‐update. Eur Urol. 2013;65(1):124‐137. [DOI] [PubMed] [Google Scholar]
- 61. Eid K, Krughoff K, Stoimenova D, et al. Validation of the urgency, weak stream, incomplete emptying, and Nocturia (UWIN) score compared with the American urological association symptoms score in assessing lower urinary tract symptoms in the clinical setting. Urology. 2014;83(1):181‐185. [DOI] [PubMed] [Google Scholar]
- 62. Ishiyama H, Hirayama T, Jhaveri P, et al. Is there an increase in genitourinary toxicity in patients treated with transurethral resection of the prostate and radiotherapy? A systematic review. Am J Clin Oncol. 2014;37(3):297‐304. [DOI] [PubMed] [Google Scholar]
- 63. Luo HL, Fang FM, Kang CH, Chuang YC, Chiang PH. Can high‐dose‐rate brachytherapy prevent the major genitourinary complication better than external beam radiation alone for patients with previous transurethral resection of prostate? Int Urol Nephrol. 2013;45(1):113‐119. [DOI] [PubMed] [Google Scholar]
- 64. Peddada AV, Jennings SB, Faricy PO, Walsh RA, White GA, Monroe AT. Low morbidity following high dose rate brachytherapy in the setting of prior transurethral prostate resection. J Urol. 2007;178(5):1963‐1967. [DOI] [PubMed] [Google Scholar]
- 65. Yamada Y, Bhatia S, Zaider M, et al. Favorable clinical outcomes of three‐dimensional computer‐optimized high‐dose‐rate prostate brachytherapy in the management of localized prostate cancer. Brachytherapy. 2006;5(3):157‐164. [DOI] [PubMed] [Google Scholar]
- 66. Sakamoto N, Akitake M, Ikoma S, et al. Clinical outcome in prostate cancer patients undergoing high‐dose‐rate brachytherapy with external beam radiotherapy in our institute. Nippon Hinyokika Gakkai Zasshi. 2011;102(4):621‐627. [DOI] [PubMed] [Google Scholar]
- 67. Yoshioka Y, Konishi K, Sumida I, et al. Monotherapeutic high‐dose‐rate brachytherapy for prostate cancer: five‐year results of an extreme hypofractionation regimen with 54 Gy in nine fractions. Int J Radiat Oncol Biol Phys. 2011;80(2):469‐475. [DOI] [PubMed] [Google Scholar]
- 68. Martin T, Röddiger S, Kurek R, et al. 3D conformal HDR brachytherapy and external beam irradiation combined with temporary androgen deprivation in the treatment of localized prostate cancer. Radiother Oncol. 2004;71(1):35‐41. [DOI] [PubMed] [Google Scholar]
- 69. Demanes DJ, Martinez AA, Ghilezan M, et al. High‐dose‐rate monotherapy: safe and effective brachytherapy for patients with localized prostate Cancer. Int J Radiat Oncol Biol Phys. 2011;81(5):1286‐1292. [DOI] [PubMed] [Google Scholar]
- 70. Dearnaley DP, Sydes MR, Graham JD, et al. Escalated‐dose versus standard‐dose conformal radiotherapy in prostate cancer: first results from the MRC RT01 randomised controlled trial. Lancet Oncol. 2007;8(6):475‐487. [DOI] [PubMed] [Google Scholar]
- 71. Peeters STH, Heemsbergen WD, Koper PCM, et al. Dose‐response in radiotherapy for localized prostate cancer: results of the Dutch multicenter randomized phase III trial comparing 68 Gy of radiotherapy with 78 Gy. J Clin Oncol. 2006;24(13):1990‐1996. [DOI] [PubMed] [Google Scholar]
- 72. Pollack A, Zagars GK, Starkschall G, et al. Prostate cancer radiation dose response: results of the M. D. Anderson phase III randomized trial. Int J Radiat Oncol Biol Phys. 2002;53(5):1097‐1105. [DOI] [PubMed] [Google Scholar]
- 73. Zietman AL, Bae K, Slater JD, et al. Randomized trial comparing conventional‐dose with high‐dose conformal radiation therapy in early‐stage adenocarcinoma of the prostate: long‐term results from proton radiation oncology group/american college of radiology 95‐09. J Clin Oncol. 2010;28(7):1106‐1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Hermesse J, Biver S, Jansen N, Lenaerts E, Nickers P. Dosimetric comparison of high‐dose‐rate brachytherapy and intensity‐modulated radiation therapy as a boost to the prostate. Int J Radiat Oncol Biol Phys. 2010;76(1):269‐276. [DOI] [PubMed] [Google Scholar]
- 75. Hsu IC, Pickett B, Shinohara K, Krieg R, Roach M, Phillips T. Normal tissue dosimetric comparison between HDR prostate implant boost and conformal external beam radiotherapy boost: potential for dose escalation. Int J Radiat Oncol Biol Phys. 2000;46(4):851‐858. [DOI] [PubMed] [Google Scholar]
- 76. Pieters BR, van de Kamer JB, van Herten YRJ, et al. Comparison of biologically equivalent dose‐volume parameters for the treatment of prostate cancer with concomitant boost IMRT versus IMRT combined with brachytherapy. Radiother Oncol. 2008;88(1):46‐52. [DOI] [PubMed] [Google Scholar]
- 77. Sudahar H, Kurup PGG, Murali V, Mahadev P, Velmurugan J. Analysis of high‐dose rate brachytherapy dose distribution resemblance in CyberKnife hypofractionated treatment plans of localized prostate cancer. Med Dosim. 2013;38(4):385‐389. [DOI] [PubMed] [Google Scholar]
- 78. Fuller DB, Naitoh J, Mardirossian G. Virtual HDR CyberKnife SBRT for localized prostatic carcinoma: 5‐year disease‐free survival and toxicity observations. Front Oncol. 2014;4:321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Dayes IS, Parpia S, Gilbert J, et al. Long‐term results of a randomized trial comparing iridium implant plus external beam radiation therapy with external beam radiation therapy alone in node‐negative locally advanced cancer of the prostate. Int J Radiat Oncol Biol Phys. 2017;99(1):90‐93. [DOI] [PubMed] [Google Scholar]
- 80. Morris WJ, Tyldesley S, Rodda S, et al. Androgen suppression combined with elective nodal and dose escalated radiation therapy (the ASCENDE‐RT trial): an analysis of survival endpoints for a randomized trial comparing a low‐dose‐rate brachytherapy boost to a dose‐escalated external beam boost for high‐ and intermediate‐risk prostate cancer. Int J Radiat Oncol Biol Phys. 2017;98(2):275‐285. [DOI] [PubMed] [Google Scholar]
- 81. Joseph D, Denham JW, Steigler A, et al. Radiation dose escalation or longer androgen suppression to prevent distant progression in men with locally advanced prostate Cancer: 10‐year data from the TROG 03.04 RADAR trial. Int J Radiat Oncol Biol Phys. 2020;106(4):693‐702. [DOI] [PubMed] [Google Scholar]
- 82. Hathout L, Mahmoud O, Wang Y, et al. A phase 2 randomized pilot study comparing high‐dose‐rate brachytherapy and low‐dose‐rate brachytherapy as monotherapy in localized prostate cancer. Adv Radiat Oncol. 2019;4(4):631‐640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Galalae RM, Kovács G, Schultze J, et al. Long‐term outcome after elective irradiation of the pelvic lymphatics and local dose escalation using high‐dose‐rate brachytherapy for locally advanced prostate cancer. Int J Radiat Oncol Biol Phys. 2002;52(1):81‐90. [DOI] [PubMed] [Google Scholar]
- 84. Phan TP, Syed AMN, Puthawala A, Sharma A, Khan F. High dose rate brachytherapy as a boost for the treatment of localized prostate cancer. J Urol. 2007;177(1):123‐127. discussion 127. [DOI] [PubMed] [Google Scholar]
- 85. Pellizzon ACA, Salvajoli J, Novaes P, et al. The relationship between the biochemical control outcomes and the quality of planning of high‐dose rate brachytherapy as a boost to external beam radiotherapy for locally and locally advanced prostate cancer using the RTOG‐ASTRO Phoenix definition. Int J Med Sci. 2008;5(3):113‐120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Viani GA, Pellizzon AC, Guimarães FS, Jacinto AA, dos Santos Novaes PER, Salvajoli JV. High dose rate and external beam radiotherapy in locally advanced prostate cancer. Am J Clin Oncol. 2009;32(2):187‐190. [DOI] [PubMed] [Google Scholar]
- 87. Morton GC, Loblaw DA, Sankreacha R, et al. Single‐fraction high‐dose‐rate brachytherapy and hypofractionated external beam radiotherapy for men with intermediate‐risk prostate cancer: analysis of short‐ and medium‐term toxicity and quality of life. Int J Radiat Oncol Biol Phys. 2010;77(3):811‐817. [DOI] [PubMed] [Google Scholar]
- 88. Neviani CB, Miziara MA, Carvalho A, De H. Results of high dose‐rate brachytherapy boost before 2D or 3D external beam irradiation for prostate cancer. Radiother Oncol. 2011;98(2):169‐174. 10.1016/j.radonc.2011.01.005 [DOI] [PubMed] [Google Scholar]
- 89. Noda Y, Sato M, Shirai S, et al. Efficacy and safety of high‐dose‐rate brachytherapy of single implant with two fractions combined with external beam radiotherapy for hormone‐naïve localized prostate cancer. Cancers. 2011;3(3):3585‐3600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Martinez AA, Gonzalez J, Ye H, et al. Dose escalation improves cancer‐related events at 10 years for intermediate‐ and high‐risk prostate cancer patients treated with hypofractionated high‐dose‐rate boost and external beam radiotherapy. Int J Radiat Oncol Biol Phys. 2011;79(2):363‐370. [DOI] [PubMed] [Google Scholar]
- 91. Tharp M, Hardacre M, Bennett R, Jones WT, Stuhldreher D, Vaught J. Prostate high‐dose‐rate brachytherapy as salvage treatment of local failure after previous external or permanent seed irradiation for prostate cancer. Brachytherapy. 2008;7(3):231‐236. 10.1016/j.brachy.2008.03.003 [DOI] [PubMed] [Google Scholar]
- 92. Łyczek J, Kawczyńska MM, Garmol D, et al. HDR brachytherapy as a solution in recurrences of locally advanced prostate cancer. J Contemp Brachyther. 2009;1(2):105‐108. [PMC free article] [PubMed] [Google Scholar]
- 93. Helou J, D'Alimonte L, Loblaw A, et al. High dose‐rate brachytherapy boost for intermediate risk prostate cancer: long‐term outcomes of two different treatment schedules and early biochemical predictors of success. Radiother Oncol. 2015;115(1):84‐89. [DOI] [PubMed] [Google Scholar]
- 94. Vigneault E, Mbodji K, Magnan S, et al. High‐dose‐rate brachytherapy boost for prostate cancer treatment: different combinations of hypofractionated regimens and clinical outcomes. Radiother Oncol. 2017;124(1):49‐55. [DOI] [PubMed] [Google Scholar]
- 95. Ishiyama H, Kamitani N, Kawamura H, et al. Nationwide multi‐institutional retrospective analysis of high‐dose‐rate brachytherapy combined with external beam radiotherapy for localized prostate cancer: an Asian prostate HDR‐BT consortium. Brachytherapy. 2017;16(3):503‐510. [DOI] [PubMed] [Google Scholar]
- 96. Falk AT, Demontoy S, Chamorey E, et al. High‐dose‐rate brachytherapy boost for prostate cancer: comparison of three different fractionation schemes. Brachytherapy. 2017;16(5):993‐999. [DOI] [PubMed] [Google Scholar]
- 97. Deger S, Boehmer D, Roigas J, et al. High dose rate (HDR) brachytherapy with conformal radiation therapy for localized prostate cancer. Eur Urol. 2005;47(4):441‐448. [DOI] [PubMed] [Google Scholar]
- 98. Aström L, Pedersen D, Mercke C, Holmäng S, Johansson KA. Long‐term outcome of high dose rate brachytherapy in radiotherapy of localised prostate cancer. Radiother Oncol. 2005;74(2):157‐161. [DOI] [PubMed] [Google Scholar]
- 99. Hiratsuka J, Jo Y, Yoshida K, Nagase N, Fujisawa M, Imajo Y. Clinical results of combined treatment conformal high‐dose‐rate iridium‐192 brachytherapy and external beam radiotherapy using staging lymphadenectomy for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2004;59(3):684‐690. [DOI] [PubMed] [Google Scholar]
- 100. Galalae RM, Martinez A, Nuernberg N, et al. Hypofractionated conformal HDR brachytherapy in hormone naïve men with localized prostate cancer. Is escalation to very high biologically equivalent dose beneficial in all prognostic risk groups? Strahlenther Onkol. 2006;182(3):135‐141. [DOI] [PubMed] [Google Scholar]
- 101. Izard MA, Haddad RL, Fogarty GB, Rinks A, Dobbins T, Katelaris P. Six year experience of external beam radiotherapy, brachytherapy boost with a 1Ci (192)Ir source, and neoadjuvant hormonal manipulation for prostate cancer. Int J Radiat Oncol Biol Phys. 2006;66(1):38‐47. [DOI] [PubMed] [Google Scholar]
- 102. Martinez A, Gonzalez J, Spencer W, et al. Conformal high dose rate brachytherapy improves biochemical control and cause specific survival in patients with prostate cancer and poor prognostic factors. J Urol. 2003;169(3):974‐979. [DOI] [PubMed] [Google Scholar]
- 103. Martinez AA, Demanes DJ, Galalae R, et al. Lack of benefit from a short course of androgen deprivation for unfavorable prostate cancer patients treated with an accelerated hypofractionated regime. Int J Radiat Oncol Biol Phys. 2005;62(5):1322‐1331. [DOI] [PubMed] [Google Scholar]
- 104. Vargas CE, Martinez AA, Boike TP, et al. High‐dose irradiation for prostate cancer via a high‐dose‐rate brachytherapy boost: results of a phase I to II study. Int J Radiat Oncol Biol Phys. 2006;66(2):416‐423. [DOI] [PubMed] [Google Scholar]
- 105. de Meerleer G, Vakaet L, Meersschout S, et al. Intensity‐modulated radiotherapy as primary treatment for prostate cancer: acute toxicity in 114 patients. Int J Radiat Oncol Biol Phys. 2004;60(3):777‐787. [DOI] [PubMed] [Google Scholar]
- 106. Liauw SL, Weichselbaum RR, Rash C, et al. Biochemical control and toxicity after intensity‐modulated radiation therapy for prostate cancer. Technol Cancer Res Treat. 2009;8(3):201‐206. [DOI] [PubMed] [Google Scholar]
- 107. Nguyen KH, Patel SA, Lee AK, Venkat P, Chang A. Brachytherapy use for favorable‐risk prostate cancer continues to decline in both academic and community centers despite superior survival compared to dose‐escalated external beam radiation therapy. JCO. 2019;37(7_suppl):105. [Google Scholar]
- 108. Lips IM, Dehnad H, van Gils CH, Boeken Kruger AE, van der Heide UA, van Vulpen M. High‐dose intensity‐modulated radiotherapy for prostate cancer using daily fiducial marker‐based position verification: acute and late toxicity in 331 patients. Radiat Oncol. 2008;3:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Martinez AA, Gustafson G, Gonzalez J, et al. Dose escalation using conformal high‐dose‐rate brachytherapy improves outcome in unfavorable prostate cancer. Int J Radiat Oncol Biol Phys. 2002;53(2):316‐327. [DOI] [PubMed] [Google Scholar]
- 110. Martinez AA, Kestin LL, Stromberg JS, et al. Interim report of image‐guided conformal high‐dose‐rate brachytherapy for patients with unfavorable prostate cancer: the William Beaumont phase II dose‐escalating trial. Int J Radiat Oncol Biol Phys. 2000;47(2):343‐352. [DOI] [PubMed] [Google Scholar]
- 111. Martinez AA, Pataki I, Edmundson G, Sebastian E, Brabbins D, Gustafson G. Phase II prospective study of the use of conformal high‐dose‐rate brachytherapy as monotherapy for the treatment of favorable stage prostate cancer: a feasibility report. Int J Radiat Oncol Biol Phys. 2001;49(1):61‐69. [DOI] [PubMed] [Google Scholar]
- 112. Jawad MS, Dilworth JT, Gustafson GS, et al. Outcomes associated with 3 treatment schedules of high‐dose‐rate brachytherapy monotherapy for favorable‐risk prostate Cancer. Int J Radiat Oncol Biol Phys. 2016;94(4):657‐666. [DOI] [PubMed] [Google Scholar]
- 113. Hauswald H, Kamrava MR, Fallon JM, et al. High‐dose‐rate monotherapy for localized prostate cancer: 10‐year results. Int J Radiat Oncol Biol Phys. 2016;94(4):667‐674. [DOI] [PubMed] [Google Scholar]
- 114. Yoshioka Y, Suzuki O, Isohashi F, et al. High‐dose‐rate brachytherapy as monotherapy for intermediate‐ and high‐risk prostate cancer: clinical results for a median 8‐year follow‐up. Int J Radiat Oncol Biol Phys. 2016;94(4):675‐682. [DOI] [PubMed] [Google Scholar]
- 115. Prada PJ, Cardenal J, Blanco AG, et al. High‐dose‐rate interstitial brachytherapy as monotherapy in one fraction for the treatment of favorable stage prostate cancer: toxicity and long‐term biochemical results. Radiother Oncol. 2016;119(3):411‐416. [DOI] [PubMed] [Google Scholar]
- 116. Kukiełka AM, Dąbrowski T, Walasek T, Olchawa A, Kudzia R, Dybek D. High‐dose‐rate brachytherapy as a monotherapy for prostate cancer—single‐institution results of the extreme fractionation regimen. Brachytherapy. 2015;14(3):359‐365. [DOI] [PubMed] [Google Scholar]
- 117. Mark RJ, Anderson PJ, Akins RS, Nair M. Interstitial high‐dose‐rate brachytherapy as monotherapy for early stage prostate cancer: median 8‐year results in 301 patients. Brachytherapy. 2010;9:S76. [Google Scholar]
- 118. Barkati M, Williams SG, Foroudi F, et al. High‐dose‐rate brachytherapy as a monotherapy for favorable‐risk prostate cancer: a phase II trial. Int J Radiat Oncol Biol Phys. 2012;82(5):1889‐1896. [DOI] [PubMed] [Google Scholar]
- 119. Rogers CL, Alder SC, Rogers RL, et al. High dose brachytherapy as monotherapy for intermediate risk prostate cancer. J Urol. 2012;187(1):109‐116. [DOI] [PubMed] [Google Scholar]
- 120. Martinez AA, Demanes J, Vargas C, Schour L, Ghilezan M, Gustafson GS. High‐dose‐rate prostate brachytherapy: an excellent accelerated‐hypofractionated treatment for favorable prostate cancer. Am J Clin Oncol. 2010;33(5):481‐488. [DOI] [PubMed] [Google Scholar]
- 121. Komiya A, Fujiuchi Y, Ito T, et al. Early quality of life outcomes in patients with prostate cancer managed by high‐dose‐rate brachytherapy as monotherapy. Int J Urol. 2013;20(2):185‐192. [DOI] [PubMed] [Google Scholar]
- 122. Ghadjar P, Oesch SL, Rentsch CA, et al. Late toxicity and five year outcomes after high‐dose‐rate brachytherapy as a monotherapy for localized prostate cancer. Radiat Oncol. 2014;9:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Grills IS, Martinez AA, Hollander M, et al. High dose rate brachytherapy as prostate cancer monotherapy reduces toxicity compared to low dose rate palladium seeds. J Urol. 2004;171(3):1098‐1104. [DOI] [PubMed] [Google Scholar]
- 124. Tselis N, Tunn UW, Chatzikonstantinou G, et al. High dose rate brachytherapy as monotherapy for localised prostate cancer: a hypofractionated two‐implant approach in 351 consecutive patients. Radiat Oncol. 2013;8:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Díez P, Mullassery V, Dankulchai P, et al. Dosimetric analysis of urethral strictures following HDR (192)Ir brachytherapy as monotherapy for intermediate‐ and high‐risk prostate cancer. Radiother Oncol. 2014;113(3):410‐413. [DOI] [PubMed] [Google Scholar]
- 126. Ghilezan M, Martinez A, Gustason G, et al. High‐dose‐rate brachytherapy as monotherapy delivered in two fractions within one day for favorable/intermediate‐risk prostate cancer: preliminary toxicity data. Int J Radiat Oncol Biol Phys. 2012;83(3):927‐932. [DOI] [PubMed] [Google Scholar]
- 127. Hoskin P, Rojas A, Ostler P, Hughes R, Alonzi R, Lowe G. Single‐dose high‐dose‐rate brachytherapy compared to two and three fractions for locally advanced prostate cancer. Radiother Oncol. 2017;124(1):56‐60. [DOI] [PubMed] [Google Scholar]
- 128. Krauss DJ, Ye H, Martinez AA, et al. Favorable preliminary outcomes for men with low‐ and intermediate‐risk prostate Cancer treated with 19‐Gy single‐fraction high‐dose‐rate brachytherapy. Int J Radiat Oncol Biol Phys. 2017;97(1):98‐106. [DOI] [PubMed] [Google Scholar]
- 129. Barnes JM, Gabani P, Sanders M, et al. Single fraction high‐dose‐rate brachytherapy as monotherapy for low and intermediate risk prostate cancer: toxicities and early outcomes from a single institutional experience. J Contemp Brachytherapy. 2019;11(5):399‐408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Prada PJ, Ferri M, Cardenal J, et al. High‐dose‐rate interstitial brachytherapy as monotherapy in one fraction of 20.5 Gy for the treatment of localized prostate cancer: toxicity and 6‐year biochemical results. Brachytherapy. 2018;17(6):845‐851. [DOI] [PubMed] [Google Scholar]
- 131. Siddiqui ZA, Gustafson GS, Ye H, et al. Five‐year outcomes of a single‐institution prospective trial of 19‐Gy single‐fraction high‐dose‐rate brachytherapy for low‐ and intermediate‐risk prostate cancer. Int J Radiat Oncol Biol Phys. 2019;104(5):1038‐1044. [DOI] [PubMed] [Google Scholar]
- 132. Morton G, McGuffin M, Chung HT, et al. Prostate high dose‐rate brachytherapy as monotherapy for low and intermediate risk prostate cancer: efficacy results from a randomized phase II clinical trial of one fraction of 19 Gy or two fractions of 13.5 Gy. Radiother Oncol. 2020;146:90‐96. [DOI] [PubMed] [Google Scholar]
- 133. Vargas C, Ghilezan M, Hollander M, et al. A new model using number of needles and androgen deprivation to predict chronic urinary toxicity for high or low dose rate prostate brachytherapy. J Urol. 2005;174(3):882‐887. [DOI] [PubMed] [Google Scholar]
- 134. Krauss D, Kestin L, Ye H, et al. Lack of benefit for the addition of androgen deprivation therapy to dose‐escalated radiotherapy in the treatment of intermediate‐ and high‐risk prostate cancer. Int J Radiat Oncol Biol Phys. 2011;80(4):1064‐1071. [DOI] [PubMed] [Google Scholar]
- 135. King CR, Brooks JD, Gill H, Pawlicki T, Cotrutz C, Presti JC. Stereotactic body radiotherapy for localized prostate cancer: interim results of a prospective phase II clinical trial. Int J Radiat Oncol Biol Phys. 2009;73(4):1043‐1048. [DOI] [PubMed] [Google Scholar]
- 136. King CR, Brooks JD, Gill H, Presti JC. Long‐term outcomes from a prospective trial of stereotactic body radiotherapy for low‐risk prostate cancer. Int J Radiat Oncol Biol Phys. 2012;82(2):877‐882. [DOI] [PubMed] [Google Scholar]
- 137. Freeman DE, King CR. Stereotactic body radiotherapy for low‐risk prostate cancer: five‐year outcomes. Radiat Oncol. 2011;6:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Brand DH, Tree AC, Ostler P, et al. Intensity‐modulated fractionated radiotherapy versus stereotactic body radiotherapy for prostate cancer (PACE‐B): acute toxicity findings from an international, randomised, open‐label, phase 3, non‐inferiority trial. Lancet Oncol. 2019;20(11):1531‐1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. McBride SM, Wong DS, Dombrowski JJ, et al. Hypofractionated stereotactic body radiotherapy in low‐risk prostate adenocarcinoma: preliminary results of a multi‐institutional phase 1 feasibility trial. Cancer. 2012;118(15):3681‐3690. [DOI] [PubMed] [Google Scholar]
- 140. Spratt DE, Scala LM, Folkert M, et al. A comparative dosimetric analysis of virtual stereotactic body radiotherapy to high‐dose‐rate monotherapy for intermediate‐risk prostate cancer. Brachytherapy. 2013;12(5):428‐433. [DOI] [PubMed] [Google Scholar]
- 141. Aluwini S, van Rooij P, Hoogeman M, et al. CyberKnife stereotactic radiotherapy as monotherapy for low‐ to intermediate‐stage prostate cancer: early experience, feasibility, and tolerance. J Endourol. 2010;24(5):865‐869. [DOI] [PubMed] [Google Scholar]
- 142. Bruce JY, Lang JM, McNeel DG, Liu G. Current controversies in the management of biochemical failure in prostate cancer. Clin Adv Hematol Oncol. 2012;10(11):716‐722. [PubMed] [Google Scholar]
- 143. Ward JF, Pagliaro LC, Pisters LL. Salvage therapy for radiorecurrent prostate cancer. Curr Probl Cancer. 2008;32(6):242‐271. [DOI] [PubMed] [Google Scholar]
- 144. Chade DC, Eastham J, Graefen M, et al. Cancer control and functional outcomes of salvage radical prostatectomy for radiation‐recurrent prostate cancer: a systematic review of the literature. Eur Urol. 2012;61(5):961‐971. [DOI] [PubMed] [Google Scholar]
- 145. Crouzet S, Blana A, Murat FJ, et al. Salvage high‐intensity focused ultrasound (HIFU) for locally recurrent prostate cancer after failed radiation therapy: multi‐institutional analysis of 418 patients. BJU Int. 2017;119(6):896‐904. [DOI] [PubMed] [Google Scholar]
- 146. Mouraviev V, Spiess PE, Jones JS. Salvage cryoablation for locally recurrent prostate cancer following primary radiotherapy. Eur Urol. 2012;61(6):1204‐1211. [DOI] [PubMed] [Google Scholar]
- 147. Ahmed HU, Pendse D, Illing R, Allen C, van der Meulen JHP, Emberton M. Will focal therapy become a standard of care for men with localized prostate cancer? Nat Clin Pract Oncol. 2007;4(11):632‐642. [DOI] [PubMed] [Google Scholar]
- 148. Chen CP, Weinberg V, Shinohara K, et al. Salvage HDR brachytherapy for recurrent prostate cancer after previous definitive radiation therapy: 5‐year outcomes. Int J Radiat Oncol Biol Phys. 2013;86(2):324‐329. [DOI] [PubMed] [Google Scholar]
- 149. Pound CR, Partin AW, Eisenberger MA, Chan DW, Pearson JD, Walsh PC. Natural history of progression after PSA elevation following radical prostatectomy. JAMA. 1999;281(17):1591‐1597. [DOI] [PubMed] [Google Scholar]
- 150. American Society for Therapeutic Radiology and Oncology Consensus Panel . Consensus statement: guidelines for PSA following radiation therapy. Int J Radiat Oncol Biol Phys. 1997;37(5):1035‐1041. [PubMed] [Google Scholar]
- 151. Roach M, Hanks G, Thames H, et al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG‐ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys. 2006;65(4):965‐974. [DOI] [PubMed] [Google Scholar]
- 152. Lee B, Shinohara K, Weinberg V, et al. Feasibility of high‐dose‐rate brachytherapy salvage for local prostate cancer recurrence after radiotherapy: the University of California‐San Francisco experience. Int J Radiat Oncol Biol Phys. 2007;67(4):1106‐1112. [DOI] [PubMed] [Google Scholar]
- 153. Oliai C, Yang L, Lee JY. Prospective quality of life and efficacy of high‐dose‐rate brachytherapy salvage for recurrent prostate Cancer. Int J Radiat Oncol Biol Phys. 2013;87(2):S396‐S397. [Google Scholar]
- 154. Tisseverasinghe SA, Crook JM. The role of salvage brachytherapy for local relapse after external beam radiotherapy for prostate cancer. Transl Androl Urol. 2018;7(3):414‐435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Jo Y, Fujii T, Hara R, et al. Salvage high‐dose‐rate brachytherapy for local prostate cancer recurrence after radiotherapy—preliminary results. BJU Int. 2012;109(6):835‐839. 10.1111/j.1464-410X.2011.10519.x [DOI] [PubMed] [Google Scholar]
- 156. Yamada Y, Kollmeier MA, Pei X, et al. A phase II study of salvage high‐dose‐rate brachytherapy for the treatment of locally recurrent prostate cancer after definitive external beam radiotherapy. Brachytherapy. 2014;13(2):111‐116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Kukiełka AM, Hetnał M, Dąbrowski T, et al. Salvage prostate HDR brachytherapy combined with interstitial hyperthermia for local recurrence after radiation therapy failure. Strahlenther Onkol. 2014;190(2):165‐170. [DOI] [PubMed] [Google Scholar]
- 158. Henríquez I, Sancho G, Hervás A, et al. Salvage brachytherapy in prostate local recurrence after radiation therapy. Predicting factors for control and toxicity. Radiat Oncol. 2014;9:102. 10.1186/1748-717X-9-102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Hanna N, Hauswald H, Van T, Demanes DJ, Kamrava M. Long‐term (10‐15 year) results with high‐dose‐rate‐(HDR) salvage therapy for recurrent prostate cancer. Brachytherapy. 2015;14:48. 10.1016/j.brachy.2015.02.274 [DOI] [Google Scholar]
- 160. Wojcieszek P, Szlag M, Głowacki G, et al. Salvage high‐dose‐rate brachytherapy for locally recurrent prostate cancer after primary radiotherapy failure. Radiother Oncol. 2016;119(3):405‐410. [DOI] [PubMed] [Google Scholar]
- 161. Jiang P, van der Horst C, Kimmig B, et al. Interstitial high‐dose‐rate brachytherapy as salvage treatment for locally recurrent prostate cancer after definitive radiation therapy. Toxicity and 5‐year outcome. Brachytherapy. 2017;16(1):186‐192. 10.1016/j.brachy.2016.09.008 [DOI] [PubMed] [Google Scholar]
- 162. Rutenberg MS, Meister M, Amin PP, Hussain A, Naslund MJ, Kwok Y. Salvage external beam radiotherapy for locally recurrent prostate cancer after definitive brachytherapy. Brachytherapy. 2016;15(6):722‐729. [DOI] [PubMed] [Google Scholar]
- 163. Fuller DB, Wurzer J, Shirazi R, Bridge SS, Law J, Mardirossian G. High‐dose‐rate stereotactic body radiation therapy for postradiation therapy locally recurrent prostatic carcinoma: preliminary prostate‐specific antigen response, disease‐free survival, and toxicity assessment. Pract Radiat Oncol. 2015;5(6):e615‐e623. [DOI] [PubMed] [Google Scholar]
- 164. Yamada Y, Okihara K, Iwata T, et al. Salvage brachytherapy for locally recurrent prostate cancer after external beam radiotherapy. Asian J Androl. 2015;17(6):899‐903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Gomez‐Veiga F, Mariño A, Alvarez L, et al. Brachytherapy for the treatment of recurrent prostate cancer after radiotherapy or radical prostatectomy. BJU Int. 2012;109(Suppl 1):17‐21. [DOI] [PubMed] [Google Scholar]
- 166. Chung HT, D'Alimonte L, Loblaw DA, et al. Quality of life (QOL) and acute toxicities of a pilot study of focal salvage high‐dose rate (HDR) prostate brachytherapy in patients with local recurrence after definitive external‐beam radiotherapy (XRT). JCO. 2015;33(7):79. [Google Scholar]
- 167. Murgic J, Morton G, Loblaw A, et al. Focal salvage high dose‐rate brachytherapy for locally recurrent prostate Cancer after primary radiation therapy failure: results from a prospective clinical trial. Int J Radiat Oncol Biol Phys. 2018;102(3):561‐567. [DOI] [PubMed] [Google Scholar]
- 168. Banerjee R, Park S‐J, Anderson E, Demanes DJ, Wang J, Kamrava M. From whole gland to hemigland to ultra‐focal high‐dose‐rate prostate brachytherapy: a dosimetric analysis. Brachytherapy. 2015;14(3):366‐372. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analysed in this study.


