Abstract
Toripalimab (Tuoyi™) is a selective, recombinant, humanized monoclonal antibody against programmed death protein 1 (PD-1) developed by Shanghai Junshi Bioscience Co., Ltd. Toripalimab is able to bind to PD-1 and block the interaction with its ligands. The binding of toripalimab to PD-1 is mainly attributed to the heavy chain of the former and the FG loop of the latter. Toripalimab received a conditional approval in China for the treatment of melanoma (second-line) in December, 2018. It has also received approvals to treat nasopharyngeal carcinoma (first-line and third-line) and urothelial carcinoma (second-line) in 2021. Additionally, several orphan drug designations were granted to toripalimab by the US Food and Drug Administration. Toripalimab has exhibited primary anti-tumor effects in tumors such as melanoma, lung cancer, digestive tract tumors, hepatobiliary and pancreatic tumors, neuroendocrine neoplasms, nasopharyngeal carcinoma and urothelial carcinoma. It showed a satisfactory anti-tumor effect and long-term survival benefits in Chinese melanoma patients, while the combination of axitinib with toripalimab exhibited an impressive result in metastatic mucosal melanoma. As a checkpoint inhibitor, toripalimab was generally well-tolerated in the enrolled patients. Due to different study populations, comparisons could not be made directly between toripalimab and other drugs in most cases. Nevertheless, the introduction of toripalimab may offer a valuable choice for decision-making in the treatment of tumors in the future.
Keywords: toripalimab, programmed death protein 1, programmed death ligand 1, immunotherapy, tumor, adverse effect
Introduction
T cells are important immune cells in the human body and express co-stimulating immune checkpoint proteins on their surface. Through immune checkpoints, cancer cells can block the activation of T cells and their cytotoxic effects on tumors, leading to immune evasion (1). As surface receptor proteins, immune checkpoints can be effectively inhibited by antibodies known as checkpoint inhibitors. The emergence of checkpoint inhibitors has radically changed the landscape of cancer therapy. Among all clinically applied checkpoint inhibitors, anti-programmed death protein 1 (PD-1)/programmed death ligand 1 (PD-L1) antibodies are the most successful (2). Antibody blockade of PD-1 prevents its interaction with PD-L1 and PD-L2, blocking their downstream pathways and recovering the anti-tumor response of T cells (3). There are already well-studied PD-1 inhibitors on the market such as nivolumab and pembrolizumab, two drugs that have been approved by the China National Medical Product Administration (NMPA) and US Food and Drug Administration (FDA) for use in various tumors (4).
Toripalimab (Tuoyi™) is the first domestic anti-PD-1 monoclonal antibody in China with completely independent intellectual property rights (5). Toripalimab has received a conditional approval for the treatment of unresectable or metastatic melanoma that has not responded to previous systemic therapy in December, 2018 (6). Recently, toripalimab was approved by the NMPA for the treatment of recurrent/metastatic nasopharyngeal carcinoma (NPC) and locally advanced or metastatic urothelial carcinoma (UC) (7–9). Showing an acceptable safety profile in clinical studies, it has exhibited promising anti-tumor effects in tumors like melanoma, neuroendocrine neoplasms and urothelial carcinoma, with obvious economic advantages. Here, we summarize the preclinical studies, pharmacological characteristics, anti-tumor effects, and adverse effects (AEs) of this drug in order to provide valuable information for decision-making in the treatment of tumors in the future.
Preclinical Data and Pharmacological Characteristics of Toripalimab
Toripalimab is the first monoclonal anti-PD-1 antibody approved by the China NMPA onto the market (10). It consists of two heavy chains of 452 amino acids, two light chains of 219 amino acids, and includes an N-linked glycosylation site at N302 in each heavy chain. The average molecular weight of toripalimab is 149,670 Daltons (11–14)( Figure 1 ). Toripalimab is able to bind to PD-1, efficiently blocking the interaction with its ligands; the blockade is mainly attributed to the stereospecific hindrance of the heavy chain (12). The interaction of toripalimab with PD-1 is mainly attributed to the complementarity determining regions of the heavy chain of the former and the FG loop of the latter; the light chain complementarity determining regions of toripalimab participate mainly in recognizing the epitopes on PD-1 (12, 17, 18). Comparatively, nivolumab mainly binds to the N-terminal loop of PD-1, while the binding of pembrolizumab primarily involves the C’ D loop (12, 15, 16) ( Figure 1 ).
Figure 1.
Comparison of structures of PD-1 in complex with nivolumab-Fab (A) (PDB code: 5WT9), pembrolizumab-Fab (B) (PDB code: 5JXE) and toripalimab-Fab (C) (PDB code: 6JBT). L chain, light chain; H chain, heavy chain; LCDRs, complementary determining regions of the light chain; HCDRs, complementary determining regions of the heavy chain; PD-1, programmed death protein 1. Images were acquired from Protein Data Bank and relevant research was conducted by Tan S et al., Na Z et al., and Liu H et al., respectively (12–16).
Preclinical Safety and Effectiveness
Toripalimab has low effective and inhibitory concentrations. In vitro, the effective concentration of toripalimab was determined to be 21 nmol/L and 0.89 ± 0.15 ng/mL by two studies (17, 18). In vivo, the effective concentration dose for toripalimab in the MC38 tumor model is between 0.3 and 1 mg/kg (12). In in vitro flow cytometry assays, the inhibitory concentrations of toripalimab were 3.0 nmol/L and 3.1 nmol/L for PD-1/PD-L1 and PD-1/PD-L2, respectively (18). In in vivo mouse models, by blocking the binding of PD-1 to PD-L1 or PD-L2 using a protein-based enzyme-linked immunosorbent assay, the inhibitory concentrations of toripalimab were determined to be 0.8 nmol/L for PD-L1 and 1.3 nmol/L for PD-L2. Using a cell-based flow cytometry assay, the concentrations were 1.3 nmol/L and 3.7 nmol/L for PD-L1 and PD-L2, respectively (12).
The blockage by toripalimab is effective. In vivo, toripalimab can dramatically inhibit the elevated expression of PD-1 on CD4+ and CD8+ T cells in a dose-dependent manner, while PD-1 receptor occupancy can reach up to 90% at 1 mg/kg and 100% at 10 mg/kg in cynomolgus monkeys (18). In three phase I studies, toripalimab can also bind to the PD-1 receptor on activated T lymphocytes and maintain complete PD-1 receptor occupancy (> 80%) on CD4+ and CD8+ T cells; this was observed in the majority of the patients in all dose cohorts throughout the observation period (19–21). In mice inoculated with tumor cells, toripalimab can significantly decrease tumor sizes after a 23-day treatment compared with IgG-treated controls (12). Treatment with toripalimab (0.01–10 μg/mL) can also dose-dependently stimulate human T cell proliferation, as well as IFN-γ and TNF-α secretion (18).
Immunogenicity
Toripalimab provoked only a weak immune response in animal experiments and clinical trials. In an animal experiment, 18 cynomolgus monkeys were treated with toripalimab at low (1 mg/kg), medium (10 mg/kg) and high (75 mg/kg) doses; 10% of monkeys from each group were positive for anti-drug antibodies 28 d after the first administration, indicating a low immunogenicity of this drug (18). In NCT03013101, detection of anti-drug antibodies was performed in 128 melanoma patients treated with toripalimab at 3 mg/kg intravenously once every 2 weeks; samples from 10.2% (13/128) patients were positive after toripalimab treatment and only one positive patient had a decreased toripalimab plasma concentration (22). In NCT02857166, patients were assigned to receive 0.3 mg/kg, 1 mg/kg, 3 mg/kg, 10 mg/kg or 240 mg toripalimab via intravenous infusion every 2 weeks; anti-drug antibodies were detected in one (33.3%) patient in the 0.3 mg/kg group, three (42.9%) patients in the 1 mg/kg group, and one (16.7%) patient in the 3 mg/kg group, but were not detected in the 10 mg/kg group or the 240 mg group (19). These results indicate a low immunogenicity of toripalimab in the body.
Pharmacological Characteristics
In the phase II study POLARIS-01, when toripalimab was given at 3 mg/kg per 2 weeks (Q2W), its steady-state median trough plasma concentration was 39.8 μg/mL (22). In phase I studies, the serum concentrations of toripalimab reached a maximum at about 1 h, within 2 h and within 6 h after infusion, respectively (19–21). In NCT02836795 and NCT02836834, the steady state trough concentration of toripalimab was 8.9 ± 4.4, 37.8 ± 17.5, 174.3 ± 95.6 μg/mL and 8.5 ± 2.6, 34.5 ± 10.0, and 195.8 ± 104.6 μg/mL for the 1 mg/kg, 3 mg/kg, and 10 mg/kg cohorts, respectively (20, 21). In NCT02857166, the analyses of blood samples of patients receiving toripalimab showed a serum half-life of 150–222 h after a single infusion and 188–525 h after multi-dose infusions (19). In another two studies, the average serum half-lives for the 1, 3 and 10 mg/kg cohorts were 6.2, 7.7, 9.8 days and 7.7, 8.0 and 14.2 days respectively after a single infusion; after multi-dose infusions, the half-lives were 9.5, 16.5, 13.9 days and 10.7, 12.1 20.1 days, respectively (20, 21). Based on the preclinical and pharmacological data, toripalimab was tested in therapeutic clinical trials.
Efficacy of Toripalimab in Tumors
Based on the above preclinical studies, toripalimab was tested in phase I clinical studies. The recommended phase II dose was determined to be 3 mg/kg Q2W in a first-in-human phase I trial (NCT02836795) (23). In NCT02836795, patients in the dose escalation cohorts received intravenous infusion of toripalimab at 1 mg/kg, 3 mg/kg, and 10 mg/kg Q2W; 28 days after the first dose, subjects continued to receive toripalimab at the intended dose level Q2W. The confirmed dosage of toripalimab laid the foundation for further clinical trials. The primary endpoints are usually safety and tolerability in phase I studies; overall response rate (ORR), defined as the percentage of patients achieving complete response (CR) or partial response (PR), in phase II studies; and overall survival (OS) and progression-free survival (PFS) in phase III studies (24). The basic information of toripalimab studies in these cancers was visualized in Figures 2 , 3 .
Figure 2.
A summary of prospective clinical studies of toripalimab on solid tumors, melanoma and lung cancer. Dates indicate the time when the results of the studies were available online. Dashed lines perpendicular to the y-axis in the bar graph indicate absence of corresponding available data. Dotted gray line at the edge of column means not less than the indicated number. Approval 1, approval in mucosal melanoma, an orphan designation by the FDA (plus axitinib); approval 2, approval in melanoma, by the NMPA (second-line); HDI, high-dose IFN-α2b; ORR, overall response rate; DCR, disease control rate; RFS, recurrence-free survival; PFS, progression-free survival; OS, overall survival; TRAEs, treatment-related adverse events; FDA, Food and Drug Administration; NMPA, National Medical Products Administration.
Figure 3.
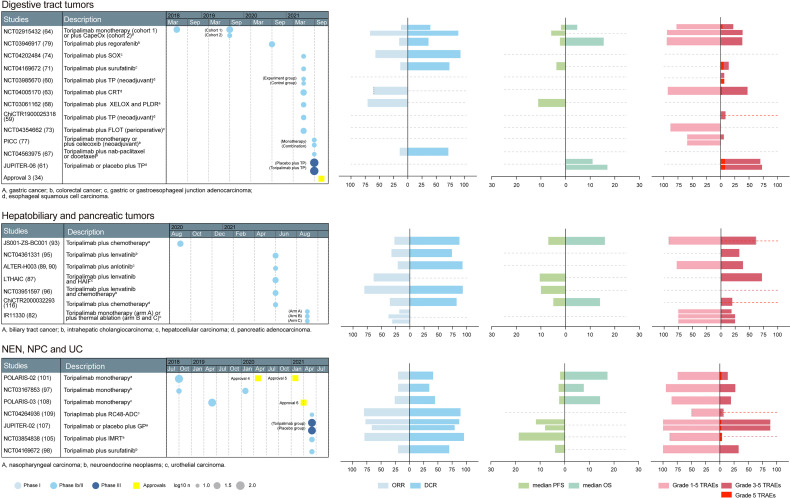
A summary of prospective clinical studies of toripalimab on digestive tract tumors, hepatobiliary and pancreatic tumors, and other tumors. Dates indicate the time when the results of the studies were available online. Dashed lines perpendicular to the y-axis in the bar graph indicate absence of corresponding available data. Dotted gray line at the edge of column means not less than the indicated number. CapeOX, capecitabine and oxaliplatin; SOX, S-1 plus oxaliplatin; TP, paclitaxel plus platinum; CRT, chemoradiotherapy; XELOX, capecitabine and oxaliplatin; PLDR, pulsed low dose rate radiotherapy; FLOT, 5-fluorouracil, leucovorin, oxaliplatin, docetaxel; HAIF,hepatic arterial infusion of oxaliplatin, 5-fluorouracil, and leucovorin; GP, gemcitabine and platinum; IMRT, intensity-modulated radiotherapy; approval 3, approval in esophageal cancer, an orphan designation by the FDA; approval 4, approval in NPC, an orphan designation by the FDA; approval 5, approval in NPC, by the NMPA (third-line); approval 6, approval in UC, by the NMPA (second-line); ORR, overall response rate; DCR, disease control rate; RFS, recurrence-free survival; PFS, progression-free survival; OS, overall survival; TRAEs, treatment-related adverse events; FDA, Food and Drug Administration; NMPA, National Medical Products Administration.
Efficacy of Toripalimab in Melanoma
Melanoma of the skin accounts for 1.7% of newly-diagnosed cancers and 0.6% of cancer-related deaths worldwide (25).Clinical trials in melanoma have largely been unsuccessful, and there are few treatment options when the disease becomes advanced (26). The first approval of toripalimab was for unresectable or metastatic melanoma that has failed to respond to previous systemic therapy, which was based on a phase II study (NCT03013101/POLARIS-01) (22). Additionally, the anti-tumor effects of nivolumab and pembrolizumab were investigated in NCT00730639 and KEYNOTE-151, respectively. The information of toripalimab studies in melanoma was visualized in Figure 2 and the comparisons between toripalimab, nivolumab and pembrolizumab in melanoma are listed in Table 1 .
Table 1.
Comparison of different therapeutic regimens for melanoma.
| Therapeutic Regimen | Toripalimab[NCT03013101/POLARIS-01 [(22, 27)] | Nivolumab[NCT00730639 (28)] | Pembrolizumab[KEYNOTE-151 (29)] | Toripalimab plus axitinib[NCT03086174 (23, 30)] | |
|---|---|---|---|---|---|
| Patient races | Chinese | Not mentioned | Chinese | Chinese | |
| ORR | total | 17.3% (22/127) | 30.8% (33/107) | 16.7% (17/102) | NA |
| cutaneous | 20.3% (16/79) | NA | 17.7% (14/79) | NA | |
| mucosal | 0 (0/22) | NA | 13.3% (2/15) | 48.3% (14/29) | |
| DCR | total | 57.5% (73/127) | 37.4% (40/107) | 38.2% (39/102) | NA |
| cutaneous | 57.0% (45/79) | NA | 42.1% (16/38) for acral | NA | |
| mucosal | 40.9% (9/22) | NA | 20.0% (3/15) | 86.2% (25/29) | |
| Median PFS | total | 3.6 months | 3.7 months | 2.8 months | NA |
| cutaneous | 3.2 months for acral, 5.5 months for NC |
NA | NA | NA | |
| mucosal | 1.9 months | NA | NA | 7.5 months | |
| Median OS | total | 22.0 months | 16.8 months | 12.1 months | NA |
| cutaneous | 16.9 months for acral, 46.1 months for NC |
NA | NA | NA | |
| mucosal | 10.3 months | NA | NA | 20.7 months | |
| Safety profiles | Grade 1-5 TRAEs | 90.6% (116/128) | 54.2% (58/107) | 84.5% (87/103) | 97.0% (32/33) |
| Grade ≥3 TRAEs | 19.5% (25/128) | 22.4% (24/107) | 10.7% (11/103) | 39.4% (13/33) | |
| Grade 5 TRAEs | 0 | 0 | 1.9% (2/103) | 0 | |
ORR, overall response rate; DCR, disease control rate; PFS, progression-free survival; OS, overall survival; NA, not available/applicable; NC, nonacral cutaneous; TRAE, treatment-related adverse event.
Overall, the toripalimab was effective in melanoma. In POLARIS-01, toripalimab was given at 3 mg/kg Q2W until disease progression or unacceptable toxicity in 128 Chinese patients who had previously received systemic therapy. The achieved ORR was 17.3% (22/127), and the disease control rate (DCR), defined as the percentage of patients achieving CR, PR, or stable disease (SD), was 57.5% (73/127); the median PFS and OS were 3.6 months and 22.2 months, respectively (22). Toripalimab has led to long-term survival benefit, and PD-L1 positive patients show markedly longer OS than PD-L1 negative patients; the data for different subtypes are shown in Table 1 (22, 27). In 22 melanoma patients from another phase I study (NCT02836795), ORR, DCR, PFS and OS were 18.2% (4/22), 45.5% (10/22), 84 days and 448 days, respectively (20). Comparatively, in KEYNOTE-151, pembrolizumab was administered in previously-treated Chinese melanoma patients; the achieved ORR was 16.7% (17/102) and the DCR was 38.2% (39/102). The ORRs were 15.8% (6/38) for acral, 19.5%(8/41) for non-acral and 13.3% (2/15) for mucosal melanoma patients, the DCRs were 42.1% (16/38) for acral melanoma and 20.0% (3/15) for mucosal melanoma, respectively (29). In NCT00730639, overall response and disease control were achieved by nivolumab in 30.8% (33/107) and 37.4% (40/107) patients, respectively; the median PFS and median OS were 3.7 months and 16.8 months (28). The ORR achieved by nivolumab appears to be the highest among all three drugs; however, the efficacies of nivolumab for each subtype are unknown, and the study population in NCT00730639 was not mentioned. In addition, adjuvant toripalimab was able to improve the recurrence-free survival of mucosal melanoma patients, but its comparison with conventional chemotherapy remained to be conducted (31).
The combination of toripalimab and axitinib, a vascular endothelial growth factor inhibitor, has shown a potent effect in treating mucosal melanoma. Vascular endothelial growth factor is an important growth factor for the growth of cancer and is upregulated in melanoma patients (32). Simultaneous blockade of PD-1 and vascular endothelial growth factor receptor 2 can induce a synergistic anti-tumor effect in mice inoculated with colon cancer cells (33). In a phase Ib study (NCT03086174), 33 Chinese patients were given axitinib 5 mg twice a day plus toripalimab 1 or 3 mg/kg every 2 weeks until confirmed disease progression, unacceptable toxicity, or voluntary withdrawal, in a dose-escalation and a cohort-expansion phase. The achieved ORR was 48.3% (14/29) and DCR was 86.2% (25/29), while the median PFS was 7.5 months (23). The combinatorial plan produced a high response rate. Thus, an orphan designation for treatment of mucosal melanoma was granted to the combinational therapy by the US FDA in March 25, 2020 (34). An updated analysis of NCT03086174 revealed a median OS of 20.7 months among these patients (30). Later, toripalimab plus axitinib combination treatment was tested in an adjuvant setting, and led to pathological complete response (pCR), defined as no viable tumor on all slides, in 14.3% (2/14) of patients (35). In addition, the effectiveness of the combination in advanced mucosal melanoma is currently being investigated in a phase II trial (registration number: NCT03941795) (36).
Toripalimab has also been tested for the treatment of melanoma in other settings. For example, the pCR achieved by toripalimab plus OrienX010, a granulocyte-macrophage colony-stimulating factor, is 14.3% (3/21) in resectable stage IIIb–IVM1a acral melanoma patients; and intravenous administration of toripalimab plus intralesional injection of OrienX010 achieved an ORR of 13.3% (2/15) in liver metastasis (37, 38). Toripalimab plus vorolanib achieved a 13.2% (5/38) ORR in advanced mucosal melanoma as a first-line therapy (39). Additionally, a randomized, multi-center, phase II study (NCT03430297) comparing toripalimab as a first-line treatment of metastatic melanoma to dacarbazine (a first-line chemotherapy for melanoma) is currently ongoing (36). The results of these studies may provide more indications for toripalimab in the future.
Efficacy of Toripalimab in Non-Small Cell Lung Cancer
Toripalimab exhibited anti-tumor effects in non-small cell lung cancer (NSCLC) as a single agent ( Figure 2 ). In a phase I study (NCT02836834), the ORR and DCR achieved by toripalimab in seven Chinese NSCLC patients were 14.3% (1/7) and 71.4% (5/7), respectively (21). In another phase I study (NCT03301688), 41 heavily-treated patients were included and treated with a single dose of toripalimab; the ORR and DCR achieved were 7.1% (2/28) and 39.3% (11/28), respectively (40). The effect of toripalimab in NSCLC was also observed in retrospective studies (41). In comparison, nivolumab and pembrolizumab monotherapy achieved ORRs of 17.1% (22/129) and 19.4% (96/495) and DCRs of 27.1% (35/129) and 41.2% (204/495) in NSCLC, as revealed by the phase I studies CHECKMATE-003 and KEYNOTE-001, respectively (42, 43). Due to the small sample size and different patient baseline characteristics such as race of NCT02836834, it is not valid to compare the effect of toripalimab with the other two drugs directly.
Toripalimab plus other therapies has also exerted anti-tumor effects in NSCLC. Multiple target cytotoxic T-lymphocyte cells can restore antitumor immunity to improve patient outcome. In NCT04193098, a combination of multiple target cytotoxic T-lymphocyte cells and toripalimab as a second-line therapy led to an ORR of 38.4% (5/13) and a DCR 76.9% (10/13) in patients with advanced NSCLC, without causing treatment-related death (44). Platinum-based chemotherapy is one of the standard systemic therapies for NSCLC (45). In NCT03513666, 40 Chinese NSCLC patients who failed to respond to first-line EGFR-TKIs and did not harbor T790M mutation were enrolled and received toripalimab combined with carboplatin and pemetrexed every 3 weeks, for up to six cycles, followed by toripalimab and pemetrexed maintenance until disease progression or unacceptable toxicity. The ORR achieved by the combination regime was 50.0% (20/40), the median PFS was 7.0 months and the median OS was 23.5 months, better than historical data for traditional chemotherapy (46). The ORR and DCR achieved by toripalimab combined with chemotherapy in a retrospective study were 39.2% and 96.2% among 79 NSCLC patients (47). In addition, a phase III clinical trial comparing the toripalimab versus placebo in combination with chemotherapy in the same setting as NCT03924050 is ongoing (48). Also, toripalimab in combination with vorolanib, a multi-target tyrosine kinase inhibitor, also exhibited anti-tumor effects as a second-line therapy (49).
A toripalimab-containing regimen in a neoadjuvant setting yielded a high major pathological response (MPR) rate and caused no treatment-related surgical delays. In a phase 2 study (NeoTPD01/NCT04304248), surgically resectable stage IIIA or T3–4N2 IIIB NSCLC patients received three cycles of neoadjuvant treatment with intravenous toripalimab plus carboplatin, and pemetrexed or nab-paclitaxel (50). MPR, defined as less than 10% residual tumor remaining at the time of surgery, was achieved in 66.7% (20/30) of patients, and pCR was achieved in 50.0% (15/30) of patients. Treatment discontinuation or dose reduction was not observed and there were no treatment-related deaths (50). In another study, neoadjuvant toripalimab plus chemotherapy led to a lower pCR rate (18.2%) and MPR rate (40.9%), but resulted in an ORR of 75.0% (30/40) and DCR of 95.0% (38/40) (51). Nivolumab and pembrolizumab monotherapy have also been tested in a neoadjuvant setting in US medical centers and an Israeli medical center; the MPRs achieved were 42.9% (9/21) and 40% (4/10), respectively (52–54). In contrast, Sheng et al. tested pembrolizumab in a Chinese setting; they enrolled 37 Chinese adults with untreated, surgically resectable stage IIB–IIIB squamous NSCLC and treated them with two cycles of pembrolizumab with albumin-bound paclitaxel plus carboplatin (55). All tumors were completely resected and MPR occurred in 24 (64.9%) resected tumors; none of the patients discontinued neoadjuvant therapy due to toxic effects and no treatment-related death was observed (55).
In addition, ongoing trials conferred potential possibilities in NSCLC treatment. For example, in NCT04238169, metastatic NSCLC patients who have previously received first-line platinum-based chemotherapy or immune checkpoint inhibitors (except toripalimab) and diagnosed with confirmed disease progression were included and treated with stereotactic body radiation therapy (30–50 Gy/5 F for 2–4 lesions) plus toripalimab (240 mg, Q3W) with or without bevacizumab (7.5 mg/kg, Q3W) until disease progression or intolerable toxicity (56). Toripalimab was also tested in different NSCLC stages in prospective studies, such as in early-stage NSCLC, and treatment-naive advanced NSCLC (57). Release of these results in the future may lay the foundations for more indications.
Efficacy of Toripalimab in Esophageal Cancer
Toripalimab plus chemotherapy is effective when used as a neoadjuvant therapy in esophageal squamous cell carcinoma (ESCC) ( Figure 3 ). Combinational fluoropyrimidine plus platinum-based chemotherapy is a recommended first-line treatment for advanced or metastatic ESCC, but confers limited survival benefits (58). In a phase II study (ChiCTR1900025318), 23 subjects with resectable ESCC were included and given toripalimab combined with paclitaxel and cisplatin as neoadjuvant treatment. Among the evaluable patients, 33.3% (6/18) achieved pCR (59). The combination regime also showed a controllable safety profile, and grade 3–4 treatment-related adverse events (TRAEs) were reported in two patients. Interestingly, when toripalimab was administrated two days after chemotherapy rather than being used simultaneously, the combinational neoadjuvant regime showed a trend toward a higher pCR rate (36.4%, 4/11 versus 7.7%, 1/13, P = 0.079) (60).
Toripalimab plus chemotherapy conferred better OS and PFS than chemotherapy alone in the first-line setting ( Figure 3 ). In the randomized, double-blind phase III study JUPITER-06, 514 Chinese patients with treatment-naive, advanced or metastatic ESCC were randomized (1:1) to receive 240 mg toripalimab or placebo in combination with paclitaxel plus cisplatin every 3 weeks for up to six cycles, followed by toripalimab or placebo maintenance (61). Incidence of fatal TRAEs was similar between the groups (8.2% vs 8.2%), while significant advantages in OS and PFS for toripalimab over placebo (HR = 0.58, HR = 0.58) were observed, with median OS being 17.0 months and 11.0 months, respectively (61). In a similar setting (KEYNOTE-590), pembrolizumab plus 5-fluorouracil and cisplatin (n = 373) also led to superior OS (12.6 months versus 9.8 months, HR = 0.72) and PFS (6.3 months versus 5.8 months, HR = 0.65) to placebo plus 5-fluorouracil and cisplatin (n = 376) in patients with advanced ESCC from 168 medical centers in 26 countries, with a lower TRAE-related death rate (7.5%, 28/373; 10.1%, 38/376) (62).
Efficacy of toripalimab was also observed in other combinatorial regimes ( Figure 3 ). For example, when combined with concurrent chemoradiotherapy, toripalimab achieved a clinical complete response rate of 60.7% (17/28) in previously untreated ESCC patients at 3 months (63). Based on the satisfactory anti-tumor effects of toripalimab shown above, an orphan designation was designated by FDA on November 8, 2021 (34).
Efficacy of Toripalimab in Gastric and Gastroesophageal Junction Cancer
The efficacy of toripalimab in advanced gastric cancer (GC) has been observed and seems promising ( Figure 3 ). In the phase Ib part of the clinical trial NCT02915432, 58 Chinese chemo-refractory advanced GC patients received toripalimab (3 mg/kg d1, Q2W) as a monotherapy plan, which resulted in an ORR of 12.1% (7/58) (64). In cohort 1 of KEYNOTE-059, 259 patients from different countries with advanced GC received pembrolizumab monotherapy; the ORR in the east Asia subgroup was 8.8% (3/34) (65). In the gastric cohort of KEYNOTE-012, pembrolizumab monotherapy was associated with an ORR of 22.2% (8/36) in patients from different countries/regions with PD-L1-positive recurrent or metastatic adenocarcinoma of the stomach or gastro-esophageal junction (66). The east Asian data of KEYNOTE-012 are unavailable. In contrast, KEYNOTE-059 is more comparable to NCT02915432.
The combination of toripalimab and chemotherapy was effective in advanced GC patients ( Figure 3 ). For example, the toripalimab plus CapeOx (capecitabine and oxaliplatin) regime (NCT02915432) as a first-line treatment in chemotherapy-naive advanced GC patients achieved an ORR and DCR of 66.7% (12/18) and 88.9% (16/18), respectively (64). In patients with advanced gastric adenocarcinoma, toripalimab plus nab-paclitaxel/docetaxel as second-line treatment resulted in confirmed PR in 1/7 patients, and SD in 4/7 patients, corresponding to an ORR of 14.2% and a DCR of 71.4% (67). The toripalimab plus XELOX (capecitabine and oxaliplatin) chemotherapy regimen and pulsed low dose rate radiotherapy also showed therapeutic effects in abdominal metastasis of advanced GC, with an ORR of 70.8% (17/24) (68). In ATTRACTION-4, nivolumab in combination with CapeOx yielded an ORR and DCR of 76.5% (13/17) and 88.2% (15/17), respectively, in treatment-naive advanced GC patients from Japan and South Korea (69). In cohort 2 of KEYNOTE-059, previously untreated advanced GC patients received pembrolizumab, cisplatin, and 5-fluorouracil (or capecitabine in Japan); the combination plan yielded an ORR of 60.0% (15/25) and a DCR of 80.0% (20/25) (70). The data of NCT02915432 and ATTRACTION-4 are more comparable because their study populations are similar.
Toripalimab showed an anti-tumor effect in gastric or gastroesophageal junction adenocarcinoma (G/GEJ) in different settings ( Figure 3 ). Surufatinib is a novel small-molecule kinase inhibitor targeting VEGFRs, FGFR and CSF-1R, and its combination with toripalimab resulted in two confirmed PR and six SD in 15 evaluable G/GEJ patients who did not respond to first-line systemic chemotherapy, with median PFS being 3.71 months (71). FLOT (docetaxel, oxaliplatin, leucovorin, 5-FU) is the standard perioperative treatment for resectable G/GEJ (72). The combination of toripalimab and FLOT produced a 25.0% pCR and 42.9% MPR among 28 G/GEJ patients receiving complete resection (73). A toripalimab plus S-1 and oxaliplatin regimen as a first-line treatment showed effectiveness (ORR 57.1%, and DCR 92.8%) among 14 patients with advanced stage G/GEJ (74).
Efficacy of Toripalimab in Colorectal Cancer
Toripalimab alone or plus celecoxib conferred therapeutic effects in colorectal cancer (CRC) patients in a neoadjuvant setting ( Figure 3 ). Cancer cell-intrinsic cyclooxygenases-2 expression contributed to resistance to immune checkpoint blockade (75). Therapeutically targeting the cyclooxygenases-2 pathway with widely used non-steroidal and steroidal anti-inflammatory drugs was able to synergize with immune checkpoint blockers and strengthen their anti-tumor effects in mouse CRC models (76). Celecoxib is a non-steroidal anti-inflammatory drug. In the phase 2 PICC study, toripalimab with or without celecoxib was tested in a neoadjuvant setting. In PICC, 34 patients with histologically-confirmed mismatch repair-deficient or microsatellite instability-high CRC, with clinical stage T3–T4 or any T with lymph node positivity were included and randomly assigned (1:1) to receive either toripalimab plus celecoxib (combination group) or toripalimab monotherapy (monotherapy group). While neither treatment caused surgical delays, pCR was achieved in 88.2% (15/17) in the combination group and 64.7% patients in the monotherapy group (77).
Toripalimab plus regorafenib showed anti-tumor efficacy in metastatic CRC ( Figure 3 ). Regorafenib is an oral tyrosine kinase inhibitor targeting angiogenesis, the tumor microenvironment and tumor immunity, and has been approved for the treatment of metastatic CRC after failing standard therapies (78). In the phase I/II study NCT03946917, regorafenib plus toripalimab was tested in relapsed or metastatic CRC patients that had failed ≥ 2 previous lines of chemotherapy or were intolerant to prior systemic treatment (79). Twelve patients were enrolled in the dose escalation phase, which identified a dose of 80 mg regorafenib plus 3 mg/kg toripalimab to be the recommended phase II dose. In the 33 evaluable patients treated with recommended phase II doses, the ORR was 15.2% (5/33), the DCR was 36.4% (12/33), the median PFS was 2.1 months and median OS was 15.5% at the data cutoff of July 12, 2020 (79). In a retrospective analysis, the ORR in these patients is 12.1% (4/33), DCR is 36.4% (12/33), and median PFS is 3.8 months (80). In a similar setting to NCT03946917, nivolumab achieved an ORR of 36.0% (9/25) in the Japanese population (81).
Efficacy of Toripalimab in Hepatobiliary Cancers
Toripalimab alone or in combination with other treatments showed promising anti-tumor effects in advanced hepatocellular carcinoma (HCC) ( Figure 3 ). Toripalimab monotherapy led to an ORR of 18.8% in previously-treated advanced HCC patients (82). Additional ablation increased the response rate in these patients, without causing treatment-related death (82, 83). Lenvatinib has been reported to improve the survival of advanced HCC patients, while hepatic arterial infusion of oxaliplatin, 5-fluorouracil, and leucovorin (HAIF) led to further survival benefit (84, 85). In a retrospective analysis, the addition of toripalimab plus HAIF prolonged PFS and OS and increased ORR in HCC patients receiving lenvatinib (86). The effect of the combination regime was also explored in a prospective phase II trial, showing an ORR of 63.9% and median PFS of 10.5 months among 36 treatment-naive advanced HCC patients (87). Anlotinib, a novel multi-targeting tyrosine kinase inhibitor, has shown promising efficacy and safety as a first- or second-line treatment strategy in advanced HCC (88). The combination of toripalimab and anlotinib conferred an ORR of 21.4% (3/14) and a DCR of 92.9% (13/14) among treatment-naive patients with advanced HCC (89, 90).
Toripalimab plus chemotherapy showed a preliminary efficacy in treatment-naive advanced biliary tract cancer (BTC) ( Figure 3 ). Combination chemotherapy with gemcitabine plus cisplatin has been regarded as the standard treatment for patients with advanced BTC, but confers only limited clinical benefit (91). In the phase II trial NCT03796429, treatment-naive patients with advanced BTC received toripalimab combined with gemcitabine and S-1 until progressive disease or unacceptable toxicity. By the data cutoff at January 24, 2021, the ORR was 27.1% (13/48) and DCR was 87.5% (42/48), median PFS was 7.0 months and median OS was 16.0 (92, 93). In a similar phase I study JapicCTI-153098, 30 chemotherapy-naive Japanese patients with unresectable or recurrent BTC were given nivolumab and cisplatin plus gemcitabine chemotherapy; in this cohort, median OS was 15.4 months, median PFS was 4.2 months, and 11 of 30 patients (36.7%) had an objective response (94).
Toripalimab plus lenvatinib showed promising efficacies in intrahepatic cholangiocarcinoma (ICC) ( Figure 3 ). In NCT04361331, 31 pathologically-confirmed advanced ICC patients were included and treated with toripalimab plus lenvatinib as a first-line therapy. The combination plan resulted in an ORR of 32.3% (10/31) and a DCR of 74.2% (23/31) (95). When the regime was added to oxaliplatin and gemcitabine chemotherapy, a standard chemotherapy regime for ICC, the ORR became 80% (24/30) and DCR 93.3% (28/30) in treatment-naive patients (96). Treatment-related death was identified in neither of the studies, which indicated an acceptable safety profile.
Efficacy of Toripalimab in Neuroendocrine Neoplasms
Toripalimab has a satisfactory efficacy when used alone or in combination with surufatinib in neuroendocrine neoplasms (NEN) ( Figure 3 ). The ORR achieved in previously-treated metastatic Chinese NEN patients was 20.0% (8/40), and DCR was 35.0% (14/40) (NCT02939651) (97). Furthermore, four patients achieved confirmed PR and 10 achieved SD among 20 tumor evaluable patients receiving toripalimab plus surufatinib, with a median PFS of 3.94 months and no treatment-related deaths (98). In contrast, pembrolizumab monotherapy resulted in ORR and DCR of 3.4% (1/29) and 24.1% (7/29), respectively, in grade 3 NEN patients refractory to first-line chemotherapy (NCT02939651) (99). It has been widely acknowledged that the prognosis of NEN based on pathological grading is dependent on the measurement of a proliferative index such as Ki-67 (100). In NCT02939651, patients had Ki-67 > 20%, while in NCT03167853, patients had Ki-67 ≥ 10%. Thus, the higher ORR achieved by toripalimab might be correlated with the lower grade of tumors in NCT03167853 to some extent. In addition, the comparison should still be made cautiously because the patient races in the two studies are different.
Efficacy of Toripalimab in Nasopharyngeal Cancer
Toripalimab was shown to be effective when used alone or with radiotherapy in treating NPC patients ( Figure 3 ). In POLARIS-02/NCT02915432, 190 treated Chinese patients with recurrent or metastatic NPC were included and received 3 mg/kg toripalimab Q2W via intravenous infusion until disease progression, unacceptable toxicity, or voluntary withdrawal (101). Toripalimab monotherapy led to an ORR of 20.5% (39/190) and a DCR of 41.6% (79/190), which led to its approval for treating patients with recurrent/metastatic NPC as a third-line therapy (8). Due to the satisfactory anti-tumor effect of toripalimab shown in NPC, an orphan designation was also given by the FDA in May 18, 2020 (34). In comparison, the ORRs achieved by nivolumab in NCI-9742/NCT02339558 and pembrolizumab in KEYNOTE-028/NCT02054806 were 20.5% (9/44) and 26.3% (5/19), respectively (102, 103). Patients in these studies have similar tumor stages and treatment status. In NCI-9742, 82.2% (37/45) of patients are Asian; in KEYNOTE-028, this number is 63.0% (17/27). Considering the small sample size and lower proportions of Asian patients in KEYNOTE-028, the comparison between NCT02915432 and NCI-9742 is more valid. Intensity-modulated radiotherapy is the most widely used radiotherapy technique for NPC and significantly improves patient survival (104). In a phase II trial in patients with recurrent NPC, toripalimab in combination with intensity-modulated radiotherapy was prescribed, achieving an ORR of 79.2% (19/24) and a DCR of 95.8% (23/24), causing one treatment-related death (4.0%) (105).
Toripalimab plus chemotherapy showed superior clinical benefit compared to chemotherapy alone in NPC patients ( Figure 3 ). Gemcitabine-cisplatin (GP) was the standard first-line chemotherapy regime for advanced or metastatic NPC (106). Recently, the phase III study JUPITOR-02 was published, making a head-to-head comparison between toripalimab plus GP and placebo plus GP (107). The study involved 289 patients with recurrent or metastatic NPC and no previous chemotherapy history. The patients were randomized (1:1) to receive toripalimab plus GP (combination group) or placebo plus GP (placebo group); longer PFS was observed in the combination group compared to the placebo group (11.7 versus 8.0 months); the data for median OS were not available yet, but a trend toward better OS was observed, with the stratified HR for OS being 0.78 (107). Due to the superior clinical benefit conferred by the combination plan, NMPA have approved the indication of toripalimab plus GP as a first-line therapy in NPC recently (7).
Efficacy of Toripalimab in Urothelial Carcinoma
Toripalimab produced a satisfactory anti-tumor effect in locally advanced or metastatic UC patients ( Figure 3 ). In a phase I study NCT02836795, toripalimab monotherapy achieved an ORR of 25.0% (2/8) and a DCR of 67.5% (5/8) among eight Chinese patients with metastatic UC (20). In the phase II study POLARIS-03/NCT03113266, 151 Chinese patients with advanced metastatic UC that had failed prior standard therapy were enrolled and treated with toripalimab until disease progression, unacceptable toxicity or voluntary withdrawal (108). By the cut-off date, there were two CR, 37 PR, and 29 SD, corresponding to an ORR of 25.8% (39/151) and a DCR of 45.0% (68/151) (65). When combined with RC48-ADC, a novel humanized anti-HER2 antibody-drug conjugate, the ORR increased to 80% (8/10) and DCR to 90% (9/10) in patients that were mostly treatment-naive (8/14) (109). The satisfactory anti-tumor effect of toripalimab shown in UC facilitated toripalimab’s approval in previously-treated locally advanced or metastatic UC (9). In CHECKMATE-275, nivolumab 3 mg/kg Q2W resulted in an ORR of 19.6% (52/265) in locally advanced or metastatic UC patients (110), and in KEYNOTE-052, pembrolizumab in treatment-naive patients yielded an ORR of 24.1% (89/370) (111). Although the patients had received prior systemic therapy, the response rate in POLARIS-03 is still higher than in KEYNOTE-052. However, it should be noted that study populations of CHECKMATE-275 and KEYNOTE-052 are different from that of POLARIS-03.
Efficacy of Toripalimab in Other Cancers
In addition to the above-mentioned tumors, toripalimab monotherapy has also shown anti-tumor effects in phase I studies on alveolar soft part sarcoma, lymphoma and renal cell carcinoma, achieving ORRs of 25.0% (3/12), 90.9% (10/11) and 33.3% (2/6), respectively (20, 21). In lymphoma patients, the ORR achieved by toripalimab (NCT02836834) is apparently higher than those achieved by nivolumab [CHECKMATE-205, 69.1% (168/243)] or pembrolizumab [KEYNOTE-087, 69.0% (145/210)]. Although the sample size in NCT02836834 is small and the patient race is different from the other two studies, the result suggests a promising anti-tumor effect of toripalimab in lymphoma that is worth exploring in future studies (21, 112, 113). As neoadjuvant therapies, toripalimab-containing regimes were claimed to be effective in penile squamous cell carcinoma and anal canal squamous carcinoma (114, 115). Toripalimab was also effective in cancers such as pancreatic adenocarcinoma and small-cell lung cancer ( Figures 2 , 3 ) (116, 117). It is interesting that the regime achieved a 100% ORR in extensive-stage small cell lung cancer (117). In addition, toripalimab in combination with YH-001, an anti-CTLA-4 monoclonal antibody, or YH-003, an anti-CD40 monoclonal antibody, were shown to be effective in phase I studies in advanced solid tumors ( Figure 2 ) (118, 119). In other retrospective studies, toripalimab was claimed to be effective when combined with other treatments in patients with soft tissue sarcoma cervical cancer, and head and neck squamous cell carcinoma (120–122). These studies have indicated a bright future for this drug in clinical applications. The efficacies of toripalimab in different clinical studies have been visualized in Figures 2 , 3 .
Adverse Effects
Although toripalimab has shown preliminary efficacy in the treatment of various tumors, there are inevitable side effects. The adverse effects have five grades, which were defined according to the Common Terminology Criteria for Adverse Events from the National Cancer Institute, referring to mild, moderate, severe and life-threatening AEs or death, in ascending order, and were coded using the Medical Dictionary for Regulatory Activities (123, 124).
Generally, the safety profile of toripalimab is acceptable; the incidence of AEs is higher than well-studied checkpoint inhibitors, but is manageable. The incidences of all grade TRAEs have been visualized in Figures 2 , 3 , and most common TRAEs are summarized in Table 2 . The safety of checkpoint inhibitors has been assessed in previous research, revealing an incidence varying between 54% and 76% for all TRAEs (125). The incidences of fatal AEs for PD-1 inhibitors and PD-L1 inhibitors were shown to be 0.361% (33/9136) and 0.63% (12/3164) in a study that comprehensively evaluated the spectrum of fatal checkpoint inhibitor-related toxic effects (126). For toripalimab, there was no dose-limiting toxicity in three phase I studies, and the pooled incidence rates of AEs and AE-related death (hereafter “the rates”) were 96.8% (91/94) and 2.1% (2/94) (19–21), respectively. The death rate appears to be higher than the average; however, the sample sizes are small, meaning that it is difficult to draw a conclusion. In the first reported phase I study of nivolumab, the rates were 69.9% (207/296) and 1.0% (3/296) (127). The rates for pembrolizumab in a phase I study were 70.9% (351/495) and 0.2% (1/495) (43). When toripalimab was used alone, TRAEs occurred in 42.9–100% patients across the studies, and the death rate ranged from 0–6.7%. In combination regimes, the highest incidence of grade 5 TRAEs was 8.2%, when combined with chemotherapy ( Figure 3 ). The incidences may not be compared directly because the baseline characteristics of patients are different.
Table 2.
Summary of most common treatment-related adverse events of all grades across 15 prospective studies with complete results.
| Trials | Tumor | Patient number | Included TRAE | Regimen | Leukopenia | Anemia | Neutropenia | Nausea | Fatigue | ALT elevation | AST elevation | Decreased appetite/Anorexia | Thrombocytopenia | Rash/Skin reaction | Vomiting | Hypothyroidism | Fever | Constipation | Diarrhea | Creatine kinase elevation | Pruritus | Hyponatremia | Hyperglycemia | Myalgia | Proteinuria | Peripheral neuropathy |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NCT03946917 (79) | CRC | 39 | All | Toripalimab plus regorafenib | 0.0% | 5.1% | 2.6% | 0.0% | 10.3% | 0.0% | 0.0% | 5.1% | 10.3% | 30.8% | 0.0% | 2.6% | 20.5% | 0.0% | 17.9% | 0.0% | 2.6% | 0.0% | 0.0% | 12.8% | 2.6% | 0.0% |
| PICC (77) (Combination) | CRC | 17 | All | Toripalimab plus celecoxib (neoadjuvant) | 0.0% | 0.0% | 0.0% | 6.0% | 12.0% | 6.0% | 18.0% | 0.0% | 0.0% | 6.0% | 0.0% | 6.0% | 0.0% | 0.0% | 0.0% | 0.0% | 12.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% |
| PICC (77) (Monotherapy) | CRC | 17 | All | Toripalimab monotherapy (neoadjuvant) | 0.0% | 0.0% | 0.0% | 18.0% | 24.0% | 6.0% | 12.0% | 6.0% | 0.0% | 18.0% | 0.0% | 0.0% | 6.0% | 0.0% | 0.0% | 0.0% | 18.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% |
| NCT02915432 (64) (Cohort 1) | GC | 58 | All (>5%) | Toripalimab monotherapy | 8.6% | 12.1% | 1.7% | 5.2% | 10.3% | 8.6% | 10.3% | 5.2% | 6.9% | 8.6% | 5.2% | 12.1% | 5.2% | 0.0% | 5.2% | 0.0% | 10.3% | 0.0% | 0.0% | 0.0% | 8.6% | 0.0% |
| NCT02915432 (64) (Cohort 2) | GC | 18 | All (>10%) | Toripalimab plus CapeOx | 38.9% | 27.8% | 38.9% | 50.0% | 16.7% | 16.7% | 33.3% | 27.8% | 22.2% | 22.2% | 38.9% | 0.0% | 11.1% | 16.7% | 27.8% | 0.0% | 22.2% | 0.0% | 0.0% | 0.0% | 22.2% | 0.0% |
| NCT03013101 (22) | Melanoma | 128 | All (>5%) | Toripalimab montherapy | 20.3% | 0.0% | 17.2% | 0.0% | 14.8% | 31.3% | 22.7% | 10.2% | 7.0% | 23.4% | 0.0% | 27.3% | 6.3% | 0.0% | 0.0% | 25.8% | 0.0% | 0.0% | 30.5% | 5.5% | 0.0% | 0.0% |
| NCT03086174 (23) | Mucosal melanoma | 33 | All (>15%) | Toripalimab plus axitinib | 27.3% | 21.2% | 24.2% | 21.2% | 48.5% | 42.4% | 33.3% | 24.2% | 0.0% | 36.4% | 0.0% | 51.5% | 0.0% | 0.0% | 60.6% | 36.4% | 0.0% | 0.0% | 33.3% | 0.0% | 57.6% | 0.0% |
| NCT03167853 (97) | NEN | 40 | All (≥10%) | Toripalimab monotherapy | 15.0% | 22.5% | 10.0% | 15.0% | 20.0% | 32.5% | 37.5% | 10.0% | 0.0% | 17.5% | 0.0% | 0.0% | 12.5% | 0.0% | 12.5% | 25.0% | 25.0% | 15.0% | 35.0% | 0.0% | 40.0% | 0.0% |
| POLARIS-02 (101) | NPC | 190 | All (≥5%) | Toripalimab monotherapy | 10.0% | 15.3% | 5.3% | 0.0% | 13.2% | 13.7% | 15.3% | 0.0% | 0.0% | 6.3% | 0.0% | 23.7% | 9.5% | 0.0% | 0.0% | 0.0% | 8.4% | 0.0% | 0.0% | 0.0% | 12.6% | 0.0% |
| NCT03854838 (105) | NPC | 25 | All | Toripalimab plus IMRT | 16.0% | 0.0% | 8.0% | 76.0% | 88.0% | 20.0% | 12.0% | 0.0% | 4.0% | 40.0% | 8.0% | 32.0% | 4.0% | 0.0% | 4.0% | 20.0% | 32.0% | 0.0% | 36.0% | 16.0% | 0.0% | 0.0% |
| JUPITER-02 (107) (Toripalimab group) | NPC | 146 | All (≥10%) | Toripalimab plus GP | 91.1% | 88.4% | 85.6% | 69.2% | 35.6% | 36.3% | 37.7% | 53.4% | 63.0% | 27.4% | 67.1% | 30.8% | 30.8% | 39.0% | 30.1% | 17.8% | 16.4% | 25.3% | 0.0% | 23.3% | 0.0% | 30.1% |
| JUPITER-02 (107) (Placebo group) | NPC | 143 | All (≥10%) | Placebo plus GP | 94.4% | 94.4% | 93.0% | 83.2% | 35.7% | 39.9% | 30.8% | 58.7% | 62.2% | 21.7% | 65.7% | 16.8% | 21.7% | 44.8% | 23.1% | 23.8% | 7.0% | 36.4% | 0.0% | 26.6% | 0.0% | 28.7% |
| NCT03301688 (40) | NSCLC | 41 | All | Toripalimab monotherapy | 2.4% | 0.0% | 0.0% | 4.9% | 2.4% | 7.3% | 12.2% | 0.0% | 0.0% | 14.6% | 0.0% | 7.3% | 0.0% | 0.0% | 0.0% | 0.0% | 2.4% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% |
| NeoTPD01 (50) | NSCLC | 33 | All | Toripalimab plus chemotherapy (neoadjuvant) | 0.0% | 51.6% | 6.1% | 30.3% | 15.2% | 0.0% | 0.0% | 12.1% | 15.2% | 18.2% | 3.0% | 18.2% | 0.0% | 0.0% | 3.0% | 0.0% | 3.0% | 0.0% | 0.0% | 15.1% | 0.0% | 15.1% |
| NCT03513666 (46) | NSCLC | 40 | All | Toripalimab plus chemotherapy | 82.5% | 67.5% | 70.0% | 47.5% | 25.0% | 50.0% | 52.5% | 37.5% | 47.5% | 15.0% | 17.5% | 0.0% | 10.0% | 27.5% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% |
| NCT02857166 (19) | ST | 25 | All | Toripalimab monotherapy | 4.0% | 0.0% | 0.0% | 8.0% | 64.0% | 4.0% | 4.0% | 16.0% | 0.0% | 24.0% | 0.0% | 12.0% | 4.0% | 0.0% | 12.0% | 0.0% | 12.0% | 0.0% | 0.0% | 0.0% | 16.0% | 0.0% |
| NCT02836834 (21) | ST | 33 | All (≥10%) | Toripalimab monotherapy | 30.0% | 18.0% | 18.0% | 0.0% | 18.0% | 18.0% | 21.0% | 0.0% | 0.0% | 21.0% | 0.0% | 0.0% | 30.0% | 0.0% | 0.0% | 3.0% | 15.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% |
| NCT02836795 (20) | ST | 36 | All (≥20%) | Toripalimab monotherapy | 25.0% | 33.3% | 0.0% | 0.0% | 22.2% | 22.2% | 25.0% | 22.2% | 0.0% | 44.4% | 0.0% | 0.0% | 38.9% | 0.0% | 0.0% | 0.0% | 25.0% | 0.0% | 58.3% | 0.0% | 50.0% | 0.0% |
CRC, colorectal cancer; GC, gastric cancer; NEN, neuroendocrine neoplasms; NPC, nasopharyngeal carcinoma; NSCLC, non-small cell lung cancer; ST, solid tumors; TRAE, treatment-related adverse events; CapeOx, capecitabine and oxaliplatin; IMRT, intensity-modulated radiotherapy; GP, gemcitabine and platinum; ALT, alanine aminotransferase; AST, aspartate aminotransferase.
Toripalimab has shown a better safety profile than pembrolizumab in melanoma. In POLARIS-01, toripalimab monotherapy resulted in 90.6% (116/128) TRAEs and 19.5% (25/128) grade 3 or 4 TRAEs, with no TRAE-related deaths, in melanoma patients (22). The rates for combination of toripalimab with axitinib were 97.0% (32/33) and 0% (23). For pembrolizumab monotherapy in melanoma, 84.5% (87/103) patients encountered TRAEs, and 8.7% (9/103) patients encountered grade 3 or 4 TRAEs; 1.9% (2/103) of patients died (one patient died of shock and one died of pulmonary embolism), although the researchers claimed that the deaths were unrelated to treatment (29). In a pooled analysis, nivolumab monotherapy caused 66.3% (57/86), 76.4% (508/665) TRAEs and 8.1% (7/86), 12.5% (83/665) grade 3 or 4 TRAEs in mucosal and cutaneous melanoma patients, without deaths. The pooled incidence of TRAEs and grade 3 or 4 TRAEs were 75.2% (565/751) and 12.0% (90/751), respectively (128). However, the analysis included patients of multiple races, compared to only Chinese patients in POLARIS-01 and KEYNOTE-151.
In other tumors, toripalimab also exhibited manageable adverse effects. Chinese patients may react differently from other populations; thus, the rates of toripalimab and the other two drugs may not be compared directly. The TRAE incidence of toripalimab in prospective clinical studies were shown and compared in Figures 2 , 3 . The safety profile of toripalimab could be analyzed systematically in future research.
Discussion
Toripalimab is the first domestic PD-1 antibody in China and has received approvals for the treatment of advanced melanoma, NPC and UC (128). The binding of toripalimab to PD-1 is mainly attributed to the heavy chain of the former and the FG loop of the latter. Toripalimab has shown preliminary anti-tumor effects comparable to pembrolizumab or nivolumab in tumors like melanoma, GC, NEN and UC, with acceptable safety profiles. Prospective clinical studies on toripalimab were shown in integrated tables and graphs in Figures 2 , 3 .
There are some disadvantages and limitations of this review. Firstly, all patients included in toripalimab studies are native Chinese, which makes it difficult to compare the anti-tumor effects with other drugs. Future studies involving other races may provide more comparable data. Secondly, although the comparisons made between studies are based on their identical primary endpoints, there is a lack of direct head-to-head comparisons. The comparisons between studies need to be made prudently, considering the different baseline characteristics of patients. Importantly, there are mainly phase 2 studies for toripalimab at present. Comparisons between drugs could be made using the indirect comparison method, which requires phase 3 randomized controlled studies with control groups (129–131). Furthermore, complete data from some studies, such as JUPITER-06 and ChiCTR1900025318, are not available. The release of complete study results will solve this problem.
Despite these limitations, there are some inherent advantages of this study. Firstly, there are more than 200 ongoing clinical trials of toripalimab, with future results expected (36). Furthermore, toripalimab has a lower cost than the other two well-studied PD-1 inhibitors (nivolumab and pembrolizumab) after the patient-assistant program; after the negotiation of Chinese government with pharmaceutical companies and the inclusion of toripalimab into the medical insurance catalogue, the cost of toripalimab further decreased (132–134). The approval of toripalimab has provided tumor patients with an extra option. Despite the decline in cost of the domestic PD-1 antibody in China, the radical change in the drug price may also exert pressure on foreign pharmaceutical companies and lead to a price decline in their PD-1/PD-L1 antibodies. Moreover, several orphan drug designations have been granted to toripalimab by the US FDA (34). Thus, it may also benefit tumor patients in other countries.
Conclusion
In conclusion, toripalimab has shown preliminary efficacy in tumors like melanoma, lung cancer, ESCC, GC, G/GEJ, CRC, HCC, BTC, ICC, NEN, NPC, and UC, with acceptable safety profiles. Specifically, it has a potent efficacy in melanoma according to data from relevant studies. Although there are some limitations, it is hopeful that the introduction of toripalimab will provide a valuable option for tumor patients. Meanwhile, our review has also provided oncologists with information on a potential choice for the treatment of cancers in the future.
Author Contributions
Conception and design: LZ. Manuscript writing: LZ and BH. Revision and language editing: LZ, ZG. Supervision: QG. All authors contributed to the article and approved the submitted version
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1. Sun C, Mezzadra R, Schumacher TN. Regulation and Function of the PD-L1 Checkpoint. Immunity (2018) 48:434–52. doi: 10.1016/j.immuni.2018.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. He X, Xu C. Immune Checkpoint Signaling and Cancer Immunotherapy. Cell Res (2020) 30:660–9. doi: 10.1038/s41422-020-0343-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Patsoukis N, Wang Q, Strauss L, Boussiotis VA. Revisiting the PD-1 Pathway. Sci Adv (2020) 6(38):eabd2712. doi: 10.1126/sciadv.abd2712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lin X, Lu X, Luo G, Xiang H. Progress in PD-1/PD-L1 Pathway Inhibitors: From Biomacromolecules to Small Molecules. Eur J Med Chem (2020) 186:111876. doi: 10.1016/j.ejmech.2019.111876 [DOI] [PubMed] [Google Scholar]
- 5. National Medical Products Administration . Toripalimab, the First Domestic Anti-PD-1 Monoclonal Antibody, Was Approved Into Market (2018). Available at: https://www.nmpa.gov.cn/directory/web/nmpa/zhuanti/ypqxgg/gggzjzh/20181217161101989.html (Accessed November 30, 2021).
- 6. Keam SJ. Toripalimab: First Global Approval. Drugs (2019) 79:573–8. doi: 10.1007/s40265-019-01076-2 [DOI] [PubMed] [Google Scholar]
- 7. National Medical Products Administration . Drug Approval Documents Released on November 26, 2021 (2021). Available at: https://www.nmpa.gov.cn/zwfw/sdxx/sdxxyp/yppjfb/20211126160047176.html (Accessed November 30, 2021).
- 8. National Medical Products Administration . Drug Approval Documents Released on February 19, 2021 (2021). Available at: https://www.nmpa.gov.cn/zwfw/sdxx/sdxxyp/yppjfb/20210219085955119.html (Accessed November 30, 2021).
- 9. National Medical Products Administration . Drug Approval Documents Released on April 12, 2021 (2021). Available at: https://www.nmpa.gov.cn/zwfw/sdxx/sdxxyp/yppjfb/20210412090005190.html (Accessed November 30, 2021).
- 10. National Medical Products Administration . The First Domestic PD-1 Antibody Toripalimab Injection was Approved Into the Market (2018). Available at: https://www.nmpa.gov.cn/directory/web/nmpa/yaowen/ypjgyw/20181217161101989.html (Accessed November 30, 2021).
- 11. Tang B, Chi Z, Guo J. Toripalimab for the Treatment of Melanoma. Expert Opin Biol Ther (2020) 20:863–9. doi: 10.1080/14712598.2020.1762561 [DOI] [PubMed] [Google Scholar]
- 12. Liu H, Guo L, Zhang J, Zhou Y, Zhou J, Yao J, et al. Glycosylation-Independent Binding of Monoclonal Antibody Toripalimab to FG Loop of PD-1 for Tumor Immune Checkpoint Therapy. MAbs (2019) 11:681–90. doi: 10.1080/19420862.2019.1596513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Burley SK, Bhikadiya C, Bi C, Bittrich S, Chen L, Crichlow GV, et al. RCSB Protein Data Bank: Powerful New Tools for Exploring 3D Structures of Biological Macromolecules for Basic and Applied Research and Education in Fundamental Biology, Biomedicine, Biotechnology, Bioengineering and Energy Sciences. Nucleic Acids Res (2021) 49:D437–51. doi: 10.1093/nar/gkaa1038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sehnal D, Bittrich S, Deshpande M, Svobodová R, Berka K, Bazgier V, et al. Mol* Viewer: Modern Web App for 3D Visualization and Analysis of Large Biomolecular Structures. Nucleic Acids Res (2021) 49:W431–7. doi: 10.1093/nar/gkab314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tan S, Zhang H, Chai Y, Song H, Tong Z, Wang Q, et al. An Unexpected N-Terminal Loop in PD-1 Dominates Binding by Nivolumab. Nat Commun (2017) 8:14369. doi: 10.1038/ncomms14369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Na Z, Yeo SP, Bharath SR, Bowler MW, Balikci E, Wang CI, et al. Structural Basis for Blocking PD-1-Mediated Immune Suppression by Therapeutic Antibody Pembrolizumab. Cell Res (2017) 27:147–50. doi: 10.1038/cr.2016.77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Huang H, Zhu H, Xie Q, Tian X, Yang X, Feng F, et al. Evaluation of 124I-JS001 for Hpd1 Immuno-PET Imaging Using Sarcoma Cell Homografts in Humanized Mice. Acta Pharm Sin B (2020) 10:1321–30. doi: 10.1016/j.apsb.2020.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fu J, Wang F, Dong LH, Zhang J, Deng CL, Wang XL, et al. Preclinical Evaluation of the Efficacy, Pharmacokinetics and Immunogenicity of JS-001, a Programmed Cell Death Protein-1 (PD-1) Monoclonal Antibody. Acta Pharmacol Sin (2017) 38:710–8. doi: 10.1038/aps.2016.161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wei XL, Ren C, Wang FH, Zhang Y, Zhao HY, Zou BY, et al. A Phase I Study of Toripalimab, an Anti-PD-1 Antibody, in Patients With Refractory Malignant Solid Tumors. Cancer Commun (2020) 40:345–54. doi: 10.1002/cac2.12068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tang B, Yan X, Sheng X, Si L, Cui C, Kong Y, et al. Safety and Clinical Activity With an Anti-PD-1 Antibody JS001 in Advanced Melanoma or Urologic Cancer Patients. J Hematol Oncol (2019) 12(1):7. doi: 10.1186/s13045-018-0693-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yang J, Dong L, Yang S, Han X, Han Y, Jiang S, et al. Safety and Clinical Efficacy of Toripalimab, a PD-1 mAb, in Patients With Advanced or Recurrent Malignancies in a Phase I Study. Eur J Cancer (2020) 130:182–92. doi: 10.1016/j.ejca.2020.01.028 [DOI] [PubMed] [Google Scholar]
- 22. Tang B, Chi Z, Chen Y, Liu X, Wu D, Chen J, et al. Safety, Efficacy, and Biomarker Analysis of Toripalimab in Previously Treated Advanced Melanoma: Results of the POLARIS-01 Multicenter Phase II Trial. Clin Cancer Res (2020) 26:4250–9. doi: 10.1158/1078-0432.CCR-19-3922 [DOI] [PubMed] [Google Scholar]
- 23. Sheng X, Yan X, Chi Z, Si L, Cui C, Tang B, et al. Axitinib in Combination With Toripalimab, a Humanized Immunoglobulin G4 Monoclonal Antibody Against Programmed Cell Death-1, in Patients With Metastatic Mucosal Melanoma: An Open-Label Phase IB Trial. J Clin Oncol (2019) 37:2987–99. doi: 10.1200/JCO.19.00210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. U.S. Food & Drug Administration . Clinical Trial Endpoints for the Approval of Cancer Drugs and Biologics Guidance for Industry (2018). Available at: https://www.fda.gov/media/71195/download (Accessed December 30, 2021).
- 25. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin (2021) 71:209–49. doi: 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 26. Schadendorf D, van Akkooi A, Berking C, Griewank KG, Gutzmer R, Hauschild A, et al. Melanoma. Lancet (2018) 392:971–84. doi: 10.1016/S0140-6736(18)31559-9 [DOI] [PubMed] [Google Scholar]
- 27. Tang B, Chi Z, Chen Y, Liu X, Wu D, Chen J, et al. Four-Year Survival Follow-Up of Toripalimab (JS001) as Salvage Therapy in Chinese Melanoma Patients. J Clin Oncol (2021) 39:e21522. doi: 10.1200/JCO.2021.39.15_suppl.e21522 [DOI] [Google Scholar]
- 28. Topalian SL, Sznol M, McDermott DF, Kluger HM, Carvajal RD, Sharfman WH, et al. Survival, Durable Tumor Remission, and Long-Term Safety in Patients With Advanced Melanoma Receiving Nivolumab. J Clin Oncol (2014) 32:1020–30. doi: 10.1200/JCO.2013.53.0105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Si L, Zhang X, Shu Y, Pan H, Wu D, Liu J, et al. A Phase Ib Study of Pembrolizumab as Second-Line Therapy for Chinese Patients With Advanced or Metastatic Melanoma (KEYNOTE-151). Transl Oncol (2019) 12:828–35. doi: 10.1016/j.tranon.2019.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sheng X, Yan X, Chi Z, Si L, Cui C, Tang B, et al. Overall Survival and Biomarker Analysis of a Phase Ib Combination Study of Toripalimab, a Humanized IgG4 mAb Against Programmed Death-1 (PD-1) With Axitinib in Patients With Metastatic Mucosal Melanoma. J Clin Oncol (2020) 38:10007. doi: 10.1200/JCO.2020.38.15_suppl.10007 [DOI] [Google Scholar]
- 31. Cui C, Lian B, Si L, Chi Z, Sheng X, Kong Y, et al. Adjuvant Anti-PD-1 Ab (Toripalimab) Versus High-Dose IFN-A2b in Resected Mucosal Melanoma: A Phase Randomized Trial. J Clin Oncol (2021) 39:9573. doi: 10.1200/JCO.2021.39.15_suppl.9573 [DOI] [Google Scholar]
- 32. Lacal PM, Graziani G. Therapeutic Implication of Vascular Endothelial Growth Factor Receptor-1 (VEGFR-1) Targeting in Cancer Cells and Tumor Microenvironment by Competitive and Non-Competitive Inhibitors. Pharmacol Res (2018) 136:97–107. doi: 10.1016/j.phrs.2018.08.023 [DOI] [PubMed] [Google Scholar]
- 33. Yasuda S, Sho M, Yamato I, Yoshiji H, Wakatsuki K, Nishiwada S, et al. Simultaneous Blockade of Programmed Death 1 and Vascular Endothelial Growth Factor Receptor 2 (VEGFR2) Induces Synergistic Anti-Tumour Effect In Vivo . Clin Exp Immunol (2013) 172:500–6. doi: 10.1111/cei.12069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. U.S. Food and Drug Administration . Search Orphan Drug Designations and Approvals (2021). Available at: https://www.accessdata.fda.gov/scripts/opdlisting/oopd/listResult.cfm (Accessed November 18, 2021).
- 35. Cui C, Wang X, Lian B, Si L, Chi Z, Sheng X, et al. A Phase 2 Clinical Trial of Neoadjuvant Anti-PD-1 Ab (Toripalimab) Plus Axitinib in Resectable Mucosal Melanoma. J Clin Oncol (2021) 39:9512. doi: 10.1200/JCO.2021.39.15_suppl.9512 [DOI] [PubMed] [Google Scholar]
- 36. U.S. National Library of Medicine . Clinical Trials on Toripalimab (2021). Available at: https://clinicaltrials.gov/ct2/results?cond=&term=toripalimab&cntry=&state=&city=&dist (Accessed November 30, 2021).
- 37. Wang X, Cui C, Si L, Li C, Dai J, Mao L, et al. A Phase Ib Clinical Trial of Neoadjuvant OrienX010, an Oncolytic Virus, in Combination With Toripalimab in Patients With Resectable Stage IIIb to Stage IVM1a Acral Melanoma. J Clin Oncol (2021) 39:9570. doi: 10.1200/JCO.2021.39.15_suppl.9570 [DOI] [Google Scholar]
- 38. Guo J, Cui C, Wang X, Lian B, Yin S, Cong Y, et al. A Phase 1b Clinical Trial of Anti-PD-1 Ab (Toripalimab) Plus Intralesional Injection of OrienX010 in Stage Melanoma With Liver Metastases. J Clin Oncol (2021) 39:9559. doi: 10.1200/JCO.2021.39.15_suppl.9559 [DOI] [Google Scholar]
- 39. Si L, Sheng X, Mao L, Li C, Wang X, Bai X, et al. A Phase II Study of Vorolanib (CM082) in Combination With Toripalimab (JS001) in Patients With Advanced Mucosal Melanoma. J Clin Oncol (2020) 38:10040. doi: 10.1200/JCO.2020.38.15_suppl.10040 [DOI] [Google Scholar]
- 40. Wang Z, Ying J, Xu J, Yuan P, Duan J, Bai H, et al. Safety, Antitumor Activity, and Pharmacokinetics of Toripalimab, a Programmed Cell Death 1 Inhibitor, in Patients With Advanced Non–Small Cell Lung Cancer. JAMA Netw Open (2020) 3:e2013770. doi: 10.1001/jamanetworkopen.2020.13770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ruan Z, Zhu B, Wang YN, Liu B, Li M, Xia G, et al. Real-World Outcomes of Toripalimab (JS001) in Advanced Non-Small Cell Lung Cancer: A Multicenter Retrospective Study. J Clin Oncol (2021) 39:e21193. doi: 10.1200/JCO.2021.39.15_suppl.e21193 [DOI] [Google Scholar]
- 42. Gettinger SN, Horn L, Gandhi L, Spigel DR, Antonia SJ, Rizvi NA, et al. Overall Survival and Long-Term Safety of Nivolumab (Anti-Programmed Death 1 Antibody, BMS-936558, ONO-4538) in Patients With Previously Treated Advanced Non-Small-Cell Lung Cancer. J Clin Oncol (2015) 33:2004–12. doi: 10.1200/JCO.2014.58.3708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, et al. Pembrolizumab for the Treatment of Non-Small-Cell Lung Cancer. N Engl J Med (2015) 372:2018–28. doi: 10.1056/NEJMoa1501824 [DOI] [PubMed] [Google Scholar]
- 44. Ren X, Zhang W. A Single-Arm Phase Ib Study of Multiple Target Cytotoxic T-Lymphocyte (MCTL) in Combination With Toripalimab as Second-Line Therapy in Advanced Non-Small Cell Lung Cancer (NSCLC). J Clin Oncol (2021) 39:2535. doi: 10.1200/JCO.2021.39.15_suppl.2535 34043455 [DOI] [Google Scholar]
- 45. National Comprehensive Cancer Network . NCCN Guidelines, Non Small Cell Lung Cancer (2021). Available at: https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1450 (Accessed November 30, 2021).
- 46. Jiang T, Wang P, Zhang J, Zhao Y, Zhou J, Fan Y, et al. Toripalimab Plus Chemotherapy as Second-Line Treatment in Previously EGFR-TKI Treated Patients With EGFR-Mutant-Advanced NSCLC: A Multicenter Phase-II Trial. Signal Transduct Target Ther (2021) 6:355. doi: 10.1038/s41392-021-00751-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Jiang M, Wang Y, Zhang X. 1310p - Toripalimab Combined With Chemotherapy as Second-Line Treatment of Advanced Non-Small Cell Lung Cancer (NSCLC): A Single Center Retrospective Study. Ann Oncol (2021) 32:S949–1039. doi: 10.1016/annonc/annonc729 [DOI] [Google Scholar]
- 48. U.S. National Library of Medicine . Search Results for Nct03924050 (2021). Available at: https://clinicaltrials.gov/ct2/results?cond=&term=NCT03924050&cntry=&state=&city=&dist (Accessed November 30, 2021).
- 49. Zhao J, Li J, Chen H, Yang X, Zhong J, Zhuo M, et al. A Phase II Study of Vorolanib in Combination With Toripalimab in Patients With Non-Small Cell Lung Cancer. J Clin Oncol (2021) 39:e21053. doi: 10.1200/JCO.2021.39.15_suppl.e21053 [DOI] [Google Scholar]
- 50. Zhao Z, Yang C, Chen S, Yu H, Lin Y, Lin Y, et al. Phase 2 Trial of Neoadjuvant Toripalimab With Chemotherapy for Resectable Stage III Non-Small-Cell Lung Cancer. Oncoimmunology (2021) 10(1):1996000. doi: 10.1080/2162402X.2021.1996000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zhu X, Sun L, Song N, Sun F, Yang Y, Duan L, et al. 1176p - Neoadjuvant PD-1 Inhibitor (Toripalimab) Plus Chemotherapy in Patients With Potentially Resectable NSCLC: An Open-Label, Single-Arm, Phase II Trial. Ann Oncol (2021) 32:S939–48. doi: 10.1016/annonc/annonc728 [DOI] [Google Scholar]
- 52. Forde PM, Chaft JE, Smith KN, Anagnostou V, Cottrell TR, Hellmann MD, et al. Neoadjuvant PD-1 Blockade in Resectable Lung Cancer. N Engl J Med (2018) 378:1976–86. doi: 10.1056/NEJMoa1716078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ben Nun A, Golan N, Ofek E, Urban D, Kamer I, Simansky D, et al. Neoadjuvant Pembrolizumab (Pembro) for Early Stage Non-Small Cell Lung Cancer (NSCLC): Initial Report of a Phase I Study, MK3475-223. Ann Oncol (2018) 29:i486. doi: 10.1093/annonc/mdy290.011 [DOI] [Google Scholar]
- 54. Bar J, Urban D, Ofek E, Ackerstein A, Redinsky I, Golan N, et al. Neoadjuvant Pembrolizumab (Pembro) for Early Stage Non-Small Cell Lung Cancer (NSCLC): Updated Report of a Phase I Study, MK3475-223. J Clin Oncol (2019) 37:8534. doi: 10.1200/JCO.2019.37.15_suppl.8534 [DOI] [Google Scholar]
- 55. Shen D, Wang J, Wu J, Chen S, Li J, Liu J, et al. Neoadjuvant Pembrolizumab With Chemotherapy for the Treatment of Stage IIB–IIIB Resectable Lung Squamous Cell Carcinoma. J Thorac Dis (2021) 13:1760–8. doi: 10.21037/jtd-21-103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sun J, Xie L, Feng Y, Qin S. 1320tip - The Efficacy and Safety of Stereotactic Body Radiation Therapy (SBRT) Plus Toripalimab With or Without Bevacizumab as Second-Line Treatment for Advanced Non-Small Cell Lung Cancer (NSCLC): A Prospective, Multicenter, Open-Label, Phase II Study. Ann Oncol (2021) 32:S949–1039. doi: 10.1016/annonc/annonc729 [DOI] [Google Scholar]
- 57. U.S. National Library of Medicine . Studies Registered for: Toripalimab and Non Small Cell Lung Cancer (2021). Available at: https://clinicaltrials.gov/ct2/results?cond=non+small+cell+lung+cancer&term=toripalimab&cntry=&state=&city=&dist (Accessed November 30, 2021).
- 58. National Comprehensive Cancer Network . NCCN Guidelines, Esophageal and Esophagogastric Junction Cancers (2021). Available at: https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1433 (Accessed November 30, 2021).
- 59. Liu D, Zhang Q, Zhu J, Qian T, Yin R, Fan Z, et al. Phase-II Study of Toripalimab Combined With Neoadjuvant Chemotherapy for the Treatment of Resectable Esophageal Squamous Cell Carcinoma. J Clin Oncol (2021) 39:e16029. doi: 10.1200/JCO.2021.39.15_suppl.e16029 [DOI] [Google Scholar]
- 60. Zhao L, Xing W, Yang Y, Zhang Y, Ma B, Fu X, et al. The Sequence of Chemotherapy and Anti-PD-1 Antibody Influence the Efficacy of Neoadjuvant Immunochemotherapy in Locally Advanced Esophageal Squamous Cell Cancer: A Phase II Study. J Clin Oncol (2021) 39:4051. doi: 10.1200/JCO.2021.39.15_suppl.4051 [DOI] [Google Scholar]
- 61. Xu R, Wang F, Cui C, Yao J, Zhang Y, Wang G, et al. 1373mo - JUPITER-06: A Randomized, Double-Blind, Phase III Study of Toripalimab Versus Placebo in Combination With First-Line Chemotherapy for Treatment Naive Advanced or Metastatic Esophageal Squamous Cell Carcinoma (ESCC). Ann Oncol (2021) 32:S1040–75. doi: 10.1016/annonc/annonc708 [DOI] [Google Scholar]
- 62. Sun J, Shen L, Shah MA, Enzinger P, Adenis A, Doi T, et al. Pembrolizumab Plus Chemotherapy Versus Chemotherapy Alone for First-Line Treatment of Advanced Oesophageal Cancer (KEYNOTE-590): A Randomised, Placebo-Controlled, Phase 3 Study. Lancet (2021) 398:759–71. doi: 10.1016/S0140-6736(21)01234-4 [DOI] [PubMed] [Google Scholar]
- 63. Xi M, Zhu Y, Li Q, Zhao L, Yang Y, Hu Y, et al. The Efficacy and Safety of Toripalimab Combined With Definitive Chemoradiotherapy for Patients With Locally Advanced Esophageal Squamous Cell Carcinoma. J Clin Oncol (2021) 39:e16043. doi: 10.1200/JCO.2021.39.15_suppl.e16043 [DOI] [Google Scholar]
- 64. Wang F, Wei XL, Wang FH, Xu N, Shen L, Dai GH, et al. Safety, Efficacy and Tumor Mutational Burden as a Biomarker of Overall Survival Benefit in Chemo-Refractory Gastric Cancer Treated With Toripalimab, a PD-1 Antibody in Phase Ib/II Clinical Trial NCT02915432. Ann Oncol (2019) 30:1479–86. doi: 10.1093/annonc/mdz197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Fuchs CS, Doi T, Jang RW, Muro K, Satoh T, Machado M, et al. Safety and Efficacy of Pembrolizumab Monotherapy in Patients With Previously Treated Advanced Gastric and Gastroesophageal Junction Cancer. JAMA Oncol (2018) 4:e180013. doi: 10.1001/jamaoncol.2018.0013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Muro K, Chung HC, Shankaran V, Geva R, Catenacci D, Gupta S, et al. Pembrolizumab for Patients With PD-L1-Positive Advanced Gastric Cancer (KEYNOTE-012): A Multicentre, Open-Label, Phase 1b Trial. Lancet Oncol (2016) 17:717–26. doi: 10.1016/S1470-2045(16)00175-3 [DOI] [PubMed] [Google Scholar]
- 67. Zhang T, Yu D, Wang J, Liu J, Ma H, Lin Z, et al. 1403p - Toripalimab Combined With Nab-Paclitaxel/Docetaxel as Second-Line Treatment in Patients With Advanced Gastric Cancer: Preliminary Results From a Single-Arm, Open-Label Phase II Trial. Ann Oncol (2021) 32:S1040–75. doi: 10.1016/annonc/annonc70 [DOI] [Google Scholar]
- 68. Yang Y, Yan J, Liu J, Li S, Gao S, Wei J, et al. Phase II Study of Combining Immunotherapy and Pulsed Low Dose Rate Radiotherapy for Abdominal Metastasis of Gastric Cancer. J Clin Oncol (2021) 39:e16099. doi: 10.1200/JCO.2021.39.15_suppl.e16099 [DOI] [Google Scholar]
- 69. Boku N, Ryu MH, Kato K, Chung HC, Minashi K, Lee KW, et al. Safety and Efficacy of Nivolumab in Combination With S-1/Capecitabine Plus Oxaliplatin in Patients With Previously Untreated, Unresectable, Advanced, or Recurrent Gastric/Gastroesophageal Junction Cancer: Interim Results of a Randomized, Phase II Trial (ATTRACTION-4). Ann Oncol (2019) 30:250–8. doi: 10.1093/annonc/mdy540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Bang Y, Kang Y, Catenacci DV, Muro K, Fuchs CS, Geva R, et al. Pembrolizumab Alone or in Combination With Chemotherapy as First-Line Therapy for Patients With Advanced Gastric or Gastroesophageal Junction Adenocarcinoma: Results From the Phase II Nonrandomized KEYNOTE-059 Study. Gastric Cancer (2019) 22:828–37. doi: 10.1007/s10120-018-00909-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Shen L, Lu M, Chen Z, Ye F, Zhang Y, Li Z, et al. Phase II Trial of Surufatinib Plus Toripalimab for Disease Progression After First-Line Chemotherapy With Platinum and Fluoropyrimidine in Advanced Gastric or Gastroesophageal Junction Adenocarcinoma. J Clin Oncol (2021) 39:e16040. doi: 10.1200/JCO.2021.39.15_suppl.e16040 [DOI] [Google Scholar]
- 72. National Comprehensive Cancer Network . NCCN Guidelines, Esophageal and Esophagogastric Junction Cancers (2021). Available at: https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1433 (Accessed November 30, 2021).
- 73. Li H, Deng J, Ge S, Zang F, Zhang L, Ren P, et al. Phase II Study of Perioperative Toripalimab in Combination With FLOT in Patients With Locally Advanced Resectable Gastric/Gastroesophageal Junction (GEJ) Adenocarcinoma. J Clin Oncol (2021) 39:4050. doi: 10.1200/JCO.2021.39.15_suppl.4050 [DOI] [Google Scholar]
- 74. Lin X, Xia Q, Han T, Zhuo M, Yang H, Qiu X, et al. Efficacy and Safety of Toripalimab Combination With SOX Regimen as a First-Line Treatment in Patients With Unresectable Locally Advanced or Recurrent/Metastatic Gastric/Gastroesophageal Junction Cancer: Preliminary Data From a Single-Armed, Exploratory Study. J Clin Oncol (2021) 39:e16015. doi: 10.1200/JCO.2021.39.15_suppl.e16015 [DOI] [Google Scholar]
- 75. Zelenay S, van der Veen AG, Böttcher JP, Snelgrove KJ, Rogers N, Acton SE, et al. Cyclooxygenase-Dependent Tumor Growth Through Evasion of Immunity. Cell (2015) 162:1257–70. doi: 10.1016/j.cell.2015.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Pelly VS, Moeini A, Roelofsen LM, Bonavita E, Bell CR, Hutton C, et al. Anti-Inflammatory Drugs Remodel the Tumor Immune Environment to Enhance Immune Checkpoint Blockade Efficacy. Cancer Discovery (2021) 11:2602–19. doi: 10.1158/2159-8290.CD-20-1815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Hu H, Kang L, Zhang J, Wu Z, Wang H, Huang M, et al. Neoadjuvant PD-1 Blockade With Toripalimab, With or Without Celecoxib, in Mismatch Repair-Deficient or Microsatellite Instability-High, Locally Advanced, Colorectal Cancer (PICC): A Single-Centre, Parallel-Group, Non-Comparative, Randomised, Phase 2 Trial. Lancet Gastroenterol Hepatol (2022) 7:38–48. doi: 10.1016/S2468-1253(21)00348-4 [DOI] [PubMed] [Google Scholar]
- 78. Loupakis F, Antonuzzo L, Bachet J, Kuan F, Macarulla T, Pietrantonio F, et al. Practical Considerations in the Use of Regorafenib in Metastatic Colorectal Cancer. Ther Adv Med Oncol (2020) 12:386357190. doi: 10.1177/1758835920956862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Wang F, He M, Yao Y, Zhao X, Wang Z, Jin Y, et al. Regorafenib Plus Toripalimab in Patients With Metastatic Colorectal Cancer: A Phase Ib/II Clinical Trial and Gut Microbiome Analysis. Cell Rep Med (2021) 2:100383. doi: 10.1016/j.xcrm.2021.100383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Yu W, Tao Q, Zhang Y, Yi F, Feng L. Efficacy and Safety of Regorafenib Combined With Toripalimab in the Third-Line and Beyond Treatment of Advanced Colorectal Cancer. J Oncol (2021) 2021:1–7. doi: 10.1155/2021/9959946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Fukuoka S, Hara H, Takahashi N, Kojima T, Kawazoe A, Asayama M, et al. Regorafenib Plus Nivolumab in Patients With Advanced Gastric or Colorectal Cancer: An Open-Label, Dose-Escalation, and Dose-Expansion Phase Ib Trial (REGONIVO, Epoc1603). J Clin Oncol (2020) 38:2053–61. doi: 10.1200/JCO.19.03296 [DOI] [PubMed] [Google Scholar]
- 82. Shi L, Zhou C, Long X, Li H, Chen C, Peng C, et al. 949p - Thermal Ablation Plus Toripalimab in Patients With Advanced Hepatocellular Carcinoma: Phase I Results From a Multicenter, Open-Label, Controlled Phase I/II Trial (IR11330). Ann Oncol (2021) 32:S818–28. doi: 10.1016/j.annonc.2021.08.169 [DOI] [Google Scholar]
- 83. Lyu N, Kong Y, Li X, Mu L, Deng H, Chen H, et al. Ablation Reboots the Response in Advanced Hepatocellular Carcinoma With Stable or Atypical Response During PD-1 Therapy: A Proof-Of-Concept Study. Front Oncol (2020) 10:580241. doi: 10.3389/fonc.2020.580241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, et al. Lenvatinib Versus Sorafenib in First-Line Treatment of Patients With Unresectable Hepatocellular Carcinoma: A Randomised Phase 3 Non-Inferiority Trial. Lancet (2018) 391:1163–73. doi: 10.1016/S0140-6736(18)30207-1 [DOI] [PubMed] [Google Scholar]
- 85. Lyu N, Kong Y, Mu L, Lin Y, Li J, Liu Y, et al. Hepatic Arterial Infusion of Oxaliplatin Plus Fluorouracil/Leucovorin vs. Sorafenib for Advanced Hepatocellular Carcinoma. J Hepatol (2018) 69:60–9. doi: 10.1016/j.jhep.2018.02.008 [DOI] [PubMed] [Google Scholar]
- 86. He M, Liang R, Zhao Y, Xu Y, Chen H, Zhou Y, et al. Lenvatinib, Toripalimab, Plus Hepatic Arterial Infusion Chemotherapy Versus Lenvatinib Alone for Advanced Hepatocellular Carcinoma. Ther Adv Med Oncol (2021) 13:386371531. doi: 10.1177/17588359211002720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. He M, Ming S, Lai Z, Li Q. A Phase II Trial of Lenvatinib Plus Toripalimab and Hepatic Arterial Infusion Chemotherapy as a First-Line Treatment for Advanced Hepatocellular Carcinoma (LTHAIC Study). J Clin Oncol (2021) 39:4083. doi: 10.1200/JCO.2021.39.15_suppl.4083 [DOI] [Google Scholar]
- 88. Sun Y, Zhou A, Zhang W, Jiang Z, Chen B, Zhao J, et al. Anlotinib in the Treatment of Advanced Hepatocellular Carcinoma: An Open-Label Phase II Study (ALTER-0802 Study). Hepatol Int (2021) 15:621–9. doi: 10.1007/s12072-021-10171-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Lin H, Ma J, Zhuo M, Zhang C, Luo J, Zhuang X, et al. Preliminary Results of the Phase II ALTER-H003 Trial: Anlotinib Plus Toripalimab as a First-Line Treatment for Patients With Unresectable Hepatocellular Carcinoma. J Clin Oncol (2021) 39:314. doi: 10.1200/JCO.2021.39.3_suppl.314 [DOI] [Google Scholar]
- 90. Lin H, Ma J, Zhuo M, Zhang C, Luo J, Zhuang X, et al. Updated Results of the Phase II ALTER-H003 Trial: Anlotinib Plus Toripalimab as a First-Line Treatment for Patients With Unresectable Hepatocellular Carcinoma. J Clin Oncol (2021) 39:e16130. doi: 10.1200/JCO.2021.39.15_suppl.e16130 [DOI] [Google Scholar]
- 91. Park K, Kim K, Park S, Chang H. Comparison of Gemcitabine Plus Cisplatin Versus Capecitabine Plus Cisplatin as First-Line Chemotherapy for Advanced Biliary Tract Cancer. Asia-Pac J Clin Onco (2017) 13:13–20. doi: 10.1111/ajco.12592 [DOI] [PubMed] [Google Scholar]
- 92. Liu L, Li W, Yu Y, Guo X, Xu X, Wang Y, et al. 53p - Toripalimab With Chemotherapy as First-Line Treatment for Advanced Biliary Tract Tumors: A Preliminary Analysis of Safety and Efficacy of an Open-Label Phase II Clinical Study. Ann Oncol (2020) 31:S260–73. doi: 10.1016/annonc/annonc259 [DOI] [Google Scholar]
- 93. Li W, Yu Y, Xu X, Guo X, Wang Y, Li Q, et al. Toripalimab With Chemotherapy as First-Line Treatment for Advanced Biliary Tract Tumors: Update Analytic Results of an Open-Label Phase II Clinical Study (JS001-ZS-Bc001). J Clin Oncol (2021) 39:e16170. doi: 10.1200/JCO.2021.39.15_suppl.e16170 [DOI] [Google Scholar]
- 94. Ueno M, Ikeda M, Morizane C, Kobayashi S, Ohno I, Kondo S, et al. Nivolumab Alone or in Combination With Cisplatin Plus Gemcitabine in Japanese Patients With Unresectable or Recurrent Biliary Tract Cancer: A Non-Randomised, Multicentre, Open-Label, Phase 1 Study. Lancet Gastroenterol Hepatol (2019) 4:611. doi: 10.1016/S2468-1253(19)30086-X [DOI] [PubMed] [Google Scholar]
- 95. Jian Z, Fan J, Shi G, Huang X, Wu D, Liang F, et al. Lenvatinib Plus Toripalimab as First-Line Treatment for Advanced Intrahepatic Cholangiocarcinoma: A Single-Arm, Phase 2 Trial. J Clin Oncol (2021) 39:4099. doi: 10.1200/JCO.2021.39.15_suppl.4099 [DOI] [Google Scholar]
- 96. Jian Z, Fan J, Shi G, Huang X, Wu D, Yang G, et al. Gemox Chemotherapy in Combination With Anti-PD1 Antibody Toripalimab and Lenvatinib as First-Line Treatment for Advanced Intrahepatic Cholangiocarcinoma: A Phase 2 Clinical Trial. J Clin Oncol (2021) 39:4094. doi: 10.1200/JCO.2021.39.15_suppl.4094 [DOI] [Google Scholar]
- 97. Lu M, Zhang P, Zhang Y, Li Z, Gong J, Li J, et al. Efficacy, Safety and Biomarkers of Toripalimab in Patients With Recurrent or Metastatic Neuroendocrine Neoplasms: A Multiple-Center Phase Ib Trial. Clin Cancer Res (2020) 626(10):2337–45. doi: 10.1158/1078-0432.CCR-19-4000 [DOI] [PubMed] [Google Scholar]
- 98. Shen L, Yu X, Lu M, Zhang X, Cheng Y, Zhang Y, et al. Surufatinib in Combination With Toripalimab in Patients With Advanced Neuroendocrine Carcinoma: Results From a Multicenter, Open-Label, Single-Arm, Phase II Trial. J Clin Oncol (2021) 39:e16199. doi: 10.1200/JCO.2021.39.15_suppl.e16199 [DOI] [Google Scholar]
- 99. Vijayvergia N, Dasari A, Deng M, Litwin S, Al-Toubah T, Alpaugh RK, et al. Pembrolizumab Monotherapy in Patients With Previously Treated Metastatic High-Grade Neuroendocrine Neoplasms: Joint Analysis of Two Prospective, non-Randomised Trials. Br J Cancer (2020) 122:1309–14. doi: 10.1038/s41416-020-0775-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Oberg K, Modlin IM, De Herder W, Pavel M, Klimstra D, Frilling A, et al. Consensus on Biomarkers for Neuroendocrine Tumour Disease. Lancet Oncol (2015) 16:e435–46. doi: 10.1016/S1470-2045(15)00186-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Wang F, Wei X, Feng J, Li Q, Xu N, Hu X, et al. Efficacy, Safety, and Correlative Biomarkers of Toripalimab in Previously Treated Recurrent or Metastatic Nasopharyngeal Carcinoma: A Phase II Clinical Trial (POLARIS-02). J Clin Oncol (2021) 38:O2002712. doi: 10.1200/JCO.20.02712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Ma B, Lim WT, Goh BC, Hui EP, Lo KW, Pettinger A, et al. Antitumor Activity of Nivolumab in Recurrent and Metastatic Nasopharyngeal Carcinoma: An International, Multicenter Study of the Mayo Clinic Phase 2 Consortium (NCI-9742). J Clin Oncol (2018) 36:1412–8. doi: 10.1200/JCO.2017.77.0388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Hsu C, Lee SH, Ejadi S, Even C, Cohen RB, Le Tourneau C, et al. Safety and Antitumor Activity of Pembrolizumab in Patients With Programmed Death-Ligand 1-Positive Nasopharyngeal Carcinoma: Results of the KEYNOTE-028 Study. J Clin Oncol (2017) 35:4050–6. doi: 10.1200/JCO.2017.73.3675 [DOI] [PubMed] [Google Scholar]
- 104. Chen YP, Chan A, Le QT, Blanchard P, Sun Y, Ma J. Nasopharyngeal Carcinoma. Lancet (2019) 394:64–80. doi: 10.1016/S0140-6736(19)30956-0 [DOI] [PubMed] [Google Scholar]
- 105. Hua Y, You R, Wang Z, Huang P, Lin M, Ouyang Y, et al. Toripalimab Plus Intensity-Modulated Radiotherapy for Recurrent Nasopharyngeal Carcinoma: An Open-Label Single-Arm, Phase II Trial. J Immunother Cancer (2021) 9:e3290. doi: 10.1136/jitc-2021-003290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Zhang L, Huang Y, Hong S, Yang Y, Yu G, Jia J, et al. Gemcitabine Plus Cisplatin Versus Fluorouracil Plus Cisplatin in Recurrent or Metastatic Nasopharyngeal Carcinoma: A Multicentre, Randomised, Open-Label, Phase 3 Trial. Lancet (2016) 388:1883–92. doi: 10.1016/S0140-6736(16)31388-5 [DOI] [PubMed] [Google Scholar]
- 107. Mai HQ, Chen QY, Chen D, Hu C, Yang K, Wen J, et al. Toripalimab or Placebo Plus Chemotherapy as First-Line Treatment in Advanced Nasopharyngeal Carcinoma: A Multicenter Randomized Phase 3 Trial. Nat Med (2021) 27:1536–43. doi: 10.1038/s41591-021-01444-0 [DOI] [PubMed] [Google Scholar]
- 108. Sheng X, Chen H, Hu B, Yao X, Liu Z, Yao X, et al. Safety, Efficacy and Biomarker Analysis of Toripalimab in Patients With Previously Treated Advanced Urothelial Carcinoma: Results From a Multicenter Phase II Trial POLARIS-03. Clin Cancer Res (2021) clincanres.2210.2021. doi: 10.1158/1078-0432.CCR-21-2210 [DOI] [PubMed] [Google Scholar]
- 109. Zhou L, Xu H, Yan X, Chi Z, Cui C, Si L, et al. RC48-ADC Combined With Toripalimab, an Anti-PD-1 Monoclonal Antibody (Ab), in Patients With Locally Advanced or Metastatic Urothelial Carcinoma (UC): Preliminary Results of a Phase Ib/II Study. J Clin Oncol (2021) 39:4534. doi: 10.1200/JCO.2021.39.15_suppl.4534 [DOI] [Google Scholar]
- 110. Sharma P, Callahan MK, Bono P, Kim J, Spiliopoulou P, Calvo E, et al. Nivolumab Monotherapy in Recurrent Metastatic Urothelial Carcinoma (CheckMate 032): A Multicentre, Open-Label, Two-Stage, Multi-Arm, Phase 1/2 Trial. Lancet Oncol (2016) 17:1590–8. doi: 10.1016/S1470-2045(16)30496-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Balar AV, Castellano D, O'Donnell PH, Grivas P, Vuky J, Powles T, et al. First-Line Pembrolizumab in Cisplatin-Ineligible Patients With Locally Advanced and Unresectable or Metastatic Urothelial Cancer (KEYNOTE-052): A Multicentre, Single-Arm, Phase 2 Study. Lancet Oncol (2017) 18:1483–92. doi: 10.1016/S1470-2045(17)30616-2 [DOI] [PubMed] [Google Scholar]
- 112. Armand P, Engert A, Younes A, Fanale M, Santoro A, Zinzani PL, et al. Nivolumab for Relapsed/Refractory Classic Hodgkin Lymphoma After Failure of Autologous Hematopoietic Cell Transplantation: Extended Follow-Up of the Multicohort Single-Arm Phase II CheckMate 205 Trial. J Clin Oncol (2018) 36:1428–39. doi: 10.1200/JCO.2017.76.0793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Chen R, Zinzani PL, Fanale MA, Armand P, Johnson NA, Brice P, et al. Phase II Study of the Efficacy and Safety of Pembrolizumab for Relapsed/Refractory Classic Hodgkin Lymphoma. J Clin Oncol (2017) 35:2125–32. doi: 10.1200/JCO.2016.72.1316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Han H, An X, Yan R, Guo S, Xue C, Liu T, et al. Immune Checkpoint Inhibitor Plus Anti-EGFR Target Therapy Plus Chemotherapy in Patients With Locally Advanced Penile Squamous Cell Carcinoma. J Clin Oncol (2021) 39:e17016. doi: 10.1200/JCO.2021.39.15_suppl.e17016 [DOI] [Google Scholar]
- 115. Xiao W, Yuan Y, Wang S, Cai P, Chen B, Zhang R, et al. Neoadjuvant PD-1 Blockade Combined With Chemotherapy Followed by Concurrent Immunoradiotherapy for Locally Advanced Anal Canal Squamous Carcinoma Patients: Antitumor Efficacy, Safety and Biomarker. J Clin Oncol (2021) 39:e15500. doi: 10.1200/JCO.2021.39.15_suppl.e15500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Cheng K, Lv W, Li X, Tian B, Cao D. Toripalimab With Nab-Paclitaxel/Gemcitabine as First-Line Treatment for Advanced Pancreatic Adenocarcinoma: Updated Results of a Single-Arm, Open-Label, Phase Ib/II Clinical Study. J Clin Oncol (2021) 39:e16213. doi: 10.1200/JCO.2021.39.15_suppl.e16213 [DOI] [Google Scholar]
- 117. Luo H, Jian D, Feng Y, Zhong L, Chen Q, Guan W, et al. Efficacy and Safety of Toripalimab With Anlotinib and Chemotherapy as First-Line Therapy in Patients With Extensive-Stage Small-Cell Lung Cancer: Preliminary Results of an Open-Label, Single-Arm, Phase II Study. J Clin Oncol (2021) 39:e20570. doi: 10.1200/JCO.2021.39.15_suppl.e20570 [DOI] [Google Scholar]
- 118. Coward J, Abed A, Nagrial A, Markman B. Phase I Open-Label, Dose Escalation of YH003, an Anti-CD40 Monoclonal Antibody in Combination With Toripalimab (Anti-PD-1 mAb) in Patients With Advanced Solid Tumors. J Clin Oncol (2021) 39:2580. doi: 10.1200/JCO.2021.39.15_suppl.2580 [DOI] [Google Scholar]
- 119. Ganju V, Cooper A, Gao B, Wilkinson K. A First-in-Human Phase I Dose Escalation of YH001, an Anti-CTLA-4 Monoclonal Antibody (mAb) in Combination With Toripalimab (Anti-PD-1 mAb) in Patients With Advanced Solid Tumors. J Clin Oncol (2021) 39:2577. doi: 10.1200/JCO.2021.39.15_suppl.2577 [DOI] [Google Scholar]
- 120. Cheng Y, Song Y, Wang C, Li X, Li Y, Zhang C. 782p - A Retrospective Study of Toripalimab Combined With Concurrent Chemoradiotherapy in Patients With Recurrent/Advanced Cervical Cancer. Ann Oncol (2021) 32:S725–72. doi: 10.1016/annonc/annonc703 [DOI] [Google Scholar]
- 121. Liu Z, Liu C, Yao W, Gao S, Wang J, Zhang P, et al. Efficacy and Safety of Toripalimab Combined With Doxorubicin as First-Line Treatment for Metastatic Soft Tissue Sarcomas. Anti-Cancer Drugs (2021) 32:962–8. doi: 10.1097/CAD.0000000000001088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Dou S, Li R, Zhang L, Jiang W, Ye L, Zhu G. Adjuvant Toripalimab or Combined With S-1 in Recurrent, Previously Irradiated Head and Neck Squamous Cell Carcinoma Treated With Salvage Surgery: A Phase II Clinical Trial (The RePASS Study). J Clin Oncol (2021) 39:6039. doi: 10.1200/JCO.2021.39.15_suppl.6039 [DOI] [Google Scholar]
- 123. National Institute of Health . Common Terminology Criteria for Adverse Events (CTCAE) (2020). Available at: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm (Accessed November 30, 2021).
- 124. Brown EG, Wood L, Wood S. The Medical Dictionary for Regulatory Activities (MedDRA). Drug Saf (1999) 20:109–17. doi: 10.2165/00002018-199920020-00002 [DOI] [PubMed] [Google Scholar]
- 125. Xu C, Chen YP, Du XJ, Liu JQ, Huang CL, Chen L, et al. Comparative Safety of Immune Checkpoint Inhibitors in Cancer: Systematic Review and Network Meta-Analysis. BMJ (2018) 363:k4226. doi: 10.1136/bmj.k4226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Wang DY, Salem J, Cohen JV, Chandra S, Menzer C, Ye F, et al. Fatal Toxic Effects Associated With Immune Checkpoint Inhibitors. JAMA Oncol (2018) 4:1721. doi: 10.1001/jamaoncol.2018.3923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, Activity, and Immune Correlates of Anti-PD-1 Antibody in Cancer. N Engl J Med (2012) 366:2443–54. doi: 10.1056/NEJMoa1200690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Angelo SP D, Larkin J, Sosman JA, Lebbé C, Brady B, Neyns B, et al. Efficacy and Safety of Nivolumab Alone or in Combination With Ipilimumab in Patients With Mucosal Melanoma: A Pooled Analysis. J Clin Oncol (2017) 35:226–35. doi: 10.1200/JCO.2016.67.9258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Song F, Xiong T, Parekh-Bhurke S, Loke YK, Sutton AJ, Eastwood AJ, et al. Inconsistency Between Direct and Indirect Comparisons of Competing Interventions: Meta-Epidemiological Study. BMJ (2011) 343:d4909. doi: 10.1136/bmj.d4909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Bucher HC, Guyatt GH, Griffith LE, Walter SD. The Results of Direct and Indirect Treatment Comparisons in Meta-Analysis of Randomized Controlled Trials. J Clin Epidemiol (1997) 50:683–91. doi: 10.1016/s0895-4356(97)00049-8 [DOI] [PubMed] [Google Scholar]
- 131. Song F, Altman DG, Glenny AM, Deeks JJ. Validity of Indirect Comparison for Estimating Efficacy of Competing Interventions: Empirical Evidence From Published Meta-Analyses. BMJ (2003) 326:472. doi: 10.1136/bmj.326.7387.472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. National Healthcare Security Administration . Public Consultation of Work Plan for the Adjustment of the National Insurance Drug List in 2020 (Draft for Comments) (2020). Available at: http://www.nhsa.gov.cn/art/2020/8/3/art_48_3394.html?from=timeline (Accessed November 30, 2021).
- 133. National Heathcare Security Administration . The Notice of Issuing <National Drug Catalogue for Basic Medical Insurance, Work-Related Injury Insurance and Maternity Insurance (2020)>, by National Health Security Administration and Ministry of Human Resouraces and Social Security of the People's Republic of China (2020). Available at: http://www.nhsa.gov.cn/art/2020/12/28/art_37_4220.html (Accessed November 30, 2021).
- 134. Central People's Government of the People's Republic of China . National Medical Insurance Catalogue (2020). Available at: http://www.gov.cn/zhengce/zhengceku/2020-12/28/5574062/files/4159212adf854117b62d0e91d445a4e2.pdf (Accessed November 30, 2021).