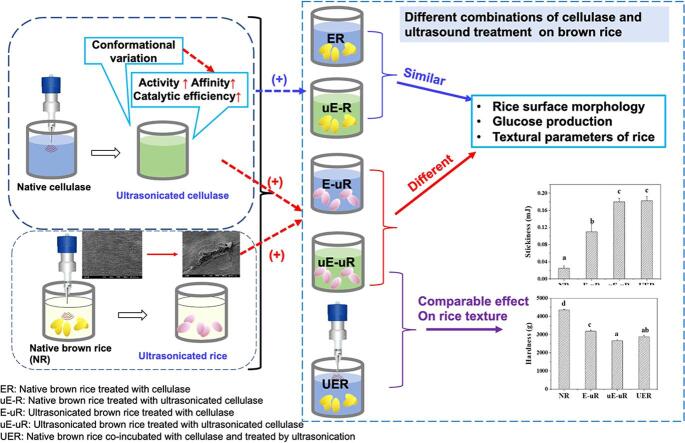
Graphical abstract
Keywords: Brown rice, Ultrasound, Cellulase, Hydrolysis effect, Bran disruption, Textural quality
Highlights
-
•
Ultrasound increases cellulase activity and alters enzyme conformation.
-
•
Ultrasonicated and untreated cellulase have comparable effect on brown rice texture.
-
•
Ultrasonicated cellulase exhibits higher hydrolysis activity than control.
-
•
Ultrasound assisted cellulase treatment is effective strategy to modify brown rice.
Abstract
Brown rice is nutritionally superior to polished white rice, as it maintains a large content of external bran that involves a series of bioactive compounds. However, the presence of bran also restricts water diffusion and results in adverse quality of brown rice. In this work, ultrasound conditions were optimized for cellulase to improve its hydrolysis effect on rice bran, and combinations of enzymatic and ultrasound treatment in different manners were conducted on brown rice, to improve the textural attributes. The results showed significant improvements in the catalytic activity and efficiency of cellulase after ultrasonication at the optimal intensity of 1.67 W cm−3 and duration of 30 min, with the conformational variation of cellulase observed from the fluorescence spectra and circular dichroism (CD). Despite the enhanced activity of ultrasonicated cellulase, it leaded to a similar rice surface morphology and a comparable amount of released glucose, and equivalent textural parameters of brown rice treated by native cellulase. However, for the pre-sonicated brown rice, the ultrasonicated cellulase showed a significantly higher hydrolysis capacity than the untreated enzyme, suggesting the important influence of ruptured bran surface on amplifying the hydrolysis effect of cellulase. Compared to the successive ultrasound stimulation on both cellulase and brown rice, ultrasound-assisted cellulase treatment on brown rice produced less glucose from rice bran, but induced similar textural properties of brown rice, possibly resulting from the simultaneously promoting effect of ultrasonication on cellulase and water diffusion. Ultimately, this study highlighted that the mild rice surface rupture is a crucial factor to display the promoted hydrolysis effect of ultrasonicated cellulase on brown rice. Ultrasound-assisted cellulase treatment potentially provides an effective strategy to improve the edible quality of brown rice.
1. Introduction
Rice, as an important staple food in Asian countries, provides essential energy and nutrition for almost half of the world's population [1], [2]. With the improving recognition and pursuits of consumers for healthy food, brown rice receives a great deal of attention. Brown rice is achieved by primarily removing the husk of rice grains, thus maintaining bran layer to a large extent. Rice bran contains dietary fibres, vitamins, minerals and functional antioxidants, and plays an important role in preventing diabetes, obesity and cardiovascular disease [3], [4]. However, rice bran also restricts water uptake during cooking, and leads to a lower palatability and harder texture of brown rice than polished white rice [5]. The undesirable sensory quality of brown rice is the main reason that limits its widespread consumption.
In order to improve the edible qualities and minimize losses of rice bran layer, various biological approaches and processing technologies have been developed and optimized[3], [6], [7], [8], [9], [10]. Among these processing methods, enzymatic treatment was widely adopted, as it enables specific transformation of targeted molecules and thus engineers food structure for obtaining novel functional and phytochemical properties [11]. According to the previous study by Das, Banerjee and Bal (2008), cellulase and xylanase were applied to whole grain brown rice before cooking, which resulted in the easier diffusion of water into the bran layer, and thus the reduced cooking time and improved edible quality [7]. Furthermore, Liu, Y. et al have also showed the improved water-holding capacity, oil-holding capacity, swelling capacity, cholesterol absorption capacity, and glucose adsorption capacity of rice bran dietary fiber after cellulase modification [12]. Despite the positive effects of enzymatic treatment on brown rice, the essential step of soaking prior to enzymatic hydrolysis accounts for nearly 90% of the total time [13], which is not acceptable in practice.
Ultrasound treatment, characterized by its eco-friendly and high efficiency properties, is another well-established technique on food processing [14], [15]. During ultrasound, ultrasonic waves generate an alternating compression and rarefaction in aqueous media in the form of cavitation bubbles, and a series of physicochemical impacts subsequently result from the rupture of these bubbles [16]. It has been proven that ultrasound treatment altered textural properties of brown rice [17]. The brown rice after soaking for 8 h and ultrasonication at 50 °C for 60 min possessed a similar hardness of cooked milled rice, and a higher content of bioactive compounds [17].
In recent decades, the application of ultrasound combining with enzymatic treatment is emerging and promising, for regulating enzymatic transformation and promoting food attributes [14]. Zhang, X et al have used ultrasound with a following cellulase incubation for brown rice processing, and resulted in the reduced cooking time, and improved odor and flavor of the cooked rice [18]. As mild ultrasound has been reported to enhance enzyme activity [19], [20], ultrasonicated cellulase and its combination with ultrasound need to be taken into consideration, to maximize its effect on brown rice processing. Additionally, the influences of ultrasound on cellulase and its resulting effects on brown rice is unclear and remains to be clarified.
In this research, ultrasound intensity and duration were firstly optimized for a food-grade cellulase, with the catalytic activity, kinetics parameters and structure changes of cellulase after ultrasound being analysed. Furthermore, ultrasonicated or native cellulase combining with ultrasound treatment was applied to brown rice in different ways, with the corresponding effect on brown rice being investigated at molecular and whole-grain levels. It was hypothesized that proper ultrasound treatment on cellulase leads to a promoted activity and conformational variation, and thus the ultrasonicated cellulase results in more bran hydrolysis and better rice texture than the native enzyme, in terms of brown rice with/without additional ultrasound treatment.
2. Materials and methods
2.1. Materials
Commercial Japonica brown rice was purchased from a local supermarket. Cellulase (Celluclast® 1.5 L) purchased from Novozymes (China) Biotech Co. (Tianjin, China) was sourced from Trichoderma reesei, and complies with the recommended purity specifications for food-grade enzymes. It was diluted with 0.1 M sodium acetate buffer (pH 4.5) into a 2% (v/v) cellulase solution, which was then used for the following enzyme activity assay and brown rice treatment. Other chemicals were analytical grades.
2.2. Ultrasound treatment for cellulase
The cellulase solution was processed using an Ultrasound horn (Biron Instrument Manufacturing Co., Shanghai, China) with probe tip diameter of 1 cm, at a frequency of 20 kHz and maximum rated power of 1000 W. The amplitudes probe was submerged 1 cm below the 150 mL of cellulase solution at 40 °C, and samples were treated at 5 s ON and 5 s OFF pulse. The effect of ultrasound intensity on cellulase activity was evaluated at 0, 0.33, 0.67, 1, 1.33, 1.67, 2, 2.33, 2.67, 3, 3.33 W·cm−3, respectively, and the effect of treatment duration (0–60 min) was also determined. Hence, the optimal conditions of ultrasound treatment were selected and used for the following measurements.
2.3. Investigations for cellulase after ultrasonication
2.3.1. Cellulase activity assay
The catalytic activity of ultrasonicated cellulase was analysed using a cellulase assay kit (K-CellG5-2 V, Megazyme, Shanghai, China), as the method provided by the manufacturer [21]. Briefly, the cellulase solution was incubated with the CellG5 solution containing β-glucosidase and blocked 4-nitrophenyl-β-D-cellopentaoside (BPNPG5) at 40 °C for 3 min. BPNPG5 was specifically hydrolysed by cellulase, and its hydrolysate was subsequently degraded by β-glucosidase present in the substrate mixture, with free 4-nitrophenol (pNP) being released and detected at the absorbance of 400 nm. The cellulase activity (one unit) was defined as 1 μmol of pNP released from CellG5 per minute.
The untreated cellulase was used as a control for all analyses related to the ultrasonicated cellulase in this study.
2.3.2. Determination of cellulase kinetics parameters
The kinetics parameters of the ultrasonicated cellulase were determined by measuring the enzyme activities at different concentrations (1, 2, 4, 6, 8, 10 mg·mL−1 H2O) of carboxymethyl cellulose (CMC). The maximal reaction rate (Vmax), Michaelis constant (Km), catalytic constant (Kcat), and specificity constant (Kcat/Km) were calculated from Lineweaver–Burk plots.
2.3.3. Fluorescence spectroscopy
Fluorescence analysis was implemented for ultrasonicated cellulase, using a fluorescence spectrophotometer (Model: SC-05, Edinburgh Instruments Ltd, UK) [22]. Enzyme samples were measured at room temperature, with excitation wavelength at 280 nm (slit width = 5 nm), emission wavelength of 300–500 nm (slit width = 5 nm), and scanning speed of 1200 nm·s−1.
2.3.4. Circular dichroism (CD)
CD spectra of ultrasound-treated cellulase were documented by a spectropolarimeter (model MOS-500, French Biological Company, Noble, France) at ambient temperature. A quartz cell with a path length of 0.1 cm was applied under constant nitrogen flow to determine the spectra from 190 to 250 nm far-UV region. An accumulation of three scans with 30 nm/min speed was conducted with 0.1 nm as band-width. The scan data were expressed as the mean residue ellipticity [θ] (deg·cm2·dmol−1), and the secondary structure content of cellulase was analysed by the DICHROWEB database [19].
2.4. Investigation of effects of various treatments on brown rice quality
2.4.1. Enzymatic and ultrasound treatments on brown rice
Native brown rice was incubated with the pre-sonicated or untreated cellulase solution in the ratio of 1:3 (w/v) at 40 °C for 30 min, to examine the effect of enzymatic treatment on rice quality improvement. As a comparison, native rice was mixed with deionized water (1:3, w/v) and subjected to ultrasonication at 1.67 W cm−3 intensity for 30 min. According to the outcomes of individual treatments on brown rice, the ultrasonicated or untreated cellulase was further incubated with the ultrasonicated brown rice, to test the joint effect of enzymatic hydrolysis and ultrasound on rice processing. Moreover, brown rice and cellulase solution were co-incubated in the ratio of 1:3 (w/v) at 40 °C with ultrasonication for 30 min, to study the effect of ultrasound-assisted enzymatic treatment.
After each treatment, while the rice solution was filtered and collected, the treated rice was rinsed with deionized water three times, dried in room temperature for 48 h, and then stored in a desiccator for the following various analyses.
2.4.2. Scan electron microscopy (SEM)
SEM was used to examine the changes on surface morphology of brown rice after different treatments. The structure of rice samples was observed at 100 × and 250 × resolution, respectively, with a scanning electron microscope (JSM-6700F, Global Micronics Corporation., Japan) at an accelerating voltage of 5 kV.
2.4.3. Chemical analyses for filtrates of brown rice after treatments
Glucose Determination. The glucose amount of the native and treated brown rice filtrates were estimated, using a D-glucose assay kit (K-GLUC, Megazyme, Shanghai, China). Briefly, 1 mL of sample solution was incubated with 3 mL of a reagent containing glucose oxidase and peroxidase at 40 °C for 20 min, with the absorbance of resultant mixture being measured at 510 nm, from where the glucose content was quantified.
Size exclusion chromatography (SEC). The collected filtrate was freeze-dried for SEC analysis, to characterise the molecular size distribution of constituents released from brown rice after treatments. Prior to the SEC, the freeze-dried samples were subjected to protease hydrolysis at 37 °C for 30 mins, followed by ethanol precipitation twice, with saccharide materials being collected and analysed. A Shimadzu SIL-20A system (Shimadzu China, Beijing) equipped with GRAM 30 and 3000 columns (PSS China, Suzhou) and a refractive index (RI) detector (RID-10A, Shimadzu China, Beijing) were adopted, with Pullulan standards with known peak weights for calibration, as detailed elsewhere [23], [24]. The molecular size distribution of polysaccharides was plotted as SEC weight distributions wbr(logRh) against the hydrodynamic radius Rh.
2.4.4. Measurement for textual properties of cooked brown rice
Cooking process was performed for brown rice grains with various treatments, as previously described [10], [25]. In brief, the rice samples were washed with deionized water three times, followed by the rice-to-water ratio being adjusted to 1:1.6 (w/w) and steam-cooking for 30 min. Subsequently, the cooked rices were cooled to room temperature and subjected to texture profile analysis (TPA) using a Texture analyzer (Brookfield Engineering Laboratories, MA, US) with a 35 mm cylindrical probe, as the method described by Zhang et al. (2019)[26].
2.5. Statistical analysis
All results were presented as mean ± standard deviation (SD). The results were statistically analysed using SPSS 20.0 software. One-way analysis of variance (ANOVA) and Student's t-test was used to compare significant differences. P values less than 0.05 were deemed significant.
3. Results and discussions
3.1. The effect of ultrasound intensity and duration on cellulase activity
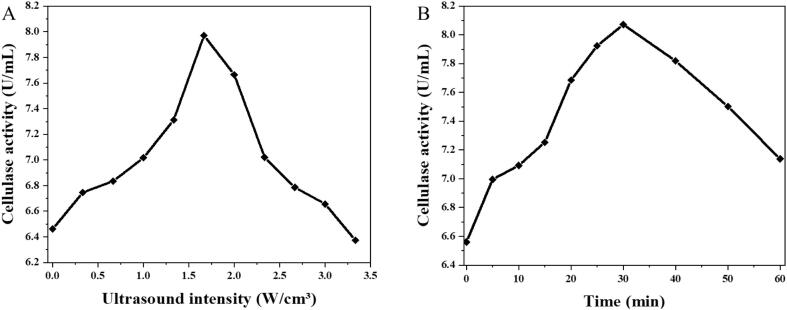
The effect of ultrasound on enzyme activity is highly dependent on intensity and duration [27]. It has been reported that low-intensity and short-duration ultrasound irradiation increased cellulase activity [22]. In this work, the cellulase activity was promoted significantly with the increasing intensity, reaching approximately 8.0 U/mL at 1.67 W·cm−3 (Fig. 1-A). However, the cellulase activity dropped with further enhancement of ultrasound intensity that was higher than 2.0 W·cm−3. Additionally, the activity of cellulase treated with intensity over 3.67 W·cm−3 was lower than that of untreated enzyme, suggesting the occurrence of enzyme deactivation [22]. In a similar tendency, the cellulase activity increased with ultrasound duration initially, reaching 8.07 U·mL−1 at 30 min, but declined gradually thereafter (Fig. 1-B). Hence, the ultrasound conditions were optimized as 1.67 W cm−3 of intensity and 30 min of duration for the following cellulase and brown rice treatment.
Fig. 1.
Ultrasound effects on cellulase activity. A-Effect of ultrasound intensity and on cellulase activity (for 30 min), B-Effect of ultrasound duration and on cellulase activity (at 1.67 W·cm−3).
3.2. Alteration of cellulase kinetics and enzyme structure induced by ultrasonication
To explore the influences of ultrasound treatment on the enzyme kinetics of cellulase, Lineweaver–Burk plot was carried out (Supplementary material, Fig. S1), with the related parameters being calculated from it and shown in Table 1. The kinetics parameters primarily include maximum rate of reaction (Vmax), Michaelis constant (Km), catalytic constant (Kcat), and specificity constant (Kcat/Km). Compared to the native cellulase, both Vmax (1.75 ± 0.05 mM min−1) and Kcat (249.89 ± 7.63 min) of treated enzymes were statistically improved, with a decreased Km value (43.48 ± 1.18 mM). Additionally, the catalytic efficiency of the ultrasonicated cellulase was further evaluated as Kcat/Km. A noted increase of Kcat/Km were observed for cellulase treated by ultrasound treatment, estimated 19.05% higher than that of untreated enzyme, suggesting the enhanced specificity of cellulase to cellulose after ultrasonication. Overall, the ultrasound treatment on cellulase accelerated the rate of enzymatic reaction and facilitated the affinity of the enzyme to cellulose, with the catalytic efficiency of enzyme being promoted significantly [28], [29].
Table 1.
Enzymatic kinetic parameters of untreated and ultrasound treatment of cellulase.
| Samples | Vmax(mM min−1) | Km(mM) | Kcat × 10-3(min) | Kcat/ Km × 10-3(mM−1·min−1) |
|---|---|---|---|---|
| Control | 1.68 ± 0.01 | 49.65 ± 1.11 | 239.74 ± 0.96 | 4.83 ± 0.13 |
| With ultrasound treatment | 1.75 ± 0.05 | 43.48 ± 1.18 | 249.89 ± 7.63 | 5.75 ± 0.02 |
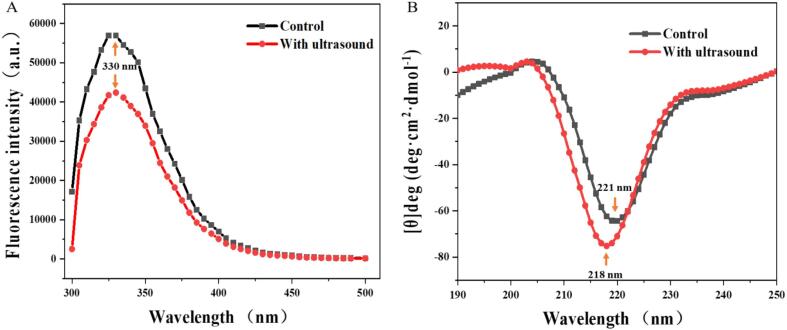
The intrinsic fluorescence was implemented for ultrasound-treated and untreated enzymes, to determine the conformational changes of cellulase. Aromatic amino acid residues of a protein, particularly tryptophan, are highly sensitive to the polarity alteration of microenvironments and the tertiary structure changes of protein, resulting in a fluorescence wavelength shift and variation of the fluorescence intensity. As shown in Fig. 2-A, the emission fluorescence intensity of control and treated cellulase (excited at 300 nm) had the maximum value (5.70 × 104 a.u.) at wavelength of 330 nm in the fluorescence spectra. In contrast, the fluorescence intensity of cellulase treated by ultrasonication (4.24 × 104 a.u.) was remarkably lower than that of control. Additionally, there was no wavelength shift of fluorescence spectra detected, possibly due to the reduction of the previous tryptophan on the surface. It indicated that the structural of cellulase treated by ultrasound was unfolded with the exposure of more internal areas in cellulase[30].
Fig. 2.
Effects of ultrasonic treatment on structure. Intrinsic fluorescence spectra (A) and CD spectra (B) for the untreated and ultrasound-treated cellulase.
The changes in secondary structure of ultrasonicated cellulase were further characterised by CD spectra (Fig. 2-B), from where the relative content of α-helix, β-chain and random coil were estimated. Compared to the control cellulase, a blue shift of the maximum emission wavelength from 221 nm to 218 nm was observed in the in the CD spectra of ultrasonicated cellulase. After ultrasound treatment, the α-helix of cellulase reduced by 73.8%, the β-chain and random coil increased by 8.0% and 4.1%, respectively (Supplementary material, Table S1). The decreased helix regions of cellulase by ultrasound treatment was suggested to be associated with the promoted cellulase activity. The breakdown of protein helix regions and improved enzyme activity caused by ultrasonication have also been observed in the other studies [31], [32], [33].
3.3. Comparable effect of ultrasonicated and native cellulase on intact brown rice
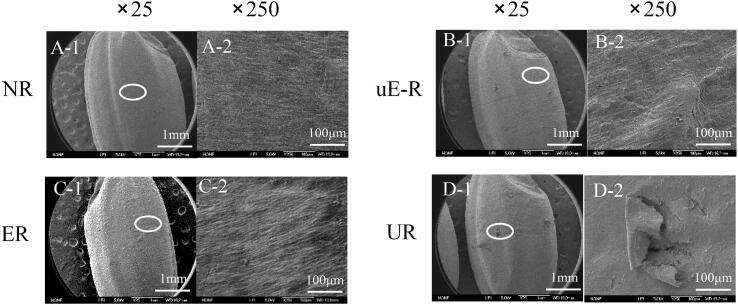
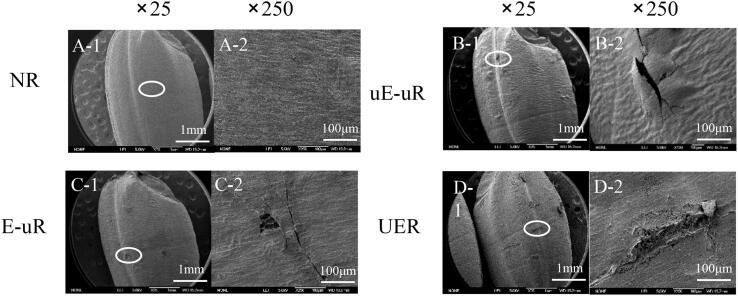
To assess influences of the ultrasonicated cellulase on brown rice processing, the microstructure of rice surface after enzyme hydrolysis was visualised under the Scanning Electron Microscopy (Fig. 3). While the control brown rice (NR) had a solid compact morphology (Fig. 3-A), the brown rice treated with cellulase (ER and u-ER) resulted in the cortical loose structure, in particular, slight folds observed on the rice surface of u-ER (Fig. 3-B&C). This might be explained by that the hydrolysis of the non-starch polysaccharides in rice bran by cellulase, which potentially caused the disruption of its epidermal structure, facilitated water penetration, and thus leaded to the loose surface of rice.
Fig. 3.
SEM image of brown rice. A-untreated brown rice (NR), B-brown rice treated with cellulase (ER), C-brown rice treated with pre-sonicated cellulase (uE-R), D-ultrasonicated rice (UR).
According to the widely accepted mechanism for enzymatic cellulose hydrolysis, cellulase performed functions of endoglucanases, exoglucanases, and β-glucosidases on cellulose and β-(1,4)-glucan chains, with glucose as the major product [34], [35]. Interestingly, the glucose concentration of filtrate from ER and u-ER were 0.319 ± 0.015 mg/mL and 0.336 ± 0.012 mg/mL (Table 2), respectively, suggesting a similar hydrolysis extent of rice cell walls hydrolysed by native and sonicated cellulase. Given the comparable hydrolysis outcome of ER and uE-R samples, it was proposed that the effect of stimulated cellulase activity on brown rice was potentially restricted by the limited accessibility of intact rice cell wall matrix.
Table 2.
The glucose concentration of filtrate from treated rice.
| Samples | Glucose concentration (mg/mL) |
|---|---|
| NR | 0.022 ± 0.002a |
| UR | 0.159 ± 0.007b |
| ER | 0.319 ± 0.015c |
| uE-R | 0.336 ± 0.012c |
| E-uR | 0.515 ± 0.001e |
| uE-uR | 0.610 ± 0.002f |
| UER | 0.420 ± 0.038d |
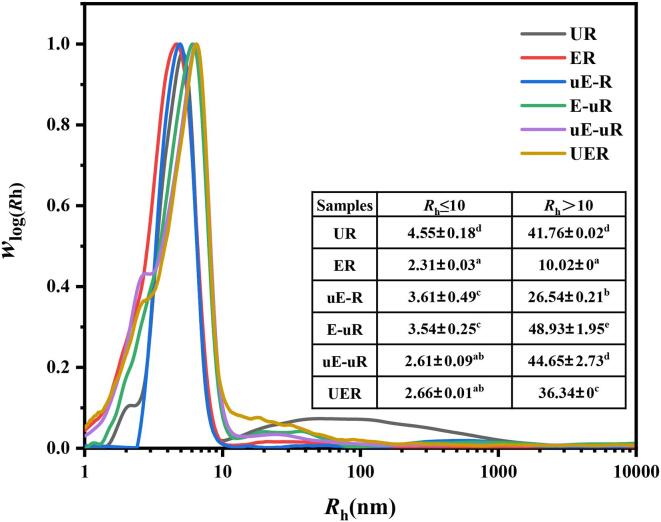
To characterise the constituents released from the treated rice, the SEC plot were shown in Fig. 4 and the corresponding representative SEC distributions of each sample were calculated. The molecular sizes of all rice samples were primarily distributed from 1 nm to 1000 nm. For ER, most components of the filtrates ranged from 1 nm to 10 nm, with a small proportion of molecules distributed from 10 nm to 100 nm. According to the specificity of cellulase on β-(1, 4)-glucose chains, the SEC peak (1–10 nm) of ER samples was inferred to represent glucose and possibly some oligosaccharides. For the small amount of components from 10 nm to100 nm, it was probably small amylopectin molecules that leached out from porosities of rice cell wall matrix caused by enzymatic treatment [36], [37]. The SEC profile of uE-R basically resembled to that of ER, with only its slightly higher Rh. of the peak from 1 nm to 10 nm than ER.
Fig. 4.
Molecular weight distributions of the constituents released from the treated rice.
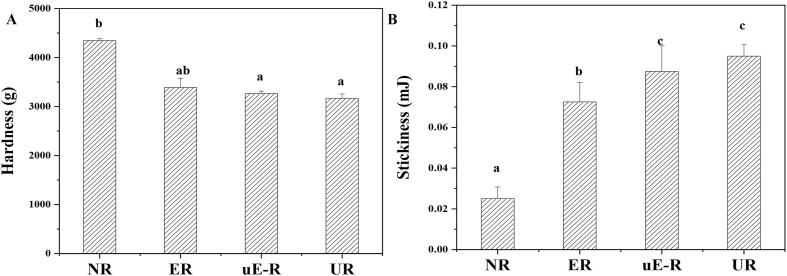
The texture of cooked rice is a crucial factor affecting consumer acceptance and preference. To evaluate the further influences of cellulase hydrolysis on rice textural quality traits, the hardness and stickiness parameters of cooked rice after enzymatic treatments were further measured. The native brown rice (NR) possessed the highest hardness (4348.0 g) and lowest stickiness (0.03 mJ) across all samples (Fig. 5). With the cellulase treatment on rice prior to cooking, a significant decrease in hardness and a drastic increase in stickiness were observed for ER and uE-R samples, leading to be a more desirable eating quality trait of rice for South-East Asian consumers [38]. It indicated that the enzymatic hydrolysis on the bran layer facilitated water penetration and starch leaching out on the rice surface, thus altered the rice stickiness and hardness [18], [39]. Notably, there was no significant difference between the hardness or stickiness of rice pre-hydrolysed by native and sonicated cellulase, implying their similar impacts on rice internal structure.
Fig. 5.
Textural properties of brown rice. A-Hardness of brown rice without treatment (NR), hydrolysed by cellulase (ER) and sonicated cellulase (uE-R) as well as with ultrasound treatment (UR), B-Stickiness of brown rice NR, ER, uE-R as well as UR.
Ultrasonication treatment was performed on brown rice, as a comparison of enzymatic treatment. As seen in Fig. 3-D, notable cracks and fissures on the rice surface were caused by acoustic cavitation, similar to the previously reported results [40], [41]. Only 15.92 ± 0.71% of free glucose was detected in the UR leachate, which was significantly lower than that of rice hydrolysed by cellulase, but remarkedly higher than that from NR (0.022 ± 0.002 mg/mL). It implicated the sonochemical destruction of rice polysaccharides [42], [43].Moreover, apart from the released glucose, some small molecular starches were proposed to leach out from rice by ultrasonication. As the UR filtrates in Fig. 4, the peak from 10 nm to 100 nm was obviously in a larger proportion than that of ER and uE-R. Additionally, the SEC weighted distributions of UR were similar to the molecular sizes of whole-grain starch, where the peak with Rh ∼1–10 nm was inferred as small amylose molecules and the peak with Rh ∼10–100 nm deemed as the small amylopectin molecules [36]. In terms of the textural properties, UR showed a lower hardness (3164.9 g) and a higher stickiness (0.03 mJ) than the native brown rice (Fig. 5). It might be ascribed to that the cracks and fissures on the surface of rice caused by ultrasonication accelerated water diffusion into the internal endosperm during the cooking process, and subsequently leaded to amylose leaching [44].
Given the remarked impacts of ultrasonication on surface disruption and textural changes of brown rice, the combination of ultrasound and cellulase treatment was further conducted on ultrasonicated rice, aiming to achieve further improvement in the textural quality of brown rice and explore the influence of bran integrity on the effectiveness of cellulase treatment.
3.4. Ultrasonicated cellulase superior to native cellulase on modification of the sonicated brown rice
The pre-sonicated brown rice with cellulase treatment possessed the morphological features of both UR and ER (Fig. 3), with apparent cracks on the loose surface of rice, as shown in the SEM images (Fig. 6). All processes involving ultrasound treatment on brown rice were shown to impart structural integrity of rice and caused the exposure of cellular content. While the sonicated rice treated with native cellulase (E-uR) had a relatively dense shell, the ultrasonicated brown rice treated with pre-treated cellulase (uE-uR) had a more loosened hull with remarked folds. This indicated that the effect of the sonicated cellulase with enhanced hydrolytic activity on rice was magnified and reflected due to the destruction of the rice cell wall matrix caused by ultrasonication, providing additional attachment points for cellulase. Moreover, different with sharp and clear cracks present on the surface of E-uR and uE-uR, ultrasound-assisted cellulase treatment on brown rice (UER) had a noticeable wider crack with erose and blur edges, reflecting distinct effects of ultrasound treatment on the co-culture of cellulase and brown rice grains.
Fig. 6.
SEM image of ultrasonicated brown rice. A-Untreated brown rice (NR), B-Ultrasonicated brown rice treated with cellulase (E-uR), C-Ultrasonicated brown rice treated with pre-sonicated cellulase (uE-uR), D-Bran rice co-cultured with cellulase (UER).
To determine the impact of ultrasonicated cellulase on hydrolysis of sonicated rice, the glucose concentration in filtrates of E-uR, uE-uR and UER were measured and compared. The glucose released from uE-uR (0.610 ± 0.002 mg/mL) were in a significantly larger amount than that from E-uR (0.515 ± 0.001 mg/mL), which was possibly attributed to the enhanced activity of cellulase after ultrasonication. In terms of UER, the glucose concentration was 0.420 ± 0.038 mg/mL, which was significantly higher than the that of uE-R (0.336 ± 0.012 mg/mL) and close to the sum of glucose of uE-R and UR (0.159 ± 0.007 mg/mL), indicating the joint promoting effect of ultrasonication on both cellulase and surface disruption of brown rice. However, UER produced statistically lesser amount of glucose, compared to E-uR and uE-uR samples, probably due to the relatively reduced surface area of UER exposed to cellulase.
For SEC weight distributions of the treated rice filtrates (Fig. 4), molecules of E-uR, uE-uR and UER were mostly in the range of 1–100 nm. Compared with E-uR, a small peak at 2 nm appeared in the SEC profile of uE-uR and UER, suggesting the increased fraction of small molecules hydrolysed from cellulose by the sonicated cellulase. While ultrasonication potentially leaded to starch leaching (UR) and release of large molecules from 10 to 100 nm, the reduced portion from 10 nm to 100 nm of E-uR, uE-uR and UER samples was possibly due to the existence of a small amount of amylase in food-grade cellulase (Supplementary materials, Fig. S2).
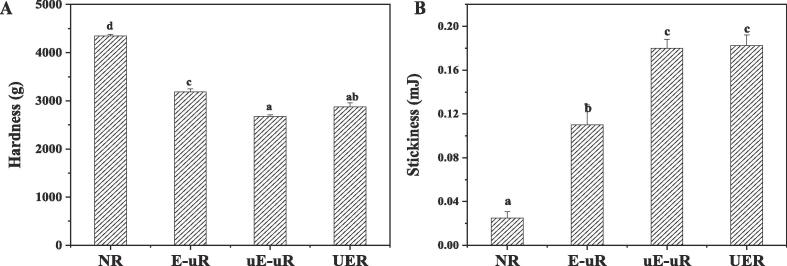
In the aspect of textural properties promotion of the ultrasonicated brown rice, the sonicated cellulase exhibited better effect than the untreated enzyme. The hardness of uE-uR (2674.5 g) was significantly lower than that of E-uR (3186.0 g), with its stickiness (0.18 mJ) remarkedly higher than that of E-uR (0.11 mJ) (Fig. 7), revealing a more favourable edible quality properties of uE-uR than E-uR. These textural differences were in accordance with the looser surface and more released glucose of uE-uR than that of E-uR (Fig. 7). While the surface disruption of brown rice by ultrasonication accelerated water transfer for both E-uR and uE-uR, the sonicated cellulase potentially degraded the rice cell wall polysaccharides to a larger extent than native cellulase, further facilitating water absorption and promoting the textural properties. Notably, although no crack was formed prior to cellulase treatment for the UER samples, the hardness and stickiness parameters of UER resembled that of uE-uR.It indicated that the ultrasound-assisted cellulase treatment on brown rice simultaneously contributed to improve cellulase activity and disrupt rice bran integrity, contributing to sufficient water diffusion and textural improvement as uE-uR. Combining the less surface rupture and glucose loss of UER than uE-uR, the similar textural properties of UER and uE-uR after cooking suggested that ultrasound-assisted cellulase treatment to be an effective strategy for brown rice modification. Compared to the previously reported plasma-treated brown rice [45], significantly more improvement in rice texture was achieved for rice with ultrasound-assisted cellulase treatment (UER), with the hardness being decreased by 33.8% and the stickiness being increased by 86.3%. This further suggests ultrasound-assisted cellulase treatment as a valuable approach for enhance brown rice textural quality [45]
Fig. 7.
Textural properties of ultrasonicated brown rice. A-Hardness of brown rice without treatment (NR), with pre-sonication treatment and hydrolysed by cellulase (E-uR), with pre-sonication treatment and hydrolysed by ultrasonicated cellulase(uE-uR), and with ultrasound-assisted cellulase treatment (UER), B-Stickiness of NR, E-uR. uE-uR and UER.
3.4.1. Implications for ultrasonicated enzyme treatment on brown rice processing
Proper intensity and duration of ultrasound treatment were of great importance to maximize the hydrolysis activity of cellulase. Under the optimum ultrasonic conditions, the catalytic kinetics of cellulase were markedly promoted, and enzymatically conformational changes were induced by the corresponding treatment, which were possibly related to the enhanced enzymatic activity. While cellulase treatment on brown rice resulted in significant changes of surface morphology, the increased cellulose hydrolysis extent, and improved textural properties, no distinct difference between the effects of native and sonicated cellulase was observed. The enhancement in cellulase activity after ultrasound treatment did not exert notably advantages over the untreated cellulase on brown rice processing. As ultrasonic modification is also a valuable approach widely used on food, the combined treatment of ultrasonication and cellulase was further conducted on brown rice. Surprisingly, compared to the native cellulase, ultrasonicated enzyme leaded to a higher amount of glucose released from the ultrasonicated rice that consequently displayed a more desirable textural trait. It was proposed to be associated with the exposure of internal bran layers of brown rice with pre-sonication, providing extra attachment points for cellulase and contributing to amplify the effect of improved cellulase activity. Furthermore, the ultrasound-assisted cellulase treatment tended to produce more glucose than the ultrasonicated cellulase treatment on native brown rice, pointing out the crucial role of sonication induced surface disruption in the enzymatic hydrolysis extent of brown rice bran. While the ultrasound-assisted cellulase treatment was less effective than the combined treatment of ultrasonication and cellulase on the cellulose degradation extent of rice bran, a comparable textural quality after rice cooking were achieved by both methods. Among all combined treatments of cellulase and ultrasonication on brown rice, the ultrasound-assisted cellulase treatment showed more practical prospect for brown rice processing due to its short treatment period and the resultant textural parameters of treated rice.
4. Conclusions
The ultrasound treatment of mild intensity and duration can enhance the cellulase activity, with the occurrence of improved kinetics parameters, as well as the tertiary and secondary structure alteration of cellulase. However, the sonicated cellulase with a promoted activity appears to hydrolyse brown rice to a similar extent to as the rice degraded by native enzyme, possibly due to the restricted surface layer of the intact bran. In contrast, for the pre-sonicated brown rice with fissures and cracks, the ultrasonicated cellulase exerted a better hydrolysis effect than the native one, leading to the increased textural quality of rice. In conclusions, to maximize the effect of enzymatic treatment on brown rice modification, the influence of bran integrity on enzymatic hydrolysis effectiveness is an essential factor for consideration, beyond purely improving enzyme activity by ultrasonication. Moreover, while the cellulase being co-cultured with brown rice and subjected to ultrasonication released less glucose than that from the pre-sonicated rice hydrolysed by ultrasonicated cellulase, both treatments lead to a similar textural quality trait of the cooked rice. Ultrasound-assisted cellulase treatment is potentially an efficient technique for whole grain processing, and the underlying mechanism is worthy to explore in the future study.
CRediT authorship contribution statement
Hongyan Li: Data curation, Validation, Supervision, Project administration. Minghao Xu: Methodology, Resources. Xu Yao: Methodology. Yangyang Wen: Methodology, Resources. Shiyi Lu: Paper draft review and revision. Jing Wang: Supervision, Project administration, Writing – review & editing. Baoguo Sun: Supervision, Project administration, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
This work was supported by National Natural Science Foundation of China (31901729, 32172236).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ultsonch.2022.105920.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Burlando B., Cornara L. Therapeutic properties of rice constituents and derivatives (Oryza sativa L.): A review update. Trends Food Sci. Technol. 2014;40(1):82–98. [Google Scholar]
- 2.Sen S., Chakraborty R., Kalita P. Rice-not just a staple food: a comprehensive review on its phytochemicals and therapeutic potential. Trends Food Sci. Technol. 2020;97:265–285. [Google Scholar]
- 3.Pang Y., Ahmed S., Xu Y., Beta T., Zhu Z., Shao Y., Bao J. Bound phenolic compounds and antioxidant properties of whole grain and bran of white, red and black rice. Food Chem. 2018;240:212–221. doi: 10.1016/j.foodchem.2017.07.095. [DOI] [PubMed] [Google Scholar]
- 4.Verma D.K., Srivastav P.P. Bioactive compounds of rice (Oryza sativa L.): review on paradigm and its potential benefit in human health. Trends Food Sci. Technol. 2020;97:355–365. [Google Scholar]
- 5.Mestres C., Ribeyre F., Pons B., Fallet V., Matencio F. Sensory texture of cooked rice is rather linked to chemical than to physical characteristics of raw grain. J. Cereal Sci. 2011;53:81–89. [Google Scholar]
- 6.Charoenthaikij P., Jangchud K., Jangchud A., Piyachomkwan K., Tungtrakul P., Prinyawiwatkul W. Germination conditions affect physicochemical properties of germinated brown rice flour. J. Food Sci. 2009;74(9):C658–C665. doi: 10.1111/j.1750-3841.2009.01345.x. [DOI] [PubMed] [Google Scholar]
- 7.Das M., Banerjee R., Bal S. Evaluation of physicochemical properties of enzyme treated brown rice (Part B) LWT-Food Sci. Technol. 2008;41:2092–2096. [Google Scholar]
- 8.Lee J.H., Woo K.S., Yong H.I., Jo C., Lee S.K., Lee B.W., Lee Y.-Y., Lee B., Kim H.-J. Physicochemical properties of brown rice according to the characteristics of cultivars treated with atmospheric pressure plasma. J. Cereal Sci. 2019;87:138–142. [Google Scholar]
- 9.Leethanapanich K., Mauromoustakos A., Wang Y.-J. Effect of soaking temperature on commingled rice properties. J. Cereal Sci. 2016;69:267–274. [Google Scholar]
- 10.Li H., Yan S., Yang L., Xu M., Ji J., Liu Y., Wang J., Sun B. High-pressure homogenization thinned starch paste and its application in improving the stickiness of cooked non-glutinous rice. LWT-Food Sci. Technol. 2020;131 [Google Scholar]
- 11.Chan C.K., Zeeb B., McClements D.J., Weiss J. Impact of laccase on the colour stability of structured oil-in-water emulsions. Food Res. Int. 2017;97:223–230. doi: 10.1016/j.foodres.2017.04.015. [DOI] [PubMed] [Google Scholar]
- 12.Liu Y., Zhang H., Yi C., Quan K., Lin B. Chemical composition, structure, physicochemical and functional properties of rice bran dietary fiber modified by cellulase treatment. Food Chem. 2021;342 doi: 10.1016/j.foodchem.2020.128352. [DOI] [PubMed] [Google Scholar]
- 13.Das M., Gupta S., Kapoor V., Banerjee R., Bal S. Enzymatic polishing of rice–A new processing technology, LWT-Food. Sci. Technol. 2008;41:2079–2084. [Google Scholar]
- 14.Singla M., Sit N. Application of ultrasound in combination with other technologies in food processing: A review. Ultrason. Sonochem. 2021;73:105506. doi: 10.1016/j.ultsonch.2021.105506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cui L., Pan Z., Yue T., Atungulu G.G., Berrios J. Effect of ultrasonic treatment of brown rice at different temperatures on cooking properties and quality. Cereal Chem. 2010;87:403–408. [Google Scholar]
- 16.Zinoviadou K.G., Galanakis C.M., Brnčić M., Grimi N., Boussetta N., Mota M.J., Saraiva J.A., Patras A., Tiwari B., Barba F.J. Fruit juice sonication: Implications on food safety and physicochemical and nutritional properties. Food Res. Int. 2015;77:743–752. [Google Scholar]
- 17.Park D.-J., Han J.-A. Quality controlling of brown rice by ultrasound treatment and its effect on isolated starch. Carbohydr. Polym. 2016;137:30–38. doi: 10.1016/j.carbpol.2015.10.045. [DOI] [PubMed] [Google Scholar]
- 18.Zhang X., Wang L., Cheng M., Wang R., Luo X., Li Y., Chen Z. Influence of ultrasonic enzyme treatment on the cooking and eating quality of brown rice. J. Cereal Sci. 2015;63:140–146. [Google Scholar]
- 19.Ma X., Wang W., Zou M., Ding T., Ye X., Liu D. Properties and structures of commercial polygalacturonase with ultrasound treatment: role of ultrasound in enzyme activation. RSC Adv. 2015;5:107591–107600. [Google Scholar]
- 20.Wang J., Cao Y., Sun B., Wang C., Mo Y. Effect of ultrasound on the activity of alliinase from fresh garlic. Ultrason. Sonochem. 2011;18:534–540. doi: 10.1016/j.ultsonch.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 21.McCleary B.V., McKie V., Draga A. Measurement of endo-1, 4-β-glucanase. Methods Enzymol. 2012;510:1–17. doi: 10.1016/B978-0-12-415931-0.00001-X. [DOI] [PubMed] [Google Scholar]
- 22.Subhedar P.B., Gogate P.R. Enhancing the activity of cellulase enzyme using ultrasonic irradiations. J. Mol. Catal. B Enzym. 2014;101:108–114. [Google Scholar]
- 23.Li H., Prakash S., Nicholson T.M., Fitzgerald M.A., Gilbert R.G. Instrumental measurement of cooked rice texture by dynamic rheological testing and its relation to the fine structure of rice starch. Carbohydr. Polym. 2016;146:253–263. doi: 10.1016/j.carbpol.2016.03.045. [DOI] [PubMed] [Google Scholar]
- 24.Mao H., Chen Z., Li J., Zhai X., Li H., Wen Y., Wang J., Sun B. Structural comparisons of pyrodextrins during thermal degradation process: The role of hydrochloric acid. Food Chem. 2021;349:129174. doi: 10.1016/j.foodchem.2021.129174. [DOI] [PubMed] [Google Scholar]
- 25.Li H., Yan S., Yang L., Xu M., Ji J., Mao H., Song Y., Wang J., Sun B. Starch gelatinization in the surface layer of rice grains is crucial in reducing the stickiness of parboiled rice. Food Chem. 2021;341 doi: 10.1016/j.foodchem.2020.128202. [DOI] [PubMed] [Google Scholar]
- 26.Zhang N., Wen Y., Yan S., Mao H., Lei N., Li H., Wang J., Chen H., Sun B. The increased stickiness of non-glutinous rice by alkali soaking and its molecular causes. Int. J. Biol. Macromol. 2019;135:394–399. doi: 10.1016/j.ijbiomac.2019.05.184. [DOI] [PubMed] [Google Scholar]
- 27.Paludo N., Alves J.S., Altmann C., Ayub M.A., Fernandez-Lafuente R., Rodrigues R.C. The combined use of ultrasound and molecular sieves improves the synthesis of ethyl butyrate catalyzed by immobilized Thermomyces lanuginosus lipase. Ultrason. Sonochem. 2015;22:89–94. doi: 10.1016/j.ultsonch.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 28.Qu W., Ma H., Jia J., He R., Luo L., Pan Z. Enzymolysis kinetics and activities of ACE inhibitory peptides from wheat germ protein prepared with SFP ultrasound-assisted processing. Ultrason. Sonochem. 2012;19:1021–1026. doi: 10.1016/j.ultsonch.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 29.Tian Z.M., Wan M.X., Wang S.P., Kang J.Q. Effects of ultrasound and additives on the function and structure of trypsin. Ultrason. Sonochem. 2004;11:399–404. doi: 10.1016/j.ultsonch.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 30.Xu B., Chen J., Chitrakar B., Li H., Wang J., Wei B., Zhou C., Ma H. Effects of flat sweep frequency and pulsed ultrasound on the activity, conformation and microstructure of mushroom polyphenol oxidase. Ultrason. Sonochem. 2022;82:105908. doi: 10.1016/j.ultsonch.2022.105908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jin J., Ma H., Wang W., Luo M., Wang B., Qu W., He R., Owusu J., Li Y. Effects and mechanism of ultrasound pretreatment on rapeseed protein enzymolysis. J. Sci. Food Agric. 2016;96:1159–1166. doi: 10.1002/jsfa.7198. [DOI] [PubMed] [Google Scholar]
- 32.Wang B., Meng T., Ma H., Zhang Y., Li Y., Jin J., Ye X. Mechanism study of dual-frequency ultrasound assisted enzymolysis on rapeseed protein by immobilized Alcalase. Ultrason. Sonochem. 2016;32:307–313. doi: 10.1016/j.ultsonch.2016.03.023. [DOI] [PubMed] [Google Scholar]
- 33.Jiang L., Wang J., Li Y., Wang Z., Liang J., Wang R., Chen Y., Ma W., Qi B., Zhang M. Effects of ultrasound on the structure and physical properties of black bean protein isolates. Food Res. Int. 2014;62:595–601. [Google Scholar]
- 34.Pasha I., Ahmad F. Monosaccharide composition and carbohydrates linkage identification in cereal brans using UHPLC/QqQ-DMRM-MS. J. Food Compos. Anal. 2021;96 [Google Scholar]
- 35.Rajnish K.N., Samuel M.S., John A., Datta S., Narendhar C., Balaji R., Jose S., Selvarajan E. Immobilization of cellulase enzymes on nano and micro-materials for breakdown of cellulose for biofuel production-a narrative review. Int. J. Biol. Macromol. 2021;182:1793–1802. doi: 10.1016/j.ijbiomac.2021.05.176. [DOI] [PubMed] [Google Scholar]
- 36.Li H., Fitzgerald M.A., Prakash S., Nicholson T.M., Gilbert R.G. The molecular structural features controlling stickiness in cooked rice, a major palatability determinant. Sci. Rep. 2017;7:1–12. doi: 10.1038/srep43713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li H., Prakash S., Nicholson T.M., Fitzgerald M.A., Gilbert R.G. The importance of amylose and amylopectin fine structure for textural properties of cooked rice grains. Food Chem. 2016;196:702–711. doi: 10.1016/j.foodchem.2015.09.112. [DOI] [PubMed] [Google Scholar]
- 38.Cuevas R.P., Pede V.O., McKinley J., Velarde O., Demont M., Yang D. Rice grain quality and consumer preferences: a case study of two rural towns in the Philippines. PLoS ONE. 2016;11(3) doi: 10.1371/journal.pone.0150345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li H., Xu M., Chen Z., Li J., Wen Y., Liu Y., Wang J. Effects of the degree of milling on starch leaching characteristics and its relation to rice stickiness. J. Cereal Sci. 2021;98:103163. doi: 10.1016/j.jcs.2021.103163. [DOI] [Google Scholar]
- 40.L.T.K. Dang, N. Therdthai, W.J.o.F.P. Ratphitagsanti, Preservation, Improvement of structure and cooking quality of brown rice using ultrasonic and enzymatic treatments, 42 (2018) e13814.
- 41.Mason T.J., Paniwnyk L., Lorimer J.P. The uses of ultrasound in food technology. Ultrason. Sonochem. 1996;3(3):S253–S260. [Google Scholar]
- 42.Xu B., Ren A., Chen J., Li H., Wei B., Wang J., Azam S.R., Bhandari B., Zhou C., Ma H. Effect of multi-mode dual-frequency ultrasound irradiation on the degradation of waxy corn starch in a gelatinized state. Food Hydrocolloids. 2021;113 [Google Scholar]
- 43.Czechowska-Biskup R., Rokita B., Lotfy S., Ulanski P., Rosiak J.M. Degradation of chitosan and starch by 360-kHz ultrasound. Carbohydr. Polym. 2005;60:175–184. [Google Scholar]
- 44.Bonto A.P., Tiozon R.N., Jr, Rojviriya C., Sreenivasulu N., Camacho D.H. Sonication increases the porosity of uncooked rice kernels affording softer textural properties, loss of intrinsic nutrients and increased uptake capacity during fortification. Ultrason. Sonochem. 2020;68 doi: 10.1016/j.ultsonch.2020.105234. [DOI] [PubMed] [Google Scholar]
- 45.Thirumdas R., Saragapani C., Ajinkya M., Deshmukh R., Annapure U. Influence of low pressure cold plasma on cooking and textural properties of brown rice. Innovative Food Sci. Emerg. Technol. 2016;37:53–60. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.