Abstract
Heart failure (HF) is the only cardiovascular disease with an ever increasing incidence. HF, through reduced functional capacity, frequent exacerbations of disease, and repeated hospitalizations, results in poorer quality of life, decreased work productivity, and significantly increased costs of the public health system. The main challenge in the treatment of HF is the availability of reliable prognostic models that would allow patients and doctors to develop realistic expectations about the prognosis and to choose the appropriate therapy and monitoring method. At this moment, there is a lack of universal parameters or scales on the basis of which we could easily capture the moment of deterioration of HF patients’ condition. Hence, it is crucial to identify such factors which at the same time will be widely available, cheap, and easy to use. We can find many studies showing different predictors of unfavorable outcome in HF patients: thorough assessment with echocardiography imaging, exercise testing (e.g., 6-min walk test, cardiopulmonary exercise testing), and biomarkers (e.g., N-terminal pro-brain type natriuretic peptide, high-sensitivity troponin T, galectin-3, high-sensitivity C-reactive protein). Some of them are very promising, but more research is needed to create a specific panel on the basis of which we will be able to assess HF patients. At this moment despite identification of many markers of adverse outcomes, clinical decision-making in HF is still predominantly based on a few basic parameters, such as the presence of HF symptoms (NYHA class), left ventricular ejection fraction, and QRS complex duration and morphology.
Keywords: Biomarker(s), Heart failure, Prognosis, Risk factor(s), Risk models, Risk prediction
Heart failure in numbers
Heart failure (HF) is a cardiovascular disease with an ever increasing incidence [1]. In the National Health and Nutrition Examination Survey (NHANES) data in USA, in 6.2 million Americans, HF was diagnosed in the period 2013–2016 compared with 5.7 million in the period 2009–2012 [2]. This disease affects an estimated 26 million people worldwide, including 1–2% of the adult population of developed countries in America and Europe, and as many as 10% in people over 70 years [1–8]. The prevalence of HF is estimated to be about 20/1000 people, and as high as 130/1000 people for those aged over 65 years [1–8], which results in more than 1 million hospitalizations annually in both the USA and Europe. About 15 million people suffer from it in the whole of Europe. In Western Europe, there are over 5 million HF patients [1, 3, 4], and in Poland nearly 1 million (about 3% of the population). Another 10 million Poles are at risk of this disease—mainly people with hypertension, coronary artery disease, obesity, diabetes, and smoking cigarettes [9–14]. In the USA, there are around 5 million HF sufferers. About 400,000 new cases of HF are diagnosed in the USA annually [2, 5]. The number of new cases of HF reported each year in Europe is approximately 2–3/1000. Among the 70–80 age group, 100/1000 people have HF every year [1, 3, 4]. By 2030, the number of HF patients will increase by half [1]. For example, in the USA, the number of HF patients will exceed 8 million people [2, 5]. By the year 2050, a quarter of the population will be older than 65 years of age in developed countries [1]. In 1950, in Europe, the average age of the population was 29.2 years, and by 1998, this had risen to 37.1 years. By 2050, the average age of the population is expected to be 47.7 years, leading to a higher prevalence of HF [15]. HF is the main cause of death worldwide [16]. Annual morbidity of HF in developed countries is 5–10 people per 1000 inhabitants [1, 3, 4]. HF is associated with high consumption of healthcare resources [4, 7]. This results in high costs of care for a patient with HF, which mostly results from repeated hospitalizations [4, 7]. HF, through reduced functional capacity, frequent exacerbations of disease, and repeated hospitalizations, results in poorer quality of life, decreased work productivity, and significantly increased costs of the public health system [16].
The importance of predictors of heart failure course
The main challenge in the treatment of HF is the availability of reliable prognostic models that would allow patients and doctors to develop realistic expectations about the prognosis and to choose the appropriate therapy and monitoring method. Prognosis assessment plays a special role in patients qualified for implantable device therapy or surgical treatment (including heart transplantation). Prognosis also plays an important role in planning terminal palliative care with the patient and his family. Not only does the predictor allow one to identify a high-risk patient in advance but it also allows one to monitor and implement individual preventive therapy. Secondly, identifying factors that contribute to poor prognosis can help develop new, targeted therapies [17]. This article begins with a review of individual markers that contribute to the risk of unfavorable outcome in HF.
Characteristics of clinically useful prognostic factors
Predictors should be easily obtainable and associated with some therapeutic and clinical results [17].
The 2016 European Society of Cardiology (ESC) guidelines on HF named over 70 predictors in HF patients [8]. A modified list is presented in Table 1.
Table 1.
Markers of unfavorable outcome in HF (according to [8], modified)
| Demographic data | Older age, male sex, low socio-economic status |
|---|---|
| Medical history | Ischemic etiology, longer HF duration, previous HF hospitalization, adequate and inadequate high-energy ICD interventions, non-compliance with evidence-based HF therapies (β-blockers, RAAS inhibitors) |
| Clinical status | Advanced NYHA class, high resting heart rate, low SBP, clinical signs of volume overload (e.g., pulmonary congestion, peripheral edema, jugular vein dilatation, hepatomegaly) and of peripheral hypoperfusion, Cheyne-Stoke ventilation, lower BMI, frailty |
| Cardiac imaging, including echocardiography | LV systolic dysfunction (low LVEF, reduced GLS), LV dilatation, LV hypertrophy, severe LV diastolic dysfunction, pseudonormal/restrictive LV filling pattern, left atrial dilatation, pulmonary hypertension, right ventricle dilatation and dysfunction, dyssynchrony, severe valvular disease, large territory of non-viable myocardium or of inducible ischemia in imaging stress testing, late gadolinium enhancement in CMR |
| Electrocardiogram | Wide QRS complex, ventricular arrhythmia, atrial fibrillation |
| Exercise testing | Short 6-min walk test distance, reduced VO2peak and high VE/VCO2slope in cardiopulmonary exercise test |
| Genetic testing | Lamin A/C—LMNA mutations (especially non-missense mutations), phospholamban (PLN) mutation |
| Non-cardiac comorbidities | Previous stroke/TIA, peripheral artery disease, diabetes, anemia, iron deficiency, COPD, sleep apnea (both central and obstructive), kidney/liver dysfunction, depression |
Abbreviations: BMI, body mass index; BUN, blood urea nitrogen; CMR, cardiac magnetic resonance; COPD, chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate; HF, heart failure; ICD, implantable cardioverter-defibrillator; LV, left ventricle; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; RAAS, renin-angiotensin-aldosterone system; RNA, ribonucleic acid; SBP, systolic blood pressure; TIA, transient ischemic attack; VE/VCO2, minute ventilation/carbon dioxide production; VO2peak, peak oxygen uptake; WBC, white blood cell count
The 2019 ACC Expert Consensus Decision Pathway on Risk Assessment, Management, and Clinical Trajectory of Patients Hospitalized With Heart Failure also named many predictors of unfavorable outcome during hospitalization in HF patients [18]. A modified list is presented in Table 2.
Table 2.
Risk factors during hospitalization in HF (according to [18], modified)
| Assessment prior to admission | |
|---|---|
|
▪ Older age ▪ Number of previous HF hospitalizations ▪ Comorbidities, especially diabetes, COPD, liver disease, cancer, dementia ▪ Frailty ▪ Known low LVEF in HFrEF ▪ RV dysfunction | |
| Assessment at admission | Reassessment at discharge |
| NYHA Class IV symptoms | Effective decongestion |
| Nonadherence to medications or salt/fluid restriction | Adherence |
| Elevated natriuretic peptide (NP) levels on admission | % reduction (> 30–60%) in NP levels Discharge NP levels |
| Elevated serum creatinine or low clearance on admission | Small increases in creatinine accompanying successful decongestion |
| High BUN on admission | High BUN at discharge |
| Low spot urine sodium after first IV diuretic dose |
Low total urinary sodium excretion Total urine output during hospitalization |
| Diuretic resistance with high outpatient doses | Diuretic resistance in-hospital High loop diuretic doses at discharge |
| Degree of congestion at admission |
Residual congestion after treatment ▪ High measured filling pressures ▪ Orthopnea ▪ Edema ▪ Composite congestion scores ▪ Lack of hemoconcentration |
| Hemodynamic profile of “cold and wet” at admission | Discharge with either “cold” or “wet” profile |
| Low systolic blood pressure | Low systolic blood pressure at discharge |
| Troponin elevation | Troponin elevation at any time during hospitalization |
| Hyponatremia | Lower sodium at discharge |
|
▪ No RAS therapy ▪ No beta blocker therapy |
Discontinuation of ACEI/ARB in hospital for hypotension or kidney dysfunction Discharge without RAS inhibition or discharge without beta-blocker |
| Unexpected in-hospital events conferring additional risks | |
|
▪ Resuscitation or intubation ▪ Intravenous inotropic therapy even if brief |
|
Abbreviations: HF – heart failure; COPD — chronic obstructive pulmonary disease; LVEF — left ventricular ejection fraction; HFrEF — heart failure with reduced ejection fraction; RV — right ventricle; NYHA — New York Heart Association; BUN — blood urea nitrogen; IV — intravenous; RAS — Renin-Angiotensin System; ACEI - Angiotensin converting enzyme inhibitors; ARB - Angiotensin II receptor blockers
However, no single risk factor is sufficient to predict prognosis in HF. Results of a few markers must be interpreted together. Still it is important to find the most important and valuable panel of a few predictors and there are still ongoing studies assessing potential new ones.
Conversation with the patient—still important
Knowledge of a patient’s demographic, medical, and clinical data could play an important role in prediction of life expectancy. Previous studies have shown that male sex is more strongly associated with left ventricular systolic dysfunction, but female sex is more strongly associated with preserved left ventricular function [19–21]. Ischemic etiology and coronary heart disease are strongly correlated with male sex [19–21]. Pathophysiological mechanisms that could explain sex-related differences can be separated into differences in bio-hormonal system activity (inflammation, oxidative stress, sympathetic nervous system, hormonal system), various cardiovascular risk factors, and various comorbidities (coronary artery disease, atrial fibrillation, hypertension, obesity, and diabetes and/or insulin resistance) [19–21]. These differences can influence mortality and morbidity differences between genders [21]. In LaMarca et al. study, animal models have shown that the sex-specific mitochondrial adaptation to effort is modulated by the estrogen receptor ERβ [22]. In the failing heart, sexual differences have been identified in the expression of genes involved in energy metabolism. Female pattern involves genes related to energy metabolism and regulation of transcription and translation while the male pattern involves genes related to muscular contraction. Failed female hearts maintain energy metabolism better than male hearts and are better protected against calcium overload. As a result, female sex can be protective against HF mortality [22].
Low socioeconomic status in adulthood and childhood is associated with worsened HF outcomes [23–25]. Low socioeconomic status in childhood is associated with worse HF risk factors in adulthood, such as smoking [26, 27], high blood pressure [28–30], obesity [31–33], and coronary heart disease [34, 35].
Physical examination
In the general population, increased systolic blood pressure (SBP) is associated with unfavorable outcomes and higher risk of development of HF. In the Framingham Heart Study population, 91% of the participants with HF had a previous diagnosis of hypertension [6]. Compared with the normotensive individuals, patients with higher SBP had 2- and 3-fold increased risk of developing HF [2]. However, in patients with heart failure with reduced ejection fraction (HFrEF), high SBP is associated with better outcomes. SBP has a U-shaped association with mortality in patients with 30%≤ LVEF< 50% and a linear association with mortality in patients with LVEF< 30%. As a result, lower SBP is associated with increased mortality in HFrEF patients [36].
In the general population, increased body mass index (BMI) predisposes to development of HF (5% increase in risk for each 1 kg/m2 increase in BMI) [37, 38]. In the Mahajan et al. meta-analysis, intentional weight loss in obese patients without HF was associated with a reduction in left atrial size (p = 0.02), a reduction in left ventricular mass index (p < 0.0001), and improvement in left ventricular diastolic function (p ≤ 0.0001) [39]. However, in the HF population, higher BMI is associated with lower risk of worsened outcomes—a 10% reduction in mortality for each 5-unit increase in BMI was observed in the Kenchaiah et al. [40] and Fonarow et al. studies [41]. In the Mahajan et al. meta-analysis, the “obesity paradox” was also observed for all-cause mortality, and for cardiovascular (CV) mortality in the overweight group (OR = 0.86 (95% CI 0.79 to 0.94), n = 11) [42].
The 2016 guidelines of the ESC identified cachexia and sarcopenia as important comorbidities of HF [8]. Cachexia (loss of body weight) develops in the course of disease in the catabolic stage. The cachectic patient may lose any type of tissue, leading to weight loss [43]. Cardiac cachexia has been observed as an independent risk factor of death in patients with HF [44, 45]. Sarcopenia (skeletal muscle wasting) is an important comorbid disease. In the Morley et al. study, sarcopenia was observed in 19.5% of all HFrEF patients [46]. The Bekfani et al. study with heart failure with preserved ejection fraction (HFpEF) patients confirmed a similar prevalence [47]. Reduced lean mass (LM) was independently associated with abnormal cardiorespiratory function and muscle strength, leading to worse prognosis and reduced quality of life in HF patients [44]. A multicenter Italian study identified sarcopenia as an important factor for prolonged hospitalization in HF patients admitted to acute care wards (5.1 days vs. 3.2 days) [48]. Many studies have also demonstrated that the loss of skeletal muscle mass is associated with loss of physical independence and, as a result, with significantly worsened prognosis and an increased risk of death in HF patients [43, 44, 46–50].
The New York Heart Association (NYHA) functional classification is still useful for assessing syndrome severity, patient’s exercise tolerance and prognosis in HF patients [51, 52]. NYHA functional class correlates with the magnitude of signs of cardiovascular impairment in these patients and has been associated with mortality in HF [51–54].
A list of other significant values from the medical history and clinical status of the patient is presented in Tables 1 and 2.
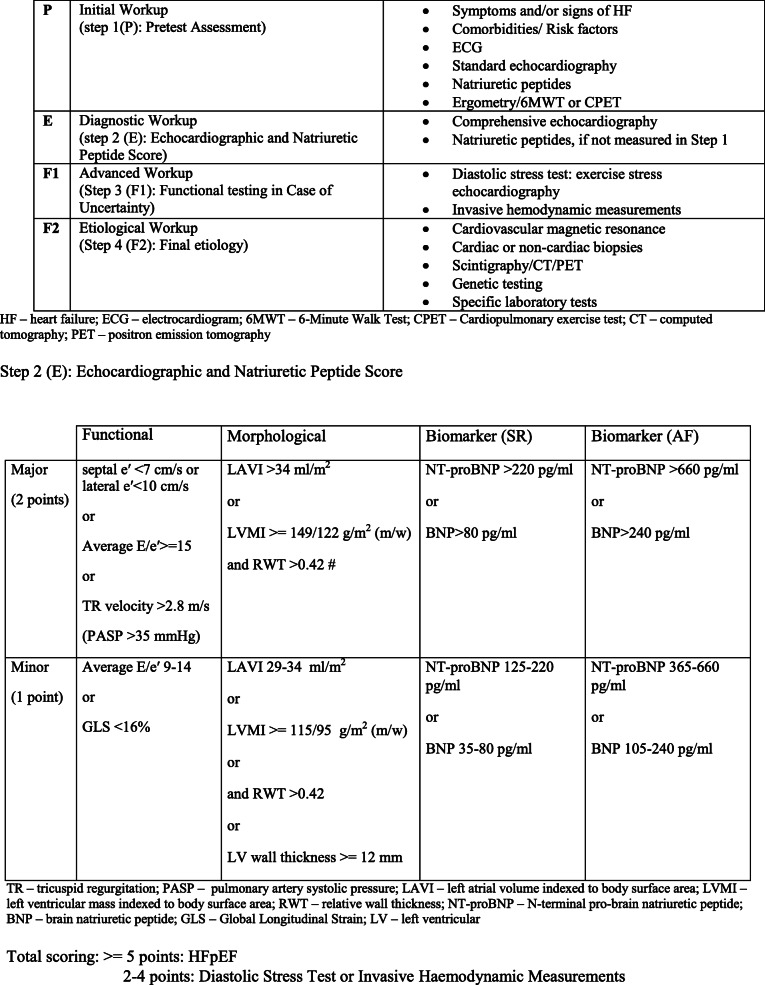
Echocardiographic imaging
Echocardiography provides detailed information regarding cardiac structure and function [8, 17]. HFrEF can be easily diagnosed by echocardiography and is understood as left ventricular ejection fraction (LVEF) < 40% [8]. Diagnostic criteria for HFpEF have been far more problematic so far. In 2019, a writing committee initiated by the HFA of the ESC therefore produced an updated consensus recommendation—the HFA–PEFF diagnostic algorithm [55]. A modified version is presented in Table 3.
Table 3.
HFA-PEFF diagnostic algorithm (according to [55], modified)
The CHARM trial [56] showed that each 10% reduction in EF was associated with a 39% increase in the risk of mortality, but this was only for EF below 45% [56]. Many measurements of structure and function of the cardiovascular system correlate with mortality in HF.
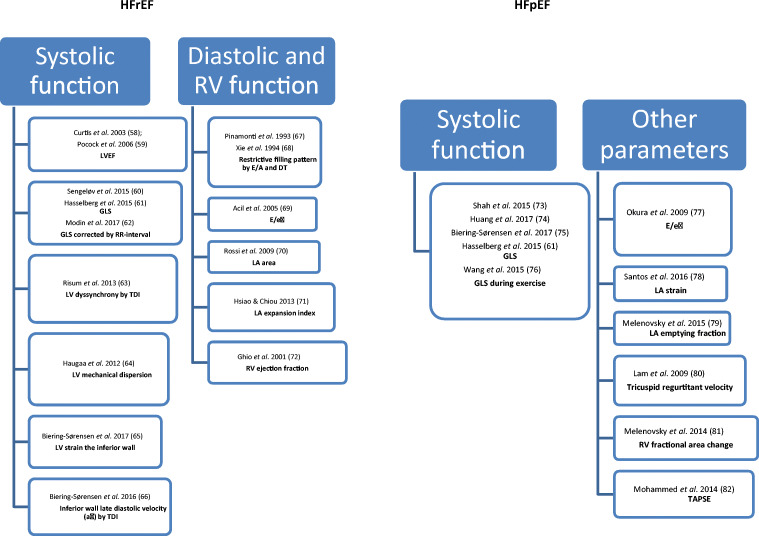
Many echocardiographic markers have prognostic value in HF (Fig. 1; Tables 1, 2, and 3).
Fig. 1.
Pathophysiological interplay between AF-HF cycle and HF–AF cycle (according to [18], modified). Abbreviations: DT, deceleration time of the E-wave; GLS, global longitudinal strain; LA, left atrial; LV, left ventricular; LVEF, left ventricular ejection fraction; RV, right ventricle; TAPSE, tricuspid annular plane systolic excursion; TDI, tissue Doppler imaging
New parameters with prognostic value in HFrEF
Mechanical dyssynchrony (the late diastolic velocity (a′)) measured by tissue Doppler imaging (TDI) and LV dyssynchrony based on global longitudinal strain (GLS) imaging seem to be important prognostic markers in HFrEF [60]. Localized areas with a changed cardiac structure (such as scarring, fibrosis, ischemia) can be missed by a global measure, such as the LVEF [57]. For example, in patients with ischemic cardiomyopathy receiving ICD, only a′ measured by TDI in the inferior wall is a predictor of VT/VF and cardiovascular death [66].
In atrial fibrillation (AF) rhythm, the varying RR interval and changing loading conditions make it difficult to measure LV systolic function. A new method of correcting GLS by the RR seems to be an important marker for evaluation of LV systolic function in HFrEF patients with AF [62, 83].
Left atrium (LA) volumes and function were the best echocardiographic markers of clinical outcomes (mortality and hospitalization) [70], as a sensitive barometer of LV filling pressure [84, 85]. The power of LA parameters measured with the LA emptying fraction and the LA expansion index (LAEI) could be useful. In the Hsiao study [71], LAEI was better compared to LA volume in predicting death and hospitalization for HF.
However, no single risk factor is sufficient to determine prognosis in HFrEF patients. The value of a few echocardiographic parameters must be interpreted together (systolic, diastolic, and RV function). Figure 1 shows many echocardiographic predictors of outcome in HFrEF (Fig. 1).
New parameters with prognostic value in HFpEF
LVEF may be correct in HFpEF, even though systolic dysfunction is already appearing. The abnormal LV contraction includes abnormal longitudinal shortening (results from dysfunctional or stressed longitudinal myofibres; measured by impaired mitral annular plane longitudinal descent and velocity, decreased GLS) [86, 87], preserved or increased circumferential shortening (measured by circumferential strain—CS; results from subendocardial fiber dysfunction with left-handed helix shortening by unbalanced subepicardial fibers) [57, 88], and increased wall thickness-to-chamber radius ratio (results from concentric hypertrophy; radial thickening; measured by radial strain—RS).
Mitral annular plane systolic excursion (MAPSE) has been suggested as a parameter for impaired longitudinal function and could provide complementary information to EF [86].
GLS measured during bicycle ergometer testing has also been identified as an important prognostic marker in HFpEF [76].
As already mentioned before, LA volumes and function are sensitive indicators of LV filling pressure [84]. Strain imaging by 2D speckle-tracking (2DS) is a new index of LA function. Recent data demonstrated that LA strain is decreased in diastolic HF [89]. This new parameter could be useful in categorizing diastolic dysfunction [89] and may have prognostic value in HFpEF [78].
Greater right ventricular (RV) afterload results in pulmonary hypertension (measured by tricuspid regurgitation (TR) velocity) and RV systolic dysfunction (measured by TAPSE) is highly prevalent in HFpEF [80]. 2DS RV free wall strain may have prognostic value in HFpEF, despite the complicated geometry of the RV [57].
In summary, LV systolic and diastolic function, LA function, and RV function have prognostic value in HFpEF. Figure 1 provides a list of studies that have identified many echocardiographic prognostic parameters in HFpEF (Fig. 1).
Exercise testing in HF
Six-min walk test
The 6-min walk test is useful in measuring functional limitation (patient’s exercise capacity) in the prognostic stratification and in evaluating the effects of therapy in children and adults with HF [90, 91]. Hsich et al. [92] observed in one study a 7% increase in mortality for each 1-min reduction in exercise capacity in HF patients. The SOLVD study [93] showed that 6MWT distance was an important and independent predictor of morbidity (heart failure hospitalization) and mortality in a logistic regression model in patients with left ventricular dysfunction. In the studies by Rostagno et al. [94], Cahalin et al. [95], and Arslan et al. [96], lower functional capacity (distance of ≤ 300 m in 6MWT) was a useful prognostic marker of death or hospitalization in patients with mild-to-moderate heart failure. The Ingle et al. study [97] also showed that the 6MWT distance is an important independent predictor of all-cause mortality in patients with HF. In the study by Boer et al. [98], 6MWT distance was a simple and feasible tool to identify children with a higher risk of death or heart transplantation in children with dilated cardiomyopathy. However, there are no data showing the prognostic usefulness of 6MWT in women, in elderly patients, or in patients with left ventricular diastolic dysfunction [99].
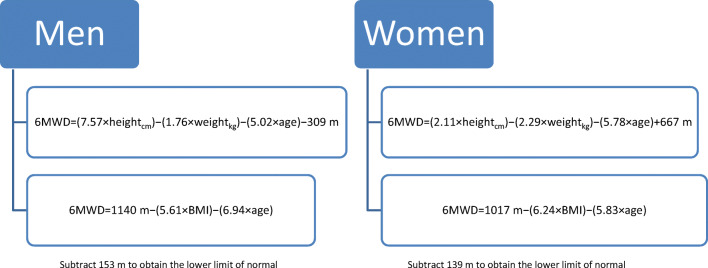
6MWT poorly correlates with hemodynamic and functional echocardiographic parameters [99]. In the Zugck et al. [100] and Opasich et al. [101] studies, only right ventricular ejection fraction correlated significantly with 6MWT distance. However, distance walked during 6MWT correlated significantly with non-cardiovascular parameters (muscular strength, postural balance, reaction time, mood, and general health) [99] and with demographic variables, such as gender (lower in women), weight and age (negative correlation) [102], and height (positive correlation) [99]. This suggests that the test result should be evaluated not only as a total distance walked in meters but also as a percentage of the predicted value (6MWD%) [103]. Figure 2 provides the reference value for the 6MWT distance corrected by anthropometric variables in a group of healthy subjects (Fig. 2).
Fig. 2.
Reference value for the 6MWT distance corrected by anthropometric variables in a group of healthy subjects (according to [99, 104], modified). Abbreviations: BMI, body mass index
6MWT distance can also be used to evaluate the effect of therapeutic interventions in patients with HF (current pharmacological therapy, program of physical training, new drugs in addition to standard therapy, ventricular assistance devices, ventricular resynchronization techniques) [99]. However, the correct total distance, percentage change from baseline, or percentage change of predicted value is not specified yet [99].
Cardiopulmonary exercise testing
Cardiopulmonary exercise testing (CPET) through measurement of peak oxygen uptake (VO2) defines maximum exercise capacity of the patient. CPET evaluation should start with maximum effort (RER > 1.0 to 1.1) [105]. Peak VO2 remains the gold standard in predicting outcome in HF. Peak VO2 < 14 ml/kg/min and < 12 ml/kg/min in patients on β-blockers, in the HFrEF population continues to be a significant prognostic factor. It is also an important predictor of death in HFpEF patients [106, 107]. For young, obese, and cachectic patients, peak VO2 should be interpreted as a percentage of predicted, with values < 50% indicating a poor prognosis [108]. Peak VO2 on effort and anaerobic threshold (AT) were used to determine the classification of the severity of HF (Table 4). For patients who do not make enough effort, oxygen uptake efficiency slope (OUES) < 1.47 l/min and VO2 at VT < 9 ml/kg/min indicate a bad prognosis [110]. Mean response time (MRT), a sensitive indicator of O2 uptake kinetics, more accurately showed the ability to increase cardiac output during low-level exercise. An MRT > 60 s correlated with a decrease in exercise right ventricular ejection fraction (RVEF) and a decrease in cardiac output (through the increased transpulmonary gradient).
Table 4.
Classification of the severity of HF depending on the CPET result (according to [109], modified)
| Class | Severity of HF | VO2 peak (ml/kg/min) | VO2-AT (ml/kg/min) |
|---|---|---|---|
| A | Mild/none | > 20 | > 14 |
| B | Mild/moderate | 16–20 | 11–14 |
| C | Moderate/severe | 10–16 | 8–11 |
| D | Severe | 6–10 | 5–8 |
| E | Very severe | < 6 | < 4 |
Failure to achieve SBP > 120 mm Hg and no increase in SBP during exercise are associated with poor prognosis [111]. Both chronotropic incompetence and slow return to normal heart rate in recovery (< 6 bpm) also indicate poor prognosis [112].
VE/VCO2 slope and exercise oscillatory ventilation (EOV) are among the strongest predictors of poor outcome in HF. VE/VCO2 slope > 34 to 36 indicates high-risk HF patients [113]. The presence of EOV is consistently associated with an annual mortality of > 20% [114].
In summary, peak oxygen uptake parameters, ventilatory efficiency or stability, and chronotropic incompetence are the main CPET factors in predicting outcome in HF patients.
CPET is not often used in chronic HF patients for a few reasons, including the limited availability of equipment (relatively expensive technology, expertise required) and patient’s inability to perform a maximal effort test (comorbidities, cognitive impairment) [99, 115]. Alternatively, severity of inspiratory muscle weakness (IMW), measured by maximal static inspiratory pressure (PImax) could be a cheap and useful parameter of inspiratory muscle strength as a marker of maximal work of breathing and can represent a reasonable alternative to pVO2 for mortality risk stratification in HF patients [115]. In previous studies [115–118], PImax was a strong and independent predictor of mortality in HFrEF patients, especially in individuals unable to perform an exercise test.
Laboratory biomarkers
Multiple impaired regulatory axes are seen in HF, including the renin-angiotensin-aldosterone system (RAAS), sympathetic regulation, neurohormonal regulation, and the cardiac stretch response. HF is associated with a chronic inflammatory state, oxidative stress, and in effect extracellular matrix remodeling [119]. Many prognostic biomarkers in HF have been identified. A classification of useful biomarkers based on their pathophysiological role in HF is presented in Table 5.
Table 5.
Classification of biomarkers based on their pathophysiological role in HF (according to [120], modified)
| Pathophysiological pathway | Biomarkers |
|---|---|
| Myocyte stress | BNP; NTpro-BNP; NTpro-ANP; MR-proADM; sST2 |
| Myocyte injury | TnT; TnI; CK-MB mass; MLCK-I; hFABP; PTX3; HSPs |
| Inflammation | hsCRP; TNF-α; sTNFR; cytokines (e.g. IL-1, IL-6, IL-18); AdipoQ, sST2; PTX3; OPG; PCT |
| Oxidative stress | oxLDL; MPO; urinary biopyrrins; IsoPs; MDA |
| Neurohormones | NE; renin; AngII; aldosterone; AVP/copeptin; EDNs; Cg; ADM; MR-proADM |
| Extracellular matrix remodeling | MMPs; TIMPs; P1NP; P3NP; Gal-3; sST2; GDF-15 |
| Cardio-renal syndrome | Serum creatinine; ACR; CysC; NGAL; BTP |
| Others | Hbg; serum albumin; RDW, VCAM |
Abbreviations: BNP, brain natriuretic peptide; NTpro-BNP, N-terminal pro-brain natriuretic peptide; NTpro-ANP, N-terminal proatrial natriuretic peptide; MR-proADM, mid-regional pro-adrenomedullin; sST2, soluble ST2; TnT, troponin T; TnI, troponin I; CK-MB mass, creatine kinase myocardial band fraction; MLCK-I, myosin light-chain kinase I; hFABP, heart-type fatty acid binding protein; PTX3, pentraxin-related protein; HSPs, heat shock proteins; hsCRP, high-sensitivity C-reactive protein; TNF-α, tumor necrosis factor α; sTNFR, soluble tumor necrosis factor receptors; IL-1, interleukin 1; IL-6, interleukin 6; IL-18, interleukin 18; AdipoQ, adiponectin; OPG, osteoprotegerin; PCT, procalcitonin; oxLDL, oxidized low-density lipoprotein; MPO, myeloperoxidase; IsoPs, isoprostanes; MDA, plasma malondialdehyde; NE, norepinephrine; AngII, angiotensin II; AVP, arginine vasopressin; EDNs, endothelins; Cg, chromogranins; ADM, adrenomedullin; MMPs, matrix metalloproteinases; TIMPs, tissue inhibitors of metalloproteinases; P1NP, procollagen type 1 N propeptide; P3NP, procollagen type 3 N propeptide; Gal-3, galectin 3; GDF-15, growth/differentiation factor 15; ACR, urine albumin to creatinine ratio; CysC, cystatin C; NGAL, neutrophil gelatinase-associated lipocalin; BTP, β-trace protein; Hbg, hemoglobin; RDW, red blood cell distribution width; VCAM, vascular cell adhesion molecule
Nowadays, natriuretic peptides are the gold standard biomarkers [8]. Brain natriuretic peptide has been shown to predict morbidity, mortality, and hospitalization from HF in clinical practice [121–123]. In the trial (PROTECT) by Januzzi et al. [124], patients who had NT-proBNP-guided therapy for heart failure benefited. Each natriuretic peptide has specific cut-off concentrations (Table 6). Plasma concentrations should be interpreted in the context of the clinical setting of the patient [125].
Table 6.
Recommended natriuretic peptide cut-offs for HF diagnosis (according to [125], modified)
| Cut-off levels (pg/ml) | ||||||
|---|---|---|---|---|---|---|
| NT-proBNP | BNP | |||||
| Age < 50 | Age 50–75 | Age > 75 | Age < 50 | Age 50–75 | Age > 75 | |
| Acute setting, patient with acute dyspnea | ||||||
| HF unlikely | < 300 | < 100 | ||||
| “Gray zone” | 300–450 | 300–900 | 300–1800 | 100–400 | ||
| HF likely | > 450 | > 900 | > 1800 | > 400 | ||
| Non-acute setting, patient with mild symptoms | ||||||
| HF unlikely | < 125 | < 35 | ||||
| “Gray zone” | 125–600 | 35–150 | ||||
| HF likely | > 600 | > 150 | ||||
Abbreviations: BNP, B-type natriuretic peptide; HF, heart failure; NT-proBNP, N-terminal proBNP
Consider reducing the cut-off levels in obese patients by 50%
Previous studies have shown different biomarker profiles between patients with HFrEF, heart failure with mid-range ejection fraction (HFmrEF), and HFpEF. For example, in the Tromp et al. study [126], HFmrEF was associated with hemoglobin, red blood cells, BNP, galectin-3, endothelin-1, and syndecan-1. In contrast, HFrEF was mostly associated with BNP, kidney injury molecule-1 (KIM-1), troponin-I (TnI), red blood cells, and hemoglobin, whereas HFpEF was associated with BNP, angiogenin, hemoglobin, galectin-3, D-dimer, and inflammation markers (pentraxin-3, RAGE) and a remodeling marker (osteopontin). In another Tromp et al. study [127], the main proteins in HFrEF were NTproBNP, growth differentiation factor-15 (GDF-15), interleukin-1 receptor type 1 (ILR-1), and activating transcription factor 2, while central proteins in HFpEF were catenin beta-1 and integrin subunit beta-2. HFrEF was related to DNA binding transcription factor activity, regulation of nitric oxide biosynthesis, and cellular protein metabolism. However, HFpEF was related to cytokine response, extracellular matrix organization, and inflammation. In addition to the above, in the Nadar et al. study [120], markers of inflammation such as high-sensitivity C-reactive protein (hsCRP), ST2, and cystatin C (CysC) levels and markers of myocardial fibrosis such as galectin-3 were identified to be increased in HFpEF patients.
In the Bielecka-Dabrowa et al. study [128], biomarkers with different pathophysiological backgrounds (NT-proBNP, CT-1, TGF-β, and CysC) gave additive prognostic value for incident HF in hypertensive patients compared to NT-proBNP alone. Michalska-Kasiczak et al. [129] also noted that a single biomarker may not be sufficient in clinical practice in a heterogeneous group of HF patients. They suggested that is necessary to use a biomarker panel. Biomarker profiles of patients with HFmrEF, HFpEF, and HFrEF are different. The biomarkers in HFpEF are mainly based on inflammation, while in HFrEF, they are more cardiac stretch based, and in HFmrEF, they are related to both inflammation and cardiac stretch. Biomarkers associated with inflammation and heart remodeling are predictive in HFmrEF and HFpEF but not in HFrEF. These data could have important therapeutic consequences for the group of HF patients and suggest that it is necessary to use a biomarker panel [120].
According to recent news, neutrophil gelatinase-associated lipocalin (NGAL), a marker of renal injury, seems to be also a good factor in the diagnosis and prognostic prediction in HF [130].
Different miRNAs (miR423-5p, miR320a, and miR22) could be increased in patients with HF [131]. A recent study suggested that miR423-5p could be the best potential biomarker [132].
Previous HF hospitalization
Hospitalization for HF within the last year has been significant risk factor of subsequent hospitalizations. The results of the QUALIFY survey showed that 30.4% of the patients had a history of at least two HF hospitalizations [133]. In the ESC-HF Pilot study, 57% of the HF patients had a history of previous hospitalizations [134] and, additionally, 24.75% of them were rehospitalized in a 1-year follow-up [135].
Prognostic factors in different multivariate predictor models
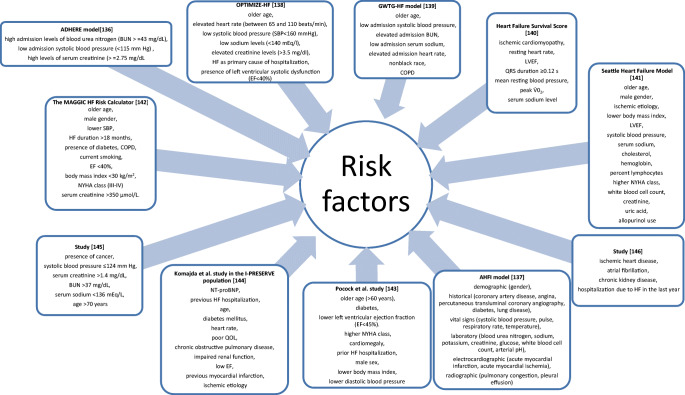
Different risk models were constructed to assess different clinical endpoints in the HF population [17]. Figure 3 provides a list of studies that have identified many prognostic values in HF (Fig. 3).
Fig. 3.
Selected risk models for the assessment of prognosis in heart failure
Conclusion
All of the presented models have shown only moderate probability in predicting death in HF [17]. Moreover, while their effects appear to be acceptable at the population level, they do not sufficiently predict outcome for an individual patient [147]. As we can see, there are also numerous parameters that may be used in clinical practice and should be used in order to determine the overall risk of our patients as accurately as possible.
Cited studies allow for the isolation of variables included in the models more often than others: sex, age, SBP, HR, NYHA class, LVEF, BUN level, serum creatinine, and sodium concentration. Other strong prognostic factors of mortality in HF, consistently reported in different models, include BNP/NT-proBNP concentration, weight or body mass index, and diabetes mellitus [17, 135–147]. Nevertheless, there is no possibility at the moment to assess and monitor HF with a single parameter or a simple scale that would apply to the whole population of patients.
Despite the identification of many markers and models of poor prognosis, clinical decisions and guidelines in HF are still based mainly on several basic parameters, such as the presence of HF symptoms (NYHA class), LVEF, and the duration and morphology of the QRS complex [17]. But considering the cited works, all potential tools for assessing the risk of HF patients should be used if possible.
Authors’ contributions
Anna Chuda: research concept and design, literature search, data analysis and interpretation, and writing the article; Maciej Banach: critical revision of the article; Marek Maciejewski: critical revision of the article; Agata Bielecka-Dabrowa: research concept and design, critical revision of the article, and final approval of the article.
Funding
The Project is financed by the Polish National Agency for Academic Exchange under the Foreign Promotion Programme
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Savarese G, Lund LH. Global public health burden of heart failure. Card Fail Rev. 2017;3(1):7–11. doi: 10.15420/cfr.2016:25:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benjamin EJ, Muntner P, Alonso A, et al. Heart disease and stroke statistics—2019 update: a report from the American Heart Association. Circulation. 2019;139:e56–e528. doi: 10.1161/CIR.0000000000000659. [DOI] [PubMed] [Google Scholar]
- 3.Mosterd A, Hoes AW. Clinical epidemiology of heart failure. Heart. 2007;93:1137–1146. doi: 10.1136/hrt.2003.025270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ambrosy AP, Fonarow GC, Butler J, et al. The global health and economic burden of hospitalizations for heart failure. J Am Coll Cardiol. 2014;63(12):1123–1133. doi: 10.1016/j.jacc.2013.11.053. [DOI] [PubMed] [Google Scholar]
- 5.Heidenreich PA, Albert NM, Allen LA, et al. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail. 2013;6(3):606–619. doi: 10.1161/HHF.0b013e318291329a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lloyd-Jones DM, Larson MG, Leip EP, et al. Lifetime risk for developing congestive heart failure: the Framingham Heart Study. Circulation. 2002;106:3068–3072. doi: 10.1161/01.cir.0000039105.49749.6f. [DOI] [PubMed] [Google Scholar]
- 7.Harper S. Economic and social implications of aging societies. Science. 2014;346(6209):587–591. doi: 10.1126/science.1254405. [DOI] [PubMed] [Google Scholar]
- 8.Ponikowski P, Voors A, Anker S, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Eur Heart J. 2016;33:1787–1847. doi: 10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]
- 9.OECD 2015 Avoidable hospital admissions in health at a glance 2015: OECD indicators. OECD Publishing. Paris. 10.1787/health_glance-2015-en.
- 10.Karasek D, Kubica A, Sinkiewicz W, Błażejewski J, Bujak R (2008) Epidemia niewydolności serca – problem zdrowotny i społeczny starzejących się społeczeństw Polski i Europy. Folia Cardiologica Excerpta 3, 5:243, 245-246.
- 11.Rywik TM, Kolodziej P, Targonski R, et al. Characteristics of the heart failure population in Poland: ZOPAN, a multicentre national programme. Kardiol Pol. 2011;69(1):24–31. [PubMed] [Google Scholar]
- 12.Rywik TM, Zielinski T, Piotrowski W, et al. Heart failure patients from hospital settings in Poland: population characteristics and treatment patterns, a multicenter retrospective study. Cardiol J. 2008;15(2):169–180. [PubMed] [Google Scholar]
- 13.Balsam P, Tyminska A, Kaplon-Cieslicka A, et al. (2015) Predictors of one-year outcome in patients hospitalized for heart failure: results from the Polish part of the Heart Failure Pilot Survey of the European Society of Cardiology. Kardiologia polska [DOI] [PubMed]
- 14.mapowanie Embaedp. [Mapping health needs in cardiology for Poland] Mapa potrzeb zdrowotnych w zakresie kardiologii dla Polski. Secondary [Mapping health needs in cardiology for Poland] Mapa potrzeb zdrowotnych w zakresie kardiologii dla Polski 2019. http://mpz.mz.gov.pl/wp-content/uploads/2019/06/MPZ_kardiologia_Polska.pdf
- 15.United Nations, Department of Economic and Social Affairs, Population Division (2019) World population prospects 2019: highlights. ST/ESA/SER.A/423
- 16.Ambrosy AP, Fonarow GC, Butler J et al (2014) The global health and economic burden of hospitalizations for heart failure: lessons learned from hospitalized heart failure registries. J Am Coll Cardiol 63(12):1123–1133. 10.1016/j.jacc.2013.11.053 indexed in PubMed: 24491689 [DOI] [PubMed]
- 17.Kapłon-Cieślicka A, Drożdż J, Filipiak KJ. Prognostic factors in heart failure — are they all equally important? Kardiol Pol. 2017;75(6):519–526. doi: 10.5603/KP.a2017.0088. [DOI] [PubMed] [Google Scholar]
- 18.Hollenberg S, Stevenson L, Vaibhav TJ, et al. 2019 ACC expert consensus decision pathway on risk assessment, management, and clinical trajectory of patients hospitalized with heart failure. J Am Coll Cardiol. 2019;74(15):1966–2011. doi: 10.1016/j.jacc.2019.08.001. [DOI] [PubMed] [Google Scholar]
- 19.Frazier CG, Alexander KP, Newby LK, Anderson S, Iverson E, Packer M, Cohn J, Goldstein S, Douglas PS. Associations of gender and etiology with outcomes in heart failure with systolic dysfunction: a pooled analysis of 5 randomized control trials. J Am Coll Cardiol. 2007;49:1450–1458. doi: 10.1016/j.jacc.2006.11.041. [DOI] [PubMed] [Google Scholar]
- 20.Parashar S, Katz R, Smith NL, Arnold AM, Vaccarino V, Wenger NK, Gottdiener JS. Race, gender and mortality in adults ≥65 years of age with incident heart failure (from the Cardiovascular Health Study) Am J Cardiol. 2009;103:1120–1127. doi: 10.1016/j.amjcard.2008.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Azad N, Kathiravelu A, Minoosepeher S, Hebert P, Fergusson D. Gender differences in the etiology of heart failure: a systematic review. J Geriatr Cardiol. 2011;8(1):15–23. doi: 10.3724/SP.J.1263.2011.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.LaMarca, B, Alexander, BT. Sex differences in cardiovascular physiology and pathophysiology. 2019. Elsevier Science. https://books.google.pl/books?id=GW2RDwAAQBAJ
- 23.Roberts CB, Couper DJ, ChangPP JSA, Rosamond WD, Heiss G. Influence of life-course socioeconomic position on incident heart failure in blacks and whites: the atherosclerosis risk in communities. Study Am J Epidemiol. 2010;172:717–727. doi: 10.1093/aje/kwq193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ingelsson E, Lind L, Arnlov J, Sundstrom J. Socioeconomic factors as predictors of incident heart failure. J Card Fail. 2006;12:540–545. doi: 10.1016/j.cardfail.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 25.Fiscella K, Tancredi D, Franks P. Adding socioeconomic status to Framingham scoring to reduce disparities in coronary risk assessment. Am Heart J. 2009;157:988–994. doi: 10.1016/j.ahj.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 26.Jefferis BJ, Power C, Graham H, et al. Effects of childhood socioeconomic circumstances on persistent smoking. Am J Public Health. 2004;94(2):279–285. doi: 10.2105/ajph.94.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lawlor DA, Batty GD, Morton SM, et al. Childhood socioeconomic position, educational attainment, and adult cardiovascular risk factors: the Aberdeen Children of the 1950s cohort study. Am J Public Health. 2005;95(7):1245–1251. doi: 10.2105/AJPH.2004.041129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Poulton R, Caspi A, Milne BJ, et al. Association between children’s experience of socioeconomic disadvantage and adult health: a life-course study. Lancet. 2002;360(9346):1640–1645. doi: 10.1016/S0140-6736(02)11602-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kivimäki M, Lawlor DA, Smith GD, et al. Early socioeconomic position and blood pressure in childhood and adulthood: the Cardiovascular Risk in Young Finns Study. Hypertension. 2006;47(1):39–44. doi: 10.1161/01.HYP.0000196682.43723.8a. [DOI] [PubMed] [Google Scholar]
- 30.Kivimäki M, Smith GD, Elovainio M, et al. Socioeconomic circumstances in childhood and blood pressure in adulthood: the Cardiovascular Risk in Young Finns Study. Ann Epidemiol. 2006;16(10):737–742. doi: 10.1016/j.annepidem.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 31.Sobal J, Stunkard AJ. Socioeconomic status and obesity: a review of the literature. Psychol Bull. 1989;105(2):260–275. doi: 10.1037/0033-2909.105.2.260. [DOI] [PubMed] [Google Scholar]
- 32.Salonen MK, Kajantie E, Osmond C, et al. Role of socioeconomic indicators on development of obesity from a life course perspective. J Environ Public Health. 2009;2009:625168. doi: 10.1155/2009/625168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parsons TJ, Power C, Logan S, et al. Childhood predictors of adult obesity: a systematic review. Int J Obes Relat Metab Disord. 1999;23(suppl 8):S1–S107. [PubMed] [Google Scholar]
- 34.Kittleson MM, Meoni LA, Wang NY, et al. Association of childhood socioeconomic status with subsequent coronary heart disease in physicians. Arch Intern Med. 2006;166(21):2356–2361. doi: 10.1001/archinte.166.21.2356. [DOI] [PubMed] [Google Scholar]
- 35.Pollitt RA, Rose KM, Kaufman JS. Evaluating the evidence for models of life course socioeconomic factors and cardiovascular outcomes: a systematic review. BMC Public Health. 2005;5:7. doi: 10.1186/1471-2458-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Levy D, Larson MG, Vasan RS, Kannel WB, Ho KK. The progression from hypertension to congestive heart failure. JAMA. 1996;275:1557–1562. [PubMed] [Google Scholar]
- 37.Ather S, Chan W, Chillar A, et al. Association of systolic blood pressure with mortality in patients with heart failure with reduced ejection fraction: a complex relationship. Am Heart J. 2011;161(3):567–573. doi: 10.1016/j.ahj.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee DS, Massaro JM, Wang TJ, et al. Antecedent blood pressure, body mass index, and the risk of incident heart failure in later life. Hypertension. 2007;50:869–876. doi: 10.1161/HYPERTENSIONAHA.107.095380. [DOI] [PubMed] [Google Scholar]
- 39.Levitan E, Yang A, Wolk A, Mittleman A. Adiposity and incidence of heart failure hospitalization and mortality: a population-based prospective study. Circ Heart Fail. 2009;2:202–208. doi: 10.1161/CIRCHEARTFAILURE.108.794099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mahajan R, Stokes M, Elliott A, et al. Complex interaction of obesity, intentional weight loss and heart failure: a systematic review and meta-analysis. Heart. 2020;106:58–68. doi: 10.1136/heartjnl-2019-314770. [DOI] [PubMed] [Google Scholar]
- 41.Kenchaiah S, Pocock SJ, Wang D i. Body mass index and prognosis in patients with chronic heart failure: insights from the Candesartan in Heart failure: assessment of reduction in mortality and morbidity (CHARM) program. Circulation. 2007;116:627–636. doi: 10.1161/CIRCULATIONAHA.106.679779. [DOI] [PubMed] [Google Scholar]
- 42.Fonarow GC, Srikanthan P, Costanzo MR, et al. An obesity paradox in acute heart failure: analysis of body mass index and inhospital mortality for 108,927 patients in the acute decompensated heart failure national registry. Am Heart J. 2007;153:74–81. doi: 10.1016/j.ahj.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 43.Haehling S. Muscle wasting and sarcopenia in heart failure: a brief overview of the current literature. ESC Heart Failure. 2018;5:1074–1082. doi: 10.1002/ehf2.12388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Carbone S, Billingsley HE, Rodriguez-Miguelez P et al (2019) Lean mass abnormalities in heart failure: the role of sarcopenia, sarcopenic obesity, and cachexia. Curr Probl Cardiol (19):30057. 10.1016/j.cpcardiol.2019.03.006 [DOI] [PMC free article] [PubMed]
- 45.Anker SD, Ponikowski P, Varney S, et al. Wasting as independent risk factor for mortality in chronic heart failure. Lancet. 1997;349:1050–1053. doi: 10.1016/S0140-6736(96)07015-8. [DOI] [PubMed] [Google Scholar]
- 46.Morley JE, Anker SD. Myopenia and precision (P4) medicine. J Cachexia Sarcopenia Muscle. 2017;8:857–863. doi: 10.1002/jcsm.12231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bekfani T, Pellicori P, Morris DA, et al. Sarcopenia in patients with heart failure with preserved ejection fraction: impact on muscle strength, exercise capacity and quality of life. Int J Cardiol. 2016;222:41–46. doi: 10.1016/j.ijcard.2016.07.135. [DOI] [PubMed] [Google Scholar]
- 48.Martone AM, Bianchi L, Abete P, et al. The GLISTEN Group Investigators. The incidence of sarcopenia among hospitalized older patients. Results from the Listen study. J Cachexia Sarcopenia Muscle. 2017;8:907–914. doi: 10.1002/jcsm.12224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kalantar-Zadeh K, Block G, Horwich T, Fonarow GC. Reverse epidemiology of conventional cardiovascular risk factors in patients with chronic heart failure. J Am Coll Cardiol. 2004;43:1439–1444. doi: 10.1016/j.jacc.2003.11.039. [DOI] [PubMed] [Google Scholar]
- 50.Bielecka-Dabrowa A, Fabis J, Mikhailidis DP, et al. Prosarcopenic effects of statins may limit their effectiveness in patients with heart failure. Trends Pharmacol Sci. 2018;39(4):331–353. doi: 10.1016/j.tips.2018.02.003. [DOI] [PubMed] [Google Scholar]
- 51.Committee of the New York Heart Association (1994) Nomenclature and criteria for diagnosis of diseases of the heart and great vessels. 9th ed. Boston: Little, Brown & Co p. 253–6.
- 52.Dickstein K, Cohen-Solal A, Filippatos G, et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the Task Force for the diagnosis and treatment of acute and chronic heart failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM) Eur J Heart Fail. 2008;10(10):933–989. doi: 10.1016/j.ejheart.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 53.Stevenson LW, Perloff JK. The limited reliability of physical signs for estimating hemodynamics in chronic heart failure. JAMA : The journal of the American Medical Association. 1989;261(6):884–888. [PubMed] [Google Scholar]
- 54.van den Broek SA, van Veldhuisen DJ, de Graeff PA, Landsman ML, Hillege H, Lie KI. Comparison between New York Heart Association classification and peak oxygen consumption in the assessment of functional status and prognosis in patients with mild to moderate chronic congestive heart failure secondary to either ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol. 1992;70(3):359–363. doi: 10.1016/0002-9149(92)90619-a. [DOI] [PubMed] [Google Scholar]
- 55.Pieske B, Tschöpe C, de Boer RA, et al. How to diagnose heart failure with preserved ejection fraction: the HFA-PEFF diagnostic algorithm: a consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC) Eur Heart J. 2019;40:3297–3317. doi: 10.1093/eurheartj/ehz641. [DOI] [PubMed] [Google Scholar]
- 56.Solomon SD, Anavekar N, Skali H, et al. Candesartan in Heart Failure Reduction in Mortality (CHARM) Investigators. Influence of ejection fraction on cardiovascular outcomes in a broad spectrum of heart failure patients. Circulation. 2005;112(24):3738–3744. doi: 10.1161/CIRCULATIONAHA.105.561423. [DOI] [PubMed] [Google Scholar]
- 57.Modin D, Andersen DM, Biering-Sørensen T. Echo and heart failure: when do people need an echo, and when do they need natriuretic peptides? Echo Res Pract. 2018;5(2):R65–R79. doi: 10.1530/ERP-18-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Curtis JP, Sokol SI, Wang Y, Rathore SS, Ko DT, Jadbabaie F, Portnay EL, Marshalko SJ, Radford MJ, Krumholz HM. The association of left ventricular ejection fraction, mortality, and cause of death in stable outpatients with heart failure. J Am Coll Cardiol. 2003;42:736–742. doi: 10.1016/S0735-1097(03)00789-7. [DOI] [PubMed] [Google Scholar]
- 59.Pocock SJ, Wang D, Pfeffer MA, Yusuf S, JJ MM, Swedberg KB, Ostergren J, Michelson EL, Pieper KS, Granger CB. Predictors of mortality and morbidity in patients with chronic heart failure. Eur Heart J. 2006;27:65–75. doi: 10.1093/eurheartj/ehi555. [DOI] [PubMed] [Google Scholar]
- 60.Sengeløv M, Jørgensen PG, Jensen JS, Bruun NE, Olsen FJ, Fritz-Hansen T, Nochioka K, Biering-Sørensen T. Global longitudinal strain is a superior predictor of all-cause mortality in heart failure with reduced ejection fraction. JACC Cardiovasc Imaging. 2015;8:1351–1359. doi: 10.1016/j.jcmg.2015.07.013. [DOI] [PubMed] [Google Scholar]
- 61.Hasselberg NE, Haugaa KH, Sarvari SI, Gullestad L, Andreassen AK, Smiseth OA, Edvardsen T. Left ventricular global longitudinal strain is associated with exercise capacity in failing hearts with preserved and reduced ejection fraction. Eur Heart J Cardiovasc Imaging. 2015;16:217–224. doi: 10.1093/ehjci/jeu277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Modin D, Sengeløv M, Jørgensen PG, Bruun NE, Olsen FJ, Dons M, Fritz Hansen T, Jensen JS, Biering-Sørensen T. Global longitudinal strain corrected by RR interval is a superior predictor of all-cause mortality in patients with systolic heart failure and atrial fibrillation. ESC Heart Failure. 2017;5:311–318. doi: 10.1002/ehf2.12220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Risum N, Williams ES, Khouri MG, Jackson KP, Olsen NT, Jons C, Storm KS, Velazquez EJ, Kisslo J, Bruun NE, et al. Mechanical dyssynchrony evaluated by tissue Doppler cross-correlation analysis is associated with long-term survival in patients after cardiac resynchronization therapy. Eur Heart J. 2013;34:48–56. doi: 10.1093/eurheartj/ehs035. [DOI] [PubMed] [Google Scholar]
- 64.Haugaa KH, Grenne BL, Eek CH, Ersbøll M, Valeur N, Svendsen JH, Florian A, Sjøli B, Brunvand H, Køber L, et al. Strain echocardiography improves risk prediction of ventricular arrhythmias after myocardial infarction. JACC Cardiovasc Imaging. 2013;6:841–850. doi: 10.1016/j.jcmg.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 65.Biering-Sorensen T, Knappe D, Pouleur AC, Claggett B, Wang PJ, Moss AJ, Solomon SD, Kutyifa V. Regional longitudinal deformation improves prediction of ventricular tachyarrhythmias in patients with heart failure with reduced ejection fraction: a MADIT-CRT Substudy (Multicenter Automatic Defibrillator Implantation Trial-Cardiac Resynchronization Therapy) Circulation: Cardiovascular Imaging. 2017;10:e005096. doi: 10.1161/CIRCIMAGING.116.005096. [DOI] [PubMed] [Google Scholar]
- 66.Biering-Sørensen T, Olsen FJ, Storm K, Fritz-Hansen T, Olsen NT, Jøns C, Vinther M, Søgaard P, Risum N. Prognostic value of tissue Doppler imaging for predicting ventricular arrhythmias and cardiovascular mortality in ischaemic cardiomyopathy. Eur Heart J Cardiovasc Imaging. 2016;17:722–731. doi: 10.1093/ehjci/jew066. [DOI] [PubMed] [Google Scholar]
- 67.Pinamonti B, Di Lenarda A, Sinagra G, Camerini F. Restrictive left ventricular filling pattern in dilated cardiomyopathy assessed by Doppler echocardiography: clinical, echocardiographic and hemodynamic correlations and prognostic implications. Heart Muscle Disease Study Group. J Am Coll Cardiol. 1993;22:808–815. doi: 10.1016/0735-1097(93)90195-7. [DOI] [PubMed] [Google Scholar]
- 68.Xie GY, Berk MR, Smith MD, Gurley JC, De Maria AN. Prognostic value of Doppler transmitral flow patterns in patients with congestive heart failure. J Am Coll Cardiol. 1994;24:132–139. doi: 10.1016/0735-1097(94)90553-3. [DOI] [PubMed] [Google Scholar]
- 69.Acil T, Wichter T, Stypmann J, Janssen F, Paul M, Grude M, Scheld HH, Breithardt G, Bruch C. Prognostic value of tissue Doppler imaging in patients with chronic congestive heart failure. Int J Cardiol. 2005;103:175–181. doi: 10.1016/j.ijcard.2004.08.048. [DOI] [PubMed] [Google Scholar]
- 70.Rossi A, Temporelli PL, Quintana M, Dini FL, Ghio S, Hillis GS, Klein AL, Marsan NA, Prior DL, Yu CM, et al. Independent relationship of left atrial size and mortality in patients with heart failure: an individual patient meta-analysis of longitudinal data (MeRGE Heart Failure) Eur J Heart Fail. 2009;11:929–936. doi: 10.1093/eurjhf/hfp112. [DOI] [PubMed] [Google Scholar]
- 71.Hsiao S-H, Chiou K-R. Left atrial expansion index predicts all-cause mortality and heart failure admissions in dyspnoea. Eur J Heart Fail. 2013;15:1245–1252. doi: 10.1093/eurjhf/hfbib87. [DOI] [PubMed] [Google Scholar]
- 72.Ghio S, Gavazzi A, Campana C, Inserra C, Klersy C, Sebastiani R, Arbustini E, Recusani F, Tavazzi L. Independent and additive prognostic value of right ventricular systolic function and pulmonary artery pressure in patients with chronic heart failure. J Am Coll Cardiol. 2001;37:183–188. doi: 10.1016/S0735-1097(00)01102-5. [DOI] [PubMed] [Google Scholar]
- 73.Shah AM, Claggett B, Sweitzer NK, Shah SJ, Anand IS, Liu L, Pitt B, Pfeffer MA, Solomon SD. Prognostic importance of impaired systolic function in heart failure with preserved ejection fraction and the impact of spironolactone. Circulation. 2015;132:402–414. doi: 10.1161/CIRCULATIONAHA.115.015884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Huang W, Chai SC, Lee SGS, MacDonald MR, Leong KTG. Prognostic factors after index hospitalization for heart failure with preserved ejection fraction. Am J Cardiol. 2017;119:2017–2020. doi: 10.1016/j.amjcard.2017.03.032. [DOI] [PubMed] [Google Scholar]
- 75.Biering-Sørensen T, Santos M, Rivero J, McCullough SD, West E, Opotowsky AR, Waxman AB, Systrom DM, Shah AM. Left ventricular deformation at rest predicts exercise-induced elevation in pulmonary artery wedge pressure in patients with unexplained dyspnoea. Eur J Heart Fail. 2017;19:101–110. doi: 10.1002/ejhf.659. [DOI] [PubMed] [Google Scholar]
- 76.Wang J, Fang F, Wai-Kwok Yip G, Sanderson JE, Feng W, Xie JM, Luo XX, Lee AP, Lam YY. Left ventricular long-axis performance during exercise is an important prognosticator in patients with heart failure and preserved ejection fraction. Int J Cardiol. 2015;178:131–135. doi: 10.1016/j.ijcard.2014.10.130. [DOI] [PubMed] [Google Scholar]
- 77.Okura H, Kubo T, Asawa K, Toda I, Yoshiyama M, Yoshikawa J, Yoshida K. Elevated E/E′ predicts prognosis in congestive heart failure patients with preserved systolic function. Circ J. 2009;73:86–91. doi: 10.1253/circj.cj-08-0457. [DOI] [PubMed] [Google Scholar]
- 78.Santos AB, Roca GQ, Claggett B, Sweitzer NK, Shah SJ, Anand IS, Fang JC, Zile MR, Pitt B, Solomon SD, et al. Prognostic relevance of left atrial dysfunction in heart failure with preserved ejection fraction. Circ Heart Fail. 2016;9:e002763. doi: 10.1161/CIRCHEARTFAILURE.115.002763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Melenovsky V, Hwang S-J, Redfield MM, Zakeri R, Lin G, Borlaug BA. Left atrial remodeling and function in advanced heart failure with preserved or reduced ejection fraction. Circ Heart Fail. 2015;8:295–303. doi: 10.1161/CIRCHEARTFAILURE.114.001667. [DOI] [PubMed] [Google Scholar]
- 80.Lam CSP, Roger VL, Rodeheffer RJ, Borlaug BA, Enders FT, Redfield MM. Pulmonary hypertension in heart failure with preserved ejection fraction: a community-based study. J Am Coll Cardiol. 2009;53:1119–1126. doi: 10.1016/j.jacc.2008.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Melenovsky V, Hwang S-J, Lin G, Redfield MM, Borlaug BA. Right heart dysfunction in heart failure with preserved ejection fraction. Eur Heart J. 2014;35:3452–3462. doi: 10.1093/eurheartj/ehu193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mohammed SF, Hussain I, AbouEzzeddine OF. Takahama H, Kwon SH, Forfia P, Roger VL, Redfield MM. Right ventricular function in heart failure with preserved ejection fraction: a community-based study. Circulation. 2014;130:2310–2320. doi: 10.1161/CIRCULATIONAHA.113.008461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Olsen FJ, Jørgensen PG, Dons M, Svendsen JH, Køber L, Jensen JS, Biering-Sørensen T. Echocardiographic quantification of systolic function during atrial fibrillation: probing the ‘ten heart cycles’ rule. Futur Cardiol. 2016;12:159–165. doi: 10.2217/fca.15.77. [DOI] [PubMed] [Google Scholar]
- 84.Von Jeinsen B, Short M, Larson M et al Prognostic significance of echocardiographic measures of cardiac remodeling. Journal of the American Society of Echocardiograph 33:72–81. 10.1016/j.echo.2019.08.001 [DOI] [PMC free article] [PubMed]
- 85.Hsiao SH, Chu KA, Wu CJ, Chiou KR. Left atrial expansion index predicts left ventricular filling pressure and adverse events in acute heart failure with severe left ventricular dysfunction. J Card Fail. 2016;22(4):272–279. doi: 10.1016/j.cardfail.2016.01.009. [DOI] [PubMed] [Google Scholar]
- 86.Yip G, Wang M, Zhang Y, Fung JWH, Ho PY, Sanderson JE. Left ventricular long axis function in diastolic heart failure is reduced in both diastole and systole: time for a redefinition? Heart. 2002;87:121–125. doi: 10.1136/heart.87.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kraigher-Krainer E, Shah AM, Gupta DK, Santos A, Claggett B, Pieske B, Zile MR, Voors AA, Lefkowitz MP, Packer M, et al. Impaired systolic function by strain imaging in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2014;63:447–456. doi: 10.1016/j.jacc.2013.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang J, Khoury DS, Yue Y, Torre-Amione G, Nagueh SF. Preserved left ventricular twist and circumferential deformation, but depressed longitudinal and radial deformation in patients with diastolic heart failure. Eur Heart J. 2008;29:1283–1289. doi: 10.1093/eurheartj/ehn141. [DOI] [PubMed] [Google Scholar]
- 89.Singh A, Addetia K, Maffessanti F, Mor-Avi V, Lang RM. LA strain categorization of LV diastolic dysfunction. JACC Cardiovasc Imaging. 2017;10:735–743. doi: 10.1016/j.jcmg.2016.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zugck C, Kruger C, Durr S, et al. Is the 6-minute walk test a reliable substitute for peak oxygen uptake in patients with dilated cardiomyopathy? Eur Heart J. 2000;21:540–549. doi: 10.1053/euhj.1999.1861. [DOI] [PubMed] [Google Scholar]
- 91.Guazzi M, Dickstein K, Vicenzi M, Arena R. Six-minute walk test and cardiopulmonary exercise testing in patients with chronic heart failure: a comparative analysis on clinical and prognostic insights. Circ Heart Fail. 2009;2:549–555. doi: 10.1161/CIRCHEARTFAILURE.109.881326. [DOI] [PubMed] [Google Scholar]
- 92.Hsich E, Gorodeski EZ, Starling RC, Blackstone EH, Ishwaran H, Lauer MS. Importance of treadmill exercise time as an initial prognostic screening tool in patients with systolic left ventricular dysfunction. Circulation. 2009;119:3189–3197. doi: 10.1161/CIRCULATIONAHA.109.848382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bittner V, Weiner DH, Yusuf S, Rogers WJ, McIntyre KM, Bangdiwala SI, et al. Prediction of mortality and morbidity with a 6-minute walk test in patients with left ventricular dysfunction. SOLVD Investigators. JAMA. 1993;270:1702–1707. [PubMed] [Google Scholar]
- 94.Rostagno C, Olivo G, Comeglio M, Boddi V, Banchelli M, Galanti G, Gensini GF. Prognostic value of 6-minute walk corridor test in patients with mild to moderate heart failure: comparison with other methods of functional evaluation. Eur J Heart Fail. 2003;5:247–252. doi: 10.1016/s1388-9842(02)00244-1. [DOI] [PubMed] [Google Scholar]
- 95.Cahalin LP, Mathier MA, Semigran MJ, Dec GW, DiSalvo TG. The six-minute walk test predicts peak oxygen uptake and survival in patients with advanced heart failure. Chest. 1996;110:325–332. doi: 10.1378/chest.110.2.325. [DOI] [PubMed] [Google Scholar]
- 96.Arslan S, Erol MK, Gundogdu F, et al. Prognostic value of 6-minute walk test in stable outpatients with heart failure. Tex Heart Inst J. 2007;34(2):166–169. [PMC free article] [PubMed] [Google Scholar]
- 97.Ingle L, Cleland JG, Clark AL. The long-term prognostic significance of 6-minute walk test distance in patients with chronic heart failure. Biomed Res Int. 2014;7:2014. doi: 10.1155/2014/505969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.den Boer SL, Flipse DHK, van der Meulen MH, et al. Six-minute walk test as a predictor for outcome in children with dilated cardiomyopathy and chronic stable heart failure. Pediatr Cardiol. 2017;38:465–471. doi: 10.1007/s00246-016-1536-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Faggiano P, D’Aloia A, Gualeni A et al (2004) The 6 minute walking test in chronic heart failure: indications, interpretation and limitations from a review of the literature. Eur J Heart Fail. 10.1016/j.ejheart.2003.11.024 [DOI] [PubMed]
- 100.Zugck C, Kruger C, Durr S. Is the 6-min walk test a reliable substitute for peak oxygen uptake in patients with dilated cardiomyopathy. Eur Heart J 200021540549 [DOI] [PubMed]
- 101.Opasich C, Pinna GD, Mazza A. Six-minute walking performance in patients with moderate-to-severe heart failure. It is a useful indicator in clinical practice. Eur Heart J 200122488496 [DOI] [PubMed]
- 102.Steffen TM, Hacker TA, Mollinger L (2002) Age- and gender-related test performance in community-dwelling elderly people: 6-min walk test, Berg balance scale, timed up and go test and gait speeds. Phys Ther [DOI] [PubMed]
- 103.ATS statement: guidelines for the 6-min walking test. Am J Respir Crit Care Med 2002; 166: 111–117 [DOI] [PubMed]
- 104.Enright PL, Sherrill DL. Reference equations for the 6-min walk in healthy adults. Am J Respir Crit Care Med 199815813841387 [DOI] [PubMed]
- 105.Malhotra R, Bakken K, D’Elia E, Lewis GD. Cardiopulmonary exercise testing in heart failure. JACC Heart Fail. 2016;4(8):607–616. doi: 10.1016/j.jchf.2016.03.022. [DOI] [PubMed] [Google Scholar]
- 106.Ritt LE, Myers J, Stein R, et al. Additive prognostic value of a cardiopulmonary exercise test score in patients with heart failure and intermediate risk. Int J Cardiol. 2015;178:262–264. doi: 10.1016/j.ijcard.2014.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.O’Neill JO, Young JB, Pothier CE, Lauer MS. Peak oxygen consumption as a predictor of death in patients with heart failure receiving beta-blockers. Circulation. 2005;111:2313–2318. doi: 10.1161/01.CIR.0000164270.72123.18. [DOI] [PubMed] [Google Scholar]
- 108.Arena R, Myers J, Abella J, et al. Determining the preferred percent-predicted equation for peak oxygen consumption in patients with heart failure. Circ Heart Fail. 2009;2:113–120. doi: 10.1161/CIRCHEARTFAILURE.108.834168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Weber KT, Kinasewitz GT, Janicki JS, et al. Oxygen utilization and ventilation during exercise in patients with chronic cardiac failure. Circulation. 1982;65:1213–1223. doi: 10.1161/01.cir.65.6.1213. [DOI] [PubMed] [Google Scholar]
- 110.Sun XG, Hansen JE, Beshai JF, Wasserman K. Oscillatory breathing and exercise gas exchange abnormalities prognosticate early mortality and morbidity in heart failure. J Am Coll Cardiol. 2010;55:1814–1823. doi: 10.1016/j.jacc.2009.10.075. [DOI] [PubMed] [Google Scholar]
- 111.Osada N, Chaitman BR, Miller LW, et al. Cardiopulmonary exercise testing identifies low risk patients with heart failure and severely impaired exercise capacity considered for heart transplantation. J Am Coll Cardiol. 1998;31:577–582. doi: 10.1016/s0735-1097(97)00533-0. [DOI] [PubMed] [Google Scholar]
- 112.Myers J, Arena R, Dewey F, et al. A cardiopulmonary exercise testing score for predicting outcomes in patients with heart failure. Am Heart J. 2008;156:1177–1183. doi: 10.1016/j.ahj.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 113.Wasserman K (2002) Cardiopulmonary exercise testing and cardiovascular health. Armonk, NY: Futura Pub. Co
- 114.Guazzi M, Raimondo R, Vicenzi M, et al. Exercise oscillatory ventilation may predict sudden cardiac death in heart failure patients. J Am Coll Cardiol. 2007;50:299–308. doi: 10.1016/j.jacc.2007.03.042. [DOI] [PubMed] [Google Scholar]
- 115.Ramalho SHR, Cipriano Junior G, Vieira PJC, et al. Inspiratory muscle strength and six-minute walking distance in heart failure: prognostic utility in a 10 years follow up cohort study. PLoS One. 2019;14(8):e0220638. doi: 10.1371/journal.pone.0220638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Meyer FJ, Borst MM, Zugck C, Kirschke A, Schellberg D, Kubler W, et al. Respiratory muscle dysfunction in congestive heart failure: clinical correlation and prognostic significance. Circulation. 2001;103(17):2153–2158. doi: 10.1161/01.cir.103.17.2153. [DOI] [PubMed] [Google Scholar]
- 117.Frankenstein L, Meyer FJ, Sigg C, Nelles M, Schellberg D, Remppis A, et al. Is serial determination of inspiratory muscle strength a useful prognostic marker in chronic heart failure? Eur J Cardiovasc Prev Rehabil. 2008;15(2):156–161. doi: 10.1097/HJR.0b013e3282f0d6ea. [DOI] [PubMed] [Google Scholar]
- 118.Frankenstein L, Nelles M, Meyer FJ, Sigg C, Schellberg D, Remppis BA, et al. (2009) Validity, prognostic value and optimal cutoff of respiratory muscle strength in patients with chronic heart failure changes with beta-blocker treatment. Eur J Cardiovasc Prev Rehabil 16(4):424–9. 10.1097/HJR.0b013e3283030a7e [DOI] [PubMed]
- 119.Ketchum E, & Levy W (2011). Establishing prognosis in heart failure: a multimarker approach. Progress in Cardiovascular Diseases, 54(2), 86-96. [DOI] [PubMed]
- 120.Nadar SK, Shaikh MM (2019) Biomarkers in routine heart failure clinical care. Card Fail Rev 5(1):50–56. 10.15420/cfr.2018.27.2 [DOI] [PMC free article] [PubMed]
- 121.Latini R, Masson S, Anand I, Salio M, Hester A, Judd D, Barlera S, Maggioni AP, Tognoni G, Cohn JN (2004) The comparative prognostic value of plasma neurohormones at baseline in patients with heart failure enrolled in Val-HeFT. Eur Heart J 25:292–299 [DOI] [PubMed]
- 122.Doust JA, Pietrzak E, Dobson A, Glasziou P (2005) How well does B-type natriuretic peptide predict death and cardiac events in patients with heart failure: systematic review, BMJ 330:625-634 [DOI] [PMC free article] [PubMed]
- 123.Maisel A, Hollander JE, Guss D, McCullough P, Nowak R, Green G, Saltzberg M, Ellison SR, Bhalla MA, Bhalla V, Clopton P, Jesse R (2004) Primary results of the Rapid Emergency Department Heart Failure Outpatient Trial (REDHOT). A multicenter study of B-type natriuretic peptide levels, emergency department decision making, and outcomes in patients presenting with shortness of breath. J Am Coll Cardiol 44:1328-1333 [DOI] [PubMed]
- 124.Januzzi JL Jr., Rehman SU, Mohammed AA et al. (2011) Use of amino-terminal pro-B-type natriuretic peptide to guide outpatient therapy of patients with chronic left ventricular systolic dysfunction. J Am Coll Cardiol 58:1881–9. 10.1016/j.jacc.2011.03.072 [DOI] [PubMed]
- 125.Mueller C, McDonald K, de Boer RA, Maisel A, Cleland JGF, Kozhuharov N, Coats AJS, Metra M, Mebazaa A, Ruschitzka F, Lainscak M, Filippatos G, Seferovic PM, Meijers WC, Bayes-Genis A, Mueller T, Richards M, Januzzi JL, Jr, on behalf of the Heart Failure Association of the European Society of Cardiology Heart Failure Association of the European Society of Cardiology practical guidance on the use of natriuretic peptide concentrations. Eur J Heart Fail. 2019;1:715–731. doi: 10.1002/ejhf.1494. [DOI] [PubMed] [Google Scholar]
- 126.Tromp J, Khan MAF, Mentz RJ, et al. (2017) Biomarker Profiles of acute heart failure patients with a mid-range ejection fraction. JACC: Heart Failure 5:507-517
- 127.Tromp JB, Westenbrink D, Ouwerkerk W, et al. (2018) Identifying pathophysiological mechanisms in heart failure with reduced versus preserved ejection fraction. J Am Coll Cardiol 72 (10) 1081-1090. [DOI] [PubMed]
- 128.Bielecka-Dabrowa A, Gluba-Brzózka A, Michalska-Kasiczak M, et al. (2015) The multi-biomarker approach for heart failure in patients with hypertension. Int J Mol Sci 16:10715-10733. [DOI] [PMC free article] [PubMed]
- 129.Michalska-Kasiczak M, Bielecka-Dabrowa A, von Haehling S et al. (2018) Biomarkers, myocardial fibrosis and co-morbidities in heart failure with preserved ejection fraction: an overview. Arch Med Sci 14:890–909. 10.5114/aoms.2018.76279. [DOI] [PMC free article] [PubMed]
- 130.Friedl A, Stoesz SP, Buckley P, Gould MN (1999) Neutrophil gelatinase-associated lipocalin in normal and neoplastic human tissues. Cell type-specific pattern of expression. Histochem J 31:433–41. 10.1023/A:1003708808934. [DOI] [PubMed]
- 131.Corsten MF, Dennert R, Jochems S et al. (2010) Circulating MicroRNA-208b and MicroRNA-499 reflect myocardial damage in cardiovascular disease. Circ Cardiovasc Genet 3:499–506. 10.1161/CIRCGENETICS.110.957415. [DOI] [PubMed]
- 132.Yan H, Ma F, Zhang Y et al. (2017) miRNAs as biomarkers for diagnosis of heart failure: a systematic review and meta-analysis. Medicine (Baltimore) 96:e6825. 10.1097/MD.0000000000006825 [DOI] [PMC free article] [PubMed]
- 133.Komajda M, Anker S, Cowie M, et al. (2016) Physician’s adherence to guideline-recommended medications in heart failure with reduced ejection fraction: data from the QUALIFY global survey. Eur J Heart Fail 18:514–522. doi:10.1002/ejhf.510. [DOI] [PubMed]
- 134.Sosnowska-Pasiarska B, Bartkowiak R, Wożakowska-Kapłon B, et al. (2013) Population of Polish patients participating in the Heart Failure Pilot Survey (ESC-HF Pilot). Kardiol Pol 71:234-240. 10.5603/KP.2013.0034. [DOI] [PubMed]
- 135.Maggioni AP, Dahlstrom U, Filippatos G, et al. (2010) EURObservational Research Programme: the Heart Failure Pilot Survey (ESC-HF Pilot). Eur J Heart Fail 12:1076-1084. 10.1093/eurjhf/hfq154. [DOI] [PubMed]
- 136.Fonarow GC, Adams KF, Abraham WT, et al. (2005) Risk stratification for in-hospital mortality in acutely decompensated heart failure: classification and regression tree analysis (ADHERE). JAMA 293(5): 572–580, 10.1001/jama.293.5.572 [DOI] [PubMed]
- 137.Auble TE, Hsieh M, Gardner W, et al. (2005) A prediction rule to identify low-risk patients with heart failure. Acad Emerg Med 12(6): 514–521, 10.1197/j.aem.2004.11.026 [DOI] [PubMed]
- 138.Abraham WT, Fonarow GC, Albert NM, et al. (2008) Predictors of in-hospital mortality in patients hospitalized for heart failure: insights from the Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF). J Am Coll Cardiol 52(5): 347–356, 10.1016/j.jacc.2008.04.028 [DOI] [PubMed]
- 139.Peterson PN, Rumsfeld JS, Liang Li, et al. (2010) American Heart Association get with the guidelines-heart failure program. A validated risk score for in-hospital mortality in patients with heart failure from the American Heart Association get with the guidelines program. Circ Cardiovasc Qual Outcomes 3(1): 25–32, 10.1161/CIRCOUTCOMES.109.854877 [DOI] [PubMed]
- 140.Aaronson KD, Schwartz JS, Chen TM, et al. (1997) Development and prospective validation of a clinical index to predict survival in ambulatory patients referred for cardiac transplant evaluation. Circulation 95(12): 2660–2667, 10.1161/01.CIR.95.12.2660 [DOI] [PubMed]
- 141.Levy WC, Mozaffarian D, Linker DT, et al. (2006) The Seattle Heart Failure Model: prediction of survival in heart failure. Circulation 113(11): 1424–1433, doi: 10.1161/CIRCULATIONAHA.105.584102 [DOI] [PubMed]
- 142.Pocock SJ, Ariti CA, McMurray JJV, et al. (2013) Meta-analysis global group in chronic heart failure. Predicting survival in heart failure: a risk score based on 39 372 patients from 30 studies. Eur Heart J 34(19): 1404–1413, 10.1093/eurheartj/ehs337 [DOI] [PubMed]
- 143.Pocock SJ, Wang D, Pfeffer MA et al. (2006) Predictors of mortality and morbidity in patients with chronic heart failure. Eur Heart J 27: 65–75 [DOI] [PubMed]
- 144.Komajda M., Carson P.E., Hetzel S. et al. (2011) Factors associated with outcome in heart failure with preserved ejection fraction: findings from the Irbesartan in Heart Failure with Preserved Ejection Fraction Study (I-PRESERVE). Circ Heart Fail 4: 27–35 [DOI] [PubMed]
- 145.Rohde LE, Goldraich L, Polanczyk CA, Borges AP, Biolo A, Rabelo E, Beck-Da-Silva L, Clausell N. A simple clinically based predictive rule for heart failure in-hospital mortality. J Card Fail. 2006;12:587–593. doi: 10.1016/j.cardfail.2006.06.475. [DOI] [PubMed] [Google Scholar]
- 146.Chuda A, Berner J, Lelonek M. The journey of the heart failure patient, based on data from a single center. Advances in Clinical and Experimental Medicine: official organ Wroclaw Medical University. 2019;28(4):489–498. doi: 10.17219/acem/78688. [DOI] [PubMed] [Google Scholar]
- 147.Allen LA, Matlock DD, Shetterly SM, et al. Use of risk models to predict death in the next year among individual ambulatory patients with heart failure. JAMA Cardiol. 2017;2(4):435–441. doi: 10.1001/jamacardio.2016.5036. [DOI] [PubMed] [Google Scholar]