Abstract
The genus Anastatus comprises a large group of parasitoids, including several biological control agents in agricultural and forest systems. The taxonomy and phylogeny of these species remain controversial. In this study, the mitogenome of A. fulloi Sheng and Wang was sequenced and characterized. The nearly full-length mitogenome of A. fulloi was 15,692 bp, compromising 13 protein-coding genes (PCGs), 2 rRNA genes, 22 tRNA genes and a control region (CR). The total A + T contents were 83.83%, 82.18%, 87.58%, 87.27%, and 82.13% in the whole mitogenome, 13 PCGs, 22 tRNA genes, 2 rRNA genes, and CR, respectively. The mitogenome presented negative AT skews and positive GC skews, except for the CR. Most PCGs were encoded on the heavy strand, started with ATN codons, and ended with TAA codons. Among the 3736 amino acid-encoding codons, TTA (Leu1), CGA (Arg), TCA (Ser2), and TCT (Ser2) were predominant. Most tRNAs had cloverleaf secondary structures, except trnS1, with the absence of a dihydrouridine (DHU) arm. Compared with mitogenomes of the ancestral insect and another parasitoid within Eupelmidae, large-scale rearrangements were found in the mitogenome of A. fulloi, especially inversions and inverse transpositions of tRNA genes. The gene arrangements of parasitoid mitogenomes within Chalcidoidea were variable. A novel gene arrangement was presented in the mitogenome of A. fulloi. Phylogenetic analyses based on the 13 protein-coding genes of 20 parasitoids indicated that the phylogenetic relationship of 6 superfamilies could be presented as Mymaridae + (Eupelmidae + (Encyrtidae + (Trichogrammatidae + (Pteromalidae + Eulophidae)))). This study presents the first mitogenome of the Anastatus genus and offers insights into the identification, taxonomy, and phylogeny of these parasitoids.
Subject terms: Evolutionary biology, Phylogenetics
Introduction
Mitochondria are double-membrane-bound organelles that are widely found in eukaryotic cells1,2. Their genomes, i.e., mitogenomes, are small in size and have the characteristics of maternal inheritance, conserved gene components, absence of introns, rare recombination, and a relatively high evolution rate3–5. Therefore, the mitogenome is a suitable molecular marker that has been widely applied in molecular identification, evolution, and phylogeny6–8. In recent years, an increasing number of mitogenomes have been sequenced, analysed, and deposited in the NCBI database9–11. These mitogenomes provide valuable information not only about nucleotide composition but also genome-level characteristics12.
In general, a typical mitogenome of an insect is a circular molecule with double strands, ranging from 14 to 19 kb in size13,14. It usually contains four components, including 13 protein-coding genes (PCGs), 2 ribosomal RNA genes (rRNAs), 22 transfer RNA genes (tRNAs), and a control region (CR)15,16. Most insect mitogenomes share an identical gene order; however, rearrangements of genes have been found in the mitogenomes of some species, and these rearrangements include transposition, inversion, inverse transposition, and tandem duplication random loss (TDRL)5,17. To date, the base composition and gene order of mitogenomes have been extensively applied to clarify evolutionary events in certain groups of insects18–20.
Most Anastatus (Hymenoptera: Chalcidoidea) species are parasitoids of numerous insect species, and several species of Anastatus have been evaluated as biological control agents for various pests in agricultural and forest systems21,22. These insects represent a large family with approximately 150 recognized species but remain largely unstudied23. The current morphology-based taxonomy of Anastatus is problematic due to early taxonomic confusion, low degrees of morphological differentiation, and morphological variation related to host and geographical origin23–25. Parasitoids within Chalcidoidea are some of the most diverse hymenopterous insects, exhibiting the characteristics of frequent gene rearrangement, high substitution rates, and a strong base composition bias in mitogenomes26,27. Although the mitogenomes of other hymenopterous insects have been applied for identification, evolution, and phylogeny, this is not the case for Anastatus parasitoids. To date, mitogenomes of Anastatus parasitoids are still not available in the NCBI database.
Next-generation sequencing (NGS) is an increasingly applied technology used to obtain large-scale genetic information. It provides the full-length sequences of insect mitogenomes practically and effectively. In the present study, we applied NGS to obtain the complete mitogenome of A. fulloi Sheng and Wang, which represented the first sequenced mitochondrial genome in the Anastatus genus. The mitogenome was characterized, including the structure of the mitogenome, gene organization, nucleotides, amino acid composition, codon usage, and tRNA secondary structure. Gene rearrangement was discussed between several mitogenomes of parasitoids and the ancestral insect. In addition, phylogenetic analyses were performed based on the 13 PCGs of 20 parasitoids within Chalcidoidea. This study provides information about the taxonomy and phylogeny of this special insect and unveils the phylogenetic position of Anastatus within Chalcidoidea.
Methods
Insect collection and DNA extraction
Adult specimens of A. fulloi were obtained from the Plant Protection Research Institute, Guangdong Academy of Agricultural Sciences, People’s Republic of China. All specimens were preserved in 100% alcohol and stored at − 20 °C. Total genomic DNA was extracted by using a DNeasy tissue kit (Qiagen, Hilden, Germany) according to the manufacturer’s protocols. Subsequently, total DNA was quantified and tested by a Qubit fluorometer with a dsDNA high-sensitivity kit (ThermoFisher, Foster City, CA, USA) and agarose gel (1%) electrophoresis.
Mitochondrial genome sequencing and assembly
At least 1 μg of DNA was used to construct the sequencing library using the TruSeq DNA Library Preparation kit according to standard protocols. The library was then sequenced by the Illumina HiSeq™4000 (Illumina, USA) platform with paired-end reads of 2 × 150 bases. A total of 4.79 Gb of clean data was obtained with a Q30 of 92.16%. SPAdes v3.10.1 (http://bioinf.spbau.ru/spades)28 and A5-miseq v2015052229 were used to assemble the clean data into contigs and scaffolding. The sequences with high sequencing coverage were extracted to identify the mitochondrial sequences by the NCBI NT library using BLASTN (BLAST v2.2.31+). MUMmer v3.130 was used to determine the contig positions and fill the gaps between contigs. Through Pilon v1.1831, the results were error-corrected to obtain the final mitochondrial sequence.
Genome annotation and analysis
The mitogenome of A. fulloi was annotated on MITOS (http://mitos.bioinf.uni-leipzig.de/index.py). The secondary structure of tRNAs was also predicted by MITOS. Annotation results were confirmed by comparison with homologous sequences in the NCBI database, and then submitted to NCBI. A mitogenome map of A. fulloi was generated by mtviz (http://pacosy.informatik.uni-leipzig.de/mtviz/mtviz).
The base composition and relative synonymous codon usage (RSCU) values of PCGs were calculated by MEGA 6.0. The AT and GC skews were counted using the following formulas: AT skew = (A − T)/(A + T) and GC skew = (G − C)/(G + C). The gene orders of parasitoid mitogenomes within Chalcidoidea were compared by CREx (http://pacosy.informatik.uni-leipzig.de/crex/form#INFO)32.
All mitogenome information from 21 species was downloaded, including 19 parasitoids within Chalcidoidea and 2 outgroups. Together with the mitogenome of A. fulloi, they were used to extract the 13 PCGs through PhyloSuite v1.2.233. These PCGs were aligned by MAFFT 7.14934 and MACSE v. 2.0335. Conserved blocks were obtained using Gblocks v0.91b36. MrBayes on XSEDE and RAxML on XSEDE (CIPRES portal) were employed to construct the bayesian inference (BI) and maximum likelihood (ML) phylogenetic trees, respectively. ModelFinder was used to evaluate the best evolutionary model37. GTR + F + I + G4 was selected by the Bayesian information criterion (BIC). The MrBayes analyses ran as 4 independent Markov chains for 3 million generations, sampled every 1000 generations. A burn-in of 25% was used to generate the consensus tree. The RAxML analysis was performed with 1000 replicates of ultrafast likelihood bootstrap. FigTree v1.4.2 was employed to edit two phylogenetic trees.
Results and discussion
General features of the mitogenome
The mitochondrial sequence of A. fulloi has been submitted to GenBank with the accession number OK545741. The nearly complete mitogenome was 15,692 bp in length, within the range of other hymenopterous mitogenomes: from 15,137 (Idris sp.) to 20,370 bp (Trachelus iudaicus)38,39. We failed to obtain the complete CR using next-generation and Sanger sequencing, which may be attributed to the comparatively high A + T content and presence of repeat units in CR19,40,41. Therefore, the A. fulloi mitogenome contained 37 genes (13 protein-coding genes, 2 rRNA genes, and 22 tRNA genes) and a partial CR (Table 1, Fig. 1). This is a common gene set for most hymenopterous mitogenomes17,42,43.
Table 1.
Characteristics of the mitochondrial genome of A. fulloi.
| Feature | Strand | Start sites | Stop sites | Size(bp) | Anticodon | Start codon | End codon | Intergenic nucleotides |
|---|---|---|---|---|---|---|---|---|
| trnV | + | 1 | 66 | 66 | UAC | − 2 | ||
| rrnS | + | 65 | 834 | 770 | 5 | |||
| trnA | + | 840 | 904 | 65 | UGC | − 18 | ||
| rrnL | + | 887 | 2252 | 1366 | 1 | |||
| trnL1 | + | 2254 | 2320 | 67 | UAG | − 3 | ||
| nad1 | + | 2318 | 3265 | 948 | ATA | TAA | 9 | |
| trnS2 | − | 3275 | 3342 | 68 | UGA | 2 | ||
| cytb | − | 3345 | 4481 | 1137 | ATG | TAA | 10 | |
| nad6 | − | 4492 | 5061 | 570 | AAC | TAA | − 2 | |
| trnT | − | 5060 | 5127 | 68 | UGU | 12 | ||
| trnP | + | 5140 | 5206 | 67 | UGG | 4 | ||
| nad4l | + | 5211 | 5498 | 288 | ATT | TAA | − 7 | |
| nad4 | + | 5492 | 6838 | 1347 | ATG | TAA | 0 | |
| trnH | + | 6839 | 6906 | 68 | GUG | − 3 | ||
| nad5 | + | 6904 | 8581 | 1678 | ATA | T | − 3 | |
| trnF | + | 8579 | 8648 | 70 | GAA | 16 | ||
| trnE | − | 8665 | 8732 | 68 | UUC | 6 | ||
| cox1 | + | 8739 | 10,280 | 1542 | ATA | TAA | 32 | |
| trnL2 | + | 10,313 | 10,379 | 67 | UAA | 0 | ||
| cox2 | + | 10,380 | 11,102 | 723 | ATT | TAA | 9 | |
| trnK | − | 11,112 | 11,182 | 71 | UUU | 13 | ||
| trnD | + | 11,196 | 11,266 | 71 | GUC | 30 | ||
| atp8 | + | 11,297 | 11,458 | 162 | ATT | TAA | − 7 | |
| atp6 | + | 11,452 | 12,123 | 672 | ATG | TAA | − 1 | |
| cox3 | + | 12,123 | 12,914 | 792 | ATT | TAA | 43 | |
| trnG | + | 12,958 | 13,022 | 65 | UCC | 18 | ||
| nad3 | + | 13,041 | 13,409 | 369 | ATA | TAG | 6 | |
| trnS1 | − | 13,416 | 13,475 | 60 | UCU | 3 | ||
| trnY | + | 13,479 | 13,549 | 71 | GUA | 82 | ||
| trnN | + | 13,632 | 13,698 | 67 | GUU | 129 | ||
| trnC | − | 13,828 | 13,889 | 62 | GCA | 53 | ||
| trnR | − | 13,943 | 14,007 | 65 | UCG | 6 | ||
| trnQ | − | 14,014 | 14,082 | 69 | UUG | − 2 | ||
| trnW | − | 14,081 | 14,145 | 65 | UCA | 2 | ||
| nad2 | − | 14,148 | 15,164 | 1017 | ATA | TAA | 29 | |
| trnI | + | 15,194 | 15,263 | 70 | GAU | 11 | ||
| trnM | − | 15,275 | 15,345 | 71 | CAU | 0 | ||
| CR | 15,346 | 15,692 | 347 |
+ represents the heavy strand; − represents the light strand.
Figure 1.
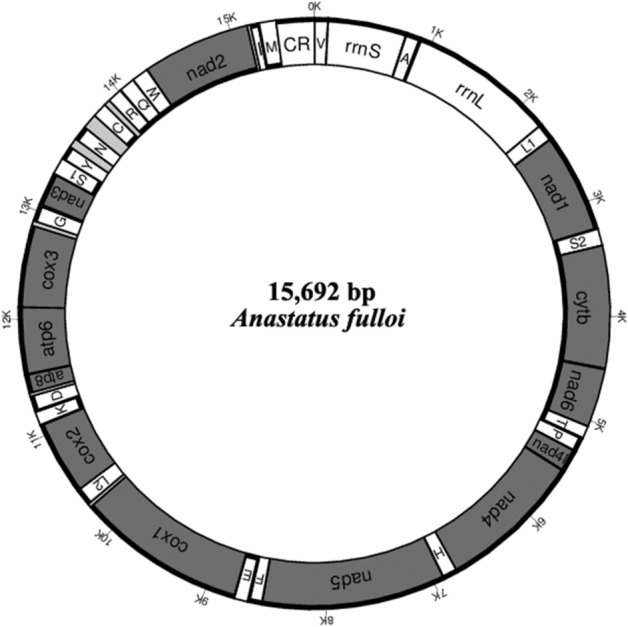
Mitochondrial map of Anastatus fulloi. PCGs are marked in grey. Genes marked with bold lines outside the circle map are encoded on the heavy strand.
Overlapping and intergenic regions are usually detected in hymenopterous mitogenomes18,20,44. Generally, the total length of overlapping regions is smaller in size than intergenic regions in most hymenopterous insects19,45,46. In the mitogenome of A. fulloi, a total of 10 overlapping regions were found, ranging in size from 1 to 18 bp (Table 1). The longest overlapping region was found between trnA and rrnL. The A. fulloi mitogenome also contained 24 intergenic spacers with a total length of 531 bp. These intergenic spacers range from 1 to 129 bp (Table 1). The longest gene spacer was located between trnC and trnN. Notably, an overlap between atp6 and atp8 was a common feature of metazoan mitogenomes47 and it was also found in A. fulloi. Moreover, an overlap between nad4 and nad4l widely occurred among mitogenomes of A. fulloi and other hymenopteran parasitoids17,18,20, which may be translated as a bicstron48.
Significant bias in nucleotide composition is typical in insect mitogenomes19 and the nucleotide compositional bias of mitogenomes is usually assessed by non-strand specific (A + T content, G + C content) and strand-specific, namely strand asymmetry (ATskew, GC-skew)49. The nucleotides of the A. fulloi mitogenome comprise A (39.06%), T (44.77%), C (6.02%), and G (10.15%) (Table 2). The A. fulloi mitogenome was highly biased towards A and T nucleotides with an A + T content of 83.83%. In addition, the mitogenome of A. fulloi had a negative AT skew (− 0.069) and a positive GC skew (0.255) (Table 2). This indicates that the mitogenome of A. fulloi contains more T than A and more G than C, as reported in other mitogenomes of hymenopterous species20,50. This common pattern of base composition in mitogenome may be attributed to the highly asymmetric effects of transcription on mutagenesis, including unequal exposure of the strands to DNA damage and the differential chance for repair51,52.
Table 2.
Nucleotide composition of A. fulloi mitochondrial genome.
| Feature | A% | T% | C% | G% | AT% | GC% | AT Skew | GC Skew |
|---|---|---|---|---|---|---|---|---|
| Protein-coding genes | 35.13 | 47.05 | 8.00 | 9.82 | 82.18 | 17.82 | − 0.145 | 0.102 |
| 1st codon position | 38.92 | 38.92 | 7.92 | 14.24 | 77.84 | 22.16 | 0.000 | 0.285 |
| 2nd codon position | 22.04 | 52.62 | 13.71 | 11.63 | 74.66 | 25.34 | − 0.410 | − 0.082 |
| 3rd codon position | 44.42 | 49.63 | 2.37 | 3.58 | 94.05 | 5.95 | − 0.055 | 0.203 |
| tRNAs | 43.08 | 44.50 | 4.59 | 7.83 | 87.58 | 12.42 | − 0.016 | 0.261 |
| rRNAs | 43.59 | 43.68 | 4.03 | 8.71 | 87.27 | 12.74 | − 0.001 | 0.367 |
| CR | 41.21 | 40.92 | 6.63 | 11.24 | 82.13 | 17.87 | 0.004 | 0.258 |
| Whole mitogenome | 39.06 | 44.77 | 6.02 | 10.15 | 83.83 | 16.17 | − 0.069 | 0.255 |
PCGs
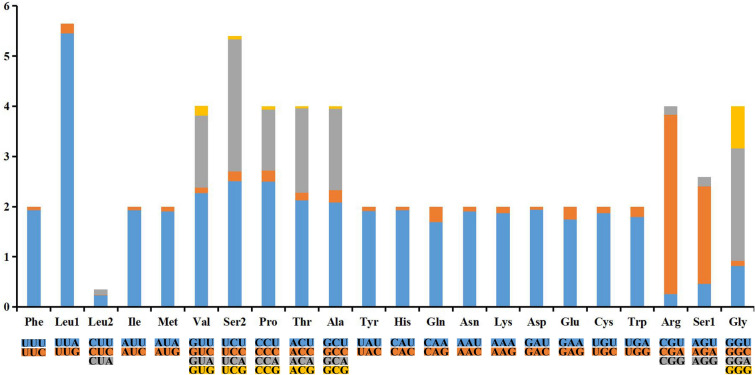
The total length of 13 PCGs was 11,245 bp, accounting for 71.66% of the whole mitogenome. This set of PCGs is conserved in animal mitogenomes, with the exception of nematodes and a bivalve that lack atp815. These PCGs range in size from 162 bp (atp8) to 1678 bp (nad5). The A + T content of all PCGs was 82.18% (Table 2). A remarkably high A + T content (94.05%) was found at the third codon sites of these PCGs, which may partly reflect the high bias towards A and T nucleotides in the mitogenome19,53,54. Meanwhile, the AT skew and GC skew of the PCGs were − 0.145 and 0.102, respectively. Of the 13 PCGs, 10 were encoded on the heavy strand, whereas cytb, nad2, and nad6 were encoded on the light strand. In insect mitogenomes, PCGs usually start with ATN codons (ATA, ATT, ATC, and ATG) and terminate with TAA or TAG55–57. However, unusual start- and termination codons were simultaneously and exclusively found, such as the start codons of TTG, CGA, GTG, and the incomplete stop codon of T58–60 In the A. fulloi mitogenome, most PCGs started with ATN, including ATA (nad1-3, nad5, and cox1), ATT (atp8, cox2, cox3, and nad4l) and ATG (atp6 and nad4). However, nad6 used an atypical starting codon of AAC, which has also been found in Cheirotonus jansoni and Prosopocoilus gracilis61,62. All PCGs terminated with TAA except nad3 (TAG) and nad5 (incomplete codon T). Previous studies have inferred that the incomplete termination codon could be completed by posttranscriptional polyadenylation15,63,64. The codon usage of PCGs was assessed by the relative synonymous codon usage (RSCU) value (Fig. 2). There is a clear preference for A or T in the third codon. Of the 3736 amino acid-encoding codons, TTA (Leu1), CGA (Arg), TCA (Ser2), and TCT (Ser2) were predominant. Codons such as CGC, AGC, and CTG were not presented.
Figure 2.
Relative synonymous codon usage of PCGs in the Anastatus fulloi mitogenome.
tRNAs, rRNAs and the control region
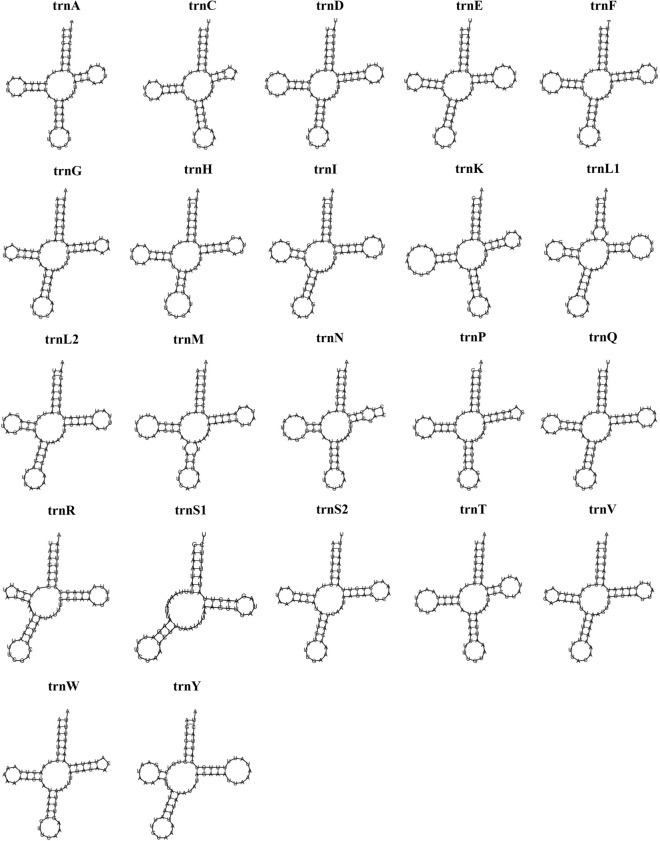
The total length of 22 tRNAs was 1481 bp, accounting for 9.44% of the whole A. fulloi mitogenome (Table 2). These tRNAs range from 60 to 71 bp, within the size range observed in hymenopteran parasitoids17,27,42 (Table 1). Among these tRNAs, 12 tRNAs were coded in the heavy strand, and the remaining 10 tRNAs were identified in the light strand. These tRNAs had a high A + T content of 87.58%, a slightly negative AT skew value (− 0.016), and a positive GC skew value (0.261). Except for the absence of a dihydrouridine (DHU) arm in trnS1, most tRNAs had a cloverleaf secondary structure (Fig. 3). The loss of the DHU arm in trnS1 is normal in insects5,65.
Figure 3.
Predicted secondary structures of the 22 typical tRNA genes in the Anastatus fulloi mitogenome.
Two rRNAs, i.e., the small ribosomal RNA (rrnS) and large ribosomal RNA (rrnL), were 770 bp and 1366 bp in length, respectively. These lengths are similar to those of most reported hymenopterous insects27,66. These rRNAs were located on the heavy strand and were separated by trnA. They consisted of A (43.59%), T (43.68%), C (4.03%), and G (8.71%), with an A + T content of 87.27%. The AT skew and GC skew were − 0.001 and 0.367, respectively.
In insect mitogenomes, the control region, i.e., the A + T-rich region, is associated with replication and transcription67,68. This region is variable not only in size but also in base composition69. The CR was 3308 bp with an A + T content of 85.9% in Pteromalus puparum70 but 578 bp with an A + T content of 93.6% in Spathius agrili18. In addition, repeat structures are usually detected in CR, which is also diverse in both type and size among insect mitogenomes69. The high A + T content and presence of repeat regions may result in the failure of obtaining CR sequence due to the inhibition of DNA polymerase40,71. Currently, the long and complex control region still presents challenges to obtaining complete mitogenomes in certain groups of parasitoids19,20,27,41,72,73. In this study, we failed to obtain the complete segment of the CR by next-generation sequencing or Sanger sequencing. The partial control region obtained for the A. fulloi mitogenome was 347 bp with an A + T content of 82.13% and was located between trnM and trnV.
Gene rearrangement
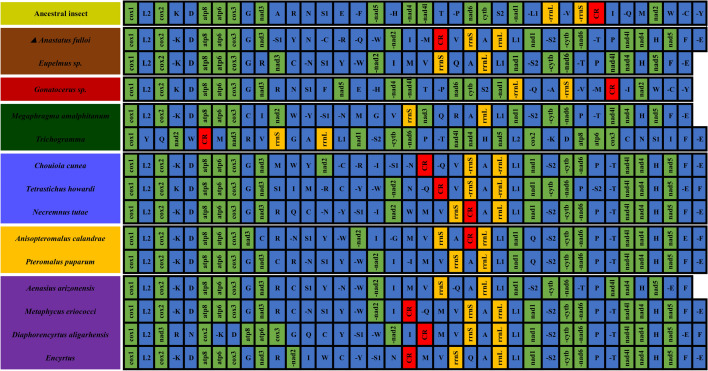
Mitochondrial gene rearrangements occur frequently in hymenopterous insects74, which are important clues to evolution and are regarded as valuable phylogenetic characters for these insects26. In this study, a comparison of gene-order data was conducted to examine the gene rearrangements. The gene orders of parasitoid mitogenomes assigned to 6 families are shown in Fig. 4. Species from different genera possessed unique gene orders. Compared with the gene order in the ancestral insect mitogenome, parasitoid mitogenomes exhibit large-scale rearrangement events for tRNA genes and PCGs, as reported in other studies of parasitoid mitogenomes17,27,75. In particular, all chalcidoid wasps exhibited an inversion gene block from nad5 to rrnL except Gonatocerus sp. Phylogenetically Gonatocerus sp. comes closer to the ancestor and agrees with the genetic order as well. Despite another relatively conserved gene block from cox1 to cox3, the remaining genes are highly rearranged. Although the gene orders of these mitogenomes from different families are variable, infrequent rearrangements were found between closely related species. Within Eupelmidae, mitogenomes of A. fulloi and Eupelmus sp. also exhibit different gene orders that are attributed to the rearrangement of a few tRNAs. Species from the same genus, such as species of Trichogramma and or Encyrtus, showed the same gene order in mitogenomes. In this study, mitochondrial gene rearrangements are diverse in chalcidoid wasps but occur infrequently in closely related taxa, which might be useful for phylogenetic analysis.
Figure 4.
Gene arrangement in the mitogenome of Chalcidoidea.
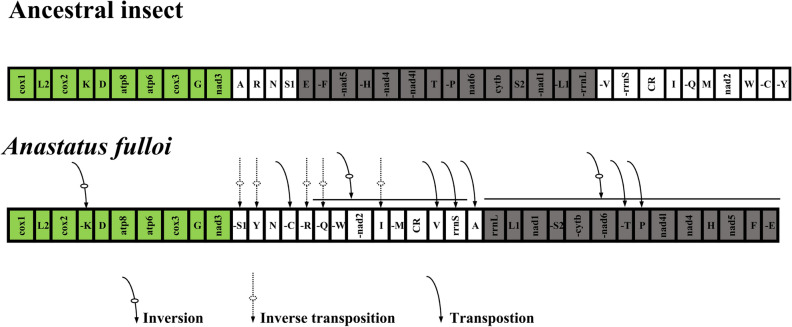
CREx analysis demonstrated that the gene order of the mitogenome of A. fulloi is novel. Compared with the gene order in the ancestral insect mitogenome, the segment between cox1 and nad3 was relatively conserved except for the inversion of trnK, which is the same as Metaphycus eriococci, Encyrtus sasakii, and Chouioia cunea76 (Fig. 5). It has been reported that the segment “trnE -trnF -nad5 -trnH -nad4 -nad4l trnT -trnP nad6 cytb” is conserved between Megraphragma and Philotrypesis44,77. However, in the mitogenome of A. fulloi, the segment “trnE -trnF -nad5 -trnH -nad4 -nad4l trnT -trnP nad6 cytb trnS2 -nad1 -trnL1 -rrnL” was inversed, and trnT -trnP have exchanged their positions. The segment “-trnV -rrnS CR trnI -trnQ trnM nad2 trnW” was highly rearranged, including the inversions of trnV, rrnS, trnM, nad2 and trnW, position exchanges of trnV and rrnS, trnM and trnI, and the transposition of trnQ (Fig. 5). In addition, trnC and trnY were transported to the segment “trnR trnN trnS1”. The gene order of these tRNA genes was changed to “-trnS1 trnY trnN -trnC -trnR”.
Figure 5.
Comparison of gene arrangement between mitogenomes of Anastatus fulloi and ancestral insects through CREx analysis. The inverted gene block is shown in grey. Conserved gene block is marked in green.
Phylogenetic analyses
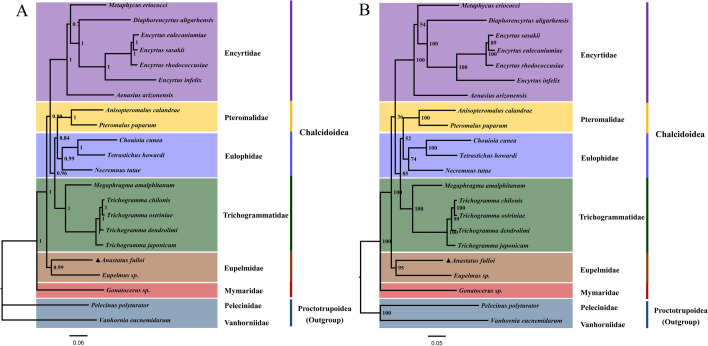
The phylogenetic relationships of 20 parasitoids within Chalcidoidea were analysed and are displayed in Fig. 6. Maximum likelihood (ML) and Bayesian inference (BI) phylogenetic trees were constructed based on nucleotide sequences of 13 PCGs of these mitogenomes in CIPRES78. Although some clades exhibited low support values, the same topological structures were found in two phylogenetic trees. Two species within Eupelmidae, A. fulloi and Eupelmus sp., were clustered together. Other species within the same families were grouped and separated from other parasitoids in different families. In Chalcidoidea, Eupelmidae is considered to be closely related to Encyrtidae, and to share several of the same features, such as an expanded acropleuron79,80. However, this family is not monophyletic, representing a grade rather than a clade24,81,82. Some species may show a close relationship to Pteromalidae79,82. In the present study, the phylogenetic relationship of parasitoids within Chalcidoidea can be presented as follows: Mymaridae + (Eupelmidae + (Encyrtidae + (Trichogrammatidae + (Pteromalidae + Eulophidae)))). This result is consistent with other reports27,43.
Figure 6.
Phylogenetic trees of Chalcidoidea inferred using MrBayes ((A) BI) and maximum likelihood ((B) ML) analyses based on 13 PCGs.
Acknowledgements
This work was financially supported by the GDAS’ Project of Science and Technology Development (Grant Nos. 2020GDASYL-20200103072 & 2020GDASYL-20200103056). We thank Xinxia Feng and Xi Yuan for their technical assistance.
Author contributions
J.Q.Y. and H.W. conceived and designed research. J.Q.Y., J.B.L., Y.F.Z., Y.J.C., J.H.L., Y.G. and Y.L.L. conducted experiments and analysed data. All Authors critically reviewed and approved the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Osellame LD, Blacker TS, Duchen MR. Cellular and molecular mechanisms of mitochondrial function. Best Pract. Res. Clin. Endocrinol. Metab. 2012;26:711–723. doi: 10.1016/j.beem.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bernt M, Braband A, Schierwater B, Stadler PF. Genetic aspects of mitochondrial genome evolution. Mol. Phylogenet. Evol. 2013;69:328–338. doi: 10.1016/j.ympev.2012.10.020. [DOI] [PubMed] [Google Scholar]
- 3.Curole JP, Kocher TD. Mitogenomics: Digging deeper with complete mitochondrial genomes. Trends Ecol. Evol. 1999;14:394–398. doi: 10.1016/s0169-5347(99)01660-2. [DOI] [PubMed] [Google Scholar]
- 4.Shao R, Dowton M, Murrell A, Barker SC. Rates of gene rearrangement and nucleotide substitution are correlated in the mitochondrial genomes of insects. Mol. Biol. Evol. 2003;20:1612–1619. doi: 10.1093/molbev/msg176. [DOI] [PubMed] [Google Scholar]
- 5.Cameron SL. Insect mitochondrial genomics: Implications for evolution and phylogeny. Annu. Rev. Entomol. 2014;59:95–117. doi: 10.1146/annurev-ento-011613-162007. [DOI] [PubMed] [Google Scholar]
- 6.Lopez-Lopez A, Vogler AP. The mitogenome phylogeny of Adephaga (Coleoptera) Mol. Phylogenet. Evol. 2017;114:166–174. doi: 10.1016/j.ympev.2017.06.009. [DOI] [PubMed] [Google Scholar]
- 7.Li Q, et al. The complete mitochondrial genomes of five important medicinal Ganoderma species: Features, evolution, and phylogeny. Int. J. Biol. Macromol. 2019;139:397–408. doi: 10.1016/j.ijbiomac.2019.08.003. [DOI] [PubMed] [Google Scholar]
- 8.Shang Y, et al. Comparative mitogenomic analysis of forensically important sarcophagid flies (Diptera: Sarcophagidae) and implications of species identification. J. Med. Entomol. 2019;56:392–407. doi: 10.1093/jme/tjy162. [DOI] [PubMed] [Google Scholar]
- 9.Riyaz M, Shah RA, Savarimuthu I, Kuppusamy S. Comparative mitochondrial genome analysis of Eudocima salaminia (Cramer, 1777) (Lepidoptera: Noctuoidea), novel gene rearrangement and phylogenetic relationship within the superfamily Noctuoidea. Mol. Biol. Rep. 2021;48:4449–4463. doi: 10.1007/s11033-021-06465-z. [DOI] [PubMed] [Google Scholar]
- 10.Ye F, Li H, Xie Q. Mitochondrial genomes from two specialized subfamilies of Reduviidae (Insecta: Hemiptera) reveal novel gene rearrangements of true Bugs. Genes. 2021;12:1134. doi: 10.3390/genes12081134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang X, Wang J, Dai R. Structural features of the mitogenome of the leafhopper genus Cladolidia (Hemiptera: Cicadellidae: Coelidiinae) and phylogenetic implications in Cicadellidae. Ecol. Evol. 2021;11:12554–12566. doi: 10.1002/ece3.8001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tyagi K, et al. Rearrangement and evolution of mitochondrial genomes in Thysanoptera (Insecta) Sci. Rep. 2020;10:695. doi: 10.1038/s41598-020-57705-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dai LS, et al. Mitochondrial genome of Diaphania indica (Saunders) (Lepidoptera: Pyraloidea) and implications for its phylogeny. Int. J. Biol. Macromol. 2018;108:981–989. doi: 10.1016/j.ijbiomac.2017.11.011. [DOI] [PubMed] [Google Scholar]
- 14.Kumar V, et al. The first complete mitochondrial genome of marigold pest thrips, Neohydatothrips samayunkur (Sericothripinae) and comparative analysis. Sci. Rep. 2019;9:191. doi: 10.1038/s41598-018-37889-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boore JL. Animal mitochondrial genomes. Nucleic Acids Res. 1999;27:1767–1780. doi: 10.1093/nar/27.8.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Du Y, Zhang C, Dietrich CH, Zhang Y, Dai W. Characterization of the complete mitochondrial genomes of Maiestas dorsalis and Japananus hyalinus (Hemiptera: Cicadellidae) and comparison with other Membracoidea. Sci. Rep. 2017;7:14197. doi: 10.1038/s41598-017-14703-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen L, et al. Extensive gene rearrangements in the mitochondrial genomes of two egg parasitoids, Trichogramma japonicum and Trichogramma ostriniae (Hymenoptera: Chalcidoidea: Trichogrammatidae) Sci. Rep. 2018;8:7034. doi: 10.1038/s41598-018-25338-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wei S, Tang P, Zheng L, Shi M, Chen X. The complete mitochondrial genome of Evania appendigaster (Hymenoptera: Evaniidae) has low A + T content and a long intergenic spacer between atp8 and atp6. Mol. Biol. Rep. 2010;37:1931–1942. doi: 10.1007/s11033-009-9640-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu JC, et al. The first two mitochondrial genomes of the family Aphelinidae with novel gene orders and phylogenetic implications. Int. J. Biol. Macromol. 2018;118:386–396. doi: 10.1016/j.ijbiomac.2018.06.087. [DOI] [PubMed] [Google Scholar]
- 20.Powell C, Caleca V, Rhode C, Teixeira DCL, van Asch B. New mitochondrial gene rearrangement in Psyttalia concolor, P. humilis and P. lounsburyi (Hymenoptera: Braconidae), three parasitoid species of economic interest. Insects. 2020;11:854. doi: 10.3390/insects11120854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stahl JM, Babendreier D, Haye T. Life history of Anastatus bifasciatus, a potential biological control agent of the brown marmorated stink bug in Europe. Biol. Control. 2019;129:178–186. [Google Scholar]
- 22.Yong-Ming C, et al. Performances of six eupelmid egg parasitoids from China on Japanese giant silkworm Caligula japonica with different host age regimes. J. Pest Sci. 2020;94:309. [Google Scholar]
- 23.Peng L, Gibson G, Tang LU, Xiang J. Review of the species of Anastatus (Hymenoptera: Eupelmidae) known from China, with description of two new species with brachypterous females. Zootaxa. 2020;4767:4763–4767. doi: 10.11646/zootaxa.4767.3.1. [DOI] [PubMed] [Google Scholar]
- 24.Peng LF, Lin NQ. Recent advances in Eupelmidae (Hymenoptera: Chalcidoidea) systematics. Fujian J. Agric. Sci. 2012;27:1269–1273. [Google Scholar]
- 25.Fusu L, Ebrahimi E, Siebold C, Villemant C. Revision of the Eupelmidae Walker, 1833 described by Jean Risbec. Part 1: The slide mounted specimens housed at the Muséum national d’Histoire naturelle in Paris. Zoosystema. 2015;37:457–480. [Google Scholar]
- 26.Feng Z, et al. Evolution of tRNA gene rearrangement in the mitochondrial genome of ichneumonoid wasps (Hymenoptera: Ichneumonoidea) Int. J. Biol. Macromol. 2020;164:540–547. doi: 10.1016/j.ijbiomac.2020.07.149. [DOI] [PubMed] [Google Scholar]
- 27.Wu Y, et al. Novel gene rearrangement in the mitochondrial genome of Pachyneuron aphidis (Hymenoptera: Pteromalidae) Int. J. Biol. Macromol. 2020;149:1207–1212. doi: 10.1016/j.ijbiomac.2020.01.308. [DOI] [PubMed] [Google Scholar]
- 28.Bankevich A, et al. SPAdes: A new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 2012;19:455–477. doi: 10.1089/cmb.2012.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coil D, Jospin G, Darling AE. A5-Miseq: An updated pipeline to assemble microbial genomes from Illumina MiSeq data. Bioinformatics. 2015;31:587–589. doi: 10.1093/bioinformatics/btu661. [DOI] [PubMed] [Google Scholar]
- 30.Kurtz S, et al. Versatile and open software for comparing large genomes. Genome Biol. 2004;5:R12. doi: 10.1186/gb-2004-5-2-r12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walker BJ, et al. Pilon: An integrated tool for comprehensive microbial variant detection and genome assembly improvement. PLoS ONE. 2014;9:e112963. doi: 10.1371/journal.pone.0112963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bernt M, et al. CREx: Inferring genomic rearrangements based on common intervals. Bioinformatics. 2007;23:2957–2958. doi: 10.1093/bioinformatics/btm468. [DOI] [PubMed] [Google Scholar]
- 33.Zhang D, et al. PhyloSuite: An integrated and scalable desktop platform for streamlined molecular sequence data management and evolutionary phylogenetics studies. Mol. Ecol. Resour. 2020;20:348–355. doi: 10.1111/1755-0998.13096. [DOI] [PubMed] [Google Scholar]
- 34.Katoh K, Standley DM. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013;30:772–780. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ranwez V, Douzery E, Cambon C, Chantret N, Delsuc F. MACSE v2: Toolkit for the alignment of coding sequences accounting for frameshifts and stop codons. Mol. Biol. Evol. 2018;35:2582–2584. doi: 10.1093/molbev/msy159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Castresana J. Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Mol. Biol. Evol. 2000;17:540–552. doi: 10.1093/oxfordjournals.molbev.a026334. [DOI] [PubMed] [Google Scholar]
- 37.Kalyaanamoorthy S, Minh BQ, Wong T, von Haeseler A, Jermiin LS. ModelFinder: Fast model selection for accurate phylogenetic estimates. Nat. Methods. 2017;14:587–589. doi: 10.1038/nmeth.4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dowton M, Cameron SL, Austin AD, Whiting MF. Phylogenetic approaches for the analysis of mitochondrial genome sequence data in the Hymenoptera—A lineage with both rapidly and slowly evolving mitochondrial genomes. Mol. Phylogenet. Evol. 2009;52:512–519. doi: 10.1016/j.ympev.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 39.Korkmaz EM, Aydemir HB, Temel B, Budak M, Başıbüyük HH. Mitogenome evolution in Cephini (Hymenoptera: Cephidae): Evidence for parallel adaptive evolution. Biochem. Syst. Ecol. 2017;71:137–146. [Google Scholar]
- 40.Wei SJ, Li Q, van Achterberg K, Chen XX. Two mitochondrial genomes from the families Bethylidae and Mutillidae: Independent rearrangement of protein-coding genes and higher-level phylogeny of the Hymenoptera. Mol. Phylogenet. Evol. 2014;77:1–10. doi: 10.1016/j.ympev.2014.03.023. [DOI] [PubMed] [Google Scholar]
- 41.Shen ZC, Chen L, Chen L, Li YX. Information from the mitochondrial genomes of two egg parasitoids, Gonatocerus sp. and Telenomus sp., reveals a controversial phylogenetic relationship between Mymaridae and Scelionidae. Genomics. 2019;111:1059–1065. doi: 10.1016/j.ygeno.2018.06.009. [DOI] [PubMed] [Google Scholar]
- 42.Yang J, Liu HX, Li YX, Wei ZM. The rearranged mitochondrial genome of Podagrion sp. (Hymenoptera: Torymidae), a parasitoid wasp of mantis. Genomics. 2019;111:436–440. doi: 10.1016/j.ygeno.2018.02.020. [DOI] [PubMed] [Google Scholar]
- 43.Xing ZP, et al. Complete mitochondrial genome of a parasitoid, Trichogramma chilonis (Hymenoptera: Chalcidoidea: Trichogrammatidae) and phylogenetic analysis. Mitochondrial DNA B Resour. 2021;6:2466–2467. doi: 10.1080/23802359.2021.1955636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xiao JH, Jia JG, Murphy RW, Huang DW. Rapid evolution of the mitochondrial genome in Chalcidoid wasps (Hymenoptera: Chalcidoidea) driven by parasitic lifestyles. PLoS ONE. 2011;6:e26645. doi: 10.1371/journal.pone.0026645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wei SJ, Shi M, He JH, Sharkey M, Chen XX. The complete mitochondrial genome of Diadegma semiclausum (Hymenoptera: Ichneumonidae) indicates extensive independent evolutionary events. Genome. 2009;52:308–319. doi: 10.1139/g09-008. [DOI] [PubMed] [Google Scholar]
- 46.Zhang QH, Huang P, Chen B, Li TJ. The complete mitochondrial genome of Orancistrocerus aterrimus aterrimus and comparative analysis in the family Vespidae (Hymenoptera, Vespidae, Eumeninae) ZooKeys. 2018;790:127–144. doi: 10.3897/zookeys.790.25356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Campbell NJ, Barker SC. The novel mitochondrial gene arrangement of the cattle tick, Boophilus microplus: Fivefold tandem repetition of a coding region. Mol. Biol. Evol. 1999;16:732–740. doi: 10.1093/oxfordjournals.molbev.a026158. [DOI] [PubMed] [Google Scholar]
- 48.Stewart JB, Beckenbach AT. Insect mitochondrial genomics: The complete mitochondrial genome sequence of the meadow spittlebug Philaenus spumarius (Hemiptera: Auchenorrhyncha: Cercopoidae) Genome. 2005;48:46–54. doi: 10.1139/g04-090. [DOI] [PubMed] [Google Scholar]
- 49.Negrisolo E, Babbucci M, Patarnello T. The mitochondrial genome of the ascalaphid owlfly Libelloides macaronius and comparative evolutionary mitochondriomics of neuropterid Insects. BMC Genomics. 2011;12:221. doi: 10.1186/1471-2164-12-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wu QL, et al. The complete mitochondrial genome of Taeniogonalos taihorina (Bischoff) (Hymenoptera: Trigonalyidae) reveals a novel gene rearrangement pattern in the Hymenoptera. Gene. 2014;543:76–84. doi: 10.1016/j.gene.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 51.Francino MP, Ochman H. Strand asymmetries in DNA evolution. Trends Genet. 1997;13:240–245. doi: 10.1016/S0168-9525(97)01118-9. [DOI] [PubMed] [Google Scholar]
- 52.Hassanin A, Leger N, Deutsch J. Evidence for multiple reversals of asymmetric mutational constraints during the evolution of the mitochondrial genome of Metazoa, and consequences for phylogenetic inferences. Syst. Biol. 2005;54:277–298. doi: 10.1080/10635150590947843. [DOI] [PubMed] [Google Scholar]
- 53.Chai HN, Du YZ. The complete mitochondrial genome of the pink stem borer, Sesamia inferens, in comparison with four other Noctuid moths. Int. J. Mol. Sci. 2012;13:10236–10256. doi: 10.3390/ijms130810236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ma Z, et al. Comparative mitogenomics of the genus Odontobutis (Perciformes: Gobioidei: Odontobutidae) revealed conserved gene rearrangement and high sequence variations. Int. J. Mol. Sci. 2015;16:25031–25049. doi: 10.3390/ijms161025031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yi J, Que S, Xin T, Xia B, Zou Z. Complete mitochondrial genome of Thitarodes pui (Lepidoptera: Hepialidae) Mitochondrial DNA A DNA Mapp. Seq. Anal. 2016;27:109–110. doi: 10.3109/19401736.2013.873926. [DOI] [PubMed] [Google Scholar]
- 56.Li J, et al. Mitochondrial genome characteristics of two Sphingidae insects (Psilogramma increta and Macroglossum stellatarum) and implications for their phylogeny. Int. J. Biol. Macromol. 2018;113:592–600. doi: 10.1016/j.ijbiomac.2018.02.159. [DOI] [PubMed] [Google Scholar]
- 57.Wang W, et al. Characterization of the complete mitochondrial genomes of two species of the genus Aphaena Guerin-Meneville (Hemiptera: Fulgoridae) and its phylogenetic implications. Int. J. Biol. Macromol. 2019;141:29–40. doi: 10.1016/j.ijbiomac.2019.08.222. [DOI] [PubMed] [Google Scholar]
- 58.Wang JJ, Yang MF, Dai RH, Li H, Wang XY. Characterization and phylogenetic implications of the complete mitochondrial genome of Idiocerinae (Hemiptera: Cicadellidae) Int. J. Biol. Macromol. 2018;120:2366–2372. doi: 10.1016/j.ijbiomac.2018.08.191. [DOI] [PubMed] [Google Scholar]
- 59.Huang Y, et al. Comparative mitochondrial genome analysis of Grammodes geometrica and other noctuid insects reveals conserved mitochondrial genome organization and phylogeny. Int. J. Biol. Macromol. 2019;125:1257–1265. doi: 10.1016/j.ijbiomac.2018.09.104. [DOI] [PubMed] [Google Scholar]
- 60.Sun Z, et al. Mitochondrial genome of Phalantus geniculatus (Hemiptera: Reduviidae): trnT duplication and phylogenetic implications. Int. J. Biol. Macromol. 2019;129:110–115. doi: 10.1016/j.ijbiomac.2019.01.205. [DOI] [PubMed] [Google Scholar]
- 61.Shao LL, et al. Complete mitochondrial genome sequence of Cheirotonus jansoni (Coleoptera: Scarabaeidae) Genet. Mol. Res. 2014;13:1047–1058. doi: 10.4238/2014.February.20.6. [DOI] [PubMed] [Google Scholar]
- 62.Wu YY, Cao YY, Fang J, Wan X. The first complete mitochondrial genome of stag beetle from China, Prosopocoilus gracilis (Coleoptera, Lucanidae) Mitochondrial DNA A DNA Mapp. Seq. Anal. 2016;27:2633–2634. doi: 10.3109/19401736.2015.1041129. [DOI] [PubMed] [Google Scholar]
- 63.Anderson S, et al. Sequence and organization of the human mitochondrial genome. Nature. 1981;290:457–465. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- 64.Ojala D, Montoya J, Attardi G. tRNA punctuation model of RNA processing in human mitochondria. Nature. 1981;290:470–474. doi: 10.1038/290470a0. [DOI] [PubMed] [Google Scholar]
- 65.Juhling F, et al. Improved systematic tRNA gene annotation allows new insights into the evolution of mitochondrial tRNA structures and into the mechanisms of mitochondrial genome rearrangements. Nucleic Acids Res. 2012;40:2833–2845. doi: 10.1093/nar/gkr1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Aydemir MN, Korkmaz EM. Comparative mitogenomics of Hymenoptera reveals evolutionary differences in structure and composition. Int. J. Biol. Macromol. 2020;144:460–472. doi: 10.1016/j.ijbiomac.2019.12.135. [DOI] [PubMed] [Google Scholar]
- 67.Taanman JW. The mitochondrial genome: Structure, transcription, translation and replication. Biochim. Biophys. Acta. 1999;1410:103–123. doi: 10.1016/s0005-2728(98)00161-3. [DOI] [PubMed] [Google Scholar]
- 68.Cameron SL, et al. Mitochondrial genome organization and phylogeny of two vespid wasps. Genome. 2008;51:800–808. doi: 10.1139/G08-066. [DOI] [PubMed] [Google Scholar]
- 69.Zhang D, Hewitt GM. Insect mitochondrial control region: A review of its structure, evolution and usefulness in evolutionary studies. Biochem. Syst. Ecol. 1997;25:99–120. [Google Scholar]
- 70.Yan Z, et al. Mitochondrial DNA and their nuclear copies in the parasitic wasp Pteromalus puparum: A comparative analysis in Chalcidoidea. Int. J. Biol. Macromol. 2019;121:572–579. doi: 10.1016/j.ijbiomac.2018.10.039. [DOI] [PubMed] [Google Scholar]
- 71.Hu M, Jex AR, Campbell BE, Gasser RB. Long PCR amplification of the entire mitochondrial genome from individual helminths for direct sequencing. Nat. Protoc. 2007;2:2339–2344. doi: 10.1038/nprot.2007.358. [DOI] [PubMed] [Google Scholar]
- 72.Mao M, Valerio A, Austin AD, Dowton M, Johnson NF. The first mitochondrial genome for the wasp superfamily Platygastroidea: The egg parasitoid Trissolcus basalis. Genome. 2012;55:194–204. doi: 10.1139/g2012-005. [DOI] [PubMed] [Google Scholar]
- 73.Oliveira DS, Gomes TM, Loreto EL. The rearranged mitochondrial genome of Leptopilina boulardi (Hymenoptera: Figitidae), a parasitoid wasp of Drosophila. Genet. Mol. Biol. 2016;39:611–615. doi: 10.1590/1678-4685-GMB-2016-0062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dowton M, Cameron SL, Dowavic JI, Austin AD, Whiting MF. Characterization of 67 mitochondrial tRNA gene rearrangements in the Hymenoptera suggests that mitochondrial tRNA gene position is selectively neutral. Mol. Biol. Evol. 2009;26:1607. doi: 10.1093/molbev/msp072. [DOI] [PubMed] [Google Scholar]
- 75.Lin ZJ, et al. Comparative analysis reveals the expansion of mitochondrial DNA control region containing unusually high G-C tandem repeat arrays in Nasonia vitripennis. Int. J. Biol. Macromol. 2021;166:1246–1257. doi: 10.1016/j.ijbiomac.2020.11.007. [DOI] [PubMed] [Google Scholar]
- 76.Tang X, et al. The mitochondrial genome of a parasitic wasp, Chouioia cunea Yang (Hymenoptera: Chalcidoidea: Eulophidae) and phylogenetic analysis. Mitochondrial DNA B Resour. 2021;6:872–874. doi: 10.1080/23802359.2021.1886008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nedoluzhko AV, et al. Mitochondrial genome of Megaphragma amalphitanum (Hymenoptera: Trichogrammatidae). Mitochondrial DNA A DNA Mapp. Seq. Anal. 2016;27:4526–4527. doi: 10.3109/19401736.2015.1101546. [DOI] [PubMed] [Google Scholar]
- 78.Miller MA, et al. A RESTful API for access to phylogenetic tools via the CIPRES science gateway. Evol. Bioinform. 2015;11:43–48. doi: 10.4137/EBO.S21501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.LaSalle J. New world Tanaostigmatidae (Hymenoptera, Chalcidoidea) Contrib. Am. Entomol. Inst. 1987;23:1–181. [Google Scholar]
- 80.Munro JB, et al. A molecular phylogeny of the Chalcidoidea (Hymenoptera) PLoS ONE. 2011;6:e27023. doi: 10.1371/journal.pone.0027023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gary APG. Phylogeny and classification of Eupelmidae, with a revision of the world genera of Calosotinae and Metapelmatinae (Hymenoptera: Chalcidoidea) Mem. Entomol. Soc. Can. 1989;121:3–121. [Google Scholar]
- 82.Gibson GA. Description of Leptoomus janzeni, N. Gen. and N. sp. (Hymenoptera: Chalcidoidea) from Baltic amber, and discussion of its relationships and classification relative to Eupelmidae, Tanaostigmatidae and Encyrtidae. Zootaxa. 2008;1730:1–26. [Google Scholar]