Abstract
INTRODUCTION:
We investigated the role of genetic risk and adherence to lifestyle factors on cognitive decline in African Americans and European Americans.
METHODS:
Utilizing data from the Chicago Health and Aging Project (1993–2012; n=3,874), we defined the genetic risk based on presence of APOE ϵ4 allele and determined a healthy lifestyle using a scoring of five factors, non-smoking, exercising, being cognitively active, having a high-quality diet, and limiting alcohol use. We used linear mixed-effects models to estimate cognitive decline by genetic risk and lifestyle score.
RESULTS:
APOE ϵ4 allele was associated with faster cognitive decline in both races. However, within APOE ϵ4 carriers, adherence to a healthy lifestyle (e.g., 4–5 healthy factors) was associated with a slower cognitive decline by 0.023 (95%CI 0.004, 0.042) units/year in African Americans and 0.044 (95%CI 0.008, 0.080) units/year in European Americans.
DISCUSSION:
A healthy lifestyle was associated with a slower cognitive decline in African and European Americans.
Keywords: lifestyle factors, apolipoprotein E, cognitive decline, genetic epidemiology, race/ethnicity, African Americans, European Americans
Introduction
A growing body of evidence suggests that adherence to a healthy lifestyle is associated with a lower risk of developing Alzheimer’s dementia [1–6]. We have shown that individuals with four or five healthy factors –including being physically and cognitively active, consuming a high-quality diet, nonsmoking, and limiting alcohol use– have 60% lower risk of Alzheimer’s dementia than individuals with none or one healthy factor [5]. In parallel, several clinical trials are ongoing around the world (World Wide FINGERS) [7], including U.S. POINTER in the United States [8], to establish causal inferences of lifestyle factors on the prevention of Alzheimer’s dementia in older adults [9]. Positive findings from these clinical trials would have paramount importance for public health because, in the absence of treatment [10], these interventions will help prevent cognitive impairment in a vulnerable population of older adults. However, a characteristic of primary prevention clinical trials, in general, is the difficulty of enrolling diverse populations, specifically African Americans [11]. When diverse individuals are underrepresented in clinical trials, it becomes challenging to generalize the findings to these populations [12,13]. It is challenging because racial differences have been identified in Alzheimer’s research, particularly between African and European Americans [14–20]. For example, the prevalence of Alzheimer’s dementia is two to three times higher among older African Americans than European Americans [20–22], although they experience a similar or slower cognitive decline [18,23]. In addition, the prevalence of the APOE ϵ4 allele, a genetic risk factor of Alzheimer’s dementia, is higher in African Americans than European Americans [14,19]. Therefore, as we generalize findings from clinical trials across different race and ethnic groups, more research is required to understand the effect of lifestyle factors in cognitive decline among diverse groups, particularly in African Americans, and how these effects compare with European Americans [24].
In the current study, we aim to determine the role of genetic risk and lifestyle factors on cognitive decline among African and European Americans enrolled in a population-based cohort study, the Chicago Health and Aging Project. This investigation will examine whether the association of lifestyle factors with cognitive decline differs between African Americans and European Americans.
Methods
Study design, settings, and population
The Chicago Health and Aging Project (CHAP) is a longitudinal population study of common chronic health problems of older people focusing on risk factors for incident Alzheimer’s dementia in a biracial neighborhood of the south side of Chicago [14]. Initiated in 1993, CHAP enrolled 10,802 participants aged 65 years or older until 2012. Through in-home interviews, data collection was conducted at the baseline and followed by successive interview cycles at approximately 3-year intervals consisting of structured questions on a wide range of characteristics, including lifestyle factors, medical history, and cognitive performance.
Of 10,802 participants, 5,807 (53.8%) individuals were randomly selected for genetic testing. Among individuals with information on APOE genotype (n=5,807), 4,089 (70.4%) had baseline data on diet and lifestyle factors, and of those, 3,886 (95%) had two or more neuropsychological assessments during follow-up. Because our study was explicitly focused on individuals of African and European ancestry, we further excluded 12 participants with other races/ethnicities. Three thousand, eight hundred seventy-four individuals comprised our study population. Compared to CHAP participants excluded from this investigation, our study participants were 3 years younger and, on average, had 1 additional year of education (12.8 vs. 11.9 years).
The Rush University Medical Center Institutional Review Board approved this study. All participants provided informed consent to be eligible to participate in this study.
APOE Genotypes, Diet, and Lifestyle Factors
The APOE genotypes were determined based on the single nucleotide polymorphisms (SNPs) of the rs7412 and rs429358 by Broad Institute Center for Genotyping (Cambridge, MA, USA) using the hME Sequenom MassARRAY platform [25]. We categorized study participants as carriers of APOE ϵ4 if they had at least one e4 allele (e2/e4, e3/e4, e4/e4) and no carriers, participants with no e4 allele (e2/e2, e3/e3, e2/e3).
Information on the dietary intake was obtained by the administration of a validated 144-item food frequency questionnaire [26]. Briefly, study participants reported the frequency, on average, of the consumption of specific foods and beverages with pre-specified portion sizes over the past year. The overall diet quality was determined by calculating the Mediterranean-DASH Diet Intervention for Neurodegenerative Delay (MIND) diet score. The MIND diet score summarizes information on 10 brain-healthy food groups (green leafy vegetables, other vegetables, nuts, berries, beans, whole grains, seafood, poultry, olive oil, red wine) and five unhealthy food groups (red meats, butter and stick margarine, cheese, pastries and sweets, and fried/fast food) [27]. In the present analysis, alcohol intake was not included in the MIND diet score because it was investigated separately as a lifestyle factor. The frequency of alcoholic beverage intake was obtained through the food frequency questionnaires, and the average gram per day was calculated. Participants reported the time spent in moderate or vigorous activities (i.e., walking for exercise, gardening or yard work, calisthenics or general exercise, bicycle riding, and swimming) through a validated questionnaire from the 1985 U.S. Health Interview Survey that was adapted for use in older adults [28]. Smoking status (current, former, or never) was obtained during the interview [29]. Cognitively stimulating activities were assessed with a structured questionnaire that gathers information on the typical time spent during the course of a year on seven activities: reading, writing letters, visiting a library, and playing games such as chess or checkers. A composite measure of participation in cognitively stimulating activities was calculated [30].
Development of the Healthy Lifestyle Score
Based on research evidence, guidelines, and expert knowledge for the health benefits of lifestyle factors in Alzheimer’s dementia, we selected five modifiable lifestyle factors and categorized them as follows: (1) MIND diet score in the top 40% of the cohort distribution; (2) cognitive activities in the top 40% of the cohort distribution; (3) non-current smoker; (4) moderate or vigorous exercise activities for at least 150 minutes per week; and (5) light-to-moderate alcohol consumption (1–15g/day in women and 1–30g/day in men – that is, up to approximately one drink a day for women and two drinks for men) [5,31–33].
In the absence of established thresholds for a healthy diet and adequate cognitive activities, we referred to previous publications that suggested a cohort-specific cutoff, such as the upper 40% of the cohort distribution [5,31–33]. In our data, the threshold for a healthy MIND diet score was 7.5, and for adequate cognitive activity was 3.43.
We evaluated each lifestyle factor separately and scored participants 1 if they met the criterion for healthy and 0 if they did not meet the criteria. The five scores were then summed to yield a final score within the range of 0–5, with higher scores indicating a healthier lifestyle.
Demographics and Health Measures
Race was defined using questions from the 1990 U.S. Census. Education was measured as the number of years of formal schooling completed. Symptoms of depression were measured using a modified 10-item version of the Center for the Epidemiological Study of Depression Scale [34]. Body mass index (BMI; weight in kilograms divided by height in meters squared) was computed from measured weight and height. History of diabetes, cancer, heart disease, and stroke was determined by self-report questions from the Established Populations for the Epidemiologic Study of the Elderly.
Assessment of Global Cognitive Function
Cognitive function was assessed during in-home visits with a battery of four cognitive tests, including two tests of episodic memory (immediate and delayed story recall)[35,36] one test of perceptual speed [37], and one test of general orientation and global cognition (the MMSE) [38].
As described previously, for each of those cognitive tests, a z-score was calculated using the baseline mean and standard deviation in the entire population [39,40]. Then, a composite score of global cognitive function was developed based on the average z-score of all four tests. The cognitive score ranges from negative values indicating poor cognitive function, 0 indicating average cognitive function, and positive values indicating higher cognitive function. Participants were followed up to 17 years with regular assessment of cognition.
Statistical Analysis
Baseline characteristics of African Americans and European Americans are presented as mean and standard deviation (SD), percentages of participants, and median and interquartile range (IQR).
Using multivariable-adjusted linear mixed-effects models, we investigated the associations between lifestyle score, APOE ϵ4 allele, and cognitive decline in African and European Americans. Models were adjusted for age (measured in years and centered at 75), sex (males versus females), education (measured by years of schooling completed and centered at 12), comorbidities (yes versus no), practice effects of cognitive testing (square root of the number of prior assessments), and their respective interactions with time. Comorbidities included heart disease, stroke, diabetes, or cancers at the baseline. The lifestyle score was examined as a continuous and categorical variable. For the continuous analysis, we computed the risk of cognitive decline per 1-point increase (e.g., one additional healthy factor) in the healthy lifestyle score. For the categorical analysis, we grouped study participants into three levels, individuals with 0 or 1, 2 or 3, and 4 or 5 healthy factors, and used as the reference category those with 0 or 1 healthy factor.
Our primary analysis focuses on determining the impact of a healthy lifestyle on cognitive decline in African Americans and European Americans. First, we examined the relationship of APOE ϵ4 allele and lifestyle score with cognitive decline in African and European Americans. In this analysis, the associations between APOE ϵ4 and healthy lifestyle score with cognitive decline are evaluated in two separate linear mixed-effects models. Next, we developed our final model in which we investigated the role of a healthy lifestyle on cognitive decline among African and European Americans with and without the APOE ϵ4 allele.
Our sensitivity analysis included a series of models to test the robustness of our primary findings. First, in addition to primary adjustment, we controlled for depressive symptoms and body mass index. We included those variables in a sensitivity analysis because both depression and weight have complex relations with cognition; they are identified as risk factors (depression and obesity) as well as outcomes of dementia (depressive symptoms and weight loss). Second, while our primary analysis adjusted for comorbidities (e.g., diabetes, heart disease, stroke, and cancers) because comorbidities could influence adherence to a healthy lifestyle and cognitive decline, we also conducted a sensitivity analysis without adjusting for baseline comorbidities to quantify the impact of comorbidities in our associations. Third, to explore the possibility of reverse causality due to impaired cognitive functioning, which ultimately can impact reporting accuracy for lifestyle behaviors, we excluded participants with a cognitive score in the lowest 10% of the cohort distribution. Fourth, to account for possible changes in lifestyle factors during the study period, we restricted the study’s follow-up time up to 10 years and repeated our primary analysis. Fifth, while we included light-moderate alcohol use in the healthy score (according to dietary guidelines) [41], individuals who do not drink should not be encouraged to start alcohol consumption. Therefore, we created a new lifestyle score without alcohol consumption. In this analysis, we adjusted for alcohol intake in the multivariable model. Sixth, since we focus on modifiable lifestyle factors, we determined former smokers as a healthy lifestyle factor to encourage current smokers to quit. However, because former smokers may have an underlying risk of cognitive impairment due to previous smoking history, we developed a new score using “never smokers” as a healthy lifestyle factor. Seventh, racial disparities have been identified in the level/quality of education, engagement in late-life reading/cognitive activity, access to social resources, and experiences of discrimination. These disparities may contribute to differences in cognition and cognitive decline experienced by African and European Americans. To explore the role of these racially patterned risk factors on cognition, we computed a propensity score matching analysis using Nearest Neighbor Matching (full details of this method are available elsewhere [42–44]). In short, we randomly selected 500 European Americans and matched 500 African Americans based on age, gender, education, adherence to lifestyle factors, and cognitive score at the baseline. As a result, we created two comparable groups of African and European Americans regarding sample size (n=500) and baseline characteristics (demographic, lifestyle, and cognition) (Supplementary Table 1 A–C). We then conducted our primary regression analysis in each group and compared their beta estimates. Lastly, we stratified our analysis by the adherence to healthy lifestyle score and race, and in each group, we evaluated the association between APOE ϵ4 and cognitive decline.
We used linear mixed-effects models to compute the predicted probability for the rate of change in global cognition and developed trajectories according to the presence of APOE ϵ4 allele and adherence to a healthy lifestyle in African and European Americans. We examined both the linear and non-linear changes in cognitive function. To account for the non-linear relationship, we included quadratic terms of time in the multivariate model.
All the analyses were performed in R software CRAN version 4.0.0 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Table 1 shows the baseline characteristics of African Americans (n=2,359, 60.9%) and European Americans (1,515, 39.1%). Compared to European Americans, African Americans were, on average, younger (71y vs. 74y at the enrollment/baseline), had a higher proportion of the APOE ϵ4 allele (36.7% vs. 26.5%), and fewer years of education (12y vs. 14y). African Americans participated less in late-life cognitive activities and physical activities compared to European Americans. Each lifestyle factor’s contribution to the score in African and European Americans is presented in the Supplementary Table 2.
Table 1.
Demographics, genetic, lifestyle, and clinical characteristics of African Americans and European Americans
| African Americans (n=2359) | European Americans (n=1515) | |
|---|---|---|
| Demographics | ||
| Age at baseline, mean (SD), y | 70.8 (5.2) | 73.7 (6.7) |
| Sex, N (%) | ||
| Men | 857 (36.3) | 584 (38.5) |
| Women | 1502 (63.7) | 931 (61.5) |
| Education, mean (SD), y | 11.8 (3.2) | 14.3 (3.2) |
| Genetic | ||
| APOE ϵ4 non-carriers, N (%) | 1493 (63.3) | 1113 (73.5) |
| APOE ϵ4 carriers, N (%) | 866 (36.7) | 402 (26.5) |
| Lifestyle factors | ||
| MIND diet, mean (SD), composite score | 7.0 (1.6) | 6.9 (1.7) |
| Cognitive activities, mean (SD), composite score | 3.1 (0.7) | 3.5 (0.6) |
| Physical activity moderate-vigorous intensity, median (IQR), min/week | 50 (0, 210) | 150 (30, 345) |
| Smoking, N (%) | ||
| Never | 1116 (47.3) | 766 (50.6) |
| Former | 950 (40.3) | 624 (41.2) |
| Current | 293 (12.4) | 125 (8.3) |
| Alcohol intake, mean (SD), g/day | 2.3 (9.0) | 7.0 (12.4) |
| Comorbidities | ||
| Diabetes, N (%) | 570 (24.2) | 184 (12.1) |
| Heart disease, N (%) | 303 (12.9) | 199 (13.1) |
| Stroke, N (%) | 217 (9.2) | 115 (7.6) |
| Cancers, N (%) | 423 (17.9) | 369 (24.4) |
| Body mass index, mean (SD), kg/m2 | 26.6 (5.0) | 28.8 (6.0) |
| Depression symptoms, mean (SD), 10-item CES-D | 1.6 (1.9) | 1.0 (1.5) |
Abbreviations: APOE, apolipoprotein E; IQR, interquartile.
Data is presented as mean and standard deviation, median interquartile, or number and proportion.
Table 2 shows the association of APOE ϵ4 allele and lifestyle score with cognitive decline in African and European Americans. APOE ϵ4 allele was associated with faster cognitive decline by −0.023 z-score units per year (95%CI −0.029, −0.017) in African Americans and by −0.037 units/year (95%CI −0.046, −0.028) in European Americans. Healthy lifestyle score was associated with a slower cognitive decline by 0.005 (95%CI 0.002, 0.008) units/year –per 1 additional healthy behavior in the score– in African Americans and 0.008 (0.005, 0.012) in European Americans.
Table 2.
Association of APOE ϵ4 and healthy lifestyle score with cognitive decline in African Americans and European Americans.
| African Americans | European Americans | |||
|---|---|---|---|---|
| N (%) | β (95%CI) | N (%) | β (95%CI) | |
| APOE ϵ4 allele | ||||
| Non-Carriers | 1493 (63.3) | reference | 1113 (73.5) | reference |
| Carriers | 866 (36.7) | −0.023 (−0.029, −0.017) | 402 (26.5) | −0.037 (−0.046, −0.028) |
| Lifestyle Score | ||||
| Continuous | 2359 (100) | 0.005 (0.002, 0.008) | 1515 (100) | 0.008 (0.005, 0.012) |
| Categorical | ||||
| 0–1 factor | 709 (30.1) | reference | 210 (13.9) | reference |
| 2–3 factors | 1363 (57.8) | 0.006 (0.000, 0.013) | 784 (51.7) | 0.021 (0.007, 0.034) |
| 4–5 factors | 287 (12.1) | 0.015 (0.005, 0.025) | 521 (34.4) | 0.031 (0.017, 0.046) |
Abbreviations: APOE, apolipoprotein E; N, number; β, beta estimate; CI, confidence interval.
The associations between APOE ϵ4 and healthy lifestyle score with cognitive decline are evaluated in two separate linear mixed-effects models, and not in one mutually adjusted model. Models are adjusted by age, sex, education, comorbidities (heart disease, stroke, diabetes, or cancers), and practice effects, and their interaction with time.
Continuous refers to 1 additional healthy behavior in the lifestyle score.
Table 3 presents the results of the association between lifestyle score with cognitive decline in African and European Americans stratified by APOE ϵ4 carrier status. A healthy lifestyle score was associated with a slower cognitive decline in APOE ϵ4 carriers and non-carriers, and these associations were much more robust in European than African Americans and APOE ϵ4 carriers than non-carriers. In APOE ϵ4 carriers, 1 additional healthy behavior in the score was associated with 0.007 units/year (95%CI 0.002, 0.013) slower cognitive decline in African Americans and 0.014 units/year (95%CI 0.005, 0.023) in European Americans. In APOE ϵ4 carriers, adherence to 4–5 healthy lifestyle factors was associated with slower cognitive decline by 0.023 units/year (95% 0.004, 0.042) in African Americans and 0.044 (95% 0.008, 0.080) in European Americans as compared to counterparts with 0–1 healthy factors.
Table 3.
Association of healthy lifestyle score with cognitive decline in African Americans and European Americans stratified by their APOE ϵ4 status
| African Americans | European Americans | |||
|---|---|---|---|---|
| N (%) | β (95%CI) | N (%) | β (95%CI) | |
| APOE ϵ4 Non-Carriers | ||||
| Lifestyle Score | ||||
| Continuous | 1493 (100) | 0.003 (0.000, 0.006) | 1113 (100) | 0.007 (0.003, 0.011) |
| Categorical | ||||
| 0–1 factor | 455 (30.5) | reference | 165 (14.8) | reference |
| 2–3 factors | 871 (58.3) | 0.004 (−0.003, 0.012) | 592 (53.2) | 0.020 (0.006, 0.033) |
| 4–5 factors | 167 (11.2) | 0.009 (−0.002, 0.020) | 356 (32.0) | 0.027 (0.012, 0.042) |
| APOE ϵ4 Carriers | ||||
| Lifestyle Score | ||||
| Continuous | 866 (100) | 0.007 (0.002, 0.013) | 402 (100) | 0.014 (0.005, 0.023) |
| Categorical | ||||
| 0–1 factor | 254 (29.3) | reference | 45 (11.2) | reference |
| 2–3 factors | 492 (56.8) | 0.008 (−0.005, 0.021) | 192 (47.8) | 0.017 (−0.017, 0.052) |
| 4–5 factors | 120 (13.9) | 0.023 (0.004, 0.042) | 165 (41.0) | 0.044 (0.008, 0.080) |
Abbreviations: APOE, apolipoprotein E; N, number; β, beta estimate; CI, confidence interval;
Adjusted for age, sex, education, comorbidities (heart disease, stroke, diabetes, or cancers), and practice effects, and their interaction with time.
Continuous refers to 1 additional healthy behavior in the lifestyle score.
The strength and magnitude of associations between the lifestyle score with cognitive decline stratified by APOE ϵ4 carrier status and race remained largely unchanged in a series of sensitivity analyses (Supplementary Table 3 Models A–G). Results were similar to the primary analysis when we additionally adjusted for body mass index and depressive symptoms (Supplementary Table 3 Model A), and when we did not adjust for comorbidities (Supplementary Table 3 Model B), and when excluded individuals (n=388) within the lowest 10% of the cognitive score at the baseline (Supplementary Table 3 Model C), and when we limited the follow-up to 10 years (Supplementary Table 3 Model D). A healthy lifestyle score without alcohol as a lifestyle factor in the score was associated with cognitive decline in African and European Americans, and β-estimates per 1 additional healthy behavior were similar to the primary analysis: 0.008 (0.002, 0.013) for African Americans and 0.014 (0.004, 0.024) for European Americans (Supplementary Table 3 Model E). Similar findings resulted when we used never smoking as a healthy lifestyle factor in the score (Supplementary Table 3 Model F). When we matched African Americans (n=500) to the baseline characteristics of European Americans (n=500), we noted that the association of healthy lifestyle score and cognitive decline became stronger among African Americans and were similar to European Americans estimates (Supplementary Table 3 Model G). In APOE ϵ4 non-carriers, 1 additional healthy behavior in the lifestyle score was associated with 0.009 units/year (95%CI 0.001, 0.016) slower cognitive decline in African Americans and 0.009 units/year (95%CI 0.001, 0.016) in European Americans. In the analysis stratified by lifestyle score and race (Supplementary Table 4), APOE ϵ4 allele was associated with a faster cognitive decline in each stratum, except for African Americans with 4–5 healthy lifestyle factors [β −0.013 (95%CI −0.028, 0.002)].
Figure 1 visually summarizes the relationship between healthy lifestyle score and the rate of cognitive decline stratified by APOE ϵ4 and race. We modeled the predicted rate of change in global cognition over the 17-years for a typical African and European American participant in our study. The figure shows that while African Americans had lower predicted scores on the global cognition composite at baseline, their average rate of decline by lifestyle and APOE ϵ4 status was slower than European Americans. Results were similar when we included quadratic terms of time in the multivariate linear mixed-effects models (Supplementary Figure 1).
Figure 1. Predicted 17-year rates of change in global cognition for a typical African American and typical European American according to their APOE ϵ4 status and adherence to a healthy lifestyle.
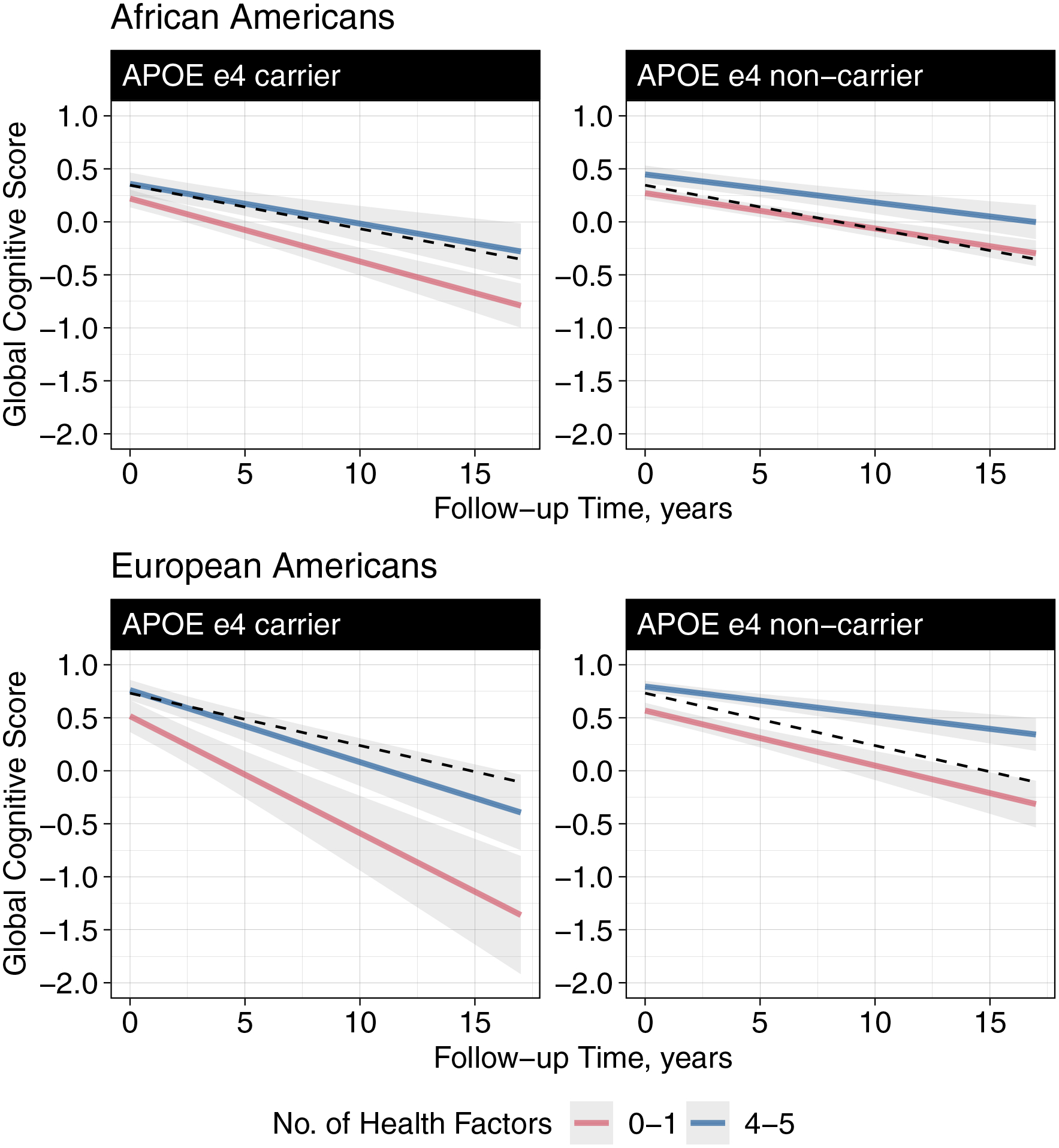
Abbreviations: APOE, apolipoprotein E; No. number;
Dotted line shows the overall (not stratified by APOE ϵ4 status or lifestyle score) predicted rate of cognitive decline for a typical African American and European American. Models were adjusted for age, sex, education, comorbidities (heart disease, stroke, diabetes, or cancers) and their interaction with time.
Discussion
In a large, biracial population-based study of older adults, we examined the role of genetic risk and a healthy lifestyle score on cognitive decline among African Americans and European Americans. Genetic risk, determined by the presence of the APOE ϵ4 allele, was associated with a faster cognitive decline, while adherence to a healthy lifestyle was associated with a slower cognitive decline in both African and European Americans. In addition, adherence to a healthy lifestyle was associated with a slower cognitive decline in African and European Americans regardless of the genetic risk. The relationship between a healthy lifestyle and slower cognitive decline was more substantial in European Americans than African Americans.
Racial differences in cognition, cognitive decline, and the risk of Alzheimer’s dementia have been the subject of several investigations [14–20]. Consistent with our findings, earlier studies have shown that the rate of cognitive decline in African Americans is slightly slower compared to European Americans [20,22,23,45]. While the association of race with cognitive decline deserves careful examination, we noted that in comparison to European Americans, African Americans scored lower on cognitive tests at baseline. A lower score at baseline, accompanied by a reduced cognitive decline during the follow-up, while contradictory, could suggest that “floor effects” may have artifactually reduced our estimate of lifestyle factors on cognitive decline among African Americans [23]. However, this explanation is unlikely because our sensitivity analysis demonstrated similar findings to the primary analysis when we excluded individuals with lower cognitive scores at baseline or limited follow-up time to 10 years. We think a more likely explanation could be attributed to individual risk factors distributed differently in African and European Americans [46]. For example, racial disparities over the life course have been identified in the level/quality of education, engagement in late-life reading/cognitive activity, access to social resources, and experiences of discrimination [46–48]. These risk factors contribute to the racial differences in the baseline level of cognition, with older European Americans, on average, having a higher score than African Americans. A higher cognitive score at baseline in European Americans could be seen as more to lose (i.e., farther to fall) cognitively than the lower-scoring African Americans [18]. However, this is not to say that African Americans will develop a fewer cases of dementia than European Americans given their slower rate of decline because, with a lower cognitive function at baseline, they are closer to the threshold for a dementia diagnosis and may disproportionately develop the disease earlier [49]. Our analysis in the matched sample of African and European Americans is further evidence that racially patterned risk factors may explain the racial differences detected in the association between lifestyle and cognition. When we matched African Americans to the baseline characteristics of European Americans –creating sub-groups of African Americans with similar age, education level, healthy lifestyle score, and cognition to European Americans–, we found identical estimates for the association of a healthy lifestyle score with cognitive decline. Overall, these findings suggest that racial differences in lifestyle and cognition are explained by individual risk factors that are socially patterned and vary by race. Nevertheless, whether adherence to a healthy lifestyle is an individual decision or a consequence of societal structures that systematically disadvantage a racial/ethnic group requires further investigation.
Our results align with most [1,2,6], but not all [3], of the studies in the literature reporting a favorable impact of a healthy lifestyle on cognition in carriers of the APOE ϵ4 allele. While these studies focus primarily on non-Hispanic whites [2,3], we determined that the favorable effect of a healthy lifestyle on cognitive decline is also present among African American carriers of the APOE ϵ4 allele. However, whether disclosure of genetic risk (e.g., APOE ϵ4 carriership) can motivate behavioral change and these behavioral changes will result in slowing cognitive decline requires further investigation.
Confidence in these findings are strengthened by several factors. First, we determined cognitive functioning by using objective assessments of cognition that were administrated by trained technicians. Second, we followed participants for up to 17 years with repeated cognitive testing to ensure a measurable rate of change in cognition, to characterize the linear and nonlinear changes in cognitive functioning, and further to account for practice effects. Third, we accounted for important potential confounders and conducted a series of sensitivity analyses to test the robustness of our findings. There are limitations to this study. First, we were limited to a well-established genetic polymorphism and selected modifiable lifestyle factors. Including a polygenic risk score or additional risk factors (e.g., sleep, socioeconomic status) may have provided additional information in our associations. Third, survival bias may impact our results because individuals with an unhealthy lifestyle have an increased risk of premature mortality and may have died before participating in our study. Similarly, carriers of the APOE ϵ4 allele have a higher risk of mortality [19], and differences in life expectancy have been noted between African and European Americans [50]. Overall, this type of bias will lead to underestimation (bias toward null) of the true associations because the rate of cognitive decline is strongly dependent on participant age. Fourth, while we used propensity score matching to balance African and European Americans on demographics, lifestyle factors, and cognitive scores and explored the role of socially patterned risk factors, residual confounding remains an issue; therefore, this approach should not be considered for making causal inferences. In addition, selecting African Americans based on European Americans’ risk factors (e.g., education, lifestyle, cognitive score) has a potential selection bias and may limit external validity.
In conclusion, after examining the role of genetic and lifestyle risk on cognition among African and European Americans, we found that adherence to a healthy lifestyle was associated with a slower cognitive decline in both races regardless of the genetic risk. Future research should study the impact of lifestyle on cognition in other race/ethnicity groups, including Asian, Latino, and Indigenous populations.
Supplementary Material
Acknowledgements
The authors thank all participants of the Chicago Health and Aging Project and study staff for their commitment to the study.
Research reported in this publication was supported by the National Institute On Aging of the National Institutes of Health, Grant/Award (R21AG070287, R01AG057532, R01AG051635, R01AG058679, and R01AG11101). The content is solely the responsibility of the authors and does not necessary represent the official views of the National Insitutes of Health.
Conflict of Interest
Drs. Dhana, Barnes, Krueger, Holland, Halloway, Evans, and Rajan report no conflicts of interest. Dr. Liu reports consultancy/speaker fees from The Peanut Institute and the Kaplan Fox & Kilsheimer, LLP, outside of submitted work. Dr. Agarwal reports philanthropic research support, for the institution, from the Michael J Fox Foundation, Consolidated anti-aging foundation, and California Strawberry Commission outside of submitted work. Dr. Desai reports receiving consultancy fees from the AllianceChicago, outside of submitted work. Dr. Aggarwal is a DSMB member and Safety Officer in grants funded by the National Institutes of Health, Consultant for ATW Health Inc, Member of the Biogen Brain Health Study Advisory Board, and the CDC BOLD Public Health Center of Excellence on Dementia Risk Reduction, outside of submitted work.
Data Availability Statement
De-identified data are available on request for qualified investigators from www.riha.rush.edu/dataportal.html
References
- [1].Kivipelto M, Rovio S, Ngandu T, Kåreholt I, Eskelinen M, Winblad B, et al. Apolipoprotein e epsilon4 magnifies lifestyle risks for dementia: A population-based study. Journal of Cellular and Molecular Medicine 2008;12:2762–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Lourida I, Hannon E, Littlejohns TJ, Langa KM, Hyppönen E, Kuzma E, et al. Association of lifestyle and genetic risk with incidence of dementia. JAMA 2019;322:430–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Licher S, Ahmad S, Karamuji’c-Čomi’c H, Voortman T, Leening MJG, Ikram MA, et al. Genetic predisposition, modifiable-risk-factor profile and long-term dementia risk in the general population. Nature Medicine 2019;25:1364–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Livingston G, Huntley J, Sommerlad A, Ames D, Ballard C, Banerjee S, et al. Dementia prevention, intervention, and care: 2020 report of the lancet commission. Lancet (London, England) 2020;396:413–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Dhana K, Evans DA, Rajan KB, Bennett DA, Morris MC. Healthy lifestyle and the risk of alzheimer dementia: Findings from 2 longitudinal studies. Neurology 2020;95:e374–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Dhana K, Aggarwal NT, Rajan KB, Barnes LL, Evans DA, Morris MC. Impact of the apolipoprotein E4 allele on the relationship between healthy lifestyle and cognitive decline: A population-based study. American Journal of Epidemiology 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Rosenberg A, Mangialasche F, Ngandu T, Solomon A, Kivipelto M. Multidomain interventions to prevent cognitive impairment, alzheimer’s disease, and dementia: From FINGER to world-wide FINGERS. The Journal of Prevention of Alzheimer’s Disease 2020;7:29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Baker LD, Beavers DP, Cleveland M, Day CE, Decarli C, Espeland MA, et al. US POINTER: STUDY DESIGN AND LAUNCH. Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association 2019;15:P1262–3. [Google Scholar]
- [9].Neurology TL. Pointing the way to primary prevention of dementia. The Lancet Neurology 2017;16:677. [DOI] [PubMed] [Google Scholar]
- [10].Sugino H, Watanabe A, Amada N, Yamamoto M, Ohgi Y, Kostic D, et al. Global trends in alzheimer disease clinical development: Increasing the probability of success. Clinical Therapeutics 2015;37:1632–42. [DOI] [PubMed] [Google Scholar]
- [11].Zhou Y, Elashoff D, Kremen S, Teng E, Karlawish J, Grill JD. African americans are less likely to enroll in preclinical alzheimer’s disease clinical trials. Alzheimer’s & Dementia (New York, N Y) 2017;3:57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Olin JT, Dagerman KS, Fox LS, Bowers B, Schneider LS. Increasing ethnic minority participation in alzheimer disease research. Alzheimer Disease and Associated Disorders 2002;16 Suppl 2:S82–5. [DOI] [PubMed] [Google Scholar]
- [13].Rabinowitz YG, Gallagher-Thompson D. Recruitment and retention of ethnic minority elders into clinical research. Alzheimer Disease and Associated Disorders 2012;24 Suppl:S35–41. [PubMed] [Google Scholar]
- [14].Evans DA, Bennett DA, Wilson RS, Bienias JL, Morris MC, Scherr PA, et al. Incidence of alzheimer disease in a biracial urban community: Relation to apolipoprotein e allele status. Archives of Neurology 2003;60:185–9. [DOI] [PubMed] [Google Scholar]
- [15].Mayeda ER, Glymour MM, Quesenberry CP, Whitmer RA. Inequalities in dementia incidence between six racial and ethnic groups over 14 years. Alzheimer’s & Dementia : The Journal of the Alzheimer’s Association 2016;12:216–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Shadlen M-F, Siscovick D, Fitzpatrick AL, Dulberg C, Kuller LH, Jackson S. Education, cognitive test scores, and black-white differences in dementia risk. Journal of the American Geriatrics Society 2006;54:898–905. [DOI] [PubMed] [Google Scholar]
- [17].Yaffe K, Falvey C, Harris TB, Newman A, Satterfield S, Koster A, et al. Effect of socioeconomic disparities on incidence of dementia among biracial older adults: Prospective study. BMJ (Clinical Research Ed) 2013;347:f7051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Weuve J, Barnes LL, de Leon CFM, Rajan KB, Beck T, Aggarwal NT, et al. Cognitive aging in black and white americans: Cognition, cognitive decline, and incidence of alzheimer disease dementia. Epidemiology (Cambridge, Mass) 2018;29:151–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Rajan KB, Barnes LL, Wilson RS, McAninch EA, Weuve J, Sighoko D, et al. Racial differences in the association between apolipoprotein e risk alleles and overall and total cardiovascular mortality over 18 years. Journal of the American Geriatrics Society 2017;65:2425–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Rajan KB, McAninch EA, Wilson RS, Weuve J, Barnes LL, Evans DA. Race, APOEɛ4, and long-term cognitive trajectories in a biracial population sample. Journal of Alzheimer’s Disease : JAD 2019;72:45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Potter GG, Plassman BL, Burke JR, Kabeto MU, Langa KM, Llewellyn DJ, et al. Cognitive performance and informant reports in the diagnosis of cognitive impairment and dementia in african americans and whites. Alzheimer’s & Dementia : The Journal of the Alzheimer’s Association 2009;5:445–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Barnes LL, Bennett DA. Alzheimer’s disease in african americans: Risk factors and challenges for the future. Health Affairs (Project Hope) 2014;33:580–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Barnes LL, Wilson RS, Li Y, Aggarwal NT, Gilley DW, McCann JJ, et al. Racial differences in the progression of cognitive decline in alzheimer disease. The American Journal of Geriatric Psychiatry : Official Journal of the American Association for Geriatric Psychiatry 2005;13:959–67. [DOI] [PubMed] [Google Scholar]
- [24].Babulal GM, Quiroz YT, Albensi BC, Arenaza-Urquijo E, Astell AJ, Babiloni C, et al. Perspectives on ethnic and racial disparities in alzheimer’s disease and related dementias: Update and areas of immediate need. Alzheimer’s & Dementia : The Journal of the Alzheimer’s Association 2019;15:292–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Wenham PR, Price WH, Blandell G. Apolipoprotein e genotyping by one-stage PCR. Lancet (London, England) 1991;337:1158–9. [DOI] [PubMed] [Google Scholar]
- [26].Morris MC, Tangney CC, Bienias JL, Evans DA, Wilson RS. Validity and reproducibility of a food frequency questionnaire by cognition in an older biracial sample. American Journal of Epidemiology 2003;158:1213–7. [DOI] [PubMed] [Google Scholar]
- [27].Morris MC, Tangney CC, Wang Y, Sacks FM, Bennett DA, Aggarwal NT. MIND diet associated with reduced incidence of alzheimer’s disease. Alzheimer’s & Dementia : The Journal of the Alzheimer’s Association 2015;11:1007–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].McPhillips JB, Pellettera KM, Barrett-Connor E, Wingard DL, Criqui MH. Exercise patterns in a population of older adults. Am J Prev Med 1989;5:65–72. [PubMed] [Google Scholar]
- [29].Aggarwal NT, Bienias JL, Bennett DA, Wilson RS, Morris MC, Schneider JA, et al. The relation of cigarette smoking to incident alzheimer’s disease in a biracial urban community population. Neuroepidemiology 2006;26:140–6. [DOI] [PubMed] [Google Scholar]
- [30].Wilson RS, Scherr PA, Schneider JA, Tang Y, Bennett DA. Relation of cognitive activity to risk of developing alzheimer disease. Neurology 2007;69:1911–20. [DOI] [PubMed] [Google Scholar]
- [31].Stampfer MJ, Hu FB, Manson JE, Rimm EB, Willett WC. Primary prevention of coronary heart disease in women through diet and lifestyle. The New England Journal of Medicine 2000;343:16–22. [DOI] [PubMed] [Google Scholar]
- [32].Hu FB, Manson JE, Stampfer MJ, Colditz G, Liu S, Solomon CG, et al. Diet, lifestyle, and the risk of type 2 diabetes mellitus in women. The New England Journal of Medicine 2001;345:790–7. [DOI] [PubMed] [Google Scholar]
- [33].Li Y, Schoufour J, Wang DD, Dhana K, Pan A, Liu X, et al. Healthy lifestyle and life expectancy free of cancer, cardiovascular disease, and type 2 diabetes: Prospective cohort study. BMJ (Clinical Research Ed) 2020;368:l6669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Radloff LS. The CES-d scale: A self-report depression scale for research in the general population. Applied Psychological Measurement 1977;1:385–401. [Google Scholar]
- [35].Albert M, Smith LA, Scherr PA, Taylor JO, Evans DA, Funkenstein HH. Use of brief cognitive tests to identify individuals in the community with clinically diagnosed alzheimer’s disease. The International Journal of Neuroscience 1991;57:167–78. [DOI] [PubMed] [Google Scholar]
- [36].Wilson RS, Bennett DA, Bienias JL, Aggarwal NT, De Leon CFM, Morris MC, et al. Cognitive activity and incident AD in a population-based sample of older persons. Neurology 2002;59:1910–4. [DOI] [PubMed] [Google Scholar]
- [37].Smith A Symbol digit modalities test (SDMT) manual (revised) western psychological services. Los Angeles 1982. [Google Scholar]
- [38].Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research 1975;12:189–98. [DOI] [PubMed] [Google Scholar]
- [39].Wilson RS, Hebert LE, Scherr PA, Dong X, Leurgens SE, Evans DA. Cognitive decline after hospitalization in a community population of older persons. Neurology 2012;78:950–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Wilson RS, Rajan KB, Barnes LL, Hebert LE, de Leon CFM, Evans DA. Cognitive aging and rate of hospitalization in an urban population of older people. The Journals of Gerontology Series A, Biological Sciences and Medical Sciences 2014;69:447–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].U.S. Department of Agriculture and U.S. Department of Health and Human Services. Dietary Guidelines for Americans, 2020–2025. 9th edition. December 2020. https://www.dietaryguidelines.gov/ (accessed March 29, 2021). [Google Scholar]
- [42].Ho DE, Imai K, King G, Stuart EA. MatchIt: Nonparametric preprocessing for parametric causal inference. Journal of Statistical Software 2011;42:1–28. [Google Scholar]
- [43].Stuart EA. Matching methods for causal inference: A review and a look forward. Statistical Science : A Review Journal of the Institute of Mathematical Statistics 2010;25:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Sekhon JS. Multivariate and propensity score matching software with automated balance optimization: The matching package for r. Journal of Statistical Software, Forthcoming 2008. [Google Scholar]
- [45].Wilson RS, Capuano AW, Sytsma J, Bennett DA, Barnes LL. Cognitive aging in older black and white persons. Psychology and Aging 2015;30:279–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Wilson RS, Rajan KB, Barnes LL, Weuve J, Evans DA. Factors related to racial differences in late-life level of cognitive function. Neuropsychology 2016;30:517–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Glymour MM, Manly JJ. Lifecourse social conditions and racial and ethnic patterns of cognitive aging. Neuropsychology Review 2008;18:223–54. [DOI] [PubMed] [Google Scholar]
- [48].Bell ML, Ebisu K. Environmental inequality in exposures to airborne particulate matter components in the united states. Environmental Health Perspectives 2012;120:1699–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Rajan KB, Wilson RS, Weuve J, Barnes LL, Evans DA. Cognitive impairment 18 years before clinical diagnosis of alzheimer disease dementia. Neurology 2015;85:898–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Harper S, Rushani D, Kaufman JS. Trends in the black-white life expectancy gap, 2003–2008. JAMA 2012;307:2257–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
De-identified data are available on request for qualified investigators from www.riha.rush.edu/dataportal.html


