Abstract
Objectives:
Although primary motor cortex (M1) transcranial direct current stimulation (tDCS) has an analgesic effect in fibromyalgia (FM), its neural mechanism remains elusive. We investigated whether M1-tDCS modulates a regional temporal variability of blood-oxygenation-level-dependent (BOLD) signals, an indicator of the brain’s flexibility and efficiency and if this change is associated with pain improvement.
Materials and Methods:
In a within-subjects cross-over design, 12 female FM patients underwent sham and active tDCS on 5 consecutive days, respectively. Each session was performed with an anode placed on the left M1 and a cathode on the contralateral supraorbital region. The subjects also participated in resting-state functional magnetic resonance imaging (fMRI) at baseline and after sham and active tDCS. We compared the BOLD signal variability (SDBOLD), defined as the standard deviation of the BOLD time-series, between the tDCS conditions. Baseline SDBOLD was compared to 15 healthy female controls.
Results:
At baseline, FM patients showed reduced SDBOLD in the ventromedial prefrontal cortex (vmPFC), lateral PFC, and anterior insula and increased SDBOLD in the posterior insula compared to healthy controls. After active tDCS, compared to sham, we found an increased SDBOLD in the left rostral anterior cingulate cortex (rACC), lateral PFC, and thalamus. After sham tDCS, compared to baseline, we found a decreased SDBOLD in the dorsomedial PFC and posterior cingulate cortex/precuneus. Interestingly, after active tDCS compared to sham, pain reduction was correlated with an increased SDBOLD in the rACC/vmPFC but with a decreased SDBOLD in the posterior insula.
Conclusion:
Our findings suggest that M1-tDCS might revert temporal variability of fMRI signals in the rACC/vmPFC and posterior insula linked to FM pain. Changes in neural variability would be part of the mechanisms underlying repetitive M1-tDCS analgesia in FM.
Keywords: tDCS, brain stimulation, fibromyalgia, resting-state fMRI, brain signal variability
Introduction
Fibromyalgia (FM) is a nociplastic (i.e., centralized) pain syndrome characterized by chronic widespread musculoskeletal pain accompanied by fatigue and cognitive and emotional disturbances2. The core explanation for its pathophysiology is the sensitized central nervous system with inefficient pain modulation and sensory integration, leading to hypersensitivity to painful and non-painful stimuli3–9. Moreover, studies have suggested an imbalance between excitatory and inhibitory neurotransmission, such as elevated glutamate/glutamine and decreased gamma-aminobutyric acid (GABA) concentration, which contributed to heightened pain10–12. Although our understanding of FM’s neural basis has advanced considerably over the last decade, most available treatments are often inadequate, associated with debilitating side effects, and in the case of opioids, can even lead to addiction13, 14.
Transcranial direct current stimulation (tDCS) over the primary motor cortex (M1) has emerged as a promising treatment and has been reported to alleviate pain for FM patients who exhibited unsatisfactory responses to pharmacological interventions15, 16. tDCS is designed to flow an electric current between two points where two electrodes (anode and cathode) are placed and modulates cortical excitability non-invasively by depolarizing or hyperpolarizing neuronal cells using a weak constant current (1–2 mA)17. The electric current, however, extends to other subcortical and cortical areas beyond the stimulated region18. Indeed, the previous computational study analyzing current flow (electric field) during tDCS demonstrated that significant electric fields were generated in the insula, anterior cingulate cortex (ACC), thalamus, and even brainstem regions19. Moreover, one of the consistent findings so far is that M1-tDCS activates top-down modulatory pathways, including the ACC and periaqueductal gray (PAG) in a molecular (e.g., mu-opioid and glutamate neurotransmission) and functional level even with a single tDCS20, 21. For instance, M1-tDCS session applied to trigeminal neuropathic pain patient during the positron emission tomography with a μ-opioid receptor (μOR) selective radiotracer, [11C]carfentanil, induced a decreased μOR binding (endogenous μ-opioid release) in the pain-related areas including the ACC18.
Previous studies, including our group, have reported reduced clinical pain and/or negative affect after repetitive M1-tDCS to FM patients1, 15, 22, 23. We also provided evidence that anodal M1-tDCS lowered Glx (glutamate + glutamine) concentration in the ACC and thalamus1. Further, we demonstrated a decreased resting-state functional connectivity of the thalamus associated with pain reduction after M1-tDCS24. A recent study with healthy participants demonstrated that M1-tDCS decreased central sensitization-related secondary hyperalgesia by increasing the activities of the descending pain inhibitory system20. However, we still lack knowledge of how tDCS modulates to achieve pain relief of FM. For the better use of brain stimulation in research and clinical settings, it is essential to enhance our understanding of how tDCS alters brain function to reduce pain and elucidate brain markers predicting tDCS efficacy.
As a step towards these goals, we applied resting-state blood-oxygen-level-dependent (BOLD) signal variability measures to the same FM patients, which we have previously investigated the tDCS effect on brain metabolites and functional connectivity1, 24. The BOLD signal variability, calculated as the standard deviation of the BOLD time-course (SDBOLD), was once regarded as a noisy signal but is currently accepted as a sensitive and reliable marker of cognitive function and pain modulation25–28. In a study with healthy participants, higher BOLD signal variability was related to lower pain sensitivity and better cognitive performance even during painful stimulation29. By contrast, chronic pain patients showed higher BOLD signal variability in the ascending pain pathway and default mode network (DMN)30–32, suggesting the importance of an optimal range of variability in the context of pain processing. Another critical aspect of BOLD signal variability is that it reflects the neural system’s readiness in response to external challenges25. Further, it is related to the brain’s modulatory capacity, making the neural system more resilient and responsive to therapeutic intervention, which might be ultimately favorable to pain modulation33, 34.
In a typical resting-state functional magnetic resonance imaging (fMRI), BOLD signal fluctuations at low-frequency range (e.g., 0.01–0.1 Hz) are related to spontaneous neural activity35, 36. It has been suggested that distinct neural oscillators generate low-frequency brain fluctuations with specific physiological functions37. These low-frequency fluctuations (LFF) can be decomposed into independent frequency bands, including slow-5: 0.01–0.027 Hz, slow-4:0.027–0.073 Hz, and slow-3: 0.073–0.198 Hz38. Interestingly, LFF in specific frequency bands showed regionally specific patterns. For instance, LFF within slow-5 was shown to have higher amplitude in the ventromedial prefrontal region than within slow-4. In comparison, the LFF amplitude within slow-4 was higher in the basal ganglia and thalamus than within slow-538. Previous resting-state fMRI studies in chronic pain showed the altered amplitude of LFF at distinct frequency bands30, 39–41. Therefore, we assumed resting-state BOLD signal variability in a specific frequency could be a clinically meaningful index of clinical pain and relief induced by tDCS applications in FM patients.
We first aimed to find regional abnormalities of pre-treatment (baseline) SDBOLD in FM compared to healthy controls (HC). We then investigated changes of SDBOLD after M1-tDCS and its relationship with clinical pain improvement in FM. Previous studies have suggested that activation of the endogenous pain modulatory system would be the neural basis for M1-tDCS effects on pain20, 42. Thus, we hypothesized that SDBOLD in the anti-nociceptive regions, including the ACC and medial prefrontal cortex (mPFC)5, 43, would change after active M1-tDCS compared with sham. In this study, we examined changes in BOLD variability within slow-5 and slow-4 bands. We believe this study provides an important insight into identifying the neural substrates of repetitive M1-tDCS for FM pain.
Materials and Methods
Study participants
We initially enrolled 13 female FM patients in the study. One patient dropped out after baseline pain and magnetic resonance imaging (MRI) assessment; hence a total of 12 patients (age range: 34–64 years, mean ± SD: 49.3 ± 9.0) completed the tDCS sessions and were fed into the data analysis related to tDCS effect. Primary inclusion criteria are 1) patients who met the 1990 criteria of the American College of Rheumatology for FM44, 2) widespread chronic pain for at least 1 year, 3) continued presence of pain more than 50% of the days, and 4) willing to not to use a new medication to control FM symptoms during the study. We confirmed that none of these participants were taking any new medication throughout the study. The patients also acknowledged potential risks related to tDCS treatment. Exclusion criteria are as follows: 1) co-existing autoimmune or chronic inflammatory disease that causes pain (e.g., rheumatoid arthritis), 2) a history of psychiatric disease (e.g., major depressive disorders) and substance abuse, 3) currently taking opiates, 4) contradictions with both tDCS and MRI including pregnancy, breastfeeding, any metal implants (e.g., pacemaker), and 5) participating in any other clinical trials. The Institutional Review Board of the University of Michigan approved all study procedures. All subjects provided informed consent before participation of the study.
We used HC MRI data acquired at the University of Michigan from the Multidisciplinary Approach to the Study of Chronic Pelvic Pain (MAPP) Research Network45. Data from 15 female HC (age range: 32–61 years, mean ± SD: 43.4 ± 8.7) were included for baseline SDBOLD comparisons with 13 FM patients (age range: 27–64 years, mean ± SD: 47.6 ± 10.6). We included one patient who dropped out after sham-tDCS treatment for baseline SDBOLD comparisons. There were no significant group differences in age (independent samples t-test, p = 0.26).
Study design
This study is a longitudinal trial with a within-subjects cross-over and sham-controlled design consisting of 3 phases, including baseline, sham, and active-tDCS (Supplementary Fig. 1). At baseline, we collected clinical pain intensity and MRI (structural and functional) data. Next, sham-tDCS was performed for 5 consecutive days, followed by an MRI (structural and functional) and clinical pain assessment (4.8 ± 1.3 days apart from the last sham-tDCS). After 7 to 11 days of washout period (9.9 days on average), active-tDCS was performed for 5 consecutive days, followed by an MRI (structural and functional) and clinical pain assessment (5.4 ± 2.7 days apart from the last active-tDCS). We assessed clinical pain immediately before the MRIs. This study was not randomized to prevent a potential carry-over effect from active to sham, since the previous study reported a lasting effect of pain improvement up to two months after 5 consecutive days of tDCS over the M1 in phantom-limb pain patients46.
tDCS procedures
We followed the same tDCS parameters used in the landmark trial conducted by Fregni et al.15, which was shown to reduce pain in patients with FM. A detailed step of tDCS procedure applied to this study was fully demonstrated in the ref.47 Anode electrodes (35 cm2) were placed on the scalp overlying the left M1, and the cathode electrode was placed on the scalp overlying the right supraorbital cortex for both the sham and active tDCS sessions. Experienced investigators (A.F.D. and T.D.N.) placed electrodes over the M1 (C3) and supraorbital region (FP2) throughout the study. Electrode positions were determined and marked individually using the 10–20 international system of the electroencephalogram. The conductive rubber electrode was enclosed in a perforated sponge pocket that was soaked with saline, 6 mL for each side, and was fixed with an elastic head strap. The shorter side of the electrode sponge for the M1 was positioned parallel to the midsagittal plane, and the longer side for the supraorbital region was positioned parallel to the axial plane. We ensured that the electrode sponge covered the marked areas.
For the sham tDCS, the 2 mA current, which mimics active tDCS, was applied for only 30 seconds at the beginning and end of the session. It has been suggested that a 30 seconds application of electrical current does not induce a lasting effect and is indistinguishable from the active one; thus, it was appropriate to blind the procedures to patients48. During the active tDCS, the 2 mA current was continuously applied for 20 minutes.
Clinical pain assessment
The clinical pain was measured by using a 10-point visual analog scale (VAS) with 0 (no pain) and 10 (worst pain imaginable) and assessed at each daily sham and active-tDCS treatment (Supplementary Table 1). We asked patients to report their average pain (VAS) before each MRI session within the time range as follows: 1) the week before the baseline MRI (5.1 ± 2.3), 2) the period between the first-day sham tDCS and second MRI (4.1 ± 2.1), and 3) the period between the first day of active tDCS and final MRI (3.3 ± 2.8). We also acquired clinical pain by using the McGill Pain Questionnaire49, but only the VAS score (mean ± SD) changed after active-tDCS (3.3 ± 2.8) when compared with baseline (5.1 ± 2.3) (p = 0.04), as we reported previously24. There was a trend toward a decrease in the VAS score from baseline to sham tDCS (4.1 ± 2.1) (p = 0.10) or sham tDCS to active tDCS (p = 0.16). We found that 6 patients with FM (50 %) showed at least 30% of pain reduction after active tDCS compared to baseline, whereas only a few (n = 2, 16.7 %) responded to sham tDCS (30% pain reduction) compared to baseline.
Resting-state fMRI acquisition
All MRI data were collected with an Ingenia 3.0 T system (Philips Medical Systems, Best, the Netherlands) at the University of Michigan. Resting-state fMRI data for the FM and HC groups were acquired with the following parameters: repetition time [TR] = 2000 ms; echo time [TE] = 30 ms; flip angle = 77°; field of view = 22 cm; voxel size for the FM group = 3.44 × 3.33 × 4.00 mm; voxel size for the HC group = 3.44 × 3.44 × 4.00 mm, and number of volumes = 300. T1-weighted brain image was acquired with the following parameters: TR = 9.8 ms; TE = 4.6 ms; flip angle = 8°; voxel size = 1 × 1 × 1 mm for the FM group; TR = 6.6–7.1 ms; TE = 4.7 ms; flip angle = 8°; voxel size = 0.9 × 0.9 × 0.9 mm for the HC group.
fMRI data preprocessing
Resting-state fMRI data were preprocessed using FSL (http://www.fmrib.ox.ac.uk/fsl) and AFNI (http://afni.nimh.nih.gov/afni). The preprocessing steps were adapted from the 1000 Functional Connectomes Project (http://www.nitrc.org/projects/fcon_1000). After discarding the first five volumes, slice time correction, motion correction, grand-mean scaling, removing of nuisance signals (cerebrospinal fluid, white matter, and six motion parameters) by regression, removing of linear and quadratic trends, spatial smoothing using a Gaussian kernel of 6 mm full-width half-maximum, and temporal band-pass filtering (slow-5, 0.01–0.027 Hz; slow-4, 0.027–0.073 Hz) were applied38. The preprocessed images were then linearly registered to 2-mm Montreal Neurological Institute (MNI) 152 template. First, functional images were aligned to the anatomical image with 6 degrees of freedom affine transformation. The anatomical image was then aligned into standard MNI space with a 12 degree of freedom affine transformation. Finally, the resulting transformation matrix was applied to each participant’s functional dataset.
SDBOLD analysis
The temporal variability was calculated as the standard deviation of BOLD time-courses at each voxel in the MNI standard space. For each participant, the voxel-wise SDBOLD map was standardized into subject-level Z-score maps by subtracting the mean of SDBOLD across the entire brain (gray matter) and then dividing by the standard deviation of SDBOLD obtained for the entire brain (gray matter)30. A positive value indicates that SDBOLD is higher than the whole-brain, while a negative value indicates that SDBOLD is lower than the whole-brain.
We calculated the frame-wise displacement (FD)50 to quantify each subject’s head motion. There was no significant difference of mean FD between the groups (mean ± SD) (FM at baseline: 0.17 ± 0.05, HC: 0.20 ± 0.09, p = 0.27) or between tDCS session (FM at baseline: 0.17 ± 0.05, sham tDCS: 0.15 ± 0.07, p = 0.24; sham: 0.15 ± 0.07, active tDCS: 0.18 ± 0.06, p = 0.10). However, mean FD was included as a covariate in the statistical models to rule out a potential effect of head micromovements on the SDBOLD51.
We performed a voxel-wise group comparison between FM and HC groups using an unpaired two-sample t-test. In FM patients, changes in SDBOLD between sham tDCS and active tDCS, and baseline and sham tDCS were determined by paired t-tests. Age and mean FD were included as covariates of no interest. Multiple comparison correction was performed at the cluster-level using a family-wise error (FWE) rate of p < 0.025 (0.05/2, to account for two different frequency bands). We used an initial cluster-forming threshold p < 0.001 to avoid the low spatial specificity and better minimize the false positives52. Thus, all statistical contrast maps were thresholded at the voxel-level threshold of p < 0.001 (uncorrected), combined with a cluster-level FWE-corrected p < 0.025. Changes in SDBOLD in the area of interest (anterior cingulate regions) after active tDCS compared with sham were probed using small volume correction in the predefined cingulate mask. The cingulate mask was generated by combining the cingulate gyrus (anterior division) and paracingulate gyrus from the Harvard-Oxford cortical structural atlas. The significance threshold was set to voxel-level p < 0.001 (uncorrected), combined with a cluster-level FWE-corrected p < 0.025.
Clinical significance of baseline and changes in SDBOLD
To explore the predictability of baseline SDBOLD in tDCS-related changes in pain symptoms, we examined the relationship between the baseline SDBOLD and changes in clinical pain from baseline to active tDCS. We performed a whole-brain voxel-wise correlation analysis between baseline SDBOLD map and clinical pain changes (active minus baseline) with age and mean FD (baseline) as a covariate. The significance threshold was set to voxel-level Z > 2.3 combined with a cluster-level FWE-corrected p < 0.025 (0.05/2, to account for two different frequency bands).
Our results revealed an increased SDBOLD in the ACC region within the slow-5 frequency band after active-tDCS compared to sham. Thus, we examined associations between changes in SDBOLD and changes in clinical pain after active tDCS compared to sham in the slow-5 frequency band. We first created difference images by subtracting SDBOLD maps (active minus sham) for each subject. Next, we performed a whole-brain voxel-wise correlation analysis between subtracted SDBOLD map (active minus sham) and changes in clinical pain (active minus sham) with age and mean FD (active minus sham) as a covariate. The significance threshold was set to voxel-level Z > 2.3 combined with a cluster-level FWE-corrected p < 0.025.
Results
Baseline SDBOLD
In the slow-5 frequency band, FM patients had reduced SDBOLD in the right ventromedial prefrontal cortex (vmPFC) compared with HC subjects (p < 0.025, FWE-corrected). In the slow-4 frequency band, FM patients also exhibited lower SDBOLD in the right vmPFC, lateral PFC, and anterior insula (aINS) and higher SDBOLD in the left posterior insula (pINS) compared with HC subjects (p < 0.025, FWE-corrected) (Fig. 1A, Table 1). A whole-brain correlation analysis revealed that right vmPFC SDBOLD (slow-5 band) at baseline correlated significantly with VAS pain score changes after active tDCS compared with baseline (r = −0.770, p = 0.003). Namely, FM patients with higher SDBOLD in the vmPFC at baseline had a greater pain reduction following active treatment. Also, the SDBOLD (slow-4 band) in the left vmPFC (r = −0.881, p < 0.001) was significantly correlated with changes in VAS pain score after active tDCS compared with baseline (Fig. 1B). Other significant regions are listed in Table 2.
Fig. 1. Regional abnormalities of baseline (pre-treatment) BOLD signal variability (SDBOLD) in fibromyalgia (FM) compared to healthy controls (HC).
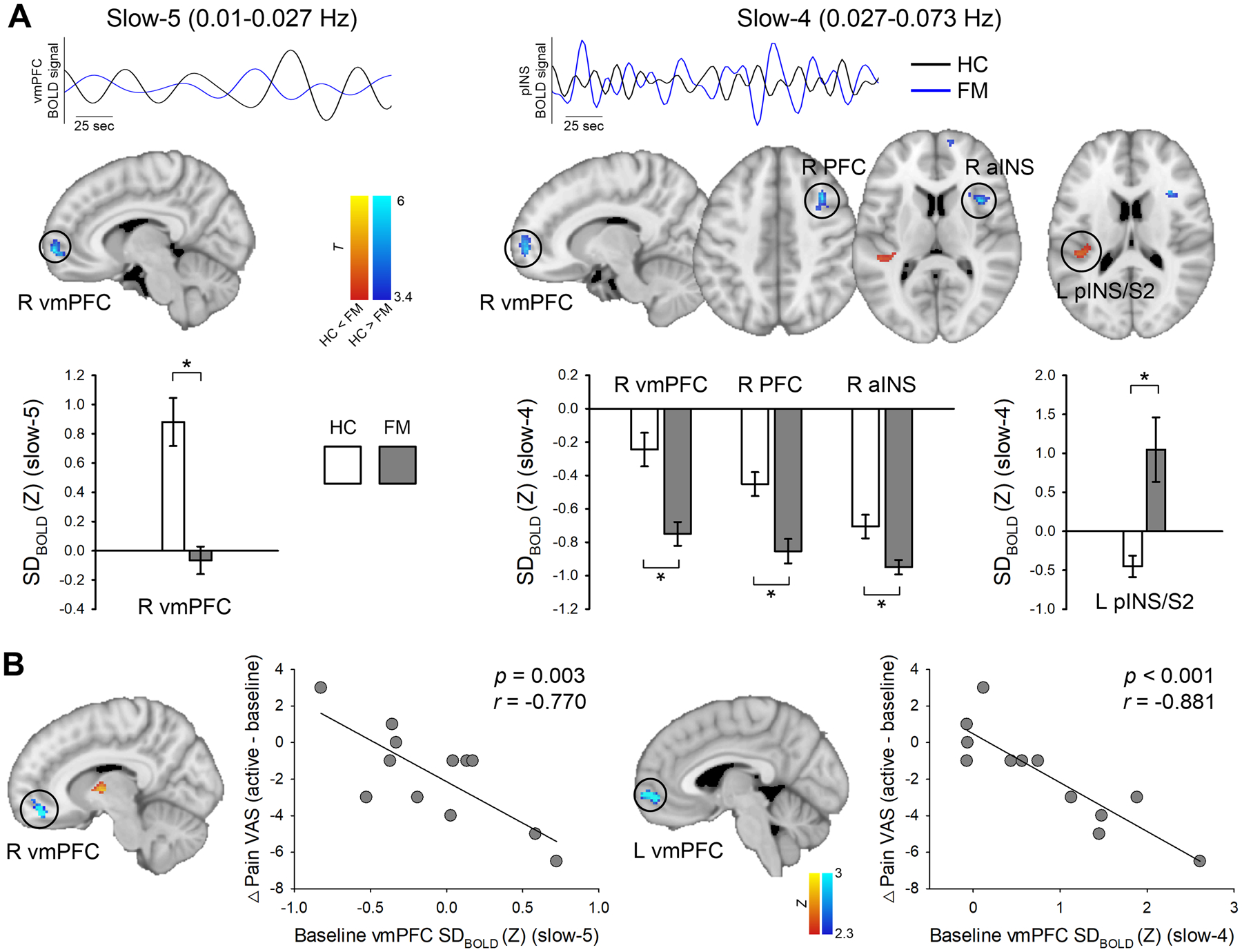
(A) Baseline group differences of resting-state SDBOLD in the slow-5 (0.01 – 0.027 Hz) and slow-4 (0.027 – 0.073 Hz) frequency bands. Blue (FM) and black lines (HC) indicate the representative time-course of band-pass filtered BOLD signal in each slow frequency band. Brain regions displaying increased (hot scale bar) and decreased (cool scale bar) SDBOLD in FM patients compared with HC were overlaid on the MNI standard brain. All statistical images are displayed with significant clusters (voxel-level threshold p < 0.001 and cluster-level extent threshold p < 0.025, FWE-corrected*). Grey bars represent FM patients (n = 12), white bars represent HC subjects (n = 15). Bar graphs were expressed as mean ± standard error of the mean. (B) Correlation between baseline SDBOLD (slow-5 and slow-4) in the ventromedial prefrontal cortex (vmPFC) and changes in VAS pain score between active-tDCS and baseline. Higher SDBOLD of the vmPFC at baseline predicted a greater reduction in clinical pain. VAS, visual analog scale; aINS, anterior insula; pINS, posterior insula; S2, secondary somatosensory cortex.
Table 1.
Brain regions with increased and decreased BOLD signal variability (SDBOLD) in fibromyalgia (FM) patients compared with healthy controls (HC).
| Frequency band | Contrast | Brain region | MNI coordinates (x, y, z) |
Number of voxels | T-value |
|---|---|---|---|---|---|
| Slow-5 (0.01–0.027 Hz) |
FM < HC | R vmPFC | 8, 62, −2 | 128 | 6.22 |
| Slow-4 (0.027–0.073 Hz) |
FM < HC | R vmPFC | 12, 60, 0 | 117 | 5.44 |
| R anterior insula | 38, 14, 10 | 95 | 5.8 | ||
| R lateral PFC | 34, 16, 44 | 111 | 5.76 | ||
| FM > HC | L posterior insula/S2 | −38, −30, 16 | 89 | 4.16 |
All statistical results were thresholded at voxel-level p < 0.001 and cluster-level p < 0.025, FWE-corrected. vmPFC, ventromedial prefrontal cortex; S2, secondary somatosensory cortex; L, left; R, Right.
Table 2.
Correlation between clinical pain changes (VAS) (active minus baseline) and baseline BOLD signal variability (SDBOLD).
| Frequency band | Brain region | MNI coordinates (x, y, z) |
Number of voxels | R-value |
|---|---|---|---|---|
| Slow-5 (0.01–0.027 Hz) |
R vmPFC | 10 48 −10 | 172 | −0.95 |
| R thalamus | 2 −8 8 | 306 | 0.88 | |
| L cerebellum | −8 −72 −38 | 249 | 0.88 | |
| Slow-4 (0.027–0.073 Hz) |
L vmPFC | −4 62 −4 | 180 | −0.89 |
| R parahippocampal gyrus | 14 −36 −10 | 220 | 0.89 |
All statistical results were thresholded at voxel-level Z > 2.3 and cluster-level p < 0.025, FWE-corrected. vmPFC, ventromedial prefrontal cortex; rACC, rostral anterior cingulate cortex; pINS, posterior insula; MCC, midcingulate cortex; SMA, supplementary motor area. L, left; R, Right.
Changes of SDBOLD between sham and active tDCS
After active tDCS, FM patients had increased SDBOLD in the left lateral prefrontal cortex (slow-5 band) and left thalamus (slow-4 band) compared with sham tDCS (p < 0.025, FWE-corrected). Separate small-volume correction in the cingulate mask revealed that FM patients displayed significantly higher SDBOLD in the left rostral ACC (rACC) (slow-5 band) (p < 0.025, FWE-small volume corrected) after active tDCS (Fig. 2, Table 3).
Fig. 2. Changes in BOLD signal variability (SDBOLD) in the slow-5 (0.01 – 0.027 Hz) (A) and slow-4 (0.027 – 0.078 Hz) (B) frequency bands after active tDCS compared with sham tDCS.
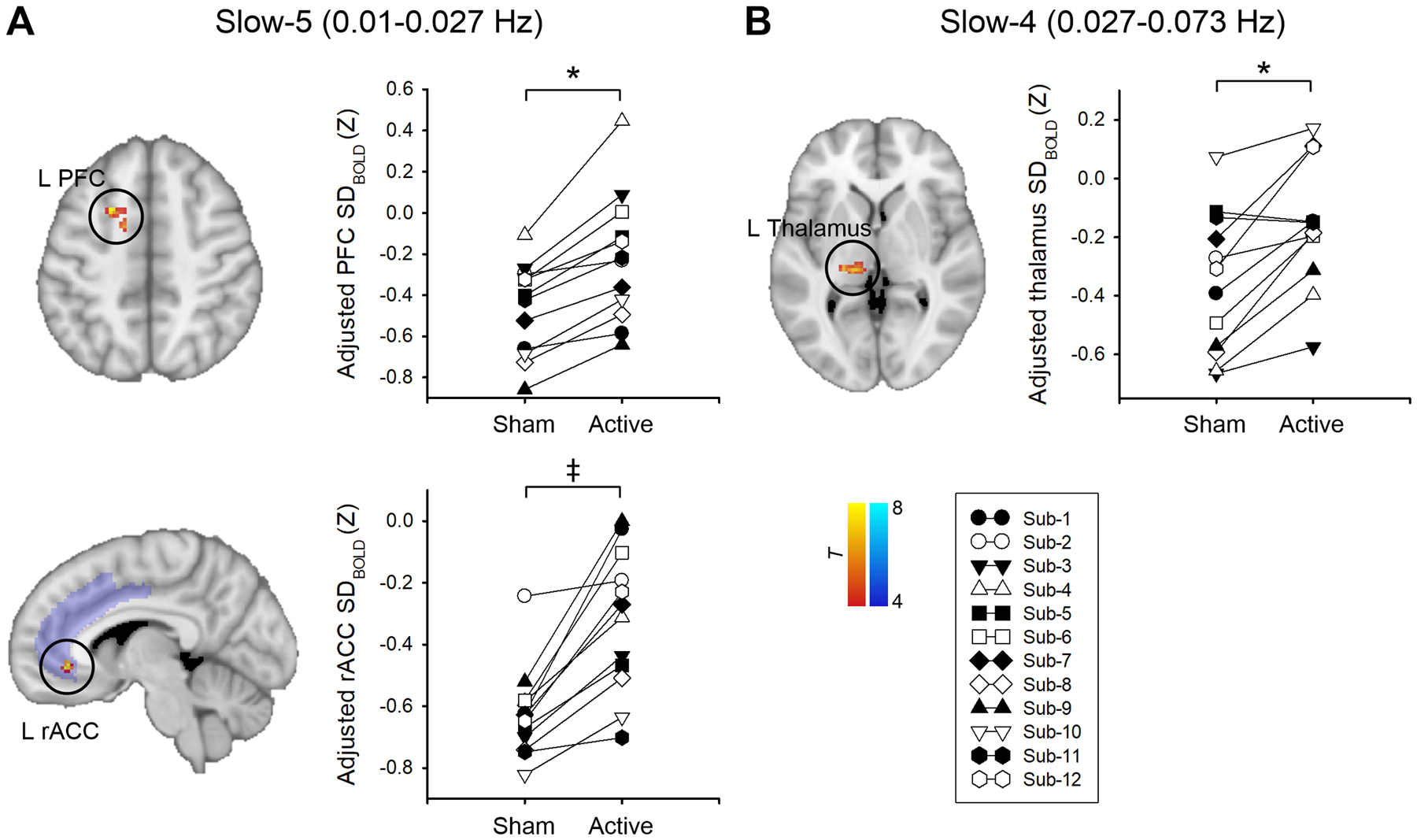
All statistical images are displayed with significant clusters (voxel-level threshold p < 0.001 and cluster-level extent threshold p < 0.025, FWE-corrected*) except for the left rostral anterior cingulate cortex (rACC). Area of interest (ACC) was probed using small volume correction in the predefined cingulate mask. The cingulate mask (purple) was derived from the Harvard-Oxford cortical structural atlases. The significance threshold was set to voxel-level p < 0.001 (uncorrected), combined with a cluster-extent threshold of p < 0.025 (FWE-small volume corrected‡). SDBOLD was adjusted (mean + residual) for age and mean frame-wise displacement. Each symbol represents an individual fibromyalgia patient. PFC, prefrontal cortex; M1, primary motor cortex.
Table 3.
Active and sham tDCS related changes in BOLD signal variability (SDBOLD).
| Frequency band | Contrast | Brain region | MNI coordinates (x, y, z) |
Number of voxels | T-value |
|---|---|---|---|---|---|
| Sham vs. active tDCS for FM | |||||
| Slow-5 (0.01–0.027 Hz) |
Sham < active | L rACC | −6, 36, −4 | 22* | 8.75 |
| L lateral PFC | −22, 12, 48 | 50 | 8.24 | ||
| Slow-4 (0.027–0.073 Hz) |
Sham < active | L thalamus | −14, −26, 2 | 66 | 6.94 |
| Baseline vs. sham tDCS for FM | |||||
| Slow-5 (0.01–0.027 Hz) |
Baseline > sham | R dmPFC | 2, 48, 34 | 73 | 8.56 |
| Slow-4 (0.027–0.073 Hz) |
Baseline > sham | R dmPFC | 0, 46, 26 | 75 | 6.94 |
| R posterior cingulate cortex/precuneus | 2, −48, 36 | 73 | 8.63 | ||
All statistical results were thresholded at voxel-level p < 0.001 and cluster-level p < 0.025, FWE-corrected.
Small volume correction in the ACC mask (voxel-level p < 0.001 and cluster level FWE-corrected p < 0.025). ACC, anterior cingulate cortex; rACC, rostral anterior cingulate cortex; dmPFC, dorsomedial prefrontal cortex; PFC, prefrontal cortex; L, left; R, Right.
Changes of SDBOLD between baseline and sham tDCS
After sham tDCS, FM patients had decreased SDBOLD in the right dorsomedial prefrontal cortex (dmPFC) (slow-5 and slow-4 bands) and right posterior cingulate cortex/precuneus (slow-4 band) constituting the DMN compared with baseline (p < 0.025, FWE-corrected) (Fig. 3, Table 3). There was no significant increase in SDBOLD after sham compared with baseline.
Fig. 3. Changes in BOLD signal variability (SDBOLD) in the slow-5 (0.01 – 0.027 Hz) (A) and slow-4 (0.027 – 0.078 Hz) (B) frequency bands after sham tDCS compared with baseline.
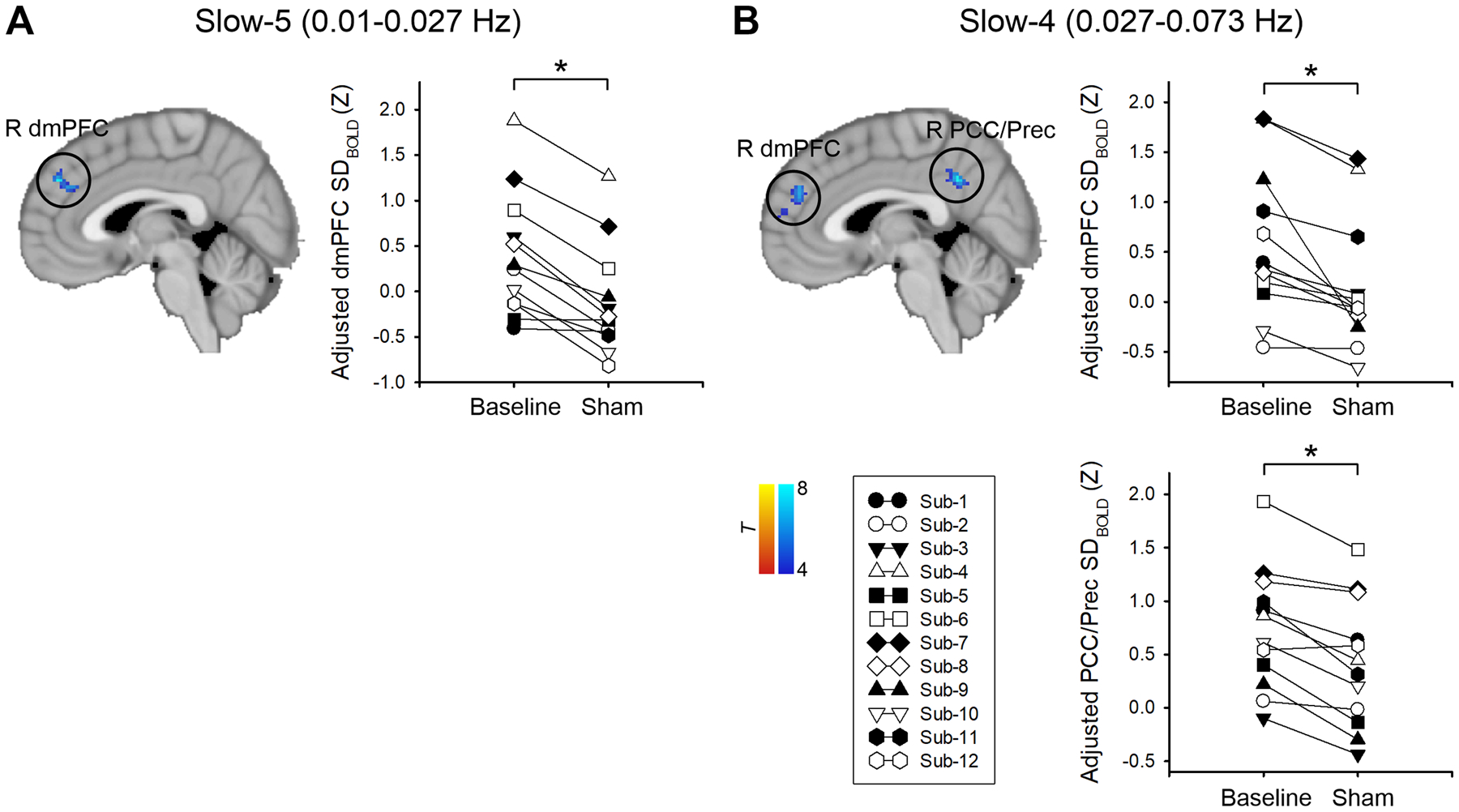
All statistical images are displayed with significant clusters (voxel-level threshold p < 0.001 and cluster-level extent threshold p < 0.025, FWE-corrected*). SDBOLD was adjusted (mean + residual) for age and mean frame-wise displacement. Each symbol represents an individual fibromyalgia patient. dmPFC, dorsomedial prefrontal cortex; PCC, posterior cingulate cortex; Prec, precuneus.
Association between clinical pain and SDBOLD
The correlation between changes in SDBOLD (slow-5) and changes in clinical pain (VAS) after active tDCS compared with sham was depicted in Fig. 4 and Table 4. We found that patients with increased SDBOLD in the vmPFC/rACC (slow-5) had a greater reduction in clinical pain (p < 0.025, FWE-corrected). Changes in SDBOLD (slow-5) in the midcingulate cortex/supplementary motor area and pINS was positively correlated with the change in clinical pain (p < 0.025, FWE-corrected). Namely, patients with decreased SDBOLD in the midcingulate cortex/supplementary motor area or pINS had a greater reduction in clinical pain after active tDCS compared with sham tDCS.
Fig. 4. Correlation between changes in BOLD signal variability (0.01 – 0.027 Hz, slow-5 band) and changes in VAS pain score after active tDCS compared with sham.
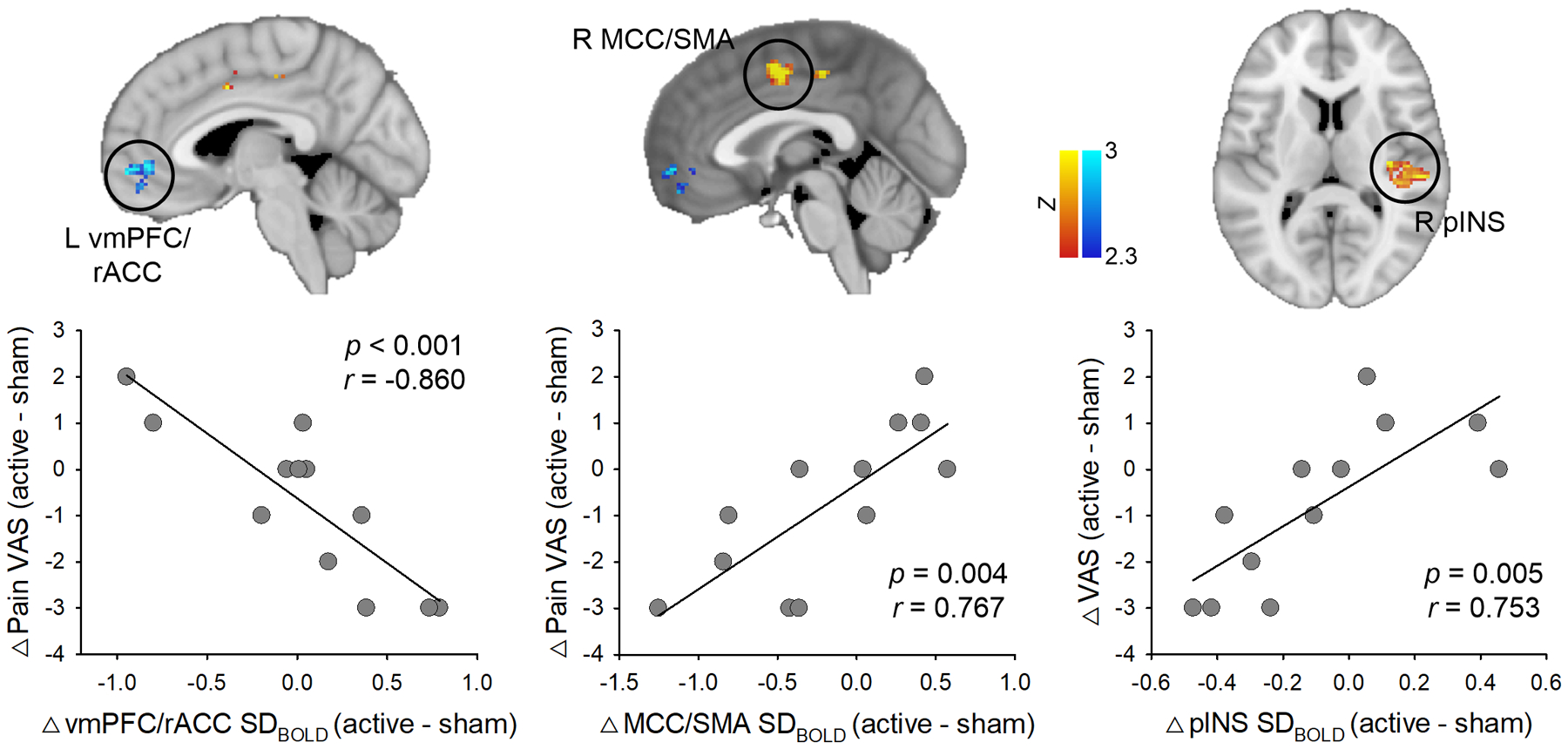
Patients with increased SDBOLD in the vmPFC/rACC had a greater reduction in clinical pain. In contrast, patients with decreased SDBOLD in the MCC/SMA or pINS had a greater reduction in clinical pain after active tDCS compared with sham tDCS. Statistical images are displayed with significant clusters (voxel-level threshold Z > 2.3 and cluster-level extent threshold p < 0.025, FWE-corrected). VAS, visual analog scale; vmPFC, ventromedial prefrontal cortex; rACC, rostral anterior cingulate cortex; MCC, midcingulate cortex; SMA, supplementary motor area; pINS, posterior insula.
Table 4.
Correlation between changes in clinical pain (VAS) (active minus sham) and changes in BOLDSV (active minus sham) in the slow-5 band (0.01–0.027 Hz).
| Brain region | MNI coordinates (x, y, z) |
Number of voxels | R-value |
|---|---|---|---|
| L vmPFC/rACC | −4, 54, −4 | 170 | −0.90 |
| L cerebellum | −20, −32, −26 | 149 | −0.92 |
| R pINS | 36, −18, 10 | 270 | 0.82 |
| MCC/SMA | 0, −2, 48 | 273 | 0.90 |
All statistical results were thresholded at voxel-level Z > 2.3 and cluster-level p < 0.025, FWE-corrected. vmPFC, ventromedial prefrontal cortex; rACC, rostral anterior cingulate cortex; pINS, posterior insula; MCC, midcingulate cortex; SMA, supplementary motor area. L, left; R, Right.
Discussion
Our results revealed that FM patients exhibited significantly lower SDBOLD in the vmPFC, lateral PFC, and aINS, and higher SDBOLD in the pINS compared with HCs. After active M1-tDCS compared with sham, we demonstrated an increased SDBOLD in the rACC/vmPFC and decreased SDBOLD in the pINS associated with clinical pain improvement in FM patients.
Previously, higher SDBOLD was thought to be favorable to cognitive function across aging53, pain sensitivity, and modulation29, but this is not always true. For example, SDBOLD of the salience network (e.g., aINS) increased linearly, whereas most other networks (e.g., DMN and sensorimotor network) decreased linearly across the lifespan27. Also, patients with ankylosing spondylitis, a form of chronic back pain, showed higher SDBOLD in the ascending nociceptive pathway and DMN, including the primary somatosensory cortex (S1), thalamus, posterior cingulate cortex, and precuneus30. These results notably highlight the importance of an optimal range of variability to perform the desired function27.
We first found that FM patients exhibited significantly lower SDBOLD in the vmPFC at pre-tDCS treatment, possibly indicating an inadequate pain modulatory function. This result is in line with the findings of attenuated activity in the rACC during provoked pain in FM patients5. It is known that the rACC/vmPFC interacts closely with PAG to exert descending pain modulation through μ-opioid transmission43, 54. In this regard, increased SDBOLD of the brain signal in the rACC (with small-volume correction), associated with pain improvement after active-tDCS compared to sham, would mean that the brain could more engage in the endogenous pain modulatory function. This hypothesis accords well with the previous studies indicating that M1-tDCS enhances the endogenous pain inhibitory system20, 21, 55, 56.
We identified that increases in SDBOLD from rACC/vmPFC variability were related to clinical pain reduction after active-tDCS compared to sham. Interestingly, functional connectivity between the rACC/mPFC and cognitive control network was increased after Tai Chi treatment, a traditional Chinese mind-body intervention, accompanied by clinical improvement in FM patients57. Since an impaired endogenous pain modulation is a critical feature of FM5, 58–61, changes in rACC/vmPFC signal involved in descending pain modulation would be essential to elicit the beneficial treatment effects on FM pain.
Moreover, we found that the higher vmPFC variability before tDCS was associated with more pain reduction after active tDCS treatment. This result indicates that individual differences in signal variability of the rACC/vmPFC, playing an essential role in the top-down regulation of pain43, 62, could serve as a substrate on how an individual responds or modulates pain after tDCS. This finding is also consistent with a recent fMRI study reporting that higher baseline variability in the left middle frontal gyrus was associated with a more significant reduction in pain unpleasantness following a delayed onset muscle soreness induction34. Given the role of SDBOLD in reflecting the brain’s modulatory capacity, resilience, and readiness to change for better performance26, we suggest that individual differences in baseline variability may predict the analgesic outcome by differentiating whether the brain is responsive to tDCS.
Previous studies with FM indicated that thalamic activities are compromised at rest and in response to painful stimuli63–65. However, we did not observe any alterations in the thalamic region at baseline. Nevertheless, our result showed that SDBOLD of the left posterior thalamus encompassing the ventral posterolateral (VPL) and pulvinar nuclei increased after active tDCS compared with sham. It is noteworthy that higher variability of the thalamus, other than any other brain regions, reflected greater large-scale functional integration of the healthy human brain66. Thus, increased SDBOLD of the thalamus after tDCS may relate to efficient thalamo-cortical integration. This hypothesis is supported by the previous study showing that M1-tDCS increased functional coupling between the thalamus and M1 in healthy participants67. Our previous tDCS study with the same patients also revealed the association between decreased VPL-pINS and VPL-M1/S1 functional connectivity and pain reduction24. In light of our preliminary finding of the endogenous μ-opioid (peptide) release of the posterior thalamus in a neuropathic pain patient immediately after a single M1-tDCS18, we speculated that μ-opioid release after M1-tDCS could contribute to changes in thalamic SDBOLD.
Our study also identified that FM showed lower aINS and higher pINS SDBOLD compared to HC at baseline. The aINS is implicated in the affective and salience component of pain68, whereas the pINS is more engaged in discriminative aspects of sensory pain69. As stated above, brain signal variability under a normal range can be interpreted as abnormal, which may be linked to disrupted salience/affective pain processing exhibited in FM70, 71. Also, greater SDBOLD of the pINS might contribute to amplified sensory and nociceptive processing, given that signal variability reflects a dynamic range of possible neuronal responses to internal or external stimuli26. Importantly, accumulating evidence has indicated that the insular metabolites, as well as their connectivity with the descending pain modulatory system and DMN, are critically implicated in the central sensitization of FM, which contributes to augmented pain perception6, 10, 11, 72, 73. A recent study using graph-theory based network analysis demonstrated altered hub topology in the aINS74. Moreover, the eigenvector centrality, indicating a hub strength of the pINS, was positively correlated with clinical pain intensity74. Thus, the functional role of the pINS in pain may explain our result that decreased SDBOLD in pINS after active tDCS was associated with pain improvement. Together, these findings suggest why we should consider the insula as a pathogenic region linked to augmented pain and therapeutic targets in FM75.
Regarding the placebo effect of sham-tDCS, we found a decreased SDBOLD in the regions consisting of DMN, including the dmPFC and posterior cingulate cortex/precuneus, while comparing sham-tDCS to baseline. This result is partly in line with our previous study, demonstrating an endogenous μ-opioid (peptide) release in the precuneus after sham-tDCS in healthy participants42. It should be acknowledged that even in a sham session, 2 mA currents were delivered during the first and last 30 seconds, which might affect patients’ expectancy. Thus, we speculated that attentional and cognitive engagement with an expectancy for pain relief, known to modify DMN activity76, likely influenced the current results.
Our study has several limitations. First, the study design was not randomized. We chose this design to prevent a potential carry-over effect of the active tDCS, which has a long-lasting effect on pain perception and brain excitability. Second, we did not perform a blinding assessment to assess whether patients noticed their treatment based on standardized documentation. Thus, the reader should consider this when interpreting our results since a lack of assessment could increase the likelihood of biased conclusions. Third, although we collected the HC data from the same scanner at the same institution, there were subtle changes in the software versions. The MR environments, including software or personnel, would unknowingly influence the BOLD signal. However, we cannot assess those potential effects on SDBOLD systemically in the current study. Lastly, a larger sample size and long-term follow-up approaches are warranted to optimize the current protocol to draw fruitful outcomes.
In conclusion, our findings suggest that M1-tDCS might revert temporal variability of fMRI signals in the rACC/vmPFC and posterior insula linked to pain improvement in FM. The rACC/vmPFC variability would have a potential role in responsiveness and readiness to tDCS treatment; thus, future studies may use this marker to deliver more tailored therapies (i.e., personalized medicine).
Supplementary Material
Acknowledgments
We acknowledge and thank Dr. Bradley R. Foerster’s contribution to the previous 1H-MRS tDCS study that was performed in FM patients, which we reported earlier1.
Sources of financial support
The original study was funded by a MICHR Clinical Trial Planning Program and CTSA high-tech funding grant, University of Michigan (Co-PIs: DaSilva, Harris). The current study employs application and processing tools developed under the National Institute of Health grants: National Institute of Dental and Craniofacial Research U01-DE025633 (DaSilva), National Institute of Neurological Disorders and Stroke R01-NS094413 (DaSilva), and National Center for Complementary and Integrative Health 1R01AT010060 (Harris, DaSilva). Healthy control participants originated from a cooperative agreement from the National Institute of Health, National Institute of Diabetes and Digestive and Kidney Diseases (Grant numbers DK082370, DK082342, DK082315, DK082344, DK082325, DK082345, DK082333, DK082316, DK103260, DK103277, and DK103271).
Footnotes
Conflict of interest statement
A. DaSilva co-created GeoPain (previously named PainTrek) and he is also the co-founder and co-owner of MoxyTech Inc., which licensed the technology from the University of Michigan. The other authors declare no competing financial interests.
References
- 1.Foerster BR, Nascimento TD, DeBoer M, et al. Excitatory and inhibitory brain metabolites as targets of motor cortex transcranial direct current stimulation therapy and predictors of its efficacy in fibromyalgia. Arthritis Rheumatol. Feb 2015;67(2):576–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clauw DJ. Fibromyalgia: a clinical review. JAMA. Apr 16 2014;311(15):1547–1555. [DOI] [PubMed] [Google Scholar]
- 3.Lopez-Sola M, Pujol J, Wager TD, et al. Altered functional magnetic resonance imaging responses to nonpainful sensory stimulation in fibromyalgia patients. Arthritis Rheumatol. Nov 2014;66(11):3200–3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lim M, Roosink M, Kim JS, et al. Disinhibition of the primary somatosensory cortex in patients with fibromyalgia. Pain. Apr 2015;156(4):666–674. [DOI] [PubMed] [Google Scholar]
- 5.Jensen KB, Kosek E, Petzke F, et al. Evidence of dysfunctional pain inhibition in Fibromyalgia reflected in rACC during provoked pain. Pain. Jul 2009;144(1–2):95–100. [DOI] [PubMed] [Google Scholar]
- 6.Pujol J, Macia D, Garcia-Fontanals A, et al. The contribution of sensory system functional connectivity reduction to clinical pain in fibromyalgia. Pain. Aug 2014;155(8):1492–1503. [DOI] [PubMed] [Google Scholar]
- 7.Lim M, Roosink M, Kim JS, et al. Augmented Pain Processing in Primary and Secondary Somatosensory Cortex in Fibromyalgia: A Magnetoencephalography Study Using Intra-Epidermal Electrical Stimulation. PLoS One. 2016;11(3):e0151776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choi W, Lim M, Kim JS, Chung CK. Habituation deficit of auditory N100m in patients with fibromyalgia. Eur J Pain. Nov 2016;20(10):1634–1643. [DOI] [PubMed] [Google Scholar]
- 9.Choe MK, Lim M, Kim JS, Lee DS, Chung CK. Disrupted Resting State Network of Fibromyalgia in Theta frequency. Sci Rep. Feb 1 2018;8(1):2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Foerster BR, Petrou M, Edden RA, et al. Reduced insular gamma-aminobutyric acid in fibromyalgia. Arthritis Rheum. Feb 2012;64(2):579–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harris RE, Sundgren PC, Craig AD, et al. Elevated insular glutamate in fibromyalgia is associated with experimental pain. Arthritis Rheum. Oct 2009;60(10):3146–3152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pomares FB, Roy S, Funck T, et al. Upregulation of cortical GABAA receptor concentration in fibromyalgia. Pain. Jan 2020;161(1):74–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fitzcharles MA, Ste-Marie PA, Gamsa A, Ware MA, Shir Y. Opioid Use, Misuse, and Abuse in Patients Labeled as Fibromyalgia. American Journal of Medicine. Oct 2011;124(10):955–960. [DOI] [PubMed] [Google Scholar]
- 14.Hauser W, Walitt B, Fitzcharles MA, Sommer C. Review of pharmacological therapies in fibromyalgia syndrome. Arthritis Research & Therapy. 2014;16(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fregni F, Gimenes R, Valle AC, et al. A randomized, sham-controlled, proof of principle study of transcranial direct current stimulation for the treatment of pain in fibromyalgia. Arthritis Rheum. Dec 2006;54(12):3988–3998. [DOI] [PubMed] [Google Scholar]
- 16.Hou WH, Wang TY, Kang JH. The effects of add-on non-invasive brain stimulation in fibromyalgia: a meta-analysis and meta-regression of randomized controlled trials. Rheumatology (Oxford). Aug 2016;55(8):1507–1517. [DOI] [PubMed] [Google Scholar]
- 17.Lefaucheur JP, Antal A, Ayache SS, et al. Evidence-based guidelines on the therapeutic use of transcranial direct current stimulation (tDCS). Clinical Neurophysiology. Jan 2017;128(1):56–92. [DOI] [PubMed] [Google Scholar]
- 18.DosSantos MF, Love TM, Martikainen IK, et al. Immediate effects of tDCS on the mu-opioid system of a chronic pain patient. Front Psychiatry. 2012;3:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dasilva AF, Mendonca ME, Zaghi S, et al. tDCS-induced analgesia and electrical fields in pain-related neural networks in chronic migraine. Headache. Sep 2012;52(8):1283–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meeker TJ, Keaser ML, Khan SA, Gullapalli RP, Seminowicz DA, Greenspan JD. Non-invasive Motor Cortex Neuromodulation Reduces Secondary Hyperalgesia and Enhances Activation of the Descending Pain Modulatory Network. Front Neurosci. 2019;13:467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DosSantos MF, Ferreira N, Toback RL, Carvalho AC, DaSilva AF. Potential Mechanisms Supporting the Value of Motor Cortex Stimulation to Treat Chronic Pain Syndromes. Front Neurosci. 2016;10:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fagerlund AJ, Hansen OA, Aslaksen PM. Transcranial direct current stimulation as a treatment for patients with fibromyalgia: a randomized controlled trial. Pain. Jan 2015;156(1):62–71. [DOI] [PubMed] [Google Scholar]
- 23.Khedr EM, Omran EAH, Ismail NM, et al. Effects of transcranial direct current stimulation on pain, mood and serum endorphin level in the treatment of fibromyalgia: A double blinded, randomized clinical trial. Brain Stimulation. Sep-Oct 2017;10(5):893–901. [DOI] [PubMed] [Google Scholar]
- 24.Cummiford CM, Nascimento TD, Foerster BR, et al. Changes in resting state functional connectivity after repetitive transcranial direct current stimulation applied to motor cortex in fibromyalgia patients. Arthritis Res Ther. Feb 3 2016;18:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garrett DD, Kovacevic N, McIntosh AR, Grady CL. The importance of being variable. J Neurosci. Mar 23 2011;31(12):4496–4503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garrett DD, Samanez-Larkin GR, MacDonald SW, Lindenberger U, McIntosh AR, Grady CL. Moment-to-moment brain signal variability: a next frontier in human brain mapping? Neurosci Biobehav Rev. May 2013;37(4):610–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nomi JS, Bolt TS, Ezie CEC, Uddin LQ, Heller AS. Moment-to-Moment BOLD Signal Variability Reflects Regional Changes in Neural Flexibility across the Lifespan. J Neurosci. May 31 2017;37(22):5539–5548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kucyi A, Davis KD. The Neural Code for Pain: From Single-Cell Electrophysiology to the Dynamic Pain Connectome. Neuroscientist. Aug 2017;23(4):397–414. [DOI] [PubMed] [Google Scholar]
- 29.Rogachov A, Cheng JC, Erpelding N, Hemington KS, Crawley AP, Davis KD. Regional brain signal variability: a novel indicator of pain sensitivity and coping. Pain. Nov 2016;157(11):2483–2492. [DOI] [PubMed] [Google Scholar]
- 30.Rogachov A, Cheng JC, Hemington KS, et al. Abnormal Low-Frequency Oscillations Reflect Trait-Like Pain Ratings in Chronic Pain Patients Revealed through a Machine Learning Approach. J Neurosci. Aug 15 2018;38(33):7293–7302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bosma RL, Kim JA, Cheng JC, et al. Dynamic pain connectome functional connectivity and oscillations reflect multiple sclerosis pain. Pain. Nov 2018;159(11):2267–2276. [DOI] [PubMed] [Google Scholar]
- 32.Lim M, Jassar H, Kim DJ, Nascimento TD, DaSilva AF. Differential alteration of fMRI signal variability in the ascending trigeminal somatosensory and pain modulatory pathways in migraine. J Headache Pain. Jan 7 2021;22(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Armbruster-Genc DJ, Ueltzhoffer K, Fiebach CJ. Brain Signal Variability Differentially Affects Cognitive Flexibility and Cognitive Stability. J Neurosci. Apr 6 2016;36(14):3978–3987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boissoneault J, Sevel L, Stennett B, Alappattu M, Bishop M, Robinson M. Regional increases in brain signal variability are associated with pain intensity reductions following repeated eccentric exercise bouts. Eur J Pain. Apr 2020;24(4):818–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. Oct 1995;34(4):537–541. [DOI] [PubMed] [Google Scholar]
- 36.Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. Sep 2007;8(9):700–711. [DOI] [PubMed] [Google Scholar]
- 37.Buzsaki G, Draguhn A. Neuronal oscillations in cortical networks. Science. Jun 25 2004;304(5679):1926–1929. [DOI] [PubMed] [Google Scholar]
- 38.Zuo XN, Di Martino A, Kelly C, et al. The oscillating brain: complex and reliable. Neuroimage. Jan 15 2010;49(2):1432–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hodkinson DJ, Wilcox SL, Veggeberg R, et al. Increased Amplitude of Thalamocortical Low-Frequency Oscillations in Patients with Migraine. J Neurosci. Jul 27 2016;36(30):8026–8036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baliki MN, Baria AT, Apkarian AV. The cortical rhythms of chronic back pain. J Neurosci. Sep 28 2011;31(39):13981–13990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lim M, Nascimento TD, Kim DJ, Ellingrod VL, DaSilva AF. Aberrant Brain Signal Variability and COMT Genotype in Chronic TMD Patients. J Dent Res. Feb 23 2021:22034521994089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.DosSantos MF, Martikainen IK, Nascimento TD, et al. Building up analgesia in humans via the endogenous mu-opioid system by combining placebo and active tDCS: a preliminary report. PLoS One. 2014;9(7):e102350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Petrovic P, Kalso E, Petersson KM, Ingvar M. Placebo and opioid analgesia-- imaging a shared neuronal network. Science. Mar 1 2002;295(5560):1737–1740. [DOI] [PubMed] [Google Scholar]
- 44.Wolfe F, Smythe HA, Yunus MB, et al. The American College of Rheumatology 1990 criteria for the classification of fibromyalgia. Report of the multicenter criteria committee. Arthritis Rheum. Feb 1990;33(2):160–172. [DOI] [PubMed] [Google Scholar]
- 45.Kutch JJ, Ichesco E, Hampson JP, et al. Brain signature and functional impact of centralized pain: a multidisciplinary approach to the study of chronic pelvic pain (MAPP) network study. Pain. Oct 2017;158(10):1979–1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bolognini N, Spandri V, Ferraro F, et al. Immediate and Sustained Effects of 5-Day Transcranial Direct Current Stimulation of the Motor Cortex in Phantom Limb Pain. J Pain. Jul 2015;16(7):657–665. [DOI] [PubMed] [Google Scholar]
- 47.DaSilva AF, Volz MS, Bikson M, Fregni F. Electrode positioning and montage in transcranial direct current stimulation. J Vis Exp. May 23 2011(51). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gandiga PC, Hummel FC, Cohen LG. Transcranial DC stimulation (tDCS): a tool for double-blind sham-controlled clinical studies in brain stimulation. Clin Neurophysiol. Apr 2006;117(4):845–850. [DOI] [PubMed] [Google Scholar]
- 49.Melzack R The short-form McGill Pain Questionnaire. Pain. Aug 1987;30(2):191–197. [DOI] [PubMed] [Google Scholar]
- 50.Power JD, Schlaggar BL, Petersen SE. Recent progress and outstanding issues in motion correction in resting state fMRI. Neuroimage. Jan 15 2015;105:536–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yan CG, Cheung B, Kelly C, et al. A comprehensive assessment of regional variation in the impact of head micromovements on functional connectomics. Neuroimage. Aug 1 2013;76:183–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Woo CW, Krishnan A, Wager TD. Cluster-extent based thresholding in fMRI analyses: pitfalls and recommendations. Neuroimage. May 1 2014;91:412–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Grady CL, Garrett DD. Understanding variability in the BOLD signal and why it matters for aging. Brain Imaging Behav. Jun 2014;8(2):274–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Eippert F, Bingel U, Schoell ED, et al. Activation of the opioidergic descending pain control system underlies placebo analgesia. Neuron. Aug 27 2009;63(4):533–543. [DOI] [PubMed] [Google Scholar]
- 55.Donnell A, T DN, Lawrence M, et al. High-Definition and Non-invasive Brain Modulation of Pain and Motor Dysfunction in Chronic TMD. Brain Stimul. Nov-Dec 2015;8(6):1085–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Duarte D, Castelo-Branco LEC, Uygur Kucukseymen E, Fregni F. Developing an optimized strategy with transcranial direct current stimulation to enhance the endogenous pain control system in fibromyalgia. Expert Rev Med Devices. Dec 2018;15(12):863–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kong J, Wolcott E, Wang Z, et al. Altered resting state functional connectivity of the cognitive control network in fibromyalgia and the modulation effect of mind-body intervention. Brain Imaging Behav. Apr 2019;13(2):482–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jensen KB, Srinivasan P, Spaeth R, et al. Overlapping structural and functional brain changes in patients with long-term exposure to fibromyalgia pain. Arthritis Rheum. Dec 2013;65(12):3293–3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.O’Brien AT, Deitos A, Trinanes Pego Y, Fregni F, Carrillo-de-la-Pena MT. Defective Endogenous Pain Modulation in Fibromyalgia: A Meta-Analysis of Temporal Summation and Conditioned Pain Modulation Paradigms. J Pain. Aug 2018;19(8):819–836. [DOI] [PubMed] [Google Scholar]
- 60.Schrepf A, Harper DE, Harte SE, et al. Endogenous opioidergic dysregulation of pain in fibromyalgia: a PET and fMRI study. Pain. Oct 2016;157(10):2217–2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Truini A, Tinelli E, Gerardi MC, et al. Abnormal resting state functional connectivity of the periaqueductal grey in patients with fibromyalgia. Clin Exp Rheumatol. Mar-Apr 2016;34(2 Suppl 96):S129–133. [PubMed] [Google Scholar]
- 62.Lim M, O’Grady C, Cane D, et al. Threat Prediction from Schemas as a Source of Bias in Pain Perception. J Neurosci. Feb 12 2020;40(7):1538–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kwiatek R, Barnden L, Tedman R, et al. Regional cerebral blood flow in fibromyalgia: single-photon-emission computed tomography evidence of reduction in the pontine tegmentum and thalami. Arthritis Rheum. Dec 2000;43(12):2823–2833. [DOI] [PubMed] [Google Scholar]
- 64.Mountz JM, Bradley LA, Modell JG, et al. Fibromyalgia in women. Abnormalities of regional cerebral blood flow in the thalamus and the caudate nucleus are associated with low pain threshold levels. Arthritis Rheum. Jul 1995;38(7):926–938. [DOI] [PubMed] [Google Scholar]
- 65.Gracely RH, Petzke F, Wolf JM, Clauw DJ. Functional magnetic resonance imaging evidence of augmented pain processing in fibromyalgia. Arthritis Rheum. May 2002;46(5):1333–1343. [DOI] [PubMed] [Google Scholar]
- 66.Garrett DD, Epp SM, Perry A, Lindenberger U. Local temporal variability reflects functional integration in the human brain. Neuroimage. Dec 2018;183:776–787. [DOI] [PubMed] [Google Scholar]
- 67.Polania R, Paulus W, Nitsche MA. Modulating cortico-striatal and thalamo-cortical functional connectivity with transcranial direct current stimulation. Human Brain Mapping. Oct 2012;33(10):2499–2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wiech K, Lin CS, Brodersen KH, Bingel U, Ploner M, Tracey I. Anterior insula integrates information about salience into perceptual decisions about pain. J Neurosci. Dec 1 2010;30(48):16324–16331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Craig AD, Chen K, Bandy D, Reiman EM. Thermosensory activation of insular cortex. Nat Neurosci. Feb 2000;3(2):184–190. [DOI] [PubMed] [Google Scholar]
- 70.Ichesco E, Puiu T, Hampson JP, et al. Altered fMRI resting-state connectivity in individuals with fibromyalgia on acute pain stimulation. Eur J Pain. Aug 2016;20(7):1079–1089. [DOI] [PubMed] [Google Scholar]
- 71.Kim J, Loggia ML, Cahalan CM, et al. The somatosensory link in fibromyalgia: functional connectivity of the primary somatosensory cortex is altered by sustained pain and is associated with clinical/autonomic dysfunction. Arthritis Rheumatol. May 2015;67(5):1395–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Napadow V, LaCount L, Park K, As-Sanie S, Clauw DJ, Harris RE. Intrinsic Brain Connectivity in Fibromyalgia Is Associated With Chronic Pain Intensity. Arthritis and Rheumatism. Aug 2010;62(8):2545–2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Harris RE, Sundgren PC, Pang YX, et al. Dynamic levels of glutamate within the insula are associated with improvements in multiple pain domains in Fibromyalgia. Arthritis and Rheumatism. Mar 2008;58(3):903–907. [DOI] [PubMed] [Google Scholar]
- 74.Kaplan CM, Schrepf A, Vatansever D, et al. Functional and neurochemical disruptions of brain hub topology in chronic pain. Pain. Apr 2019;160(4):973–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kaplan CM, Harris RE, Lee U, DaSilva AF, Mashour GA, Harte SE. Targeting network hubs with noninvasive brain stimulation in patients with fibromyalgia. Pain. Jan 2020;161(1):43–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wagner IC, Rutgen M, Hummer A, Windischberger C, Lamm C. Placebo-induced pain reduction is associated with negative coupling between brain networks at rest. Neuroimage. Jun 5 2020;219:117024. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


