Abstract
Background & Aims:
Colorectal cancer (CRC) incidence has decreased overall in the last several decades, but it has increased among younger adults. Prior studies have characterized this phenomenon in the US using only a small subset of cases. We describe CRC incidence trends using high-quality data from 92% of the US population, with an emphasis on those younger than 50 years.
Methods:
We obtained 2001-2016 data from the United States Cancer Statistics database and analyzed CRC incidence for all age groups, with a focus on individuals diagnosed at ages 20-49 years (early-onset CRC). We compared incidence trends stratified by age, as well as by race/ethnicity, sex, region, anatomic site, and stage at diagnosis.
Results:
We observed 191,659 cases of early-onset and 1,097,765 cases of late-onset CRC during the study period. Overall, CRC incidence increased in every age group from 20 to 54 years. Whites were the only racial group with a consistent increase in incidence across all younger ages, with the steepest rise seen after 2012. Hispanics also experienced smaller increases in incidence in most of the younger age groups. Asians/Pacific Islanders (APIs) and Blacks saw no increase in incidence in any age group in 2016, but Blacks continued to have the highest incidence of CRC for every age group. Greater increase in early-onset CRC incidence was observed for males, left-sided tumors, and regional and distant disease.
Conclusions:
Early-onset CRC incidence increased overall from 2001 to 2016, but the trends were markedly different for Whites, Blacks, APIs, and Hispanics. These results may inform future research on the risk factors underlying early-onset CRC.
Keywords: Colorectal cancer, young adult, early-onset, race
Introduction
Colorectal cancer (CRC) is the third most common cancer in women and men in the United States, with an estimated 149,500 new cases in 20211 The overall incidence of CRC has decreased over the last several decades, which can be attributed to widespread screening among individuals older than 50 years and a population-level reduction in modifiable dietary and lifestyle risk factors2-4
Approximately 90% of CRC cases are diagnosed in individuals aged 50 years or older, but incidence has been increasing among younger patients. CRC is already a leading cause of cancer incidence and mortality among adults younger than 50 years5,6, and it is projected to become the top cause of cancer death among individuals aged 20 to 49 years before 20307. These observations have mostly been based on data from the Surveillance, Epidemiology, and End Results (SEER) registries3,8-10. Important limitations of prior SEER studies on the incidence of early-onset CRC include that the data encompassed only 9-28% of the total US population, and that only Black and White individuals were examined.
In this study, we characterize CRC incidence trends over time for nearly the entire US population, with an emphasis on younger adults under age 50 years. We present data for the four largest racial/ethnic groups and additionally stratify results by sex, region, site, and stage at diagnosis.
Methods
Study Population
We obtained 2001-2016 data from United States Cancer Statistics (USCS), which combines the SEER and National Program of Cancer Registries (NPCR) data to include nearly all of the US population11. Our analysis includes 46 of 52 states and territories with high-quality data throughout the study period based on North American Association of Central Cancer Registries criteria12, which based on the 2010 Census encompassed 92% of the total population13. Information on sex, race/ethnicity, stage at diagnosis, and tumor site was extracted. Racial/ethnic trends were also stratified by census region. Mortality data was not available for this dataset. We compared CRC incidence in patients diagnosed between ages 20-49 years (early-onset) and 50-74 years (late-onset) during this period. Age was categorized in 5- or 10-year increments, depending on sample size of the age group. Racial categories included White, Black, and Asians/Pacific Islanders (APIs), and these were not mutually exclusive with Hispanic ethnicity. Based on the NPCR subset of the USCS database, 92% of Hispanics were White, whereas 82% of Whites were non-Hispanic11. Other racial groups, such as American Indians and Alaska Natives, had insufficient case numbers in some years and age strata and were excluded from the analysis. Tumor site was grouped by International Classification of Diseases for Oncology codes into proximal colon (C18.0, C18.2-18.4), distal colon (C18.5-18.7), and rectum (C19.9, C20.9). Cases were classified using SEER summary staging as localized, regional, or distant disease.
Statistical Analysis
Annual incidence data was obtained using SEER*Stat (version 8.3.2) and age-adjusted to the 2000 US standard population. Incidence trends were evaluated using the Joinpoint Regression Program (version 4.5.0.1) and characterized by the annual percent change (APC), which assumes change occurs at a constant percentage of the rate of the previous year. We also calculated the average annual percent change (AAPC) over the entire 2001-2016 study period. To compare cross-sectional differences between groups, we calculated incidence rate ratios. Demographic comparisons for early-onset vs. late-onset CRC were performed using the chi-squared test. Statistical significance for all tests and trends, including APC and AAPC, was defined as P < .05.
Results
We observed 191,659 cases of early-onset and 1,097,765 cases of late-onset CRC during the study period. Fifty-six percent of the overall population was male. The racial distribution was 83% White, 13% Black, 4% API, and 9% were of Hispanic ethnicity (Table 1). The anatomic distribution was 36% proximal colon, 29% distal colon, and 35% rectum. Stage at diagnosis was localized, regional, and distant in 41%, 38%, and 21% of cases.
Table 1:
Colorectal cancer patient demographics, 2001-2016
| EOCRC (n=191,659) |
LOCRC (n=1,097,765) |
P | ||
|---|---|---|---|---|
| n (%) | n (%) | |||
| Sex | Male | 101,977 (53) | 620,635 (57) | <0.001 |
| Female | 89,682 (47) | 477,130 (43) | ||
| Age, years | 20-29 | 8,336 (4) | ||
| 30-39 | 38,722 (20) | |||
| 40-44 | 51,245 (27) | |||
| 45-49 | 93,356 (49) | |||
| 50-54 | 169,023 (15) | |||
| 55-59 | 192,666 (18) | |||
| 60-64 | 221,169 (20) | |||
| 65-69 | 254,335 (23) | |||
| 70-74 | 260,572 (24) | |||
| 70-74 | 260,572 (24) | |||
| Race/Ethnicity | White | 149,207 | 902,244 | <0.001 |
| Black | 28,536 | 137,735 | ||
| Asian/Pacific Islander | 9,444 | 39,641 | ||
| Hispanic | 23,652 | 87,049 | ||
| Anatomic Site | Proximal Colon | 50,802 (27) | 411,916 (38) | <0.001 |
| Distal Colon | 59,526 (31) | 319,454 (29) | ||
| Rectum | 81,331 (42) | 366,395 (33) | ||
| Stage | Localized | 60,680 (33) | 447,531 (43) | <0.001 |
| Regional | 75,940 (41) | 393,426 (38) | ||
| Distant | 46,972 (26) | 208,942 (19) |
Abbreviations: EOCRC, early-onset colorectal cancer; LOCRC, late-onset colorectal cancer
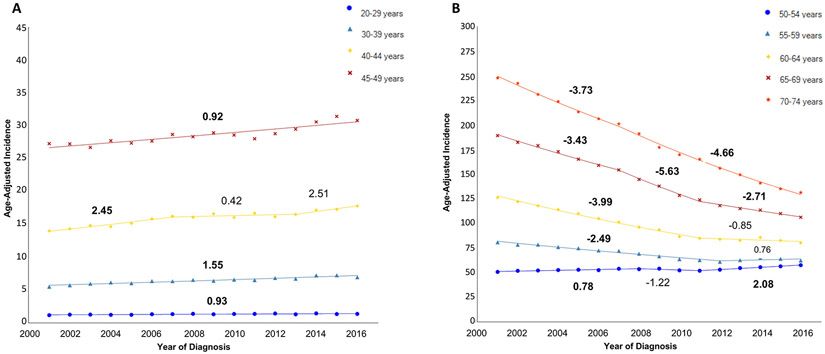
Between 2001 and 2016, early-onset CRC incidence increased in every age group, with the sharpest rise seen after 2012. The incidence increased from 1.18 to 1.38 per 100,000 persons (AAPC 0.93, P<0.05) in the 20-29 year age group, 5.52 to 6.96 per 100,000 persons (AAPC 1.55, P<0.05) in the 30-39 year age group, 14.01 to 17.81 per 100,000 persons (AAPC 1.65, P<0.05) in the 40-44 year age group, and 27.30 to 30.84 per 100,000 persons (AAPC 0.92, P<0.05) in the 45-49 year age group (Figure 1a). For late-onset cases, incidence increased slightly in the younger groups but declined dramatically in the oldest groups (Figure 1b). In the 50-54 year age group, incidence rose from 50.68 to 57.55 per 100,000 persons (AAPC 0.81, P<0.05). On the other hand, incidence decreased from 80.82 to 62.28 per 100,000 persons (AAPC −1.64, P<0.05) in the 55-59 year age group, 126.18 to 80.24 per 100,000 persons (AAPC −2.95, P<0.05) in the 60-64 year age group, 189.12 to 106.20 per 100,000 persons (AAPC −3.78, P<0.05) in the 65-69 age group, and 247.66 to 131.30 per 100,000 persons (AAPC −4.29, P<0.05) in the 70-74 age group.
Figure 1:
Incidence of colorectal cancer (2001-2016), stratified by age
A: CRC incidence, age 20-49; B: CRC incidence, age 50-74
Trends by Race/Ethnicity
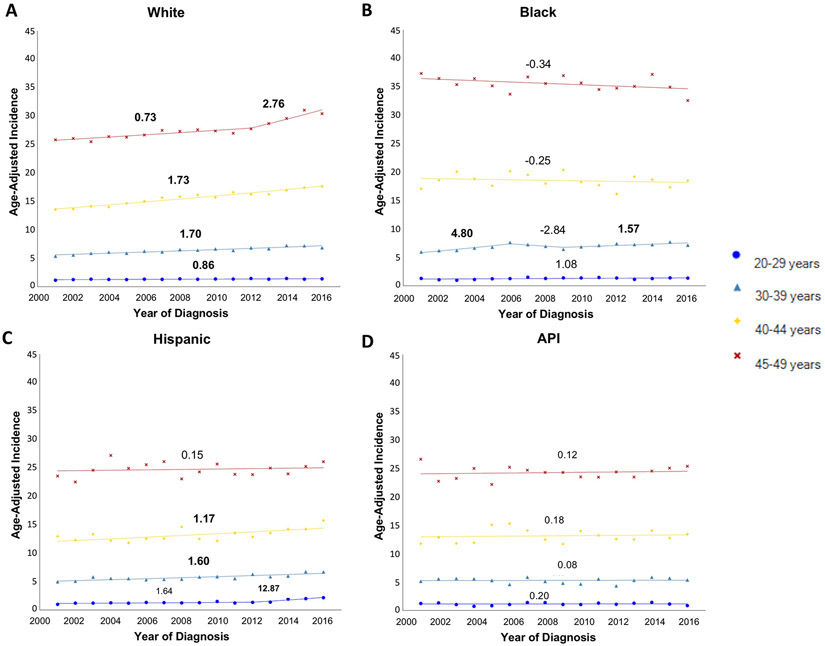
We observed substantial racial/ethnic differences in incidence trends among individuals younger than 50 years (Figure 2a-d, Supplemental Table 1). Whites were the only group with a consistent increase in incidence across all younger age groups, and a steeper increase was seen in 40-49 year-olds than 20-39 year-olds. Hispanics had an increase in incidence for the 20-44 year age groups, but there was no change in the 45-49 year age group. For APIs, there was no change in incidence in any of the younger age groups during the study period. Similarly, there was no consistent change in incidence for Blacks in the younger age groups. Although there were two periods during which Blacks aged 30-39 years saw increases, the overall trend across the study period was not statistically significant (AAPC 1.73, P>0.05). The overall increase in incidence for 40-49 year-olds was driven almost entirely by Whites. For instance, among Whites aged 45-49 years, incidence per 100,000 persons increased from 25.85 in 2001 to 30.44 per 100,000 persons in 2016 (AAPC 1.27, P<0.05). These rates were similar to the overall trend. In terms of absolute incidence, Blacks continued to have the highest rates of all racial/ethnic groups. However, among individuals younger than 50 years the gap in incidence between Blacks vs. Whites appeared to be closing, with an incidence rate ratio of 1.31 in 2001 and 1.05 in 2016 (P for trend <0.0001).
Figure 2:
Incidence of early-onset colorectal cancer (2001-2016), stratified by age and race/ethnicity
API, Asian/Pacific Islander
Early-onset CRC incidence overall appeared similar in the four census regions (Supplemental Figure 1). Whites had increasing incidence in every region (AAPC 1.21-1.83, P<0.05 for all, Supplemental Table 2), whereas Hispanics had increasing incidence only in the West (AAPC 1.64, P<0.05). Blacks saw a decrease in incidence in the South (AAPC −0.42, P<0.05)—the region with the highest incidence in 2001—but no change in the other regions. Incidence among APIs was stable in all regions.
For late-onset CRC cases, incidence decreased for all racial/ethnic groups in those aged 55 years and above from 2001 to 2016 (Supplemental Figure 2a-d, Supplemental Table 1). However, among individuals aged 55-59 years, the decline stopped among Whites after 2011 but continued for the other racial/ethnic groups. The most recent trend shows increasing incidence in the 50-54 year age group among Whites, Hispanics, and APIs, whereas Blacks had no change.
Trends by Sex, Site, and Stage
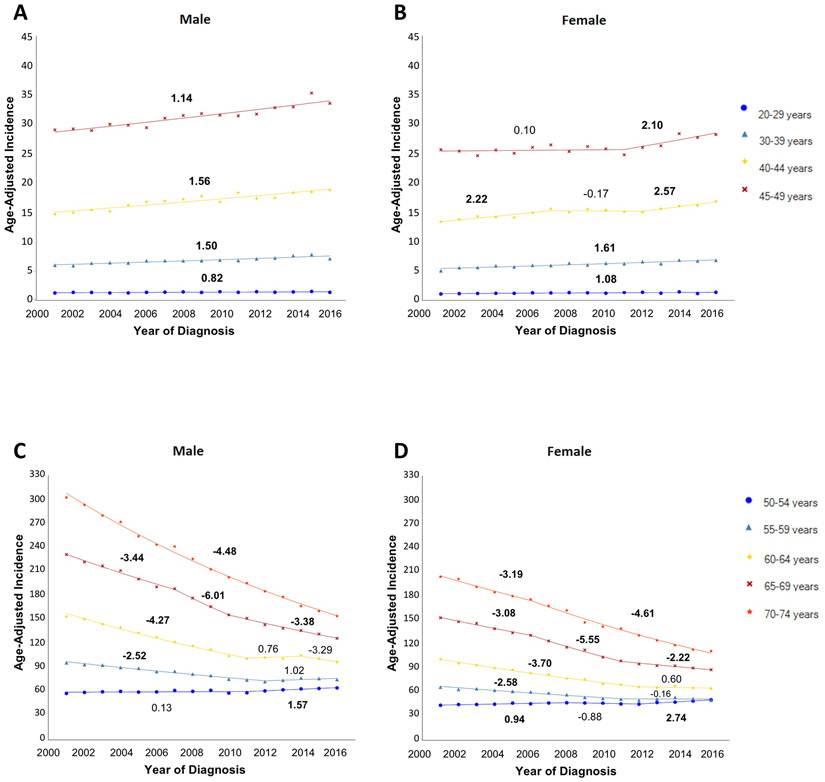
CRC incidence was higher for men than for women in all age groups, but trends over time were similar by sex (Figure 3). In 2016, incidence per 100,000 persons for men and women was 33.52 and 28.21 in the 45-49 year age group and 154.50 and 111.41 in the 70-74 year age group.
Figure 3:
Incidence of colorectal cancer (2001-2016), stratified by age and sex
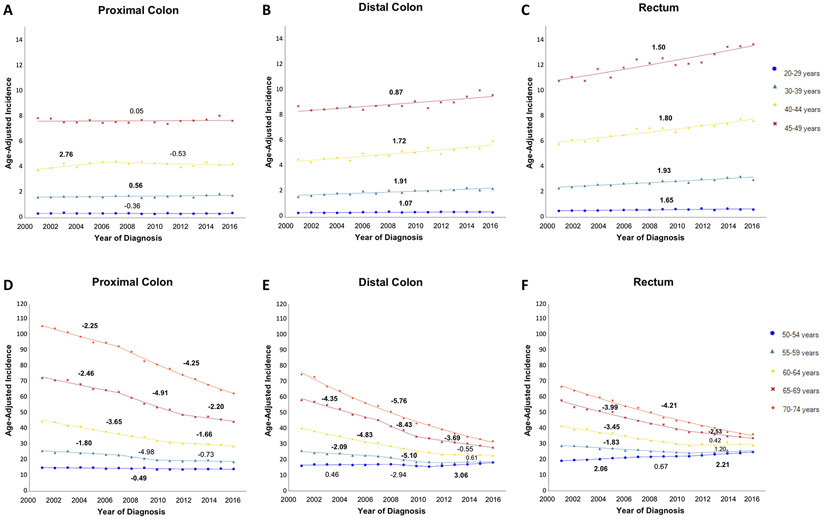
With respect to anatomic site, absolute incidence was higher for rectal cancers than cancers of the distal or proximal colon in every age group younger than 50 years (Figure 4a-c, Supplemental Table 1). Rectal and distal colon cancers both experienced an increase in incidence in all younger age groups, whereas proximal colon cancers only saw a consistent increase in incidence among 30-39 year-olds. Whites had an increase in incidence for cancers of the proximal colon (AAPC 0.27, P<0.05), distal colon (AAPC 1.70, P<0.05), and rectum (AAPC 1.87, P<0.05) in rectum. Hispanics and APIs saw smaller increases in incidence for rectal cancer (AAPC 0.81 and 0.66, P<0.05 for both), but no change for colon cancer. Blacks had no change in incidence for any site. In absolute terms, Blacks continued to have the highest incidence of proximal colon cancer, but Whites have overtaken Blacks for the highest incidence of distal colon and rectal cancer.
Figure 4:
Incidence of colorectal cancer (2001-2016), stratified by age and anatomic site
The predominance of distal cancers in adults younger than 50 years gave way to a proximal shift in distribution starting in the 60-64 year age group (Figure 4d-f). Among those in the 50-54 year age group, the incidence of rectal cancer (AAPC 1.74, P<0.05) increased from 2001 to 2016, whereas the incidence of distal colon cancer (AAPC 0.62, P>0.05) remained unchanged and the incidence of proximal colon cancer (AAPC −0.49, P<0.05) decreased. For all three sites in the 60-64 year age group, incidence dropped sharply until 2011-2012 and then plateaued. The steepest declines in incidence were seen in individuals aged 60 years and older. Among 70-74 year-olds in 2016, incidence was 62.85, 32.02, and 36.43 per 100,000 persons in the proximal colon, distal colon, and rectum.
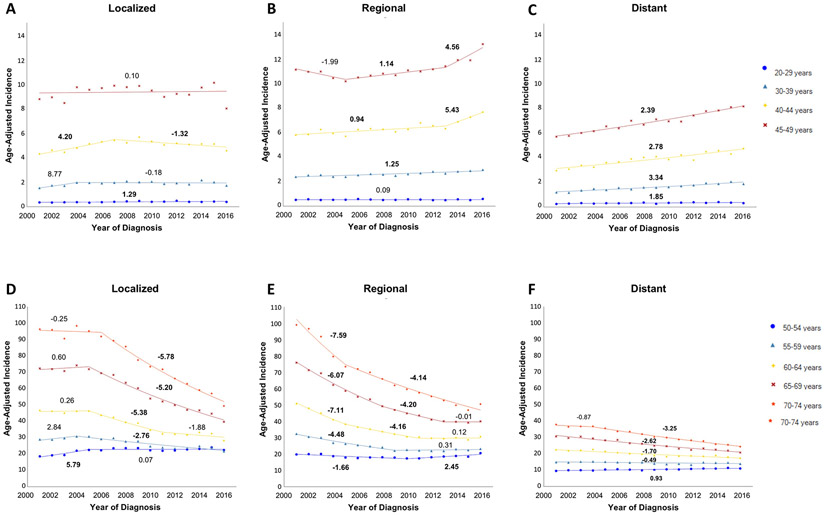
For stage at diagnosis, localized and regional cancers had comparable incidence in most age groups, whereas incidence of distant cancer was lower (Figure 5). However, recent trends show the incidence of regional and distant cancers was increasing for the 30-54 year age groups, whereas it was unchanged or decreasing for localized cancer. From 2001 to 2016, the absolute difference in incidence for localized versus distant cancers had declined from 3.04 to 0.19 per 100,000 persons for the 45-49 year age group and 59.66 to 25.07 per 100,000 persons for the 70-74 year age group.
Figure 5:
Incidence of colorectal cancer (2001-2016), stratified by age and stage of disease
Discussion
This study provides updated data on early-onset CRC that encompasses nearly the entire US population from 2001 to 2016, which includes the Hispanic and API populations. Overall, CRC incidence increased for every age group from 20 to 54 years, with the sharpest rise seen after 2012. This trend is primarily driven by Whites, although Hispanics also experienced smaller increases in incidence in most of the younger age groups. Blacks and APIs saw no increase in incidence in any age group under 50 years during the study period, but Blacks continue to have the highest incidence of both early-onset and late-onset CRC. With respect to sex, site, and stage at diagnosis, the largest increases in early-onset CRC incidence were found in men, cancers of the left colon and rectum, and regional and distant disease.
Several studies over the last decade, both in the US3,9,14 and internationally15-17, have observed similar trends in early-onset CRC. Siegel et al. observed a 22% increase in CRC incidence (from 5.9 to 7.2 per 100,000) between 2000 and 2013 in individuals younger than 50 years in the SEER 9 registries3. The largest increase was seen among non-Hispanic Whites, with an APC of 2.3 during the study period. Smaller increases were also seen in non-Hispanic Blacks and Hispanics, but not in APIs or American Indians/Alaska Natives. An earlier study from Austin et al., which examined US data from 1998 through 2009, found an increase in incidence only among non-Hispanic Whites14. More recently, Murphy et al. analyzed the SEER 13 registries data from 1992 to 2014 and concluded that the increased incidence was driven by rectal cancer, especially among Whites18. Our analysis of the overall US population confirms that the rising incidence of early-onset CRC is driven by Whites. Furthermore, by separating individuals younger than 50 years into narrower age groups, we also discovered a smaller increase among Hispanics. Similar to the Siegel et al. study, we also found that the increase in incidence was mostly restricted to cancers of the distal colon and rectum. However, whereas their analysis combining all individuals under age 50 years showed the most rapid incidence increase for distant disease, our age-stratified analysis found higher rates of regional disease in the 40-49 year age groups.
The rise in early-onset CRC is a global phenomenon. A recent study reported that 19 of 36 countries—in Asia, Oceania, North America, and Europe—experienced an increase in CRC incidence in younger adults under 50 years from 2008 to 201215. Of these 19, nine high-income countries on three continents—Australia, US, New Zealand, Canada, Slovenia, Germany, Sweden, Denmark, and the United Kingdom—had stable or declining incidence in the population 50 years or older. A Canadian study showed that from 1997 to 2010, the greatest rate of increase in incidence was in the youngest age groups, with a 6.7% per year increase in the 15-29 year age group compared to a 0.8% per year increase in the 40-49 year age group17. Similarly, a large European study encompassing 20 countries and 143 million younger adults found the average CRC incidence increase was 7.9% per year for the 20-29 year age group and 1.6% per year for the 40-49 year age group from 2004 to 201616. Our data through 2015 showed a similar relationship with age, but the addition of data from 2016 greatly reduced differences in the rate of incidence increase by age group. This demonstrates how small changes in the absolute incidence can lead to large changes in relative incidence, and therefore these values must be interpreted with caution. The availability of racial/ethnic data in our analysis, which was not available in the international studies, also provides a more nuanced understanding of the larger epidemiological trend.
The reasons behind the increasing incidence of early-onset CRC remain unclear and an area of active investigation. Several theories and risk factors have been proposed to explain this phenomenon, and these should be evaluated in the context of the racial/ethnic differences observed in the data.
Obesity is one of the most well-established modifiable risk factors of CRC, and the impact of the obesity epidemic on the younger population makes it a biologically plausible explanation for the rise in early-onset CRC. Indeed, several studies have shown increased rates of early-onset CRC associated with young adulthood obesity19-21, including a recent meta-analysis demonstrating a significant association between body mass index (BMI) and CRC in patients younger than 30 years22. Another recent study found that body fatness in childhood and adolescence, independent of adult obesity, was a risk factor for CRC in women23. However, not all studies have found an association between obesity and early-onset CRC17. Additionally, recent estimates in the US show a higher prevalence of obesity among adults and youth in Blacks and Hispanics than in Whites24, which is inconsistent with the observation that early-onset CRC trends are driven by the White population.
Other modifiable CRC risk factors have also been suggested as culprits for the rise in early-onset CRC. For example, Western diets high in processed meat and low in fiber may shift the gut microbiota towards a pro-inflammatory state25 26. Sedentary lifestyles with decreased physical activity have also been associated with increased early-onset CRC17. Health behaviors more commonly seen in populations with lower socioeconomic status (SES), such as smoking, alcohol use, and reduced healthcare access, disproportionately affect minority racial/ethnic groups27. The latter partly explains why Black individuals have the highest absolute CRC incidence in every age group28. Yet again, we are not aware of data demonstrating a higher prevalence or increase in incidence for these modifiable risk factors in the White population compared to other racial/ethnic groups.
Non-modifiable risk factors, including genetic or molecular variations, has also been raised as an explanation for the increased incidence of more distal CRC and advanced-stage cancers in young adults29-32. Although Lynch and other hereditary syndromes play a role in early-onset CRC, up to 80% of CRC in young adults do not have any identifiable germline mutation33,34. Furthermore, individuals carrying germline mutations are more likely to have proximal tumors rather than the distal disease typically seen in sporadic early-onset CRC33. Within sporadic cases, there does not seem to be any significant changes in mutation rates that would preferentially increase cancer in younger adults35-38. When considered on the evolutionary timescale, a rapid change within several decades that predominantly affects one large racial group makes a genetic basis unlikely.
The rise in early-onset CRC demonstrates a strong birth cohort effect39,40, and the latency period between exposure and carcinogenesis is likely several decades. For these reasons, it will be especially important to assess trends in early-life exposures, which have not been well-studied but may play a far larger etiological role than established risk factors for CRC. It has been shown that a shift in gut microbiota towards certain bacteria (e.g., Fusobacterium and Bacteroides) is associated with an increased risk for early-onset CRC41-43. Therefore, any factors that selectively promote gut dysbiosis in Whites could hypothetically explain early-onset CRC trends. For instance, there is some evidence that White children receive more antibiotics on average than Black children44,45. White children are also more likely to be prescribed inappropriate antibiotics for viral respiratory tract infections than children who are Black or Hispanic46. The increased incidence of inflammatory bowel disease (IBD) in young adults, which is a known risk factor for CRC and has traditionally affected Whites more than other racial/ethnic groups in industrialized countries, may also play a role47. There has also been increasing use of antibiotics in agriculture, more Cesarean deliveries, less breastfeeding, and more dietary fat intake in industrialized countries over the last several decades48. Although the racial/ethnic differences for each of these trends require further investigation, these early-life exposures could potentially lead to changes in the gut microbiota that increase susceptibility to developing cancer at a younger age.
Several national organization in the US, including the US Preventive Services Task Force, have recently changed their guidelines and lowered the general screening age to 45 years to combat the rise in early-onset CRC49-51, but the need to better understand the reasons for this trend remains as urgent as ever. Our age- and race-stratified data may serve as a helpful framework for evaluating the plausibility of candidate risk factors.
The main strengths of this study are the breadth of data source, which incorporates 92% of the US population, and more granular analysis permitted through stratification by age and race/ethnicity. Limitations include the availability of only 16 years of data as well as the lack of individual-level risk factors and mortality data. Racial groups in this dataset included individuals of Hispanic ethnicity, but because 82% of Whites are non-Hispanic in the NPCR subset of the USCS database11, it is unlikely that trends for Whites presented in this analysis would differ substantially from that of non- Hispanic Whites. In addition, although Alaska Natives have one of the highest rates of CRC incidence52, we could not include American Indians/Alaska Natives as a group in the analysis because of insufficient sample size. Finally, disaggregated data has shown differences in cancer incidence for Hispanic (e.g., Mexican, Cuban) and Asian (e.g., Chinese, Asian Indian) subgroups53,54, but these data were not available for this study.
Conclusions:
This study of nearly the entire US population showed an increase in CRC incidence in adults younger than 50 years, which is primarily driven by the trend among Whites. We found notable differences by age and race/ethnicity, as well as by sex, anatomic site, and stage at diagnosis. Whites had a consistent increase in incidence across all younger age groups, while Hispanics also saw smaller increases in incidence for most younger age groups. A consistent increase in incidence was not observed for younger APIs or Blacks. More rapid increases in early-onset CRC incidence were observed for males, left-sided tumors, and non-localized disease. These epidemiological data, especially with respect to race/ethnicity, may help guide future investigation on risk factors for early-onset CRC.
Supplementary Material
Supplemental Table 1: Average Annual Percent Change (AAPC) by age and select variables, 2001 2016
Supplemental Table 2: Average Annual Percent Change (AAPC) for early-onset colorectal cancer by select variables and race/ethnicity, 2001-2016
Supplemental Figure 1: Incidence of early-onset colorectal cancer (2001-2016), stratified by race/ethnicity and region
API, Asian/Pacific Islander
Supplemental Figure 2: Incidence of late-onset colorectal cancer (2001-2016), stratified by age and race/ethnicity
API, Asian/Pacific Islander
Supplemental Figure 3: Incidence of early-onset colorectal cancer (2001-2016), stratified by race/ethnicity and anatomic site
API, Asian/Pacific Islander
What you need to know:
Background:
Early-onset colorectal cancer (CRC) is a leading cause of cancer incidence and mortality among adults younger than 50 years. However, prior US studies on the topic have encompassed less than a third of the country’s population.
Findings:
The increase in early-onset CRC incidence is primarily driven by the trend among Whites. More rapid increases in early-onset CRC incidence were also observed for males, left-sided tumors, and non-localized disease.
Implications for patient care:
These epidemiological data provide a nuanced characterization of early-onset CRC trends and may help guide future investigation on risk factors for this condition.
Financial & Grant Support:
This study was not funded. MD is supported by grant P30 CA008748 from the NCI. PSL is supported by grant K08 CA230162 from the NCI. This material is the result of work supported in part by resources from the US Veterans Health Administration. The views expressed in this article are those of the authors and do not represent the views of the Department of Veterans Affairs.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Disclosure:
No competing interests. No support from any organization for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Writing Assistance: none
Transparency Declaration:
The guarantor of this manuscript, Peter S. Liang, affirm that the manuscript is an honest, accurate, and transparent account of the study being reported. No important aspects of the study have been omitted, and any discrepancies from the study as planned have been explained
Data sharing statement:
De-identified cancer surveillance data obtained from the United States Cancer Statistics (USCS) registry is publicly available upon request at https://www.cdc.gov/cancer/uscs/public-use/index.htm. Dissemination of the study’s results to specific individuals is not applicable.
Contributor Information
Steven H. Chang, New York University Langone Health.
Nicolas Patel, New York University Langone Health.
Mengmeng Du, Memorial Sloan Kettering Cancer Center.
Peter S. Liang, New York University Langone Health, VA New York Harbor Health Care System.
References:
- 1.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA Cancer J Clin. 2021;71(1):7–33. doi: 10.3322/caac.21654 [DOI] [PubMed] [Google Scholar]
- 2.Edwards BK, Ward E, Kohler BA, et al. Annual report to the nation on the status of cancer, 1975-2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer. 2010;116(3):544–573. doi: 10.1002/cncr.24760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siegel RL, Miller KD, Fedewa SA, et al. Colorectal cancer statistics, 2017: Colorectal Cancer Statistics, 2017. CA Cancer J Clin. 2017;67(3):177–193. doi: 10.3322/caac.21395 [DOI] [PubMed] [Google Scholar]
- 4.Johnson CM, Wei C, Ensor JE, et al. Meta-analyses of colorectal cancer risk factors. Cancer Causes Control. 2013;24(6):1207–1222. doi: 10.1007/s10552-013-0201-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7–30. doi: 10.3322/caac.21590 [DOI] [PubMed] [Google Scholar]
- 6.Bhandari A, Woodhouse M, Gupta S. Colorectal cancer is a leading cause of cancer incidence and mortality among adults younger than 50 years in the USA: a SEER-based analysis with comparison to other young-onset cancers. J Investig Med. 2017;65(2):311–315. doi: 10.1136/jim-2016-000229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rahib L, Wehner MR, Matrisian LM, Nead KT. Estimated Projection of US Cancer Incidence and Death to 2040. JAMA Netw Open. 2021;4(4):e214708. doi: 10.1001/jamanetworkopen.2021.4708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Siegel RL, Jemal A, Ward EM. Increase in Incidence of Colorectal Cancer Among Young Men and Women in the United States. Cancer Epidemiol Biomarkers Prev. 2009;18(6):1695–1698. doi: 10.1158/1055-9965.EPI-09-0186 [DOI] [PubMed] [Google Scholar]
- 9.Ansa B, Coughlin S, Alema-Mensah E, Smith S. Evaluation of Colorectal Cancer Incidence Trends in the United States (2000-2014). J Clin Med. 2018;7(2):22. doi: 10.3390/jcm7020022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bailey CE, Hu C-Y, You YN, Chang GJ. Increasing disparities in age-related incidence of colon and rectal cancer in the United States, 1975-2010. J Clin Oncol. 2014;32(3_suppl):392–392. doi: 10.1200/jco.2014.32.3_suppl.392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.National Program of Cancer Registries and Surveillance, Epidemiology, and End Results SEER*Stat Database: NPCR and SEER Incidence – U.S. Cancer Statistics 2001–2016 Public Use Research Database, November 2018 Submission (2001–2016), United States Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute. Released June 2019, Based on the November 2018 Submission. Accessed at Www.Cdc.Gov/Cancer/Uscs/Public-Use.
- 12.North American Association of Central Cancer Registries (NAACCR). Data Standards and Data Dictionary. Published online November 23, 2020. https://www.naaccr.org/data-standards-data-standards-data-dictionary/
- 13.United States Census Bureau. Change in Resident Population of the 50 States, the District of Columbia, and Puerto Rico: 1910 to 2020. Accessed June 5, 2021. https://www2.census.gov/programs-surveys/decennial/2020/data/apportionment/population-change-data-table.pdf
- 14.Austin H, Jane Henley S, King J, Richardson LC, Eheman C. Changes in colorectal cancer incidence rates in young and older adults in the United States: what does it tell us about screening. Cancer Causes Control. 2014;25(2):191–201. doi: 10.1007/s10552-013-0321-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Siegel RL, Torre LA, Soerjomataram I, et al. Global patterns and trends in colorectal cancer incidence in young adults. Gut. 2019;68(12):2179–2185. doi: 10.1136/gutjnl-2019-319511 [DOI] [PubMed] [Google Scholar]
- 16.Vuik FE, Nieuwenburg SA, Bardou M, et al. Increasing incidence of colorectal cancer in young adults in Europe over the last 25 years. Gut. Published online May 16, 2019:gutjnl-2018-317592. doi: 10.1136/gutjnl-2018-317592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patel P, De P. Trends in colorectal cancer incidence and related lifestyle risk factors in 15-49-year-olds in Canada, 1969-2010. Cancer Epidemiol. 2016;42:90–100. doi: 10.1016/j.canep.2016.03.009 [DOI] [PubMed] [Google Scholar]
- 18.Murphy CC, Wallace K, Sandler RS, Baron JA. Racial Disparities in Incidence of Young-Onset Colorectal Cancer and Patient Survival. Gastroenterology. 2019;156(4):958–965. doi: 10.1053/j.gastro.2018.11.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karahalios A, English DR, Simpson JA. Weight Change and Risk of Colorectal Cancer: A Systematic Review and Meta-Analysis. Am J Epidemiol. 2015;181(11):832–845. doi: 10.1093/aje/kwu357 [DOI] [PubMed] [Google Scholar]
- 20.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. 2008;371:10. [DOI] [PubMed] [Google Scholar]
- 21.Liu P-H, Wu K, Ng K, et al. Association of Obesity With Risk of Early-Onset Colorectal Cancer Among Women. JAMA Oncol. 2019;5(1):37. doi: 10.1001/jamaoncol.2018.4280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hidayat K, Yang C-M, Shi B-M. Body fatness at an early age and risk of colorectal cancer. Int J Cancer. 2018;142(4):729–740. doi: 10.1002/ijc.31100 [DOI] [PubMed] [Google Scholar]
- 23.Zhang X, Wu K, Giovannucci EL, et al. Early Life Body Fatness and Risk of Colorectal Cancer in U.S. Women and Men--Results from Two Large Cohort Studies. Cancer Epidemiol Biomarkers Prev. 2015;24(4):690–697. doi: 10.1158/1055-9965.EPI-14-0909-T [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arroyo-Johnson C, Mincey KD. Obesity Epidemiology Worldwide. Gastroenterol Clin North Am. 2016;45(4):571–579. doi: 10.1016/j.gtc.2016.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murphy N, Norat T, Ferrari P, et al. Dietary Fibre Intake and Risks of Cancers of the Colon and Rectum in the European Prospective Investigation into Cancer and Nutrition (EPIC). Lee JE, ed. PLoS ONE. 2012;7(6):e39361. doi: 10.1371/journal.pone.0039361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dong TS, Gupta A. Influence of Early Life, Diet, and the Environment on the Microbiome. Clin Gastroenterol Hepatol. 2019;17(2):231–242. doi: 10.1016/j.cgh.2018.08.067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Doubeni CA, Fedewa SA, Levin TR, et al. Modifiable Failures in the Colorectal Cancer Screening Process and Their Association With Risk of Death. Gastroenterology. 2019;156(1):63–74.e6. doi: 10.1053/j.gastro.2018.09.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Williams DR, Mohammed SA, Leavell J, Collins C. Race, socioeconomic status, and health: Complexities, ongoing challenges, and research opportunities: Race, SES, and health. Ann N Y Acad Sci. 2010;1186(1):69–101. doi: 10.1111/j.1749-6632.2009.05339.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kumar RR, Mittal R. Prevalence of Left-Sided Colorectal Cancer and Benefit of Flexible Sigmoidoscopy: A County Hospital Experience. Am Surg. 2007;(10):5. [PubMed] [Google Scholar]
- 30.Campos FG. Colorectal cancer in young adults: A difficult challenge. World J Gastroenterol. 2017;23(28):5041. doi: 10.3748/wjg.v23.i28.5041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leff Daniel R, Chen Alvin, Roberts David, et al. Colorectal Cancer in the Young Patient. Am Surg. 2007;73(1):42–47. [PubMed] [Google Scholar]
- 32.Yeo H, Betel D, Abelson JS, Zheng XE, Yantiss R, Shah MA. Early-onset Colorectal Cancer is Distinct From Traditional Colorectal Cancer. Clin Colorectal Cancer. 2017;16(4):293–299.e6. doi: 10.1016/j.clcc.2017.06.002 [DOI] [PubMed] [Google Scholar]
- 33.Stoffel EM, Koeppe E, Everett J, et al. Germline Genetic Features of Young Individuals With Colorectal Cancer. Gastroenterology. 2018;154(4):897–905.e1. doi: 10.1053/j.gastro.2017.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pearlman R, Frankel WL, Swanson B, et al. Prevalence and Spectrum of Germline Cancer Susceptibility Gene Mutations Among Patients With Early-Onset Colorectal Cancer. JAMA Oncol. 2017;3(4):464. doi: 10.1001/jamaoncol.2016.5194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Connell LC, Mota JM, Braghiroli MI, Hoff PM. The Rising Incidence of Younger Patients With Colorectal Cancer: Questions About Screening, Biology, and Treatment. Curr Treat Options Oncol. 2017;18(4):23. doi: 10.1007/s11864-017-0463-3 [DOI] [PubMed] [Google Scholar]
- 36.Puccini A, Lenz H, Marshall JL, et al. Impact of Patient Age on Molecular Alterations of Left-Sided Colorectal Tumors. The Oncologist. 2019;24(3):319–326. doi: 10.1634/theoncologist.2018-0117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.KI Deen, Silva H, Deen R, Chandrasinghe PC. Colorectal cancer in the young, many questions, few answers. World J Gastrointest Oncol. 2016;8(6):481. doi: 10.4251/wjgo.v8.i6.481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stigliano V Early-onset colorectal cancer: A sporadic or inherited disease? World J Gastroenterol. 2014;20(35):12420. doi: 10.3748/wjg.v20.i35.12420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Siegel RL, Fedewa SA, Anderson WF, et al. Colorectal Cancer Incidence Patterns in the United States, 1974-2013. JNCI J Natl Cancer Inst. 2017;109(8). doi: 10.1093/jnci/djw322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Murphy CC, Singal AG, Baron JA, Sandler RS. Decrease in Incidence of Young-Onset Colorectal Cancer Before Recent Increase. Gastroenterology. 2018;155(6):1716–1719.e4. doi: 10.1053/j.gastro.2018.07.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mima K, Cao Y, Chan AT, et al. Fusobacterium nucleatum in Colorectal Carcinoma Tissue According to Tumor Location: Clin Transl Gastroenterol. 2016;7(11):e200. doi: 10.1038/ctg.2016.53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Flemer B, Lynch DB, Brown JMR, et al. Tumour-associated and non-tumour-associated microbiota in colorectal cancer. Gut. 2017;66(4):633–643. doi: 10.1136/gutjnl-2015-309595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dahmus JD, Kotler DL, Kastenberg DM, Kistler CA. The gut microbiome and colorectal cancer: a review of bacterial pathogenesis. J Gastrointest Oncol. 2018;9(4):769–777. doi: 10.21037/jgo.2018.04.07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gerber JS, Prasad PA, Localio AR, et al. Racial Differences in Antibiotic Prescribing by Primary Care Pediatricians. PEDIATRICS. 2013;131(4):677–684. doi: 10.1542/peds.2012-2500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fleming-Dutra KE, Shapiro DJ, Hicks LA, Gerber JS, Hersh AL. Race, Otitis Media, and Antibiotic Selection. Pediatrics. 2014;134(6):1059–1066. doi: 10.1542/peds.2014-1781 [DOI] [PubMed] [Google Scholar]
- 46.Goyal MK, Johnson TJ, Chamberlain JM, et al. Racial and Ethnic Differences in Antibiotic Use for Viral Illness in Emergency Departments. Pediatrics. 2017;140(4):e20170203. doi: 10.1542/peds.2017-0203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Molodecky NA, Soon IS, Rabi DM, et al. Increasing Incidence and Prevalence of the Inflammatory Bowel Diseases With Time, Based on Systematic Review. Gastroenterology. 2012;142(1):46–54.e42. doi: 10.1053/j.gastro.2011.10.001 [DOI] [PubMed] [Google Scholar]
- 48.Mauri G, Sartore-Bianchi A, Russo A-G, Marsoni S, Bardelli A, Siena S. Early-onset colorectal cancer in young individuals. Mol Oncol. 2019;13(2):109–131. doi: 10.1002/1878-0261.12417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wolf AMD, Fontham ETH, Church TR, et al. Colorectal cancer screening for average-risk adults: 2018 guideline update from the American Cancer Society: ACS Colorectal Cancer Screening Guideline. CA Cancer J Clin. 2018;68(4):250–281. doi: 10.3322/caac.21457 [DOI] [PubMed] [Google Scholar]
- 50.Shaukat A, Kahi CJ, Burke CA, Rabeneck L, Sauer BG, Rex DK. ACG Clinical Guidelines: Colorectal Cancer Screening 2021. Am J Gastroenterol. 2021;116(3):458–479. doi: 10.14309/ajg.0000000000001122 [DOI] [PubMed] [Google Scholar]
- 51.US Preventive Services Task Force, Davidson KW, Barry MJ, et al. Screening for Colorectal Cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2021;325(19):1965. doi: 10.1001/jama.2021.6238 [DOI] [PubMed] [Google Scholar]
- 52.Abualkhair WH, Zhou M, Ochoa CO, et al. Geographic and intra-racial disparities in early-onset colorectal cancer in the SEER 18 registries of the United States. Cancer Med. 2020;9(23):9150–9159. doi: 10.1002/cam4.3488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Miller KD, Goding Sauer A, Ortiz AP, et al. Cancer Statistics for Hispanics/Latinos, 2018. CA Cancer J Clin. 2018;68(6):425–445. doi: 10.3322/caac.21494 [DOI] [PubMed] [Google Scholar]
- 54.Torre LA, Sauer AMG, Chen MS, Kagawa-Singer M, Jemal A, Siegel RL. Cancer statistics for Asian Americans, Native Hawaiians, and Pacific Islanders, 2016: Converging incidence in males and females: Cancer Statistics for Asian Americans, Native Hawaiians, and Pacific Islanders, 2016. CA Cancer J Clin. 2016;66(3):182–202. doi: 10.3322/caac.21335 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1: Average Annual Percent Change (AAPC) by age and select variables, 2001 2016
Supplemental Table 2: Average Annual Percent Change (AAPC) for early-onset colorectal cancer by select variables and race/ethnicity, 2001-2016
Supplemental Figure 1: Incidence of early-onset colorectal cancer (2001-2016), stratified by race/ethnicity and region
API, Asian/Pacific Islander
Supplemental Figure 2: Incidence of late-onset colorectal cancer (2001-2016), stratified by age and race/ethnicity
API, Asian/Pacific Islander
Supplemental Figure 3: Incidence of early-onset colorectal cancer (2001-2016), stratified by race/ethnicity and anatomic site
API, Asian/Pacific Islander