Abstract
INTRODUCTION:
The prognostic utility of cerebrospinal fluid (CSF) phosphorylated tau 217 (P-tau217) and P-tau181 is not understood.
METHODS:
Analyses included 753 Mayo Clinic Study on Aging participants (median age=71.6; 57% male). CSF Aβ42 and P-tau181 were measured with Elecsys® immunoassays. CSF P-tau181 and P-tau217 were also measured with Meso Scale Discovery (MSD). We used Cox proportional hazards models for risk of mild cognitive impairment (MCI) and linear mixed models for risk of global and domain-specific cognitive decline and cortical thickness. Analyses were stratified by elevated brain amyloid based on CSF Aβ42 or amyloid PET for those with imaging.
RESULTS:
CSF P-tau217 was superior to P-tau181 for the diagnosis of AD pathology. CSF MSD P-tau181 and P-tau217 were associated with risk of MCI among A+ individuals. Differences between CSF P-tau measures predicting cortical thickness were subtle.
DISCUSSION:
There are subtle differences for CSF P-tau217 and P-tau181 as prognostic AD markers.
Keywords: Cerebrospinal fluid, phosphorylated tau, biomarker, prognosis, cognitive decline, mild cognitive impairment, dementia
1. BACKGROUND
Neurofibrillary tangles, comprised of intraneuronal hyperphosphorylated tau, are one of the hallmark pathological characteristics of Alzheimer’s disease (AD), in addition to amyloid-beta (Aβ) plaques and neurodegeneration. Immunoassays to measure cerebrospinal fluid (CSF) tau phosphorylated at threonine 181 (P-tau181) have been developed as a biomarker of neurofibrillary tangles to support the diagnosis of AD dementia [1, 2]. Although CSF P-tau181 has been the primary focus in clinical and research settings to date, there are numerous tau phosphorylation sites [3], some of which are differentially enriched in the CSF compared to the brain [4].
CSF P-tau181 has consistently been shown to help in the differential diagnosis of AD dementia from the other tauopathies, suggesting that P-tau181 is more specific to AD [5, 6]. However, there is an ongoing search to identify other tau fragments or phosphorylation sites to enhance the specificity of AD dementia diagnosis and prognosis [4, 7–10]. Multiple studies now suggest that CSF phosphorylated tau 217 (P-tau217), compared with P-tau181, correlates more strongly with amyloid and tau PET and is better able to distinguish between AD and non-AD neurodegenerative diseases [11–13, 15–16]. However, the superiority of CSF P-tau217 over P-tau181 may depend on the P-tau181 antibodies used; N-terminal-directed P-tau181, but not standard mid-region P-tau181, has been shown to have similar diagnostic performance to N-terminal-directed P-tau217 [14]. Only a single study has evaluated the prognostic utility of CSF P-tau181 or P-tau217 for cognitive decline and that study was insufficiently powered to detect the association with longitudinal cognition using the mini mental state examination (MMSE) [15]. Herein, we compared P-tau217, assayed using Meso Scale Discovery platform (MSD; Meso Scale Diagnostics LLC, Rockville, MD, USA), with P-tau181, assayed using both MSD and Elecsys® immunoassay (Roche Diagnostics International Ltd, Rotkreuz, Switzerland), in relation to both cross-sectional and longitudinal measures of cognition (including risk of mild cognitive impairment [MCI] and dementia) and cortical thickness. We present results stratified by brain amyloid status (first defined by CSF Aβ42 and then by amyloid PET for the subset with this measure).
2. METHODS
2.1. Study participants
The Mayo Clinic Study of Aging (MCSA) is a prospective population-based study examining the epidemiology of cognitive aging in Olmsted County, Minnesota [17]. In 2004, residents aged between 70 and 89 years were enumerated using the Rochester Epidemiology Project medical records-linkage system in an age- and sex-stratified random sampling design [18]. The study was extended to include those aged 50 and older in 2012. The present analysis includes participants with CSF collected between November 2007 and August 2016. The study was approved by Mayo Clinic and Olmsted Medical Center Institutional Review Boards. Written informed consent was obtained.
2.2. Participant assessment
MCSA visits included an interview by a study coordinator, physician examination, and neuropsychological testing [17]. Clinic visits occurred at 15-month intervals. Participant demographics (age, sex, and years of education) were ascertained during the in-clinic examination. The cognitive battery included nine tests covering four domains: memory, language, executive function, and visuospatial. Sample-specific z-scores for all cognitive tests were calculated; domain-specific z-scores were created by averaging the z-scores for the individual tests within each domain. A global cognitive z-score was created by averaging the z-scores of the four domains. Apolipoprotein E (APOE) ε4 genotyping was performed from a blood sample.
2.3. MCI and dementia diagnostic determination
Clinical diagnoses were determined by a consensus committee of those who evaluated each participant. Cognitive performance was compared with the age-adjusted scores of cognitively unimpaired (CU) individuals previously obtained using Mayo’s Older American Normative Studies which were derived from a separate sample of individuals [19]. Participants with scores around 1.0 standard deviation (SD) below the age-specific mean in the general population were considered for possible cognitive impairment. The operational definition of MCI was based on clinical judgment including a history from the patient and informant. Published criteria were used for the diagnosis: cognitive complaint, cognitive function not normal for age, essentially normal functional activities, and no dementia [20]. A final decision was made after considering education, occupation, visual or hearing deficits, and reviewing all other participant information. The diagnosis of dementia was based on published criteria [21]. Participants who performed in the normal range and did not meet criteria for MCI or dementia were deemed CU. The consensus committee was blinded to CSF and neuroimaging results and the diagnosis of previous MCSA visits.
2.4. Lumbar punctures and CSF assays
Fasting lumbar punctures were performed in the morning in the lateral decubitus position from the L3 and L4 intravertebral space using a 20 or 22 gauge Quincke needle. CSF was collected in polypropylene tubes. Two cc were used to evaluate routine markers (glucose, protein, cell count). The remainder was divided into 0.5 cc aliquots and stored at −80 °C. Samples underwent one freeze-thaw cycle. CSF Aβ42 and P-tau181 were measured with automated electrochemiluminescence Elecsys immunoassays on a Roche COBAS 6000 E 601 module (Roche Diagnostics International Ltd, Rotkreuz, Switzerland), as described [22–24]. CSF Aβ1–42<1026 pg/mL was considered amyloid positive (A+) based on Gaussian mixture modeling. For the measurement of P-tau181, the Roche Elecsys immunoassay uses a sandwich assay principle. A biotinylated monoclonal antibody specific for phosphorylation at threonine 181 (11H5V1), binding to amino acids 170–205 of human Tau-441 with phosphorylated threonine resident at position 181 and a monoclonal Tau-specific antibody (PC1C6) labeled with a ruthenium complex react to form a sandwich complex [24]. Streptavidin-coated microparticles are added, and the interaction between biotin and streptavidin allows the complex to become bound to the solid phase. The reaction mixture is then aspirated into the measuring cell, microparticles are captured on to the electrode, and the application of voltage induces chemiluminescent emission, which is measured by a photomultiplier.
The MSD P-tau181 and P-tau217 assays were performed using a streptavidin small spot plate [11]. Briefly, anti-P-tau217 antibody (IBA413) was used as a capture antibody in the P-tau217 assay whereas anti-P-tau181 antibody (AT270) was used as a capture antibody in the P-tau181 assay. Both assays used SULFO-tagged total tau antibody (LRL) as the detection antibody (3μg/ml for the P-tau181 and at 0.5μg/ml for the P-tau217). The LRL antibody is designed with a unique specificity such that it does not bind to PNS isoforms (Figure S1). This property provides the improved specificity to brain relevant forms of tau over other tau assays. Antibodies were conjugated with Biotin (Thermo Scientific, Waltham, MA, USA, catalog number: 21329) or SULFO-TAG (Meso Scale Diagnostics LLC, Rockville, MD, USA, catalog number: R91AO-1). The assays were calibrated using a recombinant tau (4R2N) protein that was phosphorylated in vitro using a reaction with glycogen synthase kinase-3β and characterized by mass spectrometry. Samples were analyzed in duplicate and the mean was used in statistical analysis.
2.5. Neuroimaging measures
Aβ PiB-PET images were acquired using a PET/CT scanner (DRX, GE Healthcare, Chicago, IL, USA) operating in 3-dimensional mode [25]. Pittsburgh compound B (PiB)–PET scan, consisting of four 5-minute dynamic frames, was acquired from 40 to 60 minutes after injection [26, 27].
Quantitative image analysis for PiB was done using our in-house fully automated image processing pipeline [28]. A global cortical PiB-PET retention ratio was computed by calculating the median uptake over voxels in the prefrontal, orbitofrontal, parietal, temporal, anterior cingulate, and posterior cingulate/precuneus regions of interest for each participant and dividing this by the median uptake over voxels in the cerebellar crus. No partial volume correction was used. The atlas and image recognition steps were based on a 3D T1-weighted volume MRI sequence. We dichotomized participants as A+ based on a cutoff of 1.48 standard uptake value ratio (SUVR) using the reliable worsening method as previously described [29], and updated to the SPM12 processing pipeline. Briefly, the reliable worsening cut point was based on identifying a threshold baseline value beyond which the rate of change in that biomarker worsens reliably.
T1-weighted MRI scans were acquired on 3T GE scanners using a Sagittal 3D magnetization prepared rapid acquisition gradient recalled echo (MPRAGE) sequence with acquisition parameters of TR/TE/TI – 2300/3/900 ms with voxel dimensions of 1.20 × 1.015 × 1.015 mm [28]. We used Freesurfer version 5.3 for computation of cortical thicknesses and used the composite of entorhinal, inferior temporal, fusiform, and middle temporal regions [30].
2.6. Statistical analysis
Wilcoxon rank sum tests, for continuous variables, and Fisher’s exact tests, for categorical variables, were used to examine differences in participant characteristics and CSF P-tau measures by cognitive and amyloid status. Spearman correlation coefficients were used to examine the association between continuous measures of CSF P-tau and other continuous variables. Area under the curve (AUC) and De Long comparisons were used to examine significantly differences in the accuracy of the P-tau measures for CU vs. MCI, CSF A- vs. A+, and PET A- vs. A+. For model comparisons, the P-tau measures were z-scored based on the mean P-tau level of CU A- participants so that the model estimates could be directly compared. Cox proportional hazards models were used to calculate the hazard ratio (HR) and 95% confidence interval (CI) for the CSF P-tau variables and risk of MCI (among CU at baseline) or dementia (among those with MCI) using time as the timescale. Multivariable models adjusted for age, sex, education, and APOE. Linear mixed effect models were used to examine the cross-sectional and longitudinal associations between CSF P-tau variables with global- and domain-specific cognitive z-scores and cortical thickness. We specified a random intercept and random slope to account for within-subject correlation, and used an unstructured covariance matrix. Multivariable models again adjusted for age, sex, education and APOE as fixed effects. Additionally, models were summarized for model fit using the Akaike Information Criteria (AIC) in order to compare how well the models fit the data within each analysis family, the lower the AIC the better the model fit. A difference in AIC of 0–2 represents some evidence, of 4–7 considerable evidence, and >10 of very strong evidence for differences between two models [31]. Results are presented stratified by amyloid status (first defined by CSF Aβ42 and then defined by amyloid PET for the subset with this measure). A P-value<.05 was considered statistically significant. Analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA) and R version 3.6.2 [32].
3. RESULTS
3.1. Participant characteristics
The 753 participants, at the time of their lumbar puncture, had a median (range) age of 73.1 (50.7–95.3) years, education of 14.6 (0–20) years, 56.7% were men, and 27.4% had an APOE ɛ4 allele. The median (range) follow-up time was 5.6 (0–11.7) years.
The CSF P-tau measures were highly correlated (Spearman’s rho [P-value] for MSD P-tau181 versus 217=0.94[P<.001]; for MSD P-tau181 versus Elecsys P-tau181=0.86[P<.001]; and for MSD P-tau217 versus Elecsys P-tau181=0.77[P<.001]). All three markers correlated with age (Spearman’s rho [P-value] for MSD P-tau181=0.36[P<.001]; for MSD P-tau217=0.34[P<.001]; and for Elecsys P-tau181=0.38[P<.001]. None of the P-tau levels differed by sex (all P>.4). Compared to non-carriers, those with an APOE ɛ4 allele had higher levels of MSD P-tau181 (P<.001), MSD P-tau217 (P<.001), and Elecsys P-tau181 (P=.027).
3.2. CSF P-tau181 and P-tau217 by clinical diagnosis and amyloid status
Continuous measures of Elecsys P-tau181, MSD P-tau181, and MSD P-tau217 are shown in Table 1 and Figures S2-S4 by clinical diagnosis and amyloid status based on CSF Aβ42<1026 pg/ml. There were overall group differences in all P-tau measurements by clinical diagnosis and amyloid status determined by Wilcoxon rank sum tests (P<.001; Table 1). MSD P-tau 217 levels were 21% higher in CU A+ compared to CU A- (80.6 vs. 66.5 pg/ml, P =.015). However, some two-way comparisons by clinical diagnosis and amyloid status were not significant (Figures S2-S4). For example, MSD P-tau181 levels did not differ between CU A+ versus A- (82.6 vs 88.5 pg/ml, P=0.37). Interestingly, for Elecsys P-tau181 CU A- had higher levels than CU A+ (19.4 vs. 15.2, P <0.001). When comparing MCI A- to MCI A+, there was no difference in Elecsys P-tau181 whereas MSD P-tau181 was 86% higher and MSD P-tau217 was 161% higher, respectively, for MCI A+. Figure S5 shows ROC curves comparing CSF P-tau measures. Using De Long comparisons, the AUC of MSD P-tau 217 was significantly better than MSD P-tau181 (P=.002) and Elecsys P-tau181 (P=.04) for distinguishing between CU and MCI, but there was no difference between the two P-tau181 measures. When distinguishing between A+ and A- based on CSF Aβ42, the AUC of MSD P-tau217 was significantly better than MSD P-tau181 (P<.001) but not Elecsys P-tau181 (P=.58).
TABLE 1.
Characteristics by clinical diagnosis and CSF amyloid status
| CU CSF A- (N = 376) | CU CSF A+ (N = 290) | MCI CSF A- (N = 34) | MCI CSF A+ (N=47) | Dementia CSF A+ (N=6) | ||
|---|---|---|---|---|---|---|
| Characteristic | Median (IQR)/N(%) | Median (IQR)/N(%) | Median (IQR)/N(%) | Median (IQR)/N(%) | Median (IQR)/N(%) | P-value |
|
| ||||||
| Age at CSF visit (years) | 72.4 (62.9, 78.7) | 72.5 (63.8, 77.9) | 75.1 (69.1, 81.7) | 78.5 (74.5, 84.6) | 83.7 (80.4, 85.7) | < .001 |
| Male | 207 (55.1%) | 169 (58.3%) | 22 (64.7%) | 23 (48.9%) | 6 (100%) | .119 |
| Education (years) | 14 (12, 16) | 14 (12, 16) | 13 (12, 16) | 13 (12, 16) | 12 (12, 12) | .027 |
| APOE ɛ4 positive | 64 (17.1%) | 111 (38.3%) | 7 (20.6%) | 22 (46.8%) | 2 (33.3%) | < .001 |
| Global z-score | 0.23 (−0.32, 0.87) | 0.28 (−0.32, 0.85) | −1.51 (−1.72, −0.86) | −1.22 (−1.87, −0.66) | −2.42 (−2.68, −2.15) | < .001 |
| Attention z-score | 0.27 (−0.27, 0.83) | 0.23 (−0.42, 0.86) | −1.07 (−1.81, −0.45) | −0.84 (−1.38, −0.17) | −2.87 (−2.92, −2.51) | < .001 |
| Language z-score | 0.29 (−0.30, 0.83) | 0.21 (−0.32, 0.83) | −0.76 (−2.00, −0.14) | −0.95 (−1.53, −0.24) | −1.31 (−2.39, −0.81) | < .001 |
| Memory z-score | 0.21 (−0.45, 0.87) | 0.31 (−0.36, 0.78) | −1.45 (−1.81, −0.86) | −1.18 (−1.84, −0.67) | −2.24 (−2.53, −1.55) | < .001 |
| Visual-spatial z-score | 0.15 (−0.38, 0.81) | 0.15 (−0.43, 0.74) | −0.63 (−1.54, −0.08) | −0.86 (−1.44, −0.34) | −2.03 (−2.41, −0.85) | < .001 |
| Elecsys CSF P-tau181 (pg/mL) | 19.4 (16.0, 23.6) | 15.2 (12.0, 22.8) | 21.9 (16.7, 26.0) | 21.7 (15.5, 31.2) | 26.1 (16.3, 34.4) | < .001 |
| MSD CSF P-tau181 (pg/mL) | 88.5 (70.0, 112.13) | 82.6 (55.8, 149.8) | 88.5 (70.6, 118.9) | 164.4 (88.6, 303.0) | 186.2 (143.6, 270.1) | < .001 |
| MSD CSF P-tau217 (pg/mL) | 66.5 (48.3, 90.5) | 80.6 (41.3, 183.0) | 80.9 (52.8, 114.6) | 211.7 (98.3, 484.8) | 242.7 (201.7, 558.4) | < .001 |
| Elecsys CSF Aβ1–42 (pg/mL) | 1491.0 (1214.0, 1773.3) | 744.2 (595.3, 882.1) | 1508.0 (1190.0, 2036.5) | 659.4 (483.1, 796.6) | 604.0 (502.3, 681.9) | < .001 |
Abbreviations: Aβ, amyloid-beta; A+, amyloid positive; A-, amyloid negative; APOE, Apolipoprotein E; CSF, cerebrospinal fluid; CU, cognitively unimpaired; MCI, mild cognitive impairment; MSD, Mesoscale discovery platform; P-tau181, phosphorylated tau 181; P-tau217, phosphorylated tau 217. A CSF Aβ1–42 level<1026 pg/mL was considered amyloid positive (A+). See Figures S1-S3 in the Supplement for two-group comparisons.
Because the P-tau/Aβ42 ratio corresponds better to amyloid PET than CSF Aβ42 alone for defining A+, and we could not use the P-tau/Aβ42 ratio due to circularity, we replicated the above analyses but instead used amyloid PET to define amyloid status (Table S1 and Figures S6-S8). Of the participants with CSF, 356 (47.3%) had a concurrent amyloid PET scan. There were no differences between participants with versus without amyloid PET with regards to age, sex, APOE, or clinical diagnosis. Concordance (95% CI) between CSF Aβ42 and amyloid PET, measured using Cohen’s kappa, was 0.38 (0.29, 0.47). Using amyloid PET, all three CSF P-tau measures significantly (P<.001) differentiated CU PET A- and CU PET A+ as well as MCI PET A- and MCI PET A+. When comparing CU A- to CU A+, the median Elecsys P-tau181 measure was 37% higher, MSD P-tau181 was 83% higher, MSD P-tau217 was 281% higher for CU A+ participants. Similarly, when comparing MCI A- to MCI A+, median Elecsys P-tau181, MSD P-tau181, and MSD P-tau217 were 56%, 261%, 462% higher for MCI A+. Using De Long comparisons (Figure S5) for PET A+ vs. A-, the AUC of MSD P-tau217 was significantly better than MSD P-tau181 (P<.001) and Elecsys P-tau181 (P<.001). The AUC of MSD P-tau181 was also significantly better than Elecsys P-tau181 (P<.001).
3.3. Comparison of CSF P-tau181 and P-tau217 for risk of MCI and progression from MCI to dementia
Over the course of the follow-up, 106 of 621 (17%) CU participants with follow-up data were diagnosed with incident MCI. In multivariate models for risk of MCI among the entire cohort, each z-score unit increase in MSD P-tau181 (HR=1.14; 95% CI, 1.03–1.26) and MSD P-tau217 (HR=1.16; 95% CI, 1.07–1.26), but not Elecsys P-tau181 (HR=1.07; 95% CI, 0.93–1.23), was significantly associated with a greater risk of MCI (Table S2). Comparing AICs, MSD P-tau181 had the best model fit for predicting risk of MCI, followed by MSD P-tau217 then Elecsys P-tau181.
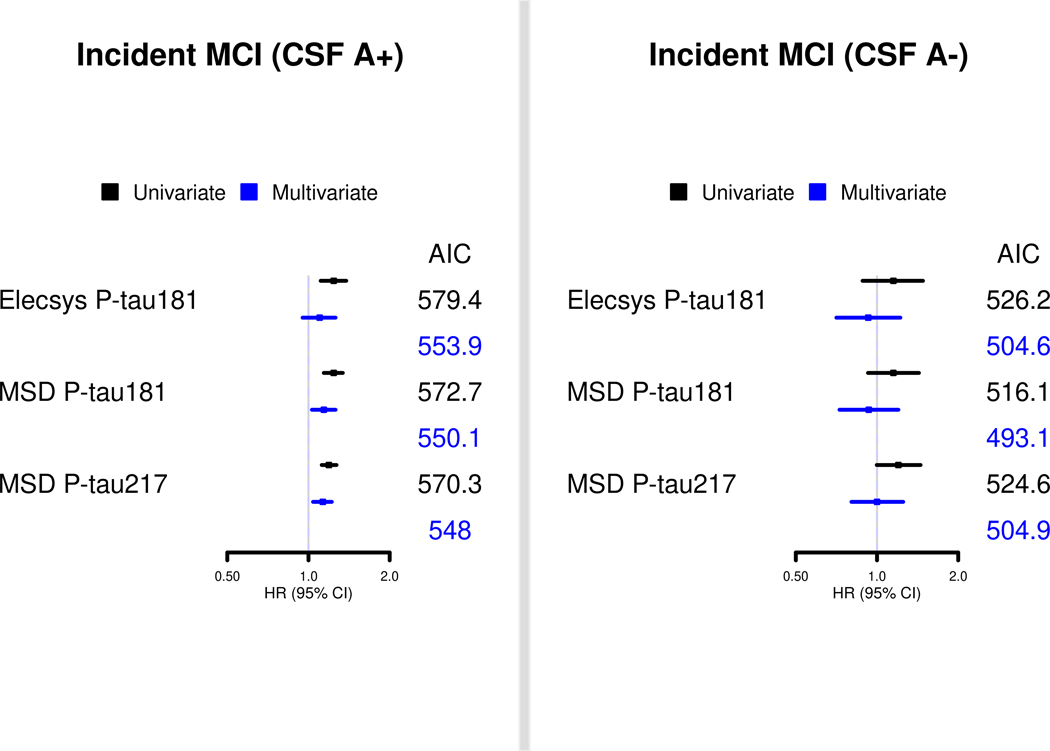
When stratified by CSF Aβ42, there was no association between any of the CSF P-tau measures and risk of MCI among those who were A- in multivariable analyses (Figure 1 and Table S2). Among A+ in multivariable analyses, MSD P-tau181 (HR=1.14; 95% CI, 1.03–1.26) and MSD P-tau217 (HR=1.13; 95% CI, 1.04–1.22), but not Elecsys P-tau181 (HR=1.10; 95% CI, 0.95–1.25), was significantly associated with risk of MCI. MSD P-tau217 had the best model fit based on AIC, but the AIC values for each P-tau measure had little overall difference.
FIGURE 1.
Comparison of CSF P-tau181 and P-tau217 markers, by CSF amyloid-beta42 status, for risk of MCI and progression from MCI to dementia in the Mayo Clinic Study on Aging. Multivariable models are presented adjusting for age, sex, education, and APOE.
We repeated the analyses restricting the sample to the 356 participants with available amyloid PET data (Table 2). Similar to the analyses with the entire cohort in multivariate models, each z-score unit increase in MSD P-tau181 (HR=1.36; 95% CI, 1.12–1.65) and MSD P-tau217 (HR=1.36; 95% CI, 1.18–1.58), but not Elecsys P-tau181 (HR=1.23; 95% CI, 0.98–1.55), was associated with a significantly greater risk of MCI. Comparing AICs, MSD P-tau217 had a slightly better model fit for predicting risk of MCI, followed by MSD P-tau181 then Elecsys P-tau181. When stratified by amyloid PET status, there was no association between any of the P-tau measures and risk of MCI among A- participants. Among A+ participants in multivariable analyses, MSD P-tau181 (HR=1.28; 95% CI, 1.01–1.63) and MSD P-tau217 (HR=1.31; 95% CI, 1.09–1.58), but not Elecsys P-tau181 (HR=1.08; 95% CI, 0.81–1.43), was significantly associated with risk of MCI. As above, MSD P-tau217 had the best model fit based on AIC, but the AIC values for each P-tau measure had little overall difference.
TABLE 2.
Comparison of CSF P-tau measures for risk of MCI among the entire cohort and stratified by elevated brain amyloid defined by amyloid PiB-PET>1.48 SUVR
| Univariate | Multivariate | |||
|---|---|---|---|---|
| Z-scored CSF P-tau measures | HR (95% CI) | AIC | HR (95% CI) | AIC |
|
| ||||
| All | ||||
| Elecsys P-tau 181 | 1.39 (1.14, 1.70) | 418.7 | 1.23 (0.98, 1.55) | 409.0 |
| MSD P-tau 181 | 1.51 (1.27, 1.79) | 410.6 | 1.36 (1.12, 1.65) | 403.7 |
| MSD P-tau 217 | 1.42 (1.26, 1.61) | 404.5 | 1.36 (1.18, 1.58) | 397.6 |
| Amyloid PET A- | ||||
| Elecsys P-tau 181 | 1.34 (0.87, 2.07) | 198.3 | 1.06 (0.66, 1.72) | 184.6 |
| MSD P-tau 181 | 1.32 (0.69, 2.50) | 199.3 | 1.06 (0.51, 2.25) | 184.7 |
| MSD P-tau 217 | 1.28 (0.57, 2.87) | 199.6 | 1.13 (0.45, 2.85) | 184.6 |
| Amyloid PET A+ | ||||
| Elecsys P-tau 181 | 1.15 (0.89, 1.49) | 159.8 | 1.08 (0.81, 1.43) | 162.0 |
| MSD P-tau 181 | 1.34 (1.07, 1.68) | 155.0 | 1.28 (1.01, 1.63) | 159.0 |
| MSD P-tau 217 | 1.34 (1.13, 1.58) | 150.6 | 1.31 (1.09, 1.58) | 154.6 |
Abbreviations: AIC, Akaike Information Criteria; CSF, cerebrospinal fluid; MCI, mild cognitive impairment; HR, hazard ratio; CI, confidence interval; MSD, Mesoscale discovery platform; P-tau181, phosphorylated tau 181; P-tau217, phosphorylated tau 217.
Multivariate models adjust for age, sex, education, and APOE.
There were 15 MCI participants who progressed to dementia. In multivariable models, only MSD P-tau217 (HR=1.17; 95% CI, 1.01–1.36), but not MSD P-tau181 (HR=1.16; 95% CI, 0.96–1.41) or Elecsys P-tau181 (HR=1.14; 95% CI, 0.82–1.58), was significantly associated with risk of progression from MCI to dementia. There were not enough incident dementia events to restrict by amyloid status based on CSF or amyloid PET.
3.4. Association between CSF P-tau181 and P-tau217 with global- and domain-specific cognitive decline and cortical thickness
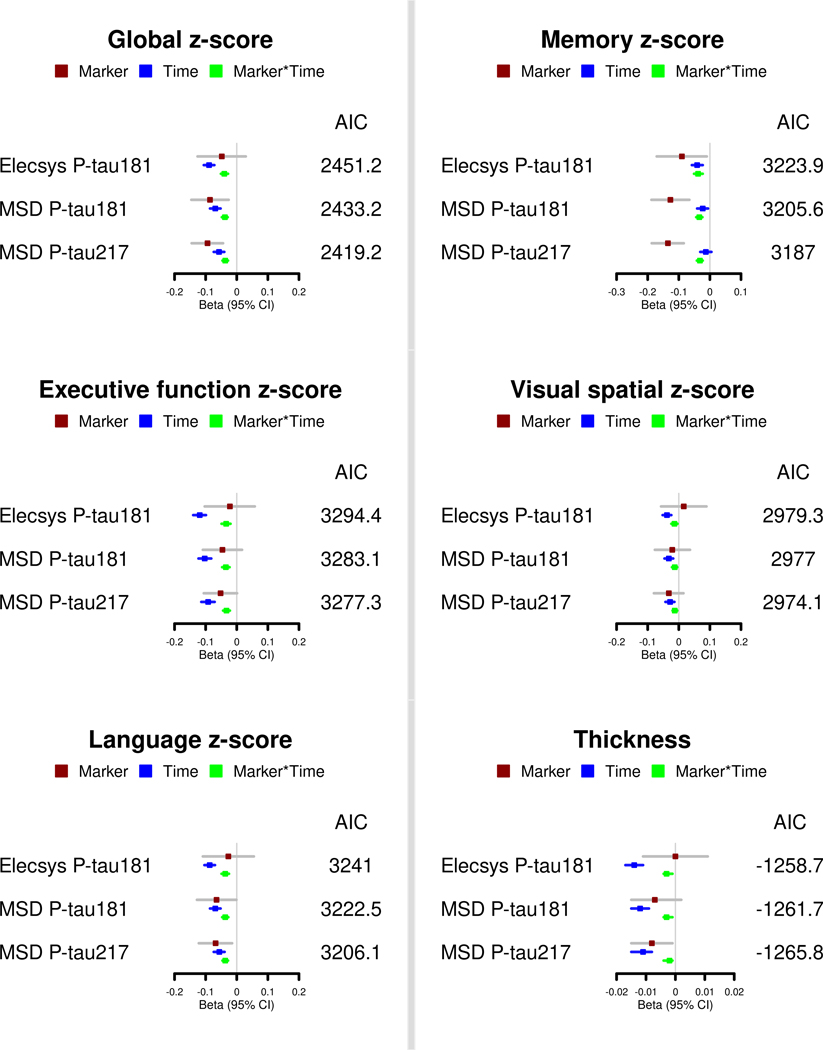
We next examined the multivariate cross-sectional and longitudinal associations of the P-tau measures with global and domain-specific cognitive decline and cortical thickness among CSF A+ (Figure 2 and Table S3) and A- (Table S4) participants. Among CSF A+ participants, MSD P-tau217 was the best fit model, based on AIC, for global cognition, all cognitive domains, and cortical thickness. However, for some outcomes such as cortical thickness, the AIC values did not differ much between the CSF P-tau measures. Among CSF A- participants, all three CSF P-tau measures were significantly associated with greater global cognitive decline and decline in all four cognitive domains, but not in cortical thickness (Table S4). Comparing the CSF P-tau measures, MSD P-tau181 had the lowest AIC and best model fit for all cognitive outcomes.
FIGURE 2.
Comparison of CSF P-tau181 and P-tau217 markers for global and domain-specific cognitive decline and cortical thickness in the Mayo Clinic Study on Aging among participants who have elevated brain amyloid. Multivariable models are presented, which adjust for age, sex, education, and APOE.
Among the subset of participants with amyloid PET, the multivariate cross-sectional and longitudinal associations of the P-tau measures with global and domain-specific cognitive decline and cortical thickness among PET A+ and PET A- are shown in Table 3 and Table S5, respectively. Among PET A+, and similar to CSF A+, MSD P-tau217 was the best fit model, based on AIC, for global cognition, all cognitive domains, and cortical thickness. However, for some outcomes such as visual-spatial performance and cortical thickness, the AIC values did not differ much between the CSF P-tau measures. There were fewer associations between the P-tau measures and cognition among the PET A- group and no association between any measure and cortical thickness. Higher levels of three CSF P-tau measures were associated with greater decline in memory but there was little difference based on AIC.
TABLE 3.
Comparison of CSF P-tau measures for global and domain-specific cognitive decline and cortical thickness among individuals with elevated brain amyloid based on amyloid PET
| Global z-score | Memory z-score | Executive function z-score | |||||||
|---|---|---|---|---|---|---|---|---|---|
| CSF Predictor | b(se) | p-value | AIC | b(se) | p-value | AIC | b(se) | p-value | AIC |
|
| |||||||||
| Elecsys P-tau 181 | 0.02 (0.07) | 0.771 | 708.8 | −0.01 (0.08) | 0.863 | 949.6 | −0.02 (0.08) | 0.840 | 1015.0 |
| time | −0.10 (0.02) | <0.0001 | −0.05 (0.02) | 0.007 | −0.13 (0.02) | <0.0001 | |||
| Elecsys P-tau 181*time | −0.04 (0.01) | 0.003 | −0.04 (0.01) | 0.001 | −0.02 (0.01) | 0.163 | |||
| MSD P-tau181 | −0.04 (0.06) | 0.448 | 698.4 | −0.12 (0.06) | 0.059 | 942.4 | −0.01 (0.06) | 0.828 | 1010.3 |
| time | −0.07 (0.02) | <0.001 | −0.02 (0.02) | 0.214 | −0.11 (0.02) | <0.0001 | |||
| MSD P-tau181*time | −0.04 (0.01) | <0.0001 | −0.04 (0.01) | <0.0001 | −0.03 (0.01) | 0.006 | |||
| MSD P-tau217 | −0.07 (0.04) | 0.116 | 687.1 | −0.13 (0.04) | 0.004 | 929.1 | −0.03 (0.05) | 0.533 | 1006.9 |
| time | −0.05 (0.02) | 0.006 | −0.002 (0.02) | 0.898 | −0.09 (0.02) | <0.001 | |||
| MSD P-tau217*time | −0.04 (0.01) | <0.0001 | −0.04 (0.01) | <0.0001 | −0.03 (0.01) | <0.001 | |||
|
| |||||||||
|
| |||||||||
| Visual-spatial z-score | Language z-score | Cortical Thickness z-score | |||||||
| CSF Predictor | b(se) | p-value | AIC | b(se) | p-value | AIC | b(se) | p-value | AIC |
|
| |||||||||
| Elecsys P-tau 181 | 0.05 (0.06) | 0.455 | 888.2 | 0.01 (0.08) | 0.890 | 1027.1 | 0.01 (0.01) | 0.421 | −359.9 |
| time | −0.06 (0.01) | <0.0001 | −0.09 (0.02) | <0.0001 | −0.02 (0.003) | <0.0001 | |||
| Elecsys P-tau 181*time | −0.01 (0.01) | 0.249 | −0.04 (0.01) | 0.006 | −0.005 (0.002) | 0.007 | |||
| MSD P-tau181 | −0.004 (0.05) | 0.932 | 887.4 | −0.04 (0.06) | 0.490 | 1016.1 | −0.002 (0.01) | 0.787 | −363.3 |
| time | −0.05 (0.02) | 0.002 | −0.06 (0.02) | 0.006 | −0.01 (0.003) | <0.0001 | |||
| MSD P-tau181*time | −0.02 (0.01) | 0.060 | −0.05 (0.01) | <0.0001 | −0.005 (0.001) | <0.0001 | |||
| MSD P-tau217 | −0.03 (0.04) | 0.450 | 886.0 | −0.05 (0.04) | 0.294 | 1005.3 | −0.01 (0.01) | 0.166 | −365.2 |
| time | −0.04 (0.02) | 0.012 | −0.03 (0.02) | 0.099 | −0.01 (0.003) | <0.001 | |||
| MSD P-tau217*time | −0.01 (0.01) | 0.019 | −0.05 (0.01) | <0.0001 | −0.004 (0.001) | <0.0001 | |||
Abbreviations: AIC, Akaike Information Criteria; CSF, cerebrospinal fluid; se, standard error; MSD, Mesoscale discovery platform; P-tau181, phosphorylated tau 181; P-tau217, phosphorylated tau 217.
Multivariate models adjust for age, sex, education, and APOE.
4. DISCUSSION
We compared the model fit of CSF P-tau217, assayed using MSD, and P-tau181, assayed using both MSD and Elecsys, in relation to cross-sectional and longitudinal measures of cognition (including risk of MCI and dementia) and cortical thickness. We examined each marker as a continuous z-scored measure to best compare coefficients and to maximize the power of the study. AIC was used to determine best model fit. In cross-sectional analyses, CSF P-tau217 better distinguished between CU and MCI groups, and between A- and A+. Thus, CSF P-tau217 is a better diagnostic marker of AD pathophysiology. Longitudinally, both CSF MSD P-tau181 and P-tau217 results were associated with risk of MCI among A+ participants. However, the best fit model varied for the two MSD assays depending upon the cognitive outcome. There were few differences between the CSF P-tau measures for change in global or domain-specific cognitive decline or cortical thickness.
Previous cross-sectional studies compared the diagnostic utility of CSF P-tau181 and P-tau217 for AD using different approaches. These studies, using multiple assay methodologies, reported that CSF P-tau217 correlated more strongly with both amyloid PET and Tau PET and was better able to distinguish between AD and non-AD neurodegenerative diseases [11–13, 15–16]. However, the superiority of CSF P-tau217 over P-tau181 may depend on the P-tau181 antibodies used; N-terminal-directed P-tau181, but not standard mid-region P-tau181, has been shown to have similar diagnostic performance to N-terminal-directed P-tau181 [14]. The current cross-sectional analysis, albeit on a larger sample size and within a population-based setting, similarly found a larger difference in CSF P-tau217 levels than P-tau181 when distinguishing between A+ and A- participants, especially among MCI. Importantly, we first defined A+ based on Aβ42<1026 pg/ml because we could not define A+ based on the CSF P-tau/Aβ42 ratio due to the circularity of comparing P-tau measures, and we did not have CSF Aβ40 assayed to determine a CSF Aβ42/Aβ40 ratio. The CSF Aβ42/Aβ40 ratio has been shown to be superior for the measurement of brain amyloid and the diagnosis of AD dementia [33, 34]. Indeed, in the present study, the Cohens kappa of 0.38 between CSF Aβ42 and amyloid PET was only fair. Concurrent amyloid PET was only available for about half of the participants. However, we did repeat the analyses among the amyloid PET subset to determine if the results were consistent. Indeed, the difference between A+ and A- was most notable and consistent when elevated brain amyloid was defined by amyloid PET instead of CSF. Thus, the misclassification of patients in the primary analysis based on CSF Aβ42 cannot be excluded.
The difference between A+ and A- in the two P-tau181 assays likely represents the contribution of unique tau protein isoform selectivity (Figure S1), although other platform or assay details cannot be ruled out. The tau protein isoform selectivity is unique compared with the N-terminal P-tau181 assay described by Karikari et al. (2020) despite using the same P-tau181 specific antibody as in Lumipulse or Innotest [14]. This unique tau isoform specificity in the Lilly P-tau assays may be important in CSF, and head-to-head comparisons of the different assays are needed. Similarly, the differences in A+ and A- between the two MSD assays highlights the contribution of P-tau217 versus P-tau181 because the only difference between the assays was the phosphorylation site-specific antibody reagent. Of note, one mass spectrometry study utilized the ratio of P-tau181 or P-tau217 peptides to unmodified peptides [16] as a way to control for background individual heterogeneity and pointed out the importance of avoiding the contribution of peripherally expressed tau.
Among serial CSF samples a mean of 3.4 years apart, a larger increase in CSF P-tau217 than CSF P-tau181 has been observed, specifically in those individuals who were A+ [11, 15]. However, due to the small sample size of these prior studies, the prognostic utility of individual P-tau assays or their comparison for cognitive decline has not been previously reported. In the present study, we compared the CSF P-tau measures for risk of MCI by amyloid status (A+ and A-). Among CSF A+, only MSD P-tau181 and P-tau217 were associated with a significant increased risk of MCI in multivariable analyses. However, there was little difference in AIC for the determination of best fit model. Among the subset of participants with concurrent amyloid PET, the results among those who were PET A+ were the same as those who were CSF A+. There were no associations between any of the CSF P-tau measures and risk of MCI among those who were CSF A- or PET A-. These results further suggest that CSF P-tau is a prognostic marker of cognitive impairment among individuals with elevated brain amyloid. For CSF A+ and PET A+, AIC was lowest for P-tau217 models, indicating best model fit, but the overall difference in AIC values between models was relatively small. Notably, both MSD assays were significantly associated with risk of MCI whereas the Elecsys P-tau181 was not.
When examining global and domain-specific cognitive decline and change in cortical thickness, MSD P-tau217 had the best fit models among A+ regardless of whether A+ was defined by CSF or PET. However, the coefficients of each P-tau measure for a given outcome were often similar and the AICs did not vary a lot. Thus, all three P-tau measures can be used to predict cognitive decline among individuals with elevated brain amyloid. In addition, we also found that all three CSF P-tau measures were similarly associated with global and domain-specific cognitive decline, but not cortical thickness, among CSF A- and PET A- participants. It is likely that these associations are driven by individuals with higher brain amyloid but who have not yet crossed the threshold to A+.
Future studies should compare these CSF P-tau measures as prognostic markers across the clinical disease spectrum and include a larger number of individuals who progress from MCI to dementia. Indeed, further understanding of the biological implications and temporality of CSF P-tau181 and P-tau217, along with P-tau231 and P-tau205 is needed. Among familiar AD mutation carriers, CSF P-tau205 was noted to increase after CSF P-tau181 and P-tau217 [35]. Other studies of sporadic AD patients suggest that CSF P-tau231 may change before P-tau181 or P-tau217 [10].
Strengths of the study include the large sample size with longitudinal follow-up, population-based setting, and robust assay methodologies. However, limitations also warrant consideration. First, we did not have available serial CSF P-tau assessments to assess change in the P-tau measures and change in cognition or cortical thickness. Second, although tau PET is now collected in the MCSA, most of the CSF was collected prior to the beginning of tau PET. Thus, we could not directly compare the CSF P-tau measures to tau PET for cognitive prognosis. Lastly, the number of incident dementia cases was low and we had insufficient power to examine associations by brain amyloid.
In conclusion, the current study with a larger sample size and population-based study design, replicate most previous findings of superior diagnostic ability of CSF P-tau217 over P-tau181 for the diagnosis of AD pathology. In addition, results suggest that CSF P-tau217 may be a better prognostic marker of MCI among cognitively unimpaired A+ individuals. In relation to cognitive decline, overall differences between the CSF P-tau measures among A+ or A- individuals are very subtle.
ACKNOWLEDGMENTS
We would like to thank Ms. Michelle Campbell, MLS(ASCP), MB(ASCP) and Ms. Tifani Flieth for running the CSF samples on the Elecsys platform on a Roche cobas 6000 e 601 module in the Department of Laboratory Medicine and Pathology at Mayo Clinic. We also thank Roche Diagnostics for providing the kits for CSF Elecsys analyses. ELECSYS, COBAS and COBAS E are registered trademark of Roche. All other product names and trademarks are the property of their respective owners’.
Funding/Support: This study was supported by funding from the National institutes of Health/National Institute on Aging grants U01 AG006786, P30 AG062677, R01 AG011378, R01 AG041851, the GHR Foundation, and was made possible by the Rochester Epidemiology Project (R01 AG034676).
Role of the Funder/Sponsor: The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study, and all authors had final responsibility for the decision to submit for publication.
CONFLICTS OF INTEREST
Dr. Mielke served as a consultant to Brain Protection Company and Biogen and receives research support from the National Institutes of Health and the Department of Defense. She is a Senior Associate Editor for Alzheimer’s and Dementia: The Journal of the Alzheimer’s Association. Mr. Aakre and Dr. Algeciras-Schimnich have nothing to disclose. Dr. Machulda receives research support from the National Institutes of Health. Mr. Proctor and Dr. Dage are employees of, and stock-holders in, Eli Lilly. Dr. Eichenlaub is an employee of Roche Diagnostics. Dr. Knopman serves on a Data Safety Monitoring Board for Biogen (fee paid to institution), the DIAN-TU study (receives personal consulting fees), Agenbio (unpaid), and an endovascular carotid reconstruction study (unpaid). He is an investigator in clinical trials sponsored by Biogen, Lilly Pharmaceuticals, and the Alzheimer’s Disease Cooperative Study; and receives research support from the National Institutes of Health. Dr. Vemuri has received speaking fees from Miller Medical Communications, Inc and receives research support from the National Institutes of Health. Dr. Graff-Radford has received payment for speaking at the American Academy of Neurology Annual meeting. Dr. Petersen is a consultant for Roche, Inc., Merck, Inc., Biogen, Inc., and Eisai, Inc. He has received payment for serving on a Data Safety Monitoring Board for Genentech, receives royalties from Oxford University Press and UpToDate, and receives research support from the National Institutes of Health. Dr. Jack serves on an independent data monitoring board for Roche, has served as a speaker for Eisai, and consulted for Biogen, but he receives no personal compensation from any commercial entity. He receives research support from NIH, the GHR Foundation and the Alexander Family Alzheimer’s Disease Research Professorship of the Mayo Clinic.
Footnotes
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of the article.
REFERENCES
- [1].Jack CR Jr., Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, et al. NIA-AA Research Framework: toward a biological definition of Alzheimer’s disease. Alzheimers Dement 2018;14:535–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Olsson B, Lautner R, Andreasson U, Ohrfelt A, Portelius E, Bjerke M, et al. CSF and blood biomarkers for the diagnosis of Alzheimer’s disease: a systematic review and meta-analysis. Lancet Neurol 2016;15:673–684. [DOI] [PubMed] [Google Scholar]
- [3].Tenreiro S, Eckermann K, Outeiro TF. Protein phosphorylation in neurodegeneration: friend or foe? Front Mol Neurosci 2014;7:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Barthelemy NR, Mallipeddi N, Moiseyev P, Sato C, Bateman RJ. Tau phosphorylation rates measured by mass spectrometry differ in the intracellular brain vs. extracellular cerebrospinal fluid compartments and are differentially affected by Alzheimer’s disease. Front Aging Neurosci 2019;11:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Hampel H, Buerger K, Zinkowski R, Teipel SJ, Goernitz A, Andreasen N, et al. Measurement of phosphorylated tau epitopes in the differential diagnosis of Alzheimer disease: a comparative cerebrospinal fluid study. Arch Gen Psychiatry 2004;61:95–102. [DOI] [PubMed] [Google Scholar]
- [6].Blennow K, Wallin A, Agren H, Spenger C, Siegfried J, Vanmechelen E. Tau protein in cerebrospinal fluid: a biochemical marker for axonal degeneration in Alzheimer disease? Mol Chem Neuropathol 1995;26:231–245. [DOI] [PubMed] [Google Scholar]
- [7].Cicognola C, Brinkmalm G, Wahlgren J, Portelius E, Gobom J, Cullen NC, et al. Novel tau fragments in cerebrospinal fluid: relation to tangle pathology and cognitive decline in Alzheimer’s disease. Acta Neuropathol 2019;137:279–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Neddens J, Temmel M, Flunkert S, Kerschbaumer B, Hoeller C, Loeffler T, et al. Phosphorylation of different tau sites during progression of Alzheimer’s disease. Acta Neuropathol Commun 2018;6:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Blennow K, Chen C, Cicognola C, Wildsmith KR, Manser PT, Bohorquez SMS, et al. Cerebrospinal fluid tau fragment correlates with tau PET: a candidate biomarker for tangle pathology. Brain 2020;143:650–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Suarez-Calvet M, Karikari TK, Ashton NJ, Lantero Rodriguez J, Mila-Aloma M, Gispert JD, et al. Novel tau biomarkers phosphorylated at T181, T217 or T231 rise in the initial stages of the preclinical Alzheimer’s continuum when only subtle changes in Abeta pathology are detected. EMBO Mol Med 2020;12:e12921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Janelidze S, Stomrud E, Smith R, Palmqvist S, Mattsson N, Airey DC, et al. Cerebrospinal fluid p-tau217 performs better than p-tau181 as a biomarker of Alzheimer’s disease. Nat Commun 2020;11:1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Barthelemy NR, Bateman RJ, Hirtz C, Marin P, Becher F, Sato C, et al. Cerebrospinal fluid phospho-tau T217 outperforms T181 as a biomarker for the differential diagnosis of Alzheimer’s disease and PET amyloid-positive patient identification. Alzheimers Res Ther 2020;12:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Hanes J, Kovac A, Kvartsberg H, Kontsekova E, Fialova L, Katina S, et al. Evaluation of a novel immunoassay to detect p-tau Thr217 in the CSF to distinguish Alzheimer disease from other dementias. Neurology 2020;95:e3026–e3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Karikari TK, Emersic A, Vrillon A, Lantero-Rodriguez J, Ashton NJ, Kramberger MG, et al. Head-to-head comparison of clinical performance of CSF phospho-tau T181 and T217 biomarkers for Alzheimer’s disease diagnosis. Alzheimers Dement 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Mattsson-Carlgren N, Andersson E, Janelidze S, Ossenkoppele R, Insel P, Strandberg O, et al. Abeta deposition is associated with increases in soluble and phosphorylated tau that precede a positive Tau PET in Alzheimer’s disease. Sci Adv 2020;6:eaaz2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Barthelemy NR, Horie K, Sato C, Bateman RJ. Blood plasma phosphorylated-tau isoforms track CNS change in Alzheimer’s disease. J Exp Med 2020;217:e20200861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Roberts RO, Geda YE, Knopman DS, Cha RH, Pankratz VS, Boeve BF, et al. The Mayo Clinic Study of Aging: design and sampling, participation, baseline measures and sample characteristics. Neuroepidemiology 2008;30:58–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].J.L. St. Sa BR Grossardt BP Yawn LJ Melton 3rd, Pankratz JJ, Brue SM, et al. Data resource profile: the Rochester Epidemiology Project (REP) medical records-linkage system. Int J Epidemiol 2012;41:1614–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Ivnik RJ, Malec JF, Smith GE, Tangalos EG, Petersen RC, Kokmen E, et al. Mayo’s Older Americans Normative Studies: updated AVLT norms for ages 56 to 97. Clinical Neuropsychologist 1992;6:83–104. [Google Scholar]
- [20].Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med 2004;256:183–194. [DOI] [PubMed] [Google Scholar]
- [21].American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-IV). 4th ed. Washington, D.C.: American Psychiatric Association; 1994. [Google Scholar]
- [22].Mielke MM, Syrjanen JA, Blennow K, Zetterberg H, Skoog I, Vemuri P, et al. Comparison of variables associated with cerebrospinal fluid neurofilament, total-tau, and neurogranin. Alzheimers Dement 2019;15:1437–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Bittner T, Zetterberg H, Teunissen CE, Ostlund RE Jr., Militello M, Andreasson U, et al. Technical performance of a novel, fully automated electrochemiluminescence immunoassay for the quantitation of beta-amyloid (1–42) in human cerebrospinal fluid. Alzheimers Dement 2016;12:517–526. [DOI] [PubMed] [Google Scholar]
- [24].Lifke V, Kollmorgen G, Manuilova E, Oelschlaegel T, Hillringhaus L, Widmann M, et al. Elecsys((R)) Total-Tau and Phospho-Tau (181P) CSF assays: Analytical performance of the novel, fully automated immunoassays for quantification of tau proteins in human cerebrospinal fluid. Clin Biochem 2019;72:30–38. [DOI] [PubMed] [Google Scholar]
- [25].Lowe VJ, Kemp BJ, Jack CR Jr., Senjem M, Weigand S, Shiung M, et al. Comparison of 18F-FDG and PiB PET in cognitive impairment. J Nucl Med 2009;50:878–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Price JC, Klunk WE, Lopresti BJ, Lu X, Hoge JA, Ziolko SK, et al. Kinetic modeling of amyloid binding in humans using PET imaging and Pittsburgh Compound-B. J Cereb Blood Flow Metab 2005;25:1528–1547. [DOI] [PubMed] [Google Scholar]
- [27].McNamee RL, Yee SH, Price JC, Klunk WE, Rosario B, Weissfeld L, et al. Consideration of optimal time window for Pittsburgh compound B PET summed uptake measurements. J Nucl Med 2009;50:348–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Jack CR Jr., Lowe VJ, Senjem ML, Weigand SD, Kemp BJ, Shiung MM, et al. 11C PiB and structural MRI provide complementary information in imaging of Alzheimer’s disease and amnestic mild cognitive impairment. Brain 2008;131:665–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Jack CR Jr., Wiste HJ, Weigand SD, Therneau TM, Lowe VJ, Knopman DS, et al. Defining imaging biomarker cut points for brain aging and Alzheimer’s disease. Alzheimers Dement 2017;13:205–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Jack CR Jr., Wiste HJ, Weigand SD, Therneau TM, Knopman DS, Lowe V, et al. Age-specific and sex-specific prevalence of cerebral beta-amyloidosis, tauopathy, and neurodegeneration in cognitively unimpaired individuals aged 50–95 years: a cross-sectional study. Lancet Neurol 2017;16:435–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Burnham KP, Anderson DR. Model Selection and Multimodal Inference: A Practical Information-Theoretic Approach. 2nd edition. New York: Springer-Verlag; 2002. [Google Scholar]
- [32].R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. 2019. https://www.R-project.org/ (accessed 05/12/2020). [Google Scholar]
- [33].Hansson O, Lehmann S, Otto M, Zetterberg H, Lewczuk P. Advantages and disadvantages of the use of the CSF Amyloid beta (Abeta) 42/40 ratio in the diagnosis of Alzheimer’s Disease. Alzheimers Res Ther 2019;11:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Janelidze S, Zetterberg H, Mattsson N, Palmqvist S, Vanderstichele H, Lindberg O, et al. CSF Abeta42/Abeta40 and Abeta42/Abeta38 ratios: better diagnostic markers of Alzheimer disease. Ann Clin Transl Neurol 2016;3:154–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Barthelemy NR, Li Y, Joseph-Mathurin N, Gordon BA, Hassenstab J, Benzinger TLS, et al. A soluble phosphorylated tau signature links tau, amyloid and the evolution of stages of dominantly inherited Alzheimer’s disease. Nat Med 2020;26:398–407. [DOI] [PMC free article] [PubMed] [Google Scholar]