Abstract
The first wave of hematopoiesis is the primitive hematopoiesis, which produces embryonic erythroid and myeloid cells. Primitive erythrocytes are thought to be generated from bipotent hemangioblasts, but the molecular basis remains unclear. Transcriptional repressors Gfi1aa and Gfi1b have been shown to cooperatively promote primitive erythrocytes differentiation from hemangioblasts in zebrafish. However, the mechanism of these repressors during the primitive wave is largely unknown. Herein, by functional analysis of zebrafish gfi1aa smu10 , gfi1b smu11 , gfi1ab smu12 single, double, and triple mutants, we found that Gfi1aa not only plays a predominant role in primitive erythropoiesis but also synergizes with Gfi1ab. To screen Gfi1aa downstream targets, we performed RNA-seq and ChIP-seq analysis and found two endothelial transcription factors, etv2 and sox7, to be repressed by Gfi1aa. Genetic analysis demonstrated Gfi1aa to promote hemangioblast differentiation into primitive erythrocytes by inhibiting both etv2 and sox7 in an Lsd1-dependent manner. Moreover, the H3K4me1 level of etv2 and sox7 were increased in gfi1aa mutant. Taken together, these results suggest that Gfi1aa/Lsd1-dependent etv2/sox7 downregulation is critical for hemangioblast differentiation during primitive hematopoiesis by inhibition of endothelial specification. The different and redundant roles for Gfi1(s), as well as their genetic and epigenetic regulation during primitive hematopoiesis, help us to better know the molecular basis of the primitive hematopoiesis and sheds light on the understanding the Gfi1(s) related pathogenesis.
Keywords: zebrafish, hemangioblast differentiation, primitive erythrocyte, Gfi1aa, etv2, sox7
Introduction
Hematopoiesis in vertebrates includes two distinct waves, the primitive wave and the definitive wave. In the primitive wave of mammals, both primitive erythroid and endothelial cells originate from the mesoderm and then aggregate and form the yolk sac blood island (Baron et al., 2012; (Garcia and Larina, 2014). In zebrafish, primitive erythroblasts originate from the lateral plate mesoderm (LPM) and then migrate to the intermediate cell mass, which is equivalent to the yolk sac blood island in mammals (Chen and Zon, 2009). Angioblasts (endothelial precursor cells) migrate to the midline from the LPM and form the vascular cord (Jin et al., 2005). Both hematopoietic and endothelial cells are thought to be derived from a common progenitor known as the hemangioblast (Lancrin et al., 2009; (Lacaud and Kouskoff, 2017), which was first proposed by Muttay in the early chick embryo (Murray, 1932). Although hemangioblasts have not been detected in mice (likely due to rare numbers), in zebrafish, a labeled gastrula-stage cell was shown to generate both hematopoietic and endothelial cells (Vogeli et al., 2006). This result suggests that the zebrafish is a model organism by which to define hemangioblast differentiation.
A series of transcription factors (e.g., Scl/Tal1 (Gering et al., 1998), Lmo2 (Patterson et al., 2007), Gata2 (Lugus et al., 2007), Etv2 (Liu and Patient, 2008), and Fli1 (Hart et al., 2000; (Spyropoulos et al., 2000; (Liu et al., 2008)) have been found that are expressed in both hematopoietic and endothelial cells. Genetic mutation of these transcription factors results in both hematopoiesis and vasculogenesis dysfunction (Gering et al., 1998; (Hart et al., 2000; (Spyropoulos et al., 2000; (Lugus et al., 2007; (Patterson et al., 2007; (Liu and Patient, 2008; (Liu et al., 2008), which provides molecular evidence for the existence of a common hemangioblast. Yet, the progression and regulation of hemangioblast differentiation, especially the molecular pathways by which hemangioblast transition to endothelial and hematopoietic cells, are largely unknown.
Gfi1 family members are reported to be involved in hemangioblast differentiation (Moore et al., 2018). Zebrafish has three Gfi1(s) paralogs: Gfi1aa and Gfi1ab are thought to be orthologs of mammalian GFI1 (Wei et al., 2008; (Cooney et al., 2013), and Gfi1b is considered to be the mammalian GFI1B’s ortholog (Cooney et al., 2013). It is reported that Gfi1aa promotes primitive erythropoiesis (Wei et al., 2008), subsequently, Gfi1b is shown synergistically with Gfi1aa to promote primitive erythroblast differentiation from hemangioblasts (Moore et al., 2018), but the molecular basis for their function is largely unclear. Gfi1ab is not expressed in primitive hematopoietic regions (Dufourcq et al., 2004), but its expression is increased in the absence of Gfi1aa (Thambyrajah et al., 2016b), suggesting the unclear role of Gfi1ab in primitive hematopoiesis. In addition, the histone demethylase, Lsd1, which demethylates mono- and di-methylated H3K4, is a co-factor of Gfi1 (Saleque et al., 2007) and critical for Gfi1aa transcription repression (Velinder et al., 2016), and its deficiency blocks primitive erythropoiesis (Takeuchi et al., 2015). Our previous study also has shown Gfi1aa inhibited cebpa expression to control neutrophil progenitor expansion was dependent upon Lsd1 (Wu et al., 2021). However, whether Gfi1aa regulates hemangioblast differentiation is dependent upon Lsd1 remains unknown. As such, the different and redundant roles for Gfi1(s), as well as their genetic and epigenetic regulation during primitive erythrocytes differentiated from hemangioblast, are not fully understood.
In this study, we assessed the role of the three zebrafish Gfi1 orthologs during primitive hematopoiesis and found that Gfi1aa, rather than Gfi1b and Gfi1ab, played a predominant role in hemangioblast differentiation to primitive erythroid cells. We screened potential Gfi1aa downstream targets by performing RNA-seq and ChIP-seq analysis and then verified genetic regulation. We found that Gfi1aa, with the help of histone demethylase Lsd1, downregulates etv2 and sox7, suppressing hemangioblast endothelial potential and promoting erythroid differentiation.
Materials and Methods
Zebrafish Husbandry
Zebrafish were raised and maintained as described (Westerfield, 2000). The following strains were used: the AB strain, the gfi1aa smu10 mutant (Wu et al., 2021), the gfi1b smu11 mutant, and the gfi1ab smu12 mutant. All zebrafish studies were approved by the South China University of Technology Animal Advisory Committee.
Generation gfi1b and gfi1ab Mutants
For the gfi1b smu11 mutant and the gfi1ab smu12 mutant, the gRNA (gfi1b: 5′- ggaggaaactctgccagctg-3′, gfi1ab: 5′- ggtactcggggtgtgaaatc-3′) was co-injected with Cas9 protein (NEB, MA, United States; M0646M) into one-cell stage embryos, the gRNAs were synthesized as described (Chang et al., 2013). The raising and screening of mutants were performed as previously described (Chang et al., 2013; (Liu et al., 2014). The genotyping primers were listed in Supplementary Table S1.
Whole Mount in situ Hybridization (WISH) and Immunofluorescence
Probes synthesis and WISH were carried out as described (Thisse and Thisse, 2008). The following probes were synthesized: gata1, alas2, scl, gata2a, fli1, etv2, sox7, and flk1. Embryos for immunofluorescence were fixed with 4% paraformaldehyde at 23 hpf and dehydrated by methanol. Then the embryos were permeabilized by acetone and stained with GFP antibody (Abcam, Cambridge, UK; ab6658).
Transgenic Zebrafish Generation and Heat Shock Treatment
For Tg (hsp70:gfi1aa-eGFP) transgenic zebrafish, the embryos injected with pTol-hsp70-eGFP construct and transposase mRNA (Wu et al., 2021) were raised to adult, then the stable transgenic lines were screened as previously described (Westerfield, 2000). To overexpress gfi1aa, 12 hpf embryos were heat shocked for 2 h at 39°C, then the GFP + embryos were picked out for subsequent experiments.
RNA Isolation and RNA-Seq
The gfi1aa smu10 mutant, gfi1b smu11 mutant, and gfi1ab smu12 mutant were generated from gfi1aa smu10/+ , gfi1b smu11/+ , and gfi1ab smu12/+ intercrossed embryos by genotyping respectively. The gfi1aa smu10 gfi1b smu11 mutant, gfi1aa smu10 gfi1ab smu12 mutant, and the gfi1aa smu10 gfi1b smu11 gfi1ab smu12 mutant were generated from gfi1aa smu10/+ gfi1b smu11 , gfi1aa smu10/+ gfi1ab smu12 , and gfi1aa smu10 gfi1b smu11 gfi1ab smu12/+ intercrossed embryos by genotyping respectively. Then, RNA from gfi1-related single, double, and triple mutants as well as WT (wild type siblings) embryos was extracted with TRIzol reagent (Invitrogen, CA, United States; 15596026). Sequencing libraries were generated using the NEBNext® UltraTM RNA Library Prep Kit for Illumina® RNA (NEB; E7770) according to the manufacturer’s instructions.
Bioinformatic Analysis
For RNA-seq data, the sequencing reads were mapped to Ensemble zebrafish reference genome (GRCz11) using STAR alignment software (Dobin et al., 2013). The differential gene expression analysis was performed by DESeq2 (Love et al., 2014). For GO enrichment analysis, the Metascape website (https://metascape.org/gp) (Zhou et al., 2019) was used.
Chromatin Immunoprecipitation-Polymerase Chain Reaction (ChIP-PCR)
Gfi1aa-GFP ChIP assay was performed as previously described (Wu et al., 2021). In detail, ∼250 WT embryos injected with the hsp-gfi1aa-eGFP plasmid or hsp-eGFP plasmid were heat-shocked and collected at 15 hpf, then the samples were performed by cross-linking, sonication, antibody binding, washing, reverse-cross linking, and ChIP DNA extraction. The ChIP DNA was assessed by qPCR with a LightCycler 96 system (Roche). The comparable WT group and gfi1aasmu10 mutant group were respectively intercrossed for H3K4me1 ChIP. About 200 embryos of each group were collected at 15 hpf and ChIP DNA was extracted as above. The etv2 ChIP-qPCR primers are used as previously described (Takeuchi et al., 2015), and sox7 ChIP-qPCR primer is listed in Supplementary Table S1.
In vivo Transient GFP Reporter Assay
For the transient GFP reporter assay, pTol-etv2-eGFP and pTol-sox7-eGFP plasmids were constructed for GFP expression under the control of etv2 or sox7 regulatory regions. For the pTol-etv2-eGFP plasmid, the 3.4 kb etv2 promoter (Veldman and Lin, 2012), containing etv2 up-1 to intron-2 region, was cloned by PCR (Primers are listed in Supplementary Table S1) from genomic DNA and inserted into the pTol vector to drive GFP. For the pTol-sox7-eGFP plasmid, the 0.7 kb promoter (containing the Gfi1aa binding peak) was cloned and constructed as above. Then, 100 ng/μL of the construct was injected into the WT control and gfi1aasmu10 mutant embryos.
Microinjection of Morpholinos (MOs)
MOs for etv2 (5′-cactgagtccttatttcactatatc-3′) (Sumanas and Lin, 2006), lsd1 (5′-gttattcacaccttgttgagatttc-3′) (Takeuchi et al., 2015), and sox7 (5′-acgcacttatcagagccgccatgtg-3′) (Cermenati et al., 2008) were synthesized by Gene Tools and dissolved in water. One-cell stage embryos were collected and injected. For double knockdown, the final concentration of 0.005 pmol etv2 MO and 0.5 pmol sox7 MO were used.
Statistical Analysis
GraphPad Prism 7.0 was used for analysis of experimental data. The Fisher’s exact test was used to compare the difference between two categorical variables. The Unpaired t-test was used to compare the mean difference of two independent groups. The p-value less than 0.05 was considered statistically significant.
Results
Gfi1ab Synergizes With Gfi1aa to Promote Primitive Erythropoiesis
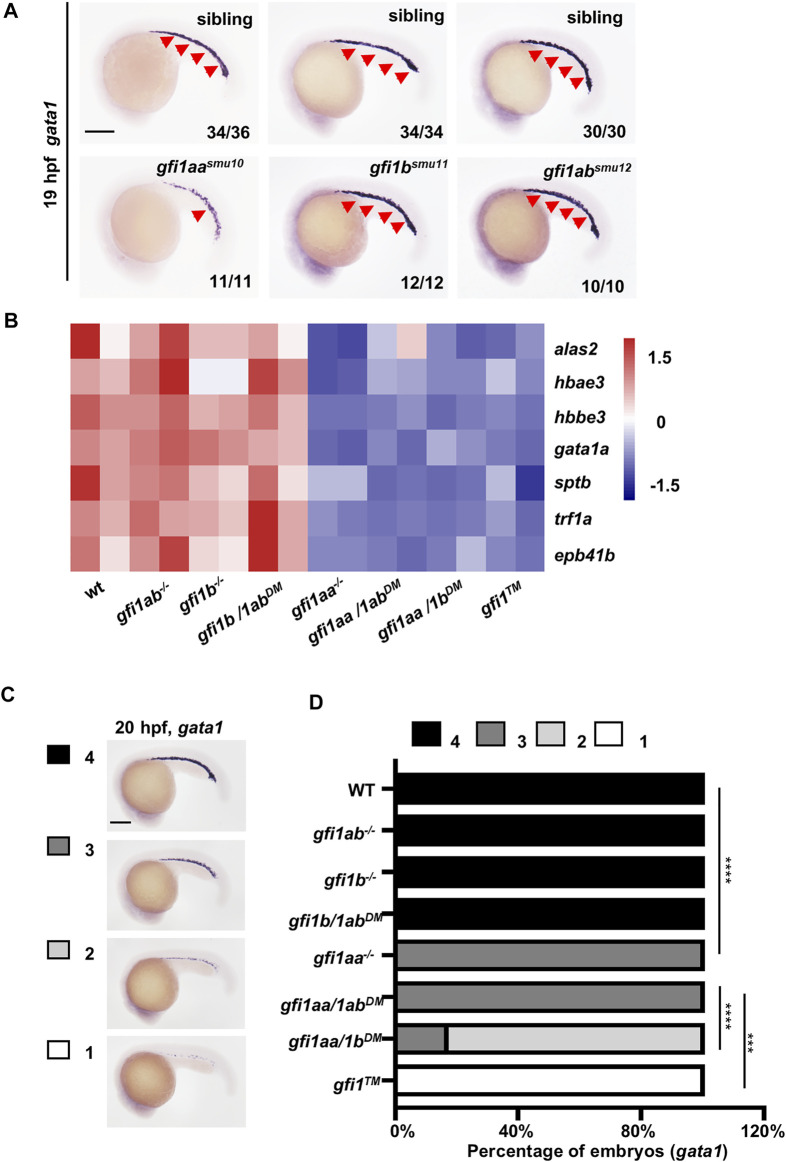
To determine the relationship of three Gfi1(s) to primitive hematopoiesis, we utilized a gfi1aa smu10 zebrafish mutant (Wu et al., 2021)) and generated gfi1b smu11 and gfi1ab smu12 zebrafish mutants with CRISPR/Cas9 technology (Supplementary Figure one). Similar to the gfi1aa smu10 mutant (Wu et al., 2021), gfi1b smu11 and gfi1ab smu12 mutants, with a 58-nt insertion (Supplementary Figure S1A) and a 1-nt deletion (Supplementary Figure S1B), respectively, were predicted to disrupt C2H2 type zinc finger domains. To identify the respective roles of Gfi1 members in primitive erythropoiesis, we compared erythroid marker, gata1, expression by WISH in each mutant. We found the expression of gata1 was decreased in gfi1aa smu10 mutant embryos compared to their siblings, while no apparent difference in the gfi1b smu11 mutant was found compared to siblings (Figure 1A), which is consistent with previously described gfi1aa qmc551 and gfi1b qmc554 mutants (Moore et al., 2018). We also monitored the phenotype of gfi1ab smu12 mutants and found gata1 expression was no altered (Figure 1A), suggesting that loss of gfi1ab does not affect primitive erythropoiesis.
FIGURE 1.
gfi1aa plays the key role in primitive erythropoiesis (A) Expression of gata1 was increased in gfi1aa smu10 mutants compared to siblings, whereas gfi1b smu11 and gfi1ab smu12 mutants show normal gata1 expression at 19 hpf by WISH. The numbers in the lower right corner indicate representative expression embryo numbers of the indicated marker. Scale bar: 200 μm (B) Heatmap of WT, gfi1aa −/− , gfi1b −/− , gfi1ab −/− signal mutant, gfi1aa/1b DM , gfi1aa/1ab DM , gfi1b/1ab DM double mutant and gfi1 TM triple mutant shows the gene expression levels of erythroid genes (alas2, hbae3, hbbe3, gata1a, sptb, trf1a, and epb41b). The color scale indicated the expression level (C,D) Expression of gata1 was decreased in gfi1aa related mutant (gfi1aa −/− , gfi1aa/1ab DM , gfi1aa/1b DM , and gfi1 TM ) compared to WT and other mutants at 20 hpf by WISH (C) The gfi1aa +/- ; gfi1b +/- ; gfi1ab +/- intercross embryos were divided into four categories according to gata1 expression (D) The percentage of WT, gfi1aa −/− , gfi1b −/− , gfi1ab −/− signal mutant, gfi1aa/1b DM , gfi1aa/1ab DM , gfi1b/1ab DM double mutant and gfi1 TM triple mutant according to the categories (****p < 0.0001, ***p < 0.001, Fisher exact tests, n ≥ 10 for each group).
To further identify the relationships among the three gfi1 members, we performed RNA-seq on wild-type (WT), gfi1aa smu10 , gfi1b smu11 , gfi1ab smu12 single mutant, gfi1aa smu10 gfi1b smu11 , gfi1aa smu10 gfi1ab smu12 , gfi1b smu11 gfi1ab smu12 double mutant and gfi1aa smu10 gfi1b smu11 gfi1ab smu12 triple mutant (hereafter referred to as gfi1aa −/− , gfi1b −/− , gfi1ab −/− , gfi1aa/1b DM , gfi1aa/1ab DM , gfi1b/1ab DM , and gfi1 TM ). As shown in the RNA-seq heatmap, we found that erythroid markers (alas2, hbae3, hbbe3, gata1a, sptb, trf1a, and epb41b) were decreased in gfi1aa related mutants (gfi1aa −/− , gfi1aa/1b DM , gfi1aa/1ab DM and gfi1 TM ) compared to WT and gfi1aa unrelated mutants (gfi1b −/− , gfi1ab −/− and gfi1b/1ab DM ) (Figure 1B). For validation, we further performed gata1 WISH on these mutants. Consistent with the RNA-seq data, the expression of gata1 was not altered in WT and gfi1aa unrelated mutants (Figures 1C,D). The expression of gata1 was decreased in gfi1aa mutants and gfi1aa/1ab DM , further decreased in gfi1aa/1b DM and the most decreased in gfi1 TM (Figures 1C,D). We then explored the genetic interplay among gfi1s and found gfi1b was decreased in gfi1aa-related mutants whereas gfi1ab was ectopic increased in gfi1aa-related mutants (Supplementary Figure S2A,B), suggesting gfi1aa dominates the expression of gfi1b and gfi1ab. These data indicate that Gfi1aa plays a predominant role in promoting primitive erythropoiesis, and that Gfi1ab, together with Gfi1b, play synergistic roles in the process.
Identification of Gfi1aa Target Genes That Promote Hemangioblast Differentiation Into Primitive Erythroid Cells
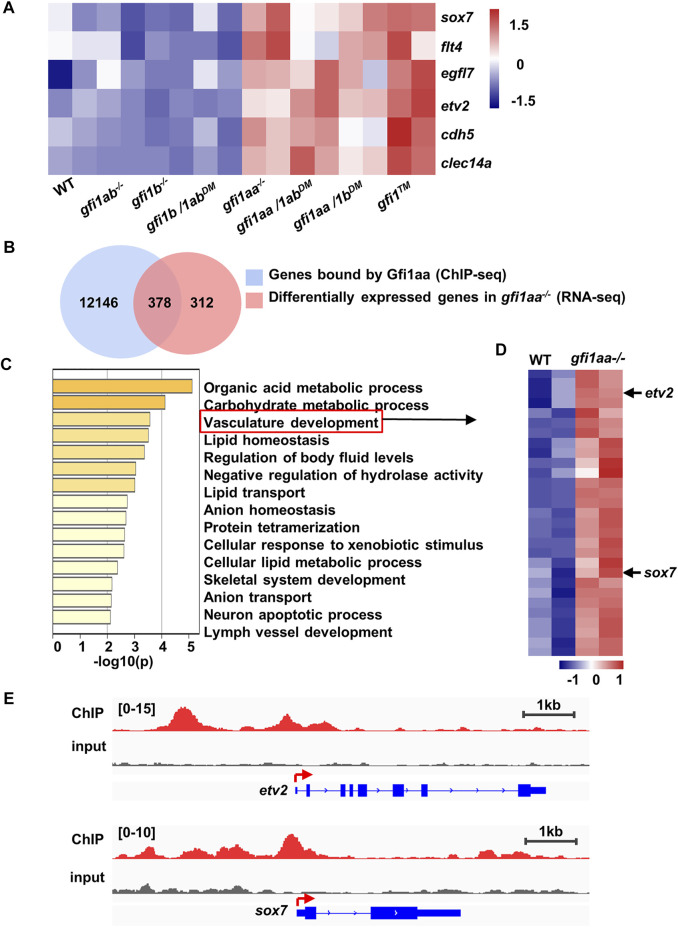
Gfi1aa and Gfi1b control primitive erythroblast differentiation by inhibition of endothelial programs (Moore et al., 2018), but the regulatory mechanisms and the key downstream factors are largely unknown. We speculated that Gfi1aa target genes probably exist in the upregulated genes of gfi1aa −/− mutant RNA-seq. Through Gene Ontology (GO) enrichment analysis of upregulated genes, we found vasculature development to be the most enriched GO term (Supplementary Figure 3A). Representative endothelial markers (including sox7, flt4, cdh5, clec14a, etv2, and egfl7 (Kaipainen et al., 1995; (Parker et al., 2004; (Sumanas et al., 2005; (Pham et al., 2007; (Cermenati et al., 2008)) were all upregulated in gfi1aa −/− mutant RNA-seq (Supplementary Figure S3B). By comparison of the differential expression of the endothelial markers among all gfi1 mutants, we found representative genes were specifically upregulated in all gfi1aa-related mutants (Figure 2A), and particularly upregulated in gfi1 TM . These data suggest that Gfi1aa, rather than Gfi1b or Gfi1ab, plays a predominant role in the inhibition of endothelial programs during hemangioblast differentiation into primitive erythrocytes.
FIGURE 2.
Gfi1aa could bind to etv2 and sox7 regulator regions (A) Endothelial genes were increased in gfi1aa related mutants. Heatmap of WT, gfi1aa −/− , gfi1b −/− , gfi1ab −/− signal mutant, gfi1aa/1b DM , gfi1aa/1ab DM , gfi1b/1ab DM double mutant and gfi1 TM triple mutant showed the gene expression levels of endothelial genes (sox7, flt4, cdh5, clec14a, etv2, and egfl7). The color scale indicated the expression level (B) Combinational analysis of gfi1aa −/− RNA-seq and Gfi1aa-eGFP ChIP-seq. 378 genes were overlapped between 690 up-regulated genes in gfi1aa −/− mutant and 12,524 genes bound by Gfi1aa (C) Go enrichment analysis of the 378 combinational genes. Vasculature development GO term was indicated by the red box (D) Heat map of WT and gfi1aa −/− mutant showed the vasculature development genes expression levels from (C). The color scale indicated the expression level (E) Visualization of Gfi1aa binding sites on etv2 (top) and sox7 (bottom) indicated by Gfi1aa ChIP-seq (red) compared to input control (grey) through integrative genomics viewer (IGV).
As Gfi1(s) function as transcription repressors, it is important to know which genes are directly targeted by Gfi1(s). By reanalyzing our previously performed Gfi1aa-eGFP ChIP-seq data (Wu et al., 2021), we found 12,524 genes bound by Gfi1aa with analyzing the peaks located 2 kb upstream and 2 kb downstream from the transcription start site (TSS) (Figure 2B). When RNA-seq upregulated genes of the gfi1aa −/− mutant were combined with the Gfi1aa ChIP targeted genes, we identified 378 candidates that may be directly targeted and transcriptionally suppressed by Gfi1aa (Figure 2B). As expected, the GO term analysis for the 378 candidate targets showed that the vasculature development pathway was highly enriched (Figure 2C). 29 endothelial associated genes were found to be involved in the pathway (Figure 2D). We then compared the differential expression of these genes among all gfi1 mutants and found sox7, flt4, egfl7, cdh5, etv2 were upregulated in gfi1aa-related mutants (Supplementary Figure S4A).
As transcription factors are thought to be critical for cell fate determination, we speculated that some transcription factors may be responsible for Gfi1aa involvement in primitive erythropoiesis. Etv2 and Sox7, two hemangioblast markers, were both highly expressed in mesodermal precursors but downregulated in differentiated hematopoietic cells (Gandillet et al., 2009; (Costa et al., 2012; (Veldman and Lin, 2012; (Sumanas and Choi, 2016). Previous studies showed that overexpression of either one promoted endothelial specification (Kataoka et al., 2011; (Costa et al., 2012). Moreover, etv2 and sox7 genes were highly bound by Gfi1aa-eGFP and their mRNAs were upregulated in gfi1aa-related mutants (Figures 2D,E, Supplementary Figure S4A). Therefore, we speculate that Gfi1aa may directly target and suppress etv2 and sox7 to promote hemangioblast differentiation into primitive erythrocytes by preventing the endothelial specification program.
Gfi1aa Directly Targets etv2 and sox7 and Suppresses Their Transcription
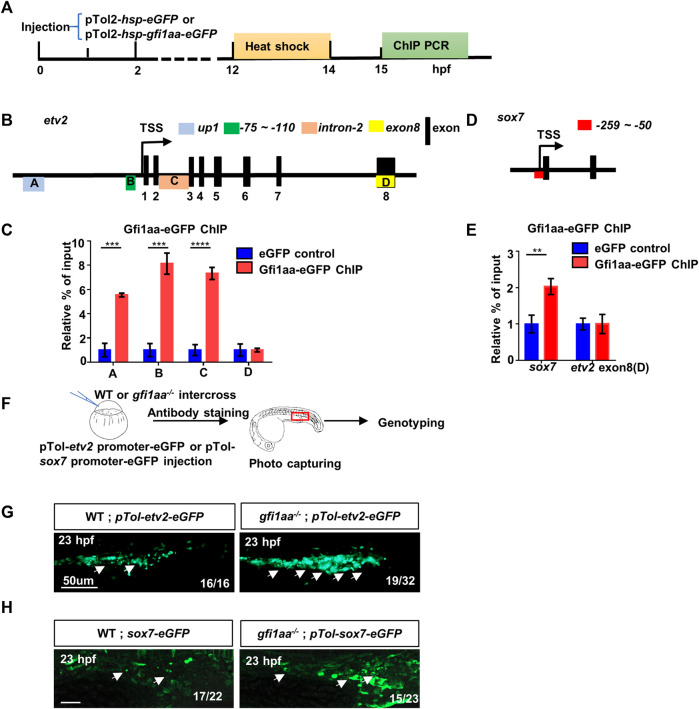
To test the hypothesis, we first validated our digital data. For validation of ChIP-seq results, we performed a ChIP-PCR assay using the pTol2-hsp-gfi1aa-eGFP construct to assess whether Gfi1aa could bind to etv2 and sox7 regulatory regions (Figure 3A). Previous data showed that three etv2 regulator regions (up1, -110 ∼ -35bp and intron-2) recapitulated etv2 expression (Veldman and Lin, 2012). ChIP PCR results showed that Gfi1aa could bind to these etv2 regulator regions (up1, -110 ∼ -35bp, intron-2) compared to the gene body control region (exon-8) (Figures 3B,C), which is consistent with the ChIP-seq data (Figure 2E). Moreover, ChIP PCR also showed an enrichment of Gfi1aa on sox7 regulatory region (-520 ∼ 180bp) (Figures 3D,E). These data suggest that the regulatory regions of etv2 and sox7 were directly bound by Gfi1aa.
FIGURE 3.
Gfi1aa directly represses etv2 and sox7 expression (A) Workflow of Gfi1aa-eGFP ChIP-PCR assay (B) Schematic diagram of etv2 gene structure. Three regulator regions up1 (Box A, blue colored), -75 ∼ -110 bp (Box B, green colored), intron-2 (Box C, orange colored) were showed on the gene structure, black boxes indicated the exons, exon-8 (Box D, yellow colored) as the control region. Box-(A–D) represented the detected region for etv2 ChIP PCR products (C) ChIP-qPCR showed Gfi1aa enriched in etv2 regulatory regions compared to eGFP control (up1, 5.5-fold; -75 ∼ -110bp, 8.1-fold; intron-2, 7.3-fold), the results were mean ± SD and generated from three independent experiments (****p < 0.0001, ***p < 0.001, t-test) (D) Schematic diagram of sox7 gene structure. Red box represented the detected region for sox7 ChIP PCR products (E) ChIP-qPCR showed 2-fold of Gfi1aa enriched in sox7 regulatory regions compared to eGFP control. The results were mean ± SD and generated from three independent experiments (**p < 0.01, t-test) (F–H) Gfi1aa was a transcription repressor for etv2 and sox7 (F) The scheme of transient GFP reporter assay for pTol-etv2-eGFP construct and pTol-sox7-eGFP construct. The red box indicated the image region (G,H) Transient expression of pTol-etv2-eGFP construct (G) and pTol-sox7-eGFP construct (H) in WT and gfi1aa −/− mutant embryos. Fluorescence in the ICM region was monitored at 23 hpf. Scale bar: 50 μm.
As etv2 and sox7 are the master regulators of hematopoietic/endothelial cell differentiation, we examined whether etv2 and sox7 were the specific downstream target genes of Gfi1aa. We detected a series of hemangioblast markers—scl, gata2, and fli1, as well as etv2 and sox7—at the beginning of primitive hematopoiesis. The results showed that etv2 and sox7 expression were markedly increased in gfi1aa −/− mutants compared to siblings, while expression of scl, gata2, and fli1 was not altered (Supplementary Figure S5A). The expression of etv2 and sox7 by qPCR also showed a similar increase in gfi1aa −/− mutants compared to WT (Supplementary Figure S5B). The WISH and qPCR results verified the RNA-seq results that etv2 and sox7 are upregulated in gfi1aa −/− mutants.
We further performed reporter assays to determine whether Gfi1aa could repress etv2 and sox7 transcription in vivo. We generated pTol-etv2-eGFP and pTol-sox7-eGFP reporter constructs and injected each construct into gfi1aa +/- intercross embryos to monitor whether GFP expression was affected by Gfi1aa (Figure 3F). The reporter assays showed that both etv2-eGFP and sox7-eGFP expression were increased in gfi1aa −/− mutants compared to their respective WT control (Figures 3G,H), suggesting a transcriptional repressive role for Gfi1aa in etv2 and sox7 regulatory regions.
The above data demonstrated that Gfi1aa targets the regulatory regions of etv2 and sox7 and suppresses their transcription.
sox7 and etv2 Cooperatively Act Downstream of Gfi1aa for Hemangioblast Differentiation
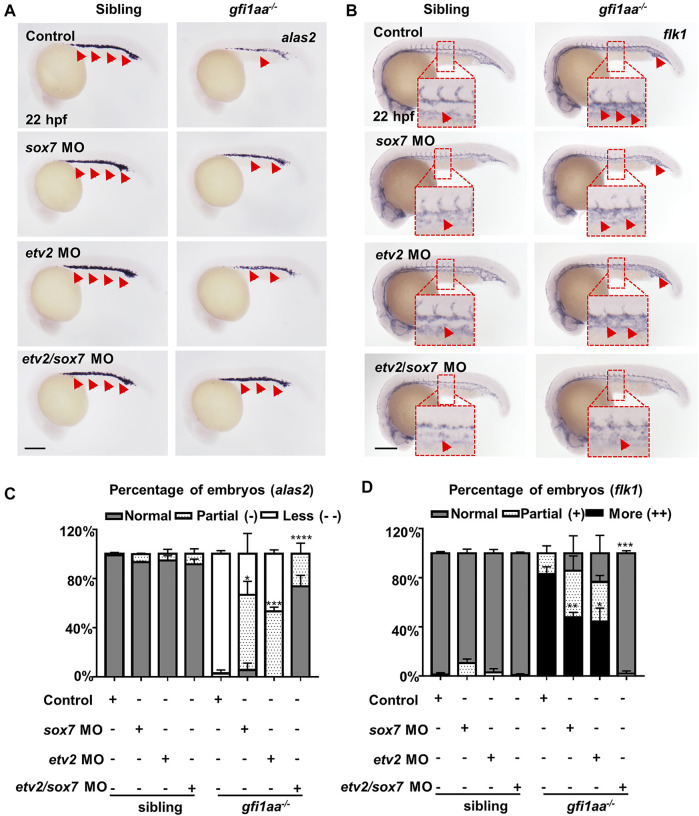
We were eager to know whether downregulation of sox7 rescued the blood deficiency of the gfi1aa −/− mutant. We injected sox7 MO into gfi1aa −/− mutants and found that alas2 + erythroid cell reduction and flk1 + endothelial cell augmentation within the intermediate cell mass (ICM) region could be partially restored (Supplementary Figures S6A–D). It has been reported that etv2 MO can also partially rescue gfi1aa mutant primitive hematopoietic defects (Moore et al., 2018). These data suggest that Gfi1aa targets not only etv2 but also sox7 to promote primitive erythrocyte differentiation from the hemangioblast.
Given the fact that either etv2 or sox7 partially rescued the primitive erythrocytes of the gfi1aa mutant, we speculated that sox7 might cooperate with etv2 for Gfi1aa regulated primitive erythropoiesis. To test this hypothesis, we knocked down both genes in gfi1aa −/− mutants to see if the hemangioblast differentiation defect could be further rescued. As a high dosage of etv2 MO could cause severe vasculature defects of developing embryos (Sumanas and Lin, 2006), the cooperative effect on endothelial cells between etv2 MO and sox7 MO would be masked. Owing to this, we decreased etv2 MO concentration and found 0.01 pmol etv2 MO was enough to partially rescue the erythroid defect in gfi1aa mutant but not affect the vasculature which concentration was comparable to sox7 MO (Supplementary Figures S7A–D). We therefore utilized the low dosage etv2 MO to involve in the double knockdown. Results showed that alas2 + erythroid cell reduction and flk1+ endothelial cells augmentation in gfi1aa −/− mutants could be almost completely restored (Figures 4A–D). These data suggest that the two transcription factors, sox7 and etv2, act cooperatively downstream of Gfi1aa during hemangioblast differentiation.
FIGURE 4.
sox7 and etv2 act cooperatively to rescue the hematopoietic defect of gfi1aa mutant (A, B) Expression of alas2 (A) and flk1 (B) in siblings and gfi1aa −/− mutants injected with 0.5 pmol sox7 MO, 0.005 pmol etv2 MO, 0.5 pmol sox7 MO with 0.005 pmol etv2 MO or control. The red arrows indicated WISH signals and the red boxes indicated the magnification of ICM region. Scale bar: 200 μm (C,D) Analysis of alas2 (C) and flk1 (D) expression in siblings and gfi1aa −/− mutants rescued by sox7 MO, etv2 MO and sox7 MO with etv2 MO. The asterisks indicate the statistical difference of the rescued proportion by MO compared to gfi1aa −/− (Three independent experiments were performed, ****p < 0.0001, ***p < 0.001, **p < 0.01, *p < 0.05, t-test, n ≥ 10 embryos for each group).
Gfi1aa Depends on Lsd1 to Repress etv2 and sox7 During Primitive Hemangioblast Differentiation
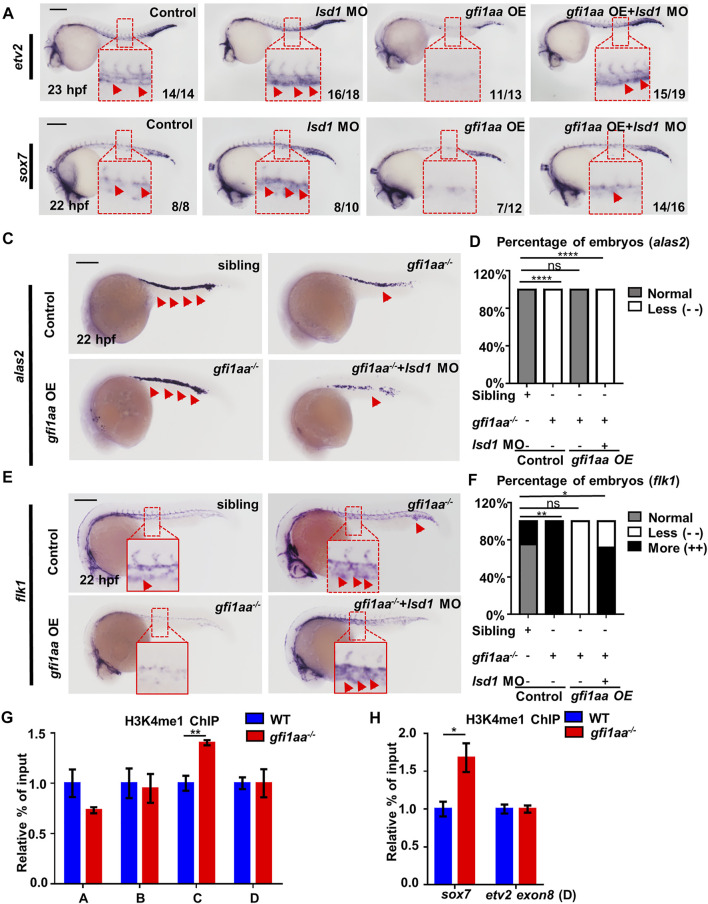
As lsd1-deficient zebrafish (Takeuchi et al., 2015) phenocopied gfi1aa −/− mutants during primitive hematopoiesis and Gfi1aa could interact with Lsd1 in zebrafish (Wu et al., 2021), we speculated that Gfi1aa regulated hemangioblast differentiation into primitive erythrocytes was dependent upon Lsd1. We first inhibited lsd1 to assess Gfi1aa repression of etv2 and sox7, and found that the repression was indeed dependent on lsd1. Inhibited etv2 and sox7 expression levels in gfi1aa-overexpressing (gfi1aa-OE) embryos were rescued by downregulating lsd1 (Figures 5A,B). This suggests that Gfi1aa requires Lsd1 to function as a transcriptional repressor. Furthermore, gfi1aa-OE rescued decreased alas2 and increased flk1 in gfi1aa −/− mutants, but downregulation of lsd1 in gfi1aa-OE gfi1aa −/− mutants showed similar expression patterns to gfi1aa −/− mutants so that counteracted the restoration by gfi1aa-OE (Figures 5C–F), suggesting that Gfi1aa requires Lsd1 to function in promotion of hemangioblast differentiation into the primitive erythroid lineage.
FIGURE 5.
Gfi1aa targets etv2 and sox7 in an Lsd1-dependent manner (A,B) Gfi1 repression activity on etv2 and sox7 transcription depends on Lsd1. Expression of etv2 (A) and sox7 (B) in WT, lsd1 MO, gfi1aa overexpression (gfi1aa-OE) embryos, and gfi1aa-OE embryos co-injected with 1 pmol lsd1 MO. gfi1aa-OE embryos were the progenies of hsp-gfi1aa-eGFP transgenic fish. The red boxes indicate the magnification of etv2 signals (A) and sox7 signals (B) in the ICM region. n ≥ 10 embryos for each group. The numbers in the bottom right corner indicate the percentage of embryos exhibiting the representative expression of indicated genes. Scale bar: 200 μm (C,D) Expression (C) and analysis (D) of erythroid marker alas2 in sibling, gfi1aa −/− mutant, gfi1aa-OE rescued gfi1aa −/− mutant and gfi1aa −/− mutant with gfi1aa-OE and lsd1-MO at 22 hpf (E,F) Expression (E) and analysis (F) of endothelial marker flk1 in sibling, gfi1aa −/− mutant, gfi1aa-OE rescued gfi1aa −/− mutant and gfi1aa −/− mutant with gfi1aa-OE and lsd1-MO at 22 hpf. The red boxes indicate the magnification of ICM region, and the red arrows indicate WISH signals (****p < 0.0001, **p < 0.01, *p < 0.05, ns, no significant, Fisher exact tests, n ≥ 10 embryos for each group). Scale bar: 200 μm (G,H) H3K4me1 levels at etv2 intron-2 locus and sox7 promoter were inhibited by Gfi1aa. ChIP-qPCR showed H3K4me1 level at etv2 gene loci (G) and sox7 promoter (H) in AB and gfi1aa −/− mutant embryos (The error bars represent three technical replicates and two independent experiments were performed, mean ± SEM; **p < 0.01; t-test).
Lsd1 is a histone demethylase that has been shown to repress etv2 by alteration of associated H3K4 methylation during zebrafish primitive hematopoiesis (Takeuchi et al., 2015). Therefore, H3K4 methylation of etv2 and sox7 in gfi1aa −/− was assessed. The results showed H3K4me1 levels (primed and active enhancers marker (Heintzman et al., 2007; (Mercer et al., 2011)) to be upregulated in the regulatory regions of the two genes in gfi1aa −/− mutants (Figures 5G,H), suggesting that Gfi1aa and Lsd1 downregulate etv2 and sox7 by suppressing their H3K4me1 levels.
The above data demonstrate Gfi1aa to depend on Lsd1 to repress downstream etv2 and sox7 by altering H3K4 methylation during primitive hemangioblast differentiation.
Discussion
In this study, we demonstrated complex roles for gfi1(s) in primitive erythropoiesis by genetic analysis of gfi1 single, double, and triple mutants. We revealed that gfi1aa played a predominant role in regulating hemangioblast differentiation, and gfi1ab, similar to gfi1b, played a compensatory role. Further, by bioinformatics assays and genetic analysis, we identified sox7 and etv2 as two key downstream targets of Gfi1aa, as Gfi1aa directly bound to the regulatory regions of the two transcription factors and suppressed their expression. Gfi1aa suppressed downstream target expressions in an Lsd1-dependent manner by altering their H3K4 methylation status. The study reveals that the Gfi1aa/Lsd1-dependent etv2 and sox7 suppression facilitates hemangioblast differentiation into primitive erythrocytes (Figure 6), which provides new insights into the generation of the first blood cells.
FIGURE 6.
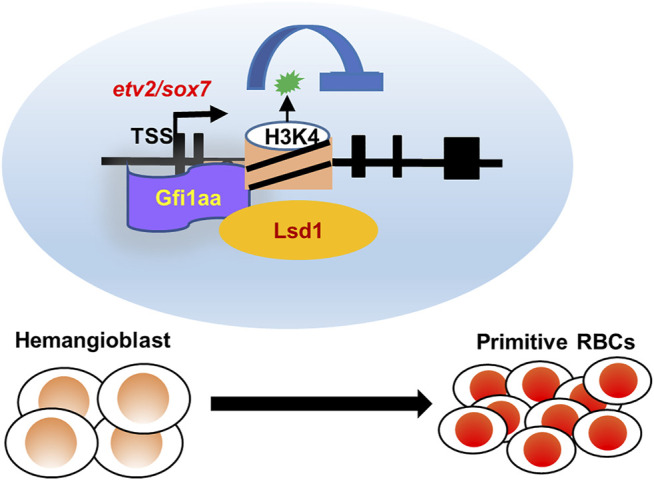
Working model of Gfi1aa/Lsd1-etv2/sox7 in primitive erythropoiesis. Gfi1aa/Lsd1-etv2/sox7 regulatory modules in hemangioblast differentiation into primitive red blood cells.
In mammals, both Gfi1 and Gfi1b are major regulators of hematopoiesis (Hock and Orkin, 2006; (van der Meer et al., 2010; (Moroy et al., 2015). Gfi1 is mainly involved in HSC self-renewal (Hock et al., 2004; (Zeng et al., 2004), lymphoid development (Yucel et al., 2003), and neutrophil differentiation (Hock et al., 2003), whereas Gfi1b is required for erythropoiesis (Saleque et al., 2002). GFI1B can compensate for GFI1 function in definitive hematopoiesis when GFI1 has lost function (Fiolka et al., 2006). Zebrafish has three Gfi1 members: Gfi1aa, Gfi1ab, and Gfi1b. By genetic analysis of gfi1 single, double, and triple mutants, we demonstrated complex roles for gfi1(s) in primitive erythropoiesis. We generated a gfi1ab −/− mutant which showed no hematopoietic defect. It is reported that gfi1ab is ectopically expressed in the ICM region of gfi1aa qmc551 mutants (Thambyrajah et al., 2016b; (Moore et al., 2018), our WISH further showed it expressed in the ICM region of all gfi1aa-related mutants, suggesting its compensatory role for gfi1aa function. With genetic evidence, we found that gfi1aa-related double and triple mutants have severe defects in primitive erythropoiesis. We hence concluded that gfi1aa played a predominant role, and gfi1ab, similar to gfi1b, played a compensatory role in regulating hemangioblast differentiation. Our results suggest differing and redundant roles for three gfi1 members in hematopoiesis.
Both Etv2 and Sox7 are hemangioblast markers that control hematopoietic and endothelial cell emergence (Gandillet et al., 2009; (Kataoka et al., 2011; (Costa et al., 2012; (Sumanas and Choi, 2016). Knockdown of Sox7 reduced both hematopoietic and endothelial cells (Gandillet et al., 2009; (Costa et al., 2012), whereas its overexpression increased endothelial markers (Costa et al., 2012). Similarly, Etv2-deficient mice (Lee et al., 2008) and etv2 zebrafish mutants (Pham et al., 2007) displayed both blood and endothelial cells disruption, while enforced expression of etv2 resulted in persistent endothelial specification (Sumanas and Lin, 2006; (Hayashi et al., 2012). Herein, we demonstrated both etv2 and sox7 to be upregulated in all gfi1aa-related mutants, while downregulation of the genes rescued the hematopoietic defect in the gfi1aa −/− mutant. Notably, both genes were directly targeted and suppressed by Gfi1aa in an lsd1-dependent manner. In previously reported lsd1 zebrafish mutant, etv2 is upregulated, and when downregulated, it rescues the hematopoietic defect of lsd1 mutants (Takeuchi et al., 2015). Moreover, lsd1 MO and gfi1aa −/− mutant exhibited a similar increase of H3K4me1 status at etv2 intron2, suggesting the co-regulation of Gfi1aa and Lsd1 on etv2. Our genetic and molecular analysis demonstrated the likely interplay among Gfi1aa, Lsd1, as well as sox7 and etv2 during primitive hematopoiesis. At the onset of primitive hematopoiesis, Gfi1aa/Lsd1 inhibits etv2 and sox7 by preventing maintenance of the endothelial characteristics of hemangioblasts. etv2 and sox7, repressed by Gfi1aa and Lsd1 cooperation, synergistically control hemangioblast differentiation. We further knocked down etv2 and sox7 in gfi1aa/1b DM and gfi1 TM mutants, whereas etv2/sox7 MO partially restored the alas2 + erythroid cells and flk1 + endothelial cells in these mutants (Supplementary Figures 8A–D), suggesting etv2 and sox7 are indeed the targets of Gfi1aa whereas other factors (e.g., flk1, cdh5, and egfl7) or pathways involve in hematopoiesis regulation remain further investigation.
During the definitive wave, hematopoietic stem cells (HSC) are derived from the hemogenic endothelium (HE) in the ventral wall of the dorsal aorta (VDA) by a process of endothelial to hematopoietic transition (EHT) (Bertrand et al., 2010). HSC-forming HE was derived from the arterial endothelium (Bonkhofer et al., 2019). For mouse embryonic HSC development, GFI1 and GFI1B, which are regulated by RUNX1 (Lancrin et al., 2012), inhibit endothelial programs to facilitate the EHT process of HSC development by recruiting the chromatin remodeler LSD1 (Thambyrajah et al., 2016a). Here, we demonstrated that Gfi1aa is dependent on Lsd1 for transcriptional suppression of endothelial factors in hemangioblast differentiation to primitive hematopoiesis. Based on current knowledge, the initial developmental processes for primitive and definitive hematopoiesis seem similar, as hematopoietic cells in two waves are both derived from bi-potential (or multi-potential) progenitors with potent endothelial specification. Since Gfi1/Lsd1 suppresses endothelial specification in both definitive and primitive waves, this suggests the regulatory module of Gfi1/Lsd1 might be a confluent of the two distinct hematopoietic waves, which may be conserved across species. It is possible that primitive hematopoietic cells, derived from hemangioblasts, share a similar molecular progression to the definitive wave of EHT. Thus, the distinct hematopoiesis waves may converge to the Gfi1(s)/Lsd1 module or even Gfi1(s)/Lsd1-etv2/sox7 involved molecular regulatory pathway.
Taken together, the results of our study demonstrate that the regulatory module Gfi1aa-Lsd1-etv2/sox7 plays a pivotal role in downregulating endothelial genes to promote hemangioblast differentiation into primitive erythrocytes. These results elucidate the genetic and epigenetic regulatory mechanisms of Gfi1(s) on the process of how primitive hematopoiesis begins with hemangioblasts. Since Gfi1/Lsd1 suppresses endothelial specification of both definitive and primitive waves, it suggests the regulatory module of Gfi1/Lsd1 might be a confluent of the two distinct hematopoietic waves. Thus, both hematopoiesis waves may converge to the Gfi1(s)/Lsd1 involved molecular regulatory pathway.
Acknowledgments
We thank Dr. Jingwei Xiong and Dr. Bo Zhang for providing CRISPR/Cas9-related plasmid (gRNA-pMD19-T) and protocol.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://www.ncbi.nlm.nih.gov/, GSE181395.
Ethics Statement
The animal study was reviewed and approved by the South China University of Technology Animal Advisory Committee.
Author Contributions
Contribution: MW and YZ designed the experiments, analyzed data and wrote the manuscript; MW performed most of the experiment. QC validated the gfi1aasmu10 mutant phenotype. YX performed the WISH and genotyping; J.Lian helped the ChIP assay. PM generated gfi1aasmu10 mutant, YL generated gfi1bsmu10 mutant and JL generated gfi1absmu12 mutant.
Funding
This work was supported by the National Key Research and Development Program of China (2018YFA0800200 and 2018YFA0801000), National Natural Science Foundation of China (31922023 and 31601172), Guangdong Province Universities and Colleges Pearl River Scholar Funded Scheme (2019), and Guangdong Natural Science Foundation (2016A030310069).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcell.2021.786426/full#supplementary-material
References
- Baron M. H., Isern J., Fraser S. T. (2012). The Embryonic Origins of Erythropoiesis in Mammals. Blood 119, 4828–4837. 10.1182/blood-2012-01-153486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand J. Y., Chi N. C., Santoso B., Teng S., Stainier D. Y. R., Traver D. (2010). Haematopoietic Stem Cells Derive Directly from Aortic Endothelium during Development. Nature 464, 108–111. 10.1038/nature08738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonkhofer F., Rispoli R., Pinheiro P., Krecsmarik M., Schneider-Swales J., Tsang I. H. C., et al. (2019). Blood Stem Cell-Forming Haemogenic Endothelium in Zebrafish Derives from Arterial Endothelium. Nat. Commun. 10, 3577. 10.1038/s41467-019-11423-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cermenati S., Moleri S., Cimbro S., Corti P., Del Giacco L., Amodeo R., et al. (2008). Sox18 and Sox7 Play Redundant Roles in Vascular Development. Blood 111, 2657–2666. 10.1182/blood-2007-07-100412 [DOI] [PubMed] [Google Scholar]
- Chang N., Sun C., Gao L., Zhu D., Xu X., Zhu X., et al. (2013). Genome Editing with RNA-Guided Cas9 Nuclease in Zebrafish Embryos. Cell Res 23, 465–472. 10.1038/cr.2013.45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen A. T., Zon L. I. (2009). Zebrafish Blood Stem Cells. J. Cel. Biochem. 108, 35–42. 10.1002/jcb.22251 [DOI] [PubMed] [Google Scholar]
- Cooney J. D., Hildick-Smith G. J., Shafizadeh E., McBride P. F., Carroll K. J., Anderson H., et al. (2013). Teleost Growth Factor independence (Gfi) Genes Differentially Regulate Successive Waves of Hematopoiesis. Developmental Biol. 373, 431–441. 10.1016/j.ydbio.2012.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa G., Mazan A., Gandillet A., Pearson S., Lacaud G., Kouskoff V. (2012). SOX7 Regulates the Expression of VE-Cadherin in the Haemogenic Endothelium at the Onset of Haematopoietic Development. Development 139, 1587–1598. 10.1242/dev.071282 [DOI] [PubMed] [Google Scholar]
- Dobin A., Davis C. A., Schlesinger F., Drenkow J., Zaleski C., Jha S., et al. (2013). STAR: Ultrafast Universal RNA-Seq Aligner. Bioinformatics 29, 15–21. 10.1093/bioinformatics/bts635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufourcq P., Rastegar S., Strähle U., Blader P. (2004). Parapineal Specific Expression of Gfi1 in the Zebrafish Epithalamus. Gene Expr. Patterns 4, 53–57. 10.1016/s1567-133x(03)00148-0 [DOI] [PubMed] [Google Scholar]
- Fiolka K., Hertzano R., Vassen L., Zeng H., Hermesh O., Avraham K. B., et al. (2006). Gfi1 and Gfi1b Act Equivalently in Haematopoiesis, but Have Distinct, Non‐overlapping Functions in Inner Ear Development. EMBO Rep. 7, 326–333. 10.1038/sj.embor.7400618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandillet A., Serrano A. G., Pearson S., Lie-A-Ling M., Lacaud G., Kouskoff V. (2009). Sox7-sustained Expression Alters the Balance between Proliferation and Differentiation of Hematopoietic Progenitors at the Onset of Blood Specification. Blood 114, 4813–4822. 10.1182/blood-2009-06-226290 [DOI] [PubMed] [Google Scholar]
- Garcia M. D., Larina I. V. (2014). Vascular Development and Hemodynamic Force in the Mouse Yolk Sac. Front. Physiol. 5, 308. 10.3389/fphys.2014.00308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gering M., Rodaway A. R. F., Göttgens B., Patient R. K., Green A. R. (1998). The SCL Gene Specifies Haemangioblast Development from Early Mesoderm. EMBO J. 17, 4029–4045. 10.1093/emboj/17.14.4029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart A., Melet F., Grossfeld P., Chien K., Jones C., Tunnacliffe A., et al. (2000). Fli-1 Is Required for Murine Vascular and Megakaryocytic Development and Is Hemizygously Deleted in Patients with Thrombocytopenia. Immunity 13, 167–177. 10.1016/s1074-7613(00)00017-0 [DOI] [PubMed] [Google Scholar]
- Hayashi M., Pluchinotta M., Momiyama A., Tanaka Y., Nishikawa S.-I., Kataoka H. (2012). Endothelialization and Altered Hematopoiesis by Persistent Etv2 Expression in Mice. Exp. Hematol. 40, 738–750. e711. 10.1016/j.exphem.2012.05.012 [DOI] [PubMed] [Google Scholar]
- Heintzman N. D., Stuart R. K., Hon G., Fu Y., Ching C. W., Hawkins R. D., et al. (2007). Distinct and Predictive Chromatin Signatures of Transcriptional Promoters and Enhancers in the Human Genome. Nat. Genet. 39, 311–318. 10.1038/ng1966 [DOI] [PubMed] [Google Scholar]
- Hock H., Hamblen M. J., Rooke H. M., Schindler J. W., Saleque S., Fujiwara Y., et al. (2004). Gfi-1 Restricts Proliferation and Preserves Functional Integrity of Haematopoietic Stem Cells. Nature 431, 1002–1007. 10.1038/nature02994 [DOI] [PubMed] [Google Scholar]
- Hock H., Hamblen M. J., Rooke H. M., Traver D., Bronson R. T., Cameron S., et al. (2003). Intrinsic Requirement for Zinc finger Transcription Factor Gfi-1 in Neutrophil Differentiation. Immunity 18, 109–120. 10.1016/s1074-7613(02)00501-0 [DOI] [PubMed] [Google Scholar]
- Hock H., Orkin S. H. (2006). Zinc-finger Transcription Factor Gfi-1: Versatile Regulator of Lymphocytes, Neutrophils and Hematopoietic Stem Cells. Curr. Opin. Hematol. 13, 1–6. 10.1097/01.moh.0000190111.85284.8f [DOI] [PubMed] [Google Scholar]
- Jin S.-W., Beis D., Mitchell T., Chen J.-N., Stainier D. Y. R. (2005). Cellular and Molecular Analyses of Vascular Tube and Lumen Formation in Zebrafish. Development 132, 5199–5209. 10.1242/dev.02087 [DOI] [PubMed] [Google Scholar]
- Kaipainen A., Korhonen J., Mustonen T., van Hinsbergh V. W., Fang G. H., Dumont D., et al. (1995). Expression of the Fms-like Tyrosine Kinase 4 Gene Becomes Restricted to Lymphatic Endothelium during Development. Proc. Natl. Acad. Sci. 92, 3566–3570. 10.1073/pnas.92.8.3566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataoka H., Hayashi M., Nakagawa R., Tanaka Y., Izumi N., Nishikawa S., et al. (2011). Etv2/ER71 Induces Vascular Mesoderm from Flk1+PDGFRα+ Primitive Mesoderm. Blood 118, 6975–6986. 10.1182/blood-2011-05-352658 [DOI] [PubMed] [Google Scholar]
- Lacaud G., Kouskoff V. (2017). Hemangioblast, Hemogenic Endothelium, and Primitive versus Definitive Hematopoiesis. Exp. Hematol. 49, 19–24. 10.1016/j.exphem.2016.12.009 [DOI] [PubMed] [Google Scholar]
- Lancrin C., Mazan M., Stefanska M., Patel R., Lichtinger M., Costa G., et al. (2012). GFI1 and GFI1B Control the Loss of Endothelial Identity of Hemogenic Endothelium during Hematopoietic Commitment. Blood 120, 314–322. 10.1182/blood-2011-10-386094 [DOI] [PubMed] [Google Scholar]
- Lancrin C., Sroczynska P., Stephenson C., Allen T., Kouskoff V., Lacaud G. (2009). The Haemangioblast Generates Haematopoietic Cells through a Haemogenic Endothelium Stage. Nature 457, 892–895. 10.1038/nature07679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D., Park C., Lee H., Lugus J. J., Kim S. H., Arentson E., et al. (2008). ER71 Acts Downstream of BMP, Notch, and Wnt Signaling in Blood and Vessel Progenitor Specification. Cell Stem Cell 2, 497–507. 10.1016/j.stem.2008.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D., Wang Z., Xiao A., Zhang Y., Li W., Zu Y., et al. (2014). Efficient Gene Targeting in Zebrafish Mediated by a Zebrafish-Codon-Optimized Cas9 and Evaluation of Off-Targeting Effect. J. Genet. Genomics 41, 43–46. 10.1016/j.jgg.2013.11.004 [DOI] [PubMed] [Google Scholar]
- Liu F., Patient R. (2008). Genome-wide Analysis of the Zebrafish ETS Family Identifies Three Genes Required for Hemangioblast Differentiation or Angiogenesis. Circ. Res. 103, 1147–1154. 10.1161/CIRCRESAHA.108.179713 [DOI] [PubMed] [Google Scholar]
- Liu F., Walmsley M., Rodaway A., Patient R. (2008). Fli1 Acts at the Top of the Transcriptional Network Driving Blood and Endothelial Development. Curr. Biol. 18, 1234–1240. 10.1016/j.cub.2008.07.048 [DOI] [PubMed] [Google Scholar]
- Love M. I., Huber W., Anders S. (2014). Moderated Estimation of Fold Change and Dispersion for RNA-Seq Data with DESeq2. Genome Biol. 15, 550. 10.1186/s13059-014-0550-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugus J. J., Chung Y. S., Mills J. C., Kim S.-I., Grass J. A., Kyba M., et al. (2007). GATA2 Functions at Multiple Steps in Hemangioblast Development and Differentiation. Development 134, 393–405. 10.1242/dev.02731 [DOI] [PubMed] [Google Scholar]
- Mercer E. M., Lin Y. C., Benner C., Jhunjhunwala S., Dutkowski J., Flores M., et al. (2011). Multilineage Priming of Enhancer Repertoires Precedes Commitment to the B and Myeloid Cell Lineages in Hematopoietic Progenitors. Immunity 35, 413–425. 10.1016/j.immuni.2011.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore C., Richens J. L., Hough Y., Ucanok D., Malla S., Sang F., et al. (2018). Gfi1aa and Gfi1b Set the Pace for Primitive Erythroblast Differentiation from Hemangioblasts in the Zebrafish Embryo. Blood Adv. 2, 2589–2606. 10.1182/bloodadvances.2018020156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Möröy T., Vassen L., Wilkes B., Khandanpour C. (2015). From Cytopenia to Leukemia: the Role of Gfi1 and Gfi1b in Blood Formation. Blood 126, 2561–2569. 10.1182/blood-2015-06-655043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray P. (1932). The Development In Vitro of the Blood of the Early Chick Embryo. Proc. R. Soc. Lond. B. 111, 497–521. 10.1098/rspb.1932.0070 [DOI] [Google Scholar]
- Parker L. H., Schmidt M., Jin S.-W., Gray A. M., Beis D., Pham T., et al. (2004). The Endothelial-Cell-Derived Secreted Factor Egfl7 Regulates Vascular Tube Formation. Nature 428, 754–758. 10.1038/nature02416 [DOI] [PubMed] [Google Scholar]
- Patterson L. J., Gering M., Eckfeldt C. E., Green A. R., Verfaillie C. M., Ekker S. C., et al. (2007). The Transcription Factors Scl and Lmo2 Act Together during Development of the Hemangioblast in Zebrafish. Blood 109, 2389–2398. 10.1182/blood-2006-02-003087 [DOI] [PubMed] [Google Scholar]
- Pham V. N., Lawson N. D., Mugford J. W., Dye L., Castranova D., Lo B., et al. (2007). Combinatorial Function of ETS Transcription Factors in the Developing Vasculature. Developmental Biol. 303, 772–783. 10.1016/j.ydbio.2006.10.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleque S., Cameron S., Orkin S. H. (2002). The Zinc-finger Proto-Oncogene Gfi-1b Is Essential for Development of the Erythroid and Megakaryocytic Lineages. Genes Dev. 16, 301–306. 10.1101/gad.959102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleque S., Kim J., Rooke H. M., Orkin S. H. (2007). Epigenetic Regulation of Hematopoietic Differentiation by Gfi-1 and Gfi-1b Is Mediated by the Cofactors CoREST and LSD1. Mol. Cel 27, 562–572. 10.1016/j.molcel.2007.06.039 [DOI] [PubMed] [Google Scholar]
- Spyropoulos D. D., Pharr P. N., Lavenburg K. R., Jackers P., Papas T. S., Ogawa M., et al. (2000). Hemorrhage, Impaired Hematopoiesis, and Lethality in Mouse Embryos Carrying a Targeted Disruption of the Fli1 Transcription Factor. Mol. Cel Biol 20, 5643–5652. 10.1128/mcb.20.15.5643-5652.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumanas S., Choi K. (2016). ETS Transcription Factor ETV2/ER71/Etsrp in Hematopoietic and Vascular Development. Curr. Top. Dev. Biol. 118, 77–111. 10.1016/bs.ctdb.2016.01.005 [DOI] [PubMed] [Google Scholar]
- Sumanas S., Jorniak T., Lin S. (2005). Identification of Novel Vascular Endothelial-specific Genes by the Microarray Analysis of the Zebrafish Cloche Mutants. Blood 106, 534–541. 10.1182/blood-2004-12-4653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumanas S., Lin S. (2006). Ets1-related Protein Is a Key Regulator of Vasculogenesis in Zebrafish. Plos Biol. 4, e10. 10.1371/journal.pbio.0040010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi M., Fuse Y., Watanabe M., Andrea C.-S., Takeuchi M., Nakajima H., et al. (2015). LSD1/KDM1A Promotes Hematopoietic Commitment of Hemangioblasts through Downregulation of Etv2. Proc. Natl. Acad. Sci. USA 112, 13922–13927. 10.1073/pnas.1517326112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thambyrajah R., Mazan M., Patel R., Moignard V., Stefanska M., Marinopoulou E., et al. (2016a). GFI1 Proteins Orchestrate the Emergence of Haematopoietic Stem Cells through Recruitment of LSD1. Nat. Cel Biol 18, 21–32. 10.1038/ncb3276 [DOI] [PubMed] [Google Scholar]
- Thambyrajah R., Ucanok D., Jalali M., Hough Y., Wilkinson R. N., McMahon K., et al. (2016b). A Gene Trap Transposon Eliminates Haematopoietic Expression of Zebrafish Gfi1aa, but Does Not Interfere with Haematopoiesis. Developmental Biol. 417, 25–39. 10.1016/j.ydbio.2016.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thisse C., Thisse B. (2008). High-resolution In Situ Hybridization to Whole-Mount Zebrafish Embryos. Nat. Protoc. 3, 59–69. 10.1038/nprot.2007.514 [DOI] [PubMed] [Google Scholar]
- van der Meer L. T., Jansen J. H., van der Reijden B. A. (2010). Gfi1 and Gfi1b: Key Regulators of Hematopoiesis. Leukemia 24, 1834–1843. 10.1038/leu.2010.195 [DOI] [PubMed] [Google Scholar]
- Veldman M. B., Lin S. (2012). Etsrp/Etv2 Is Directly Regulated by Foxc1a/b in the Zebrafish Angioblast. Circ. Res. 110, 220–229. 10.1161/CIRCRESAHA.111.251298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velinder M., Singer J., Bareyan D., Meznarich J., Tracy C. M., Fulcher J. M., et al. (2016). GFI1 Functions in Transcriptional Control and Cell Fate Determination Require SNAG Domain Methylation to Recruit LSD1. Biochem. J. 473, 3355–3369. 10.1042/BCJ20160558 [DOI] [PubMed] [Google Scholar]
- Vogeli K. M., Jin S.-W., Martin G. R., Stainier D. Y. R. (2006). A Common Progenitor for Haematopoietic and Endothelial Lineages in the Zebrafish Gastrula. Nature 443, 337–339. 10.1038/nature05045 [DOI] [PubMed] [Google Scholar]
- Wei W., Wen L., Huang P., Zhang Z., Chen Y., Xiao A., et al. (2008). Gfi1.1 Regulates Hematopoietic Lineage Differentiation during Zebrafish Embryogenesis. Cel Res 18, 677–685. 10.1038/cr.2008.60 [DOI] [PubMed] [Google Scholar]
- Westerfield M. (2000). The Zebrafish Book. A Guide for the Laboratory Use of Zebrafish (Danio rerio). 4th ed. Eugene: Univ. of Oregon Press. [Google Scholar]
- Wu M., Xu Y., Li J., Lian J., Chen Q., Meng P., et al. (2021). Genetic and Epigenetic Orchestration of Gfi1aa-Lsd1-Cebpα in Zebrafish Neutrophil Development. Development 148 (17), dev199516. 10.1242/dev.199516 [DOI] [PubMed] [Google Scholar]
- Yücel R., Karsunky H., Klein-Hitpass L., Möröy T. (2003). The Transcriptional Repressor Gfi1 Affects Development of Early, Uncommitted C-Kit+ T Cell Progenitors and CD4/CD8 Lineage Decision in the Thymus. J. Exp. Med. 197, 831–844. 10.1084/jem.20021417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng H., Yücel R., Kosan C., Klein-Hitpass L., Möröy T. (2004). Transcription Factor Gfi1 Regulates Self-Renewal and Engraftment of Hematopoietic Stem Cells. EMBO J. 23, 4116–4125. 10.1038/sj.emboj.7600419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Zhou B., Pache L., Chang M., Khodabakhshi A. H., Tanaseichuk O., et al. (2019). Metascape Provides a Biologist-Oriented Resource for the Analysis of Systems-Level Datasets. Nat. Commun. 10, 1523. 10.1038/s41467-019-09234-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://www.ncbi.nlm.nih.gov/, GSE181395.