Abstract
Background and Aims: Stenotrophomonas maltophilia is increasingly found in critically ill patients, but it is considered a pathogen of limited pathogenicity and therefore it is not often targeted. We systematically evaluated risk factors for S. maltophilia pneumonia in ICU patients for better clinical management.
Methods: Prospective and retrospective studies of S. maltophilia infection in the ICU from database establishment to August 8, 2021, were searched through PubMed, web of science, Cochrane Library Embase and CNKI. The literature was independently screened and extracted by two authors according to inclusion and exclusion criteria, evaluated for quality by the NOS scale, and meta-analyzed by stata 14.0 software.
Results: A total of eight studies with a sample size of 2,320 cases were included. Meta-analysis showed that APACHE-II score > 20 (OR = 10.98, 95% CI: 5.67 ~ 21.26), COPD (OR = 3.97, 95% CI: 2.39 ~ 6.61), malignant tumor (OR = 2.15, 95% CI: 1.03 ~ 4.50), mechanical ventilation (OR = 8.75, 95% CI: 2.59 ~ 29.58), tracheotomy (OR = 6.12, 95% CI: 2.06 ~ 18.18), endotracheal intubation (OR = 4.25, 95% CI: 2.30 ~ 7.84), β- Lactamase inhibitors (OR = 9.98, 95% CI: 1.51 ~ 65.96), aminoglycosides (OR = 4.01, 95% CI: 2.06 ~ 7.80), carbapenems (OR = 2.82, 95% CI: 1.49 ~ 5.31), and quinolones (OR = 2.17, 95% CI: 1.21 ~ 3.89) were risk factors for ICU-acquired S. maltophilia pneumonia.
Conclusion: Many risk factors are associated with S. maltophilia pneumonia in ICU patients. Clinical workers should pay more attention to assessing the risk of infection in ICU patients and enhance the prevention and management of high-risk groups, which will help reduce their risk of S. maltophilia infection.
Keywords: Stenotrophomonas maltophilia, ICU-acquired pneumonia, risk factor, meta-analysis, infection
Introduction
S. maltophilia is a non-fermentable Gram-negative bacterium that is an opportunistic agent. It is naturally resistant to many commonly used antibiotics, such as carbapenems and aminoglycosides (1, 2). It is due to such characteristics, in the context of drug resistance, that S. maltophilia is becoming an important pathogen of hospital infections in the ICU, which can lead to infections in the lungs, bloodstream and many other important parts of the body, even life-threatening (3, 4).
According to the CHINET bacterial resistance surveillance data in 2020, S. maltophilia accounted for 2.98% of all strains and ranked 9th, 7th among Gram-negative bacteria, and 3rd among non-fermentative bacteria, after Bacillus immobilis and Pseudomonas aeruginosa. S. maltophilia has the highest resistance to ceftazidime (38.5%), followed by levofloxacin (10.8%), compound sulfamethoxazole (6.7%) and tigecycline (2.7%), and the lowest resistance to minocycline (2.3%) (5). Clinically, it often causes mixed infections with other bacteria, mainly Pseudomonas aeruginosa, Klebsiella pneumoniae, and Acinetobacter baumannii, so S. maltophilia infections are difficult to be treated and the mortality rate is high. Muder et al. (6) reported a mortality rate of 21% in patients with S. maltophilia bacteremia. Paez et al. (7) reported a direct mortality rate of 26.7% due to S. maltophilia infection and a mortality rate of 21–69% associated with S. maltophilia infection.
The study of risk factors for ICU-acquired S. maltophilia pneumonia is of great practical significance for the in-depth study of the hazards of this bacterium and the adoption of appropriate preventive and control measures. Currently, Several studies at home and abroad have investigated the risk factors for the occurrence of S. maltophilia infection in ICU patients, but there are drawbacks such as small sample size and incomplete risk factor indicators, and the significance of clinical guidance is limited. This study aims to systematically evaluate the risk factors of hospital-acquired S. maltophilia pneumonia by Meta-analysis, and provide a theoretical basis for clinical formulation of prevention and control strategies to reduce the morbidity and mortality of S. maltophilia infection.
Materials and Methods
Literature Search Strategy
PubMed, Web of Science, Cochrane Library, Embase and CNKI were searched from the time of database establishment to August 8, 2021. A combination of subject terms, free words, and Boolean logical operators was used for the search terms: Stenotrophomonas maltophilia, hospital-acquired pneumonia, ventilator-associated pneumonia, intensive care, risk factors, etc. The English databases were searched for the following terms: (Stenotrophomonas maltophilia OR S. maltophilia OR SMA) AND (nosocomial infection OR hospital infection OR hospital acquired infection OR cross infection OR VAP OR ventilator associated pneumonia OR ventilator-associated pneumonia) AND (ICU OR Intensive Care OR NICU OR PICU OR CCU) AND (risk factor OR factor). A manual search of relevant content reviews and references of included literature was conducted to identify potential studies that met the inclusion criteria.
Literature Inclusion and Exclusion Criteria
The inclusion criteria were as follows: (1) The type of literature was a cohort study or case-control study published nationally and internationally; (2) The study population was divided into two groups based on whether they were infected with S. maltophilia, and the diagnosis criteria for S. maltophilia pneumonia in this study were described below; (3) Risk factors for S. maltophilia pneumonia in ICU patients were present in the literature, such as comorbid underlying diseases, invasive procedures undergone, and use of broad-spectrum antibiotics; (4) Outcome indicators for risk factors for S. maltophilia pneumonia in ICU patients could be expressed as odds ratios (OR), and their 95% confidence intervals (CI) were calculated. Studies were excluded when meeting one of the following criteria: (1) duplicate reports, conference reports, and reviews; (2) abnormal or missing data; (3) low quality of literature [Newcastle-Ottawa Scale (NOS) score ≤ 3].
In this study, infection and colonization were considered ICU-acquired if they were diagnosed more than 48 h after ICU admission. Pneumonia was defined as follows: (1) new or progressive pulmonary infiltrates. (2) Temperature > 38°C or <36.5°C, leukocyte count >12,000 μl−1 or <4000 μl−1, purulent endotracheal aspirate or sputum. (3) Positive respiratory sample. (4) Decreased oxygenation.
Data Extraction and Quality Evaluation
The retrieved literature was screened by two authors independently according to the inclusion and exclusion criteria, and the following data information was extracted: name of the first author, time of publication, source of the literature, basic characteristics of the included cases, and possible risk factors for S. maltophilia pneumonia in ICU patients. If the opinions of two reviewing authors do not agree, they discuss. If there was still disagreement after discussion, a third party opinion was sought. The quality of the included literature was also evaluated according to NOS score (8), and the evaluation items include three aspects of population selection, comparability and exposure evaluation, with a score out of 9. A score of 7 and above was considered as high-quality literature, 4–6 as moderate quality literature, and 1–3 as low-quality literature.
Data Analysis
Statistical analysis was performed by stata14.0 software. I2 was used to determine the heterogeneity of the included literature, and the fixed-effects model was used when p > 0.1 and I2 < 50%; otherwise, the random-effects model was applied. OR and its 95% CI were calculated for count data, while weighted mean difference (WMD) and its 95% CI were calculated for measurement data, and differences were considered statistically significant at p ≤ 0.05. Sensitivity analysis was performed by calculating the OR and 95% CI for both fixed-effects and random-effects models and comparing the results of the two groups. Sensitivity analysis was performed by changing the data analysis model. If there was no substantial change after the model change (no opposite conclusion was reached after changing the model), the consolidated result was considered to be stable. Begg′s test was used to test for publication bias when the number of included papers for individual risk factor analysis was ≥3.
Results
Literature Search
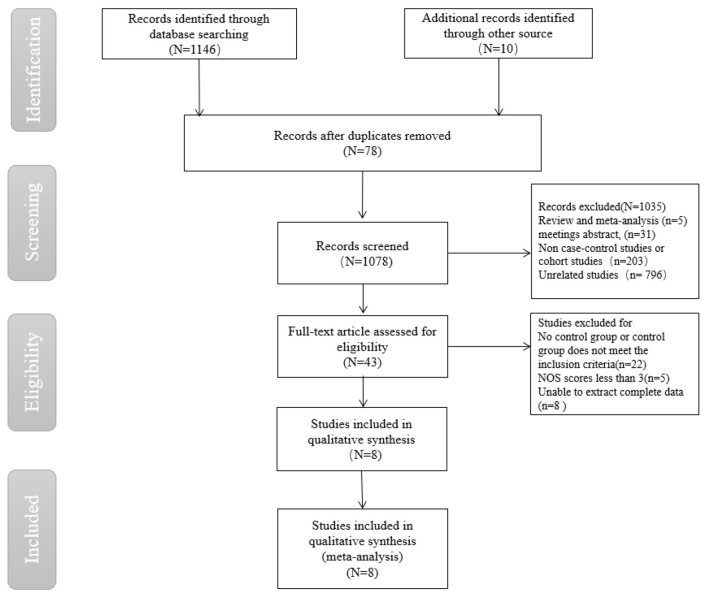
Firstly, 1,156 papers were initially searched in the database through the search strategy, and then 43 papers were selected through title, abstract and keywords, etc. Finally, eight papers (9–16) were further screened by reading the full text, including five papers in English and three papers in Chinese (Figure 1).
Figure 1.
Flow chart of literature screening for meta-analysis on risk factors for acquired Stenotrophomonas maltophilia in intensive care unit.
Baseline Characteristics of the Studies
The eight included papers included in the study were published between 2002 and 2020, seven of which were case-control studies and one was a cohort study involving 2320 patients, 306 in the S. maltophilia-infected group and 2014 in the non-infected group, and 25 exposure factors for S. maltophilia infection were extracted. The quality of the eight papers was evaluated using the NOS scale, including five high-quality papers and tree moderate-quality papers. The basic characteristics of the included literature are shown in Tables 1, 2.
Table 1.
Characteristics of the included studies.
| Study | Year | Design study | Area | Infection group | Non-infection group | Risk factors |
|---|---|---|---|---|---|---|
| Stang et al. (8) | 2002 | Case-control | USA | 26 | 137 | 01.02.03.07.20.21.22.24 |
| Hanes et al. (9) | 2006 | Cohort | France | 30 | 60 | 01.02.07.08.09.11.14.15.16.17.18.21.22.23.24.25 |
| Nseir et al. (10) | 2011 | Case-control | China | 35 | 140 | 01.02.04.11.14.15.16.19.21.23 |
| Xu et al. (11) | 2012 | Case-control | Germany | 36 | 28 | 01.02.03.08.09.10.12.13.14.15.17.18 |
| Guo et al. (12) | 2014 | Case-control | China | 42 | 84 | 01.02.04.06.08.09.11.14.15.16.19 |
| Saugel et al. (13) | 2016 | Case-control | Netherlands | 6 | 15 | 01.02.03.08.09.10.11.13.14.16 |
| Ibn Saied et al. (14) | 2019 | Case-control | China | 29 | 58 | 01.02.03.09.10.11.13.14.17.18.21.22.23.24.25 |
| Lei et al. (15) | 2020 | Case-control | USA | 102 | 1,492 | 01.02.05.06.07.11.12.13.20 |
01, Age, years; 02, Gender; 03, APACHE-II score; 04, APACHE-II score >20; 05, Glasgow score; 06, Glucocorticoid; 07, Length of ICU stay, days; 08, COPD; 09, Diabetes; 10, Malignancy; 11, Cardiovascular disease; 12, kidney dysfunction; 13, Immunosuppression; 14, Mechanical ventilation; 15, Tracheal intubation; 16, Tracheotomy; 17, Central venous catheterization; 18, Urinary catheter; 19, Nasogastric tube; 20, Operation; 21, Carbapenems; 22, β-lactamase inhibitor; 23, Aminoglycosides; 24, Quinolones; 25, Nitroimidazoles.
Table 2.
Risk of bias assessment of the included studies according to the Newcastle-Ottawa Scale (NOS).
| NOS items/Study ID | Hanes et al. | Xu et al. | Saugel et al. | Guo et al. | Scholte et al. | Shi et al. | Saied et al. |
|---|---|---|---|---|---|---|---|
| Is the case definition adequate? | * | * | * | * | * | * | |
| Representativeness of the cases | * | * | * | * | * | * | |
| Selection of controls | * | * | * | * | * | * | |
| Definition of controls | * | * | * | * | * | * | * |
| Compatibility | * | * | * | * | * | * | * |
| Ascertainment of exposure | * | * | * | * | * | * | * |
| Same method of ascertainment for cases and control | * | * | * | * | * | ||
| Non-response rate | * | * | * | * | * | ||
| Total score | 8 | 7 | 7 | 6 | 7 | 6 | 8 |
Representative studies meet this criteria.
Meta-Analysis of Exposure Factors for S. maltophilia Pneumonia
Heterogeneity was tested for exposure factors such as age, gender, APACHE-II score, length of ICU stay, Glasgow score, glucocorticoid use, diabetes, malignancy, cardiovascular disease, renal insufficiency, immunodeficiency disorders, tracheal intubation, indwelling catheters, surgery, and use of carbapenems, quinolones, and aminoglycosides. Heterogeneity was acceptable (p > 0.10, I2 < 50%), and effect sizes were combined using a fixed-effects model. heterogeneity was present for APACHE-II scores >20, COPD, tracheotomy, mechanical ventilation, indwelling nasogastric tube, central venous line, use of β-lactamase inhibitors and nitroimidazole antibiotics (p < 0.10, I2 > 50%), random-effects model combinations of effect sizes were performed.
The meta-analysis showed that risk factors for S. maltophilia pneumonia in the ICU included APACHE-II score > 20 (OR = 10.98, 95% CI: 5.67 ~ 21.26), COPD (OR = 3.97, 95% CI: 2.39 ~ 6.61), malignant tumor (OR = 2.15, 95% CI: 1.03 ~ 4.50), mechanical ventilation (OR = 8.75, 95% CI: 2.59 ~ 29.58), tracheotomy (OR = 6.12, 95% CI: 2.06 ~ 18.18), endotracheal intubation (OR = 4.25, 95% CI: 2.30 ~ 7.84), β- Lactamase inhibitors (OR = 9.98, 95% CI: 1.51 ~ 65.96), aminoglycosides (OR = 4.01, 95% CI: 2.06 ~ 7.80), carbapenems (OR = 2.82, 95% CI: 1.49 ~ 5.31), and quinolones (OR = 2.17, 95% CI: 1.21 ~ 3.89). There was no obvious correlation between risk factors and S. maltophilia pneumonia, such as age, gender, APACHE-II score, length of stay in ICU, combined diabetes, combined cardiovascular disease, combined renal insufficiency, combined immunodeficiency disease, indwelling catheter, operation, central venous line, indwelling nasogastric tube, corticosteroids, and nitroimidazole antibiotics.
Sensitivity Analysis and Publication Bias
Sensitivity analysis suggested that the results of the meta-analysis were stable for all outcome indicators except for two exposure factors, central venous placement and indwelling nasal cannula (Table 3). Begg's test was used to test for publication bias when the number of included papers for individual risk factor analysis was ≥3. The results showed p > 0.05, indicating that the publication bias of included papers was not significant.
Table 3.
Meta-analysis results of risk factors for acquired Stenotrophomonas maltophilia in intensive care unit.
| Exposure factors | Included studies | Heterogeneity | p | Fixed-effect model (FEM) | p | Random-effect model (REM) | p |
|---|---|---|---|---|---|---|---|
| General condition | |||||||
| Age, years | 8 | 0 | 0.93 | −0.76 (−2.62~1.10) | 0.42 | −0.76 (−2.62~1.10) | 0.42 |
| Gender | 8 | 0 | 0.83 | 0.77 (0.59~1.02) | 0.07 | 0.77 (0.58~1.01) | 0.06 |
| APACHE-II score | 3 | 0 | 0.83 | 2.80 (−0.31~5.82) | 0.08 | 2.80 (−0.31~5.82) | 0.08 |
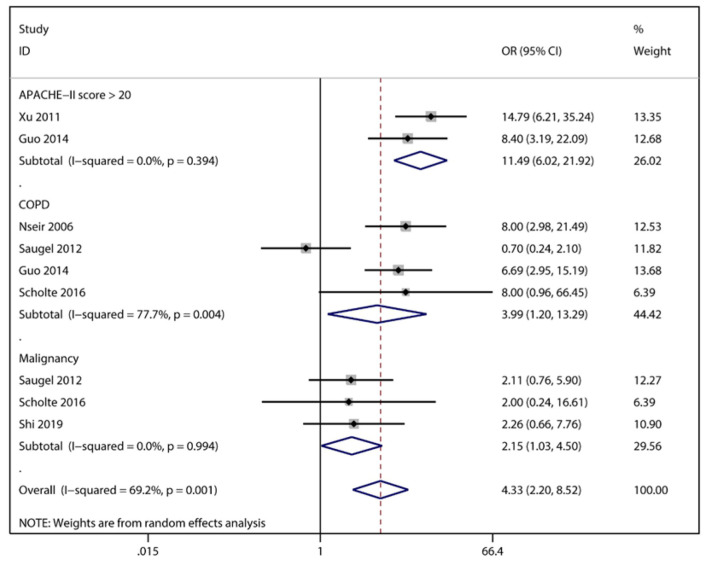
| APACHE-II score >20 | 2 | 0 | 0.39 | 10.98 (5.67~21.26) | <0.001 | 11.49 (6.02~21.92) | <0.001 |
| Glasgow score | 2 | 0 | 0.66 | −0.50 (−1.91~0.90) | 0.49 | −0.50 (−1.91~0.90) | 0.49 |
| Glucocorticoid | 3 | 42 | 0.58 | 0.91 (0.51~1.61) | 0.74 | 0.98 (0.42~2.29) | 0.97 |
| Length of ICU stay, days | 4 | 0 | 0.50 | 1.65 (0.70~2.60) | 0.001 | 1.65 (0.70~2.60) | 0.001 |
| Pre-existing medical conditions | |||||||
| COPD | 4 | 77.8 | 0.004 | 3.97 (2.39~6.61) | <0.001 | 3.99 (1.19~13.32) | 0.03 |
| Diabetes | 5 | 8.1 | 0.36 | 1.50 (0.89~2.63) | 0.13 | 1.47 (0.82~2.61) | 0.20 |
| Malignancy | 3 | 0 | 0.99 | 2.15 (1.03~4.50) | 0.04 | 2.15 (1.03~4.50) | 0.04 |
| Cardiovascular disease | 7 | 48.2 | 0.07 | 0.92 (0.66~1.29) | 0.63 | 1.0 (0.61~1.75) | 0.92 |
| kidney dysfunction | 3 | 0 | 0.87 | 1.20 (0.69~2.07) | 0.52 | 1.21 (0.70~2.07) | 0.50 |
| Immunosuppression | 3 | 49.6 | 0.14 | 1.38 (0.87~2.21) | 0.17 | 1.70 (0.38~7.69) | 0.49 |
| Invasive procedures | |||||||
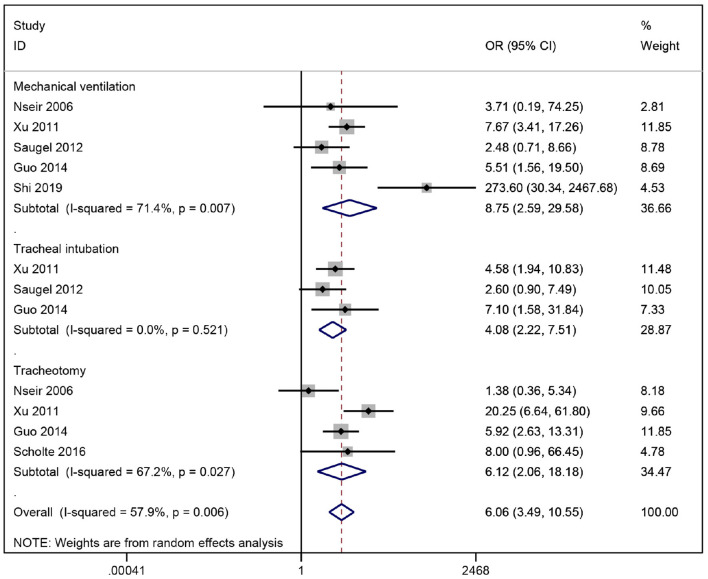
| Mechanical ventilation | 5 | 71.4 | 0.007 | 8.22 (4.82~14.03) | <0.001 | 8.75 (2.59~29.58) | <0.001 |
| Tracheal intubation | 3 | 0 | 0.52 | 4.25 (2.30~7.84) | <0.001 | 4.08 (2.22~7.51) | <0.001 |
| Tracheotomy | 4 | 67.2 | 0.03 | 6.10 (3.54~10.52) | <0.001 | 6.12 (2.06~18.18) | 0.001 |
| Central venous catheterization | 3 | 82.7 | 0.003 | 3.22 (1.62~6.42) | 0.001 | 2.30 (0.37~14.41) | 0.37 |
| Urinary catheter | 3 | 0 | 0.89 | 2.14 (0.79~5.84) | 0.14 | 2.10 (0.77~5.76) | 0.15 |
| Nasogastric tube | 3 | 78.3 | 0.03 | 3.28 (1.85~5.83) | <0.001 | 3.36 (0.95~11.87) | 0.06 |
| Operation | 3 | 0 | 0.75 | 0.78 (0.36~1.70) | 0.53 | 0.80 (0.36~1.76) | 0.57 |
| Antimicrobial agents | |||||||
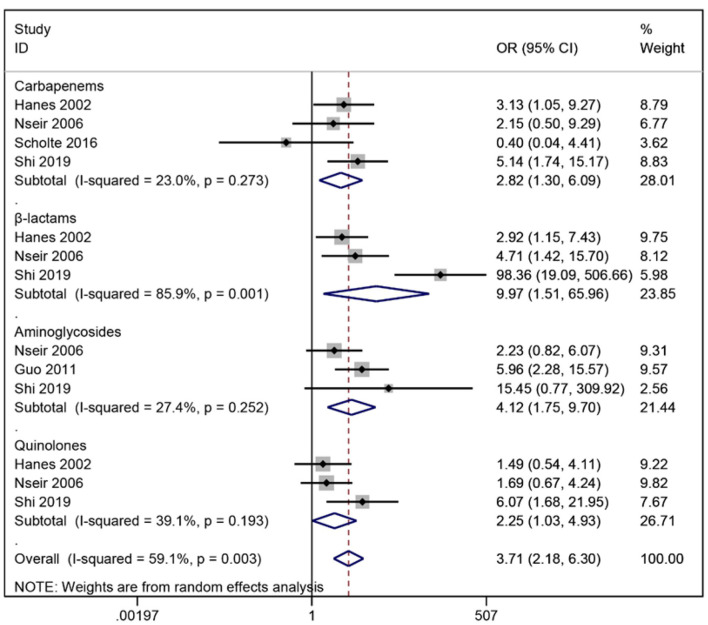
| Carbapenems | 4 | 23 | 0.27 | 2.82 (1.49~5.31) | 0.001 | 2.82 (1.30~6.09) | 0.008 |
| β-lactamase inhibitor | 3 | 85.9 | 0.001 | 7.88 (4.41~14.09) | <0.001 | 9.98 (1.51~65.96) | 0.02 |
| Aminoglycosides | 3 | 27.4 | 0.25 | 4.01 (2.06~7.81) | <0.001 | 4.12 (1.75~9.70) | 0.001 |
| Quinolones | 3 | 39.1 | 0.19 | 2.17 (1.21~3.89) | 0.009 | 2.25 (1.03~4.93) | 0.04 |
| Nitroimidazoles | 2 | 60.6 | 0.11 | 1.63 (0.43~6.24) | 0.48 | 1.75 (0.14~22.69) | 0.67 |
Discussion
Stenotrophomonas maltophilia is widely distributed in natural environments, such as soil, water, and hospital environments, and can also parasitize the human skin, respiratory and digestive tracts. It is a very common conditional pathogen. The results of studies over the past few years have shown that the detection rate of S. maltophilia pneumonia is increasing year by year and has become an important pathogen of ICU infections. ICU-acquired infections associated with S. maltophilia are an independent risk factor for mortality in the ICU, and therefore knowledge of the risk factors for S. maltophilia pneumonia in the ICU and early targeted empirical treatment are key to reducing mortality from S. maltophilia pneumonia. In this study, we conducted a meta-analysis to screen the risk factors for S. maltophilia pneumonia in the ICU regarding general condition, co-morbid underlying diseases, invasive procedures, and use of antibiotics.
Correlation of the Patient's General Condition, Co-morbid Underlying Diseases and ICU-Acquired S. maltophilia Pneumonia
S. maltophilia pneumonia was associated with the patients' underlying disease status, and among the various underlying diseases, patients with COPD had the highest risk of infection (OR = 3.99), followed by malignant tumor (OR = 2.15). In contrast, underlying diseases such as immunodeficiency disorders, diabetes mellitus, and renal insufficiency were not associated with S. maltophilia pneumonia in ICU patients (Figure 2). Severity of illness is also an important factor in S. maltophilia pneumonia, and APACHE-II score is one of the most widely used tools for critical illness assessment (17). Furthermore, by combining the APACHE-II score of the experimental and control groups, we did not find a statistical difference between the two groups, unlike that reported by a few individual studies (9, 15). However, further analysis informed that there was a hierarchical effect between APACHE-II scores and S. maltophilia pneumonia, suggesting that S. maltophilia pneumonia is closely related to the severity of the disease. In recent years, the trend of glucocorticoid abuse in clinical practice has become more serious, and related studies have reported that long-term high-dose glucocorticoid use is a high-risk factor for multi-drug-resistant bacteria and fungal infections (18–20). However, we did not find that glucocorticoids increased the incidence of S. maltophilia pneumonia in the ICU, there may be bias due to different doses of glucocorticoids, course of treatment and patients' treatment response.
Figure 2.
Meta-analysis results of impact of general condition and combined underlying diseases on risk factors for acquired Stenotrophomonas maltophilia in intensive care unit.
Invasive Procedures and ICU-Acquired S. maltophilia Pneumonia
Our meta-analysis indicated that among the risk factors involved in invasive maneuvers, mechanical ventilation was strongly associated with ICU-acquired S. maltophilia pneumonia (OR = 8.75), followed by tracheotomy (OR = 6.10) and tracheal intubation (OR = 4.25), demonstrating that invasive procedures such as mechanical ventilation, tracheal intubation and tracheotomy are high risk factors for S. maltophilia pneumonia in the ICU (Figure 3). Invasive procedures can breach the body's basic defense barriers. S. maltophilia colonized in the oral pharynx tends to form bacterial biofilms in the lining of indwelling catheters and tends to enrich at oxygen storage sites, increasing the risk of pulmonary S. maltophilia infection (21). In addition, the longer the duration of invasive procedures such as mechanical ventilation, the greater the risk of S. maltophilia infection. Guo et al. (12) have reported that the duration of invasive ventilator ventilation (>14 d) is an independent risk factor for ICU-acquired S. maltophilia infection. Therefore, clinical practitioners need to strictly follow the indications for invasive procedures and reduce unnecessary invasive operations, while tracheal intubation should be removed as early as possible when conditions allow to help reduce the risk of S. maltophilia infection. In our study, the combination of two exposure factors, central venous cannulation and indwelling nasal cannula, was more heterogeneous and less robust than other invasive procedures, and the combined results should be viewed with caution.
Figure 3.
Meta-analysis results of impact of invasive operations on risk factors for acquired Stenotrophomonas maltophilia in intensive care unit.
The Association Between Antimicrobial Drug Use and ICU-Acquired Pneumonia With S. maltophilia
At present, for the treatment of antibiotic-resistant S. maltophilia infection, Chinese experts recommended three combined treatment modes, which are based on compound sulfamethoxazole, combined with ticarcillin clavulanate potassium, cefoperazone sulbactam, fluoroquinolone, minocycline, ceftazidime or polymyxin. Or ceftazidime-based fluoroquinolones, ticarcillin clavulanate potassium or cefoperazone sulbactam regimen; Alternatively, a polymyxin-based regimen combined with ticarcillin clavulanate potassium can be adopted (22). However, the use of antibiotics is a double-edged sword. S. maltophilia is naturally resistant to carbapenems, while its AAC(6')-lz acetyltransferase and pumping system make it highly resistant to aminoglycosides, and these characteristics could explain the use of both drugs to passively screen the bacterium for hospital-acquired infections due to the proliferation of dominant bacteria (23). Nseir et al. (10) found that broad-spectrum antibiotics such as fluoroquinolones and cephalosporins can also increase the rate of S. maltophilia pneumonia and concluded that broad-spectrum antibiotic use is more significant than carbapenems alone. This study showed that the use of β-lactamase inhibitors had the largest combined OR associated with S. maltophilia pneumonia (OR = 7.88), followed by aminoglycosides (OR = 4.01) and carbapenems (OR = 2.82), suggesting that the possibility of S. maltophilia pneumonia should be considered when clinical treatment with these three drugs is not effective (Figure 4). The long-term heavy use of broad-spectrum antibiotics is an important medical factor for hospital-acquired infections of S. maltophilia. Broad-spectrum antibiotics increase the risk of infection by killing other pathogens while screening out dominant species, including S. maltophilia. A study by Xu et al. (11) concluded that the use of ≥3 antibiotics for more than 1 week was an independent risk factor for S. maltophilia pneumonia in ICU patients, and therefore care should be taken to monitor the risk of infection in ICU patients using multiple antimicrobials simultaneously.
Figure 4.
Meta-analysis results of antimicrobial drug impact of on risk factors for acquired Stenotrophomonas maltophilia in intensive care unit.
Limitation of this Study
There are a few limitations in this study: (1) Due to the limited number of domestic and international studies on risk factors for S. maltophilia pneumonia in ICU and the uneven quality of the literature. Eight papers were screened strictly according to the inclusion and exclusion criteria, including five in English and three in Chinese, which may have a certain degree of publication bias. (2) Due to limited literature, some risk factor indicators in this study were not combined effectively, which may affect the study results. (3) At present, the research on related risk factors in China is not deep enough, and there is a lack of relevant prospective cohort studies. Therefore, more rigorous design, large samples, and multicenter studies are needed to clarify the risk factors for S. maltophilia pneumonia in the ICU.
Conclusion
In recent years, the disease burden of hospital-acquired S. maltophilia pneumonia in ICU patients has been high, and resistance to the organism is increasing. S. maltophilia pneumonia occurs in patients with severe disease, comorbid COPD, malignancy, high APACHE-II scores, undergoing invasive procedures, and in ICU patients on broad-spectrum antibiotics due to a combination of host and medical factors. From the host side, these patients are characterized by impaired immune function, severe disease, and the need for prolonged hospitalization, which objectively contributes to the infection of conditional pathogens such as S. maltophilia (24). Therefore, strengthening the monitoring, prevention, and control of patients with risk factors of S. maltophilia infection is beneficial to reduce the risk of infection and death in ICU patients.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Author Contributions
NW and CT: screening, data extracting, and writing. NW, CT, and LW: analysis. NW and LW: manuscript revision. LW: manuscript finalization. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1.Zhao J, Liu YX, Wen-Tao NI, Wang R, Liu YN. Progress of study on management and clinical treatment of Stenotrophomonas maltophilia infections. Chin J Nosocomiol. (2017) 27:2397–400. 10.11816/cn.ni.2017-170297 [DOI] [Google Scholar]
- 2.Xun M, Zhang Y, Li B-L, Wu M, Zong Y, Yin Y-M. Clinical characteristics and risk factors of infections caused by Stenotrophomonas maltophilia in a hospital in northwest China. J Infect Dev Ctries. (2014) 8:1000–5. 10.3855/jidc.4236 [DOI] [PubMed] [Google Scholar]
- 3.Tian L, Sun Z, Zhang Z. Antimicrobial resistance of pathogens causing nosocomial bloodstream infection in Hubei Province, China, from 2014 to 2016: a multicenter retrospective study. BMC Public Health. (2018) 18:1121. 10.1186/s12889-018-6013-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sumida K, Chong Y, Miyake N, Akahoshi T, Yasuda M, Shimono N, et al. Risk factors associated with Stenotrophomonas maltophilia bacteremia: a matched case-control study. PLoS ONE. (2015) 10:e0133731. 10.1371/journal.pone.0133731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fupin H, Yan G, Demei Z, Fu W, Xiaofei J. CHINET surveillance of bacterial resistance: results of 2020. Chin J Infect Chemother. (2021) 21:377–87. 10.16718/j.1009-7708.2021.04.00132789009 [DOI] [Google Scholar]
- 6.Muder RR, Harris AP, Muller S, Edmond M, Chow JW, Papadakis K, et al. Bacteremia due to Stenotrophomonas (Xanthomonas) maltophilia: a prospective, multicenter study of 91 episodes. Clin Infect Dis. (1996) 22:508–12. 10.1093/clinids/22.3.508 [DOI] [PubMed] [Google Scholar]
- 7.Paez JIG, Costa SF. Risk factors associated with mortality of infections caused by Stenotrophomonas maltophilia: a systematic review. J Hosp Infect. (2008) 70:101–8. 10.1016/j.jhin.2008.05.020 [DOI] [PubMed] [Google Scholar]
- 8.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. (2010) 25:603–5. 10.1007/s10654-010-9491-z [DOI] [PubMed] [Google Scholar]
- 9.Hanes SD, Demirkan K, Tolley E, Boucher BA, Croce MA, Wood GC, et al. Risk factors for late-onset nosocomial pneumonia caused by Stenotrophomonas maltophilia in critically ill trauma patients. Clin Infect Dis. (2002) 35:228–35. 10.1086/341022 [DOI] [PubMed] [Google Scholar]
- 10.Nseir S, Di Pompeo C, Brisson H, Dewavrin F, Tissier S, Diarra M, et al. Intensive care unit-acquired Stenotrophomonas maltophilia: incidence, risk factors, and outcome. Crit Care. (2006) 10:R143. 10.1186/cc5063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu NL, Shi SJ, Lai ZS Li HR, Lian SQ, Chen YS. A case-control study on the risk factors for lower respiratory tract infection by Stenotrophomonas maltophilia in a medical intensive care unit. Chin J Tuberc Respir Dis. (2011) 34:735–8. 10.3760/cma.j.issn.1001-0939.2011.10.005 [DOI] [PubMed] [Google Scholar]
- 12.Guo L, Li H, Li Q, Juan J. Antimicrobial drug-sensitivity and clinical risk factors of stenotro-phomnas maltophilia in the neurological intensive care unit%. J Xiangnan Univ (Med Sci). (2014) 16:21–4. 10.3969/j.issn.1673-498x.2014.03.00 [DOI] [Google Scholar]
- 13.Saugel B, Eschermann K, Hoffmann R, Hapfelmeier A, Schultheiss C, Phillip V, et al. Stenotrophomonas maltophilia in the respiratory tract of medical intensive care unit patients. Eur J Clin Microbiol Infect Dis. (2012) 31:1419–28. 10.1007/s10096-011-1459-8 [DOI] [PubMed] [Google Scholar]
- 14.Ibn Saied W, Merceron S, Schwebel C, Le Monnier A, Oziel J, Garrouste-Orgeas M, et al. Ventilator-associated pneumonia due to Stenotrophomonas maltophilia: risk factors and outcome. J Infect. (2020) 80:279–85. 10.1016/j.jinf.2019.10.021 [DOI] [PubMed] [Google Scholar]
- 15.Lei S, An-hua W, Lan C, Xun H, Xiao-bei P, Chun-hui L, et al. Risk factors for healthcare-associated infection of Stenotrophomonas maltophilia in intensive care unit. Chin J Infect Control. (2019)18:403–9. 10.12138/j.issn.1671-9638.20194463 [DOI] [Google Scholar]
- 16.Scholte JBJ, Zhou TL, Bergmans DCJJ, Rohde GGU, Winkens B, Van Dessel HA, et al. Stenotrophomonas maltophilia ventilator-associated pneumonia. A retrospective matched case-control study. Infect Dis (Lond). (2016) 48:738–43. 10.1080/23744235.2016.1185534 [DOI] [PubMed] [Google Scholar]
- 17.Pathmanathan A, Waterer GW. Significance of positive Stenotrophomonas maltophilia culture in acute respiratory tract infection. Eur Respir J. (2005) 25:911–4. 10.1183/09031936.05.00096704 [DOI] [PubMed] [Google Scholar]
- 18.Yueneng Y, Waeiping Z, Xuhong J, Wenjian Q. Distribution and antibiotic resistance of ESBLs-producing Klebsiella pneumoniae and Eschedchia coil clinical isolates and risk factors of infection%. J Zhejiang Med J. (2016) 38:728–34. 10.11946/cjstp.201606230509 [DOI] [Google Scholar]
- 19.Wakino S, Imai E, Yoshioka K, Kamayachi T, Minakuchi H, Hayashi K, et al. Clinical importance of Stenotrophomonas maltophilia nosocomial pneumonia due to its high mortality in hemodialysis patients. Ther Apher Dial. (2009) 13:193–8. 10.1111/j.1744-9987.2009.00693.x [DOI] [PubMed] [Google Scholar]
- 20.Yang W, Yuan ZQ, Daikun L, Iangling WJ, Iang XJ. Clinical distribution of extended-spectrum-β-lactamase-producing Escherichia coli and Klebsiella pneumoniae and analysis of infection risk factors. Lab Med Clin. (2021) 18:1857–65. 10.3969/j.issn.1672-9455.2021.13.009 [DOI] [Google Scholar]
- 21.Kim S-H, Cha MK, Kang C-I, Ko J-H, Huh K, Cho SY, et al. Pathogenic significance of hemorrhagic pneumonia in hematologic malignancy patients with Stenotrophomonas maltophilia bacteremia: clinical and microbiological analysis. Eur J Clin Microbiol Infect Dis. (2019) 38:285–95. 10.1007/s10096-018-3425-1 [DOI] [PubMed] [Google Scholar]
- 22.Guan X, He L, Hu B, Hu J, Huang X, Lai G, et al. Laboratory diagnosis, clinical management and infection control of the infections caused by extensively drug-resistant Gram-negative bacilli: a Chinese consensus statement. Clin Microbiol Infect. (2016) 22 (Suppl. 1):S15–25. 10.1016/j.cmi.2015.11.004 [DOI] [PubMed] [Google Scholar]
- 23.Garazi M, Singer C, Tai J, Ginocchio CC. Bloodstream infections caused by Stenotrophomonas maltophilia: a seven-year review. J Hosp Infect. (2012) 81:114–8. 10.1016/j.jhin.2012.02.008 [DOI] [PubMed] [Google Scholar]
- 24.Velázquez-Acosta C, Zarco-Márquez S, Jiménez-Andrade MC, Volkow-Fernández P, Cornejo-Juárez P. Stenotrophomonas maltophilia bacteremia and pneumonia at a tertiary-care oncology center: a review of 16 years. Support Care Cancer. (2018) 26:1953–60. 10.1007/s00520-017-4032-x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.