The following was originally published in Volume 27, Number 4, pages 431–437 (DOI: https://doi.org/10.3727/096504018X15201058168730). In the original article there were some errors in Figures 3D, 4A, and 5A because of antibody contamination. Corrected versions of the figures are shown here, and the figures have been replaced with the corrected versions in the original published article in the online site (https://www.ingentaconnect.com/contentone/cog/or/2019/00000027/00000004/art00005). The authors confirm that the errors in the original figures did not have any significant impact on either the results or the conclusions reported in the study.
Abstract
MicroRNA-132 (miR-132) has been demonstrated to be a tumor suppressor in several types of tumors. However, the expression and the role of miR-132 in human thyroid cancer are still poorly understood. The aim of the present study was to examine the potential roles and molecular mechanism of miR-132 in thyroid cancer. We found that miR-132 expression levels were significantly downregulated in thyroid cancer tissues and cell lines. Function assays showed that overexpression of miR-132 in TPC1 cells inhibited cell proliferation, migration, and invasion. Forkhead box protein A1 (FOXA1) was identified as a direct target of miR-132 in thyroid cancer cells. Knockdown of FOXA1 in TPC1 cells significantly inhibited cell proliferation, migration, and invasion, which mimicked the suppressive effect induced by miR-132 overexpression. Restoration of FOXA1 expression partially reversed the suppressive effect induced by miR-132 overexpression. Taken together, these results suggested that miR-132 acts as a tumor suppressor in thyroid cancer through targeting FOXA1.
Key words: MicroRNAs, miR-132, Thyroid cancer, FOXA1
Figure 3.
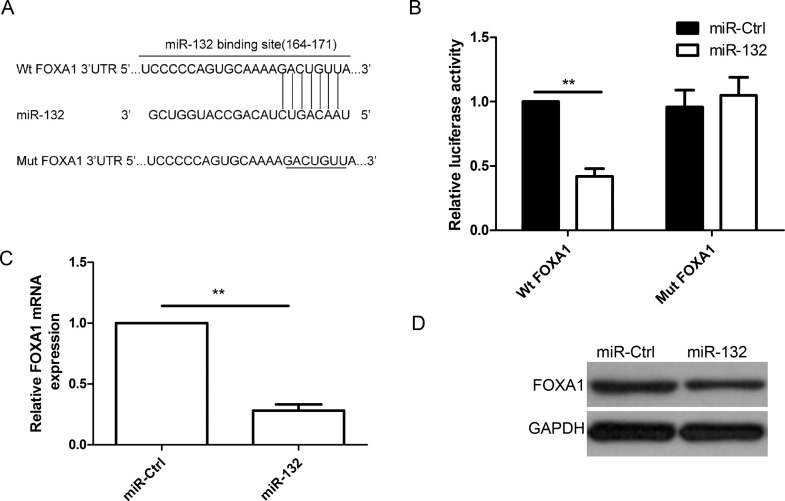
FOXA1 was a direct target of miR-132 in thyroid cancer cells. (A) The predicted binding sites for miR-132 in the 3′-untranslated region (3′-UTR) of FOXA1 (positions 164–171) and the mutations in the binding sites are shown. (B) Luciferase activities were determined in TPC1 cells 48 h after cotransfection with reporter vectors carrying either wild-type FOXA1 3′-UTR (Wt-FOXA1) or mutated (Mut-FOXA1), and miR-132 mimic or miR-Ctrl. FOXA1 expression at the mRNA level (C) and protein level (D) was detected in TPC1 cells transfected with miR-132 mimic or miR-Ctrl. GAPDH was used as the internal control. **p < 0.01.
Figure 4.
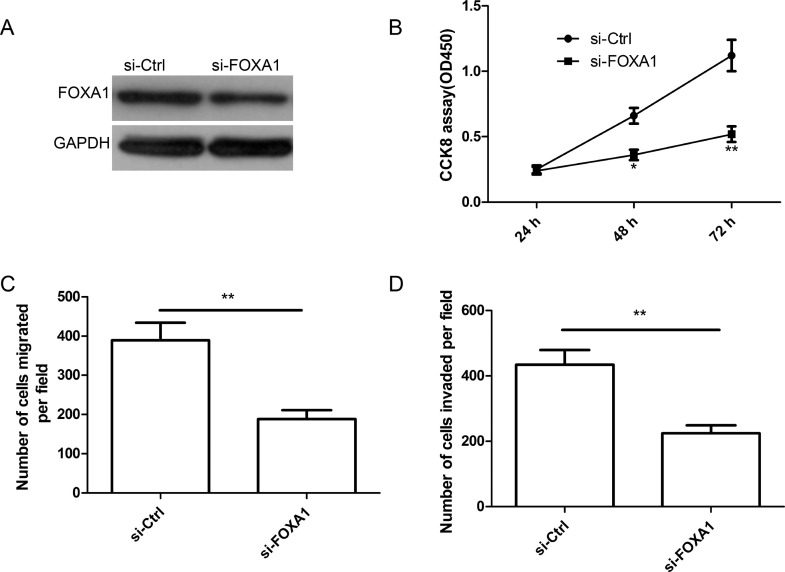
FOXA1 deletion inhibited cell proliferation, migration, and invasion in thyroid cancer cells. (A) FOXA1 protein expression was determined in TPC1 cells transfected with si-FOXA1 or si-Ctrl by Western blot. GAPDH was used as the internal control. Cell proliferation (B), migration (C), and invasion (D) were determined in TPC1 cells transfected with si-FOXA1 or si-Ctrl. *p < 0.05, **p < 0.01.
Figure 5.
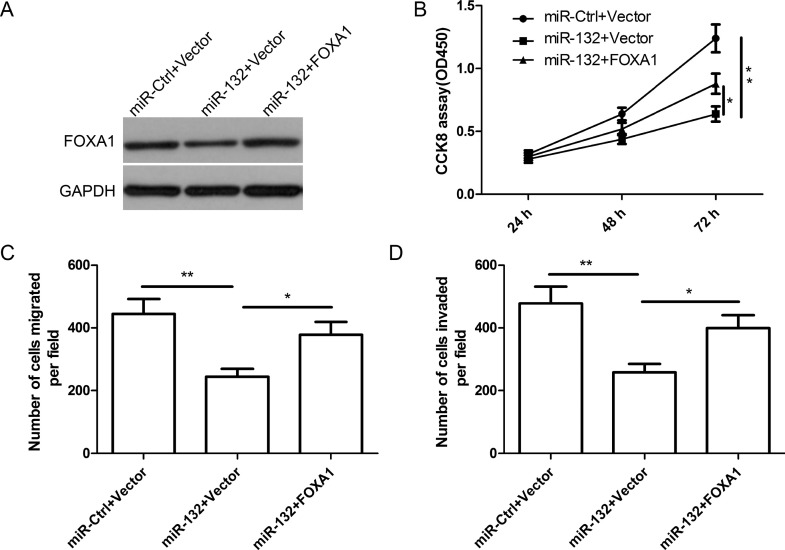
miR-132 inhibits cell proliferation, migration, and invasion in thyroid cancer cells via targeting FOXA1 expression. (A) FOXA1 protein expression was detected in TPC1 cells transfected with miR-132 mimic or miR-Ctrl, together with overexpression FOXA1 plasmids, or vector. GAPDH was used as the internal control. Cell proliferation (B), migration (C), and invasion (D) were determined in TPC1 cells transfected with miR-132 mimic or miR-Ctrl, together with overexpression FOXA1 plasmids or vector. *p < 0.05, **p < 0.01.