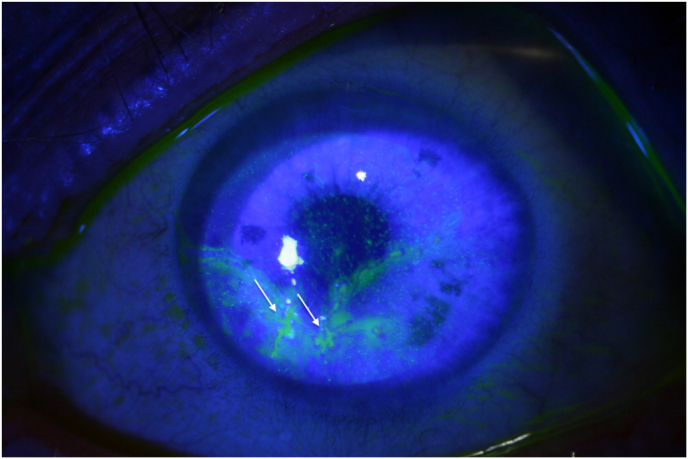
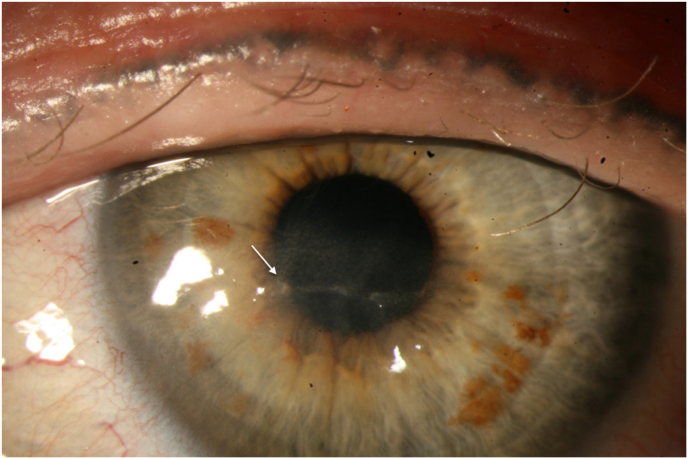
Abstract
Purpose
To strengthen the sparse evidence on acyclovir (ACV) resistance, especially in recalcitrant herpetic keratitis (HK), by describing the clinical course of 3 genotypically proven ACV resistant HK cases. An overview of mechanisms of resistance and therapeutic options currently available to ophthalmologists is provided based upon recent literature search.
Observations
Resistance to ACV due to known mutations in the gene encoding the viral thymidine kinase was confirmed in 2 cases, and a novel mutation in the UL23 gene (N202K) conferring phenotypical resistance to ACV was discovered in 1 case. Three unique therapeutic strategies finally led to epithelial closure.
Conclusions
The novel thymidine kinase mutation (N202K) should be considered to infer resistance to all molecules requiring activation by the viral thymidine kinase. Current topical alternatives in the ophthalmologist's armamentarium include trifluridine 1%, foscarnet 1,2%-1,4% or cidofovir 0,2–0,5%. Epithelial debridement, high-frequency dosing and reduction of immunosuppression are useful adjuncts.
Importance
Clinicians should perform epithelial debridement in recalcitrant HK, allowing geno- and phenotypically guided therapy, even without a history of long-term anti-viral prophylaxis or recurrent HK. This report provides mandatory knowledge allowing the reader to comprehend how therapy should be altered based upon these results. To the best of our knowledge, successful treatment of proven ACV resistant HK with topical foscarnet has not yet previously been published.
Furthermore, this paper highlights a lack of controlled studies investigating alternative topical treatments in case of viral resistance, offering opportunities for future research.
Keywords: Herpetic keratitis, Acyclovir resistance, Foscarnet, Thymidine kinase
1. Introduction
Classically, resistance to acyclovir (ACV) in Herpes Simplex (HSV) infections was considered to be a problem of the immune compromised with a prevalence varying from 3,5 to 10% up to 36% in hematopoietic stem cell transplant (HSCT) recipients, compared to a prevalence of less than 1% in the immunocompetent population.1 In 2008 however, a study reported ACV resistance in up to 6,4% of immunocompetent patients suffering from recurrent herpetic keratitis (HK).2 This implicates that the cornea, compared to other known locations prone to HSV infection, might be a site where the development of ACV resistance is facilitated.3 Interestingly, in mouse models, these ACV resistant strains seem to be less virulent than non-resistant strains.4 The immune-privilege of the cornea might allow resistant and, based on former research, less virulent strains to proliferate where they would have been suppressed at other sites by the immune system. It has been hypothesized that long-term ACV prophylaxis in recurrent HK could lead to the selection of these resistant strains.5,6 The same research group also confirmed the ability of these resistant strains to establish latency and thus possibly reactivate from the trigeminal ganglion of immunocompetent individuals.7
We want to strengthen the sparse evidence on ACV resistance, especially in recalcitrant HK, by describing the clinical characteristics of 3 cases of HK with proven resistance to acyclovir, one of which presenting a novel mutation in the UL23 gene.
2. Findings
2.1. Case 1
Our first patient, a 68-years old male, was referred by the neurologist because of a red eye preceded by a non-healing ulcer on his mouth. At that time, he was hospitalized for treatment and investigation of a myasthenia gravis exacerbation. Apart from the myasthenia gravis and a Hashimoto thyroiditis, his general history was unremarkable.
Four discrete epithelial defects were noticed and the presumption of a unilateral epithelial herpetic keratitis was made (Fig. 1). BVDU ((E)-5-(2-bromovinyl)-2′-deoxyuridine) 0,1% drops 5 times a day was prescribed.8 This first episode of HK in combination with not 1 but 4 epithelial defects (not typically dendritic) was attributed to his methylprednisolone treatment of 64 mg a day in combination with azathioprine since a few days. Due to wrongful usage of the prescribed treatment, his epithelial defects progressed to clear dendritic lesions at day 4. After 11 days, only slight improvement was noted, so systemic ACV 400 mg 5 times a day was associated. After 2 weeks of this treatment regimen and dose reduction of methylprednisolone to 16 mg, the dendritic lesions were still present. BVDU dosage was increased to 8 times a day and acyclovir was substituted for valaciclovir 500 mg 3 times a day to improve compliance and to increase bio-availability. Another 6 days later, we decided to double the valaciclovir dosage since the lesions progressed to a geographic ulcer (Fig. 2). The recalcitrant nature of the lesions under this high dosage of antiviral therapy persuaded us to perform a corneal scraping to determine viral resistance. The topical therapy was switched to ganciclovir 0,15% ointment 8 times a day. Upon control 2 weeks later we noticed epithelial closure (Fig. 3). Systemic valaciclovir was halted and topical therapy was tapered to 5 times a day until cessation of systemic steroids. Up until 7 months after halting all antivirals, no recurrence was noted. No known mutations linked to drug-resistance were identified in this sample, yet a novel amino acid substitution (N202K) was identified in the viral thymidine kinase (TK) with phenotyping showing resistance to drugs requiring the viral TK for activation (Table 1). This viral strain remained sensitive to cidofovir, foscarnet and adefovir, consistent with lack of mutation in the viral DNA polymerase. This novel amino acid substitution can be linked to drug-resistance, more specifically those drugs needing TK for activation. This, however, does not explain the good clinical response we noted upon switching to ganciclovir, as this drug also requires activation by TK. We believe several factors are at play: a concomitant reduction in methylprednisolone dosage to 8 mg allowed for a partial immune reconstitution, known to play a major role in the clinical course of herpetic disease. Secondly, the mechanical debridement of the cornea not only allows for higher intra-cellular drug concentrations to be obtained due to improved corneal penetration, but also effectively reduces the viral load. As resistance is generally acquired locally after reactivation of wild-type virus from the trigeminal ganglion, removal of cells containing mutated strains could return the patient back to a disease state where there is only wild-type virus present. (See Table 2)
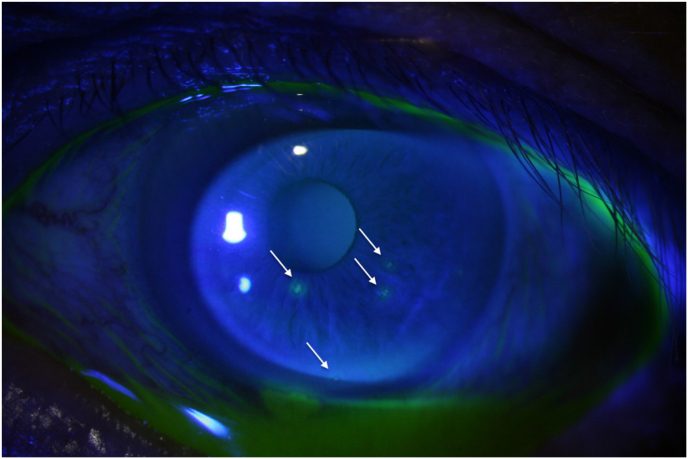
Fig. 1.
Patient 1 at initial presentation: 4 epithelial defects (not typically dendritic).
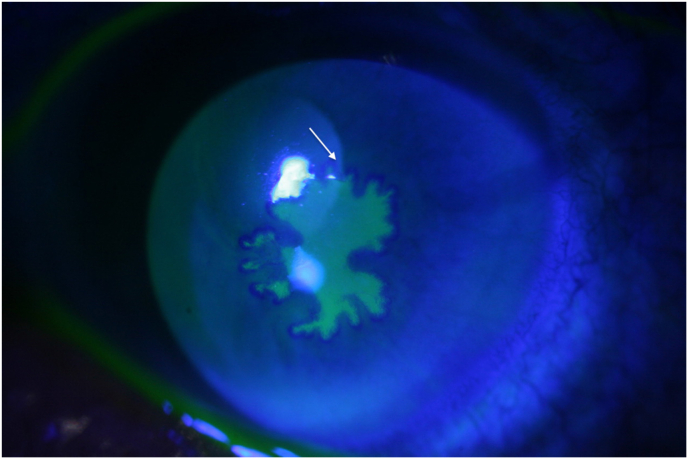
Fig. 2.
Patient 1 demonstrating a lack of response to high dose VACV po and BVDU with progression towards a geographic ulcer.
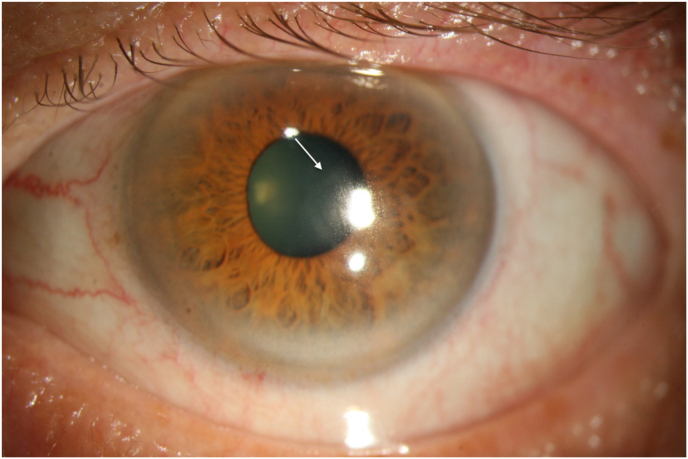
Fig. 3.
Patient 1 after switching to topical GCV demonstrating epithelial closure with remaining discrete subepithelial haze and epithelial irregularity.
Table 1.
Results of viral geno- and phenotyping
| Patient 1 | Patient 2 | Patient 3 | |
|---|---|---|---|
| Natural polymorphisms TK | G6C, P42L, R89Q, V267L, P268T, D286E, S321P, N376H | G6C, P42L, R89Q, G240E, C251G, S321P | G6C, P42L, R89Q, C251G, S321P |
| Functional mutation TK | N202K (novel) | R216C (known) | Deletion nucleotides 476–610 resulting in deletion of 11 amino acids |
| Natural polymorphisms DNA-polymerase | T566A, A646T, K700R, M905V, S1124L | T566A, K700R, H1124P | A566T, K700R |
| Functional mutation DNA-polymerase | None | None | None |
| EC50 ACV (μg/ml) | >20 (R) | NA | NA |
| EC50 PCV (μg/ml) | >20 (R) | NA | NA |
| EC50 BVDU (μg/ml) | >20 (R) | NA | NA |
| EC50 GCV (μg/ml) | >2 (R) | NA | NA |
| EC50 PFA (μg/ml) | 22,18 (S) | NA | NA |
| EC50 CDV (μg/ml) | 0,4 (S) | NA | NA |
| EC50 TFT (μg/ml) | NA | NA | NA |
EC50: 50% effective concentration or compound concentration required to reduce virus cytopathic effect by 50%. The EC50 values for the reference Kos strain were as follows: 0.055 μg/ml (ACV), 0.088 μg/ml (PCV), 0.13 μg/ml (BVDU), 0.0094 μg/ml (GCV), 40 μg/ml (PFA) and 1.79 μg/ml (CDV).
NA: not available; (R): Resistant; (S): Sensitive; TK: viral thymidine kinase; ACV: acyclovir; PCV: penciclovir; BVDU: brivudine; GCV: ganciclovir; PFA: foscarnet; CDV: cidofovir; TFT: trifluridine.
Table 2.
Patient characteristics
| Patient 1 | Patient 2 | Patient 3 | |
|---|---|---|---|
| Age at confirmed resistant Herpes Keratitis | 68 years | 42 years | 80 years |
| Number of prior recurrences | First episode | Second episode | Multiple |
| Immune status | Azathioprine 100 mg + methylprednisolone 64 mg po for myasthenia gravis exacerbation | Status post HSCT (x2) and chemotherapy for mycosis fungoides (T4N3M0B2). Treatment at diagnosis of HK: methylprednisolone 72 mg po and ruxolitinib 10 mg. | Lymphoma treated with multiple sessions of chemotherapy (R–CHOP, R–CVP) in the past, no current immunosuppressive medication. |
| Duration of antiviral prophylaxis | Sine | Intermittent Valganciclovir/Acyclovir since 9 years before HK for CMV reactivation, Herpes labialis, Herpes Zoster Ophthalmicus | Acyclovir since 13 years, necessary dosage of 3 × 800 mg to suppress viral activity. |
| Initial antiviral treatment | Topical Brivudine 5×/d | Acyclovir 5 × 800 mg po | / |
| Curative antiviral treatment | Topical Ganciclovir 0,15% 8×/d | Topical Foscarnet 1,2% 5×/d | Topical Trifluridine 1% 4×/d + IV Foscarnet |
| Duration of treatment before corneal scraping | 46 days | Immediate upon recurrence shortly after first episode (which required treatment for 11 weeks) | Immediate due to concurrent bacterial ulcer |
| Total episode duration | 64 days | 7 weeks | 5 weeks |
2.2. Case 2
Our second patient, a 41 years-old immunocompromised female, was referred by the hematologist for a red and irritated left eye since 1 day. She has a history of 2 HSC transplants (9 and 4 years ago) for Mycosis Fungoides and was hospitalized 2 weeks before her ocular complaints for IV steroids and ruxolitinib, both necessary for her presumed graft versus host induced hepatitis. All possible hepatotoxic medication was halted at that time, including ACV which she had been taking intermittently for over 9 years as anti-viral prophylaxis after several episodes of Herpes labialis and a Herpes Zoster Ophthalmicus.
Anterior segment examination revealed 2 dendritic epithelial lesions with typical terminal bulbs in her left eye. ACV 800 mg 5 times a day was initiated by the hematologist, yet as the lesions progressed and became confluent, resulting in a large geographic ulcer 4 days later(Fig. 4), topical BVDU 10 times a day was added. This combined anti-viral therapy with concomitant stepwise dose reduction of methylprednisolone to 4 mg a day led to a major reduction in the size of the epithelial defect with 2 small remaining dendritic lesions inferiorly(Fig. 5). ACV was reduced to the standard prophylactic dosage (400 mg b.i.d.)9 to prevent hepatotoxicity in this patient. BVDU was continued at the same regimen with persistence of an epithelial lesion inferiorly, suspected of viral activity, 5 weeks after initiation. Finally, 11 weeks after initial presentation, BVDU could be halted as there was only epitheliopathy without any active herpetic lesion. The topical antiviral therapy was halted and autologous serum 6 times a day, artificial tears and lubricating ointment was initiated to reduce her epitheliopathy and irregular heaped up epithelium. Persistence of this epithelial disease in combination with discrete subepithelial fibrosis convinced us to add hydrocortisone sodium phosphate 3,35 mg/ml ante nocte in combination with a bandage contact lens. Despite the anti-viral prophylaxis, she developed a recurrence of HK 7 weeks later. At this point, a corneal scraping was performed to determine viral resistance. The prophylactic anti-viral therapy was switched again to ACV 400 mg 5 times a day and topical BVDU 5 times a day. Upon control 2 weeks later, the keratitis had progressed despite treatment. The viral genotyping demonstrated ACV resistance due to a known mutation (R216C) in the gene encoding the viral TK. Phenotyping was not possible since the virus failed to grow in cell culture (Table 1). A mutation in the viral TK usually infers resistance to both ACV and BVDU.10 Based on the previous good response to high dosage BVDU and the unavailability of other topical antiviral therapy, we decided to increase BVDU dosage again to 8 times a day. Upon control 2 weeks later, the central dendritic lesion showed a reduction in size. However, the recurrence after tapering down forced us to search and start the relatively sparsely investigated foscarnet (PFA) drops.11,12 Topical PFA 1,2% 5 times a day was well supported by the patient and the toxic epitheliopathy healed without recurrence of HK(Fig. 6). However, there was an important financial burden to the patient, as these compounded drops costs ±€50 per week.
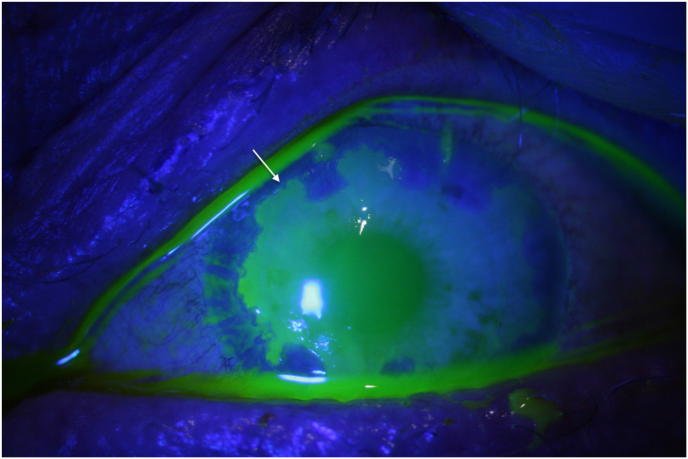
Fig. 4.
Patient 2 Development of large geographic ulcer under high-dose ACV.
Fig. 5.
Patient 2 Partial response to high-dose topical BVDU and ACV po with 2 persisting dendrites inferiorly.
Fig. 6.
Patient 2 Epithelial closure under topical PFA with residual subepithelial haze and a fine line of heaped up epithelium.
2.3. Case 3
Our third patient is an 80-years old female diagnosed with a mixed centrocytic, mixed centroblastic lymphoma stadium IIIa years back and currently in remission, for which she had received several sessions of chemotherapy. During those severely immunosuppressed periods, she suffered from HSV induced multiple organ failure and experienced her first episodes of HSV epithelial keratitis and keratouveitis. Since then, she experienced multiple recurrences of epithelial herpes keratitis under a prophylactic ACV dosage below 800 mg 2 times a day or when her immune status deteriorated for various reasons.
Thirteen years after her first episode, she presented with a painful red eye and vision loss. Visual acuity had indeed dropped to light perception and anterior segment examination revealed a central corneal ulcer with a hypopyon of 4 mm in the anterior chamber. At that time, she was still on high dosage anti-viral prophylaxis (ACV 800 mg 3 times a day) and prednisolone acetate 10 mg/ml drops 5 times a day following an episode of herpetic keratouveitis in the same eye 2 months before. A corneal scraping and anterior chamber tap were performed and both were examined by way of cultures (blood, chocolate and Sabouraud agar) and viral PCR. Awaiting the antibiogram, she was hospitalized and the ulcer was treated with both topical moxifloxacine 5mg/ml 2-hourly and chloramphenicol 0,5% hourly. Five days later, an anterior chamber washout with vancomycin was performed due to a large persisting hypopyon. The cultures confirmed the presence of Staphylococcus aureus and Staphylococcus capitis. Based on the antibiogram chloramphenicol was switched to fortified amikacine 40 mg/ml eye drops and an ointment containing tobramycine 3 mg/ml and dexamethasone 1mg/ml ante nocte was added. The recalcitrant nature of this infection indulged the association of a topical antifungal therapy, voriconazole 1% 4 times a day. The viral PCR came back positive for Herpes Simplex and revealed a deletion of nucleotides 476–610 in the UL-23 gene encoding the viral TK, resulting in the production of an inactive enzyme (Table 1). We opted to add topical trifluridine 1% 4 times daily in combination with intravenous foscarnet. It was only after adding this alternative antiviral therapy that the peripheral cornea cleared up, the infiltrate stabilized, the hypopyon resorbed and the pain disappeared. During tapering she developed a single recurrence of HK at twice-daily dosing of topical TFT, which responded well to a short-term dosage increase. We recognize that this prophylactic dosage might have been too low as the regular therapeutic regimen requires instillation up to 9 times a day, followed by a 14-day taper of 4 times a day,13 yet this patient suffered a significant physical and mental burden due to the intensive, chronic therapy and limited adherence did not allow for longer term high-frequency dosing. Further drawbacks of this therapy are the cost (±€30 per bottle) and difficulty acquiring the product, as this has to be imported in Belgium. She has been disease-free for over one year now under topical dexamethasone 1 mg/ml 4 times a day to prevent stromal recurrence.
2.4. Methods of pheno- and genotyping
All samples were sent to the Rega Institute for Medical Research, Laboratory of Virology and Chemotherapy for examination. Phenotyping was done following growth of the viral sample in human embryonic lung (HEL) fibroblasts until 100% cytopathic effect was reached. Antiviral assay was performed in HEL cells using four different viral inoculums and the reference HSV-1 Kos strain. The EC50 (Concentration required to reduce virus cytopathicity by 50%) for each drug was determined and these values were compared between the reference strain and the patient sample. Genotyping was done by PCR amplification and direct sequencing of the TK and DNA polymerase genes, aligning these against the reference strain.
3. Discussion
To comprehend clinical patterns of antiviral resistance, a basic understanding of antiviral pharmacodynamics is mandatory. Acyclovir (ACV), penciclovir (PCV) and ganciclovir (GCV) are all guanosine analogues requiring intra-cellular activation by phosphorylation to obtain their antiviral effect. This also holds true for their prodrugs valacyclovir (VACV), famciclovir (FCV) and valganciclovir (VGCV), respectively. After phosphorylation, these molecules act as a competitive inhibitor of the viral DNA polymerase slowing DNA chain elongation and, in the case of ACV, resulting in obligate chain termination. Brivudine (BVDU) is a thymidine analogue functioning similarly to the guanosine analogues, i.e. it requires intra-cellular activation by phosphorylation and will subsequently function as a competitive inhibitor of the viral DNA polymerase.8 Viral resistance to the aforementioned molecules can occur due to mutations in 2 critical viral enzymes. The first enzyme is the viral thymidine kinase (TK), which is responsible for the first phosphorylation, necessary for activation of ACV, PCV, GCV and BVDU. This enzyme is encoded by the UL-23 gene in HSV and mutations herein are responsible for 95% of ACV resistance.1 The second enzyme is the viral DNA polymerase, encoded by the UL-30 gene, the end-target of all currently available anti-herpetic drugs. These common pathways explain why a single mutation in the UL-23 or UL-30 gene often infers resistance to all guanosine analogues and to BVDU and why they are poor candidates to replace one another in the absence of clinical response.3
Foscarnet (also known as phosphonoformate, further referred to as PFA) is a direct viral DNA polymerase inhibitor, which does not need activation by the UL-23 encoded TK and is thus suggested as an alternate therapy to both guanosine and thymidine analogues when resistance is caused by a mutation in this gene. On the other hand, when resistance to these nucleoside analogues is caused by a mutation in the UL-30 encoded viral DNA-polymerase, cross-resistance with PFA is common.3
Cidofovir (CDV) is an alternate therapy when there is a mutation in UL-23 and/or UL-30. CDV is a cytosine analogue, yet it does not require activation by the viral TK because it already possesses a monophosphate group. Mutations in UL-30 associated with ACV-/PFA-resistance do not seem to reduce the sensitivity of the DNA polymerase to activated cidofovir.3
Trifluridine (TFT) was one of the first available topical anti-herpetic drugs, which inhibits thymidylate synthetase and disrupts viral DNA synthesis after incorporation into the viral DNA without the need for prior activation by the viral TK. TFT is less selective in comparison with newer anti-viral agents, thereby causing more ocular surface toxicity.13,14
Due to the lack of commercially available topical anti-viral drugs, the ophthalmologist's armamentarium is limited when confronted with HK. On the Belgian market, the only products currently available are ganciclovir 0,15% gel (Virgan® (Farmila-Théa Farmaceutici S.p.A., Settimo Milanese (MI), Italy)) and acyclovir 3% ointment (Aciclovir Agepha® (Agepha Pharma s.r.o, Senec, Slovakia)). At the University Hospitals Leuven, Belgium, BVDU drops are available as an alternative therapy.8 Logically, a TKnegative strain (i.e. complete deficiency) should be resistant to all aforementioned products, as all of them rely on activation by the viral TK. When the mutation induces only an altered substrate specificity (TKaltered) or reduced expression of viral TK (TKpartial), there may still be (intermediate) sensitivity to GCV while the strain is highly resistant to BVDU for example (and vice versa).10
Literature holds very little evidence for use of other (compounded) topical therapies. Turner and Beckingsale have reported therapeutic success with topical TFT in proven ACV resistant HK. Downsides are the necessity of frequent administration (9 times a day), significant ocular surface toxicity and unavailability in several countries.13,14 The successful topical use of CDV in ACV resistant HK has been reported in rabbits by Maugdal et al., in 1991 (Ophthalmology department University Hospitals Leuven in collaboration with REGA institute, KU Leuven, Belgium), yet to our knowledge, humane topical use has only been described in ACV resistant muco-cutaneous lesions, and not in ACV resistant HK. This might be a promising alternative as dosage was less frequent (2 times a day) then current standard of care, without ocular toxicity at a concentration of 0,2%-0,5% (ocular toxicity has been described in a pilot study on adenoviral keratoconjunctivitis at higher dosage (1% Cidofovir, 4–10 times daily)),15 faster healing (5 days on average versus 7,5 days for ACV/TFT) and shorter duration of treatment (7 days versus 14 days).13,16,17
There are only a few case reports describing successful use of intravenous Foscarnet in ACV resistant HK, as was done in our third case.18 However, to the best of our knowledge, there are no case reports available for successful topical use in ACV resistant epithelial HK. Several small studies on ACV-sensitive HK demonstrated efficacy and lack of toxicity at a concentration of 1,2%-1,4%.11,12,19
Lastly, limited anecdotal evidence supports the use of topical interferon alpha-2a as an adjunct to topical antiviral therapy in immunosuppressed patients presenting with refractory HK.20
4. Conclusions
The novel thymidine kinase mutation (N202K) should be considered to infer resistance to all molecules requiring activation by the viral thymidine kinase. Current topical alternatives in the ophthalmologist's armamentarium include trifluridine 1%, foscarnet 1,2%-1,4% or cidofovir 0,2–0,5%. Epithelial debridement, high-frequency dosing and reduction of immunosuppression are useful adjuncts. As highlighted by these 3 cases, clinicians should perform epithelial debridement in recalcitrant HK, allowing geno- and phenotypically guided therapy, even without a history of long-term anti-viral prophylaxis or recurrent HK.
Patient consent
All patients consented to publication of the case orally. This report does not contain any personal information that could lead to the identification of the patient.
Acknowledgments and disclosures
No funding or grant support
There is no conflict of interest to disclose.
None of the authors have any financial disclosures.
All authors attest that they meet the current ICMJE criteria for Authorship.
CRediT authorship contribution statement
Ivo De Clerck: Writing – original draft, Investigation, Writing – review & editing. Vincent Walgraeve: Writing – original draft. Robert Snoeck: Resources, Writing – review & editing. Graciela Andrei: Resources, Writing – review & editing. Johan Blanckaert: Resources. Evelyne Mulliez: Resources. Heleen Delbeke: Conceptualization, Resources, Writing – review & editing, Supervision.
Acknowledgements
None.
References
- 1.Piret J., Boivin G. Antiviral resistance in herpes simplex virus and varicella-zoster virus infections: diagnosis and management. Curr Opin Infect Dis. 2016;29:654–662. doi: 10.1097/QCO.0000000000000288. [DOI] [PubMed] [Google Scholar]
- 2.Duan R., de Vries R., Osterhaus A., Remeijer L., Verjans G. Acyclovir-resistant corneal HSV-1 isolates from patients with herpetic keratitis. J Infect Dis. 2008;198:659–663. doi: 10.1086/590668. [DOI] [PubMed] [Google Scholar]
- 3.Andrei G., Snoeck R. Herpes simplex virus drug-resistance. Curr Opin Infect Dis. 2013;26:551–560. doi: 10.1097/QCO.0000000000000015. [DOI] [PubMed] [Google Scholar]
- 4.Omura N., Fujii H., Yoshikawa T., et al. Association between sensitivity of viral thymidine kinase-associated acyclovir-resistant herpes simplex virus type 1 and virulence. Virol J. 2017;14:59. doi: 10.1186/s12985-017-0728-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Velzen M., van de Vijver D.A.M.C., van Loenen F.B., Osterhaus A.D.M.E., Remeijer L., Verjans G.M.G.M. Acyclovir prophylaxis predisposes to antiviral-resistant recurrent herpetic keratitis. J Infect Dis. 2013;208:1359–1365. doi: 10.1093/infdis/jit350. [DOI] [PubMed] [Google Scholar]
- 6.Rousseau A., Boutolleau D., Titier K., et al. Recurrent herpetic keratitis despite antiviral prophylaxis: a virological and pharmacological study. Antivir Res. 2017;146:205–212. doi: 10.1016/j.antiviral.2017.09.013. [DOI] [PubMed] [Google Scholar]
- 7.van Velzen M., van Loenen F.B., Meesters R.J.W., et al. Latent acyclovir-resistant herpes simplex virus type 1 in trigeminal Ganglia of immunocompetent individuals. J Infect Dis. 2012;205:1539–1543. doi: 10.1093/infdis/jis237. [DOI] [PubMed] [Google Scholar]
- 8.Maudgal P.C., Missotten L., De Clercq E., Descamps J., De Meuter E. Efficacy of (E)-5-(2-bromovinyl)-2’-deoxyuridine in the topical treatment of herpes simplex keratitis. Albrecht Von Graefes Arch Klin Exp Ophthalmol. 1981;216:261–268. doi: 10.1007/BF00455033. [DOI] [PubMed] [Google Scholar]
- 9.Group HEDS Acyclovir for the prevention of recurrent herpes simplex virus eye disease. Herpetic Eye Disease Study Group. N Engl J Med. 1998;339:300–306. doi: 10.1056/NEJM199807303390503. [DOI] [PubMed] [Google Scholar]
- 10.Andrei G., Balzarini J., Fiten P., De Clercq E., Opdenakker G., Snoeck R. Characterization of herpes simplex virus type 1 thymidine kinase mutants selected under a single round of high-dose Brivudin. J Virol. 2005;79:5863–5869. doi: 10.1128/JVI.79.9.5863-5869.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fabricius E.-M. Foscarnet eyedrops in recurence prophylaxis of herpes simplex virus keratitis. Acta Ophthalmol Scand. 1998;76:755–757. [PubMed] [Google Scholar]
- 12.Phosphonoformate Behrens-Baumann W. (foscarnet, PFA) versus trifluorthymidine in the treatment of keratitis dendritica in the human. A double-blind, randomized, preliminary trial. Acta Ophthalmol. 1992;70:690–692. doi: 10.1111/j.1755-3768.1992.tb02154.x. [DOI] [PubMed] [Google Scholar]
- 13.Romanowski E.G., Bartels S.P., Gordon Y.J. Comparative antiviral efficacies of cidofovir, trifluridine, and acyclovir in the HSV-1 rabbit keratitis model. Invest Ophthalmol Vis Sci. 1999;40:378–384. [PubMed] [Google Scholar]
- 14.Turner L.D., Beckingsale P. Acyclovir-resistant herpetic keratitis in a solid-organ transplant recipient on systemic immunosuppression. Clin Ophthalmol. 2013;7:229–232. doi: 10.2147/OPTH.S39113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hillenkamp J., Reinhard T., Ross R.S., et al. The effects of cidofovir 1% with and without cyclosporin a 1% as a topical treatment of acute adenoviral keratoconjunctivitis: a controlled clinical pilot study. Ophthalmology. 2002;109:845–850. doi: 10.1016/s0161-6420(02)00992-2. [DOI] [PubMed] [Google Scholar]
- 16.Gordon Y.J., Romanowski E.G., Araullo-Cruz T. HPMPC, a broad-spectrum topical antiviral agent, inhibits herpes simplex virus type 1 replication and promotes healing of dendritic keratitis in the New Zealand rabbit ocular model. Cornea. 1994;13:516–520. [PubMed] [Google Scholar]
- 17.Maudgal P.C., De Clercq E. (S)-1-(3-hydroxy-2-phosphonyl-methoxypropyl)cytosine in the therapy of thymidine kinase-positive and -deficient herpes simplex virus experimental keratitis. Invest Ophthalmol Vis Sci. 1991;32:1816–1820. [PubMed] [Google Scholar]
- 18.Choong K., Walker N.J., Apel A.J.G., Whitby M. Aciclovir resistant herpes keratitis. Clin Exp Ophthalmol. 2010;38:309–313. doi: 10.1111/j.1442-9071.2010.02209.x. [DOI] [PubMed] [Google Scholar]
- 19.Yu J., Zhang M.-C. Clinical investigation of foscarnet sodium eye drops for the treatment of epithelial herpes simplex keratitis. Int Eye Sci. 2012;12:899–901. [Google Scholar]
- 20.Minkovitz J.B., Pepose J.S. Topical interferon alpha-2a treatment of herpes simplex keratitis resistant to multiple antiviral medications in an immunosuppressed patient. Cornea. 1995;14:326–330. doi: 10.1097/00003226-199505000-00017. [DOI] [PubMed] [Google Scholar]