Abstract
Colon cancer is the third most common malignancy and the fifth most frequent cause of death from neoplastic disease worldwide. At the time of diagnosis, more than 20% of patients already have metastatic disease. In the last 20 years, the natural course of the disease has changed due to major changes in the management of metastatic disease such as the advent of novel surgical and local therapy approaches as well as the introduction of novel chemotherapy drugs and targeted agents such as anti-epidermal growth factor receptor, anti-BRAF and antiangiogenics. Angiogenesis is a complex biological process of new vessel formation from existing ones and is an integral component of tumor progression supporting cancer cells to grow, proliferate and metastasize. Many molecules are involved in this proangiogenic process, such as vascular endothelial growth factor and its receptors on endothelial cells. A well-standardized methodology that is applied to assess angiogenesis in the tumor microenvironment is microvascular density by using immunohistochemistry with antibodies against endothelial CD31, CD34 and CD105 antigens. Even smaller molecules, such as the microRNAs, which are small non-coding RNAs, are being studied for their usefulness as surrogate biomarkers of angiogenesis and prognosis. In this review, we will discuss recent advances regarding the investigation of angiogenesis, the crosstalk between elements of the immune microenvironment and angiogenesis and how a disorganized tumor vessel network affects the trafficking of CD8+ T cells in the tumor bed. Furthermore, we will present recent data from clinical trials that combine antiangiogenic therapies with immune checkpoint inhibitors in colorectal cancer.
Keywords: Vascular endothelial growth factor, Circulating tumor cells, Colorectal cancer, MicroRNAs, Microvascular density, Crosstalk, Angiogenesis, Immunity
Core Tip: Colon cancer is one of the most common malignancies with a poor prognosis in patients with metastatic disease. Because of the need to find more effective treatments, researchers are focusing on deciphering the mechanisms used by the cancer cell for survival, food and metastasis. The main events in this process are neoangiogenesis and immune escape through the interplay of growth factors involved in both pathways. This review presents the events involved in these pathways with a focus on their prognostic and predictive value.
INTRODUCTION
Colorectal cancer (CRC), the third most common cancer in both genders, accounts for 9% of new cancer diagnoses in men and 8% in women and is the third leading cause of cancer death in both sexes. Although it is more common in those over the age of 70, a significant proportion of patients are of middle age. The 5-year survival rate of patients with localized disease CRC is 90%. However, this rate is significantly lower in patients with metastatic disease, reaching 14% and 15% in those with colon and rectal cancer, respectively[1].
Due to the poor prognosis of metastatic CRC (mCRC), the need for novel therapeutic approaches in these patients is urgently needed. A step towards this direction was made possible by the introduction of antiangiogenetic agents, but there are several unmet needs to better define patient profiles benefiting from such an approach. Moreover, despite this and other new treatments, the prognosis of patients with mCRC is still poor, and research is focusing on biomarkers with predictive and prognostic value (Table 1). Despite extensive research, in everyday practice only mutations in RAS, BRAF, NTRK and HER2 genes as well as the level of microsatellite instability have found application in the targeted therapy of mCRC[2,3]. As the complex process of carcinogenesis and metastasis is continuously defined, this knowledge is expected to lead to the discovery of new therapies.
Table 1.
Factors related to angiogenesis and immunity and studied as biomarkers in colorectal cancer
|
Factor
|
Biologic material
|
Pathway
|
Significance
|
Ref.
|
| VEGF | Tissue, blood | Angiogenesis | Prognostic & predictive | Bendardaf et al[63] 2017, Des Guetz et al[7] 2006, Ferroni et al[64] 2006, Pascual et al[65] 2018, Tsai et al[66] 2013, Tsai et al[67] 2015, Boussios et al[68] 2019, Zygoń et al[23] 2017, Mohamed et al[69] 2019 |
| VEGF polymorphism | Tissue, blood | Angiogenesis | Prognostic & predictive | Mousa et al[9] 2015 |
| HIF-1α | Tissue | Angiogenesis | Prognostic | Baba et al[5] 2010 |
| CTCs | Blood | Angiogenesis | Prognostic & predictive | Arrazubi et al[34] 2019, Burz et al[25] 2018, Cabel et al[28] 2017, Tan et al[33] 2018, Wang et al[35] 2019, Zhang et al[70] 2017 |
| CTCs | Blood | Angiogenesis | Predictive | Nakamura et al[3] 2018 |
| MicroRNA | Tissue, blood, stools | Angiogenesis | Prognostic & predictive | Balacescu et al[40] 2018, Boussios et al[68] 2019, Peng et al[41] 2017, To et al[38] 2018 |
| MVD | Tissue | Immunity | Prognostic | den Uil et al[71] 2019, Des Guetz et al[7] 2006, Mohammed et al[72] 2020, Zhu et al[19] 2017, Zygoń et al[23] 2017 |
VEGF: Vascular endothelial growth factor; HIF-1α: Hypoxia–inducible factor 1-alpha; CTCs: Circulating tumor cells; MVD: Microvascular density.
In this review, we will discuss recent advances in CRC regarding the investigation of angiogenesis, the crosstalk between the immune microenvironment and angiogenesis and the ways through which cancer cells escape the host immune system. Furthermore, we will present recent data from clinical trials that combine antiangiogenic agents with immune checkpoint inhibitors.
ANGIOGENESIS
Vascular endothelial growth factor
Angiogenesis is a complex mechanism of new vessel production that the cancer cell uses to ensure the supply of oxygen and nutrients and thus to multiply and generate evolving solid tumors with distant metastases[4].
There are two main regulators of angiogenesis that are essential for the development of CRC, hypoxia factor-1α and vascular endothelial growth factor (VEGF). Hypoxia factor-1α is a proangiogenic factor and is found in the tumor microenvironment. It is secreted by the cancer cell under hypoxic conditions and affects a wide variety of signaling pathways, including the upregulation of the VEGF cascade[4-6]. VEGF has several important functions, the most important one being the increase of vascular permeability and the induction of new blood vessels through its binding to endothelial cells and by promoting their proliferation[7,8].
VEGF comprises a group of glycoproteins that, together with placental growth factor, interact with three VEGF receptors (VEGFR1, VEGFR2, VEGFR3) and two neuropilin co-receptors (NRP1, NRP2). VEGFRs are tyrosine kinase receptors found in endothelial vascular cells. The binding of the glycoprotein to its receptor results in the initiation of a sequence of events that ultimately result in the formation of new vessels[3].The ligation of VEGF-A with VEGFR-2 is the most important step in the activation of angiogenesis in CRC[9].
Bevacizumab is a monoclonal antibody targeting VEGF-A and the first antiangiogenic agent to be used against metastatic cancer. Bevacizumab was approved in 2004 in the United States and in 2005 in Europe for use in patients with mCRC. Its mechanism of action is mediated through the inhibition of the interaction of VEGF-A with VEGFR, and thus bevacizumab inhibits the signaling pathway that promotes neovascularization[10]. Finding biomarkers that could predict the response to antiangiogenic therapy so that it could be used only in patients who would benefit from its administration is a currently unmet need.
Due to the dominant role of VEGF in angiogenesis, researchers investigated whether the expression of this factor could be a predictive biomarker for patients receiving antiangiogenic therapy. One study indicated that high VEGF baseline levels associated with worse response to bevacizumab treatment and progression-free survival[11]. In 2013 Hegde et al[12] showed that there is no statistically significant relationship between plasma VEGF-A levels and the clinical response to bevacizumab. Therefore, it has no predictive value in metastatic colon cancer. Another exploratory analysis investigating epithelial and stromal VEGF expression, assessed by in situ hybridization and immunohistochemistry on tissue microarrays and whole tumor tissue sections, suggested that in patients with mCRC the addition of bevacizumab to chemotherapy improves survival regardless of the level of VEGF expression[13]. Mavericc was the first prospective mCRC study using gene expression data from blood (plasma VEGF-A protein levels) to evaluate the efficacy of mCRC chemotherapy regimens indicating that high plasma VEGF levels were associated with shorter treatment duration of response and progression-free survival[14]. More interesting, VEGF polymorphisms have also been studied, and it appears that they could possibly be used as predictive agents in mCRC in patients treated with irinotecan and bevacizumab[15]. In another study, VEGF-A (c.*237C>T) was associated with a significantly better time to treatment failure[16]. Another study investigating the predictive role of VEGF-A indicated a significant association of rs833061 single nucleotide polymorphism with the overall response rate in advanced CRC patients treated with cytotoxic chemotherapy plus bevacizumab[17].
Microvascular density
An important indicator used in translational studies to assess the degree of neovascularization of the tumor is the microvascular density (MVD). MVD appears to increase as it progresses from normal mucosa to adenoma and from adenoma to cancer, and this is explained by the intense angiogenesis that aims to meet the neoplastic cells need for oxygen[18]. MVD was found to be higher in primary tumors than in metastases[5,18], while its levels within the tumor were associated with an increased risk of distant metastases[19]. The assessment of MVD includes pan-endothelial cell markers, also expressed in normal tissues, such as CD31 and CD34, as well as endothelial markers expressed on the surface of proliferating endothelial cells, such as CD105[18,20]. Endoglin is expressed mainly in vascular endothelial cells during active angiogenesis, while it is only weakly expressed or absent in pre-existing vascular endothelial cells, making this marker an important indicator of neoangiogenesis[18].
A systematic review and meta-analysis have indicated that increased VEGF and MVD expression markers are associated with an increased incidence of metastasis in CRC patients treated with surgery and chemotherapy[21]. An attempt was also made to correlate MVD with clinicopathologic features, such as sex, age, location, grade of differentiation, infiltrated lymph nodes and distant metastases, but with contradictory results. A negative correlation was found in two studies that investigated MVD in relation to the above variables[22,23], but in two other studies MVD staining was positively associated with tumor invasion, lymph node metastases[18] and distant metastases[19].
Since MVD is a biomarker for the quantification of angiogenesis, the question arises whether it can be used as a predictor of the treatment outcome with the antiangiogenic agent bevacizumab.
In 2006, Jubb et al[13], reported a clinical study of 813 patients with mCRC and found no association between elevated MVD or VEGF expression and the clinical outcome in relation to bevacizumab treatment. Although the predictive value of MVD in relation to bevacizumab response has been recognized in other cancers such as advanced ovarian cancer[24], in mCRC this has not yet been demonstrated.
CIRCULATING TUMOR CELLS
It has been postulated that cancer cells circulate in the peripheral blood of patients with metastatic disease[25,26]. It is reasonable to expect that the isolation and study of these cells can provide information about the metastatic potential of primary disease and an assessment of their value as prognostic and predictive biomarkers[25].
The mechanism by which cancer cells enter the circulation and acquire the ability to metastasize is not fully understood. However, this process appears to be activated by tumor hypoxia, which also activates angiogenesis[27].
It has been estimated that the frequency of circulating tumor cells (CTC) is about 1 per 1 mL of peripheral blood[28] or otherwise 1 g of tumor releases 106 cells into the bloodstream[25]. Despite the large number of cells released into the bloodstream daily, a small number can be detected and isolated. This is partly due to the fact that these cells are covered by platelets and coagulation factors[29]. However, with the advent of new methods, it is now more feasible to isolate circulating cancer cells and study them[30]. Liquid biopsy, the isolation of CTCs or tumor cell-free DNA from peripheral blood is only minimally invasive compared to tumor biopsy and can be repeated many times for the monitoring of genomic changes that contribute to cancer progression and/or resistance to chemotherapy[31].
Although CTCs have been isolated in the blood of patients with polyps of the colon, the number of CTCs measured in the blood of patients with colon cancer is statistically significantly higher[28]. Furthermore, a smaller number of CTCs is detected in well-differentiated tumors compared to the less differentiated counterparts. The number of CTCs does not seem to be related to the tumoral histologic subtype, whereas it seems to be related to the anatomical location, being higher in cancer of the rectum and sigmoid colon compared to other sites[32]. Circulating cancer cells is an independent prognostic factor for the survival of patients with CRC[33]. In patients with mCRC and liver secondaries treated with complete resection of the primary tumor site and liver metastases, the presence of two or more CTCs/7.5 mL of blood preoperatively was an indicator of poor disease outcome and low survival[34]. Furthermore, according to another recent study, the CTC-positivity rate was an independent predictive factor of progression-free survival and overall survival in patients with advanced disease treated with chemotherapy. In addition, the CTC concentration was related to the pathological stage of the disease, the presence of metastatic disease, the depth of tumor invasion, the presence of lymphatic invasion and high serum carcinoembryonic antigen levels[35].
MicroRNAs
In recent years microRNAs, have been studied as biomarkers for diagnosis, prognosis and treatment resistance in patients with CRC. MicroRNAs are small non-coding molecules consisting of 18 to 25 nucleotides that control the expression of many target genes, either by inhibiting their expression or by stimulating it. Thus, by affecting the expression of oncogenes it is possible to either inhibit or promote oncogenesis[36]. These molecules can be detected not only in tissues but also in the serum and feces of cancer patients. They are found extracellularly either as a result of cancer cell death or due to extracellular secretion by cancer cells[37]. MicroRNAs target the 3’ untranslated region of target genes, thereby degrading and controlling their expression[36]. MicroRNA interaction with target genes and their mRNA is affected by single nucleotide polymorphisms in the 3’ untranslated region of these target genes, which also affect their expression. These polymorphisms have been studied to predict treatment outcomes, such as resistance to chemotherapy[38].
MicroRNAs are extremely stable molecules because they are stored in extracellular structures or bound to lipoproteins[38]. This feature and the fact that they do not require invasive methods for their detection make them potential ideal diagnostic and prognostic biomarkers.
The association of microRNAs with CRC was first described by Michael et al[39] in 2003. In this study, the authors showed that microRNA-143 and microRNA-145 levels were reduced in precancerous adenomatous lesions and CRC compared with normal mucosa. Since then, several research studies and meta-analyses have been published, emphasizing the importance of microRNAs in cancer[40].
In addition to oncogenesis, there are microRNAs that target regulatory molecules that lead to angiogenesis. These molecules, known as “angiomiRs,” either promote or suppress angiogenesis, thereby indirectly affecting tumor formation and metastasis.
MicroRNA-21 is the most representative of neoangiogenesis as it has been studied in many types of cancer and by several researchers. In a meta-analysis published in 2017, Peng et al[41] analyzed data from 57 studies and concluded that microRNA-21 has a diagnostic sensitivity of 64% and a specificity of 85%, making it a potential prognostic indicator for patient survival. According to this study, peripheral blood microRNA-21 levels can be used as an indicator of CRC detection, and tissue levels can be an indicator to predict patient survival.
In addition to microRNA-21, there are many other microRNAs that target regulatory molecules leading to angiogenesis. Such molecules are microRNA-126, microRNA-30, microRNA-182, microRNA-194, microRNA-23b, microRNA-27a, microRNA-27b, microRNA-29b, microRNA-143, microRNA-145 and the complexes microRNA17-92, microRNA15a/16-1, microRNA-885-3p and microRNA885-3p[42].
MicroRNAs in the stool are the least studied but have been proven stable enough to correlate with the stage of the disease and have a high sensitivity and specificity in distinguishing patients from healthy individuals[38].
Long non-coding RNAs are made up of about 200 nucleotides and have also been studied as prognostic biomarkers. Although not translated into proteins, they act competitively by binding to common microRNA binding sequences and trapping them to alter the expression of their target genes. Available data suggest that long non-coding RNAs play a role not only in CRC development but also in metastasis[43].
THE CROSSTALK BETWEEN ANGIOGENESIS AND IMMUNITY
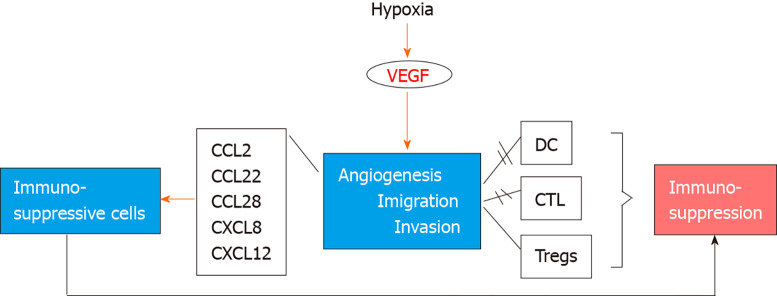
Tumor development and progression are highly dependent on the vascular network that penetrates the tumor bed and supplies proliferating malignant cells with oxygen and nutrients[44]. Although several mechanisms contribute to the constant development of the new vascular network, i.e., neoangiogenesis, most new vessels are considered to be formed by the sprouting from parental ones[45]. The process of neoangiogenesis is triggered by hypoxia and deprivation of nutrients and is regulated by many proangiogenic and antiangiogenic factors such as VEGF-A, fibroblast growth factor, platelet-derived growth factor, transforming growth factor and others[45-47]. Compared to normal tissue vasculature, tumor neoangiogenesis is characterized by abnormalities in structure and function, driven by the imbalance between proangiogenic, mainly VEGF, and antiangiogenic factors in the tumor microenvironment[48]. The abnormal structure and function of the tumor vasculature significantly affect the anti-tumor immunity, facilitating immune evasion in many different aspects (Figure 1). Overexpression of VEGF, produced by tumor cells, platelets and inflammatory cells such as neutrophils and monocytes, promotes the formation of an immature vascular network with increased leakiness, which in combination with the increased physical compression in the tumor bed leads to impaired blood perfusion and reduction of delivering oxygen and cytotoxic T cells in the tumor area[8,49]. Moreover, hypoxia/acidosis induced growth factors and cytokines such as transforming growth factor-β and VEGF suppress the activity of cytotoxic T cells, suppress the antigen presenting capacity of dendritic cells, reprogram macrophages into a protumorigenic phenotype and upregulate the expression of programmed cell death-ligand 1 by tumor cells, myeloid-derived suppressor cells and dendritic cells and macrophages, further increasing immune evasion in the tumor microenvironment[8,50-52]. Of note, hypoxia-induced chemokines such as C-C motif chemokine ligand 2, C-C motif chemokine ligand 22, C-C motif chemokine ligand 28, C-X-C motif chemokine ligand 8 and C-X-C motif chemokine ligand 12 recruit immunosuppressive cells in the tumor microenvironment such as myeloid-derived suppressor cells, regulatory T cells and M2 macrophages[53] (Figure 1). In addition, tumor endothelial cells, in contrast to normal vasculature, express FasL and acquire the ability to kill effector CD8+ T cells but not regulatory T cells[54,55].
Figure 1.
Τhe sequence of events following hypoxia and vascular endothelial growth factor secretion leading to immune system escape and carcinogenesis. VEGF: Vascular endothelial growth factor; CCL: C-C motif chemokine ligand; CXCL12: C-X-C motif chemokine ligand 12; DC: Dendritic cells; CTL: Cytotoxic T lymphocytes; Tregs: Regulatory T cells.
Immunotherapy is now a key therapeutic weapon in the treatment of many cancers, such as melanoma, lung and urothelial cancer and has significantly improved patients’ prognosis. Immunotherapies target immune checkpoints that are abnormally expressed in many patients and aim to kill the tumor indirectly by boosting the anti-tumor immune responses. Cytotoxic T-lymphocyte-associated protein 4 and programmed cell death protein 1 with its ligand programmed cell death-ligand 1 are primarily involved in inhibitory immune signaling and are essential regulators of cancer immune evasion. Current clinical practice includes mainly two types of immune checkpoint inhibitors such as anti-cytotoxic T-lymphocyte-associated protein 4 (ipilimumab and tremelimumab) and anti-programmed cell death protein 1/programmed cell death-ligand 1 (nivolumab, atezolizumab, pembrolizumab) monoclonal antibodies[56]. However, in CRC these therapies have not proved to mediate similar effects, except in tumors with microsatellite instability[57].
As the immunosuppressive tumor microenvironment is additionally induced in part by the dysfunctional vascular network, a window for therapeutic application opens for the combination of immunotherapies and antiangiogenics. This strategy has been exploited in several clinical trials for different tumor types[51], such as non-small cell lung cancer (atezolizumab and bevacizumab)[58], renal cell carcinoma (axitinib and pembrolizumab or cabozantinib and nivolumab)[59,60], endometrial cancer (lenvatinib and pembrolizumab)[61] and hepatocellular carcinoma (atezolizumab and bevacizumab)[62].
Regarding CRC, ongoing clinical studies (Table 2) are investigating the effectiveness of combinations of antiangiogenic agents and immune checkpoint inhibitors. It is possible that such combinations could be applied in the future treatment of mCRC.
Table 2.
Clinical trials related to antiangiogenic agent therapy and immunotherapy in colorectal cancer
|
Status
|
Study title
|
Drugs
|
Country
|
| Recruiting | A study evaluating the efficacy and safety of multiple immunotherapy-based treatment combinations in patients with metastatic colorectal cancer (Morpheus-CRC) | Regorafenib, atezolizumab | United States |
| Recruiting | Study of chemotherapy combination with autologous cell | Bevacizumab, oxaliplatin, capecitabine; Biological component: PD1-T cells | China |
| Recruiting | Treatment of colorectal liver metastases with immunotherapy and bevacizumab | Atezolizumab, bevacizumab, oxaliplatin | Korea |
| Recruiting | Neoadjuvant treatment in rectal cancer with radiotherapy followed by atezolizumab and bevacizumab (TARZAN) | Atezolizumab, bevacizumab | Netherlands |
| Not yet recruiting | Chemotherapy and immunotherapy as treatment for MSS metastatic | Capecitabine, oxaliplatin, bevacizumab, pembrolizumab | France |
| Not yet recruiting | QL1101 in combination with JS001 in patients with pMMR/MSS refractory metastatic | Bevacizumab, tripleitriumab | China |
| Not yet recruiting | Comparison of sintilimab to XELOX | Sintilimab vs XELOX + bevacizumab | China |
CRC: Colorectal cancer; PD-1: Programmed cell death protein 1.
CONCLUSION
Due to the poor prognosis of patients with mCRC, research has focused not only on finding prognostic and predictive factors but also on new therapeutic combinations. Immunohistochemistry methods have been instrumental in finding molecules that could be used as predictors, but molecular biology and immunology have been most informative in dissecting the mechanisms by which the cancer cell survives and spreads. Understanding how the immune and vascular microenvironments interact has opened new horizons in cancer treatment. Although such combination therapies for CRC have not yet been approved, the results of clinical trials are eagerly awaited.
Finding new molecular targets for different approaches including immunotherapy may enrich treatment options for CRC in the future.
ACKNOWLEDGEMENTS
Part of the expenses and materials for this narrative review were provided by the Hellenic Society of Medical Oncology and the Hellenic Study Group of Psychoneuroimmunology in Cancer. The authors express their gratitude to Professor Dimitrios T Boumpas for expert revision of the manuscript.
Footnotes
Conflict-of-interest statement: All authors declare no conflicts-of interest related to this article.
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: May 30, 2021
First decision: July 31, 2021
Article in press: December 28, 2021
Specialty type: Medical laboratory technology
Country/Territory of origin: Greece
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Bogach J, Valiveti CK S-Editor: Wang JJ L-Editor: Filipodia P-Editor: Wang JJ
Contributor Information
Katerina Kampoli, Hematology Oncology Unit, The Fourth Department of Internal Medicine, School of Medicine, National and Kapodistrian University of Athens, Attikon University Hospital, Haidari 12462, Athens, Greece.
Periklis G Foukas, The Second Department of Pathology, School of Medicine, National and Kapodistrian University of Athens, Attikon University Hospital, Haidari 12462, Athens, Greece.
Anastasios Ntavatzikos, Hematology Oncology Unit, The Fourth Department of Internal Medicine, School of Medicine, National and Kapodistrian University of Athens, Attikon University Hospital, Haidari 12462, Athens, Greece.
Nikolaos Arkadopoulos, The Fourth Surgical Clinic, School of Medicine, National and Kapodistrian University of Athens, Attikon University Hospital, Haidari 12462, Athens, Greece.
Anna Koumarianou, Hematology Oncology Unit, The Fourth Department of Internal Medicine, School of Medicine, National and Kapodistrian University of Athens, Attikon University Hospital, Haidari 12462, Athens, Greece. akoumari@yahoo.com.
References
- 1.Siegel RL, Miller KD, Goding Sauer A, Fedewa SA, Butterly LF, Anderson JC, Cercek A, Smith RA, Jemal A. Colorectal cancer statistics, 2020. CA Cancer J Clin. 2020;70:145–164. doi: 10.3322/caac.21601. [DOI] [PubMed] [Google Scholar]
- 2.DeStefanis RA, Kratz JD, Emmerich PB, Deming DA. Targeted Therapy in Metastatic Colorectal Cancer: Current Standards and Novel Agents in Review. Curr Colorectal Cancer Rep. 2019;15:61–69. doi: 10.1007/s11888-019-00430-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nakamura Y, Yoshino T. Clinical Utility of Analyzing Circulating Tumor DNA in Patients with Metastatic Colorectal Cancer. Oncologist. 2018;23:1310–1318. doi: 10.1634/theoncologist.2017-0621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sun W. Angiogenesis in metastatic colorectal cancer and the benefits of targeted therapy. J Hematol Oncol. 2012;5:63. doi: 10.1186/1756-8722-5-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baba Y, Nosho K, Shima K, Irahara N, Chan AT, Meyerhardt JA, Chung DC, Giovannucci EL, Fuchs CS, Ogino S. HIF1A overexpression is associated with poor prognosis in a cohort of 731 colorectal cancers. Am J Pathol. 2010;176:2292–2301. doi: 10.2353/ajpath.2010.090972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yin L, Li J, Ma D, Li D, Sun Y. Angiogenesis in primary colorectal cancer and matched metastatic tissues: Biological and clinical implications for anti-angiogenic therapies. Oncol Lett. 2020;19:3558–3566. doi: 10.3892/ol.2020.11450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Des Guetz G, Uzzan B, Nicolas P, Cucherat M, Morere JF, Benamouzig R, Breau JL, Perret GY. Microvessel density and VEGF expression are prognostic factors in colorectal cancer. Meta-analysis of the literature. Br J Cancer. 2006;94:1823–1832. doi: 10.1038/sj.bjc.6603176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee WS, Yang H, Chon HJ, Kim C. Combination of anti-angiogenic therapy and immune checkpoint blockade normalizes vascular-immune crosstalk to potentiate cancer immunity. Exp Mol Med. 2020;52:1475–1485. doi: 10.1038/s12276-020-00500-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mousa L, Salem ME, Mikhail S. Biomarkers of Angiogenesis in Colorectal Cancer. Biomark Cancer. 2015;7:13–19. doi: 10.4137/BIC.S25250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garcia J, Hurwitz HI, Sandler AB, Miles D, Coleman RL, Deurloo R, Chinot OL. Bevacizumab (Avastin®) in cancer treatment: A review of 15 years of clinical experience and future outlook. Cancer Treat Rev. 2020;86:102017. doi: 10.1016/j.ctrv.2020.102017. [DOI] [PubMed] [Google Scholar]
- 11.Jürgensmeier JM, Schmoll HJ, Robertson JD, Brooks L, Taboada M, Morgan SR, Wilson D, Hoff PM. Prognostic and predictive value of VEGF, sVEGFR-2 and CEA in mCRC studies comparing cediranib, bevacizumab and chemotherapy. Br J Cancer. 2013;108:1316–1323. doi: 10.1038/bjc.2013.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hegde PS, Jubb AM, Chen D, Li NF, Meng YG, Bernaards C, Elliott R, Scherer SJ, Chen DS. Predictive impact of circulating vascular endothelial growth factor in four phase III trials evaluating bevacizumab. Clin Cancer Res. 2013;19:929–937. doi: 10.1158/1078-0432.CCR-12-2535. [DOI] [PubMed] [Google Scholar]
- 13.Jubb AM, Hurwitz HI, Bai W, Holmgren EB, Tobin P, Guerrero AS, Kabbinavar F, Holden SN, Novotny WF, Frantz GD, Hillan KJ, Koeppen H. Impact of vascular endothelial growth factor-A expression, thrombospondin-2 expression, and microvessel density on the treatment effect of bevacizumab in metastatic colorectal cancer. J Clin Oncol. 2006;24:217–227. doi: 10.1200/JCO.2005.01.5388. [DOI] [PubMed] [Google Scholar]
- 14.Parikh AR, Lee FC, Yau L, Koh H, Knost J, Mitchell EP, Bosanac I, Choong N, Scappaticci F, Mancao C, Lenz HJ. MAVERICC, a Randomized, Biomarker-stratified, Phase II Study of mFOLFOX6-Bevacizumab versus FOLFIRI-Bevacizumab as First-line Chemotherapy in Metastatic Colorectal Cancer. Clin Cancer Res. 2019;25:2988–2995. doi: 10.1158/1078-0432.CCR-18-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koutras AK, Antonacopoulou AG, Eleftheraki AG, Dimitrakopoulos FI, Koumarianou A, Varthalitis I, Fostira F, Sgouros J, Briasoulis E, Bournakis E, Bafaloukos D, Bompolaki I, Galani E, Kalogeras KT, Pectasides D, Fountzilas G, Kalofonos HP. Vascular endothelial growth factor polymorphisms and clinical outcome in colorectal cancer patients treated with irinotecan-based chemotherapy and bevacizumab. Pharmacogenomics J. 2012;12:468–475. doi: 10.1038/tpj.2011.37. [DOI] [PubMed] [Google Scholar]
- 16.Sibertin-Blanc C, Mancini J, Fabre A, Lagarde A, Del Grande J, Levy N, Seitz JF, Olschwang S, Dahan L. Vascular Endothelial Growth Factor A c.*237C>T polymorphism is associated with bevacizumab efficacy and related hypertension in metastatic colorectal cancer. Dig Liver Dis. 2015;47:331–337. doi: 10.1016/j.dld.2014.12.013. [DOI] [PubMed] [Google Scholar]
- 17.Sohn BS, Park SJ, Kim JE, Kim KP, Hong YS, Suh C, Kim YS, Kim SY, Im SA, Kim JH, Ahn JB, Park YS, Kim TW. Single-nucleotide polymorphisms in the vascular endothelial growth factor pathway and outcomes of patients treated with first-line cytotoxic chemotherapy combined with bevacizumab for advanced colorectal cancer. Oncology. 2014;87:280–292. doi: 10.1159/000365593. [DOI] [PubMed] [Google Scholar]
- 18.Hasan J, Byers R, Jayson GC. Intra-tumoural microvessel density in human solid tumours. Br J Cancer. 2002;86:1566–1577. doi: 10.1038/sj.bjc.6600315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu B, Zhou L, Yu L, Wu S, Song W, Gong X, Wang D. Evaluation of the correlation of vasculogenic mimicry, ALDH1, KAI1 and microvessel density in the prediction of metastasis and prognosis in colorectal carcinoma. BMC Surg. 2017;17:47. doi: 10.1186/s12893-017-0246-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dallas NA, Samuel S, Xia L, Fan F, Gray MJ, Lim SJ, Ellis LM. Endoglin (CD105): a marker of tumor vasculature and potential target for therapy. Clin Cancer Res. 2008;14:1931–1937. doi: 10.1158/1078-0432.CCR-07-4478. [DOI] [PubMed] [Google Scholar]
- 21.Wang Y, Yao X, Ge J, Hu F, Zhao Y. Can vascular endothelial growth factor and microvessel density be used as prognostic biomarkers for colorectal cancer? ScientificWorldJournal. 2014;2014:102736. doi: 10.1155/2014/102736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hutajulu SH, Paramita DK, Santoso J, Sani MIA, Amalia A, Wulandari G, Ghozali A, Kurnianda J. Correlation between vascular endothelial growth factor-A expression and tumor location and invasion in patients with colorectal cancer. J Gastrointest Oncol. 2018;9:1099–1108. doi: 10.21037/jgo.2018.07.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zygoń J, Szajewski M, Kruszewski WJ, Rzepko R. VEGF, Flt-1, and microvessel density in primary tumors as predictive factors of colorectal cancer prognosis. Mol Clin Oncol. 2017;6:243–248. doi: 10.3892/mco.2016.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bais C, Mueller B, Brady MF, Mannel RS, Burger RA, Wei W, Marien KM, Kockx MM, Husain A, Birrer MJ NRG Oncology/Gynecologic Oncology Group. Tumor Microvessel Density as a Potential Predictive Marker for Bevacizumab Benefit: GOG-0218 Biomarker Analyses. J Natl Cancer Inst. 2017;109 doi: 10.1093/jnci/djx066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burz C, Pop VV, Buiga R, Daniel S, Samasca G, Aldea C, Lupan I. Circulating tumor cells in clinical research and monitoring patients with colorectal cancer. Oncotarget. 2018;9:24561–24571. doi: 10.18632/oncotarget.25337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Wit S, van Dalum G, Terstappen LW. Detection of circulating tumor cells. Scientifica (Cairo) 2014;2014:819362. doi: 10.1155/2014/819362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Donato C, Kunz L, Castro-Giner F, Paasinen-Sohns A, Strittmatter K, Szczerba BM, Scherrer R, Di Maggio N, Heusermann W, Biehlmaier O, Beisel C, Vetter M, Rochlitz C, Weber WP, Banfi A, Schroeder T, Aceto N. Hypoxia Triggers the Intravasation of Clustered Circulating Tumor Cells. Cell Rep. 2020;32:108105. doi: 10.1016/j.celrep.2020.108105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cabel L, Proudhon C, Gortais H, Loirat D, Coussy F, Pierga JY, Bidard FC. Circulating tumor cells: clinical validity and utility. Int J Clin Oncol. 2017;22:421–430. doi: 10.1007/s10147-017-1105-2. [DOI] [PubMed] [Google Scholar]
- 29.Plaks V, Koopman CD, Werb Z. Cancer. Circulating tumor cells. Science. 2013;341:1186–1188. doi: 10.1126/science.1235226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sharma S, Zhuang R, Long M, Pavlovic M, Kang Y, Ilyas A, Asghar W. Circulating tumor cell isolation, culture, and downstream molecular analysis. Biotechnol Adv. 2018;36:1063–1078. doi: 10.1016/j.biotechadv.2018.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gerdtsson AS, Thiele JA, Shishido SN, Zheng S, Schaffer R, Bethel K, Curley S, Lenz HJ, Hanna DL, Nieva J, Kolatkar A, Ruiz C, Rodriguez-Lee M, Oakley Iii GJ, Lee JSH, Hicks J, Kuhn P. Single cell correlation analysis of liquid and solid biopsies in metastatic colorectal cancer. Oncotarget. 2019;10:7016–7030. doi: 10.18632/oncotarget.27271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang C, Zhuang W, Hu Y, Zhu L. Clinical significance of peripheral circulating tumor cell counts in colorectal polyps and non-metastatic colorectal cancer. World J Surg Oncol. 2018;16:13. doi: 10.1186/s12957-017-1305-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tan Y, Wu H. The significant prognostic value of circulating tumor cells in colorectal cancer: A systematic review and meta-analysis. Curr Probl Cancer. 2018;42:95–106. doi: 10.1016/j.currproblcancer.2017.11.002. [DOI] [PubMed] [Google Scholar]
- 34.Arrazubi V, Mata E, Antelo ML, Tarifa A, Herrera J, Zazpe C, Teijeira L, Viudez A, Suárez J, Hernández I, Vera R. Circulating Tumor Cells in Patients Undergoing Resection of Colorectal Cancer Liver Metastases. Clinical Utility for Long-Term Outcome: A Prospective Trial. Ann Surg Oncol. 2019;26:2805–2811. doi: 10.1245/s10434-019-07503-8. [DOI] [PubMed] [Google Scholar]
- 35.Wang L, Zhou S, Zhang W, Wang J, Wang M, Hu X, Liu F, Zhang Y, Jiang B, Yuan H. Circulating tumor cells as an independent prognostic factor in advanced colorectal cancer: a retrospective study in 121 patients. Int J Colorectal Dis. 2019;34:589–597. doi: 10.1007/s00384-018-03223-9. [DOI] [PubMed] [Google Scholar]
- 36.Fadaka AO, Pretorius A, Klein A. Biomarkers for Stratification in Colorectal Cancer: MicroRNAs. Cancer Control. 2019;26:1073274819862784. doi: 10.1177/1073274819862784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gmerek L, Martyniak K, Horbacka K, Krokowicz P, Scierski W, Golusinski P, Golusinski W, Schneider A, Masternak MM. MicroRNA regulation in colorectal cancer tissue and serum. PLoS One. 2019;14:e0222013. doi: 10.1371/journal.pone.0222013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.To KK, Tong CW, Wu M, Cho WC. MicroRNAs in the prognosis and therapy of colorectal cancer: From bench to bedside. World J Gastroenterol. 2018;24:2949–2973. doi: 10.3748/wjg.v24.i27.2949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Michael MZ, O' Connor SM, van Holst Pellekaan NG, Young GP, James RJ. Reduced accumulation of specific microRNAs in colorectal neoplasia. Mol Cancer Res. 2003;1:882–891. [PubMed] [Google Scholar]
- 40.Balacescu O, Sur D, Cainap C, Visan S, Cruceriu D, Manzat-Saplacan R, Muresan MS, Balacescu L, Lisencu C, Irimie A. The Impact of miRNA in Colorectal Cancer Progression and Its Liver Metastases. Int J Mol Sci. 2018;19 doi: 10.3390/ijms19123711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peng Q, Zhang X, Min M, Zou L, Shen P, Zhu Y. The clinical role of microRNA-21 as a promising biomarker in the diagnosis and prognosis of colorectal cancer: a systematic review and meta-analysis. Oncotarget. 2017;8:44893–44909. doi: 10.18632/oncotarget.16488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Salinas-Vera YM, Marchat LA, Gallardo-Rincón D, Ruiz-García E, Astudillo-De La Vega H, Echavarría-Zepeda R, López-Camarillo C. AngiomiRs: MicroRNAs driving angiogenesis in cancer (Review) Int J Mol Med. 2019;43:657–670. doi: 10.3892/ijmm.2018.4003. [DOI] [PubMed] [Google Scholar]
- 43.Wang L, Cho KB, Li Y, Tao G, Xie Z, Guo B. Long Noncoding RNA (lncRNA)-Mediated Competing Endogenous RNA Networks Provide Novel Potential Biomarkers and Therapeutic Targets for Colorectal Cancer. Int J Mol Sci. 2019;20 doi: 10.3390/ijms20225758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 45.Weis SM, Cheresh DA. Tumor angiogenesis: molecular pathways and therapeutic targets. Nat Med. 2011;17:1359–1370. doi: 10.1038/nm.2537. [DOI] [PubMed] [Google Scholar]
- 46.Lanitis E, Irving M, Coukos G. Targeting the tumor vasculature to enhance T cell activity. Curr Opin Immunol. 2015;33:55–63. doi: 10.1016/j.coi.2015.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lugano R, Ramachandran M, Dimberg A. Tumor angiogenesis: causes, consequences, challenges and opportunities. Cell Mol Life Sci. 2020;77:1745–1770. doi: 10.1007/s00018-019-03351-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.De Bock K, Cauwenberghs S, Carmeliet P. Vessel abnormalization: another hallmark of cancer? Curr Opin Genet Dev. 2011;21:73–79. doi: 10.1016/j.gde.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 49.Voron T, Marcheteau E, Pernot S, Colussi O, Tartour E, Taieb J, Terme M. Control of the immune response by pro-angiogenic factors. Front Oncol. 2014;4:70. doi: 10.3389/fonc.2014.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jain RK. Antiangiogenesis strategies revisited: from starving tumors to alleviating hypoxia. Cancer Cell. 2014;26:605–622. doi: 10.1016/j.ccell.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Leong A, Kim M. The Angiopoietin-2 and TIE Pathway as a Therapeutic Target for Enhancing Antiangiogenic Therapy and Immunotherapy in Patients with Advanced Cancer. Int J Mol Sci. 2020;21 doi: 10.3390/ijms21228689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Taube JM, Galon J, Sholl LM, Rodig SJ, Cottrell TR, Giraldo NA, Baras AS, Patel SS, Anders RA, Rimm DL, Cimino-Mathews A. Implications of the tumor immune microenvironment for staging and therapeutics. Mod Pathol. 2018;31:214–234. doi: 10.1038/modpathol.2017.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fukumura D, Kloepper J, Amoozgar Z, Duda DG, Jain RK. Enhancing cancer immunotherapy using antiangiogenics: opportunities and challenges. Nat Rev Clin Oncol. 2018;15:325–340. doi: 10.1038/nrclinonc.2018.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brighi N, Farolfi A, Conteduca V, Gurioli G, Gargiulo S, Gallà V, Schepisi G, Lolli C, Casadei C, De Giorgi U. The Interplay between Inflammation, Anti-Angiogenic Agents, and Immune Checkpoint Inhibitors: Perspectives for Renal Cell Cancer Treatment. Cancers (Basel) 2019;11 doi: 10.3390/cancers11121935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Motz GT, Santoro SP, Wang LP, Garrabrant T, Lastra RR, Hagemann IS, Lal P, Feldman MD, Benencia F, Coukos G. Tumor endothelium FasL establishes a selective immune barrier promoting tolerance in tumors. Nat Med. 2014;20:607–615. doi: 10.1038/nm.3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Seidel JA, Otsuka A, Kabashima K. Anti-PD-1 and Anti-CTLA-4 Therapies in Cancer: Mechanisms of Action, Efficacy, and Limitations. Front Oncol. 2018;8:86. doi: 10.3389/fonc.2018.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, Skora AD, Luber BS, Azad NS, Laheru D, Biedrzycki B, Donehower RC, Zaheer A, Fisher GA, Crocenzi TS, Lee JJ, Duffy SM, Goldberg RM, de la Chapelle A, Koshiji M, Bhaijee F, Huebner T, Hruban RH, Wood LD, Cuka N, Pardoll DM, Papadopoulos N, Kinzler KW, Zhou S, Cornish TC, Taube JM, Anders RA, Eshleman JR, Vogelstein B, Diaz LA Jr. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med. 2015;372:2509–2520. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Socinski MA, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, Nogami N, Rodríguez-Abreu D, Moro-Sibilot D, Thomas CA, Barlesi F, Finley G, Kelsch C, Lee A, Coleman S, Deng Y, Shen Y, Kowanetz M, Lopez-Chavez A, Sandler A, Reck M IMpower150 Study Group. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N Engl J Med. 2018;378:2288–2301. doi: 10.1056/NEJMoa1716948. [DOI] [PubMed] [Google Scholar]
- 59.Bedke J, Albiges L, Capitanio U, Giles RH, Hora M, Lam TB, Ljungberg B, Marconi L, Klatte T, Volpe A, Abu-Ghanem Y, Dabestani S, Fernández-Pello S, Hofmann F, Kuusk T, Tahbaz R, Powles T, Bex A. Updated European Association of Urology Guidelines on Renal Cell Carcinoma: Nivolumab plus Cabozantinib Joins Immune Checkpoint Inhibition Combination Therapies for Treatment-naïve Metastatic Clear-Cell Renal Cell Carcinoma. Eur Urol. 2021;79:339–342. doi: 10.1016/j.eururo.2020.12.005. [DOI] [PubMed] [Google Scholar]
- 60.Rini BI, Plimack ER, Stus V, Gafanov R, Hawkins R, Nosov D, Pouliot F, Alekseev B, Soulières D, Melichar B, Vynnychenko I, Kryzhanivska A, Bondarenko I, Azevedo SJ, Borchiellini D, Szczylik C, Markus M, McDermott RS, Bedke J, Tartas S, Chang YH, Tamada S, Shou Q, Perini RF, Chen M, Atkins MB, Powles T KEYNOTE-426 Investigators. Pembrolizumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N Engl J Med. 2019;380:1116–1127. doi: 10.1056/NEJMoa1816714. [DOI] [PubMed] [Google Scholar]
- 61.Makker V, Taylor MH, Aghajanian C, Oaknin A, Mier J, Cohn AL, Romeo M, Bratos R, Brose MS, DiSimone C, Messing M, Stepan DE, Dutcus CE, Wu J, Schmidt EV, Orlowski R, Sachdev P, Shumaker R, Casado Herraez A. Lenvatinib Plus Pembrolizumab in Patients With Advanced Endometrial Cancer. J Clin Oncol. 2020;38:2981–2992. doi: 10.1200/JCO.19.02627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kudo M. Scientific Rationale for Combined Immunotherapy with PD-1/PD-L1 Antibodies and VEGF Inhibitors in Advanced Hepatocellular Carcinoma. Cancers (Basel) 2020;12 doi: 10.3390/cancers12051089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bendardaf R, El-Serafi A, Syrjänen K, Collan Y, Pyrhönen S. The effect of vascular endothelial growth factor-1 expression on survival of advanced colorectal cancer patients. Libyan J Med. 2017;12:1290741. doi: 10.1080/19932820.2017.1290741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ferroni P, Palmirotta R, Spila A, Martini F, Formica V, Portarena I, Del Monte G, Buonomo O, Roselli M, Guadagni F. Prognostic value of carcinoembryonic antigen and vascular endothelial growth factor tumor tissue content in colorectal cancer. Oncology. 2006;71:176–184. doi: 10.1159/000106072. [DOI] [PubMed] [Google Scholar]
- 65.Pascual M, Alonso S, Salvans S, Mayol X, Mojal S, Gil MJ, Grande L, Pera M. Postoperative serum Vascular Endothelial Growth Factor is an independent prognostic factor of disease free survival and overall survival in patients with non metastatic colon cancer. Am J Surg. 2018;216:255–259. doi: 10.1016/j.amjsurg.2017.06.037. [DOI] [PubMed] [Google Scholar]
- 66.Tsai HL, Yang IP, Lin CH, Chai CY, Huang YH, Chen CF, Hou MF, Kuo CH, Juo SH, Wang JY. Predictive value of vascular endothelial growth factor overexpression in early relapse of colorectal cancer patients after curative resection. Int J Colorectal Dis. 2013;28:415–424. doi: 10.1007/s00384-012-1570-z. [DOI] [PubMed] [Google Scholar]
- 67.Tsai HL, Lin CH, Huang CW, Yang IP, Yeh YS, Hsu WH, Wu JY, Kuo CH, Tseng FY, Wang JY. Decreased peritherapeutic VEGF expression could be a predictor of responsiveness to first-line FOLFIRI plus bevacizumab in mCRC patients. Int J Clin Exp Pathol. 2015;8:1900–1910. [PMC free article] [PubMed] [Google Scholar]
- 68.Boussios S, Ozturk MA, Moschetta M, Karathanasi A, Zakynthinakis-Kyriakou N, Katsanos KH, Christodoulou DK, Pavlidis N. The Developing Story of Predictive Biomarkers in Colorectal Cancer. J Pers Med. 2019;9 doi: 10.3390/jpm9010012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mohamed SY, Mohammed HL, Ibrahim HM, Mohamed EM, Salah M. Role of VEGF, CD105, and CD31 in the Prognosis of Colorectal Cancer Cases. J Gastrointest Cancer. 2019;50:23–34. doi: 10.1007/s12029-017-0014-y. [DOI] [PubMed] [Google Scholar]
- 70.Zhang D, Zhao L, Zhou P, Ma H, Huang F, Jin M, Dai X, Zheng X, Huang S, Zhang T. Circulating tumor microemboli (CTM) and vimentin+ circulating tumor cells (CTCs) detected by a size-based platform predict worse prognosis in advanced colorectal cancer patients during chemotherapy. Cancer Cell Int. 2017;17:6. doi: 10.1186/s12935-016-0373-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.den Uil SH, van den Broek E, Coupé VMH, Vellinga TT, Delis-van Diemen PM, Bril H, Belt EJT, Kranenburg O, Stockmann HBAC, Belien JAM, Meijer GA, Fijneman RJA. Prognostic value of microvessel density in stage II and III colon cancer patients: a retrospective cohort study. BMC Gastroenterol. 2019;19:146. doi: 10.1186/s12876-019-1063-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mohammed AA, Arif SH, Pity IS. P53 expression and micro-vessel density in relation with 5-year survival in patients with colorectal cancer. Ann Med Surg (Lond) 2020;57:311–314. doi: 10.1016/j.amsu.2020.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]