Abstract
Cholangiocarcinoma (CCA) is the second most common liver cancer with a median survival of 12-24 mo without treatment. It is further classified based on its location into intrahepatic CCA (iCCA), perihilar CCA (pCCA), and distal CCA. Surgical resection is the mainstay of treatment, but up to 70% of these tumors are inoperable at the time of diagnosis. CCA was previously an absolute contraindication for liver transplantation (LT) due to poor outcomes primary due to early recurrent disease. However, improvement in patient selection criteria and neoadjuvant treatment protocols have improved outcomes for inoperable pCCA patients with recent studies reporting LT may improve survival in iCCA. Future advances in the treatment of CCA should include refining patient selection criteria and organ allocation for all subtypes of CCA, determining effective immunotherapies and the evolving role of personalized medicine in patients ineligible for surgical resection or LT. Our article reviews the current status of LT in CCA, along with future directions in managing patients with CCA.
Keywords: Intrahepatic cholangiocarcinoma, Perihilar cholangiocarcinoma, Liver transplantation, Immunotherapy, Chemotherapy, Transplant
Core Tip: Perihilar cholangiocarcinoma (pCCA) is an accepted indication for liver transplantation (LT) using a strict selection process and standardized neoadjuvant treatment protocol with pre-operative disease staging. Intrahepatic cholangiocarcinoma (iCCA) has historically been a contraindication for LT due to poor reported outcomes. With improved tumor detection, patient selection, and neoadjuvant treatment, recent studies have reported improved survival in iCCA patients with LT. No standardized protocol exists for the treatment of iCCA using LT. Our review analyzes the history and current literature on the treatment of pCCA and iCCA, along with gaps in knowledge and future perspectives.
INTRODUCTION
Cholangiocarcinoma (CCA) is a malignant tumor that arises from the bile duct epithelium[1]. It is further classified based on its location into intrahepatic CCA (iCCA), perihilar CCA (pCCA), and distal CCA (dCCA) with the Whipple procedure the treatment of choice for dCCA[2]. In the past 20 years, liver transplantation (LT) has evolved to become the treatment of choice for carefully selected patients with unresectable pCCA[1]. Since 2009, a standard model for end-stage liver disease (MELD) exception point is available for patients listed for LT for pCCA[3]. In addition, a clinical trial is currently studying if LT is superior to surgical resection for “resectable” pCCA[4]. For iCCA, a recent prospective study incorporating neoadjuvant chemotherapy vs chemoradiation for selected patients with locally advanced iCCA followed by LT reported 5-year survival of 83%[5]. This has increased interest in LT for iCCA and further studies are ongoing. The aim of this article is to review the current role of LT in the management of CCA, specifically pCCA and iCCA.
SURGICAL RESECTION
Surgical resection is the mainstay of CCA treatment. Predictors of poor outcomes are size, positive margins, multiple lesions, and nodal metastasis[1]. However, resection is not always possible due to either large size or underlying cirrhosis and recurrence is common leaving LT as a possible option.
CCA is diagnosed with a dominant stricture on cholangiography and one or more of the following criteria positive cytology by endoscopic brushing or biopsy, fluorescence in situ hybridization polysomy, or elevated carbohydrate antigen 19-9 > 100 U/mL in the absence of cholangitis[1,6,7]. iCCA is commonly diagnosed with magnetic resonance imaging or computed tomography which demonstrates peripheral rim arterial phase enhancement followed by centripetal hyperenhancement on venous/delayed phase[2,8]. However, controversy exists surrounding the diagnosis of CCA given the frequency of incidentally found CCA that was suspected to be hepatocellular carcinoma (HCC) pre-operatively[8]. Biopsy may be required to differentiate CCA from HCC, but this carries a risk of tumor seeding.
The treatment and prognosis of CCA is dependent on its location along the biliary tree and likelihood of being completely resected with negative margins[9-11]. Surgical resection has been well-established as the standard treatment of CCA. Advances in surgical technique have improved outcomes in CCA patients over the past 20 years due to: (1) Extending the tumor resection to the hepatic parenchyma including caudate lobe, extended R-sided resection; (2) Extending tumor resection to the pancreatic head; (3) Performing vascular resections; (4) Performing lymphadenectomy to remove lymphatic pathways that may disseminate disease; and (5) Preoperative biliary drainage[1]. With complete resection and negative margins, 5-year survival rates are approximately 40%[1]. However, up to 70% of patients with hilar CCA are inoperable because of the extent of disease at presentation, therefore have a 5-year survival of 0%[2].
LT FOR PCCA
History of LT for pCCA
Historically, pCCA was a contraindication to LT. In the 1980s and early 1990s, LT was performed for pCCA in both Europe and the United States, but 5-year survival was 25%-30% with recurrence occurring in up to 60%[12]. The Mayo Protocol for pCCA was subsequently developed in 1993 and is outlined in Figure 1. With a 55% 5-year survival with LT, this has become the standard of care for LT in pCCA[13]. Downsides of this protocol were radiation-related injury which could affect surgery and the higher rates of vascular complications resulting in a greater need for vascular grafts[1]. Despite these difficulties, refining surgical and neoadjuvant protocol techniques have led to better long-term outcomes with survival increasing to 65% at 5 years and 59% at 10 years[14-16]. Since the development of the Mayo protocol in 1993, multicenter studies have validated this protocol and reported 5-year survival of 53%[16]. In 2002, Sudan et al[17] reported their experience with a neoadjuvant treatment protocol — using brachytherapy and 5-fluorouracil prior to LT for pCCA, this single center study reported a 45% survival over a median follow-up of 7.5 years[17]. Figure 2 illustrates the history of LT for pCCA. Subsequent studies have highlighted the improved overall survival (OS) of patients undergoing LT vs surgical resection, with age and comorbidity-matched patients having better outcomes with LT (3 and 5-year survival 72% vs 33% and 64% vs 18%, respectively)[18,19].
Figure 1.
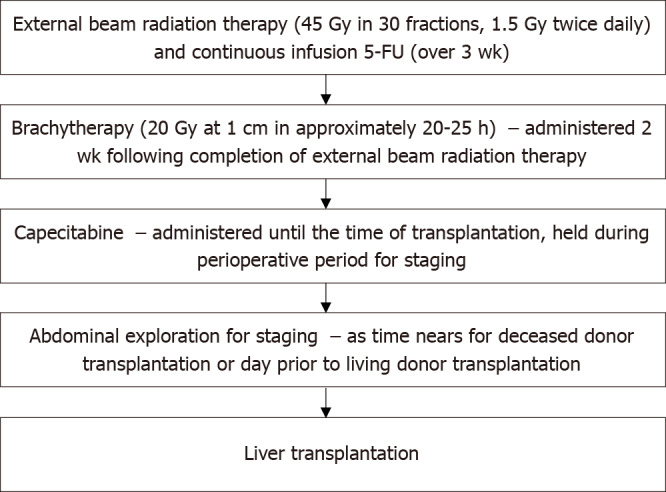
Mayo clinic protocol for neoadjuvant chemoradiation and staging laparoscopy prior to liver transplantation. Gy: Gray units of ionizing radiation; 5-FU: 5-Fluorouracil.
Figure 2.
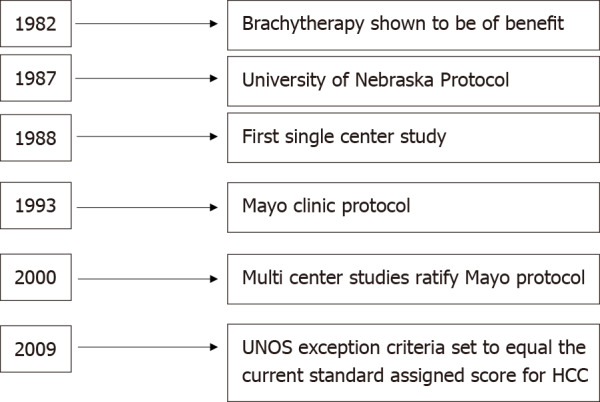
History of liver transplantation in perihilar cholangiocarcinoma, including the development of the original transplantation protocols, United Network for Organ Sharing approval, and standard exception point for liver transplantation. UNOS: United Network for Organ Sharing; HCC: Hepatocellular carcinoma.
Despite the significant improvement in survival for pCCA with LT, disagreement exists regarding the need for neoadjuvant therapy. A retrospective study of 28 patients in the European Liver Transplant registry from 1990-2010 reported 5-year survival without neoadjuvant therapy was 59%, highlighting the importance of patient selection pre-transplant as opposed to universal neoadjuvant treatment[20]. However, concern was raised about selection bias in this study. Multiple other studies have found poor outcomes in patients who do not receive neoadjuvant treatment[16]. A recent multicenter prospective study found that patients with unresectable pCCA treated with neoadjuvant therapy and LT had superior 5-year survival (64% vs 18%) than those patients treated with LT alone[18]. These results remained significant when controlling for tumor size, nodal status, and presence of primary sclerosing cholangitis (PSC).
Negative surgical margins are critically important as the most common cause of death after LT in CCA patients is abdominal tumor recurrence[1]. This is further enhanced by the need for immunosuppression after transplant[21-23]. Additional research has identified risk factors for waitlist dropout and disease recurrence, which has helped validate current selection criteria as well as identify patients who would be good candidates for future investigational therapies.
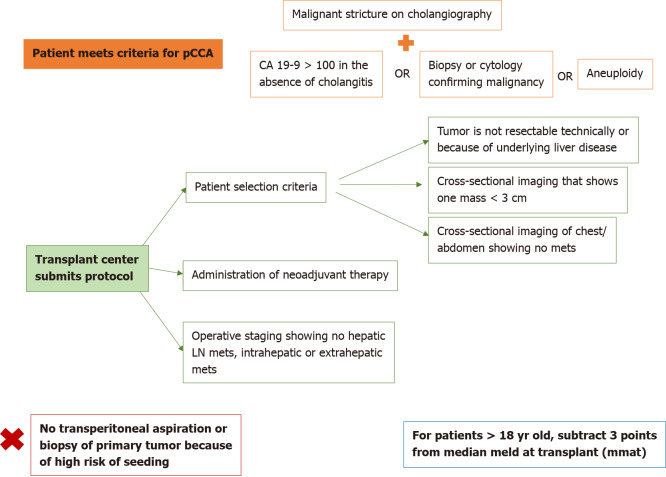
Standard MELD exception point
The standard MELD exception point for pCCA is currently set at Median MELD at transplant (MMaT) minus 3 points[3]. To qualify for standard MELD exception points, a patient must have unresectable disease due to either locally advanced tumor with extensive vascular and/or biliary invasion precluding complete resection, or poor hepatic functional reserve from underlying liver disease. It must be a single tumor < 3 cm in diameter with no evidence of intra- or extrahepatic metastasis and patients treated with neoadjuvant therapy at a center with an approved protocol. Further details on the MELD exception for CCA are found in Figure 3. Due to the increased risk of tumor seeding, it is important that transperitoneal aspiration or biopsy (i.e., endoscopic ultrasound-guided biopsy or percutaneous biopsy) of the primary tumor is not performed[24]. Due to these limitations together with the long waitlist for LT, living donor liver transplant (LDLT) provides a timely opportunity for access to transplantation, which reduces the risk of waitlist morbidity and mortality[1,2].
Figure 3.
Model for end-stage liver disease exception point for perihilar cholangiocarcinoma, as developed by the United Network for Organ Sharing. pCCA: Perihilar cholangiocarcinoma; CA 19-9: Cancer-antigen 19-9; LN: Lymph node.
The current protocol for pCCA treatment is external beam radiotherapy plus brachytherapy with a continuous infusion of 5-fluorouracil, followed by oral capecitabine until transplant (Figure 1). Other protocols have reported the use of stereotactic beam radiotherapy with gemcitabine plus cisplatin[25,26]. However, there are no comparative studies between these different regimens.
Future directions
A prospective multicenter randomized trial in France is currently comparing neoadjuvant therapy + LT vs liver and extrahepatic bile duct resection for “resectable” pCCA, with 5 year survival as the primary outcome[4].
LT FOR ICCA
Initial experience regarding LT for iCCA occurred in patient’s undergoing LT for suspected HCC which was subsequently diagnosed as iCCA after histologic evaluation of the explant[27]. One- and five-year OS in iCCA patients compared to HCC was shown to be 63.6% vs 90% and 63.6% vs 70.3% in a retrospective study of 44 patients with iCCA on explant LT for HCC[27]. A review of studies completed on LT in iCCA is reviewed in Table 1.
Table 1.
Studies assessing patient survival and disease-free survival after receiving a liver transplant for intrahepatic cholangiocarcinoma
|
Ref.
|
Study type
|
Number of LT patients
|
Overall survival (%)
|
DFS at 5-yr (%)
|
Comments | ||
|
1-yr
|
3-yr
|
5-yr
|
No
|
||||
| iCCA | |||||||
| O’Grady et al[51], 1988 | Retrospective | 13 | 38 | 10 | 10 | - | |
| Yokoyama et al[52], 1990 | Retrospective | 2 | 50 | 0 | - | - | |
| Meyer et al[53], 2000 | Retrospective Multicenter | 207 | 72 | 48 | 23 | - | 84% DFS at 25 mo |
| Shimoda et al[54], 2001 | Retrospective | 16 | 62 | 39 | - | 35 | |
| Robles et al[55], 2004 | Retrospective multicenter | 23 | 77 | 65 | 42 | - | 2 yr DFS 35% |
| Sotiropoulos et al[56], 2009 | Retrospective | 10 | 70 | 50 | 33 | - | |
| Fu et al[57], 2011 | Retrospective | 11 | 50.5 | 50.5 | 3 yr DFS 51.9% | ||
| Hong et al[8], 2011 | Retrospective | 25 | - | 38 | 32 | 33 | |
| Vallin et al[58], 2013 | Retrospective multicenter | 10 | 80 | 60 | 24 | - | |
| Facciuto et al[29], 2015 | Retrospective | 7 iCCA; 9 iCCA + HCC; 16 iCCA-HCC | 71 | - | 57 | 44 | |
| Vilchez et al[59], 2016 | Retrospective multicenter | 440 | 79 | 58 | 47 | - | |
| Very early iCCA (< 2 cm) | |||||||
| Sapisochin et al[28], 2014 | Retrospective multicenter | 27 | 78 | 66 | 51 | 36 | |
| Sapisochin et al[30], 2016 | Retrospective multicenter | 15 single < 2 cm; 33 multiple or > 2 cm | 93; 79 | 84; 50 | 65; 45 | 82; 39 | |
| Locally advanced iCCA with sustained response to chemotherapy | |||||||
| Lunsford et al[5], 2018 | Prospective single-arm | 6 | 100 | 83.3 | 83.3 | 50 | |
LT: Liver transplant; DFS: Disease free survival; iCCA: Intrahepatic cholangiocarcinoma; HCC: Hepatocellular carcinoma.
Very-early iCCA in cirrhosis
Although surgical resection is the ideal treatment for iCCA, up to 70% of iCCA is unresectable at diagnosis with a median survival of 12 mo even with chemoradiation[1,8]. Historically, LT for iCCA carries a high risk of recurrence and thus has not been considered an indication for LT.
In 2014, a Spanish multi-center retrospective trial of 2301 patients undergoing LT for HCC found 8 patients had iCCA in the explant. These patients had a 73% 5-year survival[28]. A single-center retrospective study of LT for HCC from New York of 32 patients found 7 patients had iCCA in the explant. OS of these patients was 57%[29]. An international multi-center retrospective trial of 48 iCCA patients which included 15 patients with tumors < 2 cm and 32 patients with > 2 cm tumors reported that patients with < 2 cm tumors had a 65% 5-year survival, and the > 2 cm tumor group had a 45% 5-year survival[30]. A multi-center retrospective French study of patients examined outcomes of LT vs local resection for iCCA or iCCA-HCC for tumors < 2 cm and 2-5 cm. Better outcomes were found for LT in terms of OS and recurrence free survival[31]. These studies have laid the foundation for a multi-center prospective trial in France which is assessing outcomes for LT in iCCA < 2 cm and 2-5 cm[32].
Locally advanced iCCA
A single center prospective case series analysis at Methodist Houston of 6 patients with large locally advanced unresectable iCCA were treated with neoadjuvant chemotherapy followed by LT[5]. The average total tumor burden was 10 cm in size with 4 lesions. Outcomes were positive with 80% 3-year survival and 50% recurrence free survival[5]. However, as this was only a small single center study, the investigators are developing a multi-center trial to determine if this may be a feasible treatment option for the future.
Similar to neoadjuvant and adjuvant protocols for pCCA, centers that have performed LT for iCCA have used regimens including fluorouracil or capecitabine combined with oxcaliplatin, leucovorin, and gemcitabine[8].
Risk factors for recurrent iCCA after LT
Patients with multifocal tumors, perineural invasion, infiltrative tumor subtypes, and a lack of neoadjuvant and adjuvant therapies have been associated with high risk of recurrence and poor outcomes after LT for iCCA[8]. Interestingly, tumor size did not predict the risk of recurrence.
Risks for recurrent iCCA after surgical resection
Recurrence of iCCA has been shown to occur in approximately 66% of patients who undergo curative resection[33]. Risk factors that increase the likelihood for recurrence include surgical margin < 10 mm, female sex, and presence of liver cirrhosis[33].
Currently, iCCA has no standard MELD exception. The options are to transplant based on calculated MELD score, or to use a LDLT. Although it is possible for a clinician to appeal to the National Liver Review Board (NLRB), there is no current policy or guidance regarding iCCA (unlike what exists for HCC or hCCA), which makes it challenging for NLRB to make decisions on allocation.
Future direction
Until iCCA has an established, suitable indication for MELD exception, surgical resection will remain the standard of care. However, retrospective data suggests patients with small iCCA (< 2 cm) may have good outcomes with LT. The role of neoadjuvant chemoradiotherapy and LT for iCCA > 2 cm in non-cirrhotic patients remains to be defined.
ALTERNATIVE TREATMENT STRATEGIES
Downsizing
Rayar et al[34] treated 45 patients with Yttrium-90 + chemotherapy and were able to downgrade 8 (18%) patients for resection. Given organ scarcity, using chemotherapy to downgrade to resection may be another option to LT[35].
Immunotherapy and personalized medicine
Historically, advanced, unresectable CCA has been treated with gemcitabine-based chemotherapy[1,26]. Recent advances in oncology have focused on the identification of biomarkers and molecular profiles that may be used as novel targets for chemotherapy[36-38]. In vitro and in vivo studies have suggested significant heterogeneity exists in biomarkers and molecular targets for CCA, especially iCCA[39]. This is further influenced by genetic variation, as well as the etiology for iCCA (e.g., PSC, liver-fluke, viral hepatitis)[38]. Treatments currently under evaluation include T-cells, antibodies, oncolytic viruses, cancer vaccines, and combinations of traditional chemotherapy with immunotherapy. These treatments are designed to target unique pathobiological pathways involved in CCA[40]. For example, patients with fibroblast growth factor receptor (FGFR) mutations (seen in 30% of patients with iCCA) are diagnosed at a younger age but typically have a more indolent course vs those with Kirsten rat sarcoma (KRAS) and p53 mutations which are more aggressive with poorer prognosis[41-46]. These genes are being evaluated as targets for future treatment to inhibit tumor growth[40,41,47,48]. Chemotherapy and immune checkpoint inhibitors have synergistic effects, which may increase tumor cell destruction while also decreasing the dosage of chemotherapy needed which may improve side effect profiles[41]. Radiotherapy is known to increase the sensitivity of the immune system to tumors, which in combination with immunotherapy has been efficacious for CCA. There are ongoing trials assessing the efficacy of immunotherapy, alone or in combination with chemotherapy to treat CCA. Additional promising tumor markers currently being evaluated for CCA include isocitrate dehydrogenase, programmed cell death protein 1, epidermal growth factor receptor, mechanistic target of rapamycin, mitogen-activated protein kinase and breast cancer pathways[41,49]. The identification of novel therapeutic pathways for CCA would provide a promising paradigm shift in the treatment of patients who are not candidates for resection or LT[50].
CONCLUSION
CCA is becoming increasingly prevalent worldwide. Typically presenting at advanced stages that are inoperable, there has been a rapid evolution of treatments for unresectable CCA, including LT and new immunotherapies. Future research will evaluate the efficacy of novel pharmacotherapies in treating advanced CCA. Continuing to refine patient selection criteria for LT in CCA as well as optimizing neoadjuvant treatment regimens will be helpful. If LT is established as an acceptable therapy for iCCA, determining universal criteria for referral as well as organ allocation such as MELD exceptions will be crucial. Additionally, given the presence of iCCA in explanted livers suspected to be HCC, refining pre-transplant tumor staging and radiologic identification of iCCA will be helpful.
Footnotes
Conflict-of-interest statement: There is no conflict of interest associated with any of the senior author or other coauthors contributed their efforts in this manuscript.
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: American Gastroenterological Association; American Association for the Study of Liver Diseases.
Peer-review started: April 7, 2021
First decision: June 23, 2021
Article in press: December 28, 2021
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Zheng H S-Editor: Gao CC L-Editor: A P-Editor: Gao CC
Contributor Information
Patrick Twohig, Department of Internal Medicine, Division of Gastroenterology and Transplant Hepatology, University of Nebraska Medical Center, Omaha, NE 68198, United States.
Thoetchai Bee Peeraphatdit, Department of Internal Medicine, Division of Gastroenterology and Transplant Hepatology, University of Nebraska Medical Center, Omaha, NE 68198, United States.
Sandeep Mukherjee, Department of Internal Medicine, Division of Gastroenterology, Creighton University, Omaha, NE 68124, United States. sandeep.mukherjee@alegent.org.
References
- 1.Sapisochin G, Javle M, Lerut J, Ohtsuka M, Ghobrial M, Hibi T, Kwan NM, Heimbach J. Liver Transplantation for Cholangiocarcinoma and Mixed Hepatocellular Cholangiocarcinoma: Working Group Report From the ILTS Transplant Oncology Consensus Conference. Transplantation. 2020;104:1125–1130. doi: 10.1097/TP.0000000000003212. [DOI] [PubMed] [Google Scholar]
- 2.Zamora-Valdes D, Heimbach JK. Liver Transplant for Cholangiocarcinoma. Gastroenterol Clin North Am. 2018;47:267–280. doi: 10.1016/j.gtc.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 3.Organ Procurement and Transplantation Network Policy. Department of Health and Human Services, Health Resources and Services Administration, Healthcare Systems Bureau, Division of Transplantation, Rockville, MD. 2021. [cited 25 Jan 2021]. In: Organ Procurement and Transplantation Network Policy [Internet]. Available from: https://optn.transplant.hrsa.gov/media/1200/optn_policies.pdf . [Google Scholar]
- 4.Vibert E. Liver Resection Versus Radio-chemotherapy-Transplantation for Hilar Cholangiocarcinoma (TRANSPHIL). [accessed 2021 Jan 25]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT02232932. ClinicalTrials.gov Identifier: NCT02232932.
- 5.Lunsford KE, Javle M, Heyne K, Shroff RT, Abdel-Wahab R, Gupta N, Mobley CM, Saharia A, Victor DW, Nguyen DT, Graviss EA, Kaseb AO, McFadden RS, Aloia TA, Conrad C, Li XC, Monsour HP, Gaber AO, Vauthey JN, Ghobrial RM Methodist–MD Anderson Joint Cholangiocarcinoma Collaborative Committee (MMAJCCC) Liver transplantation for locally advanced intrahepatic cholangiocarcinoma treated with neoadjuvant therapy: a prospective case-series. Lancet Gastroenterol Hepatol. 2018;3:337–348. doi: 10.1016/S2468-1253(18)30045-1. [DOI] [PubMed] [Google Scholar]
- 6.Rizvi S, Gores GJ. Pathogenesis, diagnosis, and management of cholangiocarcinoma. Gastroenterology. 2013;145:1215–1229. doi: 10.1053/j.gastro.2013.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saha SK, Zhu AX, Fuchs CS, Brooks GA. Forty-Year Trends in Cholangiocarcinoma Incidence in the U.S.: Intrahepatic Disease on the Rise. Oncologist. 2016;21:594–599. doi: 10.1634/theoncologist.2015-0446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hong JC, Jones CM, Duffy JP, Petrowsky H, Farmer DG, French S, Finn R, Durazo FA, Saab S, Tong MJ, Hiatt JR, Busuttil RW. Comparative analysis of resection and liver transplantation for intrahepatic and hilar cholangiocarcinoma: a 24-year experience in a single center. Arch Surg. 2011;146:683–689. doi: 10.1001/archsurg.2011.116. [DOI] [PubMed] [Google Scholar]
- 9.Burke EC, Jarnagin WR, Hochwald SN, Pisters PW, Fong Y, Blumgart LH. Hilar Cholangiocarcinoma: patterns of spread, the importance of hepatic resection for curative operation, and a presurgical clinical staging system. Ann Surg. 1998;228:385–394. doi: 10.1097/00000658-199809000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klempnauer J, Ridder GJ, von Wasielewski R, Werner M, Weimann A, Pichlmayr R. Resectional surgery of hilar cholangiocarcinoma: a multivariate analysis of prognostic factors. J Clin Oncol. 1997;15:947–954. doi: 10.1200/JCO.1997.15.3.947. [DOI] [PubMed] [Google Scholar]
- 11.Washburn WK, Lewis WD, Jenkins RL. Aggressive surgical resection for cholangiocarcinoma. Arch Surg. 1995;130:270–276. doi: 10.1001/archsurg.1995.01430030040006. [DOI] [PubMed] [Google Scholar]
- 12.Rosen CB, Heimbach JK, Gores GJ. Liver transplantation for cholangiocarcinoma. Transpl Int. 2010;23:692–697. doi: 10.1111/j.1432-2277.2010.01108.x. [DOI] [PubMed] [Google Scholar]
- 13.Rea DJ, Heimbach JK, Rosen CB, Haddock MG, Alberts SR, Kremers WK, Gores GJ, Nagorney DM. Liver transplantation with neoadjuvant chemoradiation is more effective than resection for hilar cholangiocarcinoma. Ann Surg. 2005;242:451–8; discussion 458. doi: 10.1097/01.sla.0000179678.13285.fa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coelen RJS, Gaspersz MP, Labeur TA, van Vugt JLA, van Dieren S, Willemssen FEJA, Nio CY, IJzermans JNM, Klümpen HJ, Groot Koerkamp B, van Gulik TM. Validation of the Mayo Clinic Staging System in Determining Prognoses of Patients With Perihilar Cholangiocarcinoma. Clin Gastroenterol Hepatol. 2017;15:1930–1939.e3. doi: 10.1016/j.cgh.2017.04.044. [DOI] [PubMed] [Google Scholar]
- 15.Rosen CB, Darwish Murad S, Heimbach JK, Nyberg SL, Nagorney DM, Gores GJ. Neoadjuvant therapy and liver transplantation for hilar cholangiocarcinoma: is pretreatment pathological confirmation of diagnosis necessary? J Am Coll Surg. 2012;215:31–8; discussion 38. doi: 10.1016/j.jamcollsurg.2012.03.014. [DOI] [PubMed] [Google Scholar]
- 16.Darwish Murad S, Kim WR, Harnois DM, Douglas DD, Burton J, Kulik LM, Botha JF, Mezrich JD, Chapman WC, Schwartz JJ, Hong JC, Emond JC, Jeon H, Rosen CB, Gores GJ, Heimbach JK. Efficacy of neoadjuvant chemoradiation, followed by liver transplantation, for perihilar cholangiocarcinoma at 12 US centers. Gastroenterology. 2012;143:88–98.e3; quiz e14. doi: 10.1053/j.gastro.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sudan D, DeRoover A, Chinnakotla S, Fox I, Shaw B Jr, McCashland T, Sorrell M, Tempero M, Langnas A. Radiochemotherapy and transplantation allow long-term survival for nonresectable hilar cholangiocarcinoma. Am J Transplant. 2002;2:774–779. doi: 10.1034/j.1600-6143.2002.20812.x. [DOI] [PubMed] [Google Scholar]
- 18.Ethun CG, Lopez-Aguiar AG, Anderson DJ, Adams AB, Fields RC, Doyle MB, Chapman WC, Krasnick BA, Weber SM, Mezrich JD, Salem A, Pawlik TM, Poultsides G, Tran TB, Idrees K, Isom CA, Martin RCG, Scoggins CR, Shen P, Mogal HD, Schmidt C, Beal E, Hatzaras I, Shenoy R, Cardona K, Maithel SK. Transplantation Versus Resection for Hilar Cholangiocarcinoma: An Argument for Shifting Treatment Paradigms for Resectable Disease. Ann Surg. 2018;267:797–805. doi: 10.1097/SLA.0000000000002574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moris D, Kostakis ID, Machairas N, Prodromidou A, Tsilimigras DI, Ravindra KV, Sudan DL, Knechtle SJ, Barbas AS. Comparison between liver transplantation and resection for hilar cholangiocarcinoma: A systematic review and meta-analysis. PLoS One. 2019;14:e0220527. doi: 10.1371/journal.pone.0220527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mantel HT, Westerkamp AC, Adam R, Bennet WF, Seehofer D, Settmacher U, Sánchez-Bueno F, Fabregat Prous J, Boleslawski E, Friman S, Porte RJ European Liver and Intestine Transplant Association (ELITA) Strict Selection Alone of Patients Undergoing Liver Transplantation for Hilar Cholangiocarcinoma Is Associated with Improved Survival. PLoS One. 2016;11:e0156127. doi: 10.1371/journal.pone.0156127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iwatsuki S, Todo S, Marsh JW, Madariaga JR, Lee RG, Dvorchik I, Fung JJ, Starzl TE. Treatment of hilar cholangiocarcinoma (Klatskin tumors) with hepatic resection or transplantation. J Am Coll Surg. 1998;187:358–364. doi: 10.1016/s1072-7515(98)00207-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Urego M, Flickinger JC, Carr BI. Radiotherapy and multimodality management of cholangiocarcinoma. Int J Radiat Oncol Biol Phys. 1999;44:121–126. doi: 10.1016/s0360-3016(98)00509-4. [DOI] [PubMed] [Google Scholar]
- 23.Flickinger JC, Epstein AH, Iwatsuki S, Carr BI, Starzl TE. Radiation therapy for primary carcinoma of the extrahepatic biliary system. An analysis of 63 cases. Cancer. 1991;68:289–294. doi: 10.1002/1097-0142(19910715)68:2<289::aid-cncr2820680213>3.0.co;2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heimbach JK, Sanchez W, Rosen CB, Gores GJ. Trans-peritoneal fine needle aspiration biopsy of hilar cholangiocarcinoma is associated with disease dissemination. HPB (Oxford) 2011;13:356–360. doi: 10.1111/j.1477-2574.2011.00298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Loveday BPT, Knox JJ, Dawson LA, Metser U, Brade A, Horgan AM, Gallinger S, Greig PD, Moulton CA. Neoadjuvant hyperfractionated chemoradiation and liver transplantation for unresectable perihilar cholangiocarcinoma in Canada. J Surg Oncol. 2018;117:213–219. doi: 10.1002/jso.24833. [DOI] [PubMed] [Google Scholar]
- 26.Valle JW, Wasan H, Johnson P, Jones E, Dixon L, Swindell R, Baka S, Maraveyas A, Corrie P, Falk S, Gollins S, Lofts F, Evans L, Meyer T, Anthoney A, Iveson T, Highley M, Osborne R, Bridgewater J. Gemcitabine alone or in combination with cisplatin in patients with advanced or metastatic cholangiocarcinomas or other biliary tract tumours: a multicentre randomised phase II study - The UK ABC-01 Study. Br J Cancer. 2009;101:621–627. doi: 10.1038/sj.bjc.6605211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee DD, Croome KP, Musto KR, Melendez J, Tranesh G, Nakhleh R, Taner CB, Nguyen JH, Patel T, Harnois DM. Liver transplantation for intrahepatic cholangiocarcinoma. Liver Transpl. 2018;24:634–644. doi: 10.1002/lt.25052. [DOI] [PubMed] [Google Scholar]
- 28.Sapisochin G, Rodríguez de Lope C, Gastaca M, Ortiz de Urbina J, Suarez MA, Santoyo J, Castroagudín JF, Varo E, López-Andujar R, Palacios F, Sanchez Antolín G, Perez B, Guiberteau A, Blanco G, González-Diéguez ML, Rodriguez M, Varona MA, Barrera MA, Fundora Y, Ferron JA, Ramos E, Fabregat J, Ciria R, Rufian S, Otero A, Vazquez MA, Pons JA, Parrilla P, Zozaya G, Herrero JI, Charco R, Bruix J. "Very early" intrahepatic cholangiocarcinoma in cirrhotic patients: should liver transplantation be reconsidered in these patients? Am J Transplant. 2014;14:660–667. doi: 10.1111/ajt.12591. [DOI] [PubMed] [Google Scholar]
- 29.Facciuto ME, Singh MK, Lubezky N, Selim MA, Robinson D, Kim-Schluger L, Florman S, Ward SC, Thung SN, Fiel M, Schiano TD. Tumors with intrahepatic bile duct differentiation in cirrhosis: implications on outcomes after liver transplantation. Transplantation. 2015;99:151–157. doi: 10.1097/TP.0000000000000286. [DOI] [PubMed] [Google Scholar]
- 30.Sapisochin G, Facciuto M, Rubbia-Brandt L, Marti J, Mehta N, Yao FY, Vibert E, Cherqui D, Grant DR, Hernandez-Alejandro R, Dale CH, Cucchetti A, Pinna A, Hwang S, Lee SG, Agopian VG, Busuttil RW, Rizvi S, Heimbach JK, Montenovo M, Reyes J, Cesaretti M, Soubrane O, Reichman T, Seal J, Kim PT, Klintmalm G, Sposito C, Mazzaferro V, Dutkowski P, Clavien PA, Toso C, Majno P, Kneteman N, Saunders C, Bruix J iCCA International Consortium. Liver transplantation for "very early" intrahepatic cholangiocarcinoma: International retrospective study supporting a prospective assessment. Hepatology. 2016;64:1178–1188. doi: 10.1002/hep.28744. [DOI] [PubMed] [Google Scholar]
- 31.De Martin E, Rayar M, Golse N, Dupeux M, Gelli M, Gnemmi V, Allard MA, Cherqui D, Sa Cunha A, Adam R, Coilly A, Antonini TM, Guettier C, Samuel D, Boudjema K, Boleslawski E, Vibert E. Analysis of Liver Resection Versus Liver Transplantation on Outcome of Small Intrahepatic Cholangiocarcinoma and Combined Hepatocellular-Cholangiocarcinoma in the Setting of Cirrhosis. Liver Transpl. 2020;26:785–798. doi: 10.1002/lt.25737. [DOI] [PubMed] [Google Scholar]
- 32.Sapisochin G, Bruix J. Liver transplantation for early intrahepatic cholangiocarcinoma (LT for iCCA). [accessed 2021 Jan 25]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT02878473?term=sapisochin. ClinicalTrials.gov Identifier: NCT02878473.
- 33.Hu LS, Zhang XF, Weiss M, Popescu I, Marques HP, Aldrighetti L, Maithel SK, Pulitano C, Bauer TW, Shen F, Poultsides GA, Soubrane O, Martel G, Koerkamp BG, Itaru E, Pawlik TM. Recurrence Patterns and Timing Courses Following Curative-Intent Resection for Intrahepatic Cholangiocarcinoma. Ann Surg Oncol. 2019;26:2549–2557. doi: 10.1245/s10434-019-07353-4. [DOI] [PubMed] [Google Scholar]
- 34.Rayar M, Sulpice L, Edeline J, Garin E, Levi Sandri GB, Meunier B, Boucher E, Boudjema K. Intra-arterial yttrium-90 radioembolization combined with systemic chemotherapy is a promising method for downstaging unresectable huge intrahepatic cholangiocarcinoma to surgical treatment. Ann Surg Oncol. 2015;22:3102–3108. doi: 10.1245/s10434-014-4365-3. [DOI] [PubMed] [Google Scholar]
- 35.Le Roy B, Gelli M, Pittau G, Allard MA, Pereira B, Serji B, Vibert E, Castaing D, Adam R, Cherqui D, Sa Cunha A. Neoadjuvant chemotherapy for initially unresectable intrahepatic cholangiocarcinoma. Br J Surg. 2018;105:839–847. doi: 10.1002/bjs.10641. [DOI] [PubMed] [Google Scholar]
- 36.Jiao Y, Pawlik TM, Anders RA, Selaru FM, Streppel MM, Lucas DJ, Niknafs N, Guthrie VB, Maitra A, Argani P, Offerhaus GJA, Roa JC, Roberts LR, Gores GJ, Popescu I, Alexandrescu ST, Dima S, Fassan M, Simbolo M, Mafficini A, Capelli P, Lawlor RT, Ruzzenente A, Guglielmi A, Tortora G, de Braud F, Scarpa A, Jarnagin W, Klimstra D, Karchin R, Velculescu VE, Hruban RH, Vogelstein B, Kinzler KW, Papadopoulos N, Wood LD. Exome sequencing identifies frequent inactivating mutations in BAP1, ARID1A and PBRM1 in intrahepatic cholangiocarcinomas. Nat Genet. 2013;45:1470–1473. doi: 10.1038/ng.2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chan-On W, Nairismägi ML, Ong CK, Lim WK, Dima S, Pairojkul C, Lim KH, McPherson JR, Cutcutache I, Heng HL, Ooi L, Chung A, Chow P, Cheow PC, Lee SY, Choo SP, Tan IB, Duda D, Nastase A, Myint SS, Wong BH, Gan A, Rajasegaran V, Ng CC, Nagarajan S, Jusakul A, Zhang S, Vohra P, Yu W, Huang D, Sithithaworn P, Yongvanit P, Wongkham S, Khuntikeo N, Bhudhisawasdi V, Popescu I, Rozen SG, Tan P, Teh BT. Exome sequencing identifies distinct mutational patterns in liver fluke-related and non-infection-related bile duct cancers. Nat Genet. 2013;45:1474–1478. doi: 10.1038/ng.2806. [DOI] [PubMed] [Google Scholar]
- 38.Ong CK, Subimerb C, Pairojkul C, Wongkham S, Cutcutache I, Yu W, McPherson JR, Allen GE, Ng CC, Wong BH, Myint SS, Rajasegaran V, Heng HL, Gan A, Zang ZJ, Wu Y, Wu J, Lee MH, Huang D, Ong P, Chan-on W, Cao Y, Qian CN, Lim KH, Ooi A, Dykema K, Furge K, Kukongviriyapan V, Sripa B, Wongkham C, Yongvanit P, Futreal PA, Bhudhisawasdi V, Rozen S, Tan P, Teh BT. Exome sequencing of liver fluke-associated cholangiocarcinoma. Nat Genet. 2012;44:690–693. doi: 10.1038/ng.2273. [DOI] [PubMed] [Google Scholar]
- 39.Ahn DH, Javle M, Ahn CW, Jain A, Mikhail S, Noonan AM, Ciombor K, Wu C, Shroff RT, Chen JL, Bekaii-Saab T. Next-generation sequencing survey of biliary tract cancer reveals the association between tumor somatic variants and chemotherapy resistance. Cancer. 2016;122:3657–3666. doi: 10.1002/cncr.30247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.French DM, Lin BC, Wang M, Adams C, Shek T, Hötzel K, Bolon B, Ferrando R, Blackmore C, Schroeder K, Rodriguez LA, Hristopoulos M, Venook R, Ashkenazi A, Desnoyers LR. Targeting FGFR4 inhibits hepatocellular carcinoma in preclinical mouse models. PLoS One. 2012;7:e36713. doi: 10.1371/journal.pone.0036713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.O'Rourke CJ, Munoz-Garrido P, Andersen JB. Molecular Targets in Cholangiocarcinoma. Hepatology. 2021;73 Suppl 1:62–74. doi: 10.1002/hep.31278. [DOI] [PubMed] [Google Scholar]
- 42.Javle M, Lowery M, Shroff RT, Weiss KH, Springfeld C, Borad MJ, Ramanathan RK, Goyal L, Sadeghi S, Macarulla T, El-Khoueiry A, Kelley RK, Borbath I, Choo SP, Oh DY, Philip PA, Chen LT, Reungwetwattana T, Van Cutsem E, Yeh KH, Ciombor K, Finn RS, Patel A, Sen S, Porter D, Isaacs R, Zhu AX, Abou-Alfa GK, Bekaii-Saab T. Phase II Study of BGJ398 in Patients With FGFR-Altered Advanced Cholangiocarcinoma. J Clin Oncol. 2018;36:276–282. doi: 10.1200/JCO.2017.75.5009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lowery MA, Abou-Alfa GK, Burris HA, Janku F, Shroff RT, Cleary JM, Azad NS, Goyal L, Maher EA, Gore L, Hollebecque A, Beeran M, Trent JC, Jiang L, Ishii Y, Auer J, Gliser C, Agresta SV, Pandya SS, Zhu AX. Phase I study of AG-120, an IDH1 mutant enzyme inhibitor: results from the cholangiocarcinoma dose escalation and expansion cohorts. J Clin Oncol. 2017;35(Suppl 15):4015. [Google Scholar]
- 44.Lavingia V, Fakih M. Impressive response to dual BRAF and MEK inhibition in patients with BRAF mutant intrahepatic cholangiocarcinoma-2 case reports and a brief review. J Gastrointest Oncol. 2016;7:E98–E102. doi: 10.21037/jgo.2016.09.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jain A, Borad MJ, Kelley RK, Wang Y, Abdel-Wahab R, Meric-Bernstam F, Baggerly KA, Kaseb AO, Al-shamsi HO, Ahn DH, DeLeon T, Bocobo AG, Bekaii-Saab T, Shroff RT, Javle M. Cholangiocarcinoma with FGFR genetic aberrations: A unique clinical phenotype. JCO Precis Oncol . 2018;2:1–12. doi: 10.1200/PO.17.00080. [DOI] [PubMed] [Google Scholar]
- 46.Li F, Peiris MN, Donoghue DJ. Functions of FGFR2 corrupted by translocations in intrahepatic cholangiocarcinoma. Cytokine Growth Factor Rev. 2020;52:56–67. doi: 10.1016/j.cytogfr.2019.12.005. [DOI] [PubMed] [Google Scholar]
- 47.Goyal L, Saha SK, Liu LY, Siravegna G, Leshchiner I, Ahronian LG, Lennerz JK, Vu P, Deshpande V, Kambadakone A, Mussolin B, Reyes S, Henderson L, Sun JE, Van Seventer EE, Gurski JM Jr, Baltschukat S, Schacher-Engstler B, Barys L, Stamm C, Furet P, Ryan DP, Stone JR, Iafrate AJ, Getz G, Porta DG, Tiedt R, Bardelli A, Juric D, Corcoran RB, Bardeesy N, Zhu AX. Polyclonal Secondary FGFR2 Mutations Drive Acquired Resistance to FGFR Inhibition in Patients with FGFR2 Fusion-Positive Cholangiocarcinoma. Cancer Discov. 2017;7:252–263. doi: 10.1158/2159-8290.CD-16-1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hagel M, Miduturu C, Sheets M, Rubin N, Weng W, Stransky N, Bifulco N, Kim JL, Hodous B, Brooijmans N, Shutes A, Winter C, Lengauer C, Kohl NE, Guzi T. First Selective Small Molecule Inhibitor of FGFR4 for the Treatment of Hepatocellular Carcinomas with an Activated FGFR4 Signaling Pathway. Cancer Discov. 2015;5:424–437. doi: 10.1158/2159-8290.CD-14-1029. [DOI] [PubMed] [Google Scholar]
- 49.Moeini A, Sia D, Bardeesy N, Mazzaferro V, Llovet JM. Molecular Pathogenesis and Targeted Therapies for Intrahepatic Cholangiocarcinoma. Clin Cancer Res. 2016;22:291–300. doi: 10.1158/1078-0432.CCR-14-3296. [DOI] [PubMed] [Google Scholar]
- 50.Fong ZV, Brownlee SA, Qadan M, Tanabe KK. The Clinical Management of Cholangiocarcinoma in the United States and Europe: A Comprehensive and Evidence-Based Comparison of Guidelines. Ann Surg Oncol. 2021;28:2660–2674. doi: 10.1245/s10434-021-09671-y. [DOI] [PubMed] [Google Scholar]
- 51.O'Grady JG, Polson RJ, Rolles K, Calne RY, Williams R. Liver transplantation for malignant disease. Results in 93 consecutive patients. Ann Surg. 1988;207:373–379. doi: 10.1097/00000658-198804000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yokoyama I, Todo S, Iwatsuki S, Starzl TE. Liver transplantation in the treatment of primary liver cancer. Hepatogastroenterology. 1990;37:188–193. [PMC free article] [PubMed] [Google Scholar]
- 53.Meyer CG, Penn I, James L. Liver transplantation for cholangiocarcinoma: results in 207 patients. Transplantation. 2000;69:1633–1637. doi: 10.1097/00007890-200004270-00019. [DOI] [PubMed] [Google Scholar]
- 54.Shimoda M, Farmer DG, Colquhoun SD, Rosove M, Ghobrial RM, Yersiz H, Chen P, Busuttil RW. Liver transplantation for cholangiocellular carcinoma: analysis of a single-center experience and review of the literature. Liver Transpl. 2001;7:1023–1033. doi: 10.1053/jlts.2001.29419. [DOI] [PubMed] [Google Scholar]
- 55.Robles R, Figueras J, Turrión VS, Margarit C, Moya A, Varo E, Calleja J, Valdivieso A, Valdecasas JC, López P, Gómez M, de Vicente E, Loinaz C, Santoyo J, Fleitas M, Bernardos A, Lladó L, Ramírez P, Bueno FS, Jaurrieta E, Parrilla P. Spanish experience in liver transplantation for hilar and peripheral cholangiocarcinoma. Ann Surg. 2004;239:265–271. doi: 10.1097/01.sla.0000108702.45715.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sotiropoulos GC, Bockhorn M, Sgourakis G, Brokalaki EI, Molmenti EP, Neuhäuser M, Radtke A, Wohlschlaeger J, Baba HA, Broelsch CE, Lang H. R0 Liver resections for primary malignant liver tumors in the noncirrhotic liver: a diagnosis-related analysis. Dig Dis Sci. 2009;54:887–894. doi: 10.1007/s10620-008-0408-6. [DOI] [PubMed] [Google Scholar]
- 57.Fu BS, Zhang T, Li H, Yi SH, Wang GS, Xu C, Yang Y, Cai CJ, Lu MQ, Chen GH. The role of liver transplantation for intrahepatic cholangiocarcinoma: a single-center experience. Eur Surg Res. 2011;47:218–221. doi: 10.1159/000332827. [DOI] [PubMed] [Google Scholar]
- 58.Vallin M, Sturm N, Lamblin G, Guillaud O, Hilleret MN, Hervieu V, Joubert J, Abergel A, Leroy V, Boillot O, Dumortier J, Scoazec JY. Unrecognized intrahepatic cholangiocarcinoma: an analysis of 993 adult cirrhotic liver explants. Clin Transplant. 2013;27:403–409. doi: 10.1111/ctr.12108. [DOI] [PubMed] [Google Scholar]
- 59.Vilchez V, Shah MB, Daily MF, Pena L, Tzeng CW, Davenport D, Hosein PJ, Gedaly R, Maynard E. Long-term outcome of patients undergoing liver transplantation for mixed hepatocellular carcinoma and cholangiocarcinoma: an analysis of the UNOS database. HPB (Oxford) 2016;18:29–34. doi: 10.1016/j.hpb.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]