Abstract
Many animals show sexually divergent foraging behaviours reflecting different physiological constraints or energetic needs. We used a bioenergetics approach to examine sex differences in foraging behaviour of the sexually monomorphic northern gannet. We derived a relationship between dynamic body acceleration and energy expenditure to quantify the energetic cost of prey capture attempts (plunge dives). Fourteen gannets were tracked using GPS, time depth recorders (TDR) and accelerometers. All plunge dives in a foraging trip represented less than 4% of total energy expenditure, with no significant sex differences in expenditure. Despite females undertaking significantly more dives than males, this low energetic cost resulted in no sex differences in overall energy expenditure across a foraging trip. Bayesian stable isotope mixing models based on blood samples highlighted sex differences in diet; however, calorific intake from successful prey capture was estimated to be similar between sexes. Females experienced 10.28% higher energy demands, primarily due to unequal chick provisioning. Estimates show a minimum of 19% of dives have to be successful for females to meet their daily energy requirements, and 26% for males. Our analyses suggest northern gannets show sex differences in foraging behaviour primarily related to dive rate and success rather than the energetic cost of foraging or energetic content of prey.
Keywords: northern gannet, isotope ecology, movement ecology, bioenergetics, accelerometry
1. Introduction
Many animals show sex-specific foraging differences, though it is often difficult to explore the mechanisms behind these differences—particularly in free-ranging predators. Sex differences in foraging are often pronounced in sexually dimorphic species [1,2]. These differences may be due to competitive exclusion [3], or where different sexes may have access to different foraging areas due to their size [3,4] or foraging habitat preference [5]. Divergent sexual behaviours may also represent differences in nutrient requirements or prey preferences [6,7], levels of parental care [8] or in the energetic demands of locomotion [9,10]. Differences can also arise because a dominant sex will outcompete or displace the other, resulting in sexual segregation [11,12], niche expansion and reduced intraspecific competition [13]. For example: giant petrels, Macronectes giganteus, where females weigh 80% the mass of males, show spatially segregated foraging areas [14], a pattern that holds true across a wide variety of taxa [15–18]. Although sex differences in foraging tend to be less obvious in sexually monomorphic species, they still occur [19].
In monomorphic species, sex-specific foraging behaviours can be driven by differing energy requirements between the sexes [20]. Foraging in different locations will provide different resources that may be required in different amounts between the sexes. For example, Barau's petrel (Pterodroma baraui) is a monomorphic seabird where males and females forage in different locations early in the breeding season, as females must restore body condition after egg production [20]. There is also evidence to suggest that sex-specific foraging strategies in sexually monomorphic species may be driven by intraspecific competition causing one sex to be displaced spatially or to forage in different niches [21]. For example, brown boobies (Sula leucogaster) are considered to show sex differences in foraging, as competition for resources suggests that males exclude females from foraging on squid, and this exclusion may change with different levels of foraging resources available. [22] Foraging theory states that animals attempt to intake food in the most optimal manner possible [23–25] to ensure that net energy gain exceeds gross energy expenditure. However, accurately measuring energy intake and expenditure remains a challenge, especially in free-ranging animals [26,27].
Measuring energetic expenditure has previously involved the use of double-labelled water, respirometry chambers or heart rate loggers [28]. Though heart loggers can be used to investigate behaviour-specific energy costs [29] and respirometers can provide resting metabolic rates and calibration for other field measurements [30], these techniques can be invasive. In recent years, accelerometry studies on free-ranging individuals have explored energetic expenditure at a much finer scale [31]. These studies can use measures of dynamic body acceleration (DBA) as a proxy for energy expenditure, due to a strong correlation with the volume of oxygen consumed by muscles during a given time period (VO2) [32–34]. However, developing a complete understanding of how accelerometry signals relate to energy use and the corresponding energy budgets of an individual animal requires knowledge of diet and energetic intake.
Net energy intake is determined by the energy gained from successful foraging against the energy expended for basal metabolism and for activities such as locomotion. Quantifying energy gained through diet in free-ranging animals can be difficult without invasive techniques such as stomach content analysis [35] or direct observation of prey capture [36]. However, stable isotope analysis (SIA) is a minimally invasive technique that can provide diet information and, in seabird studies, is known to correlate well with these other more direct methods such as regurgitate sampling and direct observation of foraging [37–39]. Isotopic ratios of 12C/13C and 14N/15N can be used to infer prey species consumed by an individual [40]. Both carbon and nitrogen can be considered as indicators of the trophic level an animal is foraging at [41]. Nitrogen isotopes enrich at a faster rate in predators than carbon isotopes, but the ratio between them can inform trophic level, trophic niche width and diet [42]. Using SIA to predict predator diet can therefore provide insight into the energetics of foraging.
The northern gannet (Morus bassanus), hereafter gannet, is sexually monomorphic with no significant morphological differences between adult males and females [43,44]. While females are marginally heavier than males on average, (average 200 g—approximately 6% difference [45]), there is considerable overlap, and mass alone cannot be used to sex individuals [45]. Despite the lack of overt sexual dimorphism, all populations of the species studied thus far show strong sexually divergent foraging strategies. Female gannets are more selective in choosing foraging grounds [46] and undertake longer trips, further offshore than males, a pattern that is thought to arise from habitat segregation [47]. From a dietary perspective, male gannets consume higher proportions of fisheries discards than females, a division thought to derive from the competitive exclusion of female gannets from vessels [48], and is a distinction only present in breeding adults [44]. Females which specialize on fisheries discards travel shorter distances than females which specialize on forage fish; however, this distinction is not apparent among males [49]. At present, there is no clear evidence for whether male and female gannets target different-sized prey items. A lack of strong sexual dimorphism in gannets suggests that sex differences in foraging strategies and diet may derive from different energetic demands between the sexes caused by differential responsibilities during chick rearing [50], a previously untested hypothesis.
In the present study, we used GPS, accelerometry and SIA data to gain a better understanding of how gannets engage in foraging and how different demands upon the sexes may affect foraging strategies. Specifically, we explore sex differences in foraging of gannets in terms of diet, dive types, frequency of prey capture attempts and the energetic cost of prey capture attempts. Additionally, we quantify the energetic requirements of each sex, taking into account energy expended during foraging and, using data from published studies, energetic demands of feeding offspring. Finally, we consider minimum dive success rates necessary for male and female gannets to meet their energy demands.
Specifically we aim to test the following hypotheses:
-
(1)
Sex differences in the foraging ecology of gannets derive from the different energetic demands placed upon the sexes by differential responsibilities during chick rearing.
-
(2)
Due to limited sexual dimorphism, there is no difference in the cost of similar prey capture attempts between the sexes.
-
(3)
Due to differing energy demands and foraging behaviour, the sexes have different prey capture success rates.
2. Methods
All data collected as part of this study are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.zs7h44j88 [51].
2.1. Data collection
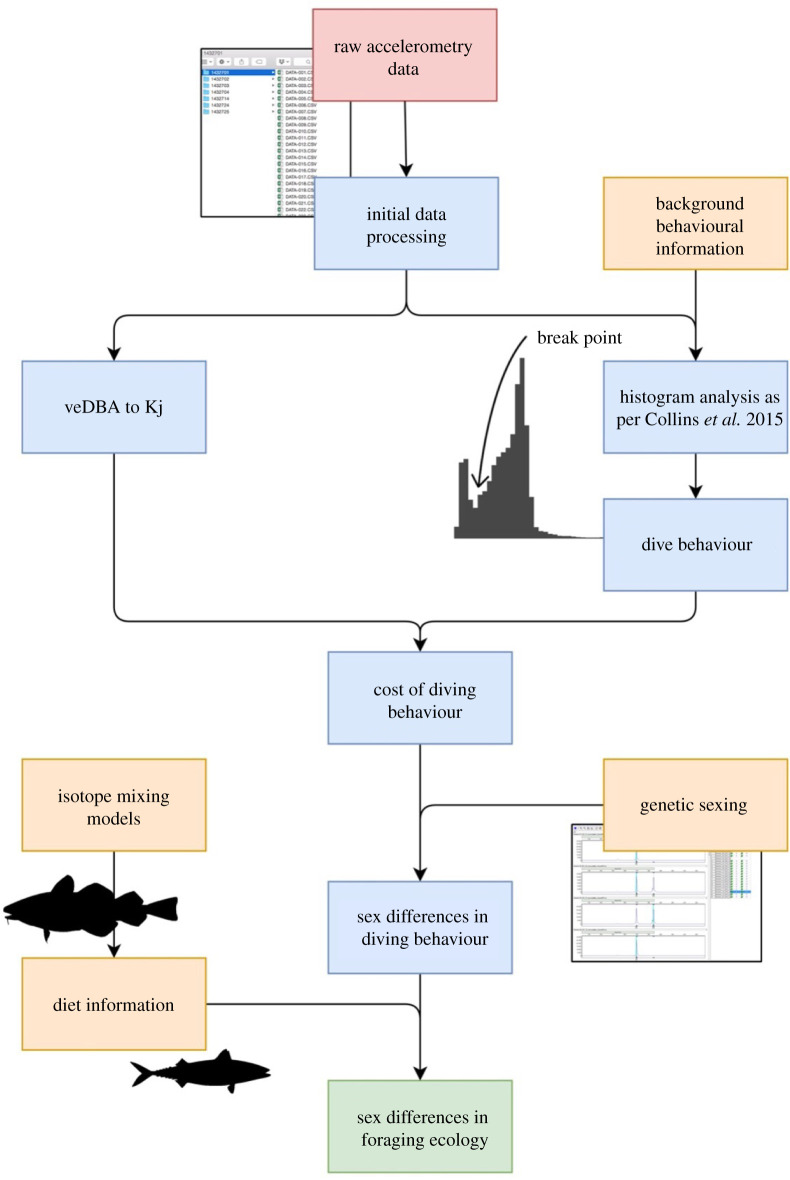
A visual diagram of the methodology is presented in figure 1. Breeding adult gannets (n = 8 in 2017, n = 6 in 2018) attending three- to five-week-old chicks were tracked from Great Saltee, southeast Ireland (52°7′37.92″ N, 6°35′45.6″ W). In 2017, three female, four male and one unknown gannets were tagged; four males and two females were then tagged in 2018. Birds were equipped with tags for an average of 3.70 ±1.39 days. To reduce potential impact on a breeding pair, only one individual of a pair was tagged for this study. Birds were caught using an 8–10 m pole with a metal crook, weighed and equipped with a combination of dataloggers. GPS loggers (i-gotU GT-120, Mobile Action Technology Inc., Taipei, Taiwan, 14 g, dimensions: 4 × 2 × 1 cm) recorded locations every 3 min; time depth recorders (TDR, CEFAS G5, 2.5 g, dimensions: 2 × 1 × 1 cm) recorded depth at 4 Hz after exceeding depth threshold of either 0.5 m or 1 m depending upon tag set-up; tri-axial accelerometers (Gulf Coast Data Concepts X16-mini, 17 g, dimensions: 6 × 2 × 1 cm) recorded g-forces (1 g = 9.807 m s−2) at 50 Hz. GPS and TDR loggers were attached ventrally to two–three central tail feathers using strips of waterproof Tesa tape. Accelerometers were attached to 10–15 mantle feathers between the wings. Three birds in 2017 and six birds in 2018 were equipped with GPS, TDR and accelerometers, while the remaining birds were equipped with only GPS and accelerometers. Total instrument mass was less than 2% of body mass and positioned to minimize impact on gannet movement, both aerodynamic and hydrodynamic [52]. It is important to consider effect of tag attachment [53]. Previous studies of gannets have employed similar devices [48,54,55]; the relatively small load placed upon gannets during these studies means that it is unlikely that gannet behaviour would be impacted from tag attachment of this magnitude. A table of deployment weights can be seen in electronic supplementary material, table S1, showing that many gannets gained weight or lost very small amounts during the tracking period. Blood samples were taken from 47 birds (n = 19 in 2017 and n = 28 in 2018), including the accelerometer-equipped birds, and used to construct a population model of dietary intake from isotope analysis (see section ‘Isotopic analysis for diet composition’ below). Between 1 and 1.5 ml of blood was sampled from the tarsus vein for SIA (see below), and two–three breast feathers were plucked for genetic sexing following the method outlined by Griffiths et al. [56]. Though we do not have data on reproductive success, all pairs were observed to continue in normal chick feeding behaviour, and chicks were observed to be alert and healthy, during and after the study period.
Figure 1.
Conceptual diagram of a methodology for data processing and the steps required to explore the sex differences in the foraging of northern gannets. The process starts at top with the red box labelled ‘raw accelerometry data’ and ends with the green box ‘sex differences in foraging ecology’. Blue boxes represent the methodology for analysing data and orange boxes represent additional analysis.
2.2. Data processing and dive behaviour definition
Behaviour classification from accelerometry data used a thresholding approach. Thresholds were determined using protocols and guidance set out by Collins et al. [57] and Shepard et al. [58]. Diving events occurred when average acceleration (running average of 2 s) in the X-axis (also known as the surge axis) was less than 0g and standard deviation (s.d.) in the mean X-axis was greater than 1.4g. The end of a dive was defined by a 1 second lagged maximum of pitch change within a 60 s period from the start of a dive. Take-off events were defined with a threshold where, following a dive, the s.d. of the Z-axis (also known as the heave axis) was greater than 1.8g and the s.d. of the X-axis was greater than 1g. Take-off events were considered to have ended and returned to normal flight when the s.d. of the Z-axis resolved to less than 1.4g and the s.d. of the X-axis was less than 1.4g. Data from a subset of birds (n = 9) tagged with both TDRs and accelerometers were used to validate accelerometer-derived dive events by visually comparing timestamps to TDR-confirmed dives; this required each dive to be manually viewed and checked to compare with a dive from a TDR and all dives from between accelerometry and TDRs were matched successfully. Accelerometer-derived dives had a bimodal distribution and were split into plunge dives and pursuit dives based on a distinct break within the frequency distribution at 5 s (see electronic supplementary material, figure S1); plunge dives are dives followed by an almost immediate rise to the surface, while a pursuit dive is characterized by sustained chase of prey underwater.
2.3. Energetics from accelerometry
DBA is a relative metric that can be used as a proxy for energetic expenditure from animal movement [59] and can be used to develop highly accurate activity budgets [60]. We used vectorial DBA (VeDBA) to account for any variation in tag alignment [61]. VeDBA was calculated for every second within the tracking period. The best practice to estimate energetic expenditure from DBA is to have species and behaviour-specific relationships between the rates of these quantities [62]. However, such calibration relationships do not exist for the overwhelming majority of species, requiring an alternative approach to be adopted that we outline here. This approach is based on the observation that the relationship between the rate of energy expenditure (kJ) and instantaneous VeDBA is linear among a variety of animal taxa, including mammals, reptiles and birds [26,33,62], with slope k. Using published allometric estimates of energy expenditure, it is possible to produce estimates of kilojoules expended in the movement for given values of VeDBA, via the process outlined in figure 2. The basis of the process is the assumption that energy expended in movement is equal to an animal's field metabolic rate (FMR) minus basal metabolic rate (BMR) for any given period; also known as the activity metabolic rate, auxiliary energy expenditure (AEE) or daily energetic scope [63,64]; here, we use AEE. AEE is then the energy expended, in kilojoules, for a given 24 h period in movement alone. The total sum of VeDBA over a 24 h period (VeDBA24) is then equivalent in energy to the AEE and we assume that where VeDBA is equal to 0, then energy expended in locomotion must equal 0 kJ, which would be the case for a completely inactive animal. We can then construct a simple relationship between AEE, VeDBA24 and the origin (where energy and movement are both 0) that allows the prediction of kilojoules expended from each unit of VeDBA expended in that day. Here, we used FMR estimates for northern gannets provided by the seabird FMR calculator [65,66], corrected for individual bird mass and colony latitude (52° N), and BMR estimates provided by allometric equations from Schreiber and Burger [67] to produce VeDBA to kJ gradients for each individual. VeDBA24 was calculated for each complete 24 h period for each bird (range 1–5) and a mean value calculated per bird, which was used in the predictive equation along with individual-specific estimates of BMR and FMR from that bird. This allowed for incorporation of an individual's mass which improved precision and allowed an estimate of the gradient between VeDBA and energy expenditure (constant k in figure 2, but also electronic supplementary material, figure S2 for individual gradients) to be estimated for each individual. To assess the effectiveness of the calculations underlying this approach, we used the individual relationships produced in this methodology to predict AEE from VeDBA24 for each bird. As this was successful (see electronic supplementary material, table S2), we accepted the use of this methodology. It is important to note that this method only allows for the prediction of energy expended by movement and assumes that all acceleration is due to movement; at present, it is not possible to effectively account for incidental records of acceleration not due to movement, such as periods of rest on water where sea swell may be detected by the accelerometer. However, if future work could filter out such incidental acceleration from acceleration due to movement, then the methodology would be further enhanced. Furthermore, the approach assumes BMR and FMR to be constant throughout the tracking period and driven by mass (and latitude in the case of FMR) alone. This ignores likely inter-individual variation in both FMR and BMR as well as any sex-specific differences that might exist in these quantities other than those accounted for by mass. However, since the relevant allometric equations do not incorporate sex effects, they are most effective for predicting population-level estimates for equal proportions of males and females. For that reason, the approach is only applicable for groups of individuals, including single-sex groups, rather than individual rates of energy expenditure, as is the case in the present study.
Figure 2.
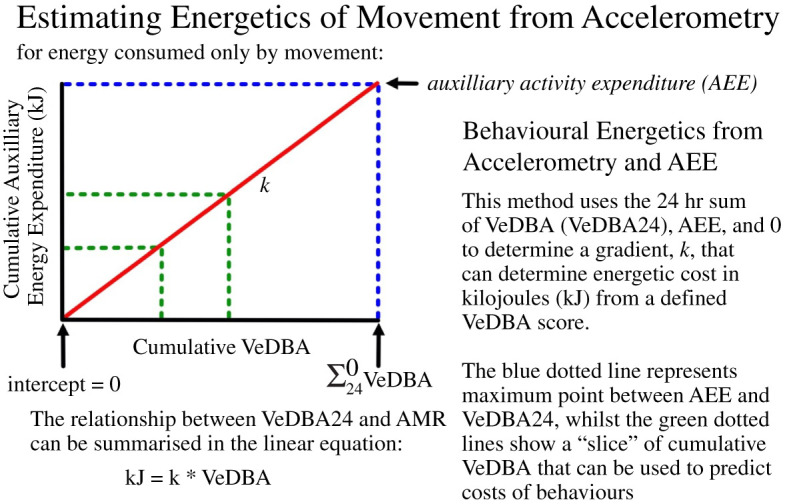
Conceptual diagram demonstrating how to estimate the AEE (in kJ) for a given quantity of VeDBA units within a dataset where it is assumed that energy above basal metabolism is consumed only by movement.
We used the individual gradient between VeDBA and energy expenditure shown in figure 2 to estimate total energetic expenditure for an individual bird from the time it left the colony, to the point of recapture. Gannet trips may range from one to several days, and so this approach allowed our predictions to account for a full range of behaviours, from in colony, transiting and foraging. This also allowed for energy expenditure to exceed FMR, which would be the case for a bird which spends more time resting than the ‘average’ bird used in population-level estimates of FMR used to build allometric relationships. As gannet foraging trips may last several days, they incur increasing energetic costs during a foraging trip, such as feeding chicks upon return. We have included this in the analysis by considering energetic differences in individual AEE per 24 h period. We then calculated total energetic demands (TED) by adding to this value the energetic demand of raising a four-week-old chick of 1397.14 kJ day−1 [50]. Due to unequal parenting roles, this cost was split with females retaining 60% of this cost and males 40% [50,68]. For multi-day trips, chick demands were multiplied by the appropriate length of time for energetic demand, and then also including BMR estimates for each 24 h period. Though it would be most appropriate to have information on feeding rates of chicks in this study, we do not have this information and instead consider the overall energy requirements of chicks which act as a proxy to feeding rates. This produced a value of TED for each gannet for each complete 24-hour period (range 1–5 days).
To calculate the energetic cost of dives, VeDBA was summed over the time frame of a dive (from initiation of the dive to completion of the subsequent take-off) and multiplied by that individual's value of k (figure 2) to estimate energy expended in kilojoules for each dive event for each gannet.
2.4. Isotopic analysis for diet composition
Blood samples taken during tag deployment were centrifuged for 10 min to separate red blood cells (RBC) from plasma. While RBC therefore represent diet prior to the deployment, preliminary sampling showed that isotopic signatures do not differ significantly between blood samples collected on deployment and recovery of devices approximately one week apart (unpublished data). Stable isotope analyses were performed at Elemtex UK (Stable Isotope and & Elemental Analysis Expertise), using a Thermoquest EA1110 Elemental Analyser linked to a Sercon 2020 stable isotope ratio mass spectrometer running in continuous flow mode. Accuracy and precision were monitored through laboratory internal standards and an in-house comparison standard nested within samples.
Prey stable isotope values were obtained from a published dataset of Celtic Sea fish samples [69]. Jennings & Cogan [69] conducted SIA of samples without lipid extraction; therefore, the δ13C data included in the published dataset are not corrected for differences in lipid content, but the % C and N data was used to make the required corrections following Logan et al. [70]. As recommended by Phillips et al. [71], a reduced prey dataset was used and included only those species previously recorded in more than 3% of the diet for Great Saltee gannets [72]. These species can be seen in electronic supplementary material, table S3.
Using Bayesian isotopic mixed models, it was possible to compare blood values to reference prey values to reconstruct the diet of gannets. The model was run on ‘long’ settings (chains = 3, length = 300 000, burn-in = 2 000 000, thinning = 100), using average diet-to-tissue discrimination factors (2.25 ± 0.61‰ for δ15N and 0.24 ± 0.79‰ for δ13C) from various studies of piscivorous birds [73–76]. Model convergence was assessed with the Gelman–Rubin diagnostic [77]. Sex-based diet estimates were obtained through Bayesian mixing models using the R package ‘MixSIAR’ [78]. We fit several models of diet with fixed and random effects as covariates and evaluated the relative support for each model using LOO (leave-one-out cross-validation) weights [79]. Model outputs were then used to construct prey proportions in the diet of males and females in 2017 and 2018.
We assumed the sizes of individual prey species were similar to those in Lewis et al. [72], a study from the same colony that did not identify any difference in the size of fish caught between the sexes. The size and mass of the fish were then used to calculate the energetic content (in kJ) of each fish species (using allometric equations referenced by Lewis et al. [72] and assuming a 76.1% assimilation efficiency following Cooper [80], see electronic supplementary material, table S3). For each sex-specific diet, the energy content (kJ) of each fish was multiplied by the proportions of species in the diet, and these proportional values were summed to provide an average kJ intake value (KIV) for a successful dive (a dive resulting in prey capture) for each individual gannet, assuming a successful dive results in the capture of one prey item.
2.5. Statistical analysis
A Mann–Whitney–Wilcoxon test was used to test weight differences between the sexes. An unpaired t-test was also undertaken to test the differences in dive length between males and females. To explore sex differences in the overall cost of prey capture attempts (dive and subsequent take-off), a linear mixed effect regression (LMER) was used to test for sex differences in dive and take-off characteristics. Factors included year, sex, mass, dive type and the interaction between sex and dive type with energy expenditure (kJ) as the dependent variable. Individual was included as a random effect to account for repeated measures of the same individual. The interaction between sex and dive type was included to explore if the different masses of the sexes (approximately 200 g [45]) impacted the cost of a dive type. To select the most parsimonious model, the dredge function from the ‘MuMin’ package was used [81]. Using the model averaging function in the MuMin package, any models within six AIC values were kept and model averaging undertaken [82]. A difference in dive rate (dives per day) between females and males was tested using a general linear model using the dive rate as a response variable for each individual and sex as a predicting factor. To determine if sex influences AEE plus chick energetic demands, an LMER was used to predict AEE (per day) from sex and year, with ID as a random effect to account for repeated measures from individuals.
A Mann–Whitney–Wilcoxon test was used to test for differences in KIV between sexes. For each gannet, TED was divided by KIV to determine how many successful dives were required to maintain body condition, forage and provision for a chick, assuming no change in body mass. The number of successful dives required was then considered as a proportion of the number of dives undertaken, therefore presenting a minimum percentage of dives which must have been successful for each individual gannet to maintain body mass and conduct its role in chick provisioning.
3. Results
Of the 14 gannets tracked, five were female, and eight were male. The DNA test of one individual was inconclusive and so was recorded as unknown sex; this individual was not included in the analysis of sex differences. Male gannets were on average lighter than females; male mass was 2.70 kg ±0.19 with females weighing 2.99 kg ±0.15 (Wilcoxon test: W = 35.5, r = 0.88, p = 0.025).
3.1. Sex differences in dive behaviour
We detected 1046 visually validated dives and subsequent take-off events. Of these dives, 24% were pursuit dives with females having a slight tendency towards pursuit dives compared with males. Female dives were 5.19 ± 3.81 s long, and male dives were 5.04 ± 3.53 s long; an unpaired t-test confirmed no significant differences in dive length between male and female dives (t = −0.53, d.f. = 496.78, p = 0.59). Combined cost of a single prey capture attempt (dive + take-off) in females was 1.94 ± 0 0.65 kJ s.d., while for males, it was 1.74 ± 0.83 kJ s.d., suggesting that male dives are 11.2% less costly than female dives. An averaged LMER indicated a significant effect of dive type and year on energy expenditure associated with dives, while sex was retained as a non-significant factor (table 1, and see electronic supplementary material, table S4 for model averaging table). The estimates of the total cost of all prey capture attempts represent less than 4% of the daily total energy expenditure for each individual (electronic supplementary material, table S5). Accounting for unequal provisioning of the chick, and the cost of foraging, daily energetic demands were 10.28% higher for females than males (female TED = 4209 kJ ±110.48 s.d.; male TED = 3817 kJ ±256.78 s.d., Wilcoxon test: W = 6, p < 0.05, total number of female days: 14.84, total number of male days: 31.88).
Table 1.
Conditional model summary from the averaged mixed effect linear regression used to predict kilojoules (kJ) expended during a prey capture attempt. Input variables were year (2017 and 2018), sex (male and female), dive type (pursuit or plunge) and mass. The interaction between sex and dive type was also included. Dive type (plunge) and sex (female) were absorbed into the intercept.
| dive energetics model | coefficient | s.e. | adjusted s.e. | z-value | p-value |
|---|---|---|---|---|---|
| intercept | −1.334 | 1.485 | 1.487 | 0.898 | 0.369 |
| type (pursuit) | 0.537 | 0.0429 | 0.0429 | 12.517 | <0.001 |
| year (2018) | 0.915 | 0.303 | 0.303 | 3.020 | <0.01 |
| mass | 0.167 | 0.782 | 0.783 | 0.214 | 0.8306 |
| sex (male) | −0.0823 | 0.375 | 0.375 | 0.219 | 0.8264 |
The daily dive rate of females was significantly greater than that of males (25.9 and 17.3, respectively, GLM F13 = 8.63, p < 0.01). However, because the cost of individual prey capture attempts is so low, a LMER predicting the AEE (kJ) per day for each individual from sex and year, with ID as a random effect, found no significant effect of sex on AEE (LMER F38 = 0.0018, p = 0.96)
3.2. Isotopic analysis
The isotope mixing model predicted that the most consumed prey species were Atlantic mackerel (Scomber scombrus) (51.07% ±4.34 s.d.) and European sprat (Sprattus sprattus) (9.42% ±4.93 s.d.) followed by lesser sandeel (Ammodytes marinus) (8.82% ±4.99 s.d.) and Atlantic herring (Clupea harengus) (4.77% ±1.98 s.d.). The remaining species included in the models were each predicted to contribute less than 8% to the overall diet. Seven different models were tested (table 2) and the best model included Year as a covariate (model weight: 76.8%, model 4). The second-best model included Sex and Year as variables with a relative weight of 23.1% and was used to predict sex-specific diets in each study year. There was no support for a model using individual ID only. Diet between the sexes was similar in both years (table 3), though mackerel made a higher contribution to male diet (difference of 3.4% in 2017 and 4.3% in 2018).
Table 2.
Bayesian mixed effect model outputs to determine predictors of diet in northern gannets. The best model lent support for a Year only model; however, the second-best model was Sex + Year with a model weight of 23.1%. This model was used to predict diet of the sexes. Leave-one-out cross-validation information criteria (LOOic) were used to assessed model suitability.
| model | variables | LOOic | standard error LOOic | delta LOOic | standard error delta LOOic | weight |
|---|---|---|---|---|---|---|
| 4 | Year | 87.5 | 11.8 | 0 | NA | 0.768 |
| 6 | Sex + Year | 89.9 | 11.6 | 2.4 | 3 | 0.231 |
| 5 | Year (by ID) | 106.8 | 8.6 | 19.3 | 6.4 | 0 |
| 2 | Sex | 109.7 | 10.9 | 22.2 | 6 | 0 |
| 1 | Null | 110.7 | 11 | 23.2 | 5.5 | 0 |
| 7 | ID | 139.2 | 10 | 51.7 | 9.5 | 0 |
| 3 | Sex (by ID) | 140.4 | 9.9 | 52.9 | 9.9 | 0 |
Table 3.
The diet composition (%) of male and female northern gannets in 2017 and 2018 as predicted by Bayesian mixed effects modelling, reported in table 2.
| species name | common name | 2017 |
2018 |
||
|---|---|---|---|---|---|
| female (%) | male (%) | female (%) | male (%) | ||
| Ammodytes spp. | sandeels | 13.3 | 13 | 4.5 | 4.5 |
| Callionymus spp. | dragonet | 4.4 | 5.5 | 5.8 | 7.7 |
| Chelidonichthys cuculus | red gurnard | 3.8 | 4.9 | 2.2 | 3 |
| Clupea harengus | Atlantic herring | 6 | 6.9 | 2.8 | 3.4 |
| Merlangius merlangus | whiting | 6.4 | 8.3 | 1.6 | 2.2 |
| Merluccius merluccius | hake | 6 | 6.9 | 4.2 | 4.6 |
| Pleuronectes platessa | plaice | 2.5 | 3 | 2.4 | 3.3 |
| Scomber scombrus | mackerel | 37.3 | 33.9 | 68.7 | 64.4 |
| Sprattus sprattus | sprat | 15 | 12.1 | 5.8 | 4.8 |
| Trisopterus esmarkii | Norway pout | 5.1 | 5.6 | 2 | 2.2 |
Applying average energy content of prey in proportion to its occurrence in the diet, a successful dive was estimated to have a KIV of 1006 kJ for females and 1005 kJ for males in 2017. In 2018, this figure rose with changing diet to 1563 kJ for females and 1553 kJ for males.
Based on the number of dives performed and average energy content of prey in proportion to their occurrence in sex-specific diets, female minimum feeding success rate was calculated as 19.39% ±7.71 s.d., while the male rate was 26.60% ±13.81 s.d. (figure 3). A summary of all results including dives, energy expenditure and success rates can be seen in table 4.
Figure 3.
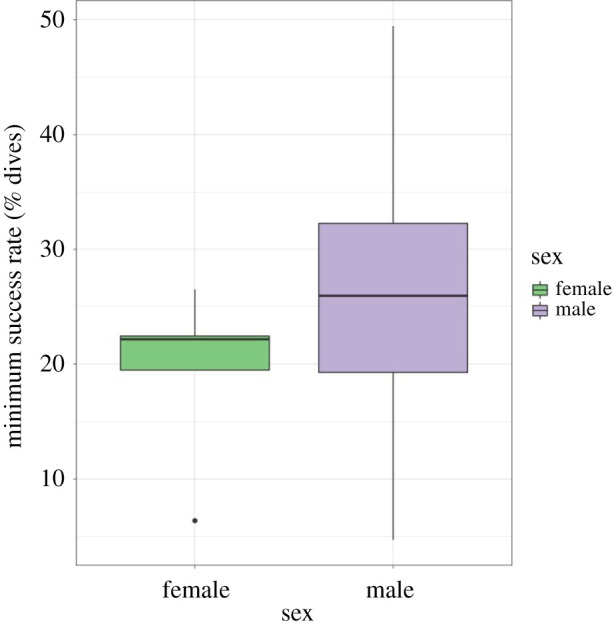
Minimum feeding success rates between the sexes to maintain body mass and provision a chick. Males were predicted to require a higher feeding success rate due to the lower numbers of dives undertaken. The middle horizontal line of the boxplot represents the median of the data range, boxes represent the 25th and 75th percentile with lines showing the remaining range of data (with outliers shown as dots).
Table 4.
Summary of results from tracked northern gannets between 2017 and 2018. Individuals were tracked using a combination of accelerometry, time depth recorders and GPS. Energy expenditure is calculated from the approach outlined in figure 2 and chick demands are accounted for by the amount of energy required by a four-week-old chick. Modelled average kJ per successful dive includes results from a Bayesian mixed model from isotope analysis and is produced as a figure for each sex per year. The kJ value of a successful dive, the number of dives undertaken and the overall energetic demands are then used to consider how many dives must be successful for a northern gannet to survive and raise a chick.
| bird ID | sex | year of study | tracking duration (days) | number of dives | dives per day | total energy expenditure during tracking (kJ) | total energy expenditure during tracking plus chick demands (kJ) | energy expenditure per day with chick demands (kJ) | modelled average kJ per successful dive | number of successful dives to meet energy demands | per cent of recorded dives needed to be successful |
|---|---|---|---|---|---|---|---|---|---|---|---|
| D01 | male | 2017 | 4.9 | 113 | 23.06 | 17 024 |
19 763 |
4033 | 1005.04 | 19.66 | 17.4 |
| D02 | male | 2017 | 2.86 | 36 | 12.56 | 8940 | 10 538 | 3684 | 1005.04 | 10.48 | 29.13 |
| D03 | unknown | 2017 | 5.08 | 189 | 37.15 | 15 840 | NA | NA | NA | NA | NA |
| D04 | female | 2017 | 0.97 | 65 | 66.89 | 3341 | 4154 | 4282 | 1005.96 | 4.13 | 6.35 |
| D05 | female | 2017 | 1.83 | 39 | 21.31 | 6109 | 7643 | 4176 | 1005.96 | 7.6 | 19.48 |
| D12 | female | 2017 | 4.68 | 90 | 19.19 | 16 147 | 20 070 | 4288 | 1005.96 | 19.95 | 22.17 |
| D13 | male | 2017 | 4.72 | 87 | 18.44 | 14 761 | 17 399 | 3686 | 1005.04 | 17.31 | 19.9 |
| D16 | male | 2017 | 1.99 | 18 | 9.06 | 6063 | 7175 | 3605 | 1005.04 | 7.14 | 39.66 |
| D25 | female | 2018 | 3.04 | 37 | 12.17 | 10 436 | 12 984 | 4271 | 1563.34 | 8.31 | 22.45 |
| D26 | male | 2018 | 2.92 | 36 | 12.31 | 11 096 | 12 728 | 4359 | 1552.61 | 8.2 | 22.77 |
| D28 | male | 2018 | 4.61 | 230 | 49.84 | 14 155 | 16 731 | 3629 | 1552.61 | 10.78 | 4.68 |
| D41 | male | 2018 | 4.83 | 39 | 8.07 | 15 351 | 18 051 | 3737 | 1552.61 | 11.63 | 29.81 |
| D52 | female | 2018 | 4.32 | 42 | 9.72 | 13 786 | 17 408 | 4029 | 1563.34 | 11.14 | 26.51 |
| D53 | male | 2018 | 5.05 | 25 | 4.95 | 16 375 | 19 198 | 3801 | 1552.61 | 12.37 | 49.46 |
4. Discussion
Here we show that, for gannets, sex differences in foraging behaviour are not the result of divergent energetic costs of foraging or different energetic content of consumed prey. We instead suggest that sex differences in foraging behaviour are likely to have arisen from unequal energetic demands between the sexes coupled with resource partitioning to avoid intraspecific competition. SIA indicated sex-specific diets, but there was no difference in energy intake between the sexes, despite the difference in mass. The cost of individual prey capture attempts associated with differing diets was low compared with total energetic expenditure, and despite females diving more than males and being heavier, there was no difference in auxilliary energetic expenditure per day between the sexes.
The methodology presented here represents a simple and easily accessible way of calculating the energetic cost of specific behaviours from accelerometry data where allometric estimates of BMR and FMR are available. DBA is an established proxy measure of energy expenditure [83], though difficulties remain in converting DBA to a true measure of energy expenditure [26]. Studies comparing DBA with energy expenditure must ensure that summed values of energy expenditure must not simply be regressed against summed values of DBA through time, a problem known as the time trap [84,85]. In this study, we accounted for time by considering complete 24 h periods, allowing for meaningful estimates of energy expenditure per unit of DBA and conversion to temporal periods based on this conversion rate and total DBA. Though we do not account for the error of environmental influences, we have assumed that this variance is equal between individuals.
The resulting energetic cost of prey capture events was low, even after including the cost of take-off from the sea surface following a dive, most likely due to the very low daily dive rate and short duration of this behaviour. For all individuals, prey capture attempts across the time tagged accounted for less than 4% of total energy expenditure. This suggests that the cost of diving probably does not limit the number of prey capture attempts in gannets from Great Saltee during our study period. This further suggests that gannets are not currently foraging at the limit of their energy demands. By contrast, little auks feeding on copepods were found to be required to feed upon six copepods a second to meet energy requirements [86].
Despite females undertaking an average of eight more dives per day, the low cost of prey capture attempts contributed to no differences in daily energetic expenditure between males and females. Year and dive type (plunge versus pursuit dive) had the largest effect on energetic cost of diving, reflecting yearly differences in diet noted in SIA analysis, that are probably related to the proportion of different dive types.
The cost of individual prey capture attempts may be slightly greater in females, as they spend more time underwater. However, energy expenditure can be affected by the medium an animal moves through [87], and this then may affect the sexes unevenly, though this is unlikely given the proportionally low energetic costs of diving. The increased cost of underwater pursuit following a ‘failed’ plunge dive suggests a cost-benefit trade-off, and Machovsky-Capuska et al. [88] noted higher feeding success in pursuit dives in Australasian gannets, Morus serrator, that would support this hypothesis. Alternatively, females may have to dive more as they are not as initially successful in the plunge dives; though our methodology only allows for minimum success rate to be calculated and this remains unknown. Intraspecific competition is expected to be higher with increasing proximity to a breeding colony [54,89] and this competition may drive sexually divergent foraging behaviour in gannets. Several studies report that male gannets forage closer to breeding colonies while females travel further [44,46]. This may be due to male gannets outcompeting females, forcing them to travel further and undertake different dive behaviour as they are forced to forage in different habitat to males [47,48] and this may also be a contributing factor to the different dive costs between the sexes reported in this study.
Gannets forage on a wide variety of prey [90], and SIA models indicated divergent diets between males and females, consistent with previous studies [44,48]. Prey proportions from our SIA models were similar to those previously reported by Lewis et al. [72] at the same site, and we found females took proportionately more mackerel and less whiting, Norway pout, and herring compared with males. After applying the average calorific content of prey species to sex-specific diets, energetic gain per dive did not differ between sexes. However, females make a greater contribution to chick provisioning [50], which may require a proportionate increase in targeting of smaller sized prey for chick consumption. While this has been observed in other seabird species [91], there is little evidence to suggest this is the case in northern gannets. Our results support that divergent diet is not the result of differing energetic cost of prey capture, or energy content of prey. Instead, sex differences in diet may be a result of intersexual competition, as previously demonstrated in this population of gannets [48].
Female gannets dived more frequently than males which may be reflective of differing provisioning roles [92,93], with female gannets estimated to have a 9.6% higher TED than male gannets, largely because of their greater contribution to chick feeding [50]. After accounting for the increased energetic demands in females, the energetic cost of foraging, the mean calorific content of prey in sex-specific diets and the number of dives performed, males were predicted to have a higher minimum feeding success rate than females (19% of dives in females and 26% of dives in males). These estimates of feeding success are lower than estimates of approximately 50–66% for Australasian gannets based on identifying prey captures from bird-borne cameras [94,95]. Our estimates reflect minimum success rates required to meet energy demands, and the discrepancy suggests that gannets may routinely catch more food than required to meet minimum energy demands. Any energy surplus may then be invested in chick provisioning, or be expended engaging in energetically demanding activities around and within the colony such as preening and aggression [96]. Energy surplus may then also be turned into body mass [97] or other costly procedures such as moulting and growth of new feathers.
Energy acquisition and allocation provide a useful framework to study ecological questions, including management and evolution [98]. This study highlights how DBA can be used to estimate the energetic costs of discrete short-lived behaviours, providing insights into the foraging ecology of free-ranging animals. While gannets are sexually monomorphic, they show divergent foraging behaviour and diet, which our results suggest are not the result of differing cost of foraging or energy content of prey. Instead, such sexually divergent foraging strategies in monomorphic species are thought to be driven by intersexual competition [48] or differing energy demands such as unequal parenting roles between the sexes [20]. Female gannets meet this additional need through increasing their dive rate, a strategy that has no appreciable additional cost given the small overall cost of individual dives and may be an adapted strategy to account for competitive exclusion. Over the course of a breeding season, this extra energetic expenditure equates to approximately 1567 kJ, less than the energy provided by one mackerel. However, after accounting for the cost of dives, the energetic content of prey, and the number of dives performed, females appear to have lower overall success rates to meet energetic requirements, suggesting some subtle difference in foraging behaviour that may represent competitive exclusion between the sexes [44,48] or another mechanism that remains unknown.
Our methodology and results have highlighted that in northern gannets, a sexually monomorphic species, the sexes show differences in foraging behaviour primarily related to dive rate and feeding success rather than the energetic cost of foraging. Evaluating sex differences in foraging behaviour from an energetic perspective may provide a clearer picture for understanding sexually divergent foraging strategies in both sexually monomorphic and dimorphic species. Future research should consider an energetics approach in exploring the fine-scale behavioural differences between sexes. It would be interesting to see this study replicated using dimorphic species, where differences between the sexes are more clearly pronounced, to see if sex differences in foraging behaviour may change with corresponding differences in energetic expenditure beyond those due to mass alone [99]. A further opportunity of study would be to consider how the sexes may differ in their energy expenditure with changing resources [100].
Supplementary Material
Acknowledgements
We are grateful to the Neale family for access to Great Saltee where this work was undertaken. All research was approved by the University College Cork Animal Ethics Committee and was conducted under licence from the Health Products Regulatory Authority, the National Parks and Wildlife Service and the British Trust for Ornithology.
Ethics
All research was approved by the University College Cork Animal Ethics Committee and was conducted under licence from the Health Products Regulatory Authority, the National Parks and Wildlife Service and the British Trust for Ornithology.
Data accessibility
All data collected as part of this study are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.zs7h44j88 [51].
The data are provided in the electronic supplementary material [101].
Authors' contributions
A.B.: conceptualization, formal analysis, funding acquisition, investigation, methodology, writing—original draft and writing—review and editing; J.G.: formal analysis, methodology and writing—review and editing; J.L.Q.: funding acquisition, methodology, supervision and writing—review and editing; J.A.G.: investigation, methodology, validation and writing—review and editing; M.J.: conceptualization, data curation, funding acquisition, investigation, writing—original draft and writing—review and editing.
All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Competing interests
We declare we have no competing interests.
Funding
A.B. was funded by the Irish Research Council Postgraduate Scholarship (Project ID: GOIPG/2016/503).
References
- 1.Ginnett TF, Demment MW. 1997. Sex differences in giraffe foraging behavior at two spatial scales. Oecologia 110, 291-300. ( 10.1007/s004420050162) [DOI] [PubMed] [Google Scholar]
- 2.Beck CA, Bowen WD, McMillan JI, Iverson SJ. 2003. Sex differences in diving at multiple temporal scales in a size-dimorphic capital breeder. J. Anim. Ecol. 72, 979-993. ( 10.1046/j.1365-2656.2003.00761.x) [DOI] [Google Scholar]
- 3.Phillips R, Silk J, Phalan B, Catry P, Croxall J. 2004. Seasonal sexual segregation in two Thalassarche albatross species: competitive exclusion, reproductive role specialization or foraging niche divergence? Proc. R. Soc. Lond. B 271, 1283-1291. ( 10.1098/rspb.2004.2718) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patrick SC, Weimerskirch H. 2014. Consistency pays: sex differences and fitness consequences of behavioural specialization in a wide-ranging seabird. Biol. Lett. 10, 20140630. ( 10.1098/rsbl.2014.0630) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wanless S, Harris M, Morris J. 1995. Factors affecting daily activity budgets of South Georgian shags during chick rearing at Bird Island, South Georgia. Condor 97, 550-558. ( 10.2307/1369040) [DOI] [Google Scholar]
- 6.Maklakov AA, Simpson SJ, Zajitschek F, Hall MD, Dessmann J, Clissold F, Raubenheimer D, Bonduriansky R, Brooks RC. 2008. Sex-specific fitness effects of nutrient intake on reproduction and lifespan. Curr. Biol. 18, 1062-1066. ( 10.1016/j.cub.2008.06.059) [DOI] [PubMed] [Google Scholar]
- 7.Reddiex AJ, Gosden TP, Bonduriansky R, Chenoweth SF. 2013. Sex-specific fitness consequences of nutrient intake and the evolvability of diet preferences. Am. Nat. 182, 91-102. ( 10.1086/670649) [DOI] [PubMed] [Google Scholar]
- 8.Kokko H, Jennions MD. 2012. Sex differences in parental care: the evolution of parental care. Oxford, UK: Oxford University Press. [Google Scholar]
- 9.Rogowitz GL, Chappell MA. 2000. Energy metabolism of eucalyptus-boring beetles at rest and during locomotion: gender makes a difference. J. Exp. Biol. 203, 1131-1139. ( 10.1242/jeb.203.7.1131) [DOI] [PubMed] [Google Scholar]
- 10.Lees JJ, Nudds RL, Folkow LP, Stokkan KA, Codd JR. 2011. Understanding sex differences in the cost of terrestrial locomotion. Proc. R. Soc. B 279, 826-832. ( 10.1098/rspb.2011.1334) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Biggerstaff MT, Lashley MA, Chitwood MC, Moorman CE, DePerno CS. 2017. Sexual segregation of forage patch use: support for the social-factors and predation hypotheses. Behav. Processes 136, 36-42. ( 10.1016/j.beproc.2017.01.003) [DOI] [PubMed] [Google Scholar]
- 12.Galezo AA, Krzyszczyk E, Mann J. 2017. Sexual segregation in Indo-Pacific bottlenose dolphins is driven by female avoidance of males. Behav. Ecol. 29, 377-386. ( 10.1093/beheco/arx177) [DOI] [Google Scholar]
- 13.Pincheira-Donoso D, Tregenza T, Butlin RK, Hodgson DJ. 2018. Sexes and species as rival units of niche saturation during community assembly. Glob. Ecol. Biogeogr. 27, 593-603. ( 10.1111/geb.12722) [DOI] [Google Scholar]
- 14.González-Solís J, Croxall JP, Wood AG. 2000. Sexual dimorphism and sexual segregation in foraging strategies of northern giant petrels, Macronectes halli, during incubation. Oikos 90, 390-398. ( 10.1034/j.1600-0706.2000.900220.x) [DOI] [Google Scholar]
- 15.Ruckstuhl K, Neuhaus P. 2000. Sexual segregation in ungulates: a new approach. Behaviour 137, 361-377. ( 10.1163/156853900502123) [DOI] [Google Scholar]
- 16.Salton M, Kirkwood R, Slip D, Harcourt R. 2019. Mechanisms for sex-based segregation in foraging behaviour by a polygynous marine carnivore. Mar. Ecol. Prog. Ser. 624, 213-226. ( 10.3354/meps13036) [DOI] [Google Scholar]
- 17.Ehl S, Hostert K, Korsch J, Gros P, Schmitt T. 2018. Sexual dimorphism in the alpine butterflies Boloria pales and Boloria napaea: differences in movement and foraging behavior (Lepidoptera: Nymphalidae). Insect Sci. 25, 1089-1101. ( 10.1111/1744-7917.12494) [DOI] [PubMed] [Google Scholar]
- 18.Tina F, Jaroensutasinee M, Jaroensutasinee K. 2015. Effects of sexual dimorphism and body size on feeding behaviour of the fiddler crab, Uca bengali Crane, 1975. Crustaceana 88, 231-242. ( 10.1163/15685403-00003405) [DOI] [Google Scholar]
- 19.Wearmouth VJ, Sims DW. 2008. Sexual segregation in marine fish, reptiles, birds and mammals: behaviour patterns, mechanisms and conservation implications. Adv. Mar. Biol. 54, 107-170. ( 10.1016/S0065-2881(08)00002-3) [DOI] [PubMed] [Google Scholar]
- 20.Pinet P, Jaquemet S, Phillips RA, Le Corre M. 2012. Sex-specific foraging strategies throughout the breeding season in a tropical, sexually monomorphic small petrel. Anim. Behav. 83, 979-989. ( 10.1016/j.anbehav.2012.01.019) [DOI] [Google Scholar]
- 21.Elliott K, Gaston A, Crump D. 2010. Sex-specific behavior by a monomorphic seabird represents risk partitioning. Behav. Ecol. 21, 1024-1032. ( 10.1093/beheco/arq076) [DOI] [Google Scholar]
- 22.Miller MG, Silva FR, Machovsky-Capuska GE, Congdon BC. 2018. Sexual segregation in tropical seabirds: drivers of sex-specific foraging in the brown booby Sula leucogaster. J. Ornithol. 159, 425-437. ( 10.1007/s10336-017-1512-1) [DOI] [Google Scholar]
- 23.MacArthur RH, Pianka ER. 1966. On optimal use of a patchy environment. Am. Nat. 100, 603-609. ( 10.1086/282454) [DOI] [Google Scholar]
- 24.Tullock G. 1971. The coal tit as a careful shopper. Am. Nat. 105, 77-80. ( 10.1086/282704) [DOI] [Google Scholar]
- 25.Pulliam HR. 1974. On the theory of optimal diets. Am. Nat. 108, 59-74. ( 10.1086/282885) [DOI] [Google Scholar]
- 26.Wilson RP, et al. 2019. Estimates for energy expenditure in free-living animals using acceleration proxies: a reappraisal. J. Anim. Ecol. 89, 161-172. ( 10.1111/1365-2656.13040) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brown DD, Kays R, Wikelski M, Wilson R, Klimley AP. 2013. Observing the unwatchable through acceleration logging of animal behavior. Anim. Biotelemetry 1, 20. ( 10.1186/2050-3385-1-20) [DOI] [Google Scholar]
- 28.Butler PJ, Green JA, Boyd I, Speakman J. 2004. Measuring metabolic rate in the field: the pros and cons of the doubly labelled water and heart rate methods. Funct. Ecol. 18, 168-183. ( 10.1111/j.0269-8463.2004.00821.x) [DOI] [Google Scholar]
- 29.Green JA. 2011. The heart rate method for estimating metabolic rate: review and recommendations. Comp. Biochem. Physiol. A 158, 287-304. ( 10.1016/j.cbpa.2010.09.011) [DOI] [PubMed] [Google Scholar]
- 30.Frappell P, Blevin H, Baudinette R. 1989. Understanding respirometry chambers: what goes in must come out. J. Theor. Biol. 138, 479-494. ( 10.1016/S0022-5193(89)80046-3) [DOI] [PubMed] [Google Scholar]
- 31.Shepard EL, et al. 2008. Identification of animal movement patterns using tri-axial accelerometry. Endangered Species Res. 10, 47-60. ( 10.3354/esr00084) [DOI] [Google Scholar]
- 32.Gleiss AC, Wilson RP, Shepard EL. 2011. Making overall dynamic body acceleration work: on the theory of acceleration as a proxy for energy expenditure. Methods Ecol. Evol. 2, 23-33. ( 10.1111/j.2041-210X.2010.00057.x) [DOI] [Google Scholar]
- 33.Halsey L, Shepard E, Quintana F, Laich AG, Green J, Wilson R. 2009. The relationship between oxygen consumption and body acceleration in a range of species. Comp. Biochem. Physiol. A 152, 197-202. ( 10.1016/j.cbpa.2008.09.021) [DOI] [PubMed] [Google Scholar]
- 34.Halsey LG, Shepard EL, Wilson RP. 2011. Assessing the development and application of the accelerometry technique for estimating energy expenditure. Comp. Biochem. Physiol. A 158, 305-314. ( 10.1016/j.cbpa.2010.09.002) [DOI] [PubMed] [Google Scholar]
- 35.Goldsworthy B, Young MJ, Seddon PJ, van Heezik Y. 2016. Stomach flushing does not affect apparent adult survival, chick hatching, or fledging success in yellow-eyed penguins (Megadyptes antipodes). Biol. Conserv. 196, 115-123. ( 10.1016/j.biocon.2016.02.009) [DOI] [Google Scholar]
- 36.Thiebault A, Semeria M, Lett C, Tremblay Y. 2016. How to capture fish in a school? Effect of successive predator attacks on seabird feeding success. J. Anim. Ecol. 85, 157-167. ( 10.1111/1365-2656.12455) [DOI] [PubMed] [Google Scholar]
- 37.Hobson KA. 1993. Trophic relationships among high Arctic seabirds: insights from tissue-dependent stable-isotope models. Mar. Ecol. Prog. Ser. 95, 7. ( 10.3354/meps095007) [DOI] [Google Scholar]
- 38.Hobson KA, Piatt JF, Pitocchelli J. 1994. Using stable isotopes to determine seabird trophic relationships. J. Anim. Ecol. 63, 786-798. ( 10.2307/5256) [DOI] [Google Scholar]
- 39.Bond AL, Jones IL. 2009. A practical introduction to stable-isotope analysis for seabird biologists: approaches, cautions and caveats. Mar. Ornithol. 37, 183-188. [Google Scholar]
- 40.Stock BC, Semmens BX. 2016. Unifying error structures in commonly used biotracer mixing models. Ecology 97, 2562-2569. ( 10.1002/ecy.1517) [DOI] [PubMed] [Google Scholar]
- 41.Swan GJ, Bearhop S, Redpath SM, Silk MJ, Goodwin CE, Inger R, McDonald RA. 2020. Evaluating Bayesian stable isotope mixing models of wild animal diet and the effects of trophic discrimination factors and informative priors. Methods Ecol. Evol. 11, 139-149. ( 10.1111/2041-210X.13311) [DOI] [Google Scholar]
- 42.Bearhop S, Adams CE, Waldron S, Fuller RA, MacLeod H. 2004. Determining trophic niche width: a novel approach using stable isotope analysis. J. Anim. Ecol. 73, 1007-1012. ( 10.1111/j.0021-8790.2004.00861.x) [DOI] [Google Scholar]
- 43.Deakin Z, et al. 2019. Sex differences in migration and demography of a wide-ranging seabird, the northern gannet. Mar. Ecol. Prog. Ser. 622, 191-201. ( 10.3354/meps12986) [DOI] [Google Scholar]
- 44.Stauss C, et al. 2012. Sex-specific foraging behaviour in northern gannets, Morus bassanus; incidence and implications. Mar. Ecol. Prog. Ser. 457, 151-162. ( 10.3354/meps09734) [DOI] [Google Scholar]
- 45.Malvat Z, Lynch S, Bennison A, Jessopp M. 2020. Evidence of links between haematological condition and foraging behaviour in northern gannets (Morus bassanus). R. Soc. Open Sci. 7, 192164. ( 10.1098/rsos.192164) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lewis S, Benvenuti S, Dall-Antonia L, Griffiths R, Money L, Sherratt T, Wanless S, Hamer KC. 2002. Sex-specific foraging behaviour in a monomorphic seabird. Proc. R. Soc. Lond. B 269, 1687-1693. ( 10.1098/rspb.2002.2083) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cleasby IR, Wakefield ED, Bodey TW, Davies RD, Patrick SC, Newton J, Votier SC, Bearhop S, Hamer KC. 2015. Sexual segregation in a wide-ranging marine predator is a consequence of habitat selection. Mar. Ecol. Prog. Ser. 518, 1-12. ( 10.3354/meps11112) [DOI] [Google Scholar]
- 48.Giménez J, et al. 2021. Sexual mismatch between vessel-associated foraging and discard consumption in a marine top predator. Front. Mar. Sci. 8, 220. ( 10.3389/fmars.2021.636468) [DOI] [Google Scholar]
- 49.Bodey TW, Cleasby IR, Votier SC, Hamer KC, Newton J, Patrick SC, Wakefield ED, Bearhop S. 2018. Frequency and consequences of individual dietary specialisation in a wide-ranging marine predator, the northern gannet. Mar. Ecol. Prog. Ser. 604, 251-262. ( 10.3354/meps12729) [DOI] [Google Scholar]
- 50.Montevecchi WA, Ricklefs R, Kirkham I, Gabaldon D. 1984. Growth energetics of nestling northern gannets (Sula bassanus). Auk 101, 334-341. ( 10.1093/auk/101.2.334) [DOI] [Google Scholar]
- 51.Bennison A, Giménez J, Quinn JL, Green JA, Jessopp M. 2022. Data associated to: a bioenergetics approach to understanding sex differences in the foraging behaviour of a sexually monomorphic species. Dryad Digital Repository. ( 10.5061/dryad.zs7h44j88) [DOI] [PMC free article] [PubMed]
- 52.Vandenabeele SP, Shepard EL, Grogan A, Wilson RP. 2012. When three per cent may not be three per cent; device-equipped seabirds experience variable flight constraints. Mar. Biol. 159, 1-14. ( 10.1007/s00227-011-1784-6) [DOI] [Google Scholar]
- 53.Bodey TW, Cleasby IR, Bell F, Parr N, Schultz A, Votier SC, Bearhop S. 2018. A phylogenetically controlled meta-analysis of biologging device effects on birds: deleterious effects and a call for more standardized reporting of study data. Methods Ecol. Evol. 9, 946-955. ( 10.1111/2041-210X.12934) [DOI] [Google Scholar]
- 54.Wakefield ED, et al. 2013. Space partitioning without territoriality in gannets. Science 341, 68-70. ( 10.1126/science.1236077) [DOI] [PubMed] [Google Scholar]
- 55.Wakefield ED, Cleasby IR, Bearhop S, Bodey TW, Davies RD, Miller PI, Newton J, Votier SC, Hamer KC. 2015. Long-term individual foraging site fidelity—why some gannets don't change their spots. Ecology 96, 3058-3074. ( 10.1890/14-1300.1) [DOI] [PubMed] [Google Scholar]
- 56.Griffiths R, Double MC, Orr K, Dawson RJ. 1998. A DNA test to sex most birds. Mol. Ecol. 7, 1071-1075. ( 10.1046/j.1365-294x.1998.00389.x) [DOI] [PubMed] [Google Scholar]
- 57.Collins PM, Green JA, Warwick-Evans V, Dodd S, Shaw PJ, Arnould JP, Halsey LG. 2015. Interpreting behaviors from accelerometry: a method combining simplicity and objectivity. Ecol. Evol. 5, 4642-4654. ( 10.1002/ece3.1660) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shepard EL, Wilson RP, Halsey LG, Quintana F, Laich AG, Gleiss AC, Liebsch N, Myers AE, Norman B. 2008. Derivation of body motion via appropriate smoothing of acceleration data. Aqu. Biol. 4, 235-241. ( 10.3354/ab00104) [DOI] [Google Scholar]
- 59.Wilson RP, White CR, Quintana F, Halsey LG, Liebsch N, Martin GR, Butler PJ. 2006. Moving towards acceleration for estimates of activity-specific metabolic rate in free-living animals: the case of the cormorant. J. Anim. Ecol. 75, 1081-1090. ( 10.1111/j.1365-2656.2006.01127.x) [DOI] [PubMed] [Google Scholar]
- 60.Patterson A, Gilchrist HG, Chivers L, Hatch S, Elliott K. 2019. A comparison of techniques for classifying behavior from accelerometers for two species of seabird. Ecol. Evol. 9, 3030-3045. ( 10.1002/ece3.4740) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Qasem L, Cardew A, Wilson A, Griffiths I, Halsey LG, Shepard EL, Gleiss AC, Wilson R. 2012. Tri-axial dynamic acceleration as a proxy for animal energy expenditure; should we be summing values or calculating the vector? PLoS ONE 7, e31187. ( 10.1371/journal.pone.0031187) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hicks O, Burthe S, Daunt F, Butler A, Bishop C, Green JA. 2017. Validating accelerometry estimates of energy expenditure across behaviours using heart rate data in a free-living seabird. J. Exp. Biol. 220, 1875-1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mathot KJ, Dingemanse NJ. 2015. Energetics and behavior: unrequited needs and new directions. Trends Ecol. Evol. 30, 199-206. ( 10.1016/j.tree.2015.01.010) [DOI] [PubMed] [Google Scholar]
- 64.Halsey LG, et al. 2019. Flexibility, variability and constraint in energy management patterns across vertebrate taxa revealed by long-term heart rate measurements. Funct. Ecol. 33, 260-272. ( 10.1111/1365-2435.13264) [DOI] [Google Scholar]
- 65.Dunn RE, White CR, Green JA. 2018. A model to estimate seabird field metabolic rates. Biol. Lett. 14, 20180190. ( 10.1098/rsbl.2018.0190) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dunn RE, White C, Green J. 2018. Seabird FMR Calculator 2018. See https://ruthedunn.shinyapps.io/seabird_fmr_calculator/.
- 67.Schreiber EA, Burger J. 2001. Biology of marine birds. Boca Raton, FL: CRC Press. [Google Scholar]
- 68.Montevecchi W, Kirkham I, Purchase R, Harvey B. 1980. Colonies of northern gannets in Newfoundland. Osprey 11, 2-8. [Google Scholar]
- 69.Jennings S, Cogan S. 2015. Nitrogen and carbon stable isotope variation in northeast Atlantic fishes and squids: ecological archives E096-226. Ecology 96, 2568. ( 10.1890/15-0299.1) [DOI] [Google Scholar]
- 70.Logan JM, Jardine TD, Miller TJ, Bunn SE, Cunjak RA, Lutcavage ME. 2008. Lipid corrections in carbon and nitrogen stable isotope analyses: comparison of chemical extraction and modelling methods. J. Anim. Ecol. 77, 838-846. ( 10.1111/j.1365-2656.2008.01394.x) [DOI] [PubMed] [Google Scholar]
- 71.Phillips DL, Inger R, Bearhop S, Jackson AL, Moore JW, Parnell AC, Semmens BX, Ward EJ. 2014. Best practices for use of stable isotope mixing models in food-web studies. Can. J. Zool. 92, 823-835. ( 10.1139/cjz-2014-0127) [DOI] [Google Scholar]
- 72.Lewis S, Sherratt TN, Hamer KC, Harris MP, Wanless S. 2003. Contrasting diet quality of northern gannets, Morus bassanus, at two colonies. Ardea 91, 167-176. [Google Scholar]
- 73.Hobson KA, Clark RG. 1992. Assessing avian diets using stable isotopes II: factors influencing diet-tissue fractionation. Condor 94, 189-197. ( 10.2307/1368808) [DOI] [Google Scholar]
- 74.Bearhop S, Waldron S, Votier SC, Furness RW. 2002. Factors that influence assimilation rates and fractionation of nitrogen and carbon stable isotopes in avian blood and feathers. Physiol. Biochem. Zool. 75, 451-458. ( 10.1086/342800) [DOI] [PubMed] [Google Scholar]
- 75.Forero MG, Tella JL, Hobson KA, Bertellotti M, Blanco G. 2002. Conspecific food competition explains variability in colony size: a test in Magellanic penguins. Ecology 83, 3466-3475. ( 10.1890/0012-9658(2002)083[3466:CFCEVI]2.0.CO;2) [DOI] [Google Scholar]
- 76.Cherel Y, Hobson KA, Hassani S. 2005. Isotopic discrimination between food and blood and feathers of captive penguins: implications for dietary studies in the wild. Physiol. Biochem. Zool. 78, 106-115. ( 10.1086/425202) [DOI] [PubMed] [Google Scholar]
- 77.Gelman A, Carlin JB, Stern HS, Dunson DB, Vehtari A, Rubin DB. 2013. Bayesian data analysis. London, UK: Chapman and Hall/CRC. [Google Scholar]
- 78.Semmens BX, et al. 2013. MixSIAR: a Bayesian stable isotope mixing model for characterizing intrapopulation niche variation. Ecol. Soc. Am. Minneapolis MN, 4-9. [Google Scholar]
- 79.Vehtari A, Gelman A, Gabry J. 2017. Practical Bayesian model evaluation using leave-one-out cross-validation and WAIC. Stat. Comput. 27, 1413-1432. ( 10.1007/s11222-016-9696-4) [DOI] [Google Scholar]
- 80.Cooper J. 1978. Energetic requirements for growth and maintenance of the Cape gannet (Aves; Sulidae). African Zool. 13, 305-317. ( 10.1080/00445096.1978.11447631) [DOI] [Google Scholar]
- 81.Barton K. 2011. MuMIn: multi-model inference. R package version 1.0. 0. Vienna, Austria: R Foundation for Statistical Computing. See http://CRANR-projectorg/package=MuMIn. [Google Scholar]
- 82.Harrison XA, Donaldson L, Correa-Cano ME, Evans J, Fisher DN, Goodwin CE, Robinson BS, Hodgson DJ, Inger R. et al. 2018. A brief introduction to mixed effects modelling and multi-model inference in ecology. PeerJ 6, e4794. ( 10.7717/peerj.4794) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Elliott K, Le Vaillant M, Kato A, Speakman J, Ropert-Coudert Y. 2013. Accelerometry predicts daily energy expenditure in a bird with high activity levels. Biol. Lett. 9, 20120919. ( 10.1098/rsbl.2012.0919) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Halsey LG. 2017. Relationships grow with time: a note of caution about energy expenditure-proxy correlations, focussing on accelerometry as an example. Funct. Ecol. 31, 1176-1183. ( 10.1111/1365-2435.12822) [DOI] [Google Scholar]
- 85.Ladds MA, Rosen DA, Slip DJ, Harcourt RG. 2017. Proxies of energy expenditure for marine mammals: an experimental test of ‘the time trap’. Sci. Rep. 7, 1-10. ( 10.1038/s41598-017-11576-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Harding AMA, Egevang C, Walkusz W, Merkel F, Blanc S, Grémillet D. 2009. Estimating prey capture rates of a planktivorous seabird, the little auk (Alle alle), using diet, diving behaviour, and energy consumption. Polar Biol. 32, 785-796. ( 10.1007/s00300-009-0581-x) [DOI] [Google Scholar]
- 87.Laich AG, Wilson RP, Gleiss AC, Shepard EL, Quintana F. 2011. Use of overall dynamic body acceleration for estimating energy expenditure in cormorants: does locomotion in different media affect relationships? J. Exp. Mar. Biol. Ecol. 399, 151-155. ( 10.1016/j.jembe.2011.01.008) [DOI] [Google Scholar]
- 88.Machovsky-Capuska GE, Vaughn RL, Würsig B, Katzir G, Raubenheimer D. 2011. Dive strategies and foraging effort in the Australasian gannet, Morus serrator, revealed by underwater videography. Mar. Ecol. Prog. Ser. 442, 255-261. ( 10.3354/meps09458) [DOI] [Google Scholar]
- 89.Ashmole NP. 1971. Seabird ecology and the marine environment. Avian Biol. 1, 223-286. [Google Scholar]
- 90.Hamer K, Humphreys E, Garthe S, Hennicke J, Peters G, Grémillet D, Phillips RA, Harris MP, Wanless S. 2007. Annual variation in diets, feeding locations and foraging behaviour of gannets in the North Sea: flexibility, consistency and constraint. Mar. Ecol. Prog. Ser. 338, 295-305. ( 10.3354/meps338295) [DOI] [Google Scholar]
- 91.Davoren GK, Burger AE. 1999. Differences in prey selection and behaviour during self-feeding and chick provisioning in rhinoceros auklets. Anim. Behav. 58, 853-863. ( 10.1006/anbe.1999.1209) [DOI] [PubMed] [Google Scholar]
- 92.Grieco F. 2001. Short-term regulation of food-provisioning rate and effect on prey size in blue tits, Parus caeruleus. Anim. Behav. 62, 107-116. ( 10.1006/anbe.2001.1736) [DOI] [Google Scholar]
- 93.Limmer B, Becker PH. 2009. Improvement in chick provisioning with parental experience in a seabird. Anim. Behav. 77, 1095-1101. ( 10.1016/j.anbehav.2009.01.015) [DOI] [Google Scholar]
- 94.Cansse T, Fauchet L, Wells M, Arnould J. 2020. Factors influencing prey capture success and profitability in Australasian gannets (Morus serrator). Biol. Open 9, bio047514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wells MR, Angel LP, Arnould JP. 2016. Habitat-specific foraging strategies in Australasian gannets. Biol. Open 5, 921-927. ( 10.1242/bio.018085) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nelson B. 2002. The Atlantic Gannet. Great Yarmouth, UK: Fenix Books. [Google Scholar]
- 97.Bryant D. 1988. Energy expenditure and body mass changes as measures of reproductive costs in birds. Funct. Ecol. 2, 23-34. ( 10.2307/2389456) [DOI] [Google Scholar]
- 98.Karasov W. 1986. Energetics, physiology and vertebrate ecology. Trends Ecol. Evol. 1, 101-104. ( 10.1016/0169-5347(86)90034-0) [DOI] [PubMed] [Google Scholar]
- 99.Weimerskirch H, Le Corre M, Gadenne H, Pinaud D, Kato A, Ropert-Coudert Y, Bost CA. 2009. Relationship between reversed sexual dimorphism, breeding investment and foraging ecology in a pelagic seabird, the masked booby. Oecologia 161, 637-649. ( 10.1007/s00442-009-1397-7) [DOI] [PubMed] [Google Scholar]
- 100.Jodice PG, Roby DD, Suryan RM, Irons DB, Turco KR, Brown ED, Thedinga JF, Visser GH. 2006. Increased energy expenditure by a seabird in response to higher food abundance. Mar. Ecol. Prog. Ser. 306, 283-293. ( 10.3354/meps306283) [DOI] [Google Scholar]
- 101.Bennison A, Giménez J, Quinn JL, Green JA, Jessopp M. 2022. A bioenergetics approach to understanding sex differences in the foraging behaviour of a sexually monomorphic species. Figshare. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Bennison A, Giménez J, Quinn JL, Green JA, Jessopp M. 2022. Data associated to: a bioenergetics approach to understanding sex differences in the foraging behaviour of a sexually monomorphic species. Dryad Digital Repository. ( 10.5061/dryad.zs7h44j88) [DOI] [PMC free article] [PubMed]
- Bennison A, Giménez J, Quinn JL, Green JA, Jessopp M. 2022. A bioenergetics approach to understanding sex differences in the foraging behaviour of a sexually monomorphic species. Figshare. [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
All data collected as part of this study are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.zs7h44j88 [51].
The data are provided in the electronic supplementary material [101].