Abstract
Reduced brain size, compared with wild individuals, is argued to be a key characteristic of domesticated mammal species, and often cited as a key component of a putative ‘domestication syndrome’. However, brain size comparisons are often based on old, inaccessible literature and in some cases drew comparisons between domestic animals and wild species that are no longer thought to represent the true progenitor species of the domestic species in question. Here we replicate studies on cranial volumes in domestic cats that were published in the 1960s and 1970s, comparing wildcats, domestic cats and their hybrids. Our data indicate that domestic cats indeed, have smaller cranial volumes (implying smaller brains) relative to both European wildcats (Felis silvestris) and the wild ancestors of domestic cats, the African wildcats (Felis lybica), verifying older results. We further found that hybrids of domestic cats and European wildcats have cranial volumes that cluster between those of the two parent species. Apart from replicating these studies, we also present new data on palate length in Felis cat skulls, showing that domestic cat palates are shorter than those of European wildcats but longer than those of African wildcats. Our data are relevant to current discussions of the causes and consequences of the ‘domestication syndrome’ in domesticated mammals.
Keywords: conservation, wildlife, hybridization, brain size, skull, cranial capacity
1. Background
Understanding differences in morphology in domestic species, their wild ancestors, closely related species and their hybrids are crucial on several fronts. First, in evaluating how domestic animals changed during the extended evolutionary process of domestication, and second, in supporting conservation efforts of wild species threatened by hybridization with domestic animals. Here we investigate the impact of domestication and hybridization on cat cranial (i.e. brain) volume by replicating results presented by Hemmer [1] and Schauenberg [2], and by discussing new results on palate length.
Over the years a vast amount of literature has accumulated, describing various characteristics of domestic mammals (e.g. curly tails, floppy ears, white patches, shorter muzzles; [3,4]). In particular, changes to cranial volume have been well documented across species, including sheep, rabbits, dogs and many more [3,5,6]. A new hypothesis may offer a unifying explanation for these typical traits of domestic mammals. The neural crest cell hypothesis describes how selection for tameness in the domestication of animals may have caused a downregulation in the migration and proliferation of neural crest cells, leading to decreased excitability and fear (tameness). However, this downregulation may also cause correlated changes to morphology, stress response and brain size [7,8]. Although this hypothesis has found a lot of research support, others are critical of it [9]. Lord and colleagues pointed out valid criticism regarding the long-term experiment to domesticate silver foxes, Vulpes vulpes, by Belyaev: first, the farmed foxes in Belyaev's domestication experiment were not truly wild but had been bred in captivity since the nineteenth century, and second, morphological comparisons are often problematic, if drawn between specific domestic animal breeds (in this case a variant of the widespread red fox) and/or wild species, which do not represent the true ancestor [10,11]. In the light of the continuing debate about the neural crest cell hypothesis, re-evaluation and replication of Hemmer's [1] and Schauenberg's [2] results are relevant.
Decreased brain size is a commonly described phenomenon in domestic mammals in comparison with their wild ancestors. In cats, a 25% reduction of cranial volume was reported between European wildcats, Felis silvestris, and domestic cats, F. catus [12,13]. However, work critical of this comparison pointed out that the European wildcat might not be the original ancestor of the domestic cat [1]. An earlier study by Klatt [14] compared cranial volumes of Felis maniculata (today Felis lybica) with those of house cats and discussed effects of feralization. More recent genetic evidence has confirmed that the African wildcat (F. lybica), more specifically the subspecies F. lybica lybica, is the ancestor to today's domestic cats [15,16].
Both Hemmer [1] and Schauenberg [2] recognized that the European wildcat was not the ancestral species to domestic cats. Schauenberg [2] formulated the cranial index, i.e. greatest length of skull divided by cranial volume, which clearly separated the skulls of European wildcats from those of domestic cats, whereby domestic cats have smaller brains (cranial index > 2.75) compared with those of European wildcats (cranial index < 2.75). Hemmer set out to compare the cranial volumes of domestic cats with those of European wildcats, various subspecies of the African wildcat and feral domestic cats. His data showed that European wildcats have the largest cranial volumes and domestic cats the smallest. Crucially, all subspecies of African wildcat (F. lybica) had cranial volumes smaller than European wildcats, yet bigger than domestic cats. Therefore, Hemmer inferred that domestication had an impact on cat cranial volume, but that effect was smaller than previously reported. To our knowledge no further work exploring this issue has been published, except Groves [17], who presented data on cranial indices of Sardinian wildcats (F. lybica) to demonstrate that they had larger cranial volumes than those of domestic cats and to support their recognition as F. lybica. It is important to note these differences in cranial volume between different domestic cat and wildcat taxa as part of the wider discussion as to whether domestic cats are truly domesticated (see Discussion).
In this study, we set out to replicate studies of cranial volume in wildcats and domestic cats as presented in Hemmer [1] and Schauenberg [2]. We hypothesize, in line with Hemmer's and Schauenberg's findings, that domestic cats have the smallest cranial volumes, European wildcats have the largest cranial volumes, African wildcats have intermediate cranial volumes and that hybrid cats (F. silvestris × F. catus) have cranial volumes that lie between those of the two parent species. We also present new data on palate length, relevant both to Hemmer's suggestion and to the neural crest cell hypothesis, whereby we hypothesize that domestication should lead to a reduction in snout dimensions, and thus palate length, in domestic cats [18].
2. Methods
2.1. Cat species classification
Cat species and subspecies names and classifications have changed over the years. We used the classification of Kitchener et al. [19] for the cat taxa used in this study from the genus Felis, including the European wildcat (F. silvestris), the African wildcat (F. lybica), the domestic cat (F. catus) and F. silvestris × F. catus hybrids from Scotland. Following Kitchener et al. [19], we view F. lybica lybica, F. lybica ornata and F. lybica cafra as subspecies of the African wildcat (F. lybica); the two subspecies of the European wildcat (F. silvestris) are F. silvestris silvestris and F. silvestris caucasica.
Our dataset comprised 19 F. lybica cats split into the following nominal subspecies (as given on museum labels): two were identified as F. l. lybica (as F. haussa), 12 as F. l. ornata, one as F. l. cafra and four as F. l. gordoni. Both F. haussa and F. l. gordoni are now included in F. l. lybica [19]. Our dataset also included 20 F. silvestris of the following nominal subspecies: 19 F. s. grampia and one F. s. silvestris, but the former is now included in the nominate subspecies. Finally, our data further included 28 domestic cats (F. catus) and 36 F. catus × F. silvestris hybrids from Scotland.
2.2. Schauenberg and Hemmer data and digitization
Schauenberg [2] measured cranial volume in two species: F. silvestris and F. catus; Hemmer [1] measured cranial volume in three species: F. silvestris, F. catus (feral and house cats) and F. lybica. It is unclear if the feral cats Hemmer presented in his study were indeed feral domestic cats or hybrids of European wildcats and domestic cats. The majority of Hemmer's feral domestic cat data has its origin in Klatt [14], who mentioned the possibility of these individuals being European wildcat/domestic hybrids. Therefore, we used skulls of known European wildcat × domestic cat hybrids (as determined by morphological and genetic criteria [20,21]) in our replication study instead of feral domesticated cat skulls.
Schauenberg's [2] cranial index is the ratio between greatest skull length and cranial volume, whereas Hemmer [1] used both basal skull length (prosthion to basion, see below) and greatest skull length. The results regarding the respective species comparisons showed identical patterns for greatest and basal skull length. Since the most complete comparison across species was presented over basal skull length by Hemmer, we decided to use basal skull measurements combined with cranial volume measurements for our own data collection in this replication study.
To allow for a direct comparison of our results with those published previously, we used the package digitize [22] to digitize the data visually presented in Schauenberg's [2] fig. 2 and Hemmer's [1] figs. 1 and 2. We updated the taxonomic classifications according to Kitchener et al. [19] as described above and provide an overview of the classification system in table 1.
Table 1.
Overview of the wildcat taxonomy updated from Hemmer [1] and Schauenberg [2], following Kitchener et al. [19].
|
Felis taxonomy | ||
|---|---|---|
| (sub)species names in Hemmer [1] and Schauenberg [2] | updated (sub)species classification [19] | common name |
| Felis silvestris silvestris | Felis silvestris | European wildcat |
| Felis silvestris f. catus | Felis catus | domestic cat |
| Felis silvestris lybica | Felis l. lybica | North African wildcat |
| Felis silvestris ornata | Felis l. ornata | Asian wildcat |
| Felis silvestris sspp. (referring to African wildcat subspecies) | Felis l. lybica/cafra | North and South African wildcats |
| Felis silvestris f. catus (feral) | Felis catus | feral domestic cat |
2.3. Original cat skull measurements
We used skulls of F. silvestris, F. lybica, F. catus and Felis catus × F. silvestris hybrids for our study, which allowed us to compare domestic cats with their known ancestor F. lybica, F. silvestris with which they are sympatric in Central and Northern Europe, as well as F. catus × F. silvestris hybrids. The hybrids (and European wildcats with unclear species identification) used in our study were classified where possible by genetic analysis in Senn et al. [21] and morphological analyses of Kitchener et al. [20]. In brief, genetic analysis involved a panel of 35 SNPs that had been developed to distinguish wildcats from domestic cats. Cats were identified as wildcat with a LBQ score of 0.75 [21]. Morphological analysis was based on seven key pelage characters with a minimum score of 19 out of 21 for wildcats [20]. In addition, five skull characters were scored [20]. Most of the hybrids originated from the hybrid swarm in Scotland and show varying levels of introgression between the parent species based on genetic and morphological data. Where there was a discrepancy between genetic and morphological evaluations, the default was to record these cats as hybrids. Where genetic data were not available, morphological characters from skins and skulls were used to classify wildcats, domestic cats and their hybrids.
We took three measurements on all cat skulls: Cranial volume, palate length and basal skull length. We measured the cranial volume by closing all openings of the skull except for the foramen magnum with clay and filled the cranium with 1 mm diameter glass beads to the edges of the foramen magnum. This methodology is well established in comparative research and has previously been used in cats [23,24]. We weighed the glass beads that filled the cranium on a balance (Mettler PM4600; Mettler PJ400). To convert the glass bead weight measurements into volumes, we established a conversion factor of 10 ml of glass beads weighing 15.37 g prior to measuring the cranial volumes. Subsequently, we were able to estimate cranial volumes from the weights of the beads.
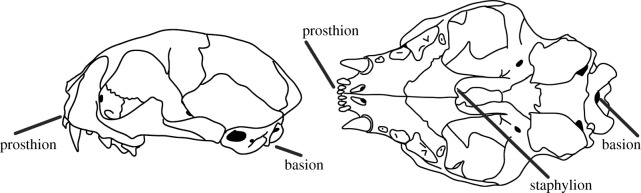
We measured palate length from the rostral-most premaxilla (prosthion) to the deepest indent of the palatine (staphylion) (figure 1), and basal length of the skull from the tip of the premaxilla to the basion (rostral-most medial indentation of the foramen magnum; figure 1). Both palate length and basal skull length were measured with digital callipers (accurate to 0.01 mm) and measurements were rounded to one decimal point prior to statistical analysis. We measured a total of 103 skulls in the collections of National Museums Scotland. We had to exclude one cat skull from our ‘domestic’ data group after data collection since its identification number came with conflicting information regarding species identification, leaving us with a total of 102 skulls (27 F. catus, 36 F. silvestris × F. catus hybrids, 19 F. lybica, 20 F. silvestris).
Figure 1.
Lateral and ventral views of a cat skull indicating the landmarks used for measurements of palate length and basal skull length. Basal skull length was measured from prosthion to basion, and palate length was measured from prosthion to staphylion.
2.4. Analysis
For our original data, we created linear models with the package lme4 for cranial volume and palate length [25]. All models included skull length in both the null and full models; species ID was added only to the full models. All full models were compared with their null models, checked for overdispersion, stability, collinearity and residual distribution. The model for palate length indicated an influential observation causing model instability. On closer inspection, this data point was revealed to be an error in recording basal skull length for this individual. Based on digital photos taken from the individual specimen, the value should probably have read 83.8 mm instead of the recorded 63.8 mm. Since equivalent accuracy in measurements from images in comparison with calliper measurements is not guaranteed, we re-ran statistics on both datasets including (see electronic supplementary material) and excluding this individual measurement. Removing the specimen with the measurement error resulted in both the palate length and cranial volume model to be stable and to fulfil all model assumptions.
3. Results
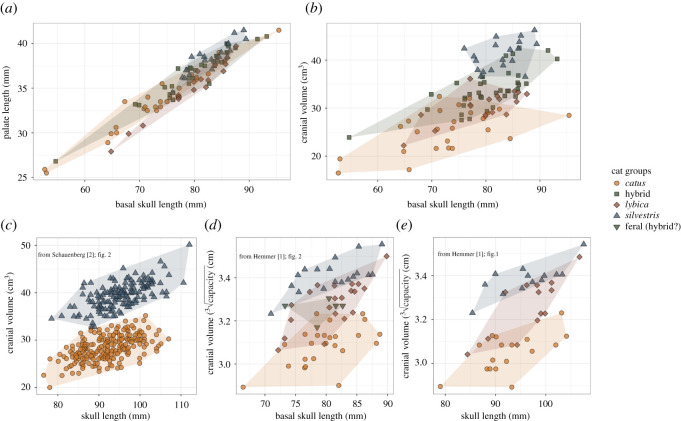
Our data support our hypothesis, as well as the results of Hemmer [1] and Schauenberg [2]. We found that domestic cats have the smallest cranial volume compared with F. silvestris, F. lybica and F. silvestris × F. catus hybrids (figure 2b). Felis silvestris cranial volumes were the largest (estimate ± standard error: 12 ± 1), and F. lybica cranial volumes were larger (estimate ± standard error: 2.5 ± 1) than those of domestic cats, but smaller than those of F. silvestris. Hybrid cats' (F. silvestris × F. catus) cranial volumes cluster between the two parent species (estimate ± standard error: 5.6 ± 0.9). While cranial volume was dependent on individuals' size, the relationship between the two is clearly species-specific (table 2).
Figure 2.
Palate length and cranial volume of Felis cat species. Domestic cats are represented by orange dots, F. catus × F. silvestris hybrids by olive squares, F. lybica by purple diamonds and F. silvestris by blue triangles. (a) Palate length of all four groups in mm over basal skull length in mm (our data). (b) Cranial volume in cm3 of all four groups over basal skull length in mm (our data). (c) Cranial volume data of F. catus and F. silvestris cats in cm3 over the total skull length from [2]. (d) Cube root of cranial volume data for all four groups in cm from [1]. (e) Cube root of cranial volume data for F. catus, F. lybica and F. silvestris cats in cm from [1].
Table 2.
Null/full model comparison and summary of the model for cranial volume. Domestic cats are included in the intercept. Residual standard error: 3.032 on 92 degrees of freedom. Multiple R-squared: 0.796, adjusted R-squared: 0.7871 F-statistic: 89.74 on 4 and 92 d.f., p-value: <2.2 × 10−16
| cranial volume | ||||
|---|---|---|---|---|
| Pr(>Chi) | ||||
| full/null comparison | <2.2 × 10−16 | |||
| estimate | s.e. | t value | Pr(>|t|) | |
|---|---|---|---|---|
| intercepta | 0.30317 | 3.28866 | 0.092 | 0.9268 |
| basal skull length | 0.3464 | 0.04445 | 7.792 | 9.71 × 10−12 |
| F. catus × F. silvestris hybrid | 5.6224 | 0.86044 | 6.534 | 3.49 × 10−9 |
| F. lybica | 2.50871 | 0.9981 | 2.513 | 0.0137 |
| F. silvestris | 12.00224 | 1.02669 | 11.69 | <2 × 10−16 |
aIntercept includes domestic cats.
Our data are less clear with regard to the hypothesis of a reduction in snout length in domestic cats. Contrary to our hypothesis the results indicate that domestic cats have longer palates compared with their ancestral species F. lybica (estimate ± standard error: −0.8 ± 0.3). Felis silvestris (estimate ± standard error: 1 ± 0.3) has longer palates than those of domestic cats, which have similar palate lengths to those of hybrid cats. While we did find statistically significant differences between species, individual body size appears to be the strongest correlate of palate length (figure 2a and table 3). Our full/null model comparison indicated the full model (including the species groups) to be significantly better than the null model, but the adjusted-R-squared value only improved marginally from the null to the full model (from 0.91 to 0.93); however, in our cranial volume models this value improved from 0.45 to 0.78 (tables 1 and 2).
Table 3.
Null/full model comparison and summary of the model for palate length. Domestic cats are included in the intercept. Residual standard error: 0.844 on 95 degrees of freedom. Multiple R-squared: 0.9401, adjusted R-squared: 0.9376. F-statistic: 373 on 4 and 95 d.f., p-value: <2.2 × 10−16
| palate length | ||||
|---|---|---|---|---|
| Pr(>Chi) | ||||
| full/null comparison | 3.44 × 10−9 | |||
| estimate | s.e. | t-value | Pr(>|t|) | |
|---|---|---|---|---|
| intercepta | 4.26124 | 0.90774 | 4.694 | 8.99 × 10−6 |
| basal skull length | 0.3994 | 0.01224 | 32.626 | <2 × 10−16 |
| F. catus × F. silvestris hybrid | 0.36457 | 0.23586 | 1.546 | 0.125506 |
| F. lybica | −0.77552 | 0.26937 | −2.879 | 0.004928 |
| F. silvestris | 0.96403 | 0.28224 | 3.416 | 0.000938 |
aIntercept includes domestic cats.
4. Discussion
In this paper, we replicated results regarding cranial volumes in domestic cats and wildcats first presented by Schauenberg [2] and Hemmer [1]. Our data support their findings that domestic cats have significantly smaller cranial volumes than those of both European wildcats (F. silvestris) and African wildcats (F. lybica). We further found that hybrids of domestic cats and European wildcats have cranial volumes that cluster between those of the two parent species. We also presented new results on palate length, a proxy for snout length, among domestic cats, European wildcats, African wildcats and hybrids (F. silvestris × F. catus). Although we found statistically significant differences among species, individual body size seems to be the main factor driving palate length.
Two characteristics often used to describe changes to domestic animals are a reduction in brain size and snout length. Wilkins et al. [7,26] suggested that a neural crest cell deficiency, caused by selection for tameness, is the underlying factor responsible for these characteristics. While a growing body of research (e.g. red junglefowl Gallus gallus [27], village dogs Canis familiaris [28] and foxes Vulpes vulpes [29]) is in line with the neural crest cell hypothesis, the hypothesis has been challenged by others [10,11,30]. Our results on cranial volume reduction in domestic cats are in line with the neural crest cell hypothesis and previous reports [1,31], but we did not find a reduction in snout length, which fails to uphold the prediction of this hypothesis. Dog data presented by Morey [32,33] also show no clear snout length reduction, but rather a relative increase in palate width. There may be several reasons for this lack of snout shortening: domestication might not have affected snout lengths after all, palate length may not be an appropriate proxy for snout length, or the neural crest cell hypothesis may be incorrect in its proposed effect on snout length during domestication.
Lord et al. [10,11] outlined two criticisms of the neural crest cell hypothesis that are potentially relevant in this context. First, they pointed out that Dmitri Belyaev's domesticated fox experiment in Siberia began not with ‘wild’ foxes, but with descendants of foxes bred in captivity in Canada for their fur since the nineteenth century and, therefore, (actively or passively) pre-selected for certain traits, such as tameness. Given the partial reliance of the neural crest cell hypothesis on the Belyaev data, Lord and colleagues argued that it is impossible to distinguish between these changes having emerged due to selection or genetic drift [10]. This potential criticism has been questioned by Zeder [34] and Trut et al. [35], who note that Belyaev and his colleagues were well aware of this fact and that all comparisons, both behavioural and morphological, were done between unselected control foxes (from this previously farmed background) and experimentally tamed foxes.
In addition to potential neural crest reductions, other factors may influence morphological changes in domestic animals. An independent factor potentially explaining reductions in brain size in domestic animals is provided by the expensive-tissue hypothesis, which explores evolutionary trade-offs between brain size and other energetically costly tissues [36]. The expensive-tissue hypothesis was originally introduced to explain brain size variability in primates with respect to a trade-off between brain volume and gut size [36]. Since then research on guppies (Poecilia reticulata) further supported the idea that brain size is negatively correlated with the size of the gut [37,38]. Further comparative experiments with domestic chicken Gallus domesticus and wild junglefowl also support the idea that a trade-off between brain size and other costly systems (e.g. reproduction) is relevant during the domestication process [39]. Therefore, brain size in domestic species might not (only) be affected by e.g. neural crest cell reduction but also by a trade-off between the relative importance of the energetic needs of the brain and other organ systems, such as the gut and/or reproductive system.
Another hypothesis with potential relevance to morphological changes in domestication research is the thyroid hormone hypothesis; this hypothesis was proposed by Crockford [40] and was named and further discussed by Wilkins [8]. This hypothesis states that domestication might have caused timing shifts in development, which potentially affect the concentration of the thyroid hormones during development. Aside from this possible connection between domestication and thyroid hormone concentration, thyroid hormones are essential in the development of craniofacial structures, e.g. deficiencies cause delayed ossification [41]. This potential mechanistic connection between thyroid hormones and craniofacial structures makes this hypothesis an additional candidate for understanding changes to cranial volume and palate length during domestication.
A potential criticism of the use of cat data in domestication research is the oft-mentioned claim that cats are not truly domesticated animals or are only ‘semi-domesticated’ (cf. [42]). We do not think that this claim is accurate, despite the fact that the cat's path to domestication is often colloquially viewed and portrayed as only beneficial to cats and not humans. Cats might not have been as ‘useful’ to humans as dogs or horses have been, but their usefulness in keeping grain harvests safe from rodents is commonly cited as a major driver in their domestication [43], and scavenging opportunities at middens may have been as important in bringing wildcats close to humans [44]. Even if wildcats were attracted to human environments because of the ready availability of food, according to Zeder [45] this conforms to the ‘commensal pathway’ to domestication, probably also relevant in early dog domestication. Furthermore, there can be little doubt that humans have selected for docility in cats, and the fact that (until recently) there has been little further selection for cooperation with humans, as is the case for dogs, or for meat or milk production, as in ungulates, in fact makes cats well-suited to this topic of research [42].
When discussing morphological data in the field of domestication research, it is also highly relevant to integrate the aspect of feralization, which is often viewed as the counter process to domestication [46]. Hemmer [1] provided cranial volumes from domestic pet cats, feral cats (both F. catus), F. silvestris and F. lybica. Hemmer's data for feral cats originated from Klatt [14], who mentioned the possibility of these individuals being hybrid offspring of F. silvestris and F. catus. Regarding brain size and feralization, research on dingoes, which are domestic dogs in Australia that became feral thousands of years ago [47], shows that brain volume did not increase again during the process of feralization [48]. Similar results have been reported for feral cats, goats, mink and pigs [49]. Thus, brain volume reduction due to domestication seems to be a permanent change that is not reversed by feralization, even after many generations [46,49–51]. This permanent change also suggests that reduced brain volume may represent an energy-budget optimization as discussed above. In light of these previous studies, our new data regarding cranial volumes of hybrids suggest that if cats do not regain their ancestral cranial volume in the course of feralization, the putatively ‘feral' cats measured and presented by Hemmer/Klatt were actually hybrid cats.
Lord and colleagues note that reports of brain size reduction, considered to be a relatively ubiquitous trait in domestic mammals, vary greatly depending on the data sources, and are sometimes based on comparison with ‘wild’ animals not representative of the true ancestral species [11]. As the current study shows, the selection of the appropriate ‘progenitor’ group for comparison with domestic variants is often highly challenging and can have crucial effects on the results.
This leads us to another key issue in comparing wild species and domestic animals: we must always acknowledge that we are comparing a now (or recently) living population of wild animals to the domestic form, and not the true ancestral population. This will always be a confounding factor since we rarely have access to the ancient population that produced our domestic animals (although ancient DNA can partially ameliorate this issue for genetic comparisons). Similarly, we should be cautious in including recently developed breeds in such comparisons. The initial process of domestication is an adaption to a new environment and, therefore, heavily influenced by both natural selection and unconscious selection by humans [26], whereas recent domestic animal breeds have mainly been subjected to conscious artificial selection pressures (e.g. consider farm cats versus Persian cats, or village dogs versus chihuahuas). Comparisons between specific domestic animal breeds and wild populations are interesting, but studies of breeds after strong artificial selection pressures may potentially warp our perception regarding the magnitude and/or type of morphological change present in early domestication [28].
An interesting recent example of the effect of specific breeds is presented by Balcarcel et al. [52,53] regarding cattle. The authors compare an impressive dataset of various cattle (Bos taurus) breeds with the ancestral aurochs (Bos primigenius), finding that all domestic cattle have smaller brains compared with their ancestors. However, among domestic cattle breeds, there are differences in brain size depending on breed temperament; bullfighting breeds have larger brain sizes, possibly related to their decreased tameness towards humans. While this is an extremely interesting finding, these differences in brain size and skull morphology among cattle breeds may also be a side effect of artificial selection pressures during breed creation and trade-offs between brain and other tissues (e.g. for meat or milk production, see above) rather than resulting specifically from the domestication process, or breed-specific selection.
To summarize, our results provide confirmation of the previously reported reduction of cranial volume in domestic cats compared with their wild ancestors. We further present new data regarding palate length, showing a lack of snout length reduction in domestic cats. Many studies documenting morphological changes foundational to domestication research are more than 40–50 years old, and in need of replication and further study in accordance with current scientific knowledge and standards (see overview across species by [52,53]). The current study is a step in this direction and helps to solidify the database for an increased understanding of domestication and its effects on morphology.
Supplementary Material
Acknowledgements
We thank Zena Timmons for her support during data collection. We thank the Negaunee Foundation for its generous support of a curatorial preparator, who prepared many of the skulls used in this study. Many of the specimens in this study were collected by Scottish Wildcat Action, which was supported by the National Lottery Heritage Fund. This article received results-blind in-principle acceptance (IPA) at Royal Society Open Science. Following IPA, the accepted Stage 1 version of the manuscript, not including results and discussion, was preregistered on the OSF (https://osf.io/hdsfq). This preregistration was performed after data analysis. All data and supplementary materials are uploaded to Dryad (https://doi.org/10.5061/dryad.zgmsbccc5).
Data accessibility
All data, code and electronic supplementary materials are publicly available on Dryad: https://doi.org/10.5061/dryad.zgmsbccc5. [54]
Authors' contributions
R.L.: conceptualization, data curation, formal analysis, investigation, methodology, project administration, software, visualization, writing—original draft, Writing—review and editing; A.C.K.: conceptualization, data curation, resources, supervision, writing—review and editing; G.H.: data curation, investigation, resources; K.K.: conceptualization, supervision, writing—review and editing; W.T.F.: conceptualization, funding acquisition, methodology, resources, supervision, writing—review and editing. All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Competing interests
We declare we have no competing interests.
Funding
Austrian Science Fund (FWF) Grant ‘Cognition & Communication 2' (#W1262-B29) to W.T.F.
References
- 1.Hemmer H. 1972. Hirngrössenvariation im Felis silvestris-Kreis. Experientia 28, 271-272. ( 10.1007/BF01928682) [DOI] [PubMed] [Google Scholar]
- 2.Schauenberg P. 1969. L'identification du chat forestier d'Europe Felis s. silvestris Schreber 1777 par une méthode ostéométrique. Rev. Suisse Zool. 76, 433-441. [PubMed] [Google Scholar]
- 3.Darwin C. 1868. The variation of animals and plants under domestication, vol. 1. London, UK: John Murray. [Google Scholar]
- 4.Kohane MJ, Parsons PA. 1988. Domestication: evolutionary change under stress. Evol. Biol. 23, 31-48. ( 10.1007/978-1-4613-1043-3_2) [DOI] [Google Scholar]
- 5.Kruska D. 1988. Mammalian domestication and its effect on brain structure and behavior. In Intelligence and evolutionary biology (eds Jerison HJ, Jerison I), pp. 211-250. Berlin, Germany: Springer. [Google Scholar]
- 6.Wright D. 2015. The genetic architecture of domestication in animals. Bioinf. Biol. Insights 9, 11-20. ( 10.4137/BBI.S28902) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilkins AS, Wrangham RW, Fitch WT. 2014. The ‘domestication syndrome’ in mammals: a unified explanation based on neural crest cell behavior and genetics. Genetics 197, 795-808. ( 10.1534/genetics.114.165423) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilkins AS. 2017. Revisiting two hypotheses on the ‘domestication syndrome’ in light of genomic data. Vavilov J. Genet. Breed. 21, 435-442. ( 10.18699/VJ17.262) [DOI] [Google Scholar]
- 9.Johnsson M, Henriksen R, Wright D. 2021. The neural crest cell hypothesis: no unified explanation for domestication. Genetics 219, 1. 10.1093/GENETICS/IYAB097) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lord KA, Larson G, Coppinger RP, Karlsson EK. et al. 2020. The history of farm foxes undermines the Animal domestication syndrome. Trends Ecol. Evol. 35, 125-136. ( 10.1016/j.tree.2019.10.011) [DOI] [PubMed] [Google Scholar]
- 11.Lord KA, Larson G, Karlsson EK. 2020. Brain size does not rescue domestication syndrome. Trends Ecol. Evol. 35, 1061-1062. ( 10.1016/j.tree.2020.10.004) [DOI] [PubMed] [Google Scholar]
- 12.Röhrs M. 1955. Vergleichende Untersuchungen an Wild- und Hauskatzen. Zool. Anz. 155, 53-69. [Google Scholar]
- 13.Herre W, Röhrs M. 1971. Die Evolution der Organismen. In Die evolution der organismen (ed. Heberer G), 3rd edn. Stuttgart, Germany: Fischer. [Google Scholar]
- 14.Klatt B. 1912. Über die Veränderung der Schädelkapazität in der Domestikation. Sitzungsberichte Ges. naturforschender Freunde 3, 153-179. [Google Scholar]
- 15.Driscoll CA, et al. 2007. The near eastern origin of cat domestication. Science 317, 519-523. ( 10.1126/science.1139518) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ottoni C, et al. 2017. The palaeogenetics of cat dispersal in the ancient world. Nat. Ecol. Evol. 1, 0139. ( 10.1038/s41559-017-0139) [DOI] [Google Scholar]
- 17.Groves CP. 1989. Feral mammals of the Mediterranean islands: documents of early domestication. In The walking larder: patterns of domestication, pastoralism and predation (ed. Juliet Clutton-Brock), pp. 46-57. London, UK: Unwin Hyman Ltd. [Google Scholar]
- 18.Herre W, Röhrs M. 1990. Anatomische Veränderungen von Einzelmerkmalen und Gefügesystemen im Hausstand. In Haustiere – zoologisch gesehen, 2, pp. 227-237. Stuttgart, Germany: Gustav Fischer Verlag. [Google Scholar]
- 19.Kitchener AC, et al. 2017. A revised taxonomy of the Felidae: the final report of the Cat Classification Task Force of the IUCN/ SSC Cat Specialist Group. CATnews. Spec. Issue 11, 80. [Google Scholar]
- 20.Kitchener AC, Yamaguchi N, Ward JM, Macdonald DW. 2005. A diagnosis for the Scottish wildcat (Felis silvestris): a tool for conservation action for a critically-endangered felid. Anim. Conserv. 8, 223-237. ( 10.1017/S1367943005002301) [DOI] [Google Scholar]
- 21.Senn HV, Ghazali M, Kaden J, Barclay D, Harrower B, Campbell RD, Macdonald DW, Kitchener AC. 2019. Distinguishing the victim from the threat: SNP-based methods reveal the extent of introgressive hybridization between wildcats and domestic cats in Scotland and inform future in situ and ex situ management options for species restoration. Evol. Appl. 12, 399-414. ( 10.1111/eva.12720) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Poisot T. 2011. The digitize package: extracting numerical data from scatterplots. R J. 3, 25-26. ( 10.32614/RJ-2011-004) [DOI] [Google Scholar]
- 23.Yamaguchi N, Driscoll CA, Kitchener A, Ward J, Macdonald D. 2004. Craniological differentiation between European wildcats (Felis silvestris silvestris), African wildcats (F. s. lybica) and Asian wildcats (F. s. ornata): implications for their evolution and conservation. Biol. J. Linnean Soc. 83, 47-63. ( 10.1111/j.1095-8312.2004.00372.x) [DOI] [Google Scholar]
- 24.Yamaguchi N, Kitchener AC, Driscoll CA, Ward JM, Macdonald DW. 2004. Craniological differentiation amongst wild-living cats in Britain and southern Africa: natural variation or the effects of hybridisation? Anim. Conser. 7, 339-351. ( 10.1017/S1367943004001520) [DOI] [Google Scholar]
- 25.Bates D, Mächler M, Bolker B, Walker S. 2014. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 48. ( 10.18637/jss.v067.i01) [DOI] [Google Scholar]
- 26.Wilkins AS, Wrangham R, Fitch WT. 2021. The neural crest/domestication syndrome hypothesis, explained: reply to Johnsson, Henriksen, and Wright. Genetics 219, iyab098. ( 10.1093/genetics/iyab098) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Agnvall B, Bélteky J, Katajamaa R, Jensen P. 2018. Is evolution of domestication driven by tameness? A selective review with focus on chickens. Appl. Anim. Behav. Sci. 205, 227-233. ( 10.1016/j.applanim.2017.09.006) [DOI] [Google Scholar]
- 28.Pendleton AL, Shen F, Taravella AM, Emery S, Veeramah KR, Boyko AR, Kidd JM. et al. 2018. Comparison of village dog and wolf genomes highlights the role of the neural crest in dog domestication. BMC Biol. 16, 1-21. ( 10.1186/s12915-018-0535-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Trut L, Oskina I, Kharlamova A. 2009. Animal evolution during domestication: the domesticated fox as a model. Bioessays 31, 349-360. ( 10.1002/bies.200800070) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hansen Wheat C, der Bijl W, Wheat CW. 2020. Morphology does not covary with predicted behavioral correlations of the domestication syndrome in dogs. Evol. Lett. 4, 189-199. ( 10.1002/evl3.168) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kruska D. 1988. Mammalian domestication and its effects on brain structure and behavior. Hum. Evol. 3, 473-485. ( 10.1007/BF02436333) [DOI] [Google Scholar]
- 32.Morey D. 1992. Size, shape, and development in the evolution of the domestic dog size, shape and development in the evolution of the domestic dog. J. Archaeol. Sci. 19, 181-204. ( 10.1016/0305-4403(92)90049-9) [DOI] [Google Scholar]
- 33.Morey DF. 1994. The early evolution of the domestic dog. Am. Sci. 82, 336-347. [Google Scholar]
- 34.Zeder MA. 2020. Straw foxes: domestication syndrome evaluation comes up short. Trends Ecol. Evol. 35, 647-649. ( 10.1016/j.tree.2020.03.001) [DOI] [PubMed] [Google Scholar]
- 35.Trut LN, Kharlamova AV, Herbeck YE. 2020. Belyaev's and PEI's foxes: a far cry. Trends Ecol. Evol. 35, 649-651. ( 10.1016/j.tree.2020.03.010) [DOI] [PubMed] [Google Scholar]
- 36.Aiello LC, Wheeler P. 1995. The expensive-tissue hypothesis. Curr. Anthropol. 36, 199-221. ( 10.1086/204350) [DOI] [Google Scholar]
- 37.Kotrschal A, Rogell BA, Bundsen A, Svensson B, Zajitschek S, Brännström I, Immler S, Maklakov AA, Kolm N. 2013. Artificial selection on relative brain size in the guppy reveals costs and benefits of evolving a larger brain. Curr. Biol. 23, 168-171. ( 10.1016/j.cub.2012.11.058) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kotrschal A, Rogell BA, Bundsen A, Svensson B, Zajitschek S, Brännström I, Immler S, Maklakov AA, Kolm N. 2013. The benefit of evolving a larger brain: big-brained guppies perform better in a cognitive task. Anim. Behav. 86, e4. ( 10.1016/j.anbehav.2013.07.011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Agnvall B, Bélteky J, Jensen P. 2017. Brain size is reduced by selection for tameness in red junglefowl-correlated effects in vital organs. Sci. Rep. 7, 1-7. ( 10.1038/s41598-017-03236-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Crockford SJ. 2004. Animal domestication and vertebrate speciation: a paradigm for the origin of species. PhD thesis, University of Victoria, Victoria, Canada.
- 41.Leitch VD, Bassett JHD, Williams GR. 2020. Role of thyroid hormones in craniofacial development. Nat. Rev. Endocrinol. 16, 147-164. ( 10.1038/s41574-019-0304-5) [DOI] [PubMed] [Google Scholar]
- 42.Montague MJ, et al. 2014. Comparative analysis of the domestic cat genome reveals genetic signatures underlying feline biology and domestication. Proc. Natl Acad. Sci. USA 111, 17 230-17 235. ( 10.1073/pnas.1410083111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Driscoll CA, Clutton-Brock J, Kitchener AC, O'Brien SJ. 2009. The taming of the cat: Genetic and archaeological findings hint that wildcats became house cats earlier—and in a different place—than previously thought. Sci. Am. 300, 68-75. ( 10.1038/scientificamerican0609-68) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Faure E, Kitchener AC. 2009. An archaeological and historical review of the relationships between felids and people. Anthrozoos 22, 221-238. ( 10.2752/175303709X457577) [DOI] [Google Scholar]
- 45.Zeder MA. 2015. Core questions in domestication research. Proc. Natl Acad. Sci. USA 112, 3191-3198. ( 10.1073/pnas.1501711112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Herre W, Röhrs M. 1990. Verwilderung - ein Gegenexperiment. In Haustiere - zoologisch gesehen, pp. 319-327. [Google Scholar]
- 47.Balme J, O'Connor S, Fallon S. 2018. New dates on dingo bones from Madura Cave provide oldest firm evidence for arrival of the species in Australia. Sci. Rep. 8, 4-9. ( 10.1038/s41598-018-28324-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schultz W. 1969. Zur Kenntnis des Hallstromhundes (Canis hallstromi, Troughton 1957). Zool. Anz. 183, 42-72. [Google Scholar]
- 49.Kruska DCT. 2005. On the evolutionary significance of encephalization in some eutherian mammals: effects of adaptive radiation, domestication, and feralization. Brain Behav. Evol. 65, 73-108. ( 10.1159/000082979) [DOI] [PubMed] [Google Scholar]
- 50.Birks J, Kitchener AC. 1999. The distribution and status of the polecat Mustela putorius in Britain in the 1990s. London, UK: The Vincent Wildlife Trust. [Google Scholar]
- 51.Price E. 1999. Behavioral development in animals undergoing domestication. Appl. Anim. Behav. Sci. 65, 245-271. ( 10.1016/S0168-1591(99)00087-8) [DOI] [Google Scholar]
- 52.Balcarcel AM, Veitschegger K, Clauss M, Sánchez-Villagra MR. 2021. Intensive human contact correlates with smaller brains: differential brain size reduction in cattle types. Proc. R. Soc. B 288, 20210813. ( 10.1098/rspb.2021.0813) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Balcarcel AM, Veitschegger K, Clauss M, Sánchez-Villagra MR. In press. The mammalian brain under domestication: discovering patterns after a century of old and new analyses. J. Exp. Zool. B: Mol. Dev. Evol. 1-24. ( 10.1002/jez.b.23105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lesch R, Kitchener AC, Hantke G, Kotrschal K, Fitch WT. 2022. Data from: Cranial volume and palate length of cats, Felis spp., under domestication, hybridization and in wild populations. Dryad Digital Repository. ( 10.5061/dryad.zgmsbccc5) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Lesch R, Kitchener AC, Hantke G, Kotrschal K, Fitch WT. 2022. Data from: Cranial volume and palate length of cats, Felis spp., under domestication, hybridization and in wild populations. Dryad Digital Repository. ( 10.5061/dryad.zgmsbccc5) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
All data, code and electronic supplementary materials are publicly available on Dryad: https://doi.org/10.5061/dryad.zgmsbccc5. [54]