Abstract
Hepatocellular carcinoma, the most common primary liver cancer, in an immunogenic tumor with a poor prognosis because these tumors are diagnosed at late stages. Although, surgical resection, ablation, liver transplant, and locoregional therapies are available for early stages; however, there are yet no effective treatment for advanced and recurrent tumors. Immune checkpoint inhibitor therapy and adoptive cell transfer therapy has gained the popularity with some positive results because these therapies overcome anergy and systemic immune suppression. However, still there is a lack of an effective treatment and thus there is an unmet need of a novel treatment. At present, the focus of the research is on oncolytic viral therapy and combination therapy where therapies including radiotherapy, immune checkpoint therapy, adoptive cell transfer therapy, and vaccines are combined to get an additive or synergistic effect enhancing the immune response of the liver with a cytotoxic effect on tumor cells. This review discusses the recent key development, the basis of drug resistance, immune evasion, immune tolerance, the available therapies based on stage of the tumor, and the ongoing clinical trials on immune checkpoint inhibitor therapy, adoptive cell transfer therapy, oncolytic viral vaccine therapy, and combination therapy.
Keywords: Hepatocellular carcinoma, Immunotherapy, Immune checkpoint inhibitors, Adoptive cell therapy, Oncolytic vaccines, Combination therapy
Core Tip: A significant proportion of patients with hepatocellular carcinoma (HCC) present with advanced disease and therapeutic strategies for such patients are limited. The tumor microenvironment mediating immune response suppression, immune tolerance, and evasion further complicate the treatment in advanced HCC. The involvement of immune response in the pathogenesis of HCC makes immunotherapy an attractive approach for the treatment of advanced HCC. Further, the recent research with beneficial results with immune checkpoint inhibition, adoptive cell transfer therapy, tumor vaccines, and combinational therapies to boost the immune response of the tumor are in development and have been discussed here.
INTRODUCTION
Hepatocellular carcinoma (HCC), an inflammation-driven cancer, is an immunogenic tumor arising from chronically inflamed liver (liver cirrhosis) caused by risk factors including alcoholic fatty liver disease, non-alcoholic fatty liver disease, and viral and non-viral pathogenesis[1]. Liver cancer is the third most common cause of cancer deaths and sixth most common cancer diagnosis worldwide. HCC has an incidence of 9.3 cases per 100000 person-years and 8.5 deaths per 100000 person-years worldwide and incidence of 9.5 per 100000 person-years in the United States[2,3]. HCC incidence has a regional variability because of relative prevalence of key risk factors. HCC has grim prognosis and increasing incidence and with a similar existing trend, HCC has a projected rate of an increase approximately 2.8% per year through 2030 particularly in countries with a high socio-demographic index[3-5]. Current therapy for HCC based on the stage of HCC comprises of surgical, locoregional, and systemic therapies. Surgical resection or liver transplantation, depending on liver function, the presence of portal hypertension, and tumor burden, is standard of therapy with a 5-year survival rate in 70% of treated patient for early stage; radiofrequency, thermal and non-thermal ablation, and trans-arterial chemoembolization (TACE) with 3-5-year survival rates in patients with nonresectable tumor and not fit for liver transplantation; and systemic therapy with tyrosine-kinase inhibitors sorafenib and regorafenib and inhibitor of vascular endothelial growth factor (VEGF) receptors lenvatinib with a very limited survival benefit due to chemoresistance and toxicities[3,6-11]. Sorafenib, targeting VEGF is standard first line systemic therapy approved for advanced HCC patients; lenvatinib is alternative first line therapy; and regorafenib, and cabozantinib are second line systemic therapy[3,5]. The lower survival rate in unresectable HCC is due to resistance to systemic treatment modalities and chemotherapy. The recent studies suggest immunotherapy as a promising modality for the treatment of advanced HCC because immunotherapy could elicit nontoxic, systemic, long-lived anti-tumor activity. Additionally, a correlation between immune response and HCC and the paucity of available therapeutic strategies supports the notion to investigate immunotherapeutic targets and designing of better therapeutics for HCC. In this review, we have discussed the basis of resistance to therapy and various modalities for the treatment of advanced cancer along with the recent updates including ongoing clinical trials.
TUMOR MICROENVIRONMENT, IMMUNOSUPPRESSION, AND IMMUNE EVASION IN HCC
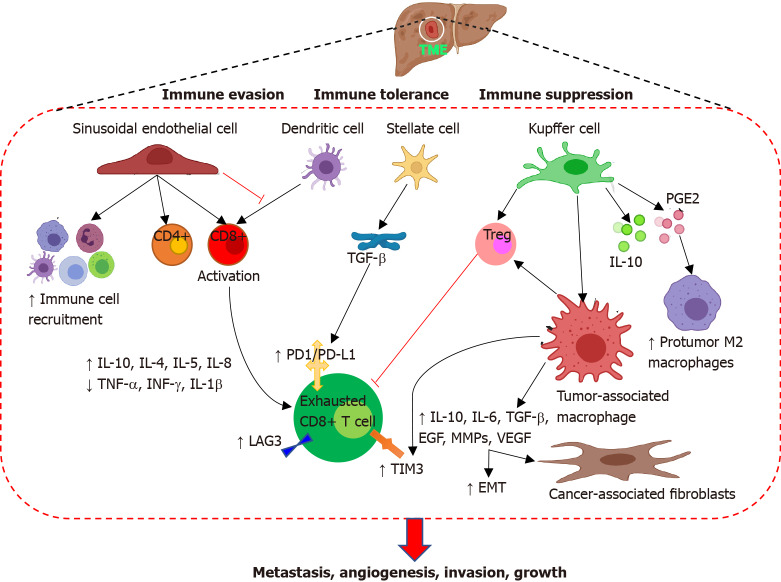
The intrinsic immune response of the liver mediated by liver sinusoidal endothelial cells (LSECs), liver resident macrophages or Kupffer cells (KCs), hepatic stellate cells (HSCs), and hepatic dendritic cells (HDCs) plays a central role in host defense, functional heterogeneity of the liver, and in the maintenance of self-tolerance (Figure 1). Natural immune response mediated by these cells to protect liver parenchyma is generated by the exposure to antigens and makes liver an immune suppressive microenvironment. LSECs, the specialized endothelial cells, are the most effective scavenger cells which also act as antigen-presenting cells (APCs) while regulating the immune response. LSECs regulate immune cell recruitment via specific integrins (αLβ2, α4β1, α4β7)[1,3,5,12,13]. LSECs prevent immune responses against gut bacterial antigens by inhibiting CD4+ and CD8+ T lymphocytes and reduce the ability of dendritic cells (DCs) to activate T cells. KCs, the non-migratory liver resident macrophages residing in the lumen of liver sinusoids, promote immunological tolerance by increasing the secretion of interleukin (IL)-10 and prostaglandins, removing the gut bacteria, attenuation of CD4+ T lymphocytes, and proliferation of inhibitory CD4+ regulatory T cells (Tregs). The roles of KCs in mediating immune response and immune tolerance, recruiting Tregs and neutrophils, stimulating T cell response to infection, and recruitment and activation of natural killer (NK) cells have been discussed in detail[5]. HSCs, the specialized fibroblasts, are present in the space of Dissé between the parenchymal cells and play an immune sentinel role. HSCs secrete transforming growth factor (TGF)-β which is an immunosuppressive cytokine involved in inflammation, liver regeneration, and liver fibrosis (Figure 1). HDCs being the poor stimulator of effector CD4+ T cells contribute to the tolerogenic microenvironment of the liver. The immune suppressive microenvironment of the liver with downregulation of immune response genes is more evident during the development and progression of HCC and results in a lower tumor immunity during advanced disease. The presence of numerous non-redundant mechanisms of immune-suppression in HCC-tumor microenvironment (TME) synergize with immunotherapy[1,3,12,13]. In addition to immune effector cells LSECs, KCs, HSCs, and HDCs; resident liver lymphocytes including NK cells and innate T-cells play a crucial role in innate immune response against intracellular bacteria, viruses, and parasites. However, a dysfunctional immune response due to higher proportion of CD4+ to CD8+ cells promote immune tolerance and a poor prognosis. A decreased T-cell activation and tumor infiltration due to lower expression of tumor antigens on liver cancer cells results in a less efficient control of tumor growth and worse clinical outcome. A hypofunctional NK cells and insufficiency of tumor-infiltrating lymphocytes (TILs) in controlling tumor growth adds to HCC progression[1,3,5].
Figure 1.
Tumor microenvironment in hepatocellular carcinoma. Tumor microenvironment comprising of liver sinusoidal endothelial cells, liver resident macrophages or Kupffer cells, hepatic stellate cells, hepatic dendritic cells, tumor-associated macrophages, cytokines, fibroblasts, infiltrating immune cells, pro-tumor M2 macrophages, and growth factors mediate immune suppression, immune tolerance, and immune evasion causing increased tumorigenicity with enhanced evasion, angiogenesis, metastasis, tumor growth. IL: Interleukin; TNF-α: Tumor necrosis factor-alpha; TGF-β: Transforming growth factor-beta; IFN-γ: Interferon-gamma; MMPs: Matrix metalloproteinases; PD-1: Programmed cell death protein 1; PD-L1: Programmed death-ligand 1; PGE2: Prostaglandin E2; EGF: Epidermal growth factor; VEGF: Vascular endothelial growth factor; TIM3: T-cell immunoglobulin and mucin-domain-containing molecule-3; LAG3: Lymphocyte-activation gene 3; EMT: Epithelial mesenchymal transition.
Although the immune suppressive microenvironment is important for the self-tolerance in the normal liver, this characteristic of the liver is a major impediment in developing an effective antitumor immunotherapy. TME helps in escaping the immunological surveillance, growth, and progression of the tumor. The decreased efficacy of antitumor treatment is also mediated by tumor evasion. The presence of cells with immune suppressive functions and higher expression of immune checkpoint molecules characterizing the TME leads to reduced activity of effector antitumor immune response and tumor immune evasion. HCC cancer cells and KCs have a higher expression of programmed death-ligand 1 (PD-L1) and an interaction between programmed cell death protein 1 (PD-1) and PD-L1 on tumor infiltrating lymphocytes and tumor cells mediate T cell exhaustion (Figure 1), tumor-specific T-cell dysfunction and immune evasion[5,14]. Immune evasion and poor prognosis in HCC are also mediated by higher expression of T-cell immunoglobulin and mucin-domain-containing molecule-3 (Tim-3) and lymphocyte-activation gene 3 (LAG-3) on CD8+ T lymphocytes from tumor (Figure 1) and not on T cells from normal liver tissue. TIM3 is also expressed on tumor-associated macrophages (TAM) which are associated with growth, angiogenesis, invasion, and metastasis of HCC[15-20]. Further, an increased expression of immunosuppressive cytokines including IL-4, IL-5, IL-8, and IL-10 and decreased levels of pro-inflammatory cytokines including tumor necrosis factor-α, interferon (IFN)-γ, and IL-1β in TME (Figure 1) contribute to immune dysfunction, immune evasion, an aggressive tumor phenotype, and poor prognosis in HCC patients[3,21,22]. The balance of effectors and immunosuppressive cells in TME as well as the ability of malignant cells to present tumor antigens to APCs are the requisite for an effective antitumor immune response by promoting the cytotoxic T cell infiltration. Increased infiltration of T cells is associated with the expression of non-specific tumor associated antigens (TAAs) and mutated antigens (neoantigens) which might be potential immunological targets because they are derived from mutation in cancer cells. Thus, identifying the immunologically relevant neoantigens present on the tumor cells surface which do not have a homology with wildtype but have a homology with pathogen-derived epitope is warranted for better therapeutics and clinical outcome[1,23].
IMMUNOTHERAPY FOR HCC
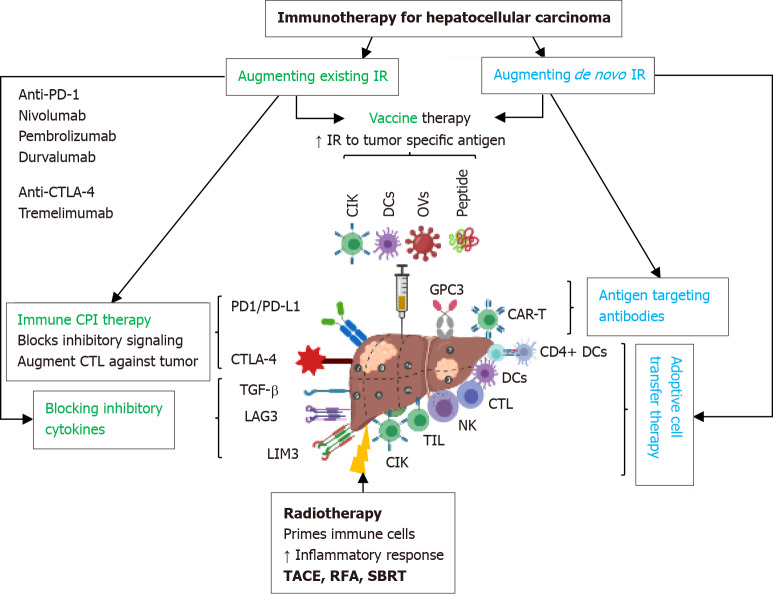
The immune-mediated pathogenesis of HCC makes it attractive for immune-based therapies. Immune dysfunction, immunosuppression, immune evasion, the presence of immune checkpoints and neoantigens underlying the pathophysiology of HCC makes immunotherapy a potential therapeutic strategy by targeting different mechanisms involved in the development and progression of HCC; however, there are limitations, and the results of various studies so far are modest. The immunotherapy strategies available for HCC are immune checkpoint inhibitors, cancer vaccines, DC-, NK cells-, cytokine-induced killer (CIK) cell-, and TILs-mediated immunotherapy, chimeric antigen receptor (CAR) T-cell immunotherapy, adoptive T cell transfer therapy, and combinational immunotherapy (Figure 2). Further, targeting molecular mediators including microRNAs, CCL2/CCR2, fibroblast growth factors (FGF), triggering receptor expressed on myeloid cells (TREMs), Wnt signaling, Smads, and TGF-β involved in the pathophysiology of HCC are additional targets[1,3,5,13,20,23-30]. The molecular mechanisms of immunotherapy for HCC based on DC, NK cells, T-cells, and Tregs cells and targeting TREMs, TLRs, folate receptor, chemokine receptor, receptor for advanced glycation end products (RAGE), and microRNAs have been described elsewhere[24]. The current immunotherapy aimed to unmask the immune response of the tumor and to stimulate a different immune response controlling the tumor growth and progression. Of these, immune checkpoint inhibitors involve targeting inhibitory receptors on T cells including PD-1, cytotoxic T-lymphocyte associated antigen 4 (CTLA-4), and immunosuppressive cytokines such as TGF-β; antibodies targeting alpha-fetoprotein (AFP) or glypican-3 (GPC3); and coupling of antibodies with T- or NK cells-mediated therapy to make it more effective[1,3,24]. PD-1/PD-L1 and CTLA-4 play an important role in the suppression of T cell activation by the tumor cells. GPC3, AFP, and heparan sulfate-based immunotherapy has been discussed in the literature[24].
Figure 2.
Immunotherapy in hepatocellular carcinoma: Potential strategies and therapeutic targets. IR: Immune response; PD-1: Programmed cell death protein 1; PD-L1: Programmed death-ligand 1; CTLA-4: Cytotoxic T-lymphocyte associated antigen 4; TGF-β: Transforming growth factor-beta; LAG3: Lymphocyte-activation gene 3; CIK: Cytokine-induced killer; TIL: Tumor-infiltrating lymphocyte; NK: Natural killer; DC: Dendritic cell; GPC3: Glypican-3.
IMMUNE CHECKPOINT INHIBITION
The systemic management of HCC has been revolutionized by the advent of immune checkpoint inhibitors. Immune checkpoint inhibition blocks the negatively regulating signals directly on T cells or on cells interacting with T cells and enhances the anti-tumor immunity. Immune checkpoint inhibitors and therapeutic monoclonal antibodies fine tunes the immune response by blocking the checkpoint proteins from binding with their partner proteins thereby helping the body to recognize and attack cancer cells by T cells leading to death of cancer cells. Immune checkpoint inhibitors are most effective in tumors with high mutagenic load[3,5,13]. Immune checkpoints are mainly expressed on B and T cells, NK cells, DC, TAMs, monocytes, and myeloid-derived suppressor cells (MDSC)[25]. CTLA-4, PD-1, LAG-3, B and T lymphocyte attenuator, and T cell immunoglobulin and mucin-domain containing (TIM-3) are the common immune checkpoints investigated in human cancer and PD-1/PD-L1 and CTLA-4 has become standard of care[31,32]. Immune checkpoint inhibitor therapy using antibodies against PD-1, CTLA-4, PD-L1, and prostatic-acid phosphatase have been shown to be safe and advantageous in treating melanoma, renal cell carcinoma, triple negative breast cancer, urothelial carcinoma, squamous cell carcinomas of the head and neck, prostate carcinoma, Merkel-cell carcinoma, non-small cell lung cancer, AIDS-related Kaposi sarcoma, and hairy cell leukemia[5,25]. PD-1 is expressed on immune cell including CD8+ T cells, CD4+ T cells, B cells, NKs, Tregs, MDSCs, and DCs. Binding of PD-1 with its ligand PD-L1 inhibits the effector T cell response and thus, PD-1 has become an attractive target for immunotherapy. PD-1 inhibitor nivolumab and pembrolizumab have been approved as second line treatment of HCC[33,34] (Table 1). Various phase I, phase II, and phase III clinical trials investigating drugs targeting PD-1 have been summarized in Table 2. Other studies including the ORIENT-32 study (NCT03794440; sintilimab, bevacizumab biosimilar vs sorafenib), the RATIONALE-301 study (NCT03412773-phase III trial), the KEYNOTE-240 study (NCT02702401-phase II trial; pembrolizumab vs placebo), NCT03713593 (pembrolizumab and lenvatinib vs lenvatinib monotherapy), NCT03764293, NCT03434379 (atezolizumab, bevacizumab), NCT03847428 (durvalumab, bevacizumab), NCT03298451 (tremelimumab, durvalumab vs sorafenib), NCT03755739 (pembrolizumab), NCT02576509 (nivolumab vs sorafenib), NCT03062358 (pembrolizumab vs placebo), and NCT03764293 (camrelizumab, apatinib) targeting PD-1/PD-L1 with VEGF inhibition have been described and summarized in the literature[3,20]. Of these NCT03298451 involves targeting CTLA-4 (tremelimumab) along with PD-1. Similarly, clinical trials of combination therapies based on PD-1/PD-L1 blockade combined with other agents (immunotherapies, antiangiogenics, targeted agents targeting TGF-β, HSP90, c-met, FGFR4, locoregional therapies including TACE and Y90) for HCC have been discussed in detail[25].
Table 1.
Immune checkpoint inhibitor therapy for hepatocellular carcinoma
|
Immune checkpoint inhibitor therapy
| |||
|
Agent
|
Type of study
|
Study details
|
Outcome
|
| Tremelimumab (anti-CTLA4)[45] | Phase II clinical trial | 21 HCC patients infected with hepatitis C virus and not eligible for surgery or locoregional therapy 15 mg/kg IV every 90 d | 17.6% patients-partial response; 58.8% patients-stable disease; Time to progression-6.48 mo; Overall survival-8.2 mo; Decreased viral load |
| TRC105 (carotuximab) antibody to CD105[46] | Phase I/II study | TRC105 (15 mg/kg) every 2 wk given with sorafenib 400 mg twice daily | Tumor ablation utilizing RFA and TACE enhance the efficacy of tremelimumab; Improves intratumoral effector CD8+ T cells infiltration |
| Nivolumab (anti-PD-1)[47] | CheckMate 040 phase I/II dose-escalation study | 182 patients with advanced HCC; Patients naive to or previously treated with sorafenib received 0.1-10 mg/kg and 3 mg/kg once every 2 wk | Durable responses with long-term survival and favorable safety in both sorafenib-naive and -experienced patients; 3.8% complete response, 14.8% partial response, and 62.6% disease control rate |
| Nivolumab (anti-PD-1)[33] | Phase I/II study NCT01658878 | 262 HCC patients; HCC patients on sorafenib | 1.4% complete response; 18.2% partial response; 83% overall survival at 6 mo |
| Pembrolizumab (anti-PD-1)[48] | KEYNOTE-224 trial | 104 advanced HCC patients on sorafenib | 1% complete response; 16% partial response; 54% overall survival at 12 mo |
| Durvalumab (PD-L1) and tremelimumab (CTLA4)[49] | Phase I/II, open-label, randomized study | For the efficacy of durvalumab combined with tremelimumab in unresectable HCC | No unexpected safety signals with durvalumab and tremelimumab seen in unresectable HCC patients |
| Tremelimumab (CTLA4)[50] | Phase II trial NCT01853618 | 32 patients with HCC with HCV; Tremelimumab at 3.5 and 10 mg/kg i.v. every 4 wk for 6 doses, followed by 3-monthly infusions; Combined with subtotal radiofrequency ablation or chemoablation at day 36 | No dose-limiting toxicities; Accumulation of intratumoral CD8+ T cells; 26% partial response |
CTLA-4: Cytotoxic T lymphocyte protein 4; PD-1: Programmed cell death protein 1; HCC: Hepatocellular carcinoma; HCV: Hepatitis C virus; RFA: Radiofrequency ablation; TACE: Transarterial chemoembolization.
Table 2.
Ongoing clinical trials for immune checkpoint inhibitor therapy
|
Identifier
|
Type of study
|
Study design
|
Status/outcome
|
| NCT02576509 (CheckMate-459) | Global phase III randomized control trial | Comparing nivolumab with sorafenib as first treatment in advanced HCC | Recruitment closed; Results awaited |
| NCT01658878 | Phase I/II dose-escalation, open-label, non-comparative study | Phase 1 to establish the safety of nivolumab at different dose; Phase 2 to compare the efficacy of nivolumab and sorafenib; To study the safety and efficacy of the combination of nivolumab plus ipilimumab and nivolumab plus cabozantinib | Active, not recruiting |
| NCT03298451 | Randomized phase III HIMALAYA trial | To compare the combination of tremelimumab (CTLA-4 inhibitor) and durvalumab (PD-L1 inhibitor) vs sorafenib | Recruiting patients |
| NCT03680508 | Phase II trial | To test efficacy of TSR-022 (cobolimab, TIM-3 binding antibody) and TSR-042 (dostarlimab, PD-1 binding antibody) on advanced HCC | Recruiting patients |
| NCT02947165 | Phase I/Ib study | Anti-TGF-β monoclonal antibody NIS793 and PD-1 inhibitor spartalizumab in breast, lung, colorectal, pancreatic, renal, and HCC | Active, not recruiting |
| NCT03412773 | Phase III randomized, open-label, multicenter, global study | To compare the efficacy and safety of tislelizumab vs sorafenib in unresectable HCC | Active, not recruiting |
| NCT03434379 (IMbrave150)[51] | Phase III study | To evaluate the efficacy and safety of atezolizumab in combination with bevacizumab compared with sorafenib in locally advanced or metastatic HCC; To determine overall survival | Atezo + Bevac showed improved survival at 18 mo (52%) with clinically meaningful treatment benefit and safety. The trial confirmed atezo + bevac as a standard of care for previously untreated, unresectable HCC |
| NCT02702401 (MK-3475-240/KEYNOTE-240) | Phase III study | Pembrolizumab (MK-3475) in advanced HCC treated systemically as a second line therapy; To determine overall survival and progression free survival | Active, not recruiting |
| NCT03062358 (MK-3475-394/KEYNOTE-394) | Phase III study | To determine the efficacy and safety of pembrolizumab or placebo with best supportive care previously systemically treated HCC | Active, not recruiting |
| NCT03383458 (CheckMate 9DX) | Phase III study | To investigate if nivolumab will improve recurrence-free survival compared to placebo in HCC undergone complete resection | Active, not recruiting |
CTLA-4: Cytotoxic T lymphocyte protein 4; PD-1: Programmed cell death protein 1; HCC: Hepatocellular carcinoma; TGF: Transforming growth factor; TIM3: T cell immunoglobulin and mucin domain-containing protein 3.
CTLA4 also known as CD152 is a membrane bound protein inhibitory receptor which keeps immune response in check and downregulates immune responses by inhibiting its binding with its ligand CD28. CTLA-4 is upregulated after T-cell activation and antagonize CD80 and CD86 co-stimulatory molecules[13]. CTLA-4 has been an attractive target for the treatment of advanced HCC and some phase I and phase II trials have shown promising results (Table 1), and some are ongoing to evaluate the effects of targeting CTLA-4 (Table 2). As discussed above, TIM-3 plays a crucial role in immune evasion and poor prognosis in HCC, TIM-3 seems to be an important target for immune checkpoint inhibitors. TIM-3 is a transmembrane protein expressed on TILs, Tregs, and CD4+ and CD8+ T-cells and increases the number and activation level of macrophages. TIM3 expression on tumor cells leads to decreased cytotoxic T lymphocytes[13,35]. LAG-3, associated with hypofunctional CD8+ response, is another membrane bound protein which binds with MHC II and suppress T cell activity and cytokine release and upregulates T cell exhaustion in chronic viral infection or cancer[36]. Efficiently augmented proliferation and cytokine production by NY-ESO-1-specific CD8(+) T cells during T-cell priming with dual blockade of LAG-3 and PD-1 in ovarian tumor indicate antitumor function of NY-ESO-1-specific CD8(+) T cells[37] and supports the notion of targeting LAG-3 in HCC as its expression is increased in HCC[1]. Increased expression of TGF-β, a membrane bound protein expressed on Tregs, is associated with suppression of CD4+ T cell response in HCC and promote tumor growth and progression[38]. Targeting PD-1 on CD4+CD69+ Tregs is another potential target for advanced HCC (Table 1). Various completed and ongoing clinical trials investigating the role of immune checkpoint inhibitors have been listed in Tables 1 and 2. Other clinical trials including CheckMate-040, CheckMate-459, Keynote-224, Keynote-524, KeyNote-240, HIMALAYA, IMbrave150, VEGF Liver 100, COSMIC312, LEAP-002 along with their details and outcomes have been summarized in the literature[13]. In addition to this, clinical trials evaluating the immune checkpoint inhibitors nivolumab (ONO-4538, MDX-1106, BMS-936558), pembrolizumab (MK-3475), tislelizumab (BGB-A317), camrelizumab (SHR-1210), and spartalizumab (PDR001) for PD-1; durvalumab (MEDI4736), atezolizumab (MPDL3280A), and avelumab (MSB0010718C) for PD-L1; and tremelimumab (CP 675206) and ipilimumab (BMS-734016, MDX-010) for CTLA-4 have also been discussed[30]. The advantage of immune checkpoint inhibitor therapy in augmenting the immune response of liver is due to its safe profile, rates of immune-related toxicity like other tumor type, and without any hepatic dysfunction as supported by the fact that the increase in aspartate aminotransferase and alanine aminotransferase with therapy was not relevant to cause its discontinuation[39,40]. The phase I and phase II clinical trials, which makes the basis for phase III trials, involving immune checkpoint inhibitors nivolumab (anti-PD1), pembrolizumab (anti-PD1), tislelizumab (anti-PD1), durvalumab (anti-PD1), tremelimumab (anti-CTLA4), and camrelizumab (anti-PD1) have been discussed in the literature[30]. Similarly, the clinical trials (NCT01658878, NCT02576509, NCT02702414, NCT02702401, NCT02715531, NCT03434379, NCT01008358, and NCT01853618) with immune checkpoint inhibitors have been summarized[20,29] (Table 2). The results of these studies and outcome of phase I and phase II clinical trials suggest that immune checkpoint inhibitor therapies can provide objective response in advanced HCC. These studies suggest that immunotherapy might be good therapeutic strategies for the treatment of HCC and combinational approach might be even more effective.
Immune check-point inhibitor therapy has been approved as first-line therapy in the cases not suitable for surgery and has been proven beneficial in stabilizing the quality of life, however, only a subset of patients has shown positive outcome and there are reports of tumor progression, worsening of liver function, and poor prognosis in others. The reason behind the equivocal results in clinical trials for immune check-point inhibitor therapy is due to the lack of biomarkers to check the tumor responsiveness during therapy[41]. Different strategies to assess the response and cut off values for the biomarkers are another reason for failure of immune check-point inhibitor therapy[42]. Decreased T-cell infiltration, expression of newer or other immune checkpoints, mutation of the immunogenicity of cancer itself, change in gut microbiota, and TME might be other causes for unresponsiveness or failure of the immune checkpoint inhibitors[43]. Limited efficacy or inability of the immune check-point inhibitors to target signaling pathways involved in tumorigenesis, as in case of monotherapy targeting PD-1/PD-L1 but not VEGF-A or Wnt/β-catenin, is another cause for failure or limited response of immune check-point inhibitor therapy[44]. Collectively, these factors are responsible for not a high response rate of immune check-point inhibitors in HCC.
ADOPTIVE CELL TRANSFER
Adoptive cell transfer therapy involves administration of autologous lymphocytes in the HCC patients. Adoptive cell transfer therapy involves infusion of NK cells, CIK cells, TILs, and CAR-T cells. NK cells form nearly 50% of the immune cells in the liver and can kill the cells without any prior activation, thus endowing the defense against infection and tumor development. The cytotoxic role of expanded NK cells on HCC in murine model resulting in reduced tumor growth and improving overall survival[29,52,53] supports the notion of using adoptive cell transfer therapy in HCC. CIK cells are T lymphocytes representing a T cell population which acquire phenotype of NK cells with NK cells surface markers through manipulation with IFN-γ, and IL-2, IL-1, and a monoclonal antibody against the T cell marker CD3 (OKT3) and represent non-MHC-restricted tumor-killing activity and inhibitory effect on tumorigenesis[3,54]. Adoptive cell transfer therapy with CIK cells represents novel immunotherapy and early randomizing trials in HCC patients following surgical resection as adjuvant therapy shows promising results with a significantly reduced risk of recurrence, but without an improvement in overall survival[3]. Improved progression and recurrence free survival has been documented by a systematic review and meta-analysis of CIK cell therapy in HCC[55]. Similarly, studies investigating the efficacy of CIK cell therapy in HCC with the results of no major adverse events after a median follow-up of 14 mo with autologous TILs, benefits of CIK cell treatment, and significant superiority in prolonging the median overall survival, progression free survival, significantly higher disease-free survival rates, and disease control rate in HCC patients has been reviewed[29]. GPC3, a member of the glypican family of heparan sulfate proteoglycans, is tumor specific and important for cell proliferation and its role in the pathogenesis of HCC has been described. Thus, targeting GPC3 and other TAAs or neoantigens with CAR-T cell therapy in HCC seems to be prospective immunotherapeutic option and has been reviewed in the literature[24,29]. The results of various clinical trial and the ongoing clinical trials for adoptive cell therapy has been summarized in Tables 3 and 4, respectively.
Table 3.
Adoptive cell therapy for hepatocellular carcinoma
|
Adoptive cell transfer
| |||
|
Agent
|
Type of study
|
Study details
|
Outcome
|
| NK cells stimulated with IL-2[60] | Phase I trial | Patients with liver cirrhosis with HCC undergoing liver transplantation | Upregulation of peripheral NK cell cytotoxicity, no adverse events |
| CIK cell therapy as adjuvant to RFA[61] | A multicenter, randomized, open label phase III trial | 230 HCC patients; CIK cell therapy as adjuvant to RFA, ethanol injection or curative resection | An improvement of 14 mo in recurrence free survival |
| Autologous TILs[62] | Phase I trial | 15 patients with HCC post-resection | Successful expansion of TILs in 88% without any evidence of disease; No serious adverse events |
| GPC3 CAR-T[63] | Phase I trial | 13 Chinese patients with r/r GPC3+ HCC | Feasible and safe for Chinese pts with r/r GPC3+ HCC; Promising antitumor potential when LDC is applied along with GPC3 CAR-T |
NK: Natural killer; IL: Interleukin; HCC: Hepatocellular carcinoma; CIK: Cytokine-induced killer; TIL: Tumor-infiltrating lymphocytes; RFA: Radiofrequency ablation; CAR: Chimeric antigen receptor.
Table 4.
Clinical trials on adoptive cell transfer therapy
|
Clinical trials #
|
Phase
|
Aim and design
|
Status
|
| NCT03563170 | Phase 1b/2 | Combining innate high-affinity natural killer (hank) cell therapy with adenoviral and yeast-based vaccines to induce t-cell responses vs sorafenib | Withdrawn |
| NCT03008343 | Phase I/II | Combination of IRE and NK cells immunotherapy vs IRE alone | Completed, no result posted |
| NCT01147380 | Phase I | Natural killer cell therapy for hepatoma liver transplantation (MIAMINK); To evaluate feasibility and safety of the adoptive transfer of activated NK cells | Completed; No adverse events reported |
| NCT02008929 | Phase II | To evaluate the safety and efficacy of injecting MG4101 (ex vivo expanded allogeneic NK cell) as a secondary treatment after curative liver resection in advanced HCC | Completed; No study results posted |
| NCT01749865 | Phase III | CIK treatment in 200 patients with HCC who underwent radical resection | Completed; No study results posted |
| NCT02723942 | Phase I/II | To evaluate the safety and efficacy of CAR-T cell immunotherapy for GPC3 positive hepatocellular carcinoma | Withdrawn due to revision of local regulations |
| NCT03198546 | Phase I | GPC3 and/or TGF-β targeting CAR-T cells in | Recruiting |
| NCT03130712 | Phase I/II | GPC3-targeted T cells by intratumor injection for advanced HCC (GPC3-CART) | Unknown |
| NCT02715362 | Phase I/II | GPC3 redirected autologous t cells for advanced HCC (GPC3-CART) | Unknown |
| NCT03013712 | Phase I/II | GPC3-targeted T cells by intratumor injection for advanced HCC (GPC3-CART) | Unknown |
| NCT03349255 | Phase I | Autologous ET1402L1-CAR T cells in AFP expressing HCC | Terminated and will study new T-cell construct |
| NCT02905188 | Phase I | To find the biggest dose of GLYCAR T cells that is safe, to see how long they last in the body, to learn what the side effects in GPC3-positive HCC | Recruiting patients; Partial response with no toxicities |
| NCT03146234 | Single arm, open-label pilot study | to determine the safety and efficacy of CAR-GPC3 T cells in patients with relapsed or refractory HCC following cyclophosphamide and fludarabine | Completed; Had a tolerable toxicity profile with no grade 3/4 neurotoxicity; Overall survival 9.1 |
| NCT02395250 | Phase I | To evaluate the safety and effectiveness of anti-GPC3 CAR T in patients with relapsed or refractory HCC | Completed, no result posted |
| NCT03980288 | Phase I | 4th generation chimeric antigen receptor T cells targeting glypican-3 (CAR-GPC3 T cells) in patients with advanced HCC | Recruiting patients |
| NCT04121273 | Phase I | GPC3-targeted CAR-T cell for treating GPC3 positive advanced HCC | Recruiting patients |
| NCT03884751 | Phase I | Clinical study of chimeric antigen receptor T cells targeting glypican-3 (CAR-GPC3 T cells) in patients with advanced HCC | Recruiting patients |
| NCT04093648 | Phase I | T cells co-expressing a second generation glypican 3-specific chimeric antigen receptor with cytokines interleukin-21 and 15 as immunotherapy for patients with liver cancer (TEGAR) | Withdrawn (the key elements of this study were incorporated into another study) |
| NCT03013712 | Phase I/II | CAR T cells targeting EpCAM positive cancer (CARTEPC); To evaluate the safety and efficacy of chimeric antigen receptor (CAR) T cells targeting EpCAM | Unknown |
NK: Natural killer; IL: Interleukin; HCC: Hepatocellular carcinoma; CIK: Cytokine-induced killer; TIL: Tumor-infiltrating lymphocytes; RFA: Radiofrequency ablation; CAR: Chimeric antigen receptor; Adenoviral and Yeast based vaccines: ETBX-011, GI-4000, avelumab, Aldoxorubicin hydrochloride, ETBX-051, ETBX-061, GI-6207, GI-6301, and N-803; IRE: Irreversible electroporation; LDC: Lymphodepleting conditioning; GLYCAR: Glypican 3-specific chimeric antigen receptor expressing T cells for hepatocellular carcinoma; HCC: Hepatocellular carcinoma.
Adoptive cell transfer therapy has emerged as a promising therapy in HCC; however, the clinical trials and clinical research is progressing slowly because of various limitations such as inactivity of the infiltrating lymphocytes due to changing TME caused by immunoediting or immunomodulation. Down regulation of MHC class I molecule, cellular heterogeneity of the cells, terminal differentiated cells and short viability of these cells, effectiveness only in in-vitro but limited efficacy in vivo, unexpected toxicity (CAR-T), specificity for a target, cytokine storm, presence of neoantigens, presence of suppressive immune cells, evolution of inhibitory ligands, variability in cells processing conditions, defects in antigen processing and presentation, evolving tumor escape mechanisms, and presence of hostile TME associates with the limited success and slow progression of adoptive cell transfer therapy[56-58]. Off-tumor effects are potential concerns related to CAR-T cell therapy and associate with limited efficacy[59]. CAR-T cells function can potentially be altered due to the interaction between CAR-T cells and host TME[58].
VACCINES
Peptide, DCs, whole-cell vaccines, oncolytic viruses, and DNA agents are the most common therapeutic vaccines used to increase immune response to tumor antigen[64]. The common peptide vaccines including AFP, multidrug resistance-associated protein 3 (MRP3), and GPC3 have been proven safe and well-tolerated and clinical trials including UMIN000001395, UMIN000005678, NCT01974661, NCT00554372, and NCT01387555 using these vaccines have been summarized in the literature[29]. As discussed above, the non-specificity of tumor antigen is a major cause of impediment in designing novel therapeutics and tumor vaccines helps in augmenting specific immune responses to tumor antigens. Thus, tumor vaccines seem to be potential therapeutics for advanced HCC, however, disappointing results from previous trials and lack of vaccine efficacy in other tumors cause scarcity of clinical trials of tumor vaccines for HCC. However, this hurdle has been renounced due to the modern techniques of identifying novel targets using RNA/DNA sequencing and bioinformatics[3]. DCs are APCs presenting the TAAs and provide secondary co-stimulation required for the priming of an effective T cell response. Peripheral DCs treated with various factors to activate and mature ex-vivo are reinfused and these primed DCs functions as vaccine via inducing recruitment of effector cells and tumor cell lysis[65]. The efficacy of the vaccines can be enhanced by optimizing the TAAs. An effective immune response in the tumor can also be induced by peptide vaccines. GPC3 peptide in increased in HCC and might be a potential target, a study by Wu et al[66] showed that GPC3 coupled lymphocytes elicit robust GPC3-specific antibody and cytotoxic T lymphocyte responses in mouse and might be precision therapeutics. A recent phase 1 clinical trial showed that after peptide vaccination in HCC patients, peptide-specific cytotoxic T lymphocytes frequency might be a predictive marker of overall survival[67]. Targeting TAA seems to be effective therapeutics, however, clinical trials showed specific T cell response rate of over 70% while targeting AFP, GPC3, and MRP3 while T cell response rate was below 40% while targeting NY-ESO-1, SSX- 2, MAGE-A, and TERT[68]. The weak efficacy of the vaccines might be due to insufficient immune activation of targeting self/tumor antigen. The use of oncolytic viruses to induce oncolysis is an important strategy in HCC because of the ability of oncolytic virus for selective infection, within tumor replication, and destruction and eradication of tumor cells. The strategies, mechanistic aspects, and the limitations of the oncolytic viruses including New-Castle disease virus (rNDV-18HL, rNDV-IL2-TRAIL, rLaSota/IL2), adenovirus [ZD55-XAF1, QG511-HA-melittin, GOLPH2-regulated GD55, AD55-Mn-SOD, Ad5-HC, Ad5-AFP (IRES), hTERT-Ad, Ad-199T, AdDE1bDVA+2’AP, Adenovirus SP-E1AE1B(D55)-TSLC1(SD55-TSLC1), Adenovirus eSurphSulf1], vaccinia virus [GLV-1h68, GLV-2b372, JX594, Pexa-Vec (JX-594)], recombinant vesicular stomatitis virus (rVSV, VSV with NSC74859), parvovirus (Recombinant H-1 PV), and measles virus (MeV-SCD) have been discussed in detail in the literature[24]. Using oncolytic viruses as vaccines is more recent approach and intra-tumoral injection of viruses is used to selectively replicate in and destroy cancer cells. Pexa-Vec is the most common virus vaccine and is a modified vaccinia poxvirus (JX-594). Various clinical trials have studied the anti-tumor effects of Pexa-Vec on HCC (Table 5) and some trials are ongoing, but results have not been posted and are awaited (Table 6). Changing tumor immunogenicity due to mutation or low tumor immunogenicity limits the response of vaccine therapy in HCC and identification of novel specific tumor epitopes is warranted to improve efficacy of HCC cancer vaccine[69].
Table 5.
Vaccine therapy for hepatocellular carcinoma
|
Vaccine
|
Phase
|
Study design
|
Outcome
|
| Autologous dendritic cells (DCs) generated ex vivo in the presence of GM-CSF and IL-4[70] | Phase I | 10 patients with unresectable primary liver cancer | Immunization well tolerated without significant toxicity |
| Mature autologous DCs[71] | Phase II | To investigate the safety and efficacy of intravenous vaccination | Safe and well tolerated with evidence of antitumor efficacy |
| Ilixadencel (pro-inflammatory allogeneic DCs stimulated by GM-CSF and IL-4)[72] | Phase I trial | 17 HCC patients; As monotherapy or in combination with sorafenib to evaluate tolerability | Increased tumor specific CD8+ T cells in peripheral blood (73%); 1 grade 3 adverse event |
| GPC3 peptide[67] | Open-label, phase I clinical trial | 33 patients with advanced HCC; To evaluate safety of GPC3 peptide, immune response, tumor response, time to tumor progression, and overall survival | GPC3 vaccination was well-tolerated; 1 patient partial response; 19 patient stable disease; 2 mo after vaccination; Measurable immune responses and antitumor efficacy |
| Pexa-Vec (modified poxvirus JX-594)[73] | Randomized phase II dose-finding trial | 30 patients with advanced HCC; 3 intra-tumoral injections; To determine the optimal JX-594 dose | Dose related survival benefit; Increased median survival of 14.1 mo compared to 6.7 mo |
| Pexa-Vec (JX-594)[74] | Phase 2, open-label, randomized dose finding study | Patients with advanced HCC; Intra-tumoral injection 3 times every 2 wk | |
| Pexa-Vec (pexastimogene devacirepvec) followed by sorafenib[75] | Global, randomized, open-label phase III trial (PHOCUS) | 459 patients will be recruited; To evaluate overall survival, time to progression, progression-free survival, overall response rate and disease control rate | Trial completed; 5% adverse events |
IL: Interleukin; GM-CSF: Granulocyte-macrophage colony-stimulating factor; HCC: Hepatocellular carcinoma.
Table 6.
Ongoing clinical trials on vaccine therapy for hepatocellular carcinoma
|
Clinical trial #
|
Phase
|
Agent/vaccine
|
Design/aim
|
Status
|
| NCT01974661 | Phase I | COMBIG-DC (ilixadencel) | Is it possible to inject the COMBIG-DC vaccine in a hepatic tumor without getting unacceptable side effects | Completed; No results posted |
| NCT01821482 | Phase II | DC-CIK | To evaluate the efficacy of DC-CIK for HCC | Unknown/not yet recruiting |
| NCT02638857 | Phase I/II | DC precision multiple antigen T cell | To evaluate the safety and efficacy of dendritic cell-precision multiple antigen T cells with TACE in HCC | Unknown/was recruiting |
| NCT02882659 | Phase I | Autologous dendritic killer cell | To evaluate the safety in patients with metastatic solid tumor; To evaluate the maximum tolerated dose | Unknown/was active, not recruiting |
| NCT03674073 | Phase I | Personalized neoantigen-based dendritic cell | A single institution, open-label, multi-arm, pilot study; DC vaccine combined with microwave ablation in HCC | Unknown/was recruiting |
| NCT03203005 | Phase I/II | Cancer vaccine called IMA970A combined with CV8102 | To investigate the safety; To check if this combination can trigger an immune response against the tumor in HCC | Completed; No results posted |
| NCT02562755 | Phase III | Pexastimogene devacirepvec (Pexa Vec) and sorafenib | To investigate if the combined treatment increases survival compared to treatment with sorafenib alone in HCC | Completed |
DCs: Dendritic cells; CIK: Cytokine-induced killer; HCC: Hepatocellular carcinoma.
COMBINATION THERAPIES
Since HCC is a multifactorial disease, targeting more than one factor involved in the pathogenesis of HCC seems to be a promising approach. Along with the ongoing clinical trials for immune checkpoint inhibitors targeting PD-1/PD-L1 and CTLA4, the recent interest is to design combination therapies. This notion is supported by significantly improved clinical response with the combination of nivolumab with ipilimumab in sorafenib-treated patients with an acceptable safety profile[76]. The combination therapies combining checkpoint inhibitors with other drugs including oncolytic virus/viral vaccines, small molecules, ablative therapies or combining multiple checkpoint inhibitors is the area of interest. The basis of the combination therapies is additive or synergistic effects of the therapy by combining systemic or radiotherapy with immunotherapy[25,28]. Radiotherapy primes the immune cells, increase inflammatory response, and when combined with immunotherapy produce synergistic effect and enhance anti-tumor effects. Various aspects of combination therapies including the primary or acquired resistance to anti-PD-1/PD-L1 therapies in melanoma, increased viral load in patients when tumor started to progress with anti-CTLA-4 therapy ultimately leading to treatment failure, the presence of low mutation rate as a cause of therapy resistance, and strategies to enhance function of effector cells have been discussed[25]. A dose-dependent increase in PD-L1 expression post irradiation in HCC cell lines mediated by IFN-γ-STAT3 signaling pathway support the notion of using combination therapy for advanced HCC[77]. An improved treatment outcome in murine model with HCC, mammary cancer in xenograft murine model, and CT26 murine colon carcinoma xenograft model while combining radiotherapy with PD-1/PD-L1 and CTLA-4 as described in[28] suggests the advantage of combining radiotherapy with immune checkpoint therapy. This rationale is also supported by the results of clinical studies documenting increased PD-1 and PD-L1 expression of T cells and tumor cells[78-80]. Additionally, the combination therapies combining checkpoint inhibitor therapies with other strategies with the mechanism of action and study details of various clinical trials (NCT01658878, NCT02519348, NCT03071094, NCT02572687, NCT03006926, NCT02856425, NCT02942329, NCT02988440, NCT02423343, NCT02859324, NCT03095781, NCT02474537, NCT02325739, NCT03143270, NCT03033446, NCT02837029, NCT03099564) have been summarized[20,25]. Similarly, promising clinical results of combining RT with immunotherapy reported by Chiang et al[81] using stereotactic body radiotherapy followed by nivolumab for large unresectable HCC and Y-90-RE and nivolumab bridging therapy prior to partial hepatectomy by[82,83] supports the notion of combination therapy for advanced HCC. The ongoing clinical trials of combination therapies for HCC has been listed in Table 7. Additionally, the clinical trials involving durvalumab + tremelimumab, nivolumab + ipilimumab, atezolizumab + bevacizumab, pembrolizumab + lenvatinib, and SHR-1210 + apatinib have been discussed[30].
Table 7.
Ongoing clinical trial for combination therapy for hepatocellular carcinoma
|
Immune checkpoint/vaccine therapy
|
Radiotherapy/other therapy
|
Phase
|
Study design
|
Status
|
Trial ID
|
| Ipilimumab | Nivolumab | Phase I/II | To assess the effects of combination treatment with nivolumab and ipilimumab pre-operatively in HCC | Recruiting patients | NCT03682276 |
| Nivolumab | Ipilimumab | Phase I | To compare the overall survival of nivolumab plus ipilimumab vs standard of care (sorafenib or lenvatinib) in patients with advanced HCC | Recruiting patients | NCT04039607 |
| Nivolumab | Ipilimumab | Phase II | Nivolumab plus Ipilimumab as neoadjuvant therapy for HCC; To test efficacy, tumor shrinkage, and objective response rate | Recruiting patients | NCT03510871 |
| Nivolumab | Ipilimumab | Phase II | Nivolumab with or without ipilimumab in treating patients with resectable liver cancer | NCT03222076 | |
| Nivolumab, ipilimumab | SBRT | Phase I | To determine the safety and tolerability of SBRT followed by nivolumab or ipilimumab in HCC | Active, not recruiting | NCT03203304 |
| Pembrolizumab | Talimogene laherparepvec (genetically modified oncolytic viral therapy) | Phase Ib/II | Multicenter, open-label, basket trial; To evaluate the safety of talimogene laherparepvec injected intra-hepatically into liver tumors alone and in combination with systemic IV administration of pembrolizumab | Recruiting patients | NCT02509507; MK-3475-611/Keynote-611 (MASTERKEY-318) |
| Nivolumab | Pexa-Vec | Phase I/II | To evaluate the safety and efficacy in HCC | Active, not recruiting | NCT03071094 |
| Modified vaccinia virus ankara vaccine expressing p53 | Pembrolizumab | Phase I | To study the side effects of vaccine therapy and in treating patients with solid tumors with metastasis | Active, not recruiting | NCT02432963 |
| GNOS-PV02 (personalized neoantigen DNA vaccine) | Plasma encoded IL-12 (INO-9012) pembrolizumab | Phase I/IIa | A single-arm, open-label, multi-site study of GNOS-PV02 and INO-9012 in combination with pembrolizumab (MK-3475) in histologically or cytologically confirmed HCC | Recruiting patients | NCT04251117 |
| DNAJB1-PRKACA fusion kinase peptide vaccine | Nivolumab and Ipilimumab | Phase I | To study the safety and tolerability of administering a vaccine targeting the DNAJB1-PRKACA fusion kinase, in combination with nivolumab and ipilimumab in unresectable or metastatic fibrolamellar HCC | Recruiting patients | NCT04248569 |
| Durvalumaband tremelimumab | Sorafenib | Phase III | To assess the efficacy and safety of durvalumab plus tremelimumab combination therapy and durvalumab monotherapy vs sorafenib in the treatment of patients with no prior systemic therapy for unresectable HCC | NCT03298451 | |
| TremelimumabDurvalumab (MEDI4736) | Radiation therapy | Phase II | To test the combination therapy as a possible treatment for HCC or biliary tract cancer | Recruiting patients | NCT03482102 |
| Nivolumab | Y90-radioembolization | Phase II | To evaluate the response rates of Y90 radioembolization in combination with nivolumab in HCC | Recruiting patients | NCT03033446 |
| Ipilimumab | SBRT | Phase I | To find the highest tolerable dose of ipilimumab and SBRT in liver and lung cancer | Completed but no results posted | NCT02239900 |
| Nivolumab | TACE | Phase II (IMMUTACE) | To evaluates the safety and the efficacy of nivolumab in combination with TACE in patients with multinodular, intermediate stage HCC as first line therapy | Active, not recruiting | NCT03572582 |
| Pembrolizumab | TACE | Phase I/II (PETAL) | Open label, single arm, multi-centre study; To determine the safety and tolerability of pembrolizumab following TACE | Recruiting patients | NCT03397654 |
| Durvalumab; Tremelimumab | TACE; RFA; Cryoablation | Phase II | To evaluate the 6-mo progression free survival with combination therapy in patients with HCC | Recruiting patients | NCT02821754 |
| Immune Checkpoint Inhibitor | TACE; SBRT | Phase II; START-FIT | Sequential TACE and SBRT with immunotherapy | Recruiting patients | NCT03817736 |
| Durvalumab | Tremelimumab | Phase II | To evaluate the safety, tolerability, antitumor activity, pharmacokinetics, pharmacodynamics, and immunogenicity of durvalumab or tremelimumab monotherapy, or durvalumab in combination with tremelimumab or bevacizumab in advanced HCC; Initial reports of concerns with safety and efficacy of the combination of durvalumab and tremelimumab in HCC | Active, not recruiting | NCT02519348 |
SBRT: Stereotactic body radiotherapy; TACE: Trans-arterial chemoembolization; HCC: Hepatocellular carcinoma; RFA: Radiofrequency ablation.
CONCLUSION
The limited efficacy of immune-based therapies is due to inherently tolerogenic character of the liver in both healthy and diseased state. Chronic inflammation of liver during the pathogenicity of HCC leads to higher tumor immunogenicity and makes a basis of immunotherapeutic approaches to treat HCC. However, strong intrinsic immune suppressive microenvironment and high immune evasion are major impediment for an effective immune response against tumor with immunogenic approach. Additionally, liver also plays a crucial role in host defense and in the maintenance of self-tolerance, it is important to design personalized immunosuppressive therapies[1]. The intrahepatic immunosuppressive TMEs play a major role in reducing the effects of immunotherapy and thus an effective therapy must be designed to counteract and target factors playing a role in immune evasion and treatment resistance. Additionally, therapeutic regimens which can amplify tumor-specific immunity and counteract immunosuppressive mechanisms might profoundly improve clinical outcomes for HCC patients. The initial results from various clinical trials involving immune checkpoint inhibitor therapy, adoptive cell transfer therapy, tumor vaccines, and combination therapy are promising but warrant more research in terms of investigating tumor specific antigens and better personalized therapies.
Footnotes
Conflict-of-interest statement: The authors have no conflicts of interests.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: American Gastroenterological Association; American Association for the Study of Liver Diseases.
Peer-review started: April 7, 2021
First decision: July 6, 2021
Article in press: December 25, 2021
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Lim SC, Xu X S-Editor: Gao CC L-Editor: A P-Editor: Gao CC
Contributor Information
Vikrant Rai, Department of Translational Research, Western University of Health Sciences, Pomona, CA 91766, United States.
Sandeep Mukherjee, Department of Medicine, Creighton University School of Medicine, Omaha, NE 68124, United States. sandeep.mukherjee@alegent.org.
References
- 1.Buonaguro L, Mauriello A, Cavalluzzo B, Petrizzo A, Tagliamonte M. Immunotherapy in hepatocellular carcinoma. Ann Hepatol. 2019;18:291–297. doi: 10.1016/j.aohep.2019.04.003. [DOI] [PubMed] [Google Scholar]
- 2.Aly A, Ronnebaum S, Patel D, Doleh Y, Benavente F. Epidemiologic, humanistic and economic burden of hepatocellular carcinoma in the USA: a systematic literature review. Hepat Oncol. 2020;7:HEP27. doi: 10.2217/hep-2020-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnston MP, Khakoo SI. Immunotherapy for hepatocellular carcinoma: Current and future. World J Gastroenterol. 2019;25:2977–2989. doi: 10.3748/wjg.v25.i24.2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Petrick JL, Kelly SP, Altekruse SF, McGlynn KA, Rosenberg PS. Future of Hepatocellular Carcinoma Incidence in the United States Forecast Through 2030. J Clin Oncol. 2016;34:1787–1794. doi: 10.1200/JCO.2015.64.7412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Keenan BP, Fong L, Kelley RK. Immunotherapy in hepatocellular carcinoma: the complex interface between inflammation, fibrosis, and the immune response. J Immunother Cancer. 2019;7:267. doi: 10.1186/s40425-019-0749-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Llovet JM, Real MI, Montaña X, Planas R, Coll S, Aponte J, Ayuso C, Sala M, Muchart J, Solà R, Rodés J, Bruix J Barcelona Liver Cancer Group. Arterial embolisation or chemoembolisation vs symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359:1734–1739. doi: 10.1016/S0140-6736(02)08649-X. [DOI] [PubMed] [Google Scholar]
- 7.Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: Chemoembolization improves survival. Hepatology. 2003;37:429–442. doi: 10.1053/jhep.2003.50047. [DOI] [PubMed] [Google Scholar]
- 8.Spinzi G, Paggi S. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:2497–8; author reply 2498. doi: 10.1056/NEJMc081780. [DOI] [PubMed] [Google Scholar]
- 9.Bruix J, Qin S, Merle P, Granito A, Huang YH, Bodoky G, Pracht M, Yokosuka O, Rosmorduc O, Breder V, Gerolami R, Masi G, Ross PJ, Song T, Bronowicki JP, Ollivier-Hourmand I, Kudo M, Cheng AL, Llovet JM, Finn RS, LeBerre MA, Baumhauer A, Meinhardt G, Han G RESORCE Investigators. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389:56–66. doi: 10.1016/S0140-6736(16)32453-9. [DOI] [PubMed] [Google Scholar]
- 10.Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, Baron A, Park JW, Han G, Jassem J, Blanc JF, Vogel A, Komov D, Evans TRJ, Lopez C, Dutcus C, Guo M, Saito K, Kraljevic S, Tamai T, Ren M, Cheng AL. Lenvatinib vs sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163–1173. doi: 10.1016/S0140-6736(18)30207-1. [DOI] [PubMed] [Google Scholar]
- 11.Eatrides J, Wang E, Kothari N, Kim R. Role of Systemic Therapy and Future Directions for Hepatocellular Carcinoma. Cancer Control. 2017;24:1073274817729243. doi: 10.1177/1073274817729243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Okrah K, Tarighat S, Liu B, Koeppen H, Wagle MC, Cheng G, Sun C, Dey A, Chang MT, Sumiyoshi T, Mounir Z, Cummings C, Hampton G, Amler L, Fridlyand J, Hegde PS, Turley SJ, Lackner MR, Huang SM. Transcriptomic analysis of hepatocellular carcinoma reveals molecular features of disease progression and tumor immune biology. NPJ Precis Oncol. 2018;2:25. doi: 10.1038/s41698-018-0068-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pinato DJ, Guerra N, Fessas P, Murphy R, Mineo T, Mauri FA, Mukherjee SK, Thursz M, Wong CN, Sharma R, Rimassa L. Immune-based therapies for hepatocellular carcinoma. Oncogene. 2020;39:3620–3637. doi: 10.1038/s41388-020-1249-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wherry EJ, Kurachi M. Molecular and cellular insights into T cell exhaustion. Nat Rev Immunol. 2015;15:486–499. doi: 10.1038/nri3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Calderaro J, Rousseau B, Amaddeo G, Mercey M, Charpy C, Costentin C, Luciani A, Zafrani ES, Laurent A, Azoulay D, Lafdil F, Pawlotsky JM. Programmed death ligand 1 expression in hepatocellular carcinoma: Relationship With clinical and pathological features. Hepatology. 2016;64:2038–2046. doi: 10.1002/hep.28710. [DOI] [PubMed] [Google Scholar]
- 16.Dai X, Xue J, Hu J, Yang SL, Chen GG, Lai PBS, Yu C, Zeng C, Fang X, Pan X, Zhang T. Positive Expression of Programmed Death Ligand 1 in Peritumoral Liver Tissue is Associated with Poor Survival after Curative Resection of Hepatocellular Carcinoma. Transl Oncol. 2017;10:511–517. doi: 10.1016/j.tranon.2017.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yan W, Liu X, Ma H, Zhang H, Song X, Gao L, Liang X, Ma C. Tim-3 fosters HCC development by enhancing TGF-β-mediated alternative activation of macrophages. Gut. 2015;64:1593–1604. doi: 10.1136/gutjnl-2014-307671. [DOI] [PubMed] [Google Scholar]
- 18.Zhou G, Sprengers D, Boor PPC, Doukas M, Schutz H, Mancham S, Pedroza-Gonzalez A, Polak WG, de Jonge J, Gaspersz M, Dong H, Thielemans K, Pan Q, IJzermans JNM, Bruno MJ, Kwekkeboom J. Antibodies Against Immune Checkpoint Molecules Restore Functions of Tumor-Infiltrating T Cells in Hepatocellular Carcinomas. Gastroenterology. 2017;153:1107–1119.e10. doi: 10.1053/j.gastro.2017.06.017. [DOI] [PubMed] [Google Scholar]
- 19.Capece D, Fischietti M, Verzella D, Gaggiano A, Cicciarelli G, Tessitore A, Zazzeroni F, Alesse E. The inflammatory microenvironment in hepatocellular carcinoma: a pivotal role for tumor-associated macrophages. Biomed Res Int. 2013;2013:187204. doi: 10.1155/2013/187204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu W, Liu K, Chen M, Sun JY, McCaughan GW, Lu XJ, Ji J. Immunotherapy for hepatocellular carcinoma: recent advances and future perspectives. Ther Adv Med Oncol. 2019;11:1758835919862692. doi: 10.1177/1758835919862692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Budhu A, Forgues M, Ye QH, Jia HL, He P, Zanetti KA, Kammula US, Chen Y, Qin LX, Tang ZY, Wang XW. Prediction of venous metastases, recurrence, and prognosis in hepatocellular carcinoma based on a unique immune response signature of the liver microenvironment. Cancer Cell. 2006;10:99–111. doi: 10.1016/j.ccr.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 22.Nishida N, Kudo M. Immunological Microenvironment of Hepatocellular Carcinoma and Its Clinical Implication. Oncology. 2017;92 Suppl 1:40–49. doi: 10.1159/000451015. [DOI] [PubMed] [Google Scholar]
- 23.Lee WC. Cell-mediated immunotherapy for hepatocellular carcinoma. J Cancer Metastasis Treat . 2017;3:244–249. [Google Scholar]
- 24.Rai V, Abdo J, Alsuwaidan AN, Agrawal S, Sharma P, Agrawal DK. Cellular and molecular targets for the immunotherapy of hepatocellular carcinoma. Mol Cell Biochem. 2018;437:13–36. doi: 10.1007/s11010-017-3092-z. [DOI] [PubMed] [Google Scholar]
- 25.Greten TF, Sangro B. Targets for immunotherapy of liver cancer. J Hepatol. 2017 doi: 10.1016/j.jhep.2017.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ghavimi S, Apfel T, Azimi H, Persaud A, Pyrsopoulos NT. Management and Treatment of Hepatocellular Carcinoma with Immunotherapy: A Review of Current and Future Options. J Clin Transl Hepatol. 2020;8:168–176. doi: 10.14218/JCTH.2020.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang L, Ding J, Li HY, Wang ZH, Wu J. Immunotherapy for advanced hepatocellular carcinoma, where are we? Biochim Biophys Acta Rev Cancer. 2020;1874:188441. doi: 10.1016/j.bbcan.2020.188441. [DOI] [PubMed] [Google Scholar]
- 28.Lee YH, Tai D, Yip C, Choo SP, Chew V. Combinational Immunotherapy for Hepatocellular Carcinoma: Radiotherapy, Immune Checkpoint Blockade and Beyond. Front Immunol. 2020;11:568759. doi: 10.3389/fimmu.2020.568759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kole C, Charalampakis N, Tsakatikas S, Vailas M, Moris D, Gkotsis E, Kykalos S, Karamouzis MV, Schizas D. Immunotherapy for Hepatocellular Carcinoma: A 2021 Update. Cancers (Basel) 2020;12 doi: 10.3390/cancers12102859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Okusaka T, Ikeda M. Immunotherapy for hepatocellular carcinoma: current status and future perspectives. ESMO Open. 2018;3:e000455. doi: 10.1136/esmoopen-2018-000455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Le Mercier I, Lines JL, Noelle RJ. Beyond CTLA-4 and PD-1, the Generation Z of Negative Checkpoint Regulators. Front Immunol. 2015;6:418. doi: 10.3389/fimmu.2015.00418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prieto J, Melero I, Sangro B. Immunological landscape and immunotherapy of hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2015;12:681–700. doi: 10.1038/nrgastro.2015.173. [DOI] [PubMed] [Google Scholar]
- 33.El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, Kim TY, Choo SP, Trojan J, Welling TH Rd, Meyer T, Kang YK, Yeo W, Chopra A, Anderson J, Dela Cruz C, Lang L, Neely J, Tang H, Dastani HB, Melero I. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389:2492–2502. doi: 10.1016/S0140-6736(17)31046-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, Roberts LR, Heimbach JK. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68:723–750. doi: 10.1002/hep.29913. [DOI] [PubMed] [Google Scholar]
- 35.Monney L, Sabatos CA, Gaglia JL, Ryu A, Waldner H, Chernova T, Manning S, Greenfield EA, Coyle AJ, Sobel RA, Freeman GJ, Kuchroo VK. Th1-specific cell surface protein Tim-3 regulates macrophage activation and severity of an autoimmune disease. Nature. 2002;415:536–541. doi: 10.1038/415536a. [DOI] [PubMed] [Google Scholar]
- 36.Blackburn SD, Shin H, Haining WN, Zou T, Workman CJ, Polley A, Betts MR, Freeman GJ, Vignali DA, Wherry EJ. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat Immunol. 2009;10:29–37. doi: 10.1038/ni.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Matsuzaki J, Gnjatic S, Mhawech-Fauceglia P, Beck A, Miller A, Tsuji T, Eppolito C, Qian F, Lele S, Shrikant P, Old LJ, Odunsi K. Tumor-infiltrating NY-ESO-1-specific CD8+ T cells are negatively regulated by LAG-3 and PD-1 in human ovarian cancer. Proc Natl Acad Sci U S A. 2010;107:7875–7880. doi: 10.1073/pnas.1003345107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Han Y, Yang Y, Chen Z, Jiang Z, Gu Y, Liu Y, Xu S, Lin C, Pan Z, Zhou W, Cao X. Human hepatocellular carcinoma-infiltrating CD4⁺CD69⁺Foxp3⁻ regulatory T cell suppresses T cell response via membrane-bound TGF-β1. J Mol Med (Berl) 2014;92:539–550. doi: 10.1007/s00109-014-1143-4. [DOI] [PubMed] [Google Scholar]
- 39.Brown ZJ, Heinrich B, Steinberg SM, Yu SJ, Greten TF. Safety in treatment of hepatocellular carcinoma with immune checkpoint inhibitors as compared to melanoma and non-small cell lung cancer. J Immunother Cancer. 2017;5:93. doi: 10.1186/s40425-017-0298-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kambhampati S, Bauer KE, Bracci PM, Keenan BP, Behr SC, Gordan JD, Kelley RK. Nivolumab in patients with advanced hepatocellular carcinoma and Child-Pugh class B cirrhosis: Safety and clinical outcomes in a retrospective case series. Cancer. 2019;125:3234–3241. doi: 10.1002/cncr.32206. [DOI] [PubMed] [Google Scholar]
- 41.Mohr R, Jost-Brinkmann F, Özdirik B, Lambrecht J, Hammerich L, Loosen SH, Luedde T, Demir M, Tacke F, Roderburg C. Lessons From Immune Checkpoint Inhibitor Trials in Hepatocellular Carcinoma. Front Immunol. 2021;12:652172. doi: 10.3389/fimmu.2021.652172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Onuma AE, Zhang H, Huang H, Williams TM, Noonan A, Tsung A. Immune Checkpoint Inhibitors in Hepatocellular Cancer: Current Understanding on Mechanisms of Resistance and Biomarkers of Response to Treatment. Gene Expr. 2020;20:53–65. doi: 10.3727/105221620X15880179864121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kalathil SG, Thanavala Y. Natural Killer Cells and T Cells in Hepatocellular Carcinoma and Viral Hepatitis: Current Status and Perspectives for Future Immunotherapeutic Approaches. Cells. 2021;10 doi: 10.3390/cells10061332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kudo M. Limited Impact of Anti-PD-1/PD-L1 Monotherapy for Hepatocellular Carcinoma. Liver Cancer. 2020;9:629–639. doi: 10.1159/000512170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sangro B, Gomez-Martin C, de la Mata M, Iñarrairaegui M, Garralda E, Barrera P, Riezu-Boj JI, Larrea E, Alfaro C, Sarobe P, Lasarte JJ, Pérez-Gracia JL, Melero I, Prieto J. A clinical trial of CTLA-4 blockade with tremelimumab in patients with hepatocellular carcinoma and chronic hepatitis C. J Hepatol. 2013;59:81–88. doi: 10.1016/j.jhep.2013.02.022. [DOI] [PubMed] [Google Scholar]
- 46.Duffy AG, Ma C, Ulahannan SV, Rahma OE, Makarova-Rusher O, Cao L, Yu Y, Kleiner DE, Trepel J, Lee MJ, Tomita Y, Steinberg SM, Heller T, Turkbey B, Choyke PL, Peer CJ, Figg WD, Wood BJ, Greten TF. Phase I and Preliminary Phase II Study of TRC105 in Combination with Sorafenib in Hepatocellular Carcinoma. Clin Cancer Res. 2017;23:4633–4641. doi: 10.1158/1078-0432.CCR-16-3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Crocenzi TS, El-Khoueiry AB, Yau T, Melero I, Sangro B, Kudo M, Hsu C, Trojan J, Kim TY, Choo SP, Meyer T, Kang YK, Yeo W, Chopra A, Baakili A, Cruz CMD, Lang L, Neely J, Welling T. Nivolumab in sorafenib-naive and-experienced patients with advanced hepatocellular carcinoma: CheckMate 040 study. J Clin Oncol. 2017;35 (suppl 15):4013. doi: 10.1016/j.annonc.2023.12.008. [DOI] [PubMed] [Google Scholar]
- 48.Zhu AX, Finn RS, Edeline J, Cattan S, Ogasawara S, Palmer D, Verslype C, Zagonel V, Fartoux L, Vogel A, Sarker D, Verset G, Chan SL, Knox J, Daniele B, Webber AL, Ebbinghaus SW, Ma J, Siegel AB, Cheng AL, Kudo M KEYNOTE-224 investigators. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol. 2018;19:940–952. doi: 10.1016/S1470-2045(18)30351-6. [DOI] [PubMed] [Google Scholar]
- 49.Kelley RK, Abou-Alfa GK, Bendell JC, Kim TY, Borad MJ, Yong WP, Morse M, Kang YK, Rebelatto M, Makowsky M, Xiao F, Morris SR, Sangro B. Phase I/II study of durvalumab and tremelimumab in patients with unresectable hepatocellular carcinoma (HCC): Phase I safety and efficacy analyses. J Clin Oncol. 2017;35 (suppl 15):4073. [Google Scholar]
- 50.Duffy AG, Ulahannan SV, Makorova-Rusher O, Rahma O, Wedemeyer H, Pratt D, Davis JL, Hughes MS, Heller T, ElGindi M, Uppala A, Korangy F, Kleiner DE, Figg WD, Venzon D, Steinberg SM, Venkatesan AM, Krishnasamy V, Abi-Jaoudeh N, Levy E, Wood BJ, Greten TF. Tremelimumab in combination with ablation in patients with advanced hepatocellular carcinoma. J Hepatol. 2017;66:545–551. doi: 10.1016/j.jhep.2016.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, Lim HY, Kudo M, Breder VV, Merle P, Kaseb AO, Li D, Verret W, Shao H, Liu J, Li L, Zhu AX, Cheng AL. IMbrave150: Updated overall survival (OS) data from a global, randomized, open-label phase III study of atezolizumab (atezo)+ bevacizumab (bev) vs sorafenib (sor) in patients (pts) with unresectable hepatocellular carcinoma (HCC) J Clin Oncol. 2021;39 (suppl 3):267. [Google Scholar]
- 52.Gao B, Jeong WI, Tian Z. Liver: An organ with predominant innate immunity. Hepatology. 2008;47:729–736. doi: 10.1002/hep.22034. [DOI] [PubMed] [Google Scholar]
- 53.Kamiya T, Chang YH, Campana D. Expanded and Activated Natural Killer Cells for Immunotherapy of Hepatocellular Carcinoma. Cancer Immunol Res. 2016;4:574–581. doi: 10.1158/2326-6066.CIR-15-0229. [DOI] [PubMed] [Google Scholar]
- 54.Schmeel LC, Schmeel FC, Coch C, Schmidt-Wolf IG. Cytokine-induced killer (CIK) cells in cancer immunotherapy: report of the international registry on CIK cells (IRCC) J Cancer Res Clin Oncol. 2015;141:839–849. doi: 10.1007/s00432-014-1864-3. [DOI] [PubMed] [Google Scholar]
- 55.Cai XR, Li X, Lin JX, Wang TT, Dong M, Chen ZH, Jia CC, Hong YF, Lin Q, Wu XY. Autologous transplantation of cytokine-induced killer cells as an adjuvant therapy for hepatocellular carcinoma in Asia: an update meta-analysis and systematic review. Oncotarget. 2017;8:31318–31328. doi: 10.18632/oncotarget.15454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Magalhaes I, Carvalho-Queiroz C, Hartana CA, Kaiser A, Lukic A, Mints M, Nilsson O, Grönlund H, Mattsson J, Berglund S. Facing the future: challenges and opportunities in adoptive T cell therapy in cancer. Expert Opin Biol Ther. 2019;19:811–827. doi: 10.1080/14712598.2019.1608179. [DOI] [PubMed] [Google Scholar]
- 57.Papaioannou NE, Beniata OV, Vitsos P, Tsitsilonis O, Samara P. Harnessing the immune system to improve cancer therapy. Ann Transl Med. 2016;4:261. doi: 10.21037/atm.2016.04.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sterner RC, Sterner RM. CAR-T cell therapy: current limitations and potential strategies. Blood Cancer J. 2021;11:69. doi: 10.1038/s41408-021-00459-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sun B, Yang D, Dai H, Liu X, Jia R, Cui X, Li W, Cai C, Xu J, Zhao X. Eradication of Hepatocellular Carcinoma by NKG2D-Based CAR-T Cells. Cancer Immunol Res. 2019;7:1813–1823. doi: 10.1158/2326-6066.CIR-19-0026. [DOI] [PubMed] [Google Scholar]
- 60.Ohira M, Nishida S, Levi D, Tekin A, Selvaggi G, Ruiz P. Adoptive immunotherapy using liver natural killer cells for preventing recurrence of hepatocellular carcinoma in cadaveric donor liver transplantation (53.20) Am Assoc Immnol . 2012 [Google Scholar]
- 61.Lee JH, Lee JH, Lim YS, Yeon JE, Song TJ, Yu SJ, Gwak GY, Kim KM, Kim YJ, Lee JW, Yoon JH. Adjuvant immunotherapy with autologous cytokine-induced killer cells for hepatocellular carcinoma. Gastroenterology. 2015;148:1383–91.e6. doi: 10.1053/j.gastro.2015.02.055. [DOI] [PubMed] [Google Scholar]
- 62.Jiang SS, Tang Y, Zhang YJ, Weng DS, Zhou ZG, Pan K, Pan QZ, Wang QJ, Liu Q, He J, Zhao JJ, Li J, Chen MS, Chang AE, Li Q, Xia JC. A phase I clinical trial utilizing autologous tumor-infiltrating lymphocytes in patients with primary hepatocellular carcinoma. Oncotarget. 2015;6:41339–41349. doi: 10.18632/oncotarget.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhai B, Shi D, Gao H, Qi X, Jiang H, Zhang Y, Chi J, Ruan H, Wang H, Ru QC, Li Z. A phase I study of anti-GPC3 chimeric antigen receptor modified T cells (GPC3 CAR-T) in Chinese patients with refractory or relapsed GPC3+ hepatocellular carcinoma (r/r GPC3+ HCC) J Clin Oncol. 2017;35 (suppl 15):3049. [Google Scholar]
- 64.Aranda F, Vacchelli E, Eggermont A, Galon J, Sautès-Fridman C, Tartour E, Zitvogel L, Kroemer G, Galluzzi L. Trial Watch: Peptide vaccines in cancer therapy. Oncoimmunology. 2013;2:e26621. doi: 10.4161/onci.26621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gustafsson K, Ingelsten M, Bergqvist L, Nyström J, Andersson B, Karlsson-Parra A. Recruitment and activation of natural killer cells in vitro by a human dendritic cell vaccine. Cancer Res. 2008;68:5965–5971. doi: 10.1158/0008-5472.CAN-07-6494. [DOI] [PubMed] [Google Scholar]
- 66.Wu Q, Pi L, Le Trinh T, Zuo C, Xia M, Jiao Y, Hou Z, Jo S, Puszyk W, Pham K, Nelson DR, Robertson K, Ostrov D, Rameshwar P, Xia CQ, Liu C. A Novel Vaccine Targeting Glypican-3 as a Treatment for Hepatocellular Carcinoma. Mol Ther. 2017;25:2299–2308. doi: 10.1016/j.ymthe.2017.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sawada Y, Yoshikawa T, Nobuoka D, Shirakawa H, Kuronuma T, Motomura Y, Mizuno S, Ishii H, Nakachi K, Konishi M, Nakagohri T, Takahashi S, Gotohda N, Takayama T, Yamao K, Uesaka K, Furuse J, Kinoshita T, Nakatsura T. Phase I trial of a glypican-3-derived peptide vaccine for advanced hepatocellular carcinoma: immunologic evidence and potential for improving overall survival. Clin Cancer Res. 2012;18:3686–3696. doi: 10.1158/1078-0432.CCR-11-3044. [DOI] [PubMed] [Google Scholar]
- 68.Sun Z, Zhu Y, Xia J, Sawakami T, Kokudo N, Zhang N. Status of and prospects for cancer vaccines against hepatocellular carcinoma in clinical trials. Biosci Trends. 2016;10:85–91. doi: 10.5582/bst.2015.01128. [DOI] [PubMed] [Google Scholar]
- 69.Buonaguro L, Petrizzo A, Tagliamonte M, Tornesello ML, Buonaguro FM. Challenges in cancer vaccine development for hepatocellular carcinoma. J Hepatol. 2013;59:897–903. doi: 10.1016/j.jhep.2013.05.031. [DOI] [PubMed] [Google Scholar]
- 70.Iwashita Y, Tahara K, Goto S, Sasaki A, Kai S, Seike M, Chen CL, Kawano K, Kitano S. A phase I study of autologous dendritic cell-based immunotherapy for patients with unresectable primary liver cancer. Cancer Immunol Immunother. 2003;52:155–161. doi: 10.1007/s00262-002-0360-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Palmer DH, Midgley RS, Mirza N, Torr EE, Ahmed F, Steele JC, Steven NM, Kerr DJ, Young LS, Adams DH. A phase II study of adoptive immunotherapy using dendritic cells pulsed with tumor lysate in patients with hepatocellular carcinoma. Hepatology. 2009;49:124–132. doi: 10.1002/hep.22626. [DOI] [PubMed] [Google Scholar]
- 72.Rizell M, Sternby Eilard M, Andersson M, Andersson B, Karlsson-Parra A, Suenaert P. Phase 1 Trial With the Cell-Based Immune Primer Ilixadencel, Alone, and Combined With Sorafenib, in Advanced Hepatocellular Carcinoma. Front Oncol. 2019;9:19. doi: 10.3389/fonc.2019.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Heo J, Reid T, Ruo L, Breitbach CJ, Rose S, Bloomston M, Cho M, Lim HY, Chung HC, Kim CW, Burke J, Lencioni R, Hickman T, Moon A, Lee YS, Kim MK, Daneshmand M, Dubois K, Longpre L, Ngo M, Rooney C, Bell JC, Rhee BG, Patt R, Hwang TH, Kirn DH. Randomized dose-finding clinical trial of oncolytic immunotherapeutic vaccinia JX-594 in liver cancer. Nat Med. 2013;19:329–336. doi: 10.1038/nm.3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Breitbach CJ, Moon A, Burke J, Hwang TH, Kirn DH. A Phase 2, Open-Label, Randomized Study of Pexa-Vec (JX-594) Administered by Intratumoral Injection in Patients with Unresectable Primary Hepatocellular Carcinoma. Methods Mol Biol. 2015;1317:343–357. doi: 10.1007/978-1-4939-2727-2_19. [DOI] [PubMed] [Google Scholar]
- 75.Abou-Alfa GK, Galle PR, Chao Y, Brown KT, Heo J, Borad MJ, Luca A, Pelusio A, Agathon D, Lusky M, Breitbach C, Burke J, Qin S. PHOCUS: A phase 3 randomized, open-label study comparing the oncolytic immunotherapy Pexa-Vec followed by sorafenib (SOR) vs SOR in patients with advanced hepatocellular carcinoma (HCC) without prior systemic therapy. J Clin Oncol. 2016;34 (suppl 15) [Google Scholar]
- 76.Yau T, Kang Y-K, Kim T-Y, El-Khoueiry AB, Santoro A, Sangro B, Melero I, Kudo M, Hou MM, Matilla A, Tovoli F, Knox JJ, He AR, El-Rayes BF, Acosta-Rivera M, Neely J, Shen Y, Baccan C, Cruz CMD, Hsu C. Nivolumab (NIVO)+ ipilimumab (IPI) combination therapy in patients (pts) with advanced hepatocellular carcinoma (aHCC): Results from CheckMate 040. J Clin Oncol. 2019;37 (suppl 15):4012. [Google Scholar]
- 77.Kim KJ, Kim JH, Lee SJ, Lee EJ, Shin EC, Seong J. Radiation improves antitumor effect of immune checkpoint inhibitor in murine hepatocellular carcinoma model. Oncotarget. 2017;8:41242–41255. doi: 10.18632/oncotarget.17168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chew V, Lee YH, Pan L, Nasir NJM, Lim CJ, Chua C, Lai L, Hazirah SN, Lim TKH, Goh BKP, Chung A, Lo RHG, Ng D, Filarca RLF, Albani S, Chow PKH. Immune activation underlies a sustained clinical response to Yttrium-90 radioembolisation in hepatocellular carcinoma. Gut. 2019;68:335–346. doi: 10.1136/gutjnl-2017-315485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dovedi SJ, Adlard AL, Lipowska-Bhalla G, McKenna C, Jones S, Cheadle EJ, Stratford IJ, Poon E, Morrow M, Stewart R, Jones H, Wilkinson RW, Honeychurch J, Illidge TM. Acquired resistance to fractionated radiotherapy can be overcome by concurrent PD-L1 blockade. Cancer Res. 2014;74:5458–5468. doi: 10.1158/0008-5472.CAN-14-1258. [DOI] [PubMed] [Google Scholar]
- 80.Cummings B, Keane T, Pintilie M, Warde P, Waldron J, Payne D, Liu FF, Bissett R, McLean M, Gullane P, O'Sullivan B. Five year results of a randomized trial comparing hyperfractionated to conventional radiotherapy over four weeks in locally advanced head and neck cancer. Radiother Oncol. 2007;85:7–16. doi: 10.1016/j.radonc.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 81.Chiang CL, Chan ACY, Chiu KWH, Kong FS. Combined Stereotactic Body Radiotherapy and Checkpoint Inhibition in Unresectable Hepatocellular Carcinoma: A Potential Synergistic Treatment Strategy. Front Oncol. 2019;9:1157. doi: 10.3389/fonc.2019.01157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wehrenberg-Klee E, Goyal L, Dugan M, Zhu AX, Ganguli S. Y-90 Radioembolization Combined with a PD-1 Inhibitor for Advanced Hepatocellular Carcinoma. Cardiovasc Intervent Radiol. 2018;41:1799–1802. doi: 10.1007/s00270-018-1993-1. [DOI] [PubMed] [Google Scholar]
- 83.Tang C, Welsh JW, de Groot P, Massarelli E, Chang JY, Hess KR, Basu S, Curran MA, Cabanillas ME, Subbiah V, Fu S, Tsimberidou AM, Karp D, Gomez DR, Diab A, Komaki R, Heymach JV, Sharma P, Naing A, Hong DS. Ipilimumab with Stereotactic Ablative Radiation Therapy: Phase I Results and Immunologic Correlates from Peripheral T Cells. Clin Cancer Res. 2017;23:1388–1396. doi: 10.1158/1078-0432.CCR-16-1432. [DOI] [PMC free article] [PubMed] [Google Scholar]