Abstract
Early-onset colorectal cancer (EOCRC) has seen an alarming rise worldwide over the past two decades. The reason for this global trend is poorly understood. EOCRC appears to have its own unique clinical and molecular features when compared with late-onset colorectal cancer. Younger patients appear to have more distal or rectal disease, a more advanced stage of disease at presentation, and more unfavorable histological features. Identifying risk factors for EOCRC is the first step in mitigating the rising burden of this disease. Here we summarize several noteworthy biological factors and environmental exposures that are postulated to be responsible culprits. This can hopefully translate in clinical practice to the development of better risk stratification tool for identifying high-risk individuals for early colorectal cancer screening, and identifying areas needed for further research to curb this rising trend.
Keywords: Early-onset colorectal cancer, Young-onset colorectal cancer, Risk factors, Environmental exposures, Microbiome, Genetics
Core Tip: The incidence of early onset colorectal cancer is on the rise. Herein, we discuss on various risk factors that have been implicated for these recent trends and point to where future research needs to be directed for better utilization of healthcare resources. Early recognition and diagnosis are essential for better outcomes of this preventable cancer.
INTRODUCTION
Colorectal cancer (CRC) is the third most common cancer and the second most common cause of cancer deaths worldwide. The International Agency for Research on Cancer estimated that there were 1.93 million new cases of CRC and 935000 deaths from CRC in 2020[1]. Early-onset CRC (EOCRC), largely defined as CRC occurring in adults younger than 50 years old, has seen an alarming rising trend in recent years[2-5].
A recent systemic review of 40 studies spanning 12 countries across five continents has found a nearly 30% increase in incidence of EOCRC around the world over the past 20 years, largely driven by increasing incidence in the United States, Australia, and Canada[6]. Since 1994, the incidence of EOCRC has been increasing by around 2% per year. This is alarming given that the overall incidence of and death from CRC has been on the decline[2]. An observational study done on CRC incidence in the United States population according to the Surveillance, Epidemiology, and End Results (SEER) registries found a steep increase in EOCRC incidence from age 49-50 years, with 92.9% of cases being invasive lesions picked up on screening[7]. This likely reflects that a significant proportion of the populations were screened too late, given that the goal of screening was to remove premalignant lesions to prevent malignant transformation. In 2018, the American Cancer Society (ACS) lowered their recommended age for average-risk adults to start screening at 45 years old[8]. Although this method allows early detection of advanced adenomas or CRC to reduce disease burden and mortality, this mass screening approach will likely lead to a substantial increase in cost and burden to the healthcare system.
EOCRC tends to have a predominantly left colonic and rectal distribution, a higher proportion of mucinous and signet ring histologic subtype, poorer cell differentiation, a higher pathologic grade, and a more advanced stage at presentation[9-11]. Although hereditary cancer syndromes and family history account for approximately 30% of EOCRC cases, the majority appear to arise sporadically[12]. To date, the underlying etiologies of this rising trend have not yet been fully elucidated. Identifying specific risk factors or causes to this trend can allow for the establishment of better risk-stratification models and more targeted screening to tackle this global phenomenon.
Multiple postulated risk factors have been identified that may be driving factors to the development of EOCRC. Exposure to many potential elements from an early age from conception to adulthood may predispose to a higher risk of EOCRC. This includes external factors such as socioeconomic background, lifestyle, diet, and antibiotic exposure; and intrinsic factors, such as genetics, gut microbiota, and oxidative stress[13].
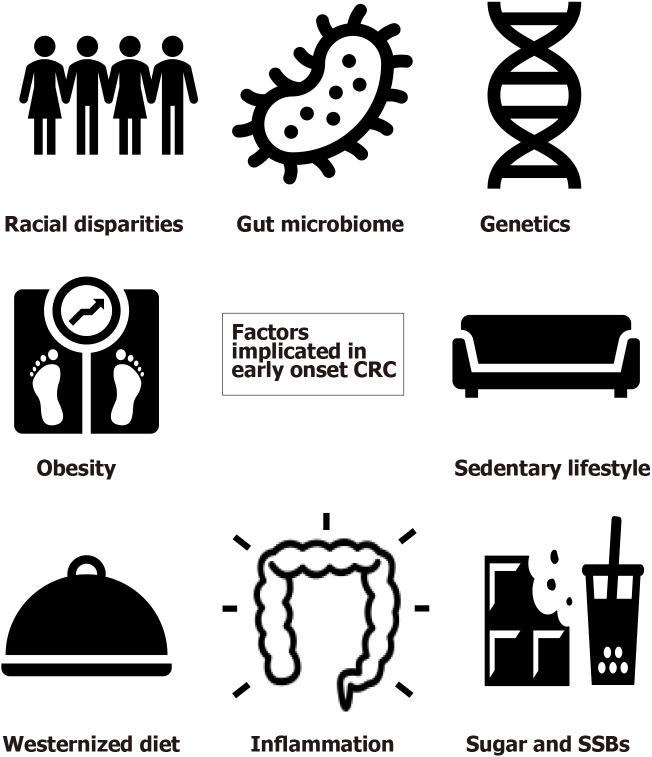
Apart from the well-established risk factors for CRC such as male gender, smoking, alcoholism, family history of CRC, type 2 diabetes, and inflammatory bowel disease, many studies have attempted to study additional demographic and environmental factors that may be specific risk factors for EOCRC[10,14,15]. A meta-analysis examining 20 studies through MEDLINE and Embase database search found that Caucasian ethnicity, obesity, and hyperlipidemia, as well as male gender, alcohol, and history of CRC in a first-degree relative, were all significantly associated with the development of EOCRC[16]. A more sedentary lifestyle or occupation, ulcerative colitis, hypertension, and diet-related factors were also found to have an association with increased risk in some studies[14]. Here we discuss in more detail some of the key suspects implicated in the development of EOCRC (Figure 1).
Figure 1.
Factors implicated in early-onset colorectal cancer. Image source for colon: https://img.icons8.com. CRC: Colorectal cancer; SSB: Sugar sweetened beverages.
RACIAL DISPARITIES
African Americans have been known to be at higher risk for the development of CRC compared with Caucasians, and this is usually associated with an earlier-onset and worse outcome[17]. Potential reasons for this disparity include lower socioeconomic status, limited access to healthcare, and lack of awareness of screening. Steps have been taken over the years to close this gap in CRC risk with the American College of Gastroenterology and American Society of Gastrointestinal Endoscopists guidelines recommending an earlier age to start CRC screening for African Americans[18].
These efforts have led to tangible results with the gap closing between Whites and Blacks[19]. In fact, the incidence of rectal cancer in Whites has now surmounted that of the Blacks and Hispanics in recent years, and the overall incidence of EOCRC is now similar in the two groups since 2015[2]. Results of a SEER analysis examining the difference in incidence of CRC amongst White and Black EOCRC patients from 1992-1996 to 2010-2014 showed that there was a 47% relative increase in CRC incidence in Whites, compared to a 1% relative increase in Blacks[20]. The rise in EOCRC is mainly due to an increase in rectal cancer, which was seen most strikingly in the White population. This suggests that rectal cancer may have its own distinct characteristics and etiological differences from colon cancer. Nevertheless, the incidence of EOCRC is still climbing steadily regardless of ethnicity, highlighting the need for further research into meaningful interventions to curb this rise.
OBESITY AND SEDENTARY LIFESTYLE
Obesity has long been associated with an increased risk of CRC[21]. According to a recent propensity-weighted analysis which included 133008 adults diagnosed with EOCRC in the United States between 1999 and 2018, there was a strong association between EOCRC and a raised body mass index (BMI) of ≥ 30 kg/m2, along with an earlier age of diabetes diagnosis[22]. A meta-analysis in 2017 found a 30% increased risk of CRC in men and a 12% increased risk of CRC in women for every 5 kg/m2 increment increase in BMI[23]. There is also an increased risk of early-onset advanced adenoma amongst obese patients[24]. The underlying mechanism behind the association between obesity and EOCRC is unclear, although it is postulated that there is an interplay between the risk of obesity, estrogen levels, and the risk of CRC, with obesity being a driver of chronic inflammation[25,26].
Of course, there are multiple confounding variables that may affect the relationship between obesity and EOCRC. This includes a reverse causality effect where CRC may induce weight loss. Obesity itself could also be a surrogate for other known risk factors for CRC. Metabolic syndrome, increased insulin resistance, raised insulin-like growth factor 1 (IGF-1), and raised low-density lipoprotein are all positively correlated with an increased risk for EOCRC[21,24].
Leading a sedentary lifestyle has also been recognized as an emerging global health problem due to increased desk work, the rising trend of e-commerce, and inactive media consumption since a young age[27]. A prospective study examining television viewing time (as a surrogate for sedentary time) in almost 90000 women aged 25 to 42 years in the United States found that more than 1 hour of daily TV viewing was associated with a 12% increased risk of CRC, particularly rectal cancer. More than 2 hours of TV viewing was associated with a 70% increase in risk. The risk appeared even higher in subgroups of patients with a high BMI, physical inactivity, and smokers[28].
Physical inactivity may result in lower energy use, higher caloric intake, and unhealthy dietary intake. It may also correlate with impaired glucose regulation or gut dysbiosis. Some studies have examined the role of increased physical activity to improve gut health by promoting certain bacterial species in the gut microbiome[29-31]. All in all, this highlights the importance of physical activity and controlling the obesity pandemic to prevent EOCRC.
WESTERN DIET
A growing adoption of a non-Mediterranean, Western diet worldwide has been consistently shown in the literature to be an important risk factor[32,33]. A diet high in red, processed meat, and low in fibre from a young age has been shown to affect the gut microbiota and drive inflammation processes[34-36]. Westernized cooking methods, such as deep-frying, grilling, or roasting, generate more advanced glycation end-products (AGEs), which are complex compounds produced from food that is rich in fat and protein[37,38]. They are involved in promoting oxidative stress and chronic inflammation, which in turn promote a microenvironment favorable for colorectal carcinogenesis. Many studies have shown that AGEs are responsible for signal pathways involved in colitis-associated colorectal carcinogenesis seen in inflammatory bowel disease[39]. Mediterranean food, on the other hand, has low AGE levels and has been found to be protective against the development of CRC[40-42].
A recent prospective cohort study, which examined dietary patterns in 29474 women who underwent colonoscopy at < 50 years of age, found that a Westernized diet was positively associated with high-risk distal or rectal adenomas, whereas healthier diets such as a prudent diet, Dietary Approaches to Stop Hypertension, Alternative Mediterranean Diet, and Alternative Healthy Eating Index were inversely associated with early onset adenomas[43]. Interestingly, some studies have found that the genetic composition of tumors associated with a Western diet tends to be KRAS wild-type, and BRAF-wild type[44]. These genetic compositions are consistent with the typical features of EOCRC.
A meta-analysis recently published suggested a strong association of higher intake of dietary fibre, calcium, and yoghurt with a reduced risk of CRC, with convincing evidence that intake of a Western diet and processed meat is associated with a higher risk of EOCRC[45]. Interestingly, the impact of yoghurt and calcium may be related with the modulation of the gut microbiome, such as the presence of lactic acid-producing bacteria, which may reduce the level of carcinogens in the gut. Yoghurt also creates a lower pH in the colon, which may be more accommodating for probiotics[46]. This supports the idea that modulating the gut microbiome with prebiotics and/or probiotics may have a potential role in preventing the development of CRC, which will be further discussed in a later section.
SUGAR
One of the other culprits in the plethora of Western food that may be a culprit for EOCRC is sugar. Refined sugars (including glucose, fructose, sucrose, and maltose) are cheap and widely available worldwide. Sugar consumption in the form of snacks, desserts, sweets, or sugar-sweetened beverages has steeply increased especially during childhood and adolescence. Over the last decade, sugar consumption globally has grown from 154 to 171 million metric tons from 2009/2010 to 2019/2020[47]. This climb was found to be most significant in developing or low-income countries[48]. In a large United States cohort study that analyzed 95464 female registered nurses’ dietary habits from the Nurses’ Health Study II, it was found that high sugar (especially fructose) intake during adolescence was significantly associated with an increased risk of colorectal adenomas. Consuming two or more, rather than one, sugar-sweetened beverages a day in adolescence further increased the risk of EOCRC by two-fold[49].
Several mechanisms that tie sugar intake to the development of CRC have been postulated. High intake of sugar can promote obesity, insulin resistance, and type 2 diabetes[50,51]. Sugar, specifically fructose, may have a direct effect on the gut microbiome, leading to chronic inflammation and a heightened susceptibility of the colorectal epithelium to cellular damage[52]. Fructose also produces AGEs, which as previously discussed, has a potentially significant role in carcinogenesis[53]. Hyperinsulinemia and elevated IGF-1 levels can stimulate cell proliferation and differentiation, inhibit apoptosis, and in turn enhance tumor development. As adolescence is a period of pronounced physiological changes that include decreased insulin sensitivity and hyperinsulinemia, this stage of development may be particularly susceptible to the effects of a high sugar intake[49].
The link between diet, nutrients, and the pathogenesis of EOCRC is complex, with a myriad of processes involving immune signaling, genetic predisposition, and alterations in the gut microbiome. Other significant food exposures that may play a role in CRC include dietary additives, nitrate-containing foods, synthetic food colorings, monosodium glutamate, etc.[13,54]. Further studies on dietary causation links will bring to light any potential preventative measures for EOCRC.
GUT MICROBIOME
It is estimated that 100 billion bacteria reside in the gastrointestinal tract (with a large proportion present in the colon), maintaining a symbiotic relationship with the human host[55]. The gut microbiota maintains gut homeostasis and functions and is often considered the first line of defense against pathogens. The composition of the gut microbiome is dynamic and subject to change by multiple factors throughout our lives. The first 1-2 years of life are pivotal for the development of the gut microbiota[56]. From birth, the microbiota composition is believed to differ significantly depending on the mode of delivery. Vaginally delivered babies tend to have more Lactobacilli, whereas Caesarean-delivered babies tend to have delayed colonization of facultative anaerobes such as Clostridium[57]. Breast-fed and bottle-fed babies also have markedly different gut microbiota composition, with breastfed babies having a much higher abundance of bacteria that are thought to be beneficial, such as Bifidobacterium and Lactobacillus species[58]. The composition of the gut microbiota stabilizes in early adulthood, but is still influenced by exposures such as diet, antibiotics, stress, and inflammation. The gut microbiome is responsible for the synthesis of many important vitamins or molecules for our human body, such as butyrate, folate, biotin, and cobalamin[59]. Some of these molecules are important in reducing bacterial translocation and promoting anti-inflammatory properties, and are essential in maintaining gut barrier integrity[60].
Alterations of gut microbiome composition (or gut dysbiosis) can lead to dysregulation of multiple pathways in the body. Extensive or prolonged antibiotics use can destroy normal gut flora and lead to colonization of unwelcome pathogens. Several microorganisms, such as Streptococcus bovis, Bacterioides fragilis, Salmonella enterica, Fusobacterium, and Escherichia coli, have been discovered to have a role in colon carcinogenesis. These pathogens can promote gut inflammation, produce cancer-associated metabolites, and activate oncogenic signaling pathways[61]. Chronic inflammation from bacterial infection or inflammatory bowel disease can cause epithelial barrier dysfunction and weaken host defenses. Different dietary exposures can lead to significant shifts in the gut microbiome, favoring organisms capable of utilizing those specific nutrients. High-fat diets can lead to accumulation of lipopolysaccharides that can promote inflammation and increase VEGF-C expression, which is a key regulator for lymphangiogenesis and lymph node metastasis in CRC[62]. One study found that a drastic increase in fibre intake over 2 wk led to a change in microbiome composition to fibre-degrading bacteria, such as Bifidobacterium and Lactobacillus, which has been associated with anti-oncogenic properties[63-65].
Probiotics have long been marketed to the general public as a dietary supplement for their potential beneficial effects on the gut[66]. The replenishment of beneficial intestinal microbial communities may help stimulate epithelial cell proliferation, reduce pathogenic overgrowth, ameliorate gut inflammation, and potentially reduce the risk of CRC[67-69]. Studies have also shown that certain strains of probiotics may be effective as an adjuvant agent to CRC treatment[70]. Yet, its effects specifically on CRC treatment are not well studied and further investigation is required.
Our diet from birth has a role in shaping our gut microbiome. Understanding the relationship between diet and gut dysbiosis teaches us that how we shape our diets at an early age could impact the development of CRC. Thus, it is important to encourage healthy eating habits from childhood to maintain a healthy microbiota. Nevertheless, it remains difficult to prove the causative link between dysbiosis in early human development and its association with EOCRC, and further research in this area is needed.
GENETIC FEATURES
Recognizing genetic alterations that can predispose to early onset of high-risk adenoma or CRC is crucial for deciding on early screening regimes and therapeutic strategies. Around 28% of EOCRC patients have a positive family history[71]. Patients with a first-degree relative of CRC have up to a four-fold increased lifetime risk of CRC[72]. Those with a known family history of a high-penetrance hereditary cancer syndrome, such as Lynch syndrome or adenomatous polyposis coli (APC), are at a particularly high risk and require an onset of colonoscopy screening at a much earlier age than the general population[73,74]. For non-hereditary cases, according to the ACS guidelines, those with a first-degree relative of CRC diagnosed before age 60 should also start colonoscopy screening from age 40, or 10 years younger than the earliest diagnosed relative[72]. However, low adherence to early screening guidelines is one of the major obstacles in EOCRC prevention. A study of 2473 patients with EOCRC found that family history-based early screening criteria were only adhered to in 25% of cases, and nearly all these patients could have had CRC diagnosed earlier or even prevented had they followed these guidelines[75]. This highlights the importance of public education on cancer screening programs.
Several studies have found that a significant proportion of EOCRC patients carrying a genetic mutation have no family history of CRC[10,71]. Apart from the well-recognized hereditary cancer syndromes accounting for around 13% of EOCRC cases, a wide spectrum of low to moderate penetrance sporadic mutations have recently been found in these patients, including some genes not traditionally associated with CRC[71,76]. A genome-wide association study found up to 140 single nucleotide polymorphisms associated with CRC[77]. Genetic mutations are much more common in EOCRC compared with those diagnosed at a later age[78] and may have a cumulative effect. However, in the absence of a positive family history, a proportion of these patients will not be enrolled into early screening programs with strategies to identify such patients being an unmet need[79].
The pathogenesis of CRC involves a complex sequence of multistep genetic alterations. There are three main genetic pathways of CRC carcinogenesis: Chromosomal instability (CIN), microsatellite instability (MSI), and CpG island methylator phenotype (CIMP) pathways[80]. Each pathway is associated with specific genetic and epigenetic alterations. The CIN pathway is characterized by an accumulation of mutations in the tumor-suppressor and oncogenes, including APC, KRAS, and TP53 amongst others, accounting for 85% of sporadic CRC cases. The MSI pathway, on the other hand, is a state of genetic hypermutability due to impaired DNA mismatch repair (MMR). MSI is the hallmark of Lynch syndrome-associated tumors, an autosomal dominant disorder characterized by the presence of DNA MMR genes (e.g., MLH1, MSH2, MSH6, and PMS2), accounting for around 8% of EOCRC cases[71,81]. Lynch syndrome increases the lifetime risk of CRC to 52%-82% depending on the pathogenic variant involved[82]. The CIMP pathway and BRAF V600E mutation are thought to be the molecular hallmark of the serrated pathway and are usually associated with proximal lesions[83].
EOCRC has distinct genetic features compared with late-onset CRC. A retrospective review of around 36000 CRC patients comparing genetic characteristics in different age groups showed that EOCRC patients are more likely to be MSI and have CTNNB1, ATM mutations, and CIMP hypermethylation. The consensus molecular subtype 1 was the most common CRC subtype in patients younger than 40 years old. There were fewer BRAF V600 mutations (< 4%) in patients less than 30 years old. KRAS, NRAS, and BRAF mutations in the mitogen-activated protein kinase pathway were lowest in the 18-29-year-old group (48%), and highest in the 70-year-old or older group (65%-70%)[84]. Hypermethylation of ESR1, GATA5, and WT1 genes were also found to be suggestive of earlier diagnosis of CRC[85].
Certain genetic mutations may infer a higher rate of progression or be predictive factors for treatment resistance. KRAS mutation confers resistance to anti-EGFR therapy. Several studies have demonstrated that MSI tumors have a lack of response to 5FU-based chemotherapy[86]. Given that around 1 in 5 patients with EOCRC have a germline mutation, broad germline testing should be considered for all EOCRC patients to guide treatment modalities, prognostication, counselling to family members, and chemoprevention strategies[76,87].
Establishing a good predictive model for risk stratification of many genetic variants predisposing to CRC is important for more targeted screening of high-risk patients. A study using a polygenic risk score (PRS) derived from 95 common genetic variants was able to predict the risk of EOCRC when testing 12197 early-onset CRC and 95865 late-onset CRC patients of European descent. A higher PRS is more strongly associated with EOCRC than late-onset patients. Those in the highest PRS quartile had a 3.7-fold increased risk of EOCRC compared with those in the lowest quartile. Interestingly, high PRS cases also had a tendency towards distal and rectal tumors[78]. PRS may therefore be a useful tool to stratify risk when used alongside the identification of other lifestyle and environmental risk factors, and may pick up some high risk patients within the average-risk screening group who would otherwise have not been identified based on conventional criteria. This may provide a more targeted and personalized approach for CRC screening than our current standard of care.
CONCLUSION
CRC is a genetic and molecularly heterogeneous disease. EOCRC represents a subgroup of CRC with unique characteristics. Genetic predisposition and multiple risk factors are being explored as potential contributors to this rising trend. Given the long process of transition from non-neoplastic cells to malignancy, exploring early-life exposures as potential culprits is important[13]. Increasing evidence has shown that obesity, sedentary lifestyle, Westernized diet, and high sugar intake are significant risk factors for EOCRC. Exposures as early as in the prenatal or perinatal stages of life, such as maternal diet or delivery methods, have been postulated to affect the composition of the gut microbiota. However, studies that prove causality remain elusive. Large epidemiological studies are still needed to further discover or verify potential causative factors.
The relationship between diet, lifestyle, and gut dysbiosis and their respective roles in colorectal carcinogenesis are complex. The composition of gut microbiome is dynamic and dependent on multiple factors including race, age, lifestyle, diet, medication use, stress, etc. There is currently no clear consensus for the definition of gut dysbiosis due to the high microbial heterogeneity in CRC[88]. Further investigations on the gut microenvironment from stool samples in CRC patients may help characterize the gut microbiome that predisposes to CRC, with emerging evidence that shows promise for its use in CRC screening and risk stratification. Future research on manipulating the gut microbiome through diet or drugs like probiotics may even play a role in cancer prevention.
Apart from the need for further research on exploring the unanswered questions of the underlying cause and mechanisms behind EOCRC, numerous barriers to the reduction of the incidence of EOCRC still exist. Poor compliance with early screening programs may be due to inadequate public awareness[89]. Information on family history may not be known to patients. Young patients and physicians alike tend to attribute early symptoms to non-sinister pathologies that may result in a delay of diagnosis. A study of young patients has shown that they present to a medical practitioner on average 294 d after the onset of rectal bleeding, which likely resulted in a more advanced stage of disease[90]. With regard to healthcare systems, there may be access, cost, or policy barriers to screening and treatment.
Steps to fight EOCRC include raising awareness of this growing threat through education and public promotion. This includes public awareness campaigns, educating the public on the dietary or lifestyle risks of CRC, and enhancing physician awareness of EOCRC. Advising young patients to stay vigilant of early symptoms, such as per-rectal bleeding, abdominal pain, weight loss, and change in bowel habits and to seek timely medical attention is also important. Promoting awareness of early colonoscopy screening for high-risk groups, and referring patients who are eligible for genetic counselling and testing are essential for early identification of at-risk individuals. Further research on predisposing genetic and epigenetic signatures is needed. In the future, we should strive for specific genetic profiling through whole-genome sequencing for better risk stratification[91]. It may be useful to see how well specific risk stratification tools including lifestyle risks or PRS perform in the real world to identify high-risk patients for a more personalized screening strategy, which in turn may allow for better allocation of resources to those most in need to combat this global rise in EOCRC.
Footnotes
Conflict-of-interest statement: All authors have no conflicts of interest to disclose.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: June 16, 2021
First decision: July 29, 2021
Article in press: November 24, 2021
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Sacdalan DL S-Editor: Yan JP L-Editor: Wang TQ P-Editor: Yan JP
Contributor Information
Claudia Wing-Kwan Wu, Division of Gastroenterology and Hepatology, Department of Medicine and Therapeutics, The Chinese University of Hong Kong, Hong Kong, China.
Rashid N Lui, Division of Gastroenterology and Hepatology, Department of Medicine and Therapeutics, The Chinese University of Hong Kong, Hong Kong, China; Department of Clinical Oncology, The Chinese University of Hong Kong, Hong Kong, China. rashidlui@cuhk.edu.hk.
References
- 1.Cancer Today. Global Cancer Observatory. International Agency for Research on Cancer. Available from: https://gco.iarc.fr/today/fact-sheets-cancers .
- 2.Surveillance , Epidemiology , and End Results. Cancer of the Colon and Rectum - Cancer Stat Facts. Available from: https://seer.cancer.gov/statfacts/html/colorect.html .
- 3.Siegel RL, Torre LA, Soerjomataram I, Hayes RB, Bray F, Weber TK, Jemal A. Global patterns and trends in colorectal cancer incidence in young adults. Gut. 2019;68:2179–2185. doi: 10.1136/gutjnl-2019-319511. [DOI] [PubMed] [Google Scholar]
- 4.Lui RN, Tsoi KKF, Ho JMW, Lo CM, Chan FCH, Kyaw MH, Sung JJY. Global Increasing Incidence of Young-Onset Colorectal Cancer Across 5 Continents: A Joinpoint Regression Analysis of 1,922,167 Cases. Cancer Epidemiol Biomarkers Prev. 2019;28:1275–1282. doi: 10.1158/1055-9965.EPI-18-1111. [DOI] [PubMed] [Google Scholar]
- 5.Sung JJY, Chiu HM, Jung KW, Jun JK, Sekiguchi M, Matsuda T, Kyaw MH. Increasing Trend in Young-Onset Colorectal Cancer in Asia: More Cancers in Men and More Rectal Cancers. Am J Gastroenterol. 2019;114:322–329. doi: 10.14309/ajg.0000000000000133. [DOI] [PubMed] [Google Scholar]
- 6.Saad El Din K, Loree JM, Sayre EC, Gill S, Brown CJ, Dau H, De Vera MA. Trends in the epidemiology of young-onset colorectal cancer: a worldwide systematic review. BMC Cancer. 2020;20:288. doi: 10.1186/s12885-020-06766-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abualkhair WH, Zhou M, Ahnen D, Yu Q, Wu XC, Karlitz JJ. Trends in Incidence of Early-Onset Colorectal Cancer in the United States Among Those Approaching Screening Age. JAMA Netw Open. 2020;3:e1920407. doi: 10.1001/jamanetworkopen.2019.20407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peterse EFP, Meester RGS, Siegel RL, Chen JC, Dwyer A, Ahnen DJ, Smith RA, Zauber AG, Lansdorp-Vogelaar I. The impact of the rising colorectal cancer incidence in young adults on the optimal age to start screening: Microsimulation analysis I to inform the American Cancer Society colorectal cancer screening guideline. Cancer. 2018;124:2964–2973. doi: 10.1002/cncr.31543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O'Connell JB, Maggard MA, Liu JH, Etzioni DA, Livingston EH, Ko CY. Rates of colon and rectal cancers are increasing in young adults. Am Surg. 2003;69:866–872. [PubMed] [Google Scholar]
- 10.Mauri G, Sartore-Bianchi A, Russo AG, Marsoni S, Bardelli A, Siena S. Early-onset colorectal cancer in young individuals. Mol Oncol. 2019;13:109–131. doi: 10.1002/1878-0261.12417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khan M, Korphaisarn K, Saif A, Foo WC, Kopetz S. Early-Onset Signet-Ring Cell Adenocarcinoma of the Colon: A Case Report and Review of the Literature. Case Rep Oncol Med. 2017;2017:2832180. doi: 10.1155/2017/2832180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wells K, Wise PE. Hereditary Colorectal Cancer Syndromes. Surg Clin North Am. 2017;97:605–625. doi: 10.1016/j.suc.2017.01.009. [DOI] [PubMed] [Google Scholar]
- 13.Hofseth LJ, Hebert JR, Chanda A, Chen H, Love BL, Pena MM, Murphy EA, Sajish M, Sheth A, Buckhaults PJ, Berger FG. Early-onset colorectal cancer: initial clues and current views. Nat Rev Gastroenterol Hepatol. 2020;17:352–364. doi: 10.1038/s41575-019-0253-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rawla P, Sunkara T, Barsouk A. Epidemiology of colorectal cancer: incidence, mortality, survival, and risk factors. Prz Gastroenterol. 2019;14:89–103. doi: 10.5114/pg.2018.81072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang Y, Ben Q, Shen H, Lu W, Zhang Y, Zhu J. Diabetes mellitus and incidence and mortality of colorectal cancer: a systematic review and meta-analysis of cohort studies. Eur J Epidemiol. 2011;26:863–876. doi: 10.1007/s10654-011-9617-y. [DOI] [PubMed] [Google Scholar]
- 16.O'Sullivan DE, Sutherland RL, Town S, Chow K, Fan J, Forbes N, Heitman SJ, Hilsden RJ, Brenner DR. Risk Factors for Early-Onset Colorectal Cancer: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2021 doi: 10.1016/j.cgh.2021.01.037. [DOI] [PubMed] [Google Scholar]
- 17.Carethers JM. Screening for colorectal cancer in African Americans: determinants and rationale for an earlier age to commence screening. Dig Dis Sci. 2015;60:711–721. doi: 10.1007/s10620-014-3443-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shaukat A, Kahi CJ, Burke CA, Rabeneck L, Sauer BG, Rex DK. ACG Clinical Guidelines: Colorectal Cancer Screening 2021. Am J Gastroenterol. 2021;116:458–479. doi: 10.14309/ajg.0000000000001122. [DOI] [PubMed] [Google Scholar]
- 19.Muller C, Ihionkhan E, Stoffel EM, Kupfer SS. Disparities in Early-Onset Colorectal Cancer. Cells. 2021;10 doi: 10.3390/cells10051018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murphy CC, Wallace K, Sandler RS, Baron JA. Racial Disparities in Incidence of Young-Onset Colorectal Cancer and Patient Survival. Gastroenterology. 2019;156:958–965. doi: 10.1053/j.gastro.2018.11.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ma Y, Yang Y, Wang F, Zhang P, Shi C, Zou Y, Qin H. Obesity and risk of colorectal cancer: a systematic review of prospective studies. PLoS One. 2013;8:e53916. doi: 10.1371/journal.pone.0053916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hussan H, Patel A, Hinton A, Ma Q, Tabung , Fred KT, Clinton S. The Associations Between Obesity and Early Onset Colorectal Cancer: A Propensity-Weighted Analysis of the National Health and Nutrition Examination Survey (NHANES) Am J Gastroenterol. 2020:115. [Google Scholar]
- 23.Kyrgiou M, Kalliala I, Markozannes G, Gunter MJ, Paraskevaidis E, Gabra H, Martin-Hirsch P, Tsilidis KK. Adiposity and cancer at major anatomical sites: umbrella review of the literature. BMJ. 2017;356:j477. doi: 10.1136/bmj.j477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim JY, Jung YS, Park JH, Kim HJ, Cho YK, Sohn CI, Jeon WK, Kim BI, Choi KY, Park DI. Different risk factors for advanced colorectal neoplasm in young adults. World J Gastroenterol. 2016;22:3611–3620. doi: 10.3748/wjg.v22.i13.3611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu PH, Wu K, Ng K, Zauber AG, Nguyen LH, Song M, He X, Fuchs CS, Ogino S, Willett WC, Chan AT, Giovannucci EL, Cao Y. Association of Obesity With Risk of Early-Onset Colorectal Cancer Among Women. JAMA Oncol. 2019;5:37–44. doi: 10.1001/jamaoncol.2018.4280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ye P, Xi Y, Huang Z, Xu P. Linking Obesity with Colorectal Cancer: Epidemiology and Mechanistic Insights. Cancers (Basel) 2020;12 doi: 10.3390/cancers12061408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park JH, Moon JH, Kim HJ, Kong MH, Oh YH. Sedentary Lifestyle: Overview of Updated Evidence of Potential Health Risks. Korean J Fam Med. 2020;41:365–373. doi: 10.4082/kjfm.20.0165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nguyen LH, Liu PH, Zheng X, Keum N, Zong X, Li X, Wu K, Fuchs CS, Ogino S, Ng K, Willett WC, Chan AT, Giovannucci EL, Cao Y. Sedentary Behaviors, TV Viewing Time, and Risk of Young-Onset Colorectal Cancer. JNCI Cancer Spectr. 2018;2:pky073. doi: 10.1093/jncics/pky073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brennan CA, Garrett WS. Gut Microbiota, Inflammation, and Colorectal Cancer. Annu Rev Microbiol. 2016;70:395–411. doi: 10.1146/annurev-micro-102215-095513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bressa C, Bailén-Andrino M, Pérez-Santiago J, González-Soltero R, Pérez M, Montalvo-Lominchar MG, Maté-Muñoz JL, Domínguez R, Moreno D, Larrosa M. Differences in gut microbiota profile between women with active lifestyle and sedentary women. PLoS One. 2017;12:e0171352. doi: 10.1371/journal.pone.0171352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mach N, Fuster-Botella D. Endurance exercise and gut microbiota: A review. J Sport Health Sci. 2017;6:179–197. doi: 10.1016/j.jshs.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Castelló A, Amiano P, Fernández de Larrea N, Martín V, Alonso MH, Castaño-Vinyals G, Pérez-Gómez B, Olmedo-Requena R, Guevara M, Fernandez-Tardon G, Dierssen-Sotos T, Llorens-Ivorra C, Huerta JM, Capelo R, Fernández-Villa T, Díez-Villanueva A, Urtiaga C, Castilla J, Jiménez-Moleón JJ, Moreno V, Dávila-Batista V, Kogevinas M, Aragonés N, Pollán M MCC-Spain researchers. Low adherence to the western and high adherence to the mediterranean dietary patterns could prevent colorectal cancer. Eur J Nutr. 2019;58:1495–1505. doi: 10.1007/s00394-018-1674-5. [DOI] [PubMed] [Google Scholar]
- 33.Feng YL, Shu L, Zheng PF, Zhang XY, Si CJ, Yu XL, Gao W, Zhang L. Dietary patterns and colorectal cancer risk: a meta-analysis. Eur J Cancer Prev. 2017;26:201–211. doi: 10.1097/CEJ.0000000000000245. [DOI] [PubMed] [Google Scholar]
- 34.Albracht-Schulte K, Islam T, Johnson P, Moustaid-Moussa N. Systematic Review of Beef Protein Effects on Gut Microbiota: Implications for Health. Adv Nutr. 2021;12:102–114. doi: 10.1093/advances/nmaa085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Larsson SC, Wolk A. Meat consumption and risk of colorectal cancer: a meta-analysis of prospective studies. Int J Cancer. 2006;119:2657–2664. doi: 10.1002/ijc.22170. [DOI] [PubMed] [Google Scholar]
- 36.Chan DS, Lau R, Aune D, Vieira R, Greenwood DC, Kampman E, Norat T. Red and processed meat and colorectal cancer incidence: meta-analysis of prospective studies. PLoS One. 2011;6:e20456. doi: 10.1371/journal.pone.0020456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang Q, Wang Y, Fu L. Dietary advanced glycation end-products: Perspectives linking food processing with health implications. Compr Rev Food Sci Food Saf. 2020;19:2559–2587. doi: 10.1111/1541-4337.12593. [DOI] [PubMed] [Google Scholar]
- 38.Omofuma OO, Turner DP, Peterson LL, Merchant AT, Zhang J, Steck SE. Dietary Advanced Glycation End-products (AGE) and Risk of Breast Cancer in the Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial (PLCO) Cancer Prev Res (Phila) 2020;13:601–610. doi: 10.1158/1940-6207.CAPR-19-0457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Azizian-Farsani F, Abedpoor N, Hasan Sheikhha M, Gure AO, Nasr-Esfahani MH, Ghaedi K. Receptor for Advanced Glycation End Products Acts as a Fuel to Colorectal Cancer Development. Front Oncol. 2020;10:552283. doi: 10.3389/fonc.2020.552283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lopez-Moreno J, Quintana-Navarro GM, Delgado-Lista J, Garcia-Rios A, Delgado-Casado N, Camargo A, Perez-Martinez P, Striker GE, Tinahones FJ, Perez-Jimenez F, Lopez-Miranda J, Yubero-Serrano EM. Mediterranean Diet Reduces Serum Advanced Glycation End Products and Increases Antioxidant Defenses in Elderly Adults: A Randomized Controlled Trial. J Am Geriatr Soc. 2016;64:901–904. doi: 10.1111/jgs.14062. [DOI] [PubMed] [Google Scholar]
- 41.Farinetti A, Zurlo V, Manenti A, Coppi F, Mattioli AV. Mediterranean diet and colorectal cancer: A systematic review. Nutrition. 2017;43-44:83–88. doi: 10.1016/j.nut.2017.06.008. [DOI] [PubMed] [Google Scholar]
- 42.Grosso G, Biondi A, Galvano F, Mistretta A, Marventano S, Buscemi S, Drago F, Basile F. Factors associated with colorectal cancer in the context of the Mediterranean diet: a case-control study. Nutr Cancer. 2014;66:558–565. doi: 10.1080/01635581.2014.902975. [DOI] [PubMed] [Google Scholar]
- 43.Zheng X, Hur J, Nguyen LH, Liu J, Song M, Wu K, Smith-Warner SA, Ogino S, Willett WC, Chan AT, Giovannucci E, Cao Y. Comprehensive Assessment of Diet Quality and Risk of Precursors of Early-Onset Colorectal Cancer. J Natl Cancer Inst. 2021;113:543–552. doi: 10.1093/jnci/djaa164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mehta RS, Song M, Nishihara R, Drew DA, Wu K, Qian ZR, Fung TT, Hamada T, Masugi Y, da Silva A, Shi Y, Li W, Gu M, Willett WC, Fuchs CS, Giovannucci EL, Ogino S, Chan AT. Dietary Patterns and Risk of Colorectal Cancer: Analysis by Tumor Location and Molecular Subtypes. Gastroenterology. 2017;152:1944–1953.e1. doi: 10.1053/j.gastro.2017.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Veettil SK, Wong TY, Loo YS, Playdon MC, Lai NM, Giovannucci EL, Chaiyakunapruk N. Role of Diet in Colorectal Cancer Incidence: Umbrella Review of Meta-analyses of Prospective Observational Studies. JAMA Netw Open. 2021;4:e2037341. doi: 10.1001/jamanetworkopen.2020.37341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zheng X, Wu K, Song M, Ogino S, Fuchs CS, Chan AT, Giovannucci EL, Cao Y, Zhang X. Yogurt consumption and risk of conventional and serrated precursors of colorectal cancer. Gut. 2020;69:970–972. doi: 10.1136/gutjnl-2019-318374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shahbandeh M. Global Sugar Consumption, 2020/21. Statista. 2021 May 27. Available from: https://www.statista.com/statistics/249681/total-consumption-of-sugar-worldwide/
- 48.OECD iLibrary. OECD-FAO Agricultural Outlook 2019-2028. OECD Publishing. 2019. Available from: https://www.oecd-ilibrary.org/agriculture-and-food/oecd-fao-agricultural-outlook-2019-2028_bdef14fa-en .
- 49.Hur J, Otegbeye E, Joh HK, Nimptsch K, Ng K, Ogino S, Meyerhardt JA, Chan AT, Willett WC, Wu K, Giovannucci E, Cao Y. Sugar-sweetened beverage intake in adulthood and adolescence and risk of early-onset colorectal cancer among women. Gut. 2021 doi: 10.1136/gutjnl-2020-323450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Slattery ML, Benson J, Berry TD, Duncan D, Edwards SL, Caan BJ, Potter JD. Dietary sugar and colon cancer. Cancer Epidemiol Biomarkers Prev. 1997;6:677–685. [PubMed] [Google Scholar]
- 51.Malik VS, Popkin BM, Bray GA, Després JP, Hu FB. Sugar-sweetened beverages, obesity, type 2 diabetes mellitus, and cardiovascular disease risk. Circulation. 2010;121:1356–1364. doi: 10.1161/CIRCULATIONAHA.109.876185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhao S, Jang C, Liu J, Uehara K, Gilbert M, Izzo L, Zeng X, Trefely S, Fernandez S, Carrer A, Miller KD, Schug ZT, Snyder NW, Gade TP, Titchenell PM, Rabinowitz JD, Wellen KE. Dietary fructose feeds hepatic lipogenesis via microbiota-derived acetate. Nature. 2020;579:586–591. doi: 10.1038/s41586-020-2101-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sotokawauchi A, Matsui T, Higashimoto Y, Yamagishi SI. Fructose causes endothelial cell damage via activation of advanced glycation end products-receptor system. Diab Vasc Dis Res. 2019;16:556–561. doi: 10.1177/1479164119866390. [DOI] [PubMed] [Google Scholar]
- 54.Crowe W, Elliott CT, Green BD. A Review of the In Vivo Evidence Investigating the Role of Nitrite Exposure from Processed Meat Consumption in the Development of Colorectal Cancer. Nutrients. 2019;11 doi: 10.3390/nu11112673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dahmus JD, Kotler DL, Kastenberg DM, Kistler CA. The gut microbiome and colorectal cancer: a review of bacterial pathogenesis. J Gastrointest Oncol. 2018;9:769–777. doi: 10.21037/jgo.2018.04.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bäckhed F, Roswall J, Peng Y, Feng Q, Jia H, Kovatcheva-Datchary P, Li Y, Xia Y, Xie H, Zhong H, Khan MT, Zhang J, Li J, Xiao L, Al-Aama J, Zhang D, Lee YS, Kotowska D, Colding C, Tremaroli V, Yin Y, Bergman S, Xu X, Madsen L, Kristiansen K, Dahlgren J, Wang J. Dynamics and Stabilization of the Human Gut Microbiome during the First Year of Life. Cell Host Microbe. 2015;17:690–703. doi: 10.1016/j.chom.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 57.Thursby E, Juge N. Introduction to the human gut microbiota. Biochem J. 2017;474:1823–1836. doi: 10.1042/BCJ20160510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Moore RE, Townsend SD. Temporal development of the infant gut microbiome. Open Biol. 2019;9:190128. doi: 10.1098/rsob.190128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.O'Keefe SJ, Ou J, Aufreiter S, O'Connor D, Sharma S, Sepulveda J, Fukuwatari T, Shibata K, Mawhinney T. Products of the colonic microbiota mediate the effects of diet on colon cancer risk. J Nutr. 2009;139:2044–2048. doi: 10.3945/jn.109.104380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen J, Vitetta L. The Role of Butyrate in Attenuating Pathobiont-Induced Hyperinflammation. Immune Netw. 2020;20:e15. doi: 10.4110/in.2020.20.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tilg H, Adolph TE, Gerner RR, Moschen AR. The Intestinal Microbiota in Colorectal Cancer. Cancer Cell. 2018;33:954–964. doi: 10.1016/j.ccell.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 62.Zhu G, Huang Q, Huang Y, Zheng W, Hua J, Yang S, Zhuang J, Wang J, Ye J. Lipopolysaccharide increases the release of VEGF-C that enhances cell motility and promotes lymphangiogenesis and lymphatic metastasis through the TLR4- NF-κB/JNK pathways in colorectal cancer. Oncotarget. 2016;7:73711–73724. doi: 10.18632/oncotarget.12449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Oliver A, Chase AB, Weihe C, Orchanian SB, Riedel SF, Hendrickson CL, Lay M, Sewall JM, Martiny JBH, Whiteson K. High-Fiber, Whole-Food Dietary Intervention Alters the Human Gut Microbiome but Not Fecal Short-Chain Fatty Acids. mSystems. 2021;6 doi: 10.1128/mSystems.00115-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bahmani S, Azarpira N, Moazamian E. Anti-colon cancer activity of Bifidobacterium metabolites on colon cancer cell line SW742. Turk J Gastroenterol. 2019;30:835–842. doi: 10.5152/tjg.2019.18451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wei H, Chen L, Lian G, Yang J, Li F, Zou Y, Lu F, Yin Y. Antitumor mechanisms of bifidobacteria. Oncol Lett. 2018;16:3–8. doi: 10.3892/ol.2018.8692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Swanson KS, Gibson GR, Hutkins R, Reimer RA, Reid G, Verbeke K, Scott KP, Holscher HD, Azad MB, Delzenne NM, Sanders ME. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of synbiotics. Nat Rev Gastroenterol Hepatol. 2020;17:687–701. doi: 10.1038/s41575-020-0344-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dikeocha IJ, Al-Kabsi AM, Hussin S, Alshawsh MA. Role of probiotics in patients with colorectal cancer: a systematic review protocol of randomised controlled trial studies. BMJ Open. 2020;10:e038128. doi: 10.1136/bmjopen-2020-038128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dos Reis SA, da Conceição LL, Siqueira NP, Rosa DD, da Silva LL, Peluzio MD. Review of the mechanisms of probiotic actions in the prevention of colorectal cancer. Nutr Res. 2017;37:1–19. doi: 10.1016/j.nutres.2016.11.009. [DOI] [PubMed] [Google Scholar]
- 69.Cruz BCS, Sarandy MM, Messias AC, Gonçalves RV, Ferreira CLLF, Peluzio MCG. Preclinical and clinical relevance of probiotics and synbiotics in colorectal carcinogenesis: a systematic review. Nutr Rev. 2020;78:667–687. doi: 10.1093/nutrit/nuz087. [DOI] [PubMed] [Google Scholar]
- 70.Sivamaruthi BS, Kesika P, Chaiyasut C. The Role of Probiotics in Colorectal Cancer Management. Evid Based Complement Alternat Med. 2020;2020:3535982. doi: 10.1155/2020/3535982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Daca Alvarez M, Quintana I, Terradas M, Mur P, Balaguer F, Valle L. The Inherited and Familial Component of Early-Onset Colorectal Cancer. Cells. 2021;10 doi: 10.3390/cells10030710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wilkins T, McMechan D, Talukder A, Herline A. Colorectal Cancer Screening and Surveillance in Individuals at Increased Risk. Am Fam Physician. 2018;97:111–116. [PubMed] [Google Scholar]
- 73.Perrod G, Rahmi G, Cellier C. Colorectal cancer screening in Lynch syndrome: Indication, techniques and future perspectives. Dig Endosc. 2021;33:520–528. doi: 10.1111/den.13702. [DOI] [PubMed] [Google Scholar]
- 74.van Leerdam ME, Roos VH, van Hooft JE, Dekker E, Jover R, Kaminski MF, Latchford A, Neumann H, Pellisé M, Saurin JC, Tanis PJ, Wagner A, Balaguer F, Ricciardiello L. Endoscopic management of polyposis syndromes: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2019;51:877–895. doi: 10.1055/a-0965-0605. [DOI] [PubMed] [Google Scholar]
- 75.Gupta S, Bharti B, Ahnen DJ, Buchanan DD, Cheng IC, Cotterchio M, Figueiredo JC, Gallinger SJ, Haile RW, Jenkins MA, Lindor NM, Macrae FA, Le Marchand L, Newcomb PA, Thibodeau SN, Win AK, Martinez ME. Potential impact of family history-based screening guidelines on the detection of early-onset colorectal cancer. Cancer. 2020;126:3013–3020. doi: 10.1002/cncr.32851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pearlman R, Frankel WL, Swanson B, Zhao W, Yilmaz A, Miller K, Bacher J, Bigley C, Nelsen L, Goodfellow PJ, Goldberg RM, Paskett E, Shields PG, Freudenheim JL, Stanich PP, Lattimer I, Arnold M, Liyanarachchi S, Kalady M, Heald B, Greenwood C, Paquette I, Prues M, Draper DJ, Lindeman C, Kuebler JP, Reynolds K, Brell JM, Shaper AA, Mahesh S, Buie N, Weeman K, Shine K, Haut M, Edwards J, Bastola S, Wickham K, Khanduja KS, Zacks R, Pritchard CC, Shirts BH, Jacobson A, Allen B, de la Chapelle A, Hampel H Ohio Colorectal Cancer Prevention Initiative Study Group. Prevalence and Spectrum of Germline Cancer Susceptibility Gene Mutations Among Patients With Early-Onset Colorectal Cancer. JAMA Oncol. 2017;3:464–471. doi: 10.1001/jamaoncol.2016.5194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Thomas M, Sakoda LC, Hoffmeister M, Rosenthal EA, Lee JK, van Duijnhoven FJB, Platz EA, Wu AH, Dampier CH, de la Chapelle A, Wolk A, Joshi AD, Burnett-Hartman A, Gsur A, Lindblom A, Castells A, Win AK, Namjou B, Van Guelpen B, Tangen CM, He Q, Li CI, Schafmayer C, Joshu CE, Ulrich CM, Bishop DT, Buchanan DD, Schaid D, Drew DA, Muller DC, Duggan D, Crosslin DR, Albanes D, Giovannucci EL, Larson E, Qu F, Mentch F, Giles GG, Hakonarson H, Hampel H, Stanaway IB, Figueiredo JC, Huyghe JR, Minnier J, Chang-Claude J, Hampe J, Harley JB, Visvanathan K, Curtis KR, Offit K, Li L, Le Marchand L, Vodickova L, Gunter MJ, Jenkins MA, Slattery ML, Lemire M, Woods MO, Song M, Murphy N, Lindor NM, Dikilitas O, Pharoah PDP, Campbell PT, Newcomb PA, Milne RL, MacInnis RJ, Castellví-Bel S, Ogino S, Berndt SI, Bézieau S, Thibodeau SN, Gallinger SJ, Zaidi SH, Harrison TA, Keku TO, Hudson TJ, Vymetalkova V, Moreno V, Martín V, Arndt V, Wei WQ, Chung W, Su YR, Hayes RB, White E, Vodicka P, Casey G, Gruber SB, Schoen RE, Chan AT, Potter JD, Brenner H, Jarvik GP, Corley DA, Peters U, Hsu L. Genome-wide Modeling of Polygenic Risk Score in Colorectal Cancer Risk. Am J Hum Genet. 2020;107:432–444. doi: 10.1016/j.ajhg.2020.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Archambault AN, Su YR, Jeon J, Thomas M, Lin Y, Conti DV, Win AK, Sakoda LC, Lansdorp-Vogelaar I, Peterse EFP, Zauber AG, Duggan D, Holowatyj AN, Huyghe JR, Brenner H, Cotterchio M, Bézieau S, Schmit SL, Edlund CK, Southey MC, MacInnis RJ, Campbell PT, Chang-Claude J, Slattery ML, Chan AT, Joshi AD, Song M, Cao Y, Woods MO, White E, Weinstein SJ, Ulrich CM, Hoffmeister M, Bien SA, Harrison TA, Hampe J, Li CI, Schafmayer C, Offit K, Pharoah PD, Moreno V, Lindblom A, Wolk A, Wu AH, Li L, Gunter MJ, Gsur A, Keku TO, Pearlman R, Bishop DT, Castellví-Bel S, Moreira L, Vodicka P, Kampman E, Giles GG, Albanes D, Baron JA, Berndt SI, Brezina S, Buch S, Buchanan DD, Trichopoulou A, Severi G, Chirlaque MD, Sánchez MJ, Palli D, Kühn T, Murphy N, Cross AJ, Burnett-Hartman AN, Chanock SJ, de la Chapelle A, Easton DF, Elliott F, English DR, Feskens EJM, FitzGerald LM, Goodman PJ, Hopper JL, Hudson TJ, Hunter DJ, Jacobs EJ, Joshu CE, Küry S, Markowitz SD, Milne RL, Platz EA, Rennert G, Rennert HS, Schumacher FR, Sandler RS, Seminara D, Tangen CM, Thibodeau SN, Toland AE, van Duijnhoven FJB, Visvanathan K, Vodickova L, Potter JD, Männistö S, Weigl K, Figueiredo J, Martín V, Larsson SC, Parfrey PS, Huang WY, Lenz HJ, Castelao JE, Gago-Dominguez M, Muñoz-Garzón V, Mancao C, Haiman CA, Wilkens LR, Siegel E, Barry E, Younghusband B, Van Guelpen B, Harlid S, Zeleniuch-Jacquotte A, Liang PS, Du M, Casey G, Lindor NM, Le Marchand L, Gallinger SJ, Jenkins MA, Newcomb PA, Gruber SB, Schoen RE, Hampel H, Corley DA, Hsu L, Peters U, Hayes RB. Cumulative Burden of Colorectal Cancer-Associated Genetic Variants Is More Strongly Associated With Early-Onset vs Late-Onset Cancer. Gastroenterology. 2020;158:1274–1286.e12. doi: 10.1053/j.gastro.2019.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mork ME, You YN, Ying J, Bannon SA, Lynch PM, Rodriguez-Bigas MA, Vilar E. High Prevalence of Hereditary Cancer Syndromes in Adolescents and Young Adults With Colorectal Cancer. J Clin Oncol. 2015;33:3544–3549. doi: 10.1200/JCO.2015.61.4503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nguyen HT, Duong HQ. The molecular characteristics of colorectal cancer: Implications for diagnosis and therapy. Oncol Lett. 2018;16:9–18. doi: 10.3892/ol.2018.8679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yurgelun MB, Kulke MH, Fuchs CS, Allen BA, Uno H, Hornick JL, Ukaegbu CI, Brais LK, McNamara PG, Mayer RJ, Schrag D, Meyerhardt JA, Ng K, Kidd J, Singh N, Hartman AR, Wenstrup RJ, Syngal S. Cancer Susceptibility Gene Mutations in Individuals With Colorectal Cancer. J Clin Oncol. 2017;35:1086–1095. doi: 10.1200/JCO.2016.71.0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jang E, Chung DC. Hereditary colon cancer: lynch syndrome. Gut Liver. 2010;4:151–160. doi: 10.5009/gnl.2010.4.2.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tapial S, Olmedillas-López S, Rueda D, Arriba M, García JL, Vivas A, Pérez J, Pena-Couso L, Olivera R, Rodríguez Y, García-Arranz M, García-Olmo D, González-Sarmiento R, Urioste M, Goel A, Perea J. Cimp-Positive Status is More Representative in Multiple Colorectal Cancers than in Unique Primary Colorectal Cancers. Sci Rep. 2019;9:10516. doi: 10.1038/s41598-019-47014-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Willauer AN, Liu Y, Pereira AAL, Lam M, Morris JS, Raghav KPS, Morris VK, Menter D, Broaddus R, Meric-Bernstam F, Hayes-Jordan A, Huh W, Overman MJ, Kopetz S, Loree JM. Clinical and molecular characterization of early-onset colorectal cancer. Cancer. 2019;125:2002–2010. doi: 10.1002/cncr.31994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Magnani G, Furlan D, Sahnane N, Reggiani Bonetti L, Domati F, Pedroni M. Molecular Features and Methylation Status in Early Onset (≤40 Years) Colorectal Cancer: A Population Based, Case-Control Study. Gastroenterol Res Pract. 2015;2015:132190. doi: 10.1155/2015/132190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ribic CM, Sargent DJ, Moore MJ, Thibodeau SN, French AJ, Goldberg RM, Hamilton SR, Laurent-Puig P, Gryfe R, Shepherd LE, Tu D, Redston M, Gallinger S. Tumor microsatellite-instability status as a predictor of benefit from fluorouracil-based adjuvant chemotherapy for colon cancer. N Engl J Med. 2003;349:247–257. doi: 10.1056/NEJMoa022289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Slomski A. Aspirin Protects Against Colorectal Cancer in Lynch Syndrome. JAMA. 2020;324:733. doi: 10.1001/jama.2020.14804. [DOI] [PubMed] [Google Scholar]
- 88.Kosumi K, Mima K, Baba H, Ogino S. Dysbiosis of the gut microbiota and colorectal cancer: the key target of molecular pathological epidemiology. J Lab Precis Med. 2018;3 doi: 10.21037/jlpm.2018.09.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hogan NM, Hanley M, Hogan AM, Sheehan M, McAnena OJ, Regan MP, Kerin MJ, Joyce MR. Awareness and uptake of family screening in patients diagnosed with colorectal cancer at a young age. Gastroenterol Res Pract. 2015;2015:194931. doi: 10.1155/2015/194931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sandhu GS, Anders R, Walde A, Leal AD, King GT, Leong S, Davis SL, Purcell WT, Goodman KA, Herter W, Meguid CL, Birnbaum EH, Ahrendt SA, Gleisner A, Schulick RD, Delchiaro M, McCarter M, Patel S, Messersmith WA, Lieu CH. High incidence of advanced stage cancer and prolonged rectal bleeding history before diagnosis in young-onset patients with colorectal cancer. J Clin Oncol. 2019;37:3576. [Google Scholar]
- 91.Valle L, de Voer RM, Goldberg Y, Sjursen W, Försti A, Ruiz-Ponte C, Caldés T, Garré P, Olsen MF, Nordling M, Castellvi-Bel S, Hemminki K. Update on genetic predisposition to colorectal cancer and polyposis. Mol Aspects Med. 2019;69:10–26. doi: 10.1016/j.mam.2019.03.001. [DOI] [PubMed] [Google Scholar]