Abstract
BACKGROUND
Colorectal cancer (CRC) is the third leading cause of cancer-related deaths in the world. Tumor removal remains the preferred frontline treatment; however, effective non-surgical interventions remain a high priority. 5-fluorouracil (5-FU) is a widely used chemotherapy agent, and molecular hydrogen (H2) has been recognized for its antioxidant and anti-inflammatory effects, with research also suggesting its potential anti-tumor effects. Therefore, H2 dissolved in water [hydrogen-rich water (HRW)], with or without 5-FU, may present itself as a novel therapeutic for CRC.
AIM
To investigate the effects of HRW, with or without 5-FU, as a novel therapeutic for CRC.
METHODS
CRC was induced in the left flank of inbred Balb/c mice. A total of 24 mice bearing tumors were randomly divided into four groups (n = 6 per group) and treated as follows: (1) Control group; (2) 5-FU group that received intraperitoneal injection of 5-FU (5 mg/kg) every other day; (3) H2 group that received HRW, created and delivered via dissolving the H2-generating tablet in the animals’ drinking water, with 200 μL also delivered by oral gavage; and (4) The combination group, H2 (administered in same way as for group three) combined with 5-FU administered same way as group two.
RESULTS
Administration of HRW + 5-FU significantly improved tumor weight, tumor size, collagen content and fibrosis as compared to the CRC control group. Specifically, HRW attenuated oxidative stress (OS) and potentiated antioxidant activity (AA), whereas 5-FU treatment exacerbated OS and blunted AA. The combination of HRW + 5-FU significantly reduced tumor weight and size, as well as reduced collagen deposition and the degree of fibrosis, while further increasing OS and decreasing AA compared to administration of 5-FU alone.
CONCLUSION
Administration of HRW, with or without 5-FU, may serve as a therapeutic for treating CRC.
Keywords: Colorectal cancer, Molecular hydrogen, 5-fluorouracil, Oxidative stress, Antioxidants, Inflammation
Core Tip: Colorectal cancer is a leading cause of death and is often treated with the chemotherapy drug, 5-fluorouracil (5-FU), which has some unwanted side effects. Molecular hydrogen (H2 gas) has antioxidant, anti-inflammatory, and anti-cancer effects. H2 gas can be dissolved in water to make hydrogen-rich water (HRW). The effects of HRW, 5-FU and the combination of HRW and 5-FU in a colorectal-cancer mouse model were examined. HRW and 5-FU decreased tumor size and weight with the combination being the most effective. In contrast to 5-FU, HRW attenuated oxidative stress and improved antioxidant activity.
INTRODUCTION
Colorectal cancer (CRC) is the third leading cause of cancer-related deaths worldwide, in which statistically 3.2% of men and 2.6% of women will die from the disease[1,2]. CRC has a survival rate of 91% if detected in stage 1. However, its overall 5-year survival rate is only 65%, according to 2020 data from the American Cancer Society published on the SEER database[3]. Surgical removal of rectal cancer remains the first-line treatment of CRC. However, non-surgical treatment options serve as important treatment tools, as rates of screening and surgery approvals between various nations can lead to differences in rate of survival[4].
Molecular hydrogen (H2) has been studied extensively as a therapeutic gas, with an estimated 1500 publications to date exploring its potential therapeutic use in 170 disease models across every organ in the mammalian body. H2 can be administered through several methods, such as H2 inhalation, dissolving H2 gas in water to make hydrogen-rich water (HRW) for oral consumption or topical application, or hydrogen-rich saline.
5-fluorouracil (5-FU) is a widely used chemotherapeutic employed during cancer treatment[5]. H2 has shown positive effects in terms of quality of life in human clinical research. For example, studies report that H2 therapy was associated with improved liver function in patients who were administered chemotherapy, as well as reduced side effects for those receiving radiation therapy, and protective effects against radiation-induced bone marrow damage in cancer patients[6-8].
H2 has been previously demonstrated to display anti-cancer properties when administered on its own. Hyperbaric H2 therapy has been examined as a potential cancer therapy, revealing potent anti-tumor effects in mice with squamous cell carcinoma[9]. In a study involving mice with colon cancer, it was shown that drinking HRW dose-dependently potentiated the tumor-inhibitory activity of 5-FU by enhancing cellular apoptosis of the cancer cells[10]. In the present study, we aimed to explore the potential effects of a higher concentration of HRW than previously utilized, to further explore the effects of high-concentration HRW compared to control, 5-FU administration on its own, as well as HRW in combination with 5-FU, for the mitigation of CRC progression and accompanying outcomes.
MATERIALS AND METHODS
Chemicals and reagents
HRW was created using H2-producing tablets (HRW Natural Health Products Inc., New Westminster BC, Canada) by dissolving it in a 500-mL beaker. HRW was made two times each day every 12 h. The concentration of HRW was > 1.5 mmol/L and remained > 0.1 mmol/L after 12 h as determined by redox titration (H2Blue; H2Sciences, Las Vegas, Nevada). 5-FU was obtained from EBEWE Pharma, Unterach, Austria. F12/Dulbecco’s Modified Eagle Medium (DMEM/F12), fetal bovine serum (FBS), penicillin (Pen) and streptomycin (Strep) were obtained from Gibco BRL, Life Technologies Inc. (Gaithersburg, MD, United States).
Cell culture
The mouse colorectal adenocarcinoma cell line CT-26 was obtained from Pasteur Institute (Tehran, Iran). CT-26 cells were cultured in DMEM/F12 medium containing 10% FBS, Pen (50 U/mL) and Strep (50 μg/mL) in a humidified atmosphere containing 5% CO2 and 95% air at 37 °C.
Xenografts in mice: Treatment and evaluation
Tumor xenograft experiments were conducted as previously described by Golovko et al[11]. In brief, 6- to 8-wk-old female inbred Balb/c mice were injected with 5 × 105 CT-26 cells (100 μL) into the left rear flank (day 0). When tumor volumes reached 80-100 mm3 (-10 d), 24 mice bearing tumors were divided randomly into four groups (n = 6 per group) and treated as follows: (1) The control group; (2) The 5-FU group received intraperitoneal injections of 5-FU (5 mg/kg) every other day; (3) The H2 group received HRW both from drinking water and by delivering 200 μL of the solution via oral gavage; and (4) The combination group, H2 (administered in same way as group three) combined with 5-FU (administered in the same way as group two). The tumor volume was calculated every other day according to the following formula: V = (length × width2)/2[12]. The animals were sacrificed on day 14 and the tumors were removed for further analysis.
Histological assay
Fixed tumor tissue samples were embedded in paraffin wax and then sectioned at 5 μm thickness with a microtome. The tumor tissue sections were deparaffinized and stained with Hematoxylin-Eosin for evaluation of tumor necrosis. Masson trichrome staining was also performed for evaluation of collagen content and fibrosis.
Tissue preparation for measurement of oxidative stress markers
The colon tissues samples were homogenized in ice with PBS and centrifuged. The supernatant was stored at -70 °C for the determination of the oxidative and antioxidative proteins.
Malondialdehyde measurement
Malondialdehyde (MDA) was measured by methods as previously described[13]. Briefly, 1 mL of homogenate was mixed with 2 mL of a solution containing thiobarbituric acid, trichloroacetic acid, and HCl in hot water (100 °C) for 45 min and centrifuged for ten minutes. The MDA levels were determined by measuring the absorbance of the solution.
Total thiol group measurement
We used di-thio nitrobenzoic acid (DTNB) reagent for measurement of total thiol group as previously described[13]. Briefly, 1 mL of Tris-EDTA buffer (pH = 8.6) was added to the colon homogenate and absorbance was measured. Similarly, 20 μL of DTNB reagents was added to the sample absorbance and the absorbance was measured again; subsequently, the total molar concentration of thiol was determined as previously described[14].
Evaluation of superoxide dismutase and catalase
Superoxide dismutase (SOD) was determined with a colorimetric assay described by Madesh et al[15]. The method is centered on the synthesis of SOD by pyrogallol auto-oxidation and inhibition of superoxide-dependent reduction of 3-(4,5-dimethyl-thiazol-2-yl) 2,5-diphenyl tetrazolium bromide (MTT) to its formazan. Catalase was measured by evaluating the kinetics of H2O2 hydrolysis at 240 nm in a buffer of sodium phosphate. The velocity of the enzyme activity can be determined by converting H2O2 to H2O and O2 within 60 s of normal conditions[15].
Ethics statement
The Mashhad University of Medical Sciences Committee on Animal Ethics has approved all animal protocols used in this research. Reference Number: 991229; Date: July 10, 2020.
Data analysis
The statistical methods of this study were reviewed by Dr. Mohammad Taghi Shakeri, a member of the Biostatistic Department of Mashad University of Medical Sciences. All the results are presented as means and standard error of the mean (mean ± SEM). The differences in the mean values among different groups were determined by a one-way analysis of variance (ANOVA) using the SPSS 22.0 program. Significance was set at values of P < 0.05.
RESULTS
H2 suppresses tumor growth and enhances the antitumor efficacy of 5-FU in a colon cancer xenograft model
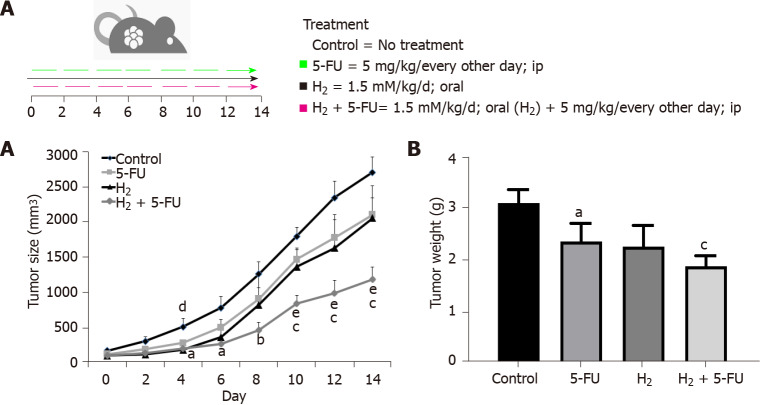
We studied the influence of H2 on tumor growth in a CRC xenograft model. Administration of HRW significantly decreased tumor growth in mice (Figure 1). The suppressive effect of HRW on tumor growth was slightly, but not statistically, more potent than 5-FU, and not as effective as the combination therapy. Specifically, the average control tumor size was 2698.85 mm3, whereas the average tumor size in the HRW group was 2047.23 mm3 (24.1% suppression compared to control), while the average tumor size in the 5-FU group was 2097.32 mm3 (22.3% suppression compared to control). The combination group of HRW + 5-FU resulted in the greatest tumor size suppression, with the average size of 1177.5 mm3 (56.4% suppression compared to control) (Figure 1A).
Figure 1.
Hydrogen, 5-fluorouracil and their combination reduced tumor growth and tumor weight in a murine model of colorectal cancer. A: Tumor size; B: Tumor weight change in mice treated with hydrogen-rich water (HRW), 5-fluorouracil (5-FU) and their combination. P < 0.05 and P < 0.001 compared to control, P < 0.01 compared to 5-FU, P < 0.05 compared to HRW and P < 0.05, P < 0.01 and P < 0.001 compared to combination groups; n = 6 per group. a: P < 0.05 compare to control; b: P < 0.01 compare to control; c: P < 0.001 compare to control; d: P < 0.05 compare to H2; e: P < 0.01 compare to 5-FU. H2: Hydrogen; 5-FU: 5-fluorouracil.
Similarly, a comparison of tumor weight between the groups showed that both 5-FU and HRW significantly reduced tumor weight (P < 0.05), and this decrease was potentiated in the combination group of HRW + 5-FU (P < 0.001) (Figure 1B). Treatment with 5-FU reached statistical significance at day 12 through 14 (P < 0.05) as compared to control, whereas the combination treatment of HRW + 5-FU reached significance by day 6 (P < 0.05) and continued its suppressive effect at day 8 (P < 0.01) and days 10-14 (P < 0.001) compared to control. Moreover, combination treatment was more effective compared to 5-FU treatment alone, reaching significance by day 8 (P < 0.05) with days 10 through 14 being even more significant (P < 0.01).
The effects of H2 and 5-FU on redox status
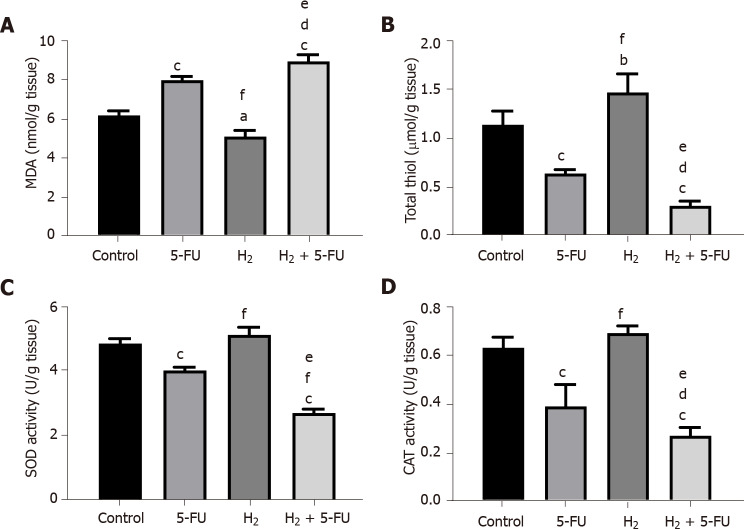
We investigated the effects of H2 administered via drinking HRW on levels of markers of OS in tissue homogenates. As shown in Figure 2, HRW treatment decreased MDA levels in tumor tissues compared to control (P < 0.05) and 5-FU treatment (P < 0.001). However, 5-FU treatment increased MDA levels compared to control (Figure 2A, P < 0.001). HRW tended to improve activity of all three antioxidant markers measured. For example, HRW increased thiol concentrations (Figure 2B) compared to control (P < 0.01) and 5-FU treatment (P < 0.001). Additionally, we observed a trend towards an increase in SOD and catalase activity following H2 treatment, although significance was only reached when compared to 5-FU treatment. Compared to the control group, there was a significant decrease in levels of all antioxidant markers following 5-FU treatment. In the combination group (HRW and 5-FU), a more prevalent rise in OS and suppression of AA was observed compared to 5-FU alone. MDA levels significantly increased in the combination group compared to control (Figures 2B, 2C and 2D; P < 0.001) and the 5-FU treatment (P < 0.05). Similarly, activity of all three antioxidants measured were suppressed by the combination compared to control and also when compared to 5-FU alone.
Figure 2.
Effects of hydrogen and 5-fluorouracil on the oxidative stress index in colorectal cancer. A: Malondialdehyde; B: Total thiol; C: Superoxide dismutase; D: Catalase activity. P < 0.05, P < 0.01 and P < 0.001 compared to control, P < 0.05 and P < 0.001 compared to 5-fluorouracil, P < 0.001 compared to hydrogen groups; n = 6 per group. a: P < 0.05 compare to control; b: P < 0.01 compare to control; c: P < 0.001 compare to control; d: P < 0.05 compare to H2; e: P < 0.001 compare to H2; f: P < 0.001 compare to 5-FU. H2: Hydrogen; 5-FU: 5-fluorouracil; SOD: Superoxide dismutase; MDA: Malondialdehyde; CAT: Catalase.
H2 and 5-FU increased necrotic areas
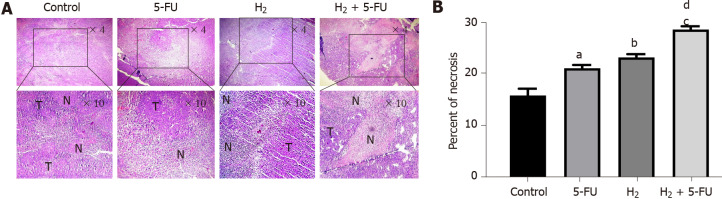
Tumor necrosis was observed under a light microscope. As illustrated in Figures 3A and 3B, treatment of H2 or 5-FU displayed interspersed tissue necrosis compared to the untreated group. In the H2 + 5-FU group, we observed larger necrotic areas than the necrotic areas in either group alone.
Figure 3.
Hydrogen and 5-fluorouracil induce necrosis in tumor tissue of colorectal cancer. A: Hematoxylin-Eosin staining of tumor sections revealed that hydrogen (H2) and 5-fluorouracil (5-FU) induce necrosis; B: Percent of tumor necrosis. P < 0.05, P < 0.01 and P < 0.001 compared to control, P < 0.001 compared to 5-FU, P < 0.01 compared to H2 groups; n = 6 per group. a: P < 0.05 compare to control; b: P < 0.01 compare to control; c: P < 0.001 compare to control; d: P < 0.001 compare to 5-FU. H2: Hydrogen; 5-FU: 5-fluorouracil; T: Tumor cells; N: Necrotic area.
H2 and 5-FU decreased tumor fibrosis in the colon cancer xenograft model
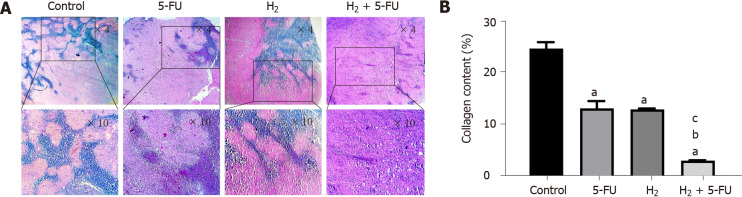
We used Masson’s trichrome staining to compare the collagen deposition in tumor tissues across the treatment and control groups. Our results demonstrate that administration of either H2 or 5-FU suppressed collagen deposition and degree of fibrosis compared to the control group (Figure 4A). The increment in percentage of collagen deposition in all treated groups was significantly decreased when compared to the control group (Figure 4B; P < 0.001). Specifically, collagen deposition percentage in the control group was 24.6%. In contrast, with both H2 and 5-FU alone, the percentage of collagen deposition in the tumor tissue was significantly reduced to about 13% (P < 0.001). However, administration of both H2 + 5-FU in combination further reduced the percentage of collagen deposition in tumor tissue (= 3%) compared to both the 5-FU group and H2 group (P < 0.001).
Figure 4.
Hydrogen and 5-fluorouracil attenuate fibrosis in tumor tissue of colorectal cancer. A: Trichrome staining of tumor samples revealed that hydrogen (H2) suppresses fibrosis in the murine model of colorectal cancer (collagen fiber accumulation appears in blue); B: Tumor fibrosis expressed as collagen content (%) in different groups. P < 0.001 compared to control, P < 0.001 compared to 5-fluorouracil, P <0.01 compared to H2 groups; n = 6 per group. a: P < 0.001 compare to control; b: P < 0.001 compare to 5-FU; c: P < 0.001 compare to H2. H2: Hydrogen; 5-FU: 5-fluorouracil.
DISCUSSION
Non-surgical treatment options to improve outcomes in CRC remains a high priority. Ideal adjuvant treatment options should aim to improve quality of life, reduce symptoms, and work synergistically with standard care. Although 5-FU remains the front-line treatment option for a variety of cancers due to its effectiveness, it also has limitations. For instance, cardiotoxicity has shown to be a serious side effect of 5-FU administration, largely due to increases in OS and suppression of endogenous antioxidant mobilization[16]. Accordingly, molecular H2 has been proposed as a novel approach for the treatment of cardiovascular disorders due to its ability to significantly reduce the effects of OS[17].
Our results demonstrate that the combination of HRW and 5-FU treatment potentiated the beneficial anti-tumor effects of both treatments on their own, such as tumor weight, size, the degree of fibrosis and collagen content in the tumor. Enigmatically, while treatment with HRW on its own significantly improved all three measured antioxidant markers while decreasing levels of MDA, the combination therapy of HRW and 5-FU significantly blunted AA and elevated MDA levels significantly above those measured with 5-FU alone. Acute temporal increases in OS after H2 administration have been noted in other studies, and it has been previously suggested that molecular H2 may act as a therapeutic hormetic agent similar to exercise[18-21]. Nogueira et al[19] (2018) examined the effects of molecular H2 administration on exercise performance and noted an acute rise in OS in the H2 treated group, followed by a greater antioxidant mobilization, leading to improved redox homeostasis shortly after the exercise period ended. Further, previously published human clinical research has demonstrated significant improvements in redox homeostasis following medium-term administration of HRW for 24 wk[22]. Since our short-term study was unable to determine the effects of H2 + 5-FU administration on OS and AA over a longer treatment course, such as has been reported in previous research on HRW[23], future research is warranted to investigate this area.
When administered on its own, HRW demonstrated similar benefits in reducing tumor size compared to 5-FU. These results corroborate earlier reports that HRW can suppress early tumor formation in rats[24]. Additionally, molecular H2 was shown to prevent tumor progression in a cell line model of lung cancer[25]. In our study, we demonstrated that HRW administration was associated with a significant decrease in pathological collagen content equivalent to that of 5-FU. In contrast to previous reports demonstrating that molecular H2 upregulates collagen biosynthesis and expression, and corresponding to the results of another study reporting that molecular H2 significantly reduced type III collagen depositions as observed via staining[26-28]. Molecular H2 has shown to both be able to promote and suppress outcomes, model dependent, for many biological processes, which may indicate that contradictory reports do not undermine our understanding of the mechanisms by which H2 operates. HRW demonstrated similar outcomes to 5-FU for visual results of fibrosis from mass trichrome staining. Further, molecular H2 has been previously demonstrated to reduce fibrosis in the lungs and abdomen[29,30].
Interestingly, the combination of 5-FU and HRW demonstrated significant reductions of tumor weight, size, collagen deposition and degree of fibrosis, while increasing markers of OS and blunting AA significantly beyond 5-FU alone. Previous studies have demonstrated that HRW generally reduces OS in most animal and human disease models when administered as a stand-alone intervention. Molecular H2 has been observed to work in an additive or synergistic capacity with several other interventions in various models, as demonstrated by a recent study, in which administration of high-concentration HRW alongside minocycline improved outcomes following ischemic stroke in rats[31]. Additionally, molecular H2 has shown to enhance the effects of photothermal therapy by inhibiting tumor progression in cell cultures and was also shown to act equivalently to sulfasalazine in a dextran sodium sulfate-induced mouse model of colitis, with the combination therapy of HRW + sulfasalazine demonstrating effects of significantly greater magnitude than either treatment on its own[32,33].
Treatment with 5-FU has been shown to result in DNA damage[34]. Alterations in various processes, such as nucleotide and amino acid metabolism, may lead to 5-FU resistance[35]. Autophagy plays an important role in nucleotide and amino acid metabolism, and H2 has been shown to both stimulate and mitigate autophagy for beneficial outcomes[36-40]. It has also been suggested that therapies which mediate DNA repair alongside 5-FU and other cancer treatments should be further explored as a therapeutic target[41]. For instance, it has been shown that H2 exerted significant protective effects against DNA damage in calf thymus tissue following exposure to radiation[42]. Future research is also needed in order to address both potential protective effects and long-term benefits of molecular H2 delivered in conjunction with 5-FU and other conventional cancer therapies, exploring outcomes related to DNA damage, cell signaling, and survival rates.
So far, little is known regarding the effects of various dosages and different administration methods of HRW, H2 inhalation, and hydrogen-rich saline on cancer cells. To date, several routes have shown potential benefits of HRW administration for cancer treatment, including the previously cited reports using inhalation studies in humans, and HRW use in murine models. Additionally, a recent report demonstrated that the use of H2-producing reactive magnesium implants was associated with significant suppression of tumor growth in a mouse model of ovarian cancer[43]. However, since H2 has demonstrated protective effects on healthy cells, it could also protect and stimulate cancer cell growth. For example, H2 administration has been demonstrated to induce the mitochondrial unfolded protein response, which is also a proliferative signal in various cancer cells[44,45]. Therefore, the effects of different dosages and administration methods of molecular H2 should be carefully analyzed to determine its effects.
CONCLUSION
Safe and well-tolerated adjuvant therapeutics with the potential to ameliorate the deleterious consequences of various cancer treatments, while simultaneously improving outcomes, are of high interest to cancer patients and the medical community. Molecular H2 therapy demonstrates potential anti-cancer properties, as well as the ability to reduce the secondary effects of various treatments. In this study, we have shown that administered on its own, HRW demonstrates anti-cancer properties and improves markers of OS and AA compared to conventional treatment (5-FU). The combination of HRW and 5-FU suppressed tumor progression in a synergistic manner; however, the addition of HRW to 5-FU treatment increased OS levels and reduced AA. Limitations of this study include that the observation period during the study was only 14 d, with rates of survival and remission not examined. As such, interpretation of these results should be evaluated cautiously. Larger, longer-term studies are highly warranted to explore HRW as an adjuvant therapy for various cancers, alongside conventional therapy, with longer observational periods needed to address unanswered questions regarding potential positive and negative effects of molecular H2 on redox homeostasis during cancer treatment.
ARTICLE HIGHLIGHTS
Research background
Colorectal cancer (CRC) is the third leading cause of cancer-related deaths worldwide. Surgical removal remains the first-line treatment for CRC; however, nonsurgical options remain important tools for treatment. Currently, treatments such as 5-fluorouracil (5-FU), a widely administered chemotherapeutic agent utilized in the treatment of CRC, presents known beneficial effects, but also significant side effects. Hydrogen-rich water (HRW) has demonstrated beneficial effects in numerous species, including humans, in many disease models, including various cancers. One attractive aspect of HRW is the high safety profile and low rates of side effects combined with its promising therapeutic effects.
Research motivation
New treatments with potential positive effects in CRC are desperately needed, particularly treatments with high safety profiles and low side effects. HRW may fit the criteria as a safe potential treatment for CRC, either as a stand-alone treatment or in combination with conventional treatments.
Research objectives
We aimed to evaluate the efficacy of HRW on a CRC model compared to 5-FU and control, as well as the combination treatment of HRW and 5-FU compared to 5-FU alone, HRW alone, or control. We measured tumor size, tumor weight, fibrosis, and collagen content, as well as oxidative stress (OS) and antioxidant activity (AA) in mice with induced CRC. These objectives allow us to determine the therapeutic efficacy and mechanistic insight of HRW with or without 5-FU, as well as determine if there are additive benefits in a combinational treatment to guide future clinical studies.
Research methods
Six- to eight-week-old female inbred Balb/c mice were injected with 5 × 105 CT-26 cells (100 μL) into the left rear flank (day 0). When tumor volumes reached 80-100 mm3, 24 mice bearing tumors were randomly divided into four groups. Mice were either left untreated (control) or treated with 5-FU (intraperitoneal injection, 5 mg/kg every other day), high-concentration HRW produced by magnesium tablets (ad libitum in drinking water, as well as by oral gavage 200 μL daily), or both HRW and 5-FU.
Research results
We report that molecular hydrogen dissolved in water (HRW) was as effective as 5-FU, with more preferential outcomes relating to higher AA and lower OS. Importantly, the combination of HRW and 5-FU was superior to either therapy on its own, presenting the possibility that HRW may be explored as an adjuvant therapy alongside conventional chemotherapeutics.
Research conclusions
HRW may be a novel safe adjuvant therapy for treating CRC, either as a stand-alone therapy, or preferably, alongside conventional chemotherapeutics.
Research perspectives
Clinical research to evaluate the effects of HRW as a treatment for CRC, both alone and in combination with 5-FU and other chemotherapeutics, is highly warranted.
ACKNOWLEDGEMENTS
We thank Mr. Alex Tarnava, CEO of HRW Natural Health Products Inc. for kindly donating Drink HRW tablets for this study and Mashhad University of Medical Sciences for their support.
Footnotes
Institutional review board statement: The Mashhad University of Medical Sciences Committee on Animal Ethics has approved all animal protocols used in this research.
Institutional animal care and use committee statement: The Mashhad University of Medical Sciences Committee on Animal Ethics has approved all animal protocols used in this research. Reference Number: 991229; Date: July 10, 2020.
Conflict-of-interest statement: Tarnava A is involved in commercial entities with interest in the marketing of hydrogen-rich water; LeBaron TW has received travel reimbursement, honoraria, and speaking and consultancy fees from various academic and commercial entities regarding molecular hydrogen. All other authors report no conflict of interest.
ARRIVE guidelines statement: The authors have read the ARRIVE Guidelines, and the manuscript was prepared and revised according to the ARRIVE Guidelines.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: May 19, 2021
First decision: June 16, 2021
Article in press: December 7, 2021
Specialty type: Oncology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Wu HL, Yao K S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ
Contributor Information
Fereshteh Asgharzadeh, Department of Physiology, Faculty of Medicine, Mashhad University of Medical Sciences, Mashhad 9177899191, Iran.
Alex Tarnava, Drink HRW, New Westminster, BC V3j0b6 Canada.
Asma Mostafapour, Department of Medical Genetics and Molecular Medicine, Faculty of Medicine, Mashhad University of Medical Sciences, Mashhad 9177899191, Iran.
Majid Khazaei, Department of Physiology, Faculty of Medicine, Mashhad University of Medical Sciences, Mashhad 9177899191, Iran.
Tyler W LeBaron, Centre of Experimental Medicine, Institute for Heart Research Slovak Academy of Sciences, Bratislava 984104, Slovakia; Department of Kinesiology and Outdoor Recreation, Southern Utah University, UT 84720, United States; Biological Research, Molecular Hydrogen Institute, UT 84721, United States; Department of Physical Science, Southern Utah University, UT 84720, United States. lebaront@molecularhydrogeninstitute.com.
Data sharing statement
No additional data are available.
References
- 1.Rawla P, Sunkara T, Barsouk A. Epidemiology of colorectal cancer: incidence, mortality, survival, and risk factors. Prz Gastroenterol. 2019;14:89–103. doi: 10.5114/pg.2018.81072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Colorectal cancer: Overview. [cited 5 May 2021] Available from: https://www.cedars-sinai.org/health-library/diseases-and-conditions/c/colorectal-cancer-overview.html .
- 3. Surveillance, Epidemiology, and End Results (SEER) Program (2021). Cancer Stat Facts: Colorectal Cancer. [cited 5 May 2021]. Available from: https://seer.cancer.gov/statfacts/html/colorect.html .
- 4.Benitez Majano S, Di Girolamo C, Rachet B, Maringe C, Guren MG, Glimelius B, Iversen LH, Schnell EA, Lundqvist K, Christensen J, Morris M, Coleman MP, Walters S. Surgical treatment and survival from colorectal cancer in Denmark, England, Norway, and Sweden: a population-based study. Lancet Oncol. 2019;20:74–87. doi: 10.1016/S1470-2045(18)30646-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Longley DB, Harkin DP, Johnston PG. 5-fluorouracil: mechanisms of action and clinical strategies. Nat Rev Cancer. 2003;3:330–338. doi: 10.1038/nrc1074. [DOI] [PubMed] [Google Scholar]
- 6.Yang Q, Ji G, Pan R, Zhao Y, Yan P. Protective effect of hydrogen-rich water on liver function of colorectal cancer patients treated with mFOLFOX6 chemotherapy. Mol Clin Oncol. 2017;7:891–896. doi: 10.3892/mco.2017.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kang KM, Kang YN, Choi IB, Gu Y, Kawamura T, Toyoda Y, Nakao A. Effects of drinking hydrogen-rich water on the quality of life of patients treated with radiotherapy for liver tumors. Med Gas Res. 2011;1:11. doi: 10.1186/2045-9912-1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hirano SI, Aoki Y, Li XK, Ichimaru N, Takahara S, Takefuji Y. Protective effects of hydrogen gas inhalation on radiation-induced bone marrow damage in cancer patients: a retrospective observational study. Med Gas Res. 2021;11:104–109. doi: 10.4103/2045-9912.314329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dole M, Wilson FR, Fife WP. Hyperbaric hydrogen therapy: a possible treatment for cancer. Science. 1975;190:152–154. doi: 10.1126/science.1166304. [DOI] [PubMed] [Google Scholar]
- 10.Runtuwene J, Amitani H, Amitani M, Asakawa A, Cheng KC, Inui A. Hydrogen-water enhances 5-fluorouracil-induced inhibition of colon cancer. PeerJ. 2015;3:e859. doi: 10.7717/peerj.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Golovko D, Kedrin D, Yilmaz ÖH, Roper J. Colorectal cancer models for novel drug discovery. Expert Opin Drug Discov. 2015;10:1217–1229. doi: 10.1517/17460441.2015.1079618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moradi-Marjaneh R, Hassanian SM, Rahmani F, Aghaee-Bakhtiari SH, Avan A, Khazaei M. Phytosomal Curcumin Elicits Anti-tumor Properties Through Suppression of Angiogenesis, Cell Proliferation and Induction of Oxidative Stress in Colorectal Cancer. Curr Pharm Des. 2018;24:4626–4638. doi: 10.2174/1381612825666190110145151. [DOI] [PubMed] [Google Scholar]
- 13.Amerizadeh F, Rezaei N, Rahmani F, Hassanian SM, Moradi-Marjaneh R, Fiuji H, Boroumand N, Nosrati-Tirkani A, Ghayour-Mobarhan M, Ferns GA, Khazaei M, Avan A. Crocin synergistically enhances the antiproliferative activity of 5-flurouracil through Wnt/PI3K pathway in a mouse model of colitis-associated colorectal cancer. J Cell Biochem. 2018;119:10250–10261. doi: 10.1002/jcb.27367. [DOI] [PubMed] [Google Scholar]
- 14.Binabaj MM, Asgharzadeh F, Avan A, Rahmani F, Soleimani A, Parizadeh MR, Ferns GA, Ryzhikov M, Khazaei M, Hassanian SM. EW-7197 prevents ulcerative colitis-associated fibrosis and inflammation. J Cell Physiol. 2019;234:11654–11661. doi: 10.1002/jcp.27823. [DOI] [PubMed] [Google Scholar]
- 15.Madesh M, Balasubramanian KA. A microtiter plate assay for superoxide using MTT reduction method. Indian J Biochem Biophys. 1997;34:535–539. [PubMed] [Google Scholar]
- 16.Polk A, Vistisen K, Vaage-Nilsen M, Nielsen DL. A systematic review of the pathophysiology of 5-fluorouracil-induced cardiotoxicity. BMC Pharmacol Toxicol. 2014;15:47. doi: 10.1186/2050-6511-15-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.LeBaron TW, Kura B, Kalocayova B, Tribulova N, Slezak J. A New Approach for the Prevention and Treatment of Cardiovascular Disorders. Molecular Hydrogen Significantly Reduces the Effects of Oxidative Stress. Molecules. 2019;24 doi: 10.3390/molecules24112076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ichihara M, Sobue S, Ito M, Hirayama M, Ohno K. Beneficial biological effects and the underlying mechanisms of molecular hydrogen - comprehensive review of 321 original articles. Med Gas Res. 2015;5:12. doi: 10.1186/s13618-015-0035-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nogueira JE, Passaglia P, Mota CMD, Santos BM, Batalhão ME, Carnio EC, Branco LGS. Molecular hydrogen reduces acute exercise-induced inflammatory and oxidative stress status. Free Radic Biol Med. 2018;129:186–193. doi: 10.1016/j.freeradbiomed.2018.09.028. [DOI] [PubMed] [Google Scholar]
- 20.Murakami Y, Ito M, Ohsawa I. Molecular hydrogen protects against oxidative stress-induced SH-SY5Y neuroblastoma cell death through the process of mitohormesis. PLoS One. 2017;12:e0176992. doi: 10.1371/journal.pone.0176992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.LeBaron TW, Laher I, Kura B, Slezak J. Hydrogen gas: from clinical medicine to an emerging ergogenic molecule for sports athletes 1. Can J Physiol Pharmacol. 2019;97:797–807. doi: 10.1139/cjpp-2019-0067. [DOI] [PubMed] [Google Scholar]
- 22.LeBaron TW, Singh RB, Fatima G, Kartikey K, Sharma JP, Ostojic SM, Gvozdjakova A, Kura B, Noda M, Mojto V, Niaz MA, Slezak J. The Effects of 24-Week, High-Concentration Hydrogen-Rich Water on Body Composition, Blood Lipid Profiles and Inflammation Biomarkers in Men and Women with Metabolic Syndrome: A Randomized Controlled Trial. Diabetes Metab Syndr Obes. 2020;13:889–896. doi: 10.2147/DMSO.S240122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sha JB, Zhang SS, Lu YM, Gong WJ, Jiang XP, Wang JJ, Qiao TL, Zhang HH, Zhao MQ, Wang DP, Xia H, Li ZW, Chen JL, Zhang L, Zhang CG. Effects of the long-term consumption of hydrogen-rich water on the antioxidant activity and the gut flora in female juvenile soccer players from Suzhou, China. Med Gas Res. 2018;8:135–143. doi: 10.4103/2045-9912.248263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li FY, Zhu SX, Wang ZP, Wang H, Zhao Y, Chen GP. Consumption of hydrogen-rich water protects against ferric nitrilotriacetate-induced nephrotoxicity and early tumor promotional events in rats. Food Chem Toxicol. 2013;61:248–254. doi: 10.1016/j.fct.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 25.Wang D, Wang L, Zhang Y, Zhao Y, Chen G. Hydrogen gas inhibits lung cancer progression through targeting SMC3. Biomed Pharmacother. 2018;104:788–797. doi: 10.1016/j.biopha.2018.05.055. [DOI] [PubMed] [Google Scholar]
- 26.Nishiwaki H, Ito M, Negishi S, Sobue S, Ichihara M, Ohno K. Molecular hydrogen upregulates heat shock response and collagen biosynthesis, and downregulates cell cycles: meta-analyses of gene expression profiles. Free Radic Res. 2018;52:434–445. doi: 10.1080/10715762.2018.1439166. [DOI] [PubMed] [Google Scholar]
- 27.Safonov M, You J, Lee , J , Safonov V L, Berman D, Zhu DH. Hydrogen generating patch improves skin cell viability, migration activity, and collagen expression. ER. 2020;1:1–5. [Google Scholar]
- 28.Terasaki Y, Ohsawa I, Terasaki M, Takahashi M, Kunugi S, Dedong K, Urushiyama H, Amenomori S, Kaneko-Togashi M, Kuwahara N, Ishikawa A, Kamimura N, Ohta S, Fukuda Y. Hydrogen therapy attenuates irradiation-induced lung damage by reducing oxidative stress. Am J Physiol Lung Cell Mol Physiol. 2011;301:L415–L426. doi: 10.1152/ajplung.00008.2011. [DOI] [PubMed] [Google Scholar]
- 29.Dong WW, Zhang YQ, Zhu XY, Mao YF, Sun XJ, Liu YJ, Jiang L. Protective Effects of Hydrogen-Rich Saline Against Lipopolysaccharide-Induced Alveolar Epithelial-to-Mesenchymal Transition and Pulmonary Fibrosis. Med Sci Monit. 2017;23:2357–2364. doi: 10.12659/MSM.900452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lu H, Chen W, Liu W, Si Y, Zhao T, Lai X, Kang Z, Sun X, Guo Z. Molecular hydrogen regulates PTEN-AKT-mTOR signaling via ROS to alleviate peritoneal dialysis-related peritoneal fibrosis. FASEB J. 2020;34:4134–4146. doi: 10.1096/fj.201901981R. [DOI] [PubMed] [Google Scholar]
- 31.Jiang Z, Alamuri TT, Muir ER, Choi DW, Duong TQ. Longitudinal multiparametric MRI study of hydrogen-enriched water with minocycline combination therapy in experimental ischemic stroke in rats. Brain Res. 2020;1748:147122. doi: 10.1016/j.brainres.2020.147122. [DOI] [PubMed] [Google Scholar]
- 32.Zhao P, Jin Z, Chen Q, Yang T, Chen D, Meng J, Lu X, Gu Z, He Q. Local generation of hydrogen for enhanced photothermal therapy. Nat Commun. 2018;9:4241. doi: 10.1038/s41467-018-06630-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barnier C, Clerissi C, Lami R, Intertaglia L, Lebaron P, Grimaud R, Urios L. Description of Palleronia rufa sp. nov., a biofilm-forming and AHL-producing Rhodobacteraceae, reclassification of Hwanghaeicola aestuarii as Palleronia aestuarii comb. nov., Maribius pontilimi as Palleronia pontilimi comb. nov., Maribius salinus as Palleronia salina comb. nov., Maribius pelagius as Palleronia pelagia comb. nov. and emended description of the genus Palleronia. Syst Appl Microbiol. 2020;43:126018. doi: 10.1016/j.syapm.2019.126018. [DOI] [PubMed] [Google Scholar]
- 34.De Angelis PM, Svendsrud DH, Kravik KL, Stokke T. Cellular response to 5-fluorouracil (5-FU) in 5-FU-resistant colon cancer cell lines during treatment and recovery. Mol Cancer. 2006;5:20. doi: 10.1186/1476-4598-5-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang N, Yin Y, Xu SJ, Chen WS. 5-Fluorouracil: mechanisms of resistance and reversal strategies. Molecules. 2008;13:1551–1569. doi: 10.3390/molecules13081551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guo JY, Teng X, Laddha SV, Ma S, Van Nostrand SC, Yang Y, Khor S, Chan CS, Rabinowitz JD, White E. Autophagy provides metabolic substrates to maintain energy charge and nucleotide pools in Ras-driven lung cancer cells. Genes Dev. 2016;30:1704–1717. doi: 10.1101/gad.283416.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen H, Mao X, Meng X, Li Y, Feng J, Zhang L, Zhang Y, Wang Y, Yu Y, Xie K. Hydrogen alleviates mitochondrial dysfunction and organ damage via autophagymediated NLRP3 inflammasome inactivation in sepsis. Int J Mol Med. 2019;44:1309–1324. doi: 10.3892/ijmm.2019.4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jiang X, Niu X, Guo Q, Dong Y, Xu J, Yin N, Qianqian Q, Jia Y, Gao L, He Q, Lv P. Corrigendum to "FoxO1-mediated autophagy plays an important role in the neuroprotective effects of hydrogen in a rat model of vascular dementia" [Behav. Brain Res. 356 (2019) 98-106] Behav Brain Res. 2019;374:111839. doi: 10.1016/j.bbr.2018.05.023. [DOI] [PubMed] [Google Scholar]
- 39.Wang H, Huo X, Chen H, Li B, Liu J, Ma W, Wang X, Xie K, Yu Y, Shi K. Hydrogen-Rich Saline Activated Autophagy via HIF-1α Pathways in Neuropathic Pain Model. Biomed Res Int. 2018;2018:4670834. doi: 10.1155/2018/4670834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gao Y, Yang H, Chi J, Xu Q, Zhao L, Yang W, Liu W. Hydrogen Gas Attenuates Myocardial Ischemia Reperfusion Injury Independent of Postconditioning in Rats by Attenuating Endoplasmic Reticulum Stress-Induced Autophagy. Cell Physiol Biochem. 2017;43:1503–1514. doi: 10.1159/000481974. [DOI] [PubMed] [Google Scholar]
- 41.Wyatt MD, Wilson DM 3rd. Participation of DNA repair in the response to 5-fluorouracil. Cell Mol Life Sci. 2009;66:788–799. doi: 10.1007/s00018-008-8557-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Abou-Hamdan M, Gardette B, Cadet J, Gharib B, De Reggi M, Douki T, Triantaphylides C. Molecular hydrogen attenuates radiation-induced nucleobase damage to DNA in aerated aqueous solutions. Int J Radiat Biol. 2016;92:536–541. doi: 10.1080/09553002.2016.1206234. [DOI] [PubMed] [Google Scholar]
- 43.Qiao S, Wang Y, Zan R, Wu H, Sun Y, Peng H, Zhang R, Song Y, Ni J, Zhang S, Zhang X. Biodegradable Mg Implants Suppress the Growth of Ovarian Tumor. ACS Biomater Sci Eng. 2020;6:1755–1763. doi: 10.1021/acsbiomaterials.9b01703. [DOI] [PubMed] [Google Scholar]
- 44.Sobue S, Inoue C, Hori F, Qiao S, Murate T, Ichihara M. Molecular hydrogen modulates gene expression via histone modification and induces the mitochondrial unfolded protein response. Biochem Biophys Res Commun. 2017;493:318–324. doi: 10.1016/j.bbrc.2017.09.024. [DOI] [PubMed] [Google Scholar]
- 45.Deng P, Haynes CM. Mitochondrial dysfunction in cancer: Potential roles of ATF5 and the mitochondrial UPR. Semin Cancer Biol. 2017;47:43–49. doi: 10.1016/j.semcancer.2017.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No additional data are available.