Abstract
BACKGROUND
As of June 1, 2020, over 370000 coronavirus disease 2019 (COVID-19) deaths have been reported to the World Health Organization. However, the risk factors for patients with moderate-to-severe or severe-to-critical COVID-19 remain unclear.
AIM
To explore the characteristics and predictive markers of severely and critically ill patients with COVID-19.
METHODS
A retrospective study was conducted at the B11 Zhongfaxincheng campus and E1-3 Guanggu campus of Tongji Hospital affiliated with Huazhong University of Science and Technology in Wuhan. Patients with COVID-19 admitted from 1st February 2020 to 8th March 2020 were enrolled and categorized into 3 groups: The moderate group, severe group and critically ill group. Epidemiological data, demographic data, clinical symptoms and outcomes, complications, laboratory tests and radiographic examinations were collected retrospectively from the hospital information system and then compared between groups.
RESULTS
A total of 126 patients were enrolled. There were 59 in the moderate group, 49 in the severe group, and 18 in the critically ill group. Multivariate logistic regression analysis showed that age [odd ratio (OR) = 1.055, 95% (confidence interval) CI: 1.099-1.104], elevated neutrophil-to-lymphocyte ratios (OR = 4.019, 95%CI: 1.045-15.467) and elevated high-sensitivity cardiac troponin I (OR = 10.126, 95%CI: 1.088 -94.247) were high-risk factors.
CONCLUSION
The following indicators can help clinicians identify patients with severe COVID-19 at an early stage: age, an elevated neutrophil-to-lymphocyte ratio and high sensitivity cardiac troponin I.
Keywords: COVID-19, SARS-CoV-2, Critically ill, Risk factors, Aspartate transaminase, Amino-terminal pro-brain natriuretic peptide, Creatinine, Calcium
Core Tip: Here, we conducted a case-control study and found out that early drug treatment is an important measure in the treatment of patients with coronavirus disease 2019 (COVID-19). And the following indicators can help clinicians identify patients with severe COVID-19 at an early stage: an elevated neutrophil-to-lymphocyte ratio; elevated aspartate transaminase, N-terminal pro b-type natriuretic peptide, and creatinine levels; as well as decreased serum calcium level.
INTRODUCTION
Since the first report of coronavirus disease 2019 (COVID-19) in Wuhan, China, in December 2019, this highly infectious respiratory disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has spread rapidly throughout the world, posing a serious threat to global health. Similar to SARS-CoV, the novel SARS-CoV-2 uses angiotensin converting enzyme II (ACE2) receptors to invade not only type II alveolar cells in the lung but also myocardial cells in the heart, proximal tubule cells in the kidney and other cells in organs with high ACE2 expression levels[1-4]. Several studies reported that a number of patients had rapid disease progression and died of acute respiratory distress syndrome and/or multiple organ failure[5-7].
According to the Chinese management guideline for COVID-19 (versions 1.0 through 7.0), the definition and classification of COVID-19 severity is divided into four levels: mild, moderate, severe and critical. The main parameters for classification are level of hypoxemia and progression of radiographic presentation[8]. Several studies have reported the mortality rates of different groups. In the latest Chinese Centers for Disease Control (CDC) report that included records from 44672 cases, patients with severe and critical disease accounted for 13.8% and 4.7% of confirmed cases, respectively; the crude case fatality rate among critically ill patients was 49%, and the fatality density was 0.325[9]. Feng et al[10] reported that the mortality rates of the moderate, severe and critically ill groups were 6.2%, 12.5% and 49.1%, respectively, in Hubei, and 0%, 0%, and 13.3%, respectively, outside of Hubei. Of 52 critically ill adult patients in the Yang et al[6] study, 32 (61.5%) had died at 28 d. The estimated mortality rates in the Li et al[5] study were 1.1% for patients with non-severe disease and 32.5% for patients with severe disease over an average of 32 d. Zhou et al[11] reported mortality rates of 0%, 18.2% and 79.2% for patients with moderate, severe and critically ill disease, respectively. Previous studies confirmed that older age, chronic disease, and D-dimer greater than 1 µg/L were important independent predictors of mortality from COVID-19.
However, the risk factors for patients with moderate-to-severe or severe-to-critical COVID-19 remain unclear. A comprehensive description of the clinical characteristics, laboratory changes, in addition to oxygen levels and radiographic examinations enable clinicians to provide more accurate prognoses and specific care which vary according to subclinical or latent severe cases. Here, we aim to explore the typical clinical characteristics and examination parameters of critically ill patients with COVID-19 at two campuses of Tongji Hospital affiliated with Huazhong University of Science and Technology in Wuhan, China.
MATERIALS AND METHODS
Study design and participants
In this retrospective study, we enrolled all inpatients who were hospitalized for COVID-19 from 1st February to 8th March 2020, at the B11 Zhongfaxincheng campus and E1-3 Guanggu campus of Tongji Hospital affiliated with Huazhong University of Science and Technology in Wuhan, China. These two campuses were designated hospitals treating mainly severely and critically ill patients, and according to the arrangement of the Chinese government, patients with mild to moderate COVID-19 were isolated from their families and communities and then transferred and admitted to Fangcang shelter hospitals[12]. All patients in our study were confirmed with throat swab specimens to extract viral RNA for laboratory confirmation of SARS-CoV-2 infection. The study was approved by the Research Ethics Commission of Beijing Hospital (2020BJYYEC-047-01), and the requirement for written informed consent was waived by the Ethics Commission for emerging infectious diseases.
Procedures
Epidemiological data, demographic data, clinical symptoms and outcomes, complications, laboratory examinations and imaging test information were extracted from electronic medical records, and clinical outcomes were followed until March 26, 2020. If data were missing from the medical records or clarification was needed, we obtained data by direct communication with attending doctors and other health-care providers. All data were checked by two researchers and two physicians from each campus.
According to COVID-19 severity defined by the Chinese management guideline for COVID-19 (version 7.0)[8], we categorized the patients into 3 groups, namely, the moderate group (level 2), severe group (level 3) and critically ill (level 4) group, to analyze the clinical features and high-risk factors of severe and critical COVID-19.
Definitions
The severity of COVID-19 (according to the Chinese management guideline for COVID- 19 (version 7.0) was classified as follows[8].
Mild (level 1): The patient had light clinical symptoms but no evidence of pneumonia on X-ray or computed tomography (CT) examination.
Moderate (level 2): The patient had fever and respiratory symptoms, and the X-ray or CT examination showed evidence of pneumonia.
Severe (level 3): Patients aged over 18 years who met the following conditions: (1) Shortness of breath, with a respiratory rate ≥ 30; (2) Resting-state oxygen saturation values from one finger of one arm of ≤ 93%; (3) Arterial partial pressure of oxygen (PaO2)/fraction of inspired oxygen (FiO2) ≤ 300 mmHg; and (4) Rapid progression of lesions over 50% within 24-48 h.
Critically ill (level 4): Patients aged over 18 years who met the following conditions: (1) Acute respiratory failure requiring mechanical ventilation support; (2) Shock; and (3) COVID-19 complicated by other organ failure and the need for critical care in the Intensive care unit.
Statistical analysis
EpiData 3.1 was used for the data collection and SPSS (version 22.0) for the analyses. Continuous variables are presented as the mean ± SD if they were normally distributed or the median (interquartile range, IQR) if they were not, and variables were compared by one-way ANOVA, the Mann-Whitney U test or Kruskal-Wallis test. Categorical variables are presented as n (%) and were compared by the χ2 test or Fisher’s exact test. A two-sided α of less than 0.05 was considered statistically significant. The high-risk factors for severe and critically ill COVID-19 were analyzed by logistic regression analysis. An ordinal logistic regression model was adopted and used with JMP15.0 software to explore potential risk factors associated with the severity of COVID-19. According to clinical significance, which was the most important measure, data completeness and the single-factor screening results of the χ2 analysis were considered. Under clinician guidance, potential collinear variables were categorized to process the collinearity diagnosis by SPSS 22.0 (IBM Corp., Armonk, NY, United States). Timeline charts of laboratory parameters were plotted using GraphPad Prism version 8.0.
RESULTS
As of March 8, 126 patients with COVID-19 were included in this study, 67 from the B11 Zhongfaxincheng campus and 59 from the E1-3 Guanggu campus. There were 59 in the moderate group, 49 in the severe group, and 18 in the critically ill group. Although more than half of the infected patients in the severe and critically ill groups were men, there was no significant difference in sex between groups; however, compared with the moderate group, the difference became significant when we merged the severe and critically ill together (P = 0.008). Men were more vulnerable to COVID-19.
The median age of the patients was 61.00 years (IQR 48.00-68.00), ranging from 24 years to 91 years. Patients in the critically ill group were significantly older than those in the moderate group (65.44 vs 54.76 years, P = 0.019). The median time from onset of symptoms to first hospital admission was 8 d (IQR 3.00-14.00), and among the three groups, this duration was longest in the moderate group. The median time from onset of symptoms to COVID-19 Laboratory confirmation (via throat swab samples) was 7.5 d (IQR 3.35-13.75), and there were no differences between groups (Table 1).
Table 1.
Baseline and clinical characteristics of patients with coronavirus disease 2019
|
Demographics and clinical characteristics
|
Total
|
Moderate
|
Severe
|
Critically ill
|
P
value
|
| Male | 67/126 (53.2) | 24/59 (40.7) | 30/49 (61.2) | 13/18 (72.2) | 0.022 |
| Age (yr) | 54.76 ± 1.73 | 59.22 ± 2.10 | 65.44 ± 3.17 | P (Critically ill vs moderate) = 0.019 | |
| Exposure history | 28/67 (41.8) | 10/19 (52.6) | 13/36 (36.1) | 5/12 (41.7) | 0.498 |
| Time from illness onset to hospital admission (d) | 16.00 (11.00-18.00) | 7.00 (3.75-11.25) | 6.00 (4.00-7.00) | P (Critically ill vs moderate) = 0.000; P (Severe vs moderate) = 0.001 | |
| Time from illness onset to laboratory confirmation (d) | 10.00 (5.00-13.00) | 7.50 (3.75-18.00) | 2.00 (0.0 -4.50) | 0.176 | |
| Comorbidity | |||||
| All | 104/126 (82.5) | 51/59 (86.4) | 35/49 (71.4) | 18/18 (100) | P (Critically ill vs severe) < 0.05 |
| COPD/CB | 6/126 (4.8) | 1/59 (1.7) | 3/49 (6.1) | 2/18 (11.1) | 0.152 |
| Cerebrovascular disease | 3/126 (2.4) | 0/59 (0) | 1/49 (2.0) | 2/18 (11.1) | P (Critically ill vs moderate) < 0.05 |
| Coronary heart disease | 7/126 (5.6) | 4/59 (6.8) | 3/49 (6.1) | 0/18 (0.0) | 0.325 |
| Chronic liver disease | 6/126 (4.8) | 0/59 (0.0) | 5/49 (10.2) | 1/18 (5.6) | P (Severe vs moderate) < 0.05 |
| Hypertension | 46/126 (36.5) | 21/59 (35.6) | 16/49 (32.7) | 9/18 (50.0) | 0.417 |
| Diabetes | 22/126 (17.5) | 7/59 (11.9) | 10/49 (20.4) | 5/18 (27.8) | 0.238 |
| Hyperlipidemia | 1/126 (0.8) | 0/59 (0.0) | 1/49 (2.0) | 0/18 (0.0) | 0.532 |
| Cancer | 11/126 (8.7) | 2/59 (3.4) | 4/49 (8.2) | 5/18 (27.8) | P (Critically ill vs moderate) < 0.05 |
| Drug history | |||||
| Aspirin | 2/67 (3.0) | 0/19 (0.0) | 1/36 (2.8) | 1/12 (8.3) | 0.406 |
| Beta blockers | 2/67 (3.0) | 1/19 (5.3) | 1/36 (2.8) | 0/12 (0.0) | 1.000 |
| Insulin | 2/67 (3.0) | 1/19 (5.3) | 0/36 (0) | 1/12 (8.3) | 0.210 |
| Stains | 2/67 (3.0) | 0/19 (0.0) | 2/36 (5.6) | 0/12 (0.0) | 0.691 |
| ACEIs | 6/126 (4.8) | 4/59 (6/8) | 2/49 (4.1) | 0/18 (0.0) | 0.623 |
| ARB | 16/126 (12.7) | 7/59 (11.9) | 9/49 (18.4) | 0/18 (0.0) | 0.045 |
| Drug treatment before admission | |||||
| All | 108/126 (85.7) | 55/59 (93.2) | 40/49 (81.6) | 13/18 (72.2) | P (Critically ill vs moderate) < 0.05 |
| Antibiotics | 33/67 (49.3) | 7/19 (36.8) | 19/36 (53.7) | 7/12 (58.3) | 0.418 |
| Traditional Chinese medicine | 83/126 (65.9) | 49/59 (83.1) | 28/49 (57.1) | 6/18 (33.3) | P (Critically ill vs moderate) < 0.05; P (Severe vs moderate) < 0.05 |
| Arbidol | 70/126 (55.6) | 43/59 (72.9) | 20/49 (40.8) | 7/18 (38.9) | P (Critically ill vs moderate) < 0.05; P (Severe vs moderate) < 0.05 |
| Oseltamivir | 28/67 (41.8) | 10/19 (52.6) | 16/36 (44.4) | 2/12 (16/7) | 0.126 |
| Lopinavir/ritonavir | 2/126 (1.6) | 1/59 (1.7) | 0/49 (0.0) | 1/18 (5.6) | 0.266 |
| Fever | 98/126 (77.8) | 44/59 (46.8) | 42/49 (85.7) | 12/18 (66.7) | 0.175 |
| Maximum body temperature | 38.90 (38.00-39.40) | 38.70 (38.00-39.00) | 39.00 (38.92-39.25) | 0.440 | |
| Rigor | 32/67 (47.8) | 7/19 (36.8) | 20/36 (55.6) | 5/12 (42.7) | 0.375 |
| Fatigue | 43/67 (64.2) | 10/19 (52.6) | 24/36 (66.7) | 9/12 (75.0) | 0.405 |
| Sore throat | 25/67 (37.3) | 6/19 (31.6) | 14/36 (38.9) | 5/12 (41.7) | 0.816 |
| Running nose | 1/67 (1.5) | 0/19 (0.0) | 1/36 (2.8) | 0/12 (0.0) | 1.000 |
| Stuffy nose | 6/67 (9.0) | 1/19 (5.3) | 5/36 (13.9) | 0/12 (0.0) | 0.169 |
| Cough | 98/126 (77.8) | 46/59 (78.0) | 40/49 (81.6) | 12/18 (66.7) | 0.426 |
| Expectoration | 39/67 (58.2) | 11/19 (57.9) | 22/36 (61.1) | 6/12 (50.0) | 0.795 |
| White sputum | 14/67 (20.9) | 1/19 (5.3) | 11/36 (30.6) | 2/12 (16.7) | 0.083 |
| Yellow sputum | 5/67 (7.5) | 1/19 (5.3) | 4/36 (11.1) | 0/12 (0.0) | 0.571 |
| Blood-stained sputum | 15/67 (22.4) | 5/19 (26.3) | 8/36 (22.2) | 2/12 (16.7) | 0.817 |
| Shortness of breath | 49/126 (38.9) | 19/59 (32.2) | 22/49 (44.9) | 8/18 (44.4) | 0.352 |
| Exertional dyspnea | 24/67 (35.8) | 6/19 (31.6) | 14/36 (38.9) | 4/12 (33.3) | 0.848 |
| Headache | 22/67 (32.8) | 7/19 (36.8) | 11/36 (30.6) | 4/12 (33.3) | 0.895 |
| Myalgia | 29/67 (43.3) | 5/19 (26.3) | 18/36 (50.0) | 6/12 (50.0) | 0.211 |
| Abdominal pain | 21/67 (31.3) | 4/19 (21.1) | 13/36 (36.1) | 4/12 (33.3) | 0.497 |
| Diarrhea | 40/126 (31.7) | 16/59 (27.1) | 20/49 (40.8) | 4/18 (22.2) | 0.202 |
| Nausea | 29/67 (43.3) | 7/19 (36.8) | 17/36 (47.2) | 5/12 (41.7) | 0.755 |
| Anorexia | 32/67 (47.8) | 6/19 (31.6) | 20/36 (55.6) | 6/12 (50.0) | 0.235 |
| Vomiting | 22/67 (32.8) | 4/19 (21.1) | 13/36 (36.1) | 5/12 (41.7) | 0.392 |
| Conjunctivitis | 0/67 (0.0) | 0/19 (0.0) | 0/36 (0.0) | 0/12 (0.0) | - |
Data are presented as n/n (%) or mean ± SD or median (interquartile range). P values were calculated by Mann-Whitney U test, χ² test, or Fisher’s exact test, as appropriate.
Of the 126 patients, 104 (82.5%) had 1 or more coexisting medical conditions. Hypertension [46 (36.5%)], diabetes [22 (17.5%)], cancer [11 (8.7%)], and coronary heart disease [7 (5.6%)] were the most common coexisting conditions. Compared with the critically ill group, the moderate group had fewer patients with cerebrovascular diseases or cancer (P < 0.05). Compared with the severe group, the moderate group had fewer patients with chronic liver disease (P < 0.05). There were more patients in the critically ill group with 1 or more underlying diseases than in the other groups. Before admission, 33 (33/67, 49.3%) patients reported having taken antibiotics, and third-generation cephalosporins, and quinolone antibiotics were the most common. Twenty-eight (28/67, 41.8%) patients reported taking oseltamivir. Two (2/126, 1.6%) patient reported taking lopinavir/ritonavir. Seventy (70/126, 55.6%) patients reported having taken arbidol. In addition, 83 patients reported having taken traditional Chinese medicine, mainly Lianhua Qingwen capsules. Regarding patients who had taken traditional Chinese medicine and arbidol before admission, they were more likely to be in the moderate group (P < 0.05) (Table 1).
Among the 126 patients in the study, the most common symptoms at disease onset were fever [98 (77.8%)], cough [98 (77.8%)], shortness of breath [49 (38.9%)] and fatigue (43/67, 64.2%). Less common symptoms included expectoration, rigor, anorexia, myalgia, and nausea (Table 1). No significant differences in the symptoms at disease onset were found between groups (Table 1).
All patients had received radiographic examination in our study. Among 86 imaging diagnostic reports, patchy shadows were found in 45.3% and 44% of patients’ early chest CT and X-ray reports, respectively, and 47.7% of the patients’ reports showed multiple area involvement. A total of 18.6% of images showed pleural adhesions, 15.1% showed emphysema, and 14.0% showed enlarged mediastinal lymph nodes. 35 patients who were admitted to the B11 Zhongfaxincheng campus of Tongji Hospital had ground-glass opacity.
All the laboratory data were collected through patients’ electronic medical records. Patient’s first laboratory results are shown in Table 2. Of all the patients, 58 (58/123, 47.2%) had hemoglobin levels below normal. In addition, 44 (44/121, 36.4%) patients had lymphocyte counts below normal, and 35 (35/125, 28.0%) patients had lower lymphocyte ratios. Seventy-eight (78/123, 63.4%) patients had decreased hematocrit levels. 107/122 (87.7%) patients had higher-than- normal levels of C-reactive protein. Regarding coagulation tests, 8 (8/66, 12.1%), 11 (11/66, 16.7%) and 39 (39/66, 59.1%) patients had elevated thrombin times, prothrombin times and fibrinogen levels, respectively; notably, 80 (80/124, 64.5%) patients showed significantly increased D-dimer levels. Additionally, we found that 71 (71/120, 59.2%) patients had increased lactate dehydrogenase, 45 (45/62, 72.6%) patients had higher ferritin levels, and 68 (68/120, 56.7%) patients had decreased calcium levels (Table 2).
Table 2.
Laboratory findings of patients with coronavirus disease 2019
|
Laboratory findings
|
Total
|
Moderate
|
Severe
|
Critically ill
|
P
value
|
| Leukocyte count, × 109/L | 6.02 ± 2.07 | 5.79 ± 0.21 | 6.09 ± 0.32 | 6.66 ± 0.71 | |
| Total | 125/125 (100) | 59/125 (47.2) | 49/125 (39.2) | 17/125 (13.6) | 0.26 |
| Lower (< 3.5) | 13/125 (10.4) | 4/59 (6.8) | 6/49 (12.2) | 3/17 (17.6) | |
| Normal (3.5-9.5) | 104/125 (83.2) | 53/59 (89.8) | 39/49 (79.6) | 12/17 (70.6) | |
| Higher (> 9.5) | 8/125 (6.4) | 2/59 (3.4) | 4/49 (8.2) | 2/17 (11.8) | |
| Neutrophil count, × 109/L | 3.78 (2.40-5.15) | 3.37 (2.40-4.19) | 4.05 (2.34-5.17) | 5.71 (2.82-7.45) | |
| Total | 121/121 (100) | 56/121 (46.3) | 47/121 (38.8) | 18/121 (14.9) | P (Moderate vs critically ill) < 0.05 |
| Lower (< 1.8) | 13/121 (10.8) | 3/56 (5.4) | 7/47 (14.9) | 3/18 (16.7) | |
| Normal (1.8-6.3) | 91/121 (75.2) | 50/56 (89.2) | 33/47 (70.2) | 8 (44.4) | |
| Higher (> 6.3) | 17/121 (14.0) | 3/56 (5.4) | 7/47 (14.9) | 7 (38.9) | |
| Neutrophil ratio | 63.70 (53.75-73.80) | 57.85 (52.80-57.85) | 68.55 (52.53-75.78) | 81.05 (72.90-87.25) | |
| Total | 124/124 (100) | 58/124 (46.8) | 48/124 (38.7) | 18/124 (14.5) | P (Moderate vs severe) < 0.05; P (Moderate vs critically ill) < 0.05; P (Severe vs critically ill) < 0.05 |
| Lower (< 40) | 4/124 (3.2) | 0/58 (0) | 4/48 (8.5) | 0/18 (0) | |
| Normal (40-75) | 89/124 (71.8) | 53/58 (91.4) | 31/48 (64.6) | 5/18 (27.8) | |
| Higher (> 75) | 31/124 (25.0) | 5/58 (8.6) | 13/48 (27.1) | 13/18 (72.2) | |
| Eosinophil count, × 109/L | 0.08 (0.03-0.15) | 0.13 (0.06-0.20) | 0.07 (0.02-0.14) | 0.00 (0.00-0.06) | |
| Total | 121/121 (100) | 56/121 (46.3) | 47/121 (38.8) | 18/121 (14.9) | P (Moderate vs critically ill) < 0.05; P (Severe vs critically ill) < 0.05 |
| Lower (< 0.02) | 25/121 (20.7) | 3/56 (5.4) | 11/47 (23.4) | 11/18 (61.1) | |
| Normal (0.02-0.52) | 95/121 (78.5) | 52/56 (92.9) | 36/47 (76.6) | 7/18 (38.9) | |
| Higher (> 0.52) | 1/121 (0.8) | 1/56 (1.8) | 0/56 (0.0) | 0/56 (0.0) | |
| Eosinophil ratio, | 1.35 (0.40-2.83) | 2.20 (1.10-3.40) | 1.25 (0.20-2.18) | 0 (0-0.65) | |
| Total | 122/122 (100) | 56/122 (45.9) | 48/122 (39.3) | 18/122 (14.8) | P (Moderate vs severe) < 0.05; P (Moderate vs critically ill) < 0.05; P (Severe vs critically ill) < 0.05 |
| Lower (< 0.4) | 29/122 (23.8) | 3/56 (5.4) | 14/48 (29.2) | 12/18 (23.8) | |
| Normal (0.4-8) | 91/122 (74.6) | 51/56 (91.1) | 34/48 (70.8) | 6/18 (74.6) | |
| Higher (> 8) | 2/122 (1.6) | 2/56 (3.6) | 0/48 (0) | 0/18 (0) | |
| Monocyte count, × 109/L | 0.53 (0.40-0.66) | 0.55 (0.46-0.66) | 0.55 (0.38-0.67) | 0.39 (0.20-0.56) | |
| Total | 123/123 (100) | 57/123 (46.3) | 48/123 (39.0) | 18/123 (14.7) | 0.266 |
| Lower (< 0.1) | 1/123 (0.8) | 0/57 (0.0) | 0/48 (0.0) | 1/18 (5.6) | |
| Normal (0.1-0.6) | 80/123 (65.0) | 37/57 (64.9) | 30/48 (62.5) | 13/18 (72.2) | |
| Higher (> 0.6) | 42/123 (34.1) | 20/57 (35.1) | 18/48 (37.5) | 4/18 (22.2) | |
| Monocyte ratio, | 9.05 (7.90-10.33) | 9.15 (8.23-11.03) | 9.3 (8.03-10.28) | 5.90 (3.75-8.80) | |
| Total | 122/122 (100) | 56/122 (45.9) | 48/122 (39.3) | 18/122 (14.8) | P (Moderate vs critically ill) < 0.05 |
| Lower (< 3) | 3/122 (2.5) | 0/56 (0) | 1/48 (2.1) | 2/18 (11.1) | |
| Normal (3-10) | 82/122 (67.2) | 35/56 (62.5) | 33/48 (68.8) | 14/18 (77.8) | |
| Higher (> 10) | 37/122 (30.3) | 21/56 (37.5) | 14/48 (29.2) | 2/18 (11.1) | |
| Lymphocyte count, × 109/L | 1.25 (0.92-1.88) | 1.79 (1.25-2.01) | 1.19 (0.90-1.44) | 0.70 (0.52-0.87) | |
| Total | 121/121 (100) | 56/121 (46.3) | 47/121 (38.8) | 18/121 (14.9) | P (Moderate vs severe) < 0.05; P (Moderate vs critically ill) < 0.05; P (Severe vs critically ill) < 0.05 |
| Lower (< 1.1) | 44/121 (36.4) | 8/56 (14.3) | 20/47 (42.6) | 16/18 (88.9) | |
| Normal (1.1-3.2) | 75/121 (62.0) | 47/56 (83.9) | 26/47 (55.3) | 2/18 (11.1) | |
| Higher (> 3.2) | 2/121 (1.7) | 1/56 (1.8) | 1/47 (2.1) | 0/18 (0.0) | |
| Lymphocyte ratio, | 24.40 (14.55-32.40) | 29.60 (23.20-34.10) | 20.15 (13.40-33.28) | 12.95 (8.50-20.15) | |
| Total | 125/125 (100) | 59/125 (47.2) | 48/125 (38.4) | 18/125 (14.4) | P (Moderate vs severe) < 0.05; P (Moderate vs critically ill) < 0.05 |
| Lower (< 20) | 35/125 (28.0) | 9/59 (15.3) | 23/48 (47.9) | 13/18 (72.2) | |
| Normal (20-50) | 77/125 (61.6) | 50/59 (84.7) | 22/48 (45.8) | 5/18 (27.8) | |
| Higher (> 50) | 3/125 (2.4) | 0/59 (0) | 3/48 (6.3) | 0/18 (0) | |
| Hemoglobin, g/L | 127.00 (114.00-136.00) | 127.00 (116.00-135.00) | 126.00 (114.75-136.75) | 123.00 (103.00-136.00) | |
| Total | 123/123 (100) | 57/123 (47.2) | 48/123 (38.4) | 18/123 (14.4) | P (Moderate vs critically ill) < 0.05 |
| Lower (< 1151, < 1302) | 58/123 (47.2) | 21/57 (36.8) | 24/48 (50) | 13/18 (72.2) | |
| Normal (115-1501, 130-1752) | 65/123 (52.8) | 36/57 (63.2) | 24/48 (50) | 5/18 (27.8) | |
| Hematocrit, | 36.50 (33.30-39.40) | 37.40 (33.75-39.60) | 36.5 (33.15-39.95) | 34.55 (29.85-38.65) | |
| Total | 123/123 (100) | 57/123 (46.4) | 48/123 (39.0) | 18/123 (14.6) | P (moderate vs critically ill) < 0.05 |
| Lower (< 341, < 402) | 78/123 (63.4) | 29/57 (50.9) | 33/48 (68.8) | 16/18 (88.9) | |
| Normal (34-451, 40-502) | 44/123 (35.8) | 27/57 (47.4) | 15/48 (31.3) | 2/18 (11.1) | |
| Higher (> 451, > 452) | 1/123 (0.8) | 1/57 (1.8) | 0/48 (0) | 0/18 (0) | |
| Platelet’s count, × 109/L | 260.00 (187.50-342.50) | 268.00 (212.50-336.75) | 287.00 (193.00-368.00) | 157.00 (67.00-230.75) | |
| Total | 122/122 (100) | 56/122 (45.9) | 48/122 (39.3) | 18/122 (14.8) | P (Moderate vs severe) < 0.05; P (Moderate vs critically ill) < 0.05 |
| Lower (< 125) | 10/122 (8.2) | 2/56 (3.6) | 2/48 (4.2) | 6/18 (33.3) | |
| Normal (125-350) | 84/122 (68.8) | 41/56 (73.2) | 32/48 (66.7) | 1118 (61.1) | |
| Higher (> 350) | 28/122 (23.0) | 13/56 (23.2) | 14/48 (29.2) | 1/18 (5.6) | |
| Neutrophil to lymphocyte ratio | 2.57 (1.57-4.82) | 1.99 (1.43-2.81) | 3.29 (1.49-5.50) | 6.21 (3.62-9.78) | |
| Total | 121/121 (100) | 55/121 (45.4) | 48/121 (39.7) | 18/121 (14.9) | P (Moderate vs severe) < 0.05; P (Moderate vs critically ill) < 0.05 |
| Normal (≤ 3.13) | 71/121 (58.7) | 46/55 (83.6) | 21/55 (43.8) | 4/55 (22.2) | |
| Higher (> 3.13) | 50/121 (41.3) | 9/55 (16.4) | 27/55 (56.3) | 14/55 (77.8) | |
| Alanine aminotransferase, U/L | 25.00 (15.00-43.00) | 22.00 (16.00-41.00) | 28.00 (13.50-45.50) | 37.00 (14.00-47.50) | |
| Total | 123/123 (100) | 57/123 (46.4) | 49/123 (39.8) | 17/123 (13.8) | 0.77 |
| Normal (≤ 331, ≤ 412) | 89/123 (73.4) | 43/57 (74.4) | 34/49 (69.4) | 12/17 (70.6) | |
| Higher (> 331, > 412) | 34/123 (27.6) | 14/57 (24.6) | 15/49 (30.6) | 5/17 (29.4) | |
| Lactate dehydrogenase, U/L | 253.0 (193.25-300.0) | 210.0 (186.0-266.5) | 262.00 (193.00-300.00) | 341.00 (286.75-497.00) | |
| Total | 120/120 (100) | 55/120 (45.8) | 57/120 (47.5) | 18/120 (14.7) | P (Moderate vs critically ill) < 0.05 |
| Lower (< 135) | 5/120 (4.2) | 4/55 (7.3) | 1/57 (2.1) | 0/18 (0) | |
| Normal (135-2141, 135-2252) | 44/120 (36.7) | 24/55 (43.6) | 18/57 (38.3) | 2/18 (11.1) | |
| Higher (>2141,>2252) | 71/120 (59.2) | 27/55 (49.1) | 28/57 (59.6) | 16/18 (88.9) | |
| Aspartate aminotransferase, U/L | 24.00 (17.00-37.00) | 22.00 (17.00-29.00) | 22.00 (17.00-37.00) | 42.00 (21.00-68.50) | |
| Total | 123/123 (100) | 57/123 (46.4) | 49/123 (39.8) | 17/123 (13.8) | P (Moderate vs critically ill) < 0.05 |
| Normal (≤ 321, ≤ 402) | 94/123 (76.4) | 48/57 (84.2) | 39/49 (79.6) | 7/17 (41.2) | |
| Higher (> 321, > 402) | 29/123 (23.6) | 9/57 (15.8) | 10/49 (20.4) | 10/17 (58.8) | |
| Serum amylase, U/L | 69.33 ± 2.65 | 66.51 ± 3.55 | 71.59 ± 5.17 | 71.92 ± 4.92 | |
| Total | 77/77 (100) | 35/77 (45.4) | 29/77 (39.7) | 13/77 (14.9) | 0.13 |
| Lower (< 28) | 1/77 (1.3) | 1/35 (2.9) | 0/29 (0) | 0/13 (0) | |
| Normal (28-100) | 66/77 (85.7) | 32/35 (91.4) | 22/29 (75.9) | 12/13 (92.3) | |
| Higher (> 100) | 10/77 (13.0) | 2/35 (5.7) | 7/29 (24.1) | 1/13 (7.7) | |
| Total bilirubin, μmol/L | 8.00 (5.90-12.00) | 7.40 (4.30-10.60) | 8.65 (5.98-12.43) | 10.45 (7.98-18.13) | |
| Total | 121/121 (100) | 55/121 (45.4) | 48/121 (39.7) | 18/121 (14.9) | 0.18 |
| Normal (≤ 211, ≤ 262) | 116/121 (95.9) | 54/55 (98.2) | 46/48 (95.8) | 16/18 (88.9) | |
| Higher (> 211, > 262) | 5/121 (4.1) | 1/55 (1.8) | 2/48 (4.2) | 2/18 (11.1) | |
| Albumin, g/L | 36.19 ± 4.87 | 39.25 ± 0.51 | 34.44 ± 0.56 | 31.49 ± 1.03 | |
| Total | 121/121 (100) | 55/121 (45.4) | 48/121 (39.7) | 18/121 (14.9) | P (Moderate vs severe) < 0.05; P (Moderate vs critically ill) < 0.05 |
| Lower (< 35) | 46/121 (38.0) | 7/55 (12.7) | 27/48 (56.3) | 12/18 (66.7) | |
| Normal (35-52) | 75/121 (62.0) | 48/55 (87.3) | 21/48 (43.8) | 6/18 (33.3) | |
| Creatinine, μmol/L | 69.00 (59.00-78.00) | 67.00 (58.00-80.00) | 69.00 (60.00-76.00) | 73.00 (54.75-87.50) | |
| Total | 121/121 (100) | 55/121 (45.4) | 48/121 (39.7) | 18/121 (14.9) | P (Moderate vs severe) < 0.05; P (Severe vs critically ill) < 0.05 |
| Lower (< 451, < 582) | 13/121 (10.7) | 2/55 (3.6) | 6/48 (12.5) | 5/18 (27.8) | |
| Normal (45-841, 59-1042) | 101/121 (83.5) | 51/55 (92.7) | 41/48 (85.4) | 9/18 (50) | |
| Higher (> 841, > 1042) | 7/121 (5.8) | 2/55 (3.6) | 1/48 (2.1) | 4/18 (22.2) | |
| C-reactive protein, mg/L | 6.55 (1.60-38.50) | 2.30 (1.20-6.70) | 12.40 (2.68-48.58) | 91.00 (37.3-131.25) | |
| Total | 122/122 (100) | 57/122 (46.7) | 48/122 (39.4) | 17/122 (13.9) | P (Moderate vs severe) < 0.05 |
| Normal (≤ 1) | 15/122 (12.3) | 12/57 (21.1) | 2/48 (4.2) | 1/17 (5.9) | |
| Higher (> 1) | 107/122 (87.7) | 45/57 (78.9) | 46/48 (95.8) | 16/17 (94.1) | |
| Creatine kinase, U/L | 46.00 (33.50-66.50) | 41.00 (34.50-58.50) | 52.00 (33.00-77.00) | 55.00 (40.00-148.00) | |
| Total | 101/101 (100) | 49/101 (48.5) | 37/101 (36.6) | 15/101 (14.9) | 0.09 |
| Normal (≤ 170) | 94/101 (93.1) | 46/49 (93.9) | 36/37 (97.3) | 12/15 (80) | |
| Higher (> 170) | 7/101 (6.9) | 3/49 (6.1) | 1/37 (2.7) | 3/15 (20) | |
| Procalcitonin3, ng/mL | |||||
| Total | 91/91 (100) | 37/91 (40.7) | 37/91 (40.7) | 17/91 (18.6) | P (Moderate vs severe) < 0.05; P (Moderate vs critically ill) < 0.05 |
| Lower (< 0.02) | 6/91 (6.6) | 0/37 (0) | 5/37 (13.5) | 1/17 (5.9) | |
| Normal (0.02-0.05) | 44/91 (48.4) | 25/37 (67.6) | 17/37 (45.9) | 2/17 (11.8) | |
| Higher (> 0.05) | 41/91 (45.0) | 12/37 (32.4) | 15/37 (40.5) | 14/17 (82.4) | |
| Potassium, mmol/L | 4.24 (3.96-4.64) | 4.24 (3.97-4.40) | 4.30 (3.92-4.75) | 4.15 (3.85-4.59) | |
| Total | 64/64 (100) | 16/64 (25.0) | 36/64 (56.3) | 12/64 (18.7) | 0.27 |
| Lower (< 3.5) | 1/64 (1.6) | 0/16 (0) | 0/36 (0) | 1/12 (8.3) | |
| Normal (3.5-5.1) | 58/64 (90.6) | 14/16 (87.5) | 34/36 (94.4) | 10/12 (83.3) | |
| Higher (> 5.1) | 5/64 (7.8) | 2/16 (12.5) | 2/36 (5.6) | 1/12 (8.3) | |
| Corrected calcium, mmol/L | 2.41 ± 0.10 | 2.41 ± 0.02 | 2.40 ± 0.02 | 2.45 ± 0.04 | |
| Total (< 2.15) | 63/63 (100) | 16/63 (25.4) | 35/63 (55.6) | 12/63 (19.0) | 1.00 |
| Normal (2.15-2.57) | 59/63 (93.7) | 15/16 (93.8) | 33/35 (94.3) | 11/12 (91.7) | |
| Higher (> 2.57) | 4/63 (6.3) | 1/26 (6.3) | 2/35 (5.7) | 1/12 (8.3) | |
| Calcium, mmol/L | 2.16 ± 0.11 | 2.20 ± 0.01 | 2.14 ± 0.01 | 2.10 ± 0.03 | |
| Total | 120/120 (100) | 54/120 (45.0) | 48/120 (40.0) | 18/120 (15.0) | P (Moderate vs severe) < 0.05; P (Moderate vs critically ill) < 0.05 |
| Lower (< 2.151, < 2.202) | 68/120 (56.7) | 17/54 (31.5) | 35/48 (72.9) | 16/18 (88.9) | |
| Normal (2.15-2.51, 2.2-2.552) | 52/120 (43.3) | 37/54 (68.5) | 13/48 (27.1) | 2/18 (11.1) | |
| Thrombin time, s | 16.25 (15.28-16.93) | 15.15 (14.53-16.18) | 16.40 (15.55-17.20) | 16.90 (14.73-19.55) | |
| Total | 66/66 (100) | 18/66 (27.3) | 36/66 (54.5) | 12/66 (18.2) | P (Moderate vs critically ill) < 0.05 |
| Lower (< 14) | 1/66 (1.5) | 0/18 (0) | 0/36 (0) | 1//36 (8.3) | |
| Normal (14-19) | 57/66 (86.4) | 18/18 (100) | 32/36 (88.9) | 7/36 (58.3) | |
| Higher (> 19) | 8/66 (12.1) | 0/18 (0) | 4/36 (11.1) | 4/36 (33.3) | |
| Prothrombin time, s | 13.65 (13.20-14.23) | 13.25 (13.18-13.60) | 13.95 (13.45-14.28) | 14.00 (13.03-15.28) | |
| Total | 66/66 (100) | 18/66 (27.3) | 36/66 (54.5) | 12/66 (18.2) | P (Moderate vs critically ill) < 0.05 |
| Normal (11.5-14.5) | 55/66 (83.3) | 18/18 (100) | 30/36 (83.3) | 7/12 (58.3) | |
| Higher (> 14.5) | 11/66 (16.7) | 0/18 (0) | 6/36 (16.7) | 5/12 (41.7) | |
| Prothrombin activity | 94.00 (86.00-100.00) | 99.50 (95.50-102.25) | 90.50 (86.25-96.75) | 88.50 (75.00-105.00) | |
| Total | 66/66 (100) | 18/66 (27.3) | 36/66 (54.5) | 12/66 (18.2) | P (Moderate vs critically ill) < 0.05 |
| Lower (< 75) | 7/66 (10.6) | 0/18 (0) | 4/36 (11.1) | 3/12 (25) | |
| Normal (75-125) | 58/66 (87.9) | 18/18 (100) | 32/36 (88.9) | 8/12 (66.7) | |
| Higher (> 125) | 1/66 (1.5) | 0/18 (0) | 0/36 (0) | 1/12 (8.3) | |
| Fibrinogen, g/L | 4.46 ± 0.18 | 3.72 ± 0.22 | 4.75 ± 0.25 | 4.68 ± 0.55 | |
| Total | 66/66 (100) | 18/66 (27.3) | 36/66 (54.5) | 12/66 (18.2) | P (Moderate vs critically ill) < 0.05 |
| Lower (< 2) | 3/66 (4.5) | 0/18 (0) | 1/36 (2.8) | 2/12 (16.7) | |
| Normal (2-4) | 24/66 (36.4) | 11/18 (61.1) | 12/36 (33.3) | 1/12 (8.3) | |
| Higher (> 4) | 39/66 (59.1) | 7/18 (38.9) | 23/36 (63.9) | 9/12 (75) | |
| D-dimer3, μg/mL | |||||
| Total | 124/124 (100) | 57/124 (46.0) | 49/124 (39.5) | 18/124 (14.5) | P (Moderate vs severe) < 0.05; P (Moderate vs critically ill) < 0.05 |
| Normal (< 0.5) | 44/124 (35.5) | 34/57 (59.6) | 10/49 (20.4) | 0/18 (0) | |
| Higher (≥ 0.5) | 80/124 (64.5) | 23/57 (40.4) | 39/49 (79.6) | 18/18 (100) | |
| High-sensitivity cardiac troponin3, pg/mL | |||||
| Total | 123/123 (100) | 56/123 (46.4) | 49/123 (39.0) | 18/123 (14.6) | P (Moderate vs severe) < 0.05; P (Moderate vs critically ill) < 0.05 |
| Normal (≤15.61, ≤34.22) | 117/123 (95.1) | 54/56 (96.4) | 49/49 (100) | 14/18 (77.8) | |
| Higher (>15.61, >34.22) | 6/123 (4.9) | 2/56 (3.6) | 0/49 (0) | 4/18 (22.2) | |
| Amino-terminal pro-brainnatriuretic peptide3, pg/mL | |||||
| Total | 123/123 (100) | 57/123 (45.5) | 49/123 (39.9) | 18/123 (14.6) | P (Moderate vs severe) < 0.05; P (Moderate vs critically ill) < 0.05; P (Severe vs critically ill) < 0.05 |
| Normal (< 2851, < 4862) | 83/123 (67.5) | 50/57 (87.7) | 29/49 (60.4) | 4/18 (22.2) | |
| Higher (≥ 2851, ≥ 4862) | 40/123 (32.5) | 7/57 (12.3) | 19/49 (39.6) | 14/18 (77.8) | |
| Ferritin, μg/L | 468.25 (292.18-1022.08) | 9.80 (4.30-18.68) | 269.05 (169.60-407.23) | 601.60 (383.5-1195.90) | |
| Total | 62/62 (100) | 18/62 (29.0) | 31/62 (50.0) | 13/62 (21.0) | 0.08 |
| Lower (< 151, < 302) | 2/62 (3.2) | 1/18 (5.6) | 1/31 (3.2) | 0/13 (0) | |
| Normal (15-1501, 30-4002) | 15/62 (24.2) | 8/18 (44.4) | 6/31 (19.4) | 1/13 (7.7) | |
| Higher (> 1501, > 4002) | 45/62 (72.6) | 9/18 (50) | 24/31 (77.4) | 12/13 (92.3) |
The normal and abnormal range applied to female patients.
The normal and abnormal range applied to male patients.
Comparisons between groups were not completed because the laboratory item was grade variables.
Data are presented as n/n (%) or mean ± SD or median (interquartile range). The level of each laboratory finding was divided as normal and abnormal levels (lower than normal or higher than normal) according to the standard normal physiological range of our research centers. Average levels of each group were described by mean ± SD or median (interquartile range). Numbers and proportions of patients with different levels of each laboratory item were presented above.
Neutrophil ratio, eosinophil ratio, lymphocyte count and amnio-terminal pro-brain natriuretic peptide are significantly different between each group in the two-group comparisons. Additionally, compared with the moderate group, there are 21 more laboratory terms are significantly different (details of the between-group comparisons are shown in Table 2).
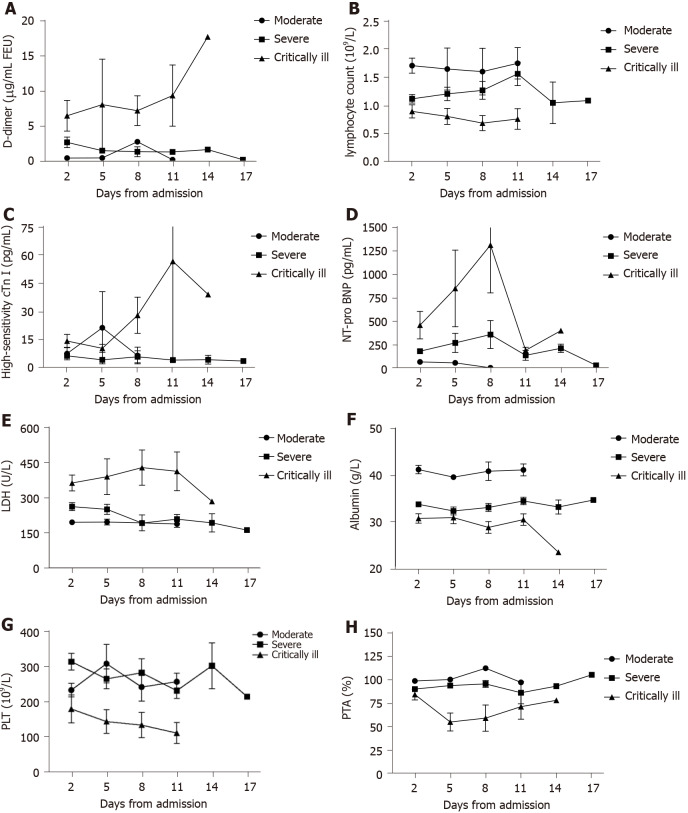
Major laboratory markers were tracked in 67 patients on the B11 Zhongfaxincheng campus beginning at admission (Figure 1). During hospitalization, most patients had marked lymphopenia, and patients in the critically ill group developed more severe lymphopenia over time. The level of D-dimer was significantly higher in the critically ill group than in the other groups and rapidly increased from day 11 after admission. Levels of high-sensitivity cardiac troponin I, amino-terminal pro-brain natriuretic peptide (NT-pro BNP), and lactate dehydrogenase were clearly elevated in the critically ill group compared with the other groups throughout the early clinical course but decreased from day 11. The level of albumin was lowest in the critically ill group and decreased with illness deterioration.
Figure 1.
Temporal changes in laboratory markers from admission in patients with coronavirus disease 2019. A-H: Timeline charts illustrate the laboratory markers changes in d-dimer, lymphocyte count, high-sensitivity cardiac troponin I, amino-terminal pro-brain natriuretic peptide, lactate dehydrogenase, albumin, platelets and prothrombin activity from admission in 67 patients with coronavirus disease 2019. cTn I: Cardiac troponin I; NT-pro BNP: Amino-terminal pro-brain natriuretic peptide; LDH: Lactate dehydrogenase; PLT: Platelets; PTA: Prothrombin activity.
An ordinal logistic regression model was adopted and conducted with JMP15.0 software to explore potential risk factors associated with the severity of COVID-19. Based on data completeness, the clinical significance and the single-factor screening results in Table 1 and Table 2, we first took all the variables proven to be significant in the χ2 tests as candidate variables while excluding the time from disease onset to hospital admission, thrombin time, prothrombin time, prothrombin activity and fibrinogen for incomplete data sets, as only one of our research centers had collected these data. Second, we categorized the remaining candidate variables as comorbidity, drug treatment before admission, coagulation system, inflammation markers and liver functional system to the process collinearity diagnosis. Thus, we excluded hemoglobin, chronic liver disease, lactate dehydrogenase and C-reactive protein. Remaining candidate variables included age, sex, cerebrovascular disease, cancer, neutrophil-to- lymphocyte ratio, monocyte ratio, creatinine, aspartate transaminase (AST), drug treatment before admission, albumin, calcium, hematocrit, procalcitonin, NT-pro BNP, platelet count, eosinophil ratio and high- sensitivity cardiac troponin I. Then, a step-by-step regression method (P for inclusion = 0.05, P for exclusion = 0.05) was applied, and 3 variables were included in the final model: age, neutrophil-to-lymphocyte ratio and high-sensitivity cardiac troponin I (Table 3).
Table 3.
Logistic regression results of potential risk factors for severity of coronavirus disease 2019
|
|
Wald χ2
|
P
value
|
OR
|
95%CI
|
|
|
Upper bound
|
Lower bound
|
||||
| Intercept (Moderate) | 1.800 | 0.180 | 9.816 | - | - |
| Intercept (Severe) | 1.122 | 0.289 | 0.179 | - | - |
| Age | 5.477 | 0.019 | 1.055 | 1.009 | 1.104 |
| High-sensitivity cardiac troponin I | |||||
| Normal (≤ 15.61, ≤ 34.22) | |||||
| Higher (> 15.61, > 34.22) | 4.094 | 0.043 | 4.019 | 1.045 | 15.467 |
| Neutrophil to lymphocyte ratio | |||||
| Higher (> 3.13) | 5.477 | 0.042 | 10.126 | 1.088 | 94.247 |
| Normal (≤ 3.13) | |||||
The normal and abnormal range applied to female patients.
The normal and abnormal range applied to male patients.
Ordinal logistic regression model was adopted in JMP15.0 software to explore the risk factors associated with severity of coronavirus disease 2019 patients. Step-by-step regression method (P for inclusion = 0.05, P for exclusion = 0.05) was applied and 3 variables were included in the final model. Results showed that total model test χ2 = 17.380, P < 0.001, which means at least one β in the equation did not equal to 0 and this model was preceded to constant 1. Good-to-fitness test showed χ2 = 59.137, P = 0.968 > 0.05, which means this model fit well. OR: Odds ratio.
The results showed that the total model test χ2 =17.380, P < 0.001, which meant at least one β in the equation did not equal 0, and the final model was preceded to a constant value. The goodness-of-fit test showed χ2 =59.137, P = 0.968 > 0.05, meaning that the final model fit well. We found that neutrophil-to-lymphocyte ratio (P = 0.042) and high-sensitivity cardiac troponin I (P = 0.043) were statistically significant independent risk factors (details in Table 3). Compared with patients who had normal neutrophil-to-lymphocyte ratios and high-sensitivity cardiac troponin I, the odd ratio (OR) for severe COVID-19 in patients with elevated neutrophil-to- lymphocyte ratios were 4.019 times higher [95% confidence interval (CI): 1.045-15.467] and elevated high-sensitivity cardiac troponin I was 10.126 times higher (95%CI: 1.088-94.247).
As of 22nd March, 2020, 114 (90.5%) of 126 patients were discharged, 8 (6.3%) patients died, and 4 (3.2%) remained hospitalized. Fitness for discharge was based on abatement of fever for at least 3 d, with the disappearance of respiratory symptoms, improvement based on chest radiographic evidence, and two successive indications (interval not less than 24 h) of viral clearance in respiratory samples obtained from the upper respiratory tract. For those who were discharged, the length of hospital stay was 18 d (IQR, 10.0-26.0). For those who died, the time from admission to death was 5.00 d (IQR 1.75-16.50).
DISCUSSION
This retrospective study discovered several clinical features and risk factors for critical illness in patients who were hospitalized with COVID-19 in Tongji Hospital. As of March 8, 2020, 126 patients with COVID-19 were included in this study: 67 from the B11 Zhongfaxincheng campus and 59 from the E1-3 Guanggu campus. Of the 59 (46.8%) patients in the moderate group, 24 were male. Of the 49 (38.8%) patients in the severe group, 30 were male. Of the 18 (14.3%) patients in the critically ill group, 13 were male. By 26th March, according to National Health Commission statistics, there were 3460 confirmed cases (2880 cases in Wuhan) and 1034 severe cases (995 cases in Wuhan) in China, and to date, 81340 cumulative cases have been confirmed, 3292 have died and 74588 have been discharged[13]. The focus of medical services has now changed to treat patients with severe disease. In the latest Chinese CDC report, 31.1% of confirmed patients were aged over 60, and the crude mortality was highest among patients ≥ 80-years-old (14.8%) and among patients with chronic underlying diseases (5.6%-10.5%)[9]. Similar results were reported in several studies in which increased age and comorbidity were associated with death among patients with COVID-19[5,6,11,14]. Older COVID-19 patients with chronic comorbidities such as hypertension, diabetes, cardiovascular disease, cancer or other coexisting medical conditions were more likely to develop disease involving multiple systems and organs and rapidly progress to poor outcomes[9]. In our study, the median age of the patients was 61.00 years (IQR 48.00-68.00). Patients in the critically ill group were significantly older than those in the moderate group (65.44 years vs 54.76 years, P = 0.019). Patients with cerebrovascular disease, chronic liver disease, and cancer also presented with more severe disease; however, we did not find a significant difference in sex between the 3 groups. When we merged the severe and critically ill group together, the men in the merged group appeared to be more vulnerable to the COVID-19 than those in the moderate group. Recently, several studies have noted that more men than women were diagnosed with severe disease and that men had a higher case fatality rate[15-17]. Channappanavar et al[18] and Ling Ma et al[19] indicated that SARS-CoV-2 may affect male gonadal function via ACE2 receptors and that estrogen receptor signaling may provide a protective effect during coronavirus infection.
In terms of laboratory tests, the most common laboratory abnormalities observed in the severe and critically ill groups were decreased lymphocytes and albumin, as well as elevated lactate dehydrogenase, C-reactive protein, fibrinogen and D-dimer. In our study, compared with the moderate group, patients in the severe and critically ill groups had numerous laboratory abnormalities, which suggests that COVID-19 may be associated with coagulation activation, liver dysfunction, acute kidney injury, cardiac injury, and immune deficiency. The dynamic change in laboratory findings was tracked in 67 patients with COVID-19. In the critically ill group, the D-dimer, high-sensitivity cardiac troponin I, NT-pro BNP and lactate dehydrogenase levels increased with disease progression, and lymphopenia markedly decreased. Our results were consistent with those of several other studies, which also confirmed that high levels of D-dimer, lactate dehydrogenase, and creatine kinase and the neutrophil-to-lymphocyte ratio were independent risk factors for mortality among hospitalized patients with COVID-19[5,11,20].
Chai et al[21] indicated that liver dysfunction in patients with COVID-19 might be induced by cholangiocyte damage rather than hepatocyte damage, which is consistent with our finding that elevated AST and decreased albumin were associated with progression to more severe disease. However, we did not find significant difference between groups in ALT and total bilirubin results. Further studies could investigate whether other causes might participate in liver injury, such as systemic inflammatory response or hypoxemia.
Compared to the moderate group, the severe and critically ill groups had more patients with abnormal myocardial zymograms and patients who presented with elevated levels of lactate dehydrogenase (59.6% and 88.9%), creatine kinase (2.1% and 22.2%), high-sensitivity cardiac troponin I (0% and 22.2%), and NT-pro BNP (39.6% and 77.8%). Myocardial injury associated with COVID-19 may be due to hypoxemia and systemic pro-inflammatory cytokine responses. In a fatal case of COVID-19 in China, interstitial mononuclear inflammatory infiltrates in heart tissue were confirmed, but parenchymal damage and viral detection were not evident[22].
Regarding the coagulation indicators, D-dimer was above the normal range in 79.6% of the patients in the severe group and in 100% of the patients in the critically ill group; the thrombin time was longer than normal in 11.1% of the patients in the severe group and in 33.3% of the patients in the critically ill group; and the prothrombin time was longer than normal in 16.7% of the patients in the severe group and 41.7% of the patients in the critically ill group, indicating the profound influence of COVID-19 on the coagulation system. Possible reasons for coagulation activity may be direct injury to endothelial cells by SARS-CoV- 2[23,24], which is also related to atherosclerotic plaque rupture induced by inflammation and the release of procoagulant factors released[11]. Basic studies also confirmed that an inflammatory cytokine storm induced by the virus could lead to lymphocyte apoptosis and that lymphocytes express ACE2 receptors, which make them direct targets of SARS-CoV- 2[25]. The elevation in proinflammatory factors may increase fibrin deposition in the pulmonary microvasculature, contributing to acute respiratory distress syndrome and disseminated intravascular coagulation and significantly increasing blood lactic acid and D-dimer levels[26,27]. Acute kidney injury is directly related to viral attack and cytokine storms, causing metabolic acidosis with elevated creatine and decreased serum calcium levels[1,2,11,17].
However, there are still some limitations to our study. First, the majority of our patients were transferred from local hospitals during the late phases of their illnesses, which caused the collection of medical history to be limited. Second, in this retrospective study, some of the laboratory tests were not routinely performed for all patients or were periodically conducted during the progression of the disease. Third, our study was conducted only on two campuses in one nation (China); not all the analyses were available simultaneously at both centers, and some comparisons were performed only on one campus.
CONCLUSION
In conclusion, people of all ages, both male and female, are susceptible to COVID-19. Early drug treatment is an important measure in the treatment of patients with COVID-19, and the following indicators can help clinicians identify patients with severe COVID-19 at an early stage: age, an elevated neutrophil-to-lymphocyte ratio and high sensitivity cardiac troponin I.
ARTICLE HIGHLIGHTS
Research background
Since it was first reported, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has spread rapidly throughout the world, posing a serious threat to global health. However, the risk factors for patients with moderate-to-severe or severe-to-critical coronavirus disease 2019 (COVID-19) remain unclear.
Research motivation
A comprehensive description of the clinical characteristics, laboratory changes, in addition to oxygen levels and radiographic examinations enable clinicians to provide more accurate prognoses and specific care which vary according to subclinical or latent severe cases.
Research objectives
This study aimed to explore the characteristics and predictive markers of severe COVID-19.
Research methods
Patients with COVID-19 admitted from 1st February 2020 to 8th March 2020 were enrolled and categorized into 3 groups: the moderate group, severe group and critically ill group. Information was extracted from hospital information systems. Epidemiological and demographic, clinical symptoms and outcomes, complications, laboratory and radiographic examinations were collected retrospectively and then compared between groups.
Research results
A total of 126 patients were enrolled. There were 59 in the moderate group, 49 in the severe group, and 18 in the critically ill group. Over 50% patients have increased levels of lactate dehydrogenase, aspartate transaminase (AST), C-reactive protein, fibrinogen, D-dimer, tumor necrosis factor-α, ferritin, as well as decreased levels of hematocrit and calcium. Compared with the moderate group, the severe and critically ill group has significant higher rates of abnormality in levels of neutrophil ratio, eosinophil ratio, lymphocyte ratio, platelets count, neutrophil to lymphocyte ratio, AST, albumin, procalcitonin, calcium, D-dimer, interleukin-6, high-sensitivity cardiac troponin, amino-terminal pro-brain natriuretic peptide (NT-pro BNP), and ferritin. Multivariate logistic regression analysis showed that no drug treatment before admission, a higher neutrophil-to-lymphocyte ratio, a higher AST level, a higher NT-pro BNP level, a higher creatinine level, and serum calcium below the normal range were high-risk factors.
Research conclusions
People of all ages, both male and female, are susceptible to COVID-19. Early drug treatment is an important measure in the treatment of patients with COVID-19, and the following indicators can help clinicians identify patients with severe COVID-19 at an early stage: an elevated neutrophil-to-lymphocyte ratio; elevated AST, NT-pro BNP, and creatinine levels; and serum calcium below the normal range.
Research perspectives
A large sample size with long-term survival data is needed in future studies.
Footnotes
Institutional review board statement: The study was approved by the Research Ethics Commission of Beijing Hospital (2020BJYYEC-047-01).
Informed consent statement: Written informed consent was waived by the Ethics.
Conflict-of-interest statement: None reported.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: September 2, 2021
First decision: September 29, 2021
Article in press: December 23, 2021
Specialty type: Infectious Diseases
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Hill TD, Kim KH S-Editor: Wang JL L-Editor: Filipodia P-Editor: Wang JL
Contributor Information
Xin Chu, Department of Surgical Intensive Care Medicine, Beijing Hospital, National Center of Gerontology; Institute of Geriatric Medicine, Chinese Academy of Medical Sciences, Beijing 100730, China.
Gui-Fang Zhang, The Key Laboratory of Geriatrics, Beijing Institute of Geriatrics, Institute of Geriatric Medicine, Chinese Academy of Medical Sciences, Beijing Hospital /National Center of Gerontology of National Health Commission, Beijing 100730, China.
Yong-Ke Zheng, Department of Intensive Care Unit, Affiliated Hangzhou First People's Hospital, Zhejiang University School of Medicine, Hangzhou 310006, Zhejiang Province, China.
Yi-Gang Zhong, Department of Cardiology, Affiliated Hangzhou First People's Hospital, Zhejiang University School of Medicine, Hangzhou 310006, Zhejiang Province, China.
Li Wen, Department of Emergency, Beijing Hospital, National Center of Gerontology; Institute of Geriatric Medicine, Chinese Academy of Medical Sciences, Beijing 100730, China.
Ping Zeng, The Key Laboratory of Geriatrics, Beijing Institute of Geriatrics, Institute of Geriatric Medicine, Chinese Academy of Medical Sciences, Beijing Hospital /National Center of Gerontology of National Health Commission, Beijing 100730, China.
Chun-Yi Fu, Department of Emergency, Beijing Hospital, National Center of Gerontology; Institute of Geriatric Medicine, Chinese Academy of Medical Sciences, Beijing 100730, China.
Xun-Liang Tong, Department of Respiratory and Critical Care Medicine, Beijing Hospital, National Center of Gerontology; Institute of Geriatric Medicine, Chinese Academy of Medical Sciences, Beijing 100730, China.
Yun-Fei Long, Department of Neurology, Beijing Hospital, National Center of Gerontology; Institute of Geriatric Medicine, Chinese Academy of Medical Sciences, Beijing 100730, China.
Jing Li, Department of Cardiology, Beijing Hospital, National Center of Gerontology; Institute of Geriatric Medicine, Chinese Academy of Medical Sciences, Beijing 100730, China.
Ya-Lin Liu, Department of Surgical Intensive Care Medicine, Beijing Hospital, National Center of Gerontology; Institute of Geriatric Medicine, Chinese Academy of Medical Sciences, Beijing 100730, China.
Zhi-Gang Chang, Department of Surgical Intensive Care Medicine, Beijing Hospital, National Center of Gerontology; Institute of Geriatric Medicine, Chinese Academy of Medical Sciences, Beijing 100730, China.
Huan Xi, Department of Geriatrics, Beijing Hospital, National Center of Gerontology, National Health Commission; Institute of Geriatric Medicine, Chinese Academy of Medical Sciences, Beijing 100730, China. xih@bjhmoh.cn.
Data sharing statement
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
References
- 1.Zou X, Chen K, Zou J, Han P, Hao J, Han Z. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front Med. 2020;14:185–192. doi: 10.1007/s11684-020-0754-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Müller MA, Drosten C, Pöhlmann S. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181:271–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu M, Wang T, Zhou Y, Zhao Y, Zhang Y, Li J. Potential Role of ACE2 in Coronavirus Disease 2019 (COVID-19) Prevention and Management. J Transl Int Med. 2020;8:9–19. doi: 10.2478/jtim-2020-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sahin M, Akkus E. Comment on "Potential Role of ACE2 in Coronavirus Disease 2019 (COVID-19) Prevention and Management". J Transl Int Med. 2020;8:199. doi: 10.2478/jtim-2020-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li X, Xu S, Yu M, Wang K, Tao Y, Zhou Y, Shi J, Zhou M, Wu B, Yang Z, Zhang C, Yue J, Zhang Z, Renz H, Liu X, Xie J, Xie M, Zhao J. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J Allergy Clin Immunol. 2020;146:110–118. doi: 10.1016/j.jaci.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, Wu Y, Zhang L, Yu Z, Fang M, Yu T, Wang Y, Pan S, Zou X, Yuan S, Shang Y. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, Xia J, Yu T, Zhang X, Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National Health Commission of the People’s Republic of China. The guidelines on the Diagnosis and Treatment of COVID-19 (version 7.0). 2020. [cited 20 April 2020]. Available from: http://www.nhc.gov.cn/yzygj/s7653p/202003/46c9294a7dfe4cef80dc7f5912eb1989.sht. ml.
- 9.Epidemiology Working Group for NCIP Epidemic Response. Chinese Center for Disease Control and Prevention. The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China. Zhonghua Liu Xing Bing Xue Za Zhi. 2020;41:145–151. doi: 10.3760/cma.j.issn.0254-6450.2020.02.003. [DOI] [PubMed] [Google Scholar]
- 10.Feng Y, Ling Y, Bai T, Xie Y, Huang J, Li J, Xiong W, Yang D, Chen R, Lu F, Lu Y, Liu X, Chen Y, Li X, Li Y, Summah HD, Lin H, Yan J, Zhou M, Lu H, Qu J. COVID-19 with Different Severities: A Multicenter Study of Clinical Features. Am J Respir Crit Care Med. 2020;201:1380–1388. doi: 10.1164/rccm.202002-0445OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen S, Zhang Z, Yang J, Wang J, Zhai X, Bärnighausen T, Wang C. Fangcang shelter hospitals: a novel concept for responding to public health emergencies. Lancet. 2020;395:1305–1314. doi: 10.1016/S0140-6736(20)30744-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.National Health Commission of the People’s Republic of China. COVID-19 outbreaks (2020-3-26). 2020. [cited 20 April 2020]. Available from: http://www.nhc.gov.cn/xcs/yqtb/202003/c521093a01734df3b3fbc156064ba19f.shtml .
- 14.Wang X, Fang J, Zhu Y, Chen L, Ding F, Zhou R, Ge L, Wang F, Chen Q, Zhang Y, Zhao Q. Clinical characteristics of non-critically ill patients with novel coronavirus infection (COVID-19) in a Fangcang Hospital. Clin Microbiol Infect. 2020;26:1063–1068. doi: 10.1016/j.cmi.2020.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS China Medical Treatment Expert Group for Covid-19. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wenham C, Smith J, Morgan R Gender and COVID-19 Working Group. COVID-19: the gendered impacts of the outbreak. Lancet. 2020;395:846–848. doi: 10.1016/S0140-6736(20)30526-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Channappanavar R, Fett C, Mack M, Ten Eyck PP, Meyerholz DK, Perlman S. Sex-Based Differences in Susceptibility to Severe Acute Respiratory Syndrome Coronavirus Infection. J Immunol. 2017;198:4046–4053. doi: 10.4049/jimmunol.1601896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ma L, Xie W, Li D, Shi L, Mao Y, Xiong Y, Zhang Y, Zhang M. Effect of SARS-CoV-2 infection upon male gonadal function: A single center-based study. 2020 Preprint. Available from: medRxiv:2020.03.21.20037267.
- 20.Liu J, Liu Y, Xiang P, Pu L, Xiong H, Li C, Zhang M, Tan J, Xu Y, Song R, Song M, Wang L, Zhang W, Han B, Yang L, Wang X, Zhou G, Zhang T, Li B, Wang Y, Chen Z. Neutrophil-to-lymphocyte ratio predicts critical illness patients with 2019 coronavirus disease in the early stage. J Transl Med. 2020;18:206. doi: 10.1186/s12967-020-02374-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chai X, Hu L, Zhang Y, Han W, Lu Z, Ke A, Zhou J, Shi G, Fang N, Fan J, Cai J, Lan F. Specific ACE2 Expression in Cholangiocytes May Cause Liver Damage After 2019-nCoV Infection. 2020 Preprint. Available from: bioRxiv:2020.02.03.931766.
- 22.Liu Q, Wang RS, Qu GQ, Wang YY, Liu P, Zhu YZ, Fei G, Ren L, Zhou YW, Liu L. Gross examination report of a COVID-19 death autopsy. Fa Yi Xue Za Zhi. 2020;36:21–23. doi: 10.12116/j.issn.1004-5619.2020.01.005. [DOI] [PubMed] [Google Scholar]
- 23.Yang M. Cell Pyroptosis, a Potential Pathogenic Mechanism of 2019-nCoV Infection. SSRN Electron J. 2020 [Google Scholar]
- 24.Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, Mehra MR, Schuepbach RA, Ruschitzka F, Moch H. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tan L, Wang Q, Zhang D, Ding J, Huang Q, Tang YQ, Miao H. Lymphopenia predicts disease severity of COVID-19: a descriptive and predictive study. Signal Transduct Target Ther. 2020;5:33. doi: 10.1038/s41392-020-0148-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang J, Hajizadeh N, Moore EE, McIntyre RC, Moore PK, Veress LA, Yaffe MB, Moore HB, Barrett CD. Tissue plasminogen activator (tPA) treatment for COVID-19 associated acute respiratory distress syndrome (ARDS): A case series. J Thromb Haemost. 2020;18:1752–1755. doi: 10.1111/jth.14828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li N, Jie Z. The Application of Corticosteroids in COVID-19: A Two-edged Sword. J Transl Int Med. 2020;8:66–70. doi: 10.2478/jtim-2020-0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.