Abstract
BACKGROUND
Tumor-infiltrating lymphocytes (TILs) constitute a prognostic factor in hepatocellular carcinoma (HCC). However, different methods of assessing TILs have various pre-analytical, analytical, and post-analytical challenges. The evaluation of TILs in hematoxylin and eosin (H&E)-stained tumor sections proposed by the International Immuno-Oncology Biomarker Working Group was demonstrated to be a reproducible, affordable and easily applied method in many tumors.
AIM
To evaluate the prognostic significance of TILs in H&E-stained slides of HCCs.
METHODS
This was a retrospective study performed in the hospital. HCC patients who underwent liver resection between 2015 and 2017 in Zhongshan Hospital were enrolled in this study. Patients who experienced recurrence or received therapy in addition to antiviral therapy before surgery at this time were excluded. A total of 204 patients were enrolled in the study. The ILs were counted manually in tumor sections stained with H&E under an optical microscope at 400 ×. The ILs were assessed separately in the center of the tumor (TILsCT), the invasive front (TILsIF), and peritumor (PILs) areas. Univariate and multivariate survival analyses were performed using a Cox regression model. P < 0.05 was considered statistically significant and all P-values were two-sided.
RESULTS
Among the 204 patients, univariate analysis indicated that macrovascular invasion (MaVI) (P = 0.001), microvascular invasion (MVI) (P = 0.012), multiple tumors (P = 0.008), large tumors (> 10 cm) (P = 0.001), absence of a tumor capsule (P = 0.026), macrotrabecular histological subtype (P = 0.001), low density of TILsCT (P = 0.039), TILsIF (P = 0.014), and PILs (P = 0.010) were predictors of progression-free survival (PFS). Cox multivariate analysis indicated that MaVI (P = 0.009), absence of a tumor capsule (P = 0.031), low-density of TILsIF (P = 0.047) and PILs (P = 0.0495) were independent predictors of PFS. A three-category analysis was carried out by combining TILsCT, TILsIF, and PILs, after which HCCs were classified into immunehigh [(TILsCT)high, (TILsIF)high, and PILshigh, 83 cases], immunemod (tumors other than immunehigh and immunelow subtypes, 94 cases), and immunelow [(TILsCT)low, (TILsIF)low, and PILslow, 27 cases)] subtypes. The immunehigh subtype had a lower rate of MVI (40.96%) than the immunemod (61.70%, P = 0.017) and immunelow (66.67%, P = 0.020) subtypes. The recurrence rates of the immunehigh, immunemod and immunelow subtypes were 10.8%, 25.5% and 33.3%, respectively.
CONCLUSION
HCC patients with high infiltrating lymphocytes tend to have a lower recurrence rate and less MVI. The evaluation of TILs in H&E-stained specimens could be a prognostic parameter for HCC.
Keywords: Lymphocytes, Tumor infiltration, Hepatocellular carcinoma, Hematoxylin and eosin-stained, Pathology
Core Tip: Successful use of immunotherapy in tumors starve for widely applicable, accessible and reliable immune-oncology biomarkers. Assessing tumor infiltrating lymphocytes (TILs) in hematoxylin and eosin (H&E)-stained tissues showed great clinical validity and utility in many solid tumors. However, barely any research on hepatocellular carcinoma (HCC) has been published. TILs evaluated in H&E-stained HCC tissues showed a great prognostic effect for recurrence in the present study and might be helpful to select patients with the highest likelihood of responding to immunotherapeutic agents. The method has low requirements in terms of technical and economic costs and can be easily applied in routine practice.
INTRODUCTION
Cancer incidence and mortality are rapidly growing globally. Hepatocellular carcinoma (HCC) is one of the most common primary malignancies of the liver, representing the third leading cause of cancer-related deaths worldwide[1]. HCC is associated with chronic inflammation and fibrosis arising from different etiologies, including hepatitis B and C and alcoholic and non-alcoholic fatty liver diseases[2]. The stromal component of tumors consists of fibroblasts, endothelial cells, and various immune cells. Together, these cells play a critical role in tumor development and response to treatment.
Many different methods have demonstrated the prognostic effect of tumor infiltrating lymphocytes (TILs) in HCC[3]. For instance, the densities of tumor-infiltrating T cells and B cells are correlated with superior survival in HCC patients[4], and patients with high-grade HCC of the predominant immune-high subtype had significantly better prognosis[5]. Different methods of assessing TILs have various pre-analytical, analytical, and post-analytical challenges. For example, semi-quantitative hematoxylin and eosin (H&E)-based scores suffer from low precision and poor interobserver reproducibility due to lack of guidance, while digital quantification of immunohistochemical (IHC)-stained sections may have varied results due to inaccurate measurement of the test variable without controlled calibration.
Furthermore, the immunoscore proposed by Jerome Galon showed great prognostic power and outperformed the tumor node metastasis classification for disease-free survival, disease-specific survival and overall survival[6,7]. However, the immunoscore requires rigorous pathology and experimental practice for the staining, and deviation from the predefined standardized operating procedure might result in improper quantification[8].
Accumulating evidence suggests that lymphocytic infiltration in tumor tissues can be assessed as a significant parameter by evaluating H&E-stained tumor sections[9], which achieved good consistency and reproducibility in pathologists, including pathology resident trainees[10]. The criteria have been assessed in many different solid tumors, including lung, colon, upper gastrointestinal tract, head and neck, genitourinary tract, gynecological organs, mesothelioma, melanoma, and primary brain tumors[11]. However, evaluating of infiltrating lymphocytes in H&E slides of HCC has rarely been studied.
The present study aimed to assess the prognostic effect and the clinicopathological correlation of TILs evaluated in H&E sections of HCC patients.
MATERIALS AND METHODS
Patients and samples
HCC samples that met the following criteria were enrolled in the present study: (1) Patients who underwent liver resection for the first time from January 2015 to December 2017 in the Department of Liver Surgery, Zhong Shan Hospital, Fudan University, China; (2) Liver resection samples diagnosed as HCC by a pathologist; and (3) Complete clinicopathological data and disease-progression information. Patients who received therapy in addition to antiviruses were excluded, e.g., transarterial chemoembolization, ablation, bland embolization, radioembolization, chemotherapy, and immunotherapy.
The study was approved by the Human Ethics Institutional Review Board of Huadong Hospital, Fudan University (approval number 2019K119), and informed consent was waived by the Review Board because of the retrospective nature of the study.
H&E staining of tumor tissue
H&E staining was performed on a high-throughput fast automatic platform (Dako coverstainer, United States) according to standard protocols.
According to the architectural growth patterns[12], distinctive and easily recognizable histological features were defined with a predominant (> 50%) architectural pattern. HCC was divided into microtrabecular/pseudoglandular, macrotrabecular, compact, and lymphoepithelioma-like subtypes[13]. The macrotrabecular subtype is classified as a predominant trabecular architectural pattern which is more than six cells thick[14].
Density of infiltrating lymphocytes
Two general pathologists and one senior pathologist were involved in this study. The density of ILs was determined based on the recommendation by the International Immuno-Oncology Biomarker Working Group[15]: (1) The number of ILs on full sections was scanned at low magnification and evaluated at higher magnification (400 ×) manually under an optical microscope; (2) ILs were assessed in the areas of the tumor center (TILsCT), the invasive front (TILsIF) and on the portal areas of the peritumour 1 cm away from the border (PILs). The “invasive front” (IF) is defined as the region centered on the border separating the host tissue from the malignant nests by 1 mm. Areas with crush artifacts, necrosis, and previous biopsy sites were excluded; and (3) All mononuclear cells, including lymphocytes and plasma cells, were counted (polymorphonuclear leukocytes were excluded from the count of ILs, and neutrophils were recorded separately from the count of ILs).
Immunohistochemistry staining
Programmed cell death-ligand 1 (PD-L1) (SP142) rabbit monoclonal primary antibody (Ventana Medical Systems Inc, Tucson, AZ, United States) was optimized for a fully automated IHC assay on the BenchMark ULTRA (Ventana Medical Systems Inc) staining platform using the OptiView DAB IHC Detection Kit and OptiView Amplification Kit (Ventana Medical Systems Inc)[16]. All the tissues were subjected to PD-L1 (SP142) IHC staining.
The expression of PD-L1 on tumor cells (TCs) was assessed as the proportion of TCs showing membrane staining of any intensity. The expression on TILs was assessed as the proportion of stromal areas occupied by PD-L1-positive TILs of any intensity (approved by the US Food and Drug Administration).
Follow-up
Patients were followed up by ultrasound, computed tomography (CT), or magnetic resonance imaging every 3-6 mo after the resection, with a maximum period of 1063 d. The primary study endpoint was progression-free survival (PFS), which refers to the duration of patient survival without any evidence of the tumor.
Statistical analyses
Univariate and multivariate survival analyses were performed using Cox regression model. A non-paired t-test was conducted to compare the clinicopathological parameters of the immune subtypes. All statistical analyses were performed using GraphPad Prism 7 software. P < 0.05 was considered statistically significant and all P-values were two-sided. The statistical methods of this study were reviewed by Xin-xin Xu from Huadong Hospital.
RESULTS
Clinical and pathological factors
A total of 204 patients were included in the present study, 91.67% of the patients were hepatitis B virus infected. Macrovascular invasion (MaVI) was presented in 21 (10.29%) tumors, while microvascular invasion (MVI) was observed in 110 (53.92%) tumors. A total of 156 patients had a single tumor and 117 tumors were capsulated. Cirrhosis was observed in 171 (83.82%) tumors (Table 1).
Table 1.
Clinicopathological data of the patients
|
Variable
|
|
No. of case
|
| Age (median) | 56 (204) | |
| Gender | Male | 174 |
| Female | 30 | |
| HBV infection | Yes | 187 |
| No | 12 | |
| Not mentioned | 5 | |
| HBV DNA | Positive | 66 |
| Negative | 108 | |
| Not mentioned | 30 | |
| MaVI | Yes | 21 |
| No | 183 | |
| MVI | Positive | 110 |
| Negative | 94 | |
| Differentiation | Moderately differentiated | 73 |
| Poorly differentiated | 131 | |
| Histological subtype | Microtrabecular/pseudoglandular | 87 |
| Macrotrabecular | 108 | |
| compact | 5 | |
| Lymphoepithelioma-like | 4 | |
| Tumor number | Single | 156 |
| Multiple (≥ 2) | 48 | |
| Largest tumor diameter | ≤ 10 cm | 189 |
| > 10 cm | 15 | |
| Capsule | Yes | 117 |
| No | 87 | |
| Cirrhosis in peritumor | Yes | 171 |
| No | 33 | |
| TILsCT | ≤ 30 | 35 |
| > 30 | 169 | |
| TILsIF | ≤ 200 | 62 |
| > 200 | 140 | |
| PILs | ≤ 200 | 113 |
| > 200 | 89 |
MaVI: Macrovascular invasion; MVI: Microvascular invasion; TILsCT: Tumor infiltrating lymphocytes in the tumor center; TILsIF: Tumor infiltrating lymphocytes in the invasive front 1 mm spacing from the malignant nests, two cases cannot assess infiltrating lymphocytes in the invasive front; PILs: Infiltrating lymphocytes in the peritumor, two cases cannot assess infiltrating lymphocytes in peritumor areas; HBV: Hepatitis B virus.
Areas with microtrabecular/pseudo-glandular, macrotrabecular, compact, and lymphoepithelioma-like histological architectural patterns were identified in 42.64%, 52.94%, 2.45%, and 1.96% of the tumors, respectively (Table 1).
A total of 42/204 (20.6%) patients experienced tumor recurrence. The univariate analysis indicated that MaVI (P = 0.001), MVI (P = 0.012), multiple tumors (P = 0.008), large tumors (> 10 cm) (P = 0.001), absence of a tumor capsule (P = 0.026), and the macrotrabecular histological subtype (P = 0.001) were independent predictors of PFS (Supplementary Figure 1 and Table 2). MaVI (P = 0.009) and absence of a capsule (P = 0.031) were multivariate analysis predictors of PFS (Table 2).
Table 2.
Results of univariate and multivariate analysis
| Variable |
Univariate analysis
|
Multivariate analysis
|
||||
|
HR
|
95% CI
|
P
value
|
HR
|
95% CI
|
P
value
|
|
| MaVI | 3.09 | 1.02-9.34 | 0.001 | 3.77 | 1.63-7.40 | 0.009 |
| MVI | 2.80 | 1.51-5.16 | 0.012 | 1.19 | 0.64-2.23 | 0.693 |
| Tumor number | 2.38 | 1.10-5.14 | 0.008 | 1.95 | 1.04-3.77 | 0.122 |
| Largest tumor diameter | 3.31 | 1.06-10.32 | 0.001 | 1.76 | 0.95-3.45 | 0.322 |
| Capsule | 1.99 | 1.07-3.70 | 0.026 | 0.42 | 0.20-0.83 | 0.031 |
| Macrotrabecular histological subtype | 3.22 | 1.77-5.86 | 0.001 | 1.89 | 1.03-3.67 | 0.104 |
| TILsCT (≤ 30) | 0.49 | 0.22-0.92 | 0.039 | 0.85 | 0.41-1.63 | 0.734 |
| TILsIF (≤ 200) | 0.37 | 0.14-0.98 | 0.014 | 0.46 | 0.25-0.86 | 0.047 |
| PILs (≤ 200) | 0.40 | 0.22-0.75 | 0.010 | 0.37 | 0.19-0.77 | 0.0495 |
MaVI: Macrovascular invasion; MVI: Microvascular invasion; TILsCT: Tumor infiltrating lymphocytes in the tumor center; TILsIF: Tumor infiltrating lymphocytes in the invasive front 1 mm spacing from the malignant nests; PILs: Infiltrating lymphocytes in the peritumor; HR: Hazard ratio; CI: Confidence interval.
Immune microenvironment was heterogeneous
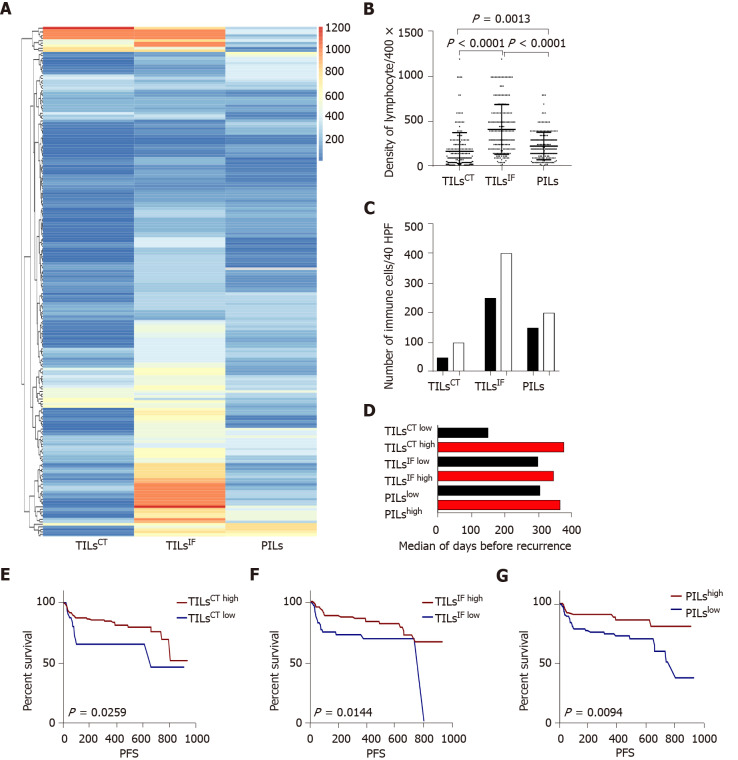
In the current study cohort, the number of TILsCT, TILsIF, and PILs was 10-1200/high power field (HPF). The ILs showed a great diversity among TILsCT, TILsIF, and PILs. Compared to the adjacent non-tumor liver tissues, the tumor microenvironment was found to be relatively inert due to a lower number of TILsCT (P = 0.001). A significantly higher proportion of TILsIF was observed compared to TILsCT and PILs (P < 0.0001) (Figure 1).
Figure 1.
The distribution and recurrence association of tumor infiltrating lymphocytes in the tumor center, invasive front, and peritumor. A: The spectrum of tumor infiltrating lymphocytes in the tumor center (TILsCT), TILs in the invasive front (TILsIF), and TILs in the peritumor (PILs) of 204 cases. TILsCT were lower than TILsIF and PILs. TILsIF was the most flaming part than the other two areas; B: Dot map of TILsCT, TILsIF, and PILs, indicating a heterogeneous distribution of inflammation; C: Comparison of the mean of TILsCT, TILsIF and PILs from patients with tumor recurrence (black bars) or without tumor recurrence (white bars); D: Median survival time for all patients, with high densities (red bars) or low densities (black bars) of TILsCT, TILsIF and PILs; E, F and G: Patients with high TILsCT, TILsIF, and PILs had a lower recurrence rate. TILsCT: Tumor infiltrating lymphocytes in the tumor center; TILsIF: Tumor infiltrating lymphocytes in the invasive front 1 mm spacing from the malignant nests; PILs: infiltrating lymphocytes in the peritumor.
Immunehigh patients had better PFS and a lower rate of MVI
Immune cell densities in the tumor center, invasive front, and peritumor regions were converted into percentiles: 0%-25% was scored as low, and 25%-100% was scored as high. Patients with high TILsCT, TILsIF, and PILs had better PFS than those with low TILsCT, TILsIF, and PILs (Figure 1). Multivariate analysis, including those variables that appeared statistically significant in the univariable analysis, showed that low TILsIF (P = 0.0495) and PILs (P = 0.047) were independent risk factors for PFS in patients with HCC.
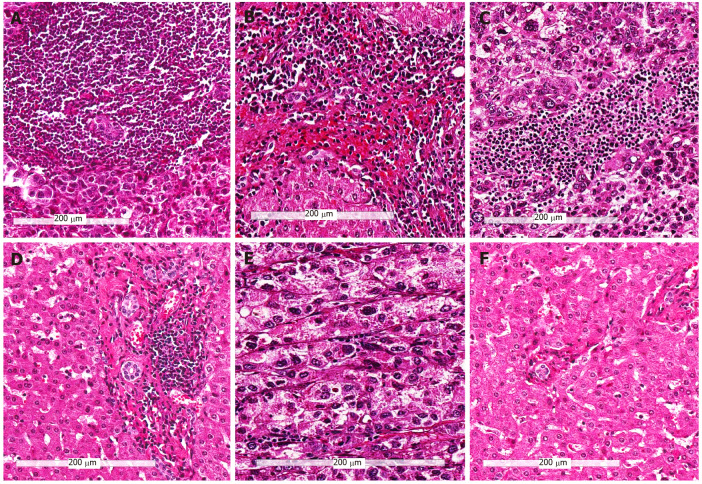
After integrating TILsCT, TILsIF, and PILs, we divided HCCs into three-category analysis: (1) Immunehigh subtype [(TILsCT)high, (TILsIF)high, and PILshigh, 83 cases]; (2) Immunemod subtype (tumours other than Immunehigh and Immunelow subtypes, 94 cases); (3) Immunelow subtype [(TILsCT)low, (TILsIF)low, and PILslow, 27 cases]. The H&E images of the three immune subtypes are illustrated in Figure 2.
Figure 2.
Representative hematoxylin and eosin images of the three immune subtypes (200 ×). A and B: Tumors with high infiltrating lymphocytes in the tumor center, invasive front and peritumor (Immunehigh) subtype; C and D: Tumors other than immunehigh and immunelow (Immunemod) subtype; E and F: Tumors with low infiltrating lymphocytes in the tumor center, invasive front and peritumor (Immunelow) subtype; A, C, and E: Immune cells in tumor center; B, D, and F: Immune cells in the peritumor region.
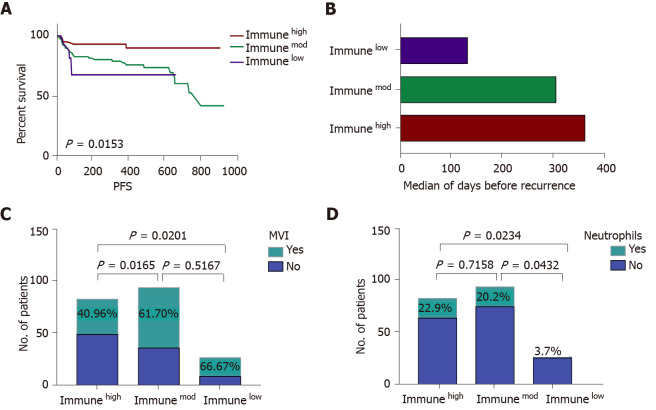
A higher number of the immunemod subtype (46.1%) HCCs was noted compared to the immunehigh subtype (40.7%), while 13.2% of the HCCs were immunelow subtype. Recurrent disease was identified in 10.8% of the immunehigh patients compared to the 25.5% of the immunemod patients and 33.3% of the immunelow patients (P = 0.0153). The immunehigh subtype had a lower rate of MVI (40.96%) than the immunemod (61.70%; P = 0.017) and immunelow (66.67%; P = 0.020) subtypes. A large number of patients had neutrophils in the microenvironment of the immunhigh and immunemod subtypes compared with the immunelow subtype (Figure 3).
Figure 3.
The comparison of immunehigh, immunemod and immunelow subtypes. A: Immune subtypes can predict patients’ progression-free survival. Immunehigh patients had a low recurrence rate, and immunelow patients experienced a high recurrence rate; B: The median survival time for all patients divided into three categories: Immunehigh (red bars), immunemod (green bars), and immune low (purple bars); C: The incidence rate of microvascular invasion (MVI) in three immune subtypes, immunehigh subtype had a lower rate of MVI compared to immunemod and immunelow subtypes; D: The presence of neutrophils in the three immune subtypes, a high incidence of neutrophils was detected in immunehigh and immunemod subtypes. Immunehigh: Tumors with high infiltrating lymphocytes in the tumor center, invasive front and peritumor; Immunemod: Tumors other than immunehigh and immunelow; Immunelow: Tumors with low infiltrating lymphocytes in the tumor center, invasive front and peritumor; PFS: Progression-free survival; MVI: Microvascular invasion.
Regarding other parameters, including MaVI, multiple tumors, tumor diameter, capsule, differentiation, histological subtype, and lymphoid follicle, PD-L1 (SP142) expression did not exhibit a significant difference between the three groups (Table 3).
Table 3.
Clinicopathological data between the three immune subtypes
|
Variable
|
Immunehigh
|
Immunemod
|
Immunelow
|
|
| HBV DNA | Positive | 30 | 30 | 5 |
| Negative | 38 | 54 | 16 | |
| Not mentioned | 15 | 10 | 6 | |
| MaVI | Yes | 8 | 10 | 3 |
| No | 75 | 84 | 24 | |
| MVI | Yes | 34 | 58 | 18 |
| No | 49 | 36 | 9 | |
| Tumor number | Single | 60 | 78 | 18 |
| Multiple (≥ 2) | 23 | 16 | 9 | |
| Largest tumor diameter | ≤ 10 | 80 | 85 | 24 |
| > 10 | 3 | 9 | 3 | |
| Capsule | Yes | 48 | 53 | 16 |
| No | 35 | 41 | 11 | |
| Neutrophils | Yes | 19 | 19 | 1 |
| No | 64 | 75 | 26 | |
| Tertiary lymphoid structures | Yes | 7 | 3 | 0 |
| No | 76 | 91 | 27 | |
| Differentiation | Moderately differentiated | 40 | 50 | 17 |
| Poorly differentiated | 43 | 44 | 10 | |
| Histological subtype | Microtrabecular/pseudoglandular | 39 | 40 | 13 |
| Macrotrabecular | 38 | 52 | 14 | |
| Compact | 3 | 2 | 0 | |
| Lymphoepithelioma-like | 3 | 0 | 0 | |
| PD-L1 expression | Tumor cells | 24 | 44 | 12 |
| TILs | 79 | 94 | 26 | |
| Recurrence | Yes | 9 | 24 | 9 |
| No | 74 | 70 | 18 | |
MaVI: Macrovascular invasion; MVI: Microvascular invasion; PD-L1: Programmed cell death-ligand 1; TIL: Tumor infiltrating lymphocyte; HBV: Hepatitis B virus.
Patients with neutrophils or tertiary lymphoid structures among the TILs had a low recurrence rate
Neutrophils and tertiary lymphoid structures (TLSs) were distinguished in the tumor microenvironment on H&E-stained slides. Therefore, we recorded the presence and density of these inflammatory cells. Patients with neutrophils among the TILs exhibited a tendency for decreased recurrence, albeit without a significant difference. The patients with TLSs in the microenvironment did not show any recurrence after a follow-up of 37-791 d.
High PD-L1 (SP142) expression on TILs was associated with better PFS
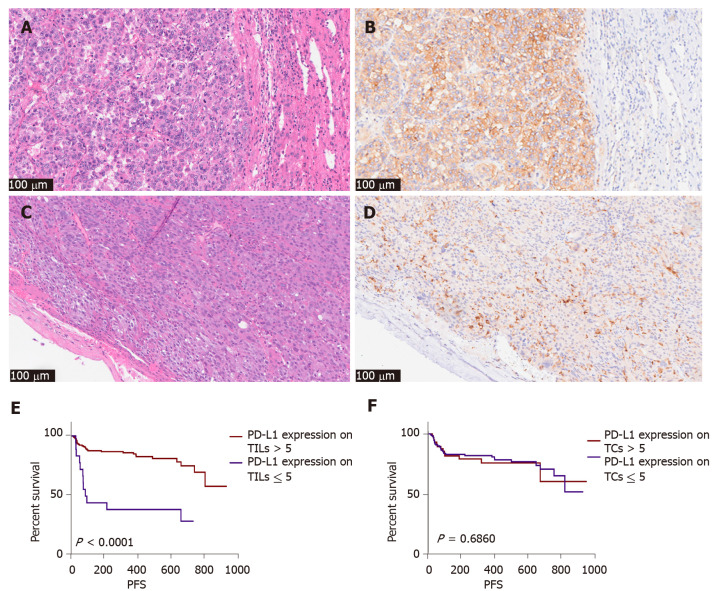
PD-L1 (SP142) was expressed on TCs in 80 patients and TILs in 200 patients. Patients with a higher expression of PD-L1 (SP142) on TILs (> 5%) had a lower recurrence rate than those with lower expression (Figure 4). The greater the number of TILs, the higher the level of PD-L1 (SP142) expression on the TILs. However, the expression of PD-L1 (SP142) on TCs was not associated with PFS or TILs in our cohort. Additionally, we observed the expression of PD-L1 (SP142) on neutrophils; however, the proportion of neutrophils in TILs was not significantly associated with the expression of PD-L1 (SP142).
Figure 4.
The pathological picture and recurrence association of programmed cell death-ligand 1 SP142 expression in tumor cells and immune cells (200 ×). A and B: Hematoxylin and eosin (H&E) and immunohistochemistry (IHC) picture of programmed cell death-ligand 1 (PD-L1) SP142 in tumor cells of one case; C and D: H&E and IHC picture of PD-L1 SP142 in immune cells of another case; E: Patients with high expression of PD-L1 (SP142) on tumor infiltrating lymphocytes tend to have less recurrence; F: Expression of PD-L1 (SP142) on tumor cells was not statistically significant. PD-L1: Programmed cell death-ligand 1; IHC: TILs: Tumor infiltrating lymphocytes; TCs: Tumor cells; PFS: Progression-free survival.
We performed the IHC assay of (SP142), (28-8), and (E1L3N) in the other cohort of HCC patients; (SP142) is a more robust PD-L1 staining reagent than (28-8) and (E1L3N) in both tumors and immune cells of HCC, while (28-8) and (E1L3N) have similar staining effect in tumor cells. Therefore, we chose (SP142) as the major reagent analyzed in this study (Supplementary Figure 2).
DISCUSSION
This study revealed that the density of infiltrating lymphocytes in H&E-stained tissues can predict the recurrence of HCC. The International Immuno-Oncology Biomarker working Group proposed that TILs should be reported separately for the stromal compartment (= % stromal TILs) and the tumor cell compartment (= % intra-tumoral TILs). The stroma of classical HCC is composed of sinusoid-like blood spaces lined by a single layer of endothelial cells, which sometimes show varying degrees of dilatation or may be difficult to recognize owing to compression by tumor cells[17]. Most classical HCCs do not induce a desmoplastic stroma, therefore the method of stromal TILs is not suitable for HCC assessment. The method of intra-tumoral TILs with tumor cell area for the denominator is hard to accomplish manually, as visual estimation is subjective and TILs are manifested as infiltrating nests in tumor area in our study; meanwhile in daily practice most pathologists will report discrete estimates, for example 13.5% will be rounded to 15%, which will result in underestimation of the difference. Therefore, we tried to distinguish the immune subtypes of HCC by recording the densities of infiltrating lymphocytes in the tumor center, invasive front and peritumor. However, this method is admittedly challenging, and inter-observer reproducibility requires particular attention. The method showed a prognostic effect for HCC recurrence and might be helpful to select patients with the highest likelihood of responding to immunotherapeutic agents.
HCC is characterized by immune tolerance and comprises numerous infiltrated immune cells, a large number of suppressive molecules, complex proinflammatory/immunoregulatory signaling and intricate interactions between different components. The immune microenvironment in HCC plays a key role in HCC progression and recurrence[18]. The immune system plays a dual role in cancer: It can not only suppress tumor growth by destroying cancer cells or inhibiting their outgrowth but also promote tumor progression either by selecting tumor cells that are more fit to survive in an immunocompetent host or by establishing conditions within the tumor microenvironment that facilitate tumor outgrowth[19]. Regulatory T cells and myeloid-derived suppressor cells are two major types of immunosuppressive leukocyte populations that play key roles in inhibiting host-protective antitumor responses. Tumor infiltration by IFN-γ-producing Th1 CD4+ T cells and CD8+ T cells and the presence of cytokines such as IFN-γ and TNF-α that promote tumor control have been associated with an improved prognosis for patients with many different cancers[20]. Therefore, tumor-promoting inflammation and protective tumor immunity are dynamically interconnected. Many different approaches are used to assess the immune infiltrate in tumors with highly variable requirements, costs and complexity[21-23]. TILs assessment of H&E sections has shown clinical validity as a prognostic marker in invasive breast carcinoma and is reproducible, affordable and widely available[24].
Here, we found that high TILs were significantly associated with less microvascular invasion. Vascular invasion has been recognized as a crucial step in metastasis and may indicate disseminated disease and unfavorable prognosis among cancer patients. Patients with high TILs experienced less recurrence, perhaps in part due to less microvascular invasion. The effect of TILs on vascular invasion needs further investigation.
Neutrophils and TLSs were associated with lower recurrence in the present study. The bulk of the clinical evidence assessing neutrophil to lymphocyte ratios (NLRs) mostly supports the notion that neutrophils promote, rather than inhibit, cancer progression[25]. In comparison with NLR, the prognostic and predictive power of intratumoral neutrophils is murkier and more variable, and positive (gastric cancer), negative (renal cancer and melanoma) or no (lung cancer) correlation with patient outcome has been observed in different studies. However, experimental studies have highlighted multifaceted and sometimes opposing roles of neutrophils in cancer[26]. Analysis of the current literature shows that the presence of TLSs is associated with a favorable clinical outcome for cancer patients, regardless of the approach used to quantify TLSs and the stage of the disease[27]. Researchers have indicated that TLSs represent a privileged area for the recruitment of lymphocytes into tumors and the generation of central memory T and B cells that circulate and limit cancer progression[28].
Different immunotherapeutic modalities have been used to treat HCC, including diverse vaccine platforms, adoptive T-cell therapy, cytokines, gene therapy and monoclonal antibodies that target immune checkpoint molecules[29]. The importance of lymphocytes has been highlighted in many studies, wherein increasing infiltration of tumors with lymphocytes has been associated with enhanced response to cytotoxic treatment and prognosis in cancer patients[30]. HCC immunogenicity is indicated by the presence of tumor-infiltrating lymphocytes and an evident reduction in relapse rates after resection and transplantation in patients with dense lymphocytic infiltration.
Nevertheless, the present study had some limitations. This was a retrospective, single-center study with a small number of patients. Additionally, this method is more challenging to implement in daily practice and has lower inter-observer reproducibility than stromal TILs. The method should be improved upon with further study undertaken and as evidence becomes available. The study lacked immune cell characterization. Understanding the types and function of immune cells as well as different cytokines will provide more insight into tumor immunology and immunotherapy.
CONCLUSION
HCC patients with high infiltrating lymphocytes tend to have a lower recurrence rate and less microvascular invasion. The evaluation of TILs in H&E-stained specimens could be a prognostic parameter for HCC.
ARTICLE HIGHLIGHTS
Research background
As successful use of immune checkpoint inhibitors and other forms of immunotherapy has become a clinical reality, the need for widely applicable, accessible and reliable biomarkers is clear. Different methods of assessing tumor infiltrating lymphocytes (TILs) have various pre-analytical, analytical, and post-analytical challenges. The evaluation of TILs in hematoxylin and eosin (H&E) stained tumor sections proposed by the International Immuno-Oncology Biomarker Working Group was demonstrated to be a reproducible, affordable and easily applied method in many tumors. However, this method has barely been conducted in hepatocellular carcinoma (HCC). The exploration of TILs in H&E sections of HCC could provide a detailed information for the selection of patients who receive the immunotherapy and evaluation of the prognostic effect of immunotherapy.
Research motivation
There have been few suggestions to evaluate HCC by examining TILs in H&E sections. The key problem is to build a method suitable for the tissue specificity of HCC. Once a consensus of the method is established, it will be helpful to manifest the inflammatory condition of the tumor and help to select patients that will experience the greatest benefit of immunotherapy as well as to gain deep insight into immunotherapy.
Research objectives
The main objective of this study was to explore whether evaluating TILs in H&E-stained sections has a prognostic effect in HCC. Based on this study, evaluating TILs in H&E-stained sections could be a prognostic method for HCC. Increasing multicenter research to validate and improve this method should be implemented in the future.
Research methods
H&E staining was performed on a high-throughput fast automatic platform (Dako coverstainer, United States) according to standard protocols. Programmed cell death-ligand 1 (PD-L1) (SP142) rabbit monoclonal primary antibody (Ventana Medical Systems Inc, Tucson, AZ, United States) was optimized for a fully automated immunohistochemical (IHC) assay on the BenchMark ULTRA (Ventana Medical Systems Inc) staining platform using the OptiView DAB IHC Detection Kit and OptiView Amplification Kit (Ventana Medical Systems Inc). The method to record TILs was described as follows: (1) The number of ILs on full sections was scanned at low magnification and evaluated manually at higher magnification (400 ×) under an optical microscope; (2) ILs were assessed in the areas of the tumor center (TILsCT), the invasive front (TILsIF) and on the portal areas of the peritumor 1 cm away from the border (PILs). The “invasive front” (IF) is defined as the region centered on the border separating the host tissue from the malignant nests by 1 mm. Areas with crush artifacts, necrosis, and previous biopsy sites were excluded; and (3) All mononuclear cells, including lymphocytes and plasma cells, were counted. Kaplan-Meier univariate and multivariate survival analyses were performed using a Cox regression model. A nonpaired t-test was conducted to compare the clinicopathological parameters of the immune subtypes.
Research results
Based on this research, low density of TILsCT (P = 0.039), TILsIF (P = 0.014), and PILs (P = 0.010) were independent predictors of progression-free survival (PFS). The immunehigh subtype [(TILsCT)high, (TILsIF)high, and PILshigh, 83 cases] had a lower rate of microvascular invasion (MVI) (40.96%) than the immunemod (tumors other than immunehigh and immunelow subtypes, 94 cases) (61.70%, P = 0.017) and immunelow [(TILsCT)low, (TILsIF)low, and PILslow, 27 cases] (66.67%, P = 0.020) subtypes. The recurrence rates of the immunehigh, immunemod and immunelow subtypes were 10.8%, 25.5% and 33.3%, respectively.
Research conclusions
This study proposed that the density of TILs in HCC tissues can predict the recurrence of the patient. The method of evaluating TILs in H&E-stained specimens may also be meaningful in HCC.
Research perspectives
Increasing multicenter research to validate and improve this method should be implemented in the future.
ACKNOWLEDGEMENTS
The authors thanks all the colleagues for their help in this study. Min Du carried out the study, Yu-Meng Cai made genuine contributions to the data collection, Yu-Lei Yin and Li Xiao helped in data analysis and modification of manuscript, Yuan Ji endorsed the data and conclusions.
Footnotes
Institutional review board statement: The study was approved by the Human Ethics Institutional Review Board of Huadong Hospital, Fudan University (approval number 2019K119).
Informed consent statement: Informed consent was waived by the Review Board because of the nature of retrospective study.
Conflict-of-interest statement: We have no financial relationships to disclose.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: August 18, 2021
First decision: September 29, 2021
Article in press: December 23, 2021
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Limaiem F, Vij M S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ
Contributor Information
Min Du, Department of Pathology, Huadong Hospital, Fudan University, Shanghai 200040, Shanghai Province, China.
Yu-Meng Cai, Department of Pathology, Zhongshan Hospital, Fudan University, Shanghai 200032, Shanghai Province, China.
Yu-Lei Yin, Department of Pathology, Huadong Hospital, Fudan University, Shanghai 200040, Shanghai Province, China.
Li Xiao, Department of Pathology, Huadong Hospital, Fudan University, Shanghai 200040, Shanghai Province, China.
Yuan Ji, Department of Pathology, Zhongshan Hospital, Fudan University, Shanghai 200032, Shanghai Province, China. ji.yuan@zs-hospital.sh.cn.
Data sharing statement
No additional data are available.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Yang YM, Kim SY, Seki E. Inflammation and Liver Cancer: Molecular Mechanisms and Therapeutic Targets. Semin Liver Dis. 2019;39:26–42. doi: 10.1055/s-0038-1676806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hsiao YW, Chiu LT, Chen CH, Shih WL, Lu TP. Tumor-Infiltrating Leukocyte Composition and Prognostic Power in Hepatitis B- and Hepatitis C-Related Hepatocellular Carcinomas. Genes (Basel) 2019;10 doi: 10.3390/genes10080630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garnelo M, Tan A, Her Z, Yeong J, Lim CJ, Chen J, Lim KH, Weber A, Chow P, Chung A, Ooi LL, Toh HC, Heikenwalder M, Ng IO, Nardin A, Chen Q, Abastado JP, Chew V. Interaction between tumour-infiltrating B cells and T cells controls the progression of hepatocellular carcinoma. Gut. 2017;66:342–351. doi: 10.1136/gutjnl-2015-310814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kurebayashi Y, Ojima H, Tsujikawa H, Kubota N, Maehara J, Abe Y, Kitago M, Shinoda M, Kitagawa Y, Sakamoto M. Landscape of immune microenvironment in hepatocellular carcinoma and its additional impact on histological and molecular classification. Hepatology. 2018;68:1025–1041. doi: 10.1002/hep.29904. [DOI] [PubMed] [Google Scholar]
- 6.Bruni D, Angell HK, Galon J. The immune contexture and Immunoscore in cancer prognosis and therapeutic efficacy. Nat Rev Cancer. 2020;20:662–680. doi: 10.1038/s41568-020-0285-7. [DOI] [PubMed] [Google Scholar]
- 7.Pagès F, Mlecnik B, Marliot F, Bindea G, Ou FS, Bifulco C, Lugli A, Zlobec I, Rau TT, Berger MD, Nagtegaal ID, Vink-Börger E, Hartmann A, Geppert C, Kolwelter J, Merkel S, Grützmann R, Van den Eynde M, Jouret-Mourin A, Kartheuser A, Léonard D, Remue C, Wang JY, Bavi P, Roehrl MHA, Ohashi PS, Nguyen LT, Han S, MacGregor HL, Hafezi-Bakhtiari S, Wouters BG, Masucci GV, Andersson EK, Zavadova E, Vocka M, Spacek J, Petruzelka L, Konopasek B, Dundr P, Skalova H, Nemejcova K, Botti G, Tatangelo F, Delrio P, Ciliberto G, Maio M, Laghi L, Grizzi F, Fredriksen T, Buttard B, Angelova M, Vasaturo A, Maby P, Church SE, Angell HK, Lafontaine L, Bruni D, El Sissy C, Haicheur N, Kirilovsky A, Berger A, Lagorce C, Meyers JP, Paustian C, Feng Z, Ballesteros-Merino C, Dijkstra J, van de Water C, van Lent-van Vliet S, Knijn N, Mușină AM, Scripcariu DV, Popivanova B, Xu M, Fujita T, Hazama S, Suzuki N, Nagano H, Okuno K, Torigoe T, Sato N, Furuhata T, Takemasa I, Itoh K, Patel PS, Vora HH, Shah B, Patel JB, Rajvik KN, Pandya SJ, Shukla SN, Wang Y, Zhang G, Kawakami Y, Marincola FM, Ascierto PA, Sargent DJ, Fox BA, Galon J. International validation of the consensus Immunoscore for the classification of colon cancer: a prognostic and accuracy study. Lancet. 2018;391:2128–2139. doi: 10.1016/S0140-6736(18)30789-X. [DOI] [PubMed] [Google Scholar]
- 8.Angell HK, Bruni D, Barrett JC, Herbst R, Galon J. The Immunoscore: Colon Cancer and Beyond. Clin Cancer Res. 2020;26:332–339. doi: 10.1158/1078-0432.CCR-18-1851. [DOI] [PubMed] [Google Scholar]
- 9.Castaneda CA, Castillo M, Aliaga K, Bernabe LA, Casavilca S, Sanchez J, Torres-Cabala CA, Gomez HL, Mas L, Dunstan J, Cotrina JM, Abugattas J, Chavez I, Ruiz E, Montenegro P, Rojas V, Orrego E, Galvez-Nino M, Felix B, Landa-Baella MP, Vidaurre T, Villa MR, Zevallos R, Taxa L, Guerra H. Level of tumor-infiltrating lymphocytes and density of infiltrating immune cells in different malignancies. Biomark Med. 2019;13:1481–1491. doi: 10.2217/bmm-2019-0178. [DOI] [PubMed] [Google Scholar]
- 10.Kojima YA, Wang X, Sun H, Compton F, Covinsky M, Zhang S. Reproducible evaluation of tumor-infiltrating lymphocytes (TILs) using the recommendations of International TILs Working Group 2014. Ann Diagn Pathol. 2018;35:77–79. doi: 10.1016/j.anndiagpath.2018.05.007. [DOI] [PubMed] [Google Scholar]
- 11.Hendry S, Salgado R, Gevaert T, Russell PA, John T, Thapa B, Christie M, van de Vijver K, Estrada MV, Gonzalez-Ericsson PI, Sanders M, Solomon B, Solinas C, Van den Eynden GGGM, Allory Y, Preusser M, Hainfellner J, Pruneri G, Vingiani A, Demaria S, Symmans F, Nuciforo P, Comerma L, Thompson EA, Lakhani S, Kim SR, Schnitt S, Colpaert C, Sotiriou C, Scherer SJ, Ignatiadis M, Badve S, Pierce RH, Viale G, Sirtaine N, Penault-Llorca F, Sugie T, Fineberg S, Paik S, Srinivasan A, Richardson A, Wang Y, Chmielik E, Brock J, Johnson DB, Balko J, Wienert S, Bossuyt V, Michiels S, Ternes N, Burchardi N, Luen SJ, Savas P, Klauschen F, Watson PH, Nelson BH, Criscitiello C, O'Toole S, Larsimont D, de Wind R, Curigliano G, André F, Lacroix-Triki M, van de Vijver M, Rojo F, Floris G, Bedri S, Sparano J, Rimm D, Nielsen T, Kos Z, Hewitt S, Singh B, Farshid G, Loibl S, Allison KH, Tung N, Adams S, Willard-Gallo K, Horlings HM, Gandhi L, Moreira A, Hirsch F, Dieci MV, Urbanowicz M, Brcic I, Korski K, Gaire F, Koeppen H, Lo A, Giltnane J, Rebelatto MC, Steele KE, Zha J, Emancipator K, Juco JW, Denkert C, Reis-Filho J, Loi S, Fox SB. Assessing Tumor-Infiltrating Lymphocytes in Solid Tumors: A Practical Review for Pathologists and Proposal for a Standardized Method from the International Immuno-Oncology Biomarkers Working Group: Part 2: TILs in Melanoma, Gastrointestinal Tract Carcinomas, Non-Small Cell Lung Carcinoma and Mesothelioma, Endometrial and Ovarian Carcinomas, Squamous Cell Carcinoma of the Head and Neck, Genitourinary Carcinomas, and Primary Brain Tumors. Adv Anat Pathol. 2017;24:311–335. doi: 10.1097/PAP.0000000000000161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Calderaro J, Ziol M, Paradis V, Zucman-Rossi J. Molecular and histological correlations in liver cancer. J Hepatol. 2019;71:616–630. doi: 10.1016/j.jhep.2019.06.001. [DOI] [PubMed] [Google Scholar]
- 13.Chan AW, Zhang Z, Chong CC, Tin EK, Chow C, Wong N. Genomic landscape of lymphoepithelioma-like hepatocellular carcinoma. J Pathol. 2019;249:166–172. doi: 10.1002/path.5313. [DOI] [PubMed] [Google Scholar]
- 14.Ziol M, Poté N, Amaddeo G, Laurent A, Nault JC, Oberti F, Costentin C, Michalak S, Bouattour M, Francoz C, Pageaux GP, Ramos J, Decaens T, Luciani A, Guiu B, Vilgrain V, Aubé C, Derman J, Charpy C, Zucman-Rossi J, Barget N, Seror O, Ganne-Carrié N, Paradis V, Calderaro J. Macrotrabecular-massive hepatocellular carcinoma: A distinctive histological subtype with clinical relevance. Hepatology. 2018;68:103–112. doi: 10.1002/hep.29762. [DOI] [PubMed] [Google Scholar]
- 15.Hendry S, Salgado R, Gevaert T, Russell PA, John T, Thapa B, Christie M, van de Vijver K, Estrada MV, Gonzalez-Ericsson PI, Sanders M, Solomon B, Solinas C, Van den Eynden GGGM, Allory Y, Preusser M, Hainfellner J, Pruneri G, Vingiani A, Demaria S, Symmans F, Nuciforo P, Comerma L, Thompson EA, Lakhani S, Kim SR, Schnitt S, Colpaert C, Sotiriou C, Scherer SJ, Ignatiadis M, Badve S, Pierce RH, Viale G, Sirtaine N, Penault-Llorca F, Sugie T, Fineberg S, Paik S, Srinivasan A, Richardson A, Wang Y, Chmielik E, Brock J, Johnson DB, Balko J, Wienert S, Bossuyt V, Michiels S, Ternes N, Burchardi N, Luen SJ, Savas P, Klauschen F, Watson PH, Nelson BH, Criscitiello C, O'Toole S, Larsimont D, de Wind R, Curigliano G, André F, Lacroix-Triki M, van de Vijver M, Rojo F, Floris G, Bedri S, Sparano J, Rimm D, Nielsen T, Kos Z, Hewitt S, Singh B, Farshid G, Loibl S, Allison KH, Tung N, Adams S, Willard-Gallo K, Horlings HM, Gandhi L, Moreira A, Hirsch F, Dieci MV, Urbanowicz M, Brcic I, Korski K, Gaire F, Koeppen H, Lo A, Giltnane J, Rebelatto MC, Steele KE, Zha J, Emancipator K, Juco JW, Denkert C, Reis-Filho J, Loi S, Fox SB. Assessing Tumor-infiltrating Lymphocytes in Solid Tumors: A Practical Review for Pathologists and Proposal for a Standardized Method From the International Immunooncology Biomarkers Working Group: Part 1: Assessing the Host Immune Response, TILs in Invasive Breast Carcinoma and Ductal Carcinoma In Situ, Metastatic Tumor Deposits and Areas for Further Research. Adv Anat Pathol. 2017;24:235–251. doi: 10.1097/PAP.0000000000000162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vennapusa B, Baker B, Kowanetz M, Boone J, Menzl I, Bruey JM, Fine G, Mariathasan S, McCaffery I, Mocci S, Rost S, Smith D, Dennis E, Tang SY, Damadzadeh B, Walker E, Hegde PS, Williams JA, Koeppen H, Boyd Z. Development of a PD-L1 Complementary Diagnostic Immunohistochemistry Assay (SP142) for Atezolizumab. Appl Immunohistochem Mol Morphol. 2019;27:92–100. doi: 10.1097/PAI.0000000000000594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Torbenson MS, Ng IOL, Park YN, Roncalli M, Sakamato M. WHO Classification of Tumors: Digestive System Tumours. 5th ed. Lyon, France: International Agency for Research on Cancer; 2019: 229-239. [Google Scholar]
- 18.Fu Y, Liu S, Zeng S, Shen H. From bench to bed: the tumor immune microenvironment and current immunotherapeutic strategies for hepatocellular carcinoma. J Exp Clin Cancer Res. 2019;38:396. doi: 10.1186/s13046-019-1396-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oura K, Morishita A, Tani J, Masaki T. Tumor Immune Microenvironment and Immunosuppressive Therapy in Hepatocellular Carcinoma: A Review. Int J Mol Sci. 2021;22 doi: 10.3390/ijms22115801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science. 2011;331:1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 21.Cancer Genome Atlas Research Network. Cancer Genome Atlas Research Network. Comprehensive and Integrative Genomic Characterization of Hepatocellular Carcinoma. Cell. 2017;169:1327–1341.e23. doi: 10.1016/j.cell.2017.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zheng C, Zheng L, Yoo JK, Guo H, Zhang Y, Guo X, Kang B, Hu R, Huang JY, Zhang Q, Liu Z, Dong M, Hu X, Ouyang W, Peng J, Zhang Z. Landscape of Infiltrating T Cells in Liver Cancer Revealed by Single-Cell Sequencing. Cell. 2017;169:1342–1356.e16. doi: 10.1016/j.cell.2017.05.035. [DOI] [PubMed] [Google Scholar]
- 23.Ding W, Xu X, Qian Y, Xue W, Wang Y, Du J, Jin L, Tan Y. Prognostic value of tumor-infiltrating lymphocytes in hepatocellular carcinoma: A meta-analysis. Medicine (Baltimore) 2018;97:e13301. doi: 10.1097/MD.0000000000013301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dieci MV, Radosevic-Robin N, Fineberg S, van den Eynden G, Ternes N, Penault-Llorca F, Pruneri G, D'Alfonso TM, Demaria S, Castaneda C, Sanchez J, Badve S, Michiels S, Bossuyt V, Rojo F, Singh B, Nielsen T, Viale G, Kim SR, Hewitt S, Wienert S, Loibl S, Rimm D, Symmans F, Denkert C, Adams S, Loi S, Salgado R International Immuno-Oncology Biomarker Working Group on Breast Cancer. Update on tumor-infiltrating lymphocytes (TILs) in breast cancer, including recommendations to assess TILs in residual disease after neoadjuvant therapy and in carcinoma in situ: A report of the International Immuno-Oncology Biomarker Working Group on Breast Cancer. Semin Cancer Biol. 2018;52:16–25. doi: 10.1016/j.semcancer.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 25.Huang Z, Fu Z, Huang W, Huang K. Prognostic value of neutrophil-to-lymphocyte ratio in sepsis: A meta-analysis. Am J Emerg Med. 2020;38:641–647. doi: 10.1016/j.ajem.2019.10.023. [DOI] [PubMed] [Google Scholar]
- 26.Jaillon S, Ponzetta A, Di Mitri D, Santoni A, Bonecchi R, Mantovani A. Neutrophil diversity and plasticity in tumour progression and therapy. Nat Rev Cancer. 2020;20:485–503. doi: 10.1038/s41568-020-0281-y. [DOI] [PubMed] [Google Scholar]
- 27.Calderaro J, Petitprez F, Becht E, Laurent A, Hirsch TZ, Rousseau B, Luciani A, Amaddeo G, Derman J, Charpy C, Zucman-Rossi J, Fridman WH, Sautès-Fridman C. Intra-tumoral tertiary lymphoid structures are associated with a low risk of early recurrence of hepatocellular carcinoma. J Hepatol. 2019;70:58–65. doi: 10.1016/j.jhep.2018.09.003. [DOI] [PubMed] [Google Scholar]
- 28.Sautès-Fridman C, Petitprez F, Calderaro J, Fridman WH. Tertiary lymphoid structures in the era of cancer immunotherapy. Nat Rev Cancer. 2019;19:307–325. doi: 10.1038/s41568-019-0144-6. [DOI] [PubMed] [Google Scholar]
- 29.Zhang L, Ding J, Li HY, Wang ZH, Wu J. Immunotherapy for advanced hepatocellular carcinoma, where are we? Biochim Biophys Acta Rev Cancer. 2020;1874:188441. doi: 10.1016/j.bbcan.2020.188441. [DOI] [PubMed] [Google Scholar]
- 30.Denkert C, von Minckwitz G, Darb-Esfahani S, Lederer B, Heppner BI, Weber KE, Budczies J, Huober J, Klauschen F, Furlanetto J, Schmitt WD, Blohmer JU, Karn T, Pfitzner BM, Kümmel S, Engels K, Schneeweiss A, Hartmann A, Noske A, Fasching PA, Jackisch C, van Mackelenbergh M, Sinn P, Schem C, Hanusch C, Untch M, Loibl S. Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: a pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol. 2018;19:40–50. doi: 10.1016/S1470-2045(17)30904-X. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No additional data are available.