Graphical abstract
Keywords: Tuberculosis, Mycobacterium tuberculosis, Granuloma, Treatment, Vascular endothelial growth factor, Anti-VEGF anti-VEGFR
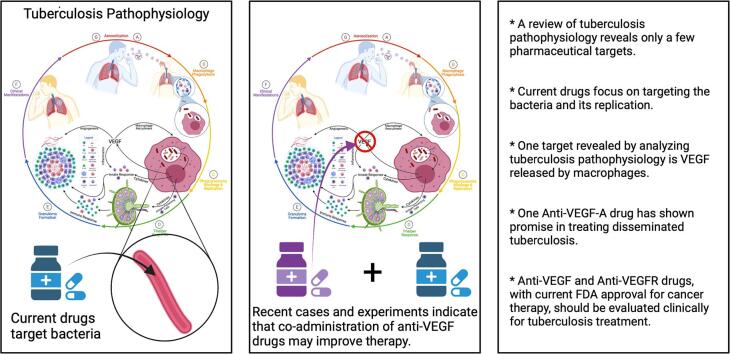
Highlights
-
•
A review of tuberculosis pathophysiology reveals only a few pharmaceutical targets.
-
•
Current drugs focus on targeting the bacteria and its replication.
-
•
One target revealed by analyzing tuberculosis pathophysiology is VEGF released by macrophages.
-
•
One Anti-VEGF-A drug has shown promise in treating disseminated tuberculosis.
-
•
We should clinically evaluate all Anti-VEGF and Anti-VEGFR drugs with current FDA approval for treating cancer for tuberculosis intervention.
Abstract
The pathophysiological understanding of tuberculosis is growing, and with this growth comes the possibility of applying established pharmaceuticals in new ways. These new ways interlude with the many mechanisms by which the intracellular pathogen, Mycobacterium tuberculosis, thrives in its human host. This article will discuss those mechanisms in the context of the pathophysiological processes associated with tuberculosis. Tuberculosis is a disease that results in systemic lesions arising from bacterial-immune interactions. The pathophysiology of this disease proceeds as aerosolization, phagocytosis, phagolysosome blockage and replication, T- helper response, granuloma formation, clinical manifestations, and concluding with active disease and transmission. Herein are the brief details of each of these processes. The conclusion of this article will be current tuberculosis treatments and future promising pharmacological directions. Particularly using the anti-vascular endothelial growth factor treatments currently used in cancer therapy, which are rationally presented with support from case studies. The purpose of this article is thus to present the pathophysiology of tuberculosis to convince the reader of the logical theory behind why anti-VEGF intervention should be used in tuberculosis treatment.
1. Introduction
The pathophysiology of Mycobacterium tuberculosis infections, known as tuberculosis, is a concert of interplay between pathogenic and physiological processes. M. tuberculosis has evolved to thrive by using the human immune system to gain access to the host and remain within the host for years. M. tuberculosis is an intracellular pathogenic bacteria that has a mycolic acid coating, is non-motile, and undergoes cell division once every 18–24 h. Tuberculosis is the disease caused by M. tuberculosis. [1], [2] This disease causes over 4,000 deaths per day, 1.2–1.5 million deaths per year, and has infected 1.7–2 billion people worldwide, [1], [2], [3], [4], [5] with as many as 13 million people in the United States having latent tuberculosis infections (LTBI). [6] 5–10% of these persons with LTBI will develop active tuberculosis. [6] There are approximately 15 million active tuberculosis cases every year, with the heaviest burden occurring in India, Indonesia, South Africa, Nigeria, the Philippines, Pakistan, Bangladesh, and China. [3], [7] Immunocompromised persons have a more significant risk for active tuberculosis - such as those with human immunodeficiency virus (HIV), organ transplants, diabetes mellitus, and silicosis, among others. [7], [8]
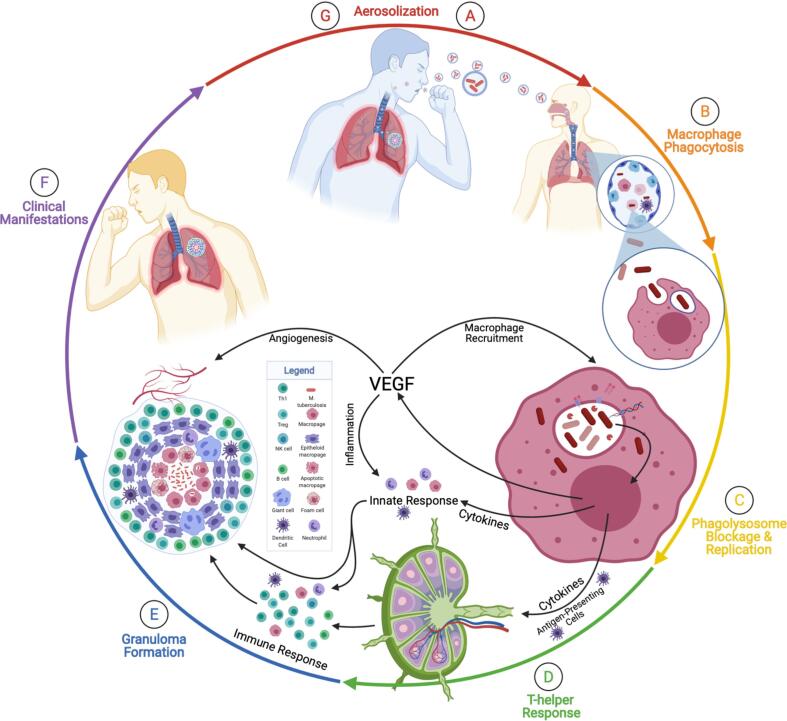
The bacteria has long co-evolved with humans as its host and has developed unique antibacterial mechanisms enabling its persistence within the host, [1] and causes the pathophysiology effects discussed in this article. This article’s purpose is to discuss the seven relative steps in active M. tuberculosis infection, tuberculosis pathophysiology, and disease transmission in the eventual context of clinical pharmacology. These steps are aerosolization, macrophage phagocytosis, phagolysosome blockage and replication, T helper type 1 (TH1) response, granuloma formation, clinical manifestations, and transmission - with lesser intermediate steps interluding (Fig. 1A-G). [9] This review will discuss these steps in context from the molecular mechanism, cellular movements and morphologies, and clinical manifestations. This review will focus on the infection of the lungs, the most common anatomically affected location. Still, the infection can spread to the skin, the nervous system, eyes, lymph nodes, joints and bones, genitourinary, and the abdomen. [7] This review will summarize current treatments with a perspective for future pharmaceutical interventions using established anti-VEGF pharmaceuticals currently available.
Fig. 1.
Seven Steps in the Pathophysiology of Active Tuberculosis. This figure demonstrates the pathophysiology of active tuberculosis. These steps are aerosolization, macrophage phagocytosis, phagolysosome blockage and replication, TH1 response, granuloma formation, clinical manifestations, and transmission. A) Aerosolization is the beginning and the end of the cycle of tuberculosis pathophysiology. Aerosolization occurs when a person with active tuberculosis forcefully expires through actions such as coughing. B) A susceptible person who breathes in the aerosolized Mycobacterium tuberculosis and droplets small enough to reach the alveolar sacs (shown in the first magnification) will encounter macrophages, dendritic cells, and monocytes. The macrophages will phagocytose the bacteria (shown in the second magnification) and attempt to destroy the invader. Dendritic cells will migrate to lymph nodes to activate T-helper cells. C) M. tuberculosis prevents the phagolysosome fusion, avoids destruction, begins replicating, and releases DNA, RNA, proteases, and lipids. Additionally, the macrophages will release cytokines and vascular endothelial growth factor (VEGF). The VEGF will trigger angiogenesis and increase vascularization to the lesion. The cytokines will initiate the innate response and recruit natural killer (NK) cells, dendritic cells (DC), neutrophils, and macrophages in different forms. D) The T-helper cell response will involve the migration of TH1, Tregs, and B cells primed in the germinal center. These cells will combine to form the granuloma (E). The granuloma is a prison to wall off the bacteria from spreading systemically. F) Later, or present, immunocompromisation prevents the granuloma from containing the bacteria. The bacteria will spread and multiply in multiple clinical manifestations. G) During this phase, the bacteria can be aerosolized by the original susceptible, now infected, host, and begin the cycle anew. Adapted from “Granuloma”, by BioRender.com (2021). Retrieved from https://app.biorender.com/biorender-templates.
The purpose of this article is two-fold. First, is to discuss the seven steps in M. tuberculosis infection, tuberculosis pathophysiology, and disease transmission. These steps are aerosolization, macrophage phagocytosis, phagolysosome blockage, TH1 response, granuloma formation, clinical manifestations, and transmission. [9] Second, is to provide the pharmaceutical perspective on tuberculosis and present the possibility of new tuberculosis interventions.
2. Tuberculosis pathophysiology
2.1. Aerosolization
This story of tuberculosis pathophysiology caused by M. tuberculosis will begin where it will end: with the transmission of infectious bacteria. The tuberculosis transmission cascade breaks down into several steps and criteria. [10] The first criteria in the transmission is that there must be a source of the bacteria - the index case. That source must generate infectious particles - that is, have primary or active tuberculosis. M. tuberculosis can then infect healthy individuals via mucous membranes, damaged dermal layers, the digestive system, and most commonly, the respiratory tract. [11] As stated, the source is a person with active tuberculosis of the lungs or larynx able to aerosolize M. tuberculosis. [10] The source generates these aerosolizations via forceful expiratory actions such as coughing, sneezing, shouting, or singing (Fig. 1A). [8], [10] M. tuberculosis is then able to survive airborne. Susceptible individuals inhale the aerosolized M. tuberculosis. Some of these droplets that are smaller than 5 µm and contain 1–3 bacilli can reach the alveolar sacs upon inhalation. [8], [12] The size of the infectious particles, however, varies from 0.65 to > 7 µm. [13] Upon reaching the alveolar sacs, the bacteria take up residence there.
2.2. Macrophage phagocytosis
Once M. tuberculosis has become resident in the alveolar sacs, the bacilli will encounter alveolar macrophages, also known as dust cells in this relative anatomical capacity, along with monocytes and dendritic cells (Fig. 1B). [2], [7] The alveolar macrophages are the dominant cell type in tuberculosis, [2] and are considered to have limited bactericidal activity due to operating in surfactant. [4] M. tuberculosis will bind with dust cells via mannose receptors, scavenger receptors, complement receptors (CR1, CR3, CR4), Fc receptors, and surfactant protein receptors (SPR). [14], [15], [16], [17] The mannose receptor is a pathogen recognition receptor that is responsible for regulating trafficking, antigen presentation, macrophage differentiation, and inflammation. [16] The mannose receptor is the most abundant receptor of human monocyte-derived macrophages. [16]
Once the M. tuberculosis has bound to the mannose receptor, the mannose receptor recruits Grb2, which activates the Rac/Pak/Cdc-42 pathway of M. tuberculosis uptake. [16] The Rac/Pak/Cdc-42 pathway is related to the uptake of M. tuberculosis and recruits Src homology 2 (SH2) domain containing protein tyrosine phosphatase 1 (SHP-1). [16] SHP-1 limits the activity of phosphatidylinositol 3-phosphate (PI3P), a trafficking phospholipid, and thereby limits the phagosome and the lysosome fusion. [16] PI3P is also eliminated from the phagosome by a secreted lipid phosphatase, secretory acid phosphatase (SapM), produced by M. tuberculosis. [17] Moreover, PI3P is a docking molecule that interacts with proteins on the lysosome. [18] Therefore, PI3P is a regulatory lipid essential in the merger of the phagosome and the lysosome that is eliminated in phagosomes containing live M. tuberculosis. [19] Thus, this Rac/Pak/Cdc-42/SHP-1/PI3P cascade promotes the growth of M. tuberculosis within the alveolar macrophages themselves. [16]
Additionally, the bacilli has a very diverse set of cell wall lipids that assist in macrophage evasion. [8] These lipids include lipoarabinomannan (LAM), phosphatidylinositol mannoside (PIM), sulfated glycoplipid (SL), trehalose dimycolate (TDM), and dimycocerosate phthiocerol (DIM). [8] LAM and PIM enhance M. tuberculosis virulence. [8] SL and TDM inhibit lysosome fusion with the phagosome. [8] DIM prevents acidification and increases the permeability of the phagosome. [8]
Once phagocytosed into the macrophages, the bacilli will also activate toll-like receptors (TLR) and release mycolyl-arabinogalactan-peptidoglycan (MAGP), deoxyribonucleic acid (DNA), and ribonucleic acid (RNA) into the cytosol. [17] These actions are accomplished through the early secreted antigenic target, 6 kDA (ESAT-6) secretion system 1 (ESX-1)/Type VII secretion system that disrupts the phagosome membrane. [2], [20] The host TLRs will activate the myeloid differentiation primary response 88 (MyD88) signalling pathway and stimulate nuclear factor kappa-light-chain enhancer of activated B cells (NF-κB) and cytokines. [17] The MAGP will be detected by nucleotide-binding oligomerization domain 2 (NOD2)/caspase recruitment domain 15 (CARD15), which will, in turn, also stimulate NF-κB and cytokines [17] such as tumor necrosis factor (TNF), interleukin 1 beta (IL-1β), interleukin (IL)-6, and Type I interferon (IFN). [1] The bacterial DNA, upon entering the cytosol, will activate the stimulator of interferon genes (STING) pathway via cyclic guanosine monophosphate (GMP)-adenosine monophosphate (AMP) synthase (cGAS) and IFN-activable protein 204 (IFI204), further increasing the activation of the NF-κB and interferon regulatory factor 3 (IRF3) cytokine expression pathways. [1] Additionally, the DNA will activate absent in melanoma 2 (AIM2), causing inflammasome activation and maturation of IL-1β and IL-18. [1] Once in the cytosol, the bacterial RNA will activate retinoic acid inducible gene 1 (RIG-1)/melanoma differentiation-associated protein 5 (MDA5)/mitochondrial antiviral signaling (MAVS), NOD2, and protein kinase R (PKR), causing an activation of IRF3 and NF-κB. [1] Furthermore, the bacterial RNA will activate inflammasomes via nucleotide-binding and oligomerization domain (NOD)-like receptor family (NLR) pyrin domain containing 3 (NLRP3), also causing maturation of IL-1β and IL-18. [1] The cytokines and NF-κB will, in turn, recruit immune cells and begin the immune response against the M. tuberculosis invader. Thus, bacterial RNA, DNA, and MAGP are all responsible for initiating the pathophysiological immune response.
Further, the bacilli in the phagosome prevent the association of vacuolar proton adenosine triphosphate synthase (ATPase), natural resistance associated membrane protein 1 (NRAMP1), and inducible nitric oxide synthase (NOS) with the phagosome. [17] This prevention enables the vesicle to avoid a drop in pH and thereby preserves the bacilli, [17] allowing for replication to occur. Moreover, to amplify the prevention of the phagosome and lysosome fusion even further, the bacteria recruits coronin, a host protein that activates phosphatase calcineurin, which, in turn, inhibits fusion. [15]
M. tuberculosis also induces the macrophage to express and secrete vascular endothelial growth factor (VEGF) into the extracellular spaces. [7] Multiple isoforms of VEGF are critical components in several granuloma processes related to the pathogenesis of mycobacterium. These processes include angiogenesis, monocyte accumulation, macrophage recruitment, and inflammation. [21], [22], [23] As stated, VEGF is responsible for the recruitment of blood vessels and vascular permeability through a physiological process known as angiogenesis. [21], [23], [24] The purpose of angiogenesis into the eventual tuberculosis granuloma is not entirely clear. Still, there exist both immunological and pathological reasons. Immunologically, the eventual blood vessels will serve as an expedient way for immune cells to reach the granuloma and attempt to fight the infection. [7] Pathologically, the eventual blood vessels will serve as a highway for the bacteria to reach systemic circulation and disseminate to other parts of the body. [7], [21] Regardless of defining the purpose of angiogenesis, the vasculature is chaotic, lacks pericytes, has an incomplete basement membrane, and is hyperpermeable. [25] Additional to angiogenesis, VEGF Receptor (VEGFR) has been associated with lymphangiogenesis and mycobacterial specific T cells. [22] The next role of VEGF is as a macrophage chemokine that contributes to the progression of tuberculosis through monocyte and macrophage recruitment in a non-angiogenic manner. [23] This recruitment enhances the bacterial infection by providing new host cells and contributes to cell death signaling related to granuloma repopulation. [23] Inflammation is the third hallmark of the VEGF contribution to mycobacterial infection and granuloma formation. This inflammation is excessive for protection and contributes to the symptomatology and lung pathology of the disease. [23] Interestingly, VEGF inhibition has been shown to reduce granulomatous inflammation, and co-treatment with corticosteroids reduces tuberculosis patient mortality by 17%. [23] Taken together, these processes of angiogenesis, macrophage recruitment, and inflammation make VEGF one of the most important contributors to the pathophysiology of tuberculosis.
Pathophysiologically, the immediately aforementioned steps represent the establishment of the bacteria, and thus, the beginning of bacteremia and the innate inflammatory response. [15] The chemokines produced by the dust cells will recruit natural killer cells, gamma delta (ɣδ)-T cells, neutrophils, and monocytes. [26]
2.3. Phagolysosome blockage & replication
M. tuberculosis replicates intracellularly within the macrophages after preventing the fusion of the phagosome and the lysosome (Fig. 1C). M. tuberculosis has a very unique form of cell division known as asymmetric cell division. [27], [28] Asymmetric cell division means that the bacilli grow preferentially from one pole, and by doing so produce a fast-growing daughter cell and a slow-growing daughter cell. [27], [28] The slow-growing daughter cell differs in many ways from the fast-growing daughter and must assemble a growth pole de novo. These differences are most striking in that the differences between the daughter cells affects both growth rate and antibiotic resistance, [27], [28] a possible reason for the prolific and persistent nature of this bacteria in humans. During this part of the latent infection, the macrophage and M. tuberculosis, either together or individually, will migrate from the alveolar space into the lung parenchyma. [2] Once in the lung parenchyma/interstitium, the immune system will begin to form a granuloma around the invader, in this instance also referred to as a tuberculoma. As the granuloma forms with the simultaneous recruitment of monocytes and immune cells, the bacteria enter the logarithmic phase of growth and must be contained. Pathologically, the anatomical translocation to the lung parenchyma is associated with inflammation of the lungs. As stated, the bacilli replicate intracellularly, and this expansion will eventually cause the destruction of the macrophage via apoptosis, pyroptosis, necroptosis, ferroptosis, and extracellular trap-associated destruction. [1], [29] Apoptosis is an initial defense mechanism against the invading bacteria, and strongly virulent strains inhibit the apoptotic process. [11] As a whole, however, apoptosis and pyroptosis restrict M. tuberculosis growth, whereas necroptosis and ferroptosis are beneficial to the bacteria's survival and success. [1]
2.4. T-helper response
The dendritic cells and the monocytes from earlier in the story will have migrated to local and regional lymph nodes to activate T-cells by way of major histocompatibility complex (MHC) class II proteins and IL-12 (Fig. 1D). [5], [8], [9] This cluster of differentiation 4 (CD4+) response occurs after the first three weeks of infection, during which time M. tuberculosis will have extensively proliferated its population and potentially spread to other organs. [4] The CD4+ T-cell response is why HIV patients are more susceptible to being unable to control tuberculosis infection, as HIV patients have a reduced CD4+ T-cell count. [2] These antigen-specific T-cells will initiate the TH1 response, which, as stated begins about three weeks after infection. [15] TH1 cells mediate the cell-mediated immune response. This response involves activating endothelium, proliferating effector T cell populations, and most relevant to granulomas, using interferon gamma (IFNɣ) and cluster of differentiation 40 (CD40) ligand to activate macrophages. [9] Natural killer cells recruited to the lesion will also release IFNɣ. [30] Cell-mediated immunity has three primary effects. [9] The first is a type IV hypersensitivity reaction - pathophysiologically, this reaction is the cause of the positive Mendel-Mantoux test with the purified protein derivative tuberculin glycerol extract. [4], [9] The second primary effect is the release of IFNɣ that is the further activation of macrophages with augmented bactericidal properties so as to combat better the invader. [4], [9] The third effect will be the development of the granuloma, [9] resulting from a large number of macrophages recruited to the initial lesion. [4] TH2 cells play a lesser perceived role in tuberculosis, and are responsible for promoting humoral immunity via secretion of IL-4, IL-5, IL-10, and IL-13. [4]
2.5. Granuloma formation
A granuloma, as an analogy, is a bacterial jail that intends to imprison a bacteria inside a wall of immune cells. [2] The IFNɣ from the TH1 response will allow the maturation of the phagolysosome in the macrophages, cause the macrophage to produce nitric oxide via nitric oxide synthase, and induce autophagy. [15] The activated macrophages, now being unable to eliminate the pathogen, will release TNF alpha (TNFα). TNFα induces differentiation of monocytes into epithelioid histiocyte cells that form caseating granulomas to contain M. tuberculosis. [9], [15] Some of these epithelioid histiocyte cells fuse to form giant cells. [15] The TNFα continues a feedback chain by recruiting more monocytes to replace the newly differentiated monocytes. [15] The granuloma itself is formed from both macrophages and lymphocytes surrounding and containing M. tuberculosis. Cells involved in the granuloma include TH1, regulatory T cells (Treg), natural killer (NK) cells, B cells, Giant cells, dendritic cells, neutrophils, macrophage, foam cells, and epithelioid macrophage (Fig. 1E). The hypoxic environment within the granuloma temporarily restricts the growth of M. tuberculosis [4], but may also further promote angiogenesis into the tuberculoma. Unfortunately, within the granuloma, the necrotic pool serves as a nutrient source and protective barrier for this pathogen. Additionally, the eventual vasculature amplifies the nutrient supply to the bacteria.
2.6. Clinical manifestations
There are two types of tuberculosis regarding clinical relevance (Fig. 1F): primary tuberculosis and secondary tuberculosis. [15] Primary tuberculosis is a novel infection; that is, the first time someone has acquired M. tuberculosis. [15] This (primary) is the infection that results when the immune system cannot control the initial infection, and is usually the case seen in immunocompromised persons. [9] The primary infection is one completion of the story. This stage is where the infected individual can generate infectious aerosolization of M. tuberculosis and infect the next susceptible individual (Fig. 1G).
Suppose the immune system and granuloma contain M. tuberculosis but do not eliminate the bacteria. In that case, the disease is said to be latent and can progress to secondary tuberculosis at a later stage. [9] During the latent stage of tuberculosis, the bacteria form protective biofilms within the necrotic tissue. [4] Subsequent immunosuppression allows the M. tuberculosis within the granuloma to reactivate and can result in pulmonary disease, extra-pulmonary disease, or miliary tuberculosis. [9] Pulmonary disease is the most common outcome following LTBI and includes the ghon complex radiographic finding and cough, hemolysis, weight loss, night sweats, anorexia, and fever. [9] Extra-pulmonary disease disseminates to the lymph nodes, genitourinary system, gastrointestinal system, pleura, and skeletal systems (with the latter resulting in tuberculosis spondylitis). [9] Miliary tuberculosis is a disease where the granuloma has spread systemically and tuberculomas are resident throughout the body. Secondary tuberculosis can also be the end of the story, as the tuberculoma can liquefy and drain upon bacterial reactivation (cavitation), and the bacilli are aerosolized via the airways. [9] To conclude, infection with M. tuberculosis, and the pathophysiology of the disease known as tuberculosis, can result in primary or secondary manifestations for clinical outcomes.
3. Pharmaceuticals
Ten drugs currently have FDA approval for active, drug-susceptible tuberculosis (as shown in Fig. 1). [31] Of these, the core tuberculosis treatments are taken from six to nine months and include isoniazid, rifampin, ethambutol, and pyrazinamide. [31], [32], [33] Currently, the treatments for LBTI are chemoprophylaxis with six to nine-month isoniazid or rifampin. [4] Isoniazid and rifampin are administered daily during these treatments. [4] Isoniazid, however, can be given bi-weekly at higher dosages. [4] Additionally, isoniazid and rifapentine can be administered to decrease the hepatotoxicity seen with isoniazid monotherapy. [4] The World Health Organization (WHO) recently approved a one-month isoniazid-rifapentine treatment and a four-month treatment for rifampin for LTBI. [5] Furthermore, a one-month isoniazid-rifapentine therapy for LTBI has recently come into use to prevent HIV-related tuberculosis. [5] Multidrug-resistant tuberculosis is treated with an oral bedaquiline regimen. [5] Otherwise, drug-resistant active tuberculosis utilizes the same drugs as drug-susceptible tuberculosis, but with different intervals and doses. [31]
Isoniazid is a small molecule antibacterial that targets two proteins in M. tuberculosis. [34] The first target is catalase-peroxidase, an enzyme that protects the bacteria from reactive oxygen species. [34] The second target is enoyl-[acyl-carrier-protein] reductase, which is involved in mycolic acid production. [34] Rifampin is an antibiotic produced by Streptomyces mediterranei. [35] Rifampin binds to and inhibits DNA-dependent RNA polymerase, thus inhibiting transcription. [33], [35] Ethambutol is bacteriostatic via targeting probable arabinosyltransferases A, B, and C, which inhibits M. tuberculosis cell wall synthesis. [33], [36] Pyrazinamide is a small molecule antituberculosis agent that targets M. tuberculosis fatty acid synthetase, causing plasma membrane disruption and intracellular acidification. [33], [37] Rifapentine is an antibiotic that also inhibits DNA-dependent RNA polymerase. [38] Bedaquiline is an antimycobacterial drug that targets ATP synthase subunit c. [39]
Future tuberculosis treatments need to further evaluate the involvement of the many anti-VEGF inhibitors and combinations currently in use for tumor treatment. [24] Many anti-VEGF and anti-VEGFR drugs exist with Food and Drug Administration (FDA) approval - both small molecules and antibodies. The logic of these treatments for use in tuberculosis are supported by the understanding that VEGF concentrations are elevated in the serum of patients with active tuberculosis, that VEGF recruits macrophage and blood and lymphatic vasculature, and VEGF contributes to inflammation. [7], [22], [23] The use of anti-VEGF and anti-VEGF Receptor (VEGFR) treatments have been shown to assist in treating tuberculosis. Demonstratively in humans, the success of anti-VEGF drugs exists in a case study that found success with intravitreal administration of bevacizumab in the clinical regression of granuloma. [40] Bevacizumab is a humanized monoclonal anti-VEGF-A IgG antibody. [41], [42] In rabbits, bevacizumab is also shown to normalize vasculature and increase delivery of small molecules. [25] The improvement of small molecule delivery seen in anti-VEGF treatment may allow the currently prescribed antibacterials to be more effective. There are at least five isoforms of VEGF, with FDA-approved drugs targeting the A and B isoforms. Additionally, there are at least three isoforms of VEGFR, with FDA-approved drugs to target all three. FDA-approved drugs include tyrosine kinase inhibitors (small molecules targeting VEGFR), monoclonal antibodies, and fusion proteins. [43] In vivo, anti-VEGF therapy abolishes the mycobacterial spread from the infection site, reduces granuloma formation, improves small molecule delivery, and can result in the clinical regression of granuloma. [21], [23], [40]
Clinicians and pharmaceutical companies may consider several concerns during evaluating FDA-approved anti-VEGF/VEGFR antibodies and small molecules in tuberculosis treatment. These concerns are anti-VEGF resistance, adverse effects, altered helper T cell function, and hypoxia. First, this approach can result in macrophage-induced resistance to anti-VEGF antibodies, as shown in tumors. [44] Combinations of anti-angiogenesis agents have been a solution to this issue in tumor treatments. [43] This resistance is a reason why anti-VEGF tumor therapy is often combined with chemotherapy. [45] Such could be an argument for the current treatment regimen recommended by the WHO in combination with anti-VEGF therapy. Anti-VEGF resistance can also include activation of alternative angiogenesis pathways. [43] Second, anti-VEGF treatment has many common side effects, including complications with wound healing, hypertension, and thromboembolism, to name a few. [42], [45] Meaning each patient should be evaluated for their potential outcomes. The foundation for anti-VEGF treatment in tumors is that the human adult normal vasculature is independent of VEGF for normal function and that the benefits of treatment outweigh the risks. [42] And while capillary regression does occur during anti-VEGF treatment in some organs, this regression is reversible after treatment. [42] Third, anti-VEGF therapies carry with them the ability to alter helper T cells. In tumor-bearing hosts, anti-VEGF therapy has been shown to decrease Tregs, while improving the TH1 cytokine response in humans. [43] Thus, potentially exacerbating inflammation. Though bevacizumab in tumor-bearing hosts has been shown to restore dendritic cell function. [43] Overall, however, the understanding of the effects of most anti-VEGF treatments in cancer treatment on T cell function remains unclear. [43] Lastly, anti-VEGF treatment can increase hypoxia in tumors, which still requires further investigation. [43] These concerns of anti-VEGF resistance, adverse effects, altered helper T cell function, and hypoxic effects need to be further evaluated when evaluating anti-VEGF as tuberculosis interventions.
Evaluating both benefits and concerns in conjunction with the millions of deaths per year and immense global burden from tuberculosis suggests that anti-VEGF therapy can be a great benefit to worldwide tuberculosis intervention if evaluated in clinical trials.
4. Concluding section
To conclude, there are many steps in the pathophysiology of tuberculosis. Pharmaceutically, these steps are difficult to target as the bacteria is evasive in hijacking the immune system. Anti-VEGF treatments offer an avenue that has only yet seen brief exploration in humans with a drug exclusive to the A isoform of VEGF (bevacizumab). As success exists with disseminated tuberculosis and anti-VEGF therapy using both small molecules and antibodies, clinical trials are warranted to evaluate all current anti-VEGF and anti-VEGFR drugs in tuberculosis treatment.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Chai Q., Wang L., Liu C.H., Ge B. New insights into the evasion of host innate immunity by Mycobacterium tuberculosis. Cell Mol Immunol. 2020;17(9):901–913. doi: 10.1038/s41423-020-0502-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pai M., Behr M.A., Dowdy D., Dheda K., Divangahi M., Boehme C.C., et al. Tuberculosis. Nat Rev Dis Primers. 2016;2(1) doi: 10.1038/nrdp.2016.76. [DOI] [PubMed] [Google Scholar]
- 3.Fortune S. Sarah Fortune’s Faculty Website. Sarah Fortune’s Faculty Website. Published 2021. Accessed April 21, 2021. https://www.hsph.harvard.edu/sarah-fortune/.
- 4.Jameson JL, Fauci AS, Kasper DL, Hauser SL, Longo DL, Loscalzo J, eds. Harrison’s Principles of Internal Medicine. 20e ed. McGraw-Hill Education; 2018.
- 5.Pai M. Tuberculosis: the story after the Primer. Nat Rev Dis Primers. 2020;6(1) doi: 10.1038/s41572-020-0161-5. [DOI] [PubMed] [Google Scholar]
- 6.Basic TB Facts | TB | CDC. Published March 27, 2020. Accessed April 26, 2021. https://www.cdc.gov/tb/topic/basics/default.htm.
- 7.Batista L.A.F., Silva K.J.S., da Costa e Silva L.M., de Moura Y.F., Zucchi F.C.R. Tuberculosis: A granulomatous disease mediated by epigenetic factors. Tuberculosis. 2020;123:101943. doi: 10.1016/j.tube.2020.101943. [DOI] [PubMed] [Google Scholar]
- 8.Bhatt S, Gour A, Singh G, Nandi U. Tuberculosis. In: Rayees S, Din I, Singh G, Malik FA, eds. Chronic Lung Diseases. Springer Singapore; 2020:87-127. doi:10.1007/978-981-15-3734-9_5.
- 9.Agosti Y, Duke P. MedMaps for Pathophysiology. Illustrated edition. LWW; 2007.
- 10.Churchyard G., Kim P., Shah N.S., Rustomjee R., Gandhi N., Mathema B., et al. What We Know About Tuberculosis Transmission: An Overview. J Infect Dis. 2017;216(suppl_6):S629–S635. doi: 10.1093/infdis/jix362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhai W., Wu F., Zhang Y., Fu Y., Liu Z. The Immune Escape Mechanisms of Mycobacterium Tuberculosis. Int J Mol Sci. 2019;20(2):340. doi: 10.3390/ijms20020340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ryndak M.B., Laal S. Mycobacterium tuberculosis Primary Infection and Dissemination: A Critical Role for Alveolar Epithelial Cells. Front Cell Infect Microbiol. 2019;9:299. doi: 10.3389/fcimb.2019.00299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bussi C., Gutierrez M.G. Mycobacterium tuberculosis infection of host cells in space and time. FEMS Microbiol Rev. 2019;43(4):341–361. doi: 10.1093/femsre/fuz006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kumar V., Abbas A., Aster J. 9th edition. Elsevier; 2014. Robbins & Cotran Pathologic Basis of Disease. [Google Scholar]
- 15.Kumar V., Aster J.C., Perkins J.A., et al. 10th Edition. Elsevier; 2021. Robbins and Cotran Pathologic Basis of Disease. [Google Scholar]
- 16.Rajaram M.V.S., Arnett E., Azad A.K., et al. M. tuberculosis-initiated human mannose receptor signaling temporally regulates macrophage recognition and vesicle trafficking by FcRγ-chain, Grb2 and SHP-1. Cell Rep. 2017;21(1):126–140. doi: 10.1016/j.celrep.2017.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sakamoto K. The pathology of Mycobacterium tuberculosis infection. Vet Pathol. 2012;49(3):423–439. doi: 10.1177/0300985811429313. [DOI] [PubMed] [Google Scholar]
- 18.Bhat K.H., Yaseen I. Mycobacterium Tuberculosis: Macrophage Takeover and Modulation of Innate Effector Responses. IntechOpen. 2018 doi: 10.5772/intechopen.75003. [DOI] [Google Scholar]
- 19.Vergne I., Chua J., Lee H.H., Lucas M., Belisle J., Deretic V. Mechanism of phagolysosome biogenesis block by viable Mycobacterium tuberculosis. Proc Natl Acad Sci U S A. 2005;102(11):4033–4038. doi: 10.1073/pnas.0409716102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Famelis N., Rivera-Calzada A., Degliesposti G., Wingender M., Mietrach N., Skehel J.M., et al. Architecture of the mycobacterial type VII secretion system. Nature. 2019;576(7786):321–325. doi: 10.1038/s41586-019-1633-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Polena H., Boudou F., Tilleul S., Dubois-Colas N., Lecointe C., Rakotosamimanana N., et al. Mycobacterium tuberculosis exploits the formation of new blood vessels for its dissemination. Sci Rep. 2016;6(1) doi: 10.1038/srep33162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harding J., Ritter A., Rayasam A., Fabry Z., Sandor M. Lymphangiogenesis Is Induced by Mycobacterial Granulomas via Vascular Endothelial Growth Factor Receptor-3 and Supports Systemic T-Cell Responses against Mycobacterial Antigen. Am J Pathol. 2015;185(2):432–445. doi: 10.1016/j.ajpath.2014.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harding J.S., Herbath M., Chen Y., Rayasam A., Ritter A., Csoka B., et al. VEGF-A from Granuloma Macrophages Regulates Granulomatous Inflammation by a Non-angiogenic Pathway during Mycobacterial Infection. Cell Reports. 2019;27(7):2119–2131.e6. doi: 10.1016/j.celrep.2019.04.072. [DOI] [PubMed] [Google Scholar]
- 24.Maison D.P. The Vascular Endothelial Growth Factor Pathway of Angiogenesis in Tumors: Associated Pharmaceutical Targets and Treatments. CMUJNS. 2018;17(4) doi: 10.12982/CMUJNS.2018.0023. [DOI] [Google Scholar]
- 25.Datta M., Via L.E., Kamoun W.S., Liu C., Chen W., Seano G., et al. Anti-vascular endothelial growth factor treatment normalizes tuberculosis granuloma vasculature and improves small molecule delivery. PNAS. 2015;112(6):1827–1832. doi: 10.1073/pnas.1424563112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ehlers S., Schaible U.E. The Granuloma in Tuberculosis: Dynamics of a Host-Pathogen Collusion. Front Immunol. 2013;3 doi: 10.3389/fimmu.2012.00411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aakre C.D., Laub M.T. Asymmetric cell division: a persistent issue? Dev Cell. 2012;22(2):235–236. doi: 10.1016/j.devcel.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aldridge B.B., Fernandez-Suarez M., Heller D., et al. Asymmetry and aging of mycobacterial cells leads to variable growth and antibiotic susceptibility. Science. 2012;335(6064):100–104. doi: 10.1126/science.1216166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wong K.W., Jacobs W.R. Mycobacterium tuberculosis Exploits Human Interferon γ to Stimulate Macrophage Extracellular Trap Formation and Necrosis. J Infect Dis. 2013;208(1):109–119. doi: 10.1093/infdis/jit097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu C.H., Liu H., Ge B. Innate immunity in tuberculosis: host defense vs pathogen evasion. Cell Mol Immunol. 2017;14(12):963–975. doi: 10.1038/cmi.2017.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.CDC. Treatment for TB Disease. Published May 9, 2019. Accessed January 8, 2022. https://www.cdc.gov/tb/topic/treatment/tbdisease.htm.
- 32.Haas M.K., Belknap R.W. Updates in the Treatment of Active and Latent Tuberculosis. Semin Respir Crit Care Med. 2018;39(3):297–309. doi: 10.1055/s-0038-1660863. [DOI] [PubMed] [Google Scholar]
- 33.Bendre A.D., Peters P.J., Kumar J. Tuberculosis: Past, present and future of the treatment and drug discovery research. Curr Res Pharmacol Drug Discov. 2021;2:100037. doi: 10.1016/j.crphar.2021.100037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.DrugBank. Isoniazid. Published January 9, 2022. Accessed April 29, 2021. https://go.drugbank.com/drugs/DB00951.
- 35.DrugBank. Rifampicin. Published January 9, 2022. Accessed April 29, 2021. https://go.drugbank.com/drugs/DB01045.
- 36.DrugBank. Ethambutol. Published January 9, 2022. Accessed January 10, 2022. https://go.drugbank.com/drugs/DB00330.
- 37.DrugBank. Pyrazinamide. Published January 9, 2022. Accessed January 10, 2022. https://go.drugbank.com/drugs/DB00339.
- 38.DrugBank. Rifapentine. Published August 7, 2021. Accessed April 29, 2021. https://go.drugbank.com/drugs/DB01201.
- 39.DrugBank. Bedaquiline. Published October 3, 2021. Accessed April 29, 2021. https://go.drugbank.com/drugs/DB08903.
- 40.Agarwal M., Gupta C., Mohan KVarsha, Upadhyay PramodK, Jha V. Correlation of vascular endothelial growth factor with the clinical regression of tubercular granuloma. Indian J Ophthalmol. 2020;68(9):2037. doi: 10.4103/ijo.IJO_1261_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ferrara N., Hillan K.J., Gerber H.P., Novotny W. Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat Rev Drug Discov. 2004;3(5):391–400. doi: 10.1038/nrd1381. [DOI] [PubMed] [Google Scholar]
- 42.Kamba T., McDonald D.M. Mechanisms of adverse effects of anti-VEGF therapy for cancer. Br J Cancer. 2007;96(12):1788–1795. doi: 10.1038/sj.bjc.6603813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bourhis M., Palle J., Galy-Fauroux I., Terme M. Direct and Indirect Modulation of T Cells by VEGF-A Counteracted by Anti-Angiogenic Treatment. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.616837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dalton H.J., Pradeep S., McGuire M., Hailemichael Y., Ma S., Lyons Y., et al. Macrophages facilitate resistance to anti-VEGF therapy by altered VEGFR expression. Clin Cancer Res. 2017;23(22):7034–7046. doi: 10.1158/1078-0432.CCR-17-0647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kazazi-Hyseni F., Beijnen J.H., Schellens J.H.M. Bevacizumab. Oncologist. 2010;15(8):819–825. doi: 10.1634/theoncologist.2009-0317. [DOI] [PMC free article] [PubMed] [Google Scholar]