Summary
Background
Non-invasive pneumococcal pneumonia causes significant morbidity and mortality in older adults. Understanding pneumococcal sero-epidemiology in adults ≥50 years is necessary to inform vaccination policies and the updating of pneumococcal vaccines.
Methods
We conducted a systematic review and random-effects meta-analysis to determine the proportion of community-acquired pneumonia (CAP) in people ≥50 years due to pneumococcus and the proportion caused by pneumococcal vaccine serotypes. We searched MEDLINE, EMBASE and PubMed from 1 January 1990 to 30 March 2021. Heterogeneity was explored by subgroup analysis according to a) patient group (stratified versus age) and depth of testing, b) detection/serotyping method, and c) continent. The protocol is registered with PROSPERO (CRD42020192002).
Findings
Twenty-eight studies were included (34,216 patients). In the period 1–5 years after introduction of childhood PCV10/13 immunisation, 18% of CAP cases (95% CI 13–24%) were attributable to pneumococcus, with 49% (43–54%) of pneumococcal CAP due to PCV13 serotypes. The estimated proportion of pneumococcal CAP was highest in one study that used 24-valent serotype-specific urinary-antigen detection (ss-UAD)(30% [28–31%]), followed by studies based on diagnostic serology (28% [24–33%]), PCR (26% [15–37%]), ss-UAD14 (17% [13–22%]), and culture alone (14% [10–19%]). A higher estimate was observed in Europe (26% [21–30%] than North America (11% [9–12%](p<0·001). PCV13-serotype estimates were also influenced by serotyping methods.
Interpretation
Non-invasive pneumococcal CAP and vaccine-type pneumococcal CAP remains a burden in older adults despite widespread introduction of pneumococcal infant immunisation. Studies heavily reliant on ss-UADs restricted to vaccine-type serotypes may overestimate the proportion of potentially vaccine-preventable pneumococcal pneumonia. Sero-epidemiological data from low-income countries are lacking.
Keywords: Pneumonia, Streptococcus pneumoniae, Pneumococcal vaccines, Serotype, Older adults
Research in context.
Evidence before this study
We searched Medline, Embase and PubMed from 1 January 1990 to 30 March 2021, with no restriction by language, for studies evaluating the proportion of non-invasive pneumococcal pneumonia in adults aged 50 years and above caused by serotypes included in pneumococcal vaccines across the world. Observational studies have differed widely in the reported contribution of vaccine-type serotypes to non-invasive disease (from 25% to 80%), likely reflecting the use of different diagnostic and serotyping techniques, regional epidemiology and the impacts of national pneumococcal vaccination programmes. Our search did not reveal any previous systematic reviews that have assessed the impact of these factors on estimates of pneumococcal pneumonia and vaccine-preventable disease in older adults globally.
Added value of this study
This systematic review and meta-analysis, conducted at the request of the WHO Pneumococcal SAGE Working Group, is the first to focus on the impact of pneumococcal serotypes on non-invasive pneumococcal CAP in adults aged 50 years and above. We included 28 eligible studies (34,216 patients) from different parts of the world and have shown that pneumococcal serotypes included in current pneumococcal vaccines still cause a persistent burden of potentially vaccine preventable non-invasive CAP in older people despite the widespread introduction of infant immunisation with pneumococcal conjugate vaccines. Furthermore, we highlight the effect of pneumococcal diagnostic/serotyping method and geographical location on the estimates of pneumococcal and vaccine-type CAP, and identify an important evidence-gap through the absence of data from low-middle income countries (LMICs) where the burden from pneumonia remains very high.
Implications of all the available evidence
Strategies to further reduce the substantial proportion of vaccine-preventable non-invasive CAP in older people, despite infant pneumococcal immunisation programmes, warrant consideration. Studies from LMICs are required to guide global pneumococcal vaccination policies as there are currently no serotype specific data for non-invasive disease from these countries. Future studies should be supported by the development of improved serotype-specific diagnostic methods which detect a wide range of serotypes not restricted to those in existing vaccines so that changes in non-vaccine serotypes can be detected, and to reduce potentially biased estimates of vaccine-preventable disease.
Alt-text: Unlabelled box
Introduction
Community-acquired pneumonia (CAP) is a significant cause of morbidity and mortality worldwide.1,2 Streptococcus pneumoniae (pneumococcus) is the most commonly implicated bacterial pathogen,3, 4, 5, 6, 7 and the spectrum of pneumococcal disease ranges from asymptomatic nasopharyngeal carriage through to localised infections and invasive pneumococcal disease (IPD), although non-invasive pneumococcal pneumonia is the most common manifestation.8 The Global Burden of Diseases Study estimated that in 2016 pneumococcal lower respiratory infections caused around 1·2 million deaths worldwide and 197 million episodes.9 The burden of pneumococcal disease follows a U-shaped curve, with greatest incidence and mortality in young children under 5 years and older adults aged 65 and above.2,9,10
With 100 documented pneumococcal serotypes, which vary in their disease severity, invasiveness and antimicrobial susceptibility, pneumococcal disease is at least partially vaccine-preventable. Many countries around the world have established infant immunisation programmes against pneumococcal disease, most using either a 13-valent or 10-valent pneumococcal conjugate vaccine (PCV). Introduction of childhood PCV immunisation has been associated with a reduction of overall and serotype-specific IPD in young children. Furthermore, infant PCV immunisation reduces nasopharyngeal carriage in those who are vaccinated thus preventing onward transmission to unvaccinated children and adults, and has resulted in reductions in both IPD and pneumococcal pneumonia across all ages.11, 12, 13, 14, 15 Some higher-income countries offer PPV23 and/or PCV13 vaccination to high-risk adults, including those over 65 years, although uptake varies between countries and is poor in many countries in which the vaccine is available.16,17
Most data on the epidemiology of vaccine-serotype pneumococcal disease is derived from invasive isolates, although fewer than 10% of pneumococcal pneumonia cases are invasive. To inform effective global pneumococcal vaccination strategies it is important to understand the true burden of non-invasive pneumonia and the impact of current vaccination programmes on the sero-epidemiology of the disease in older people. The primary objective of this systematic review and meta-analysis was to summarise the evidence on the proportion of non-invasive pneumococcal CAP due to S. pneumoniae serotypes covered by the PCV and PPV23 vaccines in people who are 50 years or older, and the secondary objective to evaluate the proportion of non-invasive pneumococcal CAP due to S. pneumoniae. This systematic review and meta-analysis was conducted at the request of the WHO Pneumococcal SAGE Working Group and initial results were presented to the Working Group in August 2020.
Methods
We conducted this systematic review and meta-analysis in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) reporting guidelines.18 The study protocol was registered with the National Institute for Health Research international prospective register of systematic reviews (PROSPERO) (CRD42020192002).19
Search strategy
We searched MEDLINE, EMBASE and PubMed from 1 January 1990 up to 30 March 2021, using search terms relating to community-acquired pneumonia, Streptococcus pneumoniae, and pneumococcal serotypes. (See Supplementary Material 1 for example of the EMBASE search strategy). We also manually searched the reference lists of included articles for relevant studies. No language restrictions were imposed.
Study selection
We included cohort studies, surveillance studies and registry studies reporting data on non-invasive pneumococcal pneumonia caused by serotypes included in the study country's national pneumococcal immunisation schedule. Studies were eligible if they had separate pneumococcal data for adults aged 50 years or above, either stratified according to age group or in which the overall mean or median age of the entire cohort was ≥50 years, with a diagnosis of pneumonia acquired in the community. We excluded experimental studies, case-control studies, editorials, reviews and meta-analyses, studies with insufficient data on the pneumococcal serotypes causing non-invasive pneumonia in people aged 50 years and older, and studies which only included patients with invasive pneumococcal disease. One author (LL) screened the titles using pre-specified inclusion and exclusion criteria. Abstract and full text screening was independently performed by two authors (LL and HL/BL/TM) with disagreements resolved by consensus.
Data extraction and analysis
Two reviewers (LL and HL/BL/TM) independently extracted data from individual studies using a predefined piloted template. We collected data on study methodology, location and setting, study population, national pneumococcal immunisation schedule including type and date of introduction, the incidence and proportion of CAP patients with pneumonia due to S. pneumoniae and the proportion of those due to vaccine serotypes, and the methods used to diagnose pneumococcal pneumonia and to identify pneumococcal serotypes. We assessed the risk of bias in the study group selection and outcome domains using a modification of the Newcastle-Ottawa Scale.20 The comparability domain was not considered relevant due to the design of the included studies.
Outcome measures
Outcome measures were the proportion of CAP caused by S. pneumoniae in people aged 50 years and over, and the proportion of pneumococcal pneumonia in patients aged 50 and over in whom a vaccine type (VT) pneumococcus was identified as the causative pathogen. The serotypes covered by each of the currently licensed pneumococcal vaccines are listed in supplement 2.
Data synthesis
We estimated the pooled proportion using the metaprop command in Stata and a random effects model.21 Variances were stabilised using the Freeman-Tukey double arcsine transformation which normalises the outcomes before pooling so that studies with proportions close to 0% or 100% were approximately estimated. For a study i, in which ri denotes the number of observations with a certain characteristic and ni is the total number of observations, this is defined as:
The asymptotic variance of the transformed variable is defined as:
The DerSimonian Laird method was used to compute pooled estimates based on the transformed values and their variances.
We assessed heterogeneity using the I2 statistic.
We estimated the proportion of a) patients aged 50 and above with CAP caused by pneumococcus; b) patients aged 50 and above with pneumococcal pneumonia caused by a serotype covered by PCV13 and PPV23. Analyses were stratified by: patient group (entire cohort aged ≥50 years or median/mean ≥50 years) and completeness of testing (with optimal testing defined as simultaneous testing by culture, BinaxNOW (non-specific pneumococcal antigen test) and serotype-specific urinary antigen detection (ss-UAD)/serotype specific serology); by serotype detection method (UAD24, UAD14, serology, PCR, respiratory culture only); and by continent (Asia, Europe and North America). Data were analysed for the periods pre- and post-introduction of childhood PCV10/13 immunisation programmes, with data for the post-PCV 10/13 period being included if collected at least one year after national introduction of PCV10/13.
All analyses were conducted in Stata 16·0 software (StataCorp. 2019. Stata Statistical Software: Release 16. College Station, TX:StatCorp LLC.).
Ethics statement
Ethical approval for this systematic review and meta-analysis is not applicable since the data utilised were collected from previously published research in the literature. All the included studies in this review had received ethical approval prior to data collection.
Role of the funding source
The funders had no role in the study design, data collection, data analysis, data interpretation, or in the writing of the manuscript.
Results
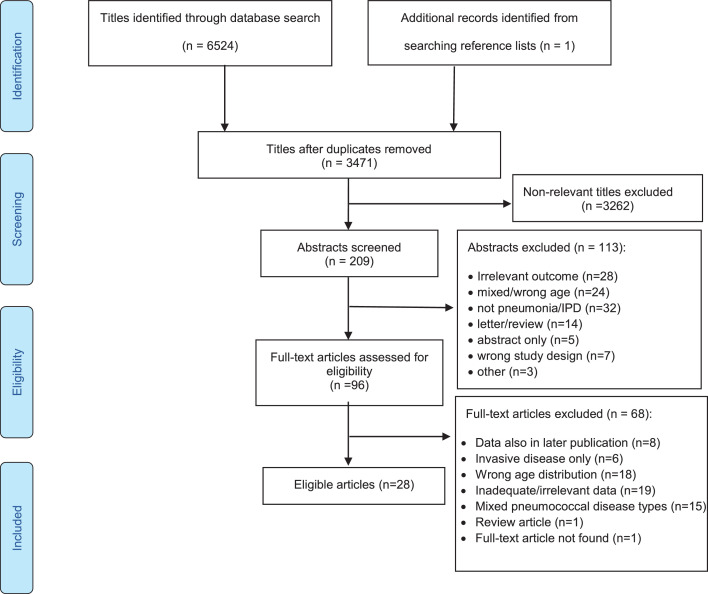
After screening 3471 papers, 28 studies were eligible for inclusion (Figure 1)15,22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48 and included data on 34,216 patients from 19 countries across Asia, Europe and North America (supplementary Table 1). All studies were from high income countries. The most common reasons for exclusion of studies were wrong age group and inadequacy of serotype data.
Figure 1.
PRISMA flow diagram for study selection.
In the modified risk of bias assessment, 8 of the 28 (28·6%) studies were judged to have some degree of selection bias as included patients may have not been truly representative of patients with CAP (Supplementary Table 2).
Proportion of CAP due to Streptococcus pneumoniae
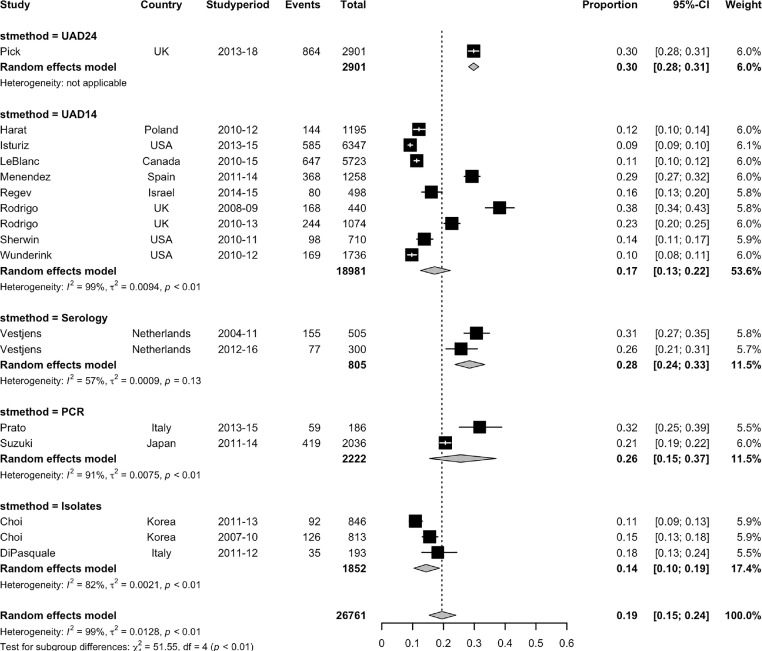
Prior to introduction of childhood PCV10/13 immunisation, the pooled estimated proportion of CAP which was due to pneumococcus in people aged 50 years and above was 22% (95% CI 15–29%, 6 studies, I2 = 97·1%),15,25,27,42,44,46 ranging from 12% to 38%. (Supplementary Figure 1) Eleven studies included data from at least one year after the introduction of PCV10/13, with a pooled proportion of 18% (95% CI 13–24%, I2= 98·9%), ranging from 9% to 32%.15,22,24,25,27,30,31,33,37,39,41 The estimated proportion of CAP due to pneumococcus was influenced by the testing method, with stratification according to pneumococcal detection method showing significant subgroup heterogeneity (p<0·001). The highest estimated proportion of CAP due to pneumococcus was seen with ss-UAD24 (30% [95% CI 28–31%], 1 study), followed by serology (28% [95% CI 24–33%], 2 datasets), PCR (26% [95% CI 15–37%], 2 studies), ss-UAD14 (17% [95% CI 13–22%, 9 datasets), and culture alone (14% [95% CI 10–19%], 3 datasets). (Figure 2)
Figure 2.
Forest plot of the pooled estimated proportion of CAP due to Streptococcus pneumoniae, stratified by the method of pneumococcal serotyping employed by included studies.
Key: UAD24: 24-valent serotype-specific urinary antigen detection,
UAD14: 14-valent serotype-specific urinary antigen detection,
PCR: polymerase chain reaction (S. pneumoniae isolated by PCR with sequential multiplex PCR using 29 serotype specific primer pairs).
Sensitivity analysis excluding 1 study (di Pasquale) considered to be at risk of some selection bias:
UAD24: 30% (28–31),
UAD14: 17% (13–22),
Serology: 28% (24–33),
PCR: 26% (15–37),
Isolates: 13% (11–15),
Overall I2=98.75%.
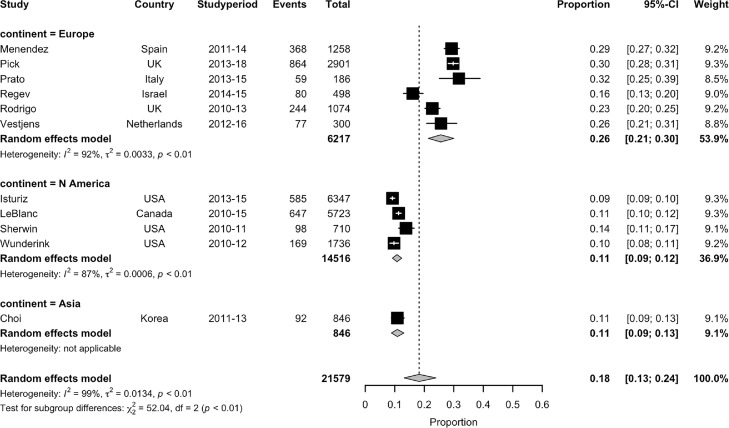
Significant heterogeneity was noted between studies stratified by continent (p<0·001)(Figure 3). In the post PCV10/13 period, the summary estimate for the proportion of CAP due to pneumococcus from six studies in Europe was 26% (95% CI 21–30%, I2=92%),15,24,25,30,33,41 whereas from four North American studies it was 11% (95% CI 9–12%, I2=87·2%).22,31,37,39 Similarly, one study from Korea also reported a proportion of 11% (95% CI 9–13%), although this was based on serotyped isolates from bacterial culture rather than ss-UAD testing so may be an underestimate of the true proportion.27
Figure 3.
Estimated proportion CAP due to S. pneumoniae stratified by continent.
No significant subgroup differences were observed when studies were grouped by definition of age groups (defined strata versus mean/median age) (p = 0·64), nor by depth of testing (optimal versus sub-optimal)(p = 0·71)(Supplementary Figures 2 and 3).
Proportion of pneumococcal CAP due to PCV13 serotypes
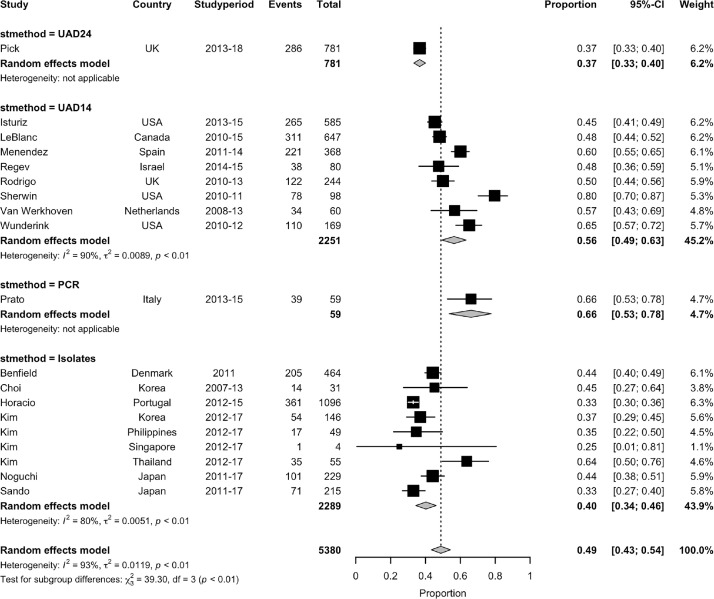
The overall pooled proportion of pneumococcal CAP which was due to PCV13 serotypes in the 1–5 years post PCV10/13 period was 49% (95% CI 43–54%, 15 studies, 19 datasets, I2=92·7%)(Supplementary Figure 4). For studies in which pneumococcal testing was optimal and which stratified by age, the pooled proportion of pneumococcal CAP due to PCV13 serotypes was similar to the overall estimate, 51% (95% CI 43–59%, 7 studies, I2=92·7%).15,22,30,31,37, 38, 39,41 The pooled proportion of all CAP due to PCV13 serotypes from these same studies was 8% (95% CI 6–11%, I2=97·1%).
Again, the estimates were influenced by the method of pneumococcal serotype detection (Figure 4). The highest estimated proportion of pneumococcal CAP due to PCV13 serotypes was observed in one study in which sequential-multiplex PCR was used for serotyping using 29 serotype-specific primer pairs (66%, 95% CI 53–78%).33 The estimated pooled proportion of pneumococcal CAP due to PCV13 serotypes from studies employing a 14-valent ss-UAD was 56% (95% CI 49–63%, 8 studies, I2=90·2%),15,22,24,30,31,37, 38, 39 and 40% (95% CI 34–46%, I2= 80·4%) from six studies in which serotyping was performed on isolates only.26, 27, 28, 29,35,43 In the one study which utilised a 24-valent ss-UAD, the proportion of pneumococcal CAP due to PCV-13 serotypes was 37% (95% CI 33–40%).41
Figure 4.
Estimated proportion of pneumococcal CAP due to PCV13 vaccine serotypes in the period after introduction of PCV10/13 immunisation programmes, stratified by the method of pneumococcal serotyping employed by included studies. Legend: Sensitivity analysis excluding 4 studies (Benfield, Horacio, Kim, Sando) considered to be at risk of some selection bias:
UAD24: 37% (33–40),
UAD14: 56% (49–63),
PCR: 66% (53–77),
Isolates: 54% (47–60),
Overall I2=92.14%.
By continent, the highest proportion of pneumococcal CAP caused by PCV13 serotypes was noted when studies from North America were pooled, all of which employed ss-UAD14 (59%, 95% CI 47–71%, 4 studies, I2=95%),22,31,37,39 followed by Europe (48%, 95% CI 40–56%, 8 studies, I2=94·3%) for which serotyping methods varied between the included studies.15,24,28,30,33,38,41,43 The pooled estimate was lowest in studies from Asia, all of which were based on serotyping of pneumococcal isolates (41%, 95% CI 34–49%, 4 studies, I2=70·1%).26,27,29,35(Supplementary Figure 5)
Proportion of pneumococcal CAP due to PPV23 serotypes
The overall pooled estimate of the proportion of pneumococcal CAP due to PPV23 serotypes, including those in PCV13, was 67% (95% CI 62–72%, 12 studies, I2=90·3%). Two studies which used PCR for serotyping had a pooled estimated proportion of 80% (95% CI 75–83%). One of these studies examined for 50 pneumococcal serotypes by nanofluidic real-time PCR and the second employed 29 primer pairs to serotype pneumococcus PCR-positive samples.33,46 The proportion of CAP due to PPV23 serotypes in the one study which used SS-UAD24 to detect pneumococci was 70% (95% CI 66–73%).41 Pooling data from the nine studies which serotyped using the Quellung method on pneumococcal isolates only gave the lowest estimated proportion of CAP due to PPV23 serotypes at 65% (95% CI 58–71%, I2=88·2%).26–29,32,35,40,43,45(Supplementary Figure 6)
Prevalent pneumococcal serotypes in CAP
The most prevalent pneumococcal serotypes for each study are presented in Table 1. Overall serotype 3 ranked as one of the top three most prevalent serotypes in 25 of 30 (83·3%) datasets for non-bacteraemic CAP, followed by 19A (53·3% of datasets), and 11A/E and 19F (both 30% of datasets).
Table 1.
Pneumococcal serotypes with the highest prevalence causing CAP in the included studies.
| Study | Country | Year | Pre PCVa/Years post PCV infant immunisation | Number of people included | Most prevalent serotypes |
|---|---|---|---|---|---|
| Europe | |||||
| Domenech45 | Spain | 2001–8 | Pre | 255 | 3, 1, 5 (bacteraemic) 3, 19F, 23F (non-bacteraemic) |
| Harat42 | Poland | 2010–12 | Pre | 1195 | 3, 23F, 18C |
| Pletz34 | Germany | 2002–11 | Pre | 391 | 7F, 3, 1 |
| Vila Corcoles40 | Spain | 2008–11 | Pre | 125 | 3, 19A, 22F |
| Horacio36 | Portugal | 1999–2011 | Pre to 1 | 225 | 3, 11A, 19F |
| Benfield43 | Denmark | 2011 | 1 | 464 | 3, 11A,19A (non-bacteraemic) 1, 7F, 3 (bacteraemic) |
| Van Werkhoven38 | Netherlands | 2011–13 | Up to 2 | 288 | 3, 7F, 19A |
| Rodrigo15 | UK | 2008–13 | Up to 3 | 1887 | 1, 7F/A, 19A |
| Menendez24 | Spain | 2011–14 | 1–3 | 1258 | 3, 7F, 19A |
| Di Pasquale44 | Italy | 2011–12 | 2–3 | 193 | 35F, 3, 24 |
| Regev30 | Israel | 2014–15 | 4 | 498 | 3, 23F, 19A |
| Vestjens25 | Netherlands | 2012–16 | 1–5 | 805 | 3, 8, 19A |
| Horacio28 | Portugal | 2012–15 | 2–5 | 1096 | 3, 11A, 19F |
| Prato33 | Italy | 2013–15 | 3–5 | 186 | 23F, 9 V, 19A |
| Quirk48 | Iceland | 2009–17 | Pre, up to 6 | 430 | 19F, 6B, 3 19F, 3, 11A |
| Pick41 | UK | 2013–18 | 3–8 | 2901 | 3, 8, 15A |
| North America | |||||
| Sherwin31 | USA | 2010–11 | 1 | 710 | 19A, 7F/A, 3 and 5 |
| Wunderink37 | USA | 2010–12 | Up to 2 | 2736 | 19A, 3, 7F |
| LeBlanc22,47 | Canada | 2011–15 | 1–5 | 5723 | 3, 7F, 19A |
| Isturiz39 | USA | 2013–16 | 3–5 | 6347 | 19A, 3, 5 |
| Asia | |||||
| Oishi32 | Japan | 2001–03 | Pre | 114 | 19F, 23F, 6B |
| Morimoto23 | Japan | 2011–13 | Pre | 1772 | 3, 11A, 19F |
| Choi27 | Korea | 2007–13 | Pre, up to 3 | 2221 | 3, 19A, 11A/E 3, 19A, 6C |
| Kim26 | China | 2008–14 | Pre | 194 | 19F, 19A, 23F |
| Korea | 2008–14 | 2–5 | 146 | 11A, 19A, 19F | |
| Philippines | 2008–14 | 2–5 | 49 | 3, 11A, 6B/19A | |
| Thailand | 2008–14 | 1–4 | 55 | 3, 23F, 6B | |
| Suzuki46 | Japan | 2011–14 | Up to 1 | 2036 | 3, 10A, 6A/B |
| Sando35 | Japan | 2011–14, 2016–17 | Pre, 3 | 438 | 3, 11A, 19 35B, 3, 6C/19A |
| Noguchi29 | Japan | 2011–17 | Up to 4 | 229 | 3, 19F, 11A/E |
Notes:
PCV10 or PCV13;
(pre) – pre-PCV 10/13 in infant immunisation programme;
(post) – post introduction of PCV 10/13 in infant immunisation.
Of the serotypes listed in the table:
PCV7 serotypes – 6B, 9 V, 18C, 19F, 23F;
PCV13Non7 serotypes – 1,3,5,6C, 7F, 19A;
PPV23NonPCV13 serotypes – 8, 22F, 10A, 11A;
Non-vaccine serotypes – 7A, 15A, 24, 35B, 35F.
Trends in vaccine-type CAP post PCV10/13 infant immunisation
Overall trends in vaccine serotype and non-vaccine serotype pneumococcal pneumonia are described in Table 2.
Table 2.
Trends in vaccine serotype and non-vaccine serotype pneumococcal pneumonia after the introduction of infant PCV immunisation.
| Pneumococcal Serotype | Temporal trend in serotypes causing pneumococcal CAP | Studies reporting |
|---|---|---|
| PCV7 | Overall decrease |
|
| Persistence |
|
|
| PCV13Non7 | Overall decrease |
|
| Persistence |
|
|
| PCV13 | Overall decrease |
|
| PCV10 | Overall decrease |
|
| PCV10Non7 | No change | |
| Non-vaccine serotypes | Overall increase | |
| No change |
|
Discussion
Our systematic review and meta-analysis indicates that in people ≥50 years, the proportion of CAP due to pneumococcus has remained substantial even after the introduction of infant immunisation programmes using PCV vaccines, ranging from 9% up to 30% in the included studies. Overall, almost 50% of serotypes causing pneumococcal CAP were covered by the PCV13, ranging from 25% to 80%, and 67% by PPV23, ranging from 50% to 82% in individual studies.
Of the factors influencing the results, the type of testing employed for pneumococcal detection and serotyping had a significant impact on the estimated proportions with lower estimates from those studies in which conventional techniques only were employed compared to newer methods such as ss-UAD and PCR. Serotype-specific multiplex urinary immunoassays have been developed which detect specific pneumococcal serotypes covered by PCV13 and/or PPV23.49, 50, 51 These ss-UADs detect additional pneumococcal cases of CAP that would otherwise be missed by traditional culture or BinaxNOW alone.37,39 These non-cultural tests (ss-UAD or PCR) have a higher sensitivity for the detection of pneumococcal infection compared to conventional culture with serotyping of isolates using the Quellung reaction. Consequently, studies that rely heavily on ss-UADs for the detection of pneumococcal cases will over-estimate the proportion of pneumococcal CAP due to the serotypes detected by the ss-UADs used; for ss-UAD14 and ss-UAD24 these correspond largely to the VTs in PCV13 and PPV23 respectively. It should also be considered that the higher sensitivity of ss-UADs and PCR tests may be detecting pneumococcal carriage and not true lower respiratory tract infection. Conversely, studies using traditional detection methods such as blood and/or sputum culture alone are likely to underestimate the true burden of pneumococcus. The diagnostic sensitivity of sputum culture is highly variable and will depend upon the quality of the sample, processing delays and prior antimicrobial therapy, and false positives from upper respiratory tract carriage may occur.52
Detection of pneumococcal C-polysaccharide antigen by the BinaxNOW urinary antigen test has been estimated to have increased diagnosis of pneumococcal CAP by 11–23% beyond conventional culture.53,54 It has an estimated sensitivity and specificity of 75% and 95% respectively,53, 54, 55, 56 although several studies found that sensitivity is higher for bacteraemic than non-bacteraemic pneumonia (77–92% and 52–78% respectively).57, 58, 59, 60 Sensitivity has also been shown to vary according to pneumococcal serotype and temporal changes in sensitivity have been associated with changes in the distribution of serotypes with time, sensitivity being highest for serotypes 9 V, 14, 18C and 20, and lowest for STs 8, 9 L/N, 11A, 23B and non-typeable serotypes.61
We observed that the proportion of CAP due to pneumococcus varied according to geographical region, with a pooled estimate of 26% from European studies compared to 11% in North America. This is in accordance with other studies which have found similar discrepancies between continents.62, 63, 64 This observation may represent a true difference in the proportion of CAP caused by pneumococcus and an increase in non-vaccine serotypes in both invasive and non-invasive pneumococcal disease in Europe which has not been seen in North America, or it may be due to the use of the 24-valent ss-UAD in the UK and thus a reflection of the types of tests employed. Other factors which may partially explain the finding include differences in the prevalence of tobacco smoking, in pneumococcal vaccine coverage, disparities between healthcare systems and access to antibiotics.65,66,67
It was notable in our review that none of the data on VT non-invasive pneumococcal pneumonia were from LMICs. Currently only invasive pneumococci are serotyped in most sub-Saharan African countries. One recent study from Malawi using the non-serotype specific BinaxNOW assay suggested that, following introduction of universal infant PCV in 2011, pneumococcal CAP still accounted for 21·4% of pneumonias in hospitalised adults. No data on serotype distribution has been described.68 A South African study confirmed that ss-UAD has high diagnostic accuracy to detect pneumococcal pneumonia and simultaneous detection of PCV13 serotypes in HIV-infected adults in South Africa, offering a potentially useful tool to determine pneumococcal pneumonia in this population.69
Of note is the persistence of ST3 as one of the most prevalent vaccine serotypes both before and after the PCV13 infant programme. A recent global genomic analysis of ST3 has identified a new antibiotic-resistant clade which is replacing the less resistant clade.70 Multiple reports suggest that the indirect protective effects of infant PCV immunisation against IPD in adults is less for ST3 than for other PCV serotypes.12,71, 72, 73, 74, 75 It has been reported that PCV13 is less effective at decreasing ST3 colonisation in children, and therefore may be insufficient to indirectly protect older adults.76
Our systematic review and meta-analysis has some limitations in addition to those already discussed. Our search strategy sought to identify studies with quantitative data on pneumococcal serotypes, so studies not reporting on serotypes would not have been included in our analysis of the overall proportion of CAP caused by S. pneumoniae. Sampling methods within populations were not standardised across studies and varied from convenience sampling to recruitment of all eligible patients in a cohort and this will affect the overall estimates of proportions. Although we focused on community-acquired infections, we cannot exclude the possibility that some included patients may have had non-community acquired infections. The true incidence of vaccine-type pneumococcal pneumonia is likely to be underestimated from studies which only included patients hospitalised with CAP; there is evidence from several studies that many cases of CAP are not hospitalised and the percentage treated as outpatients or in primary care will vary depend upon the different set up of healthcare systems in different countries.5,6,77 Some countries have also introduced adult pneumococcal vaccination policies which may affect variations in the incidence of pneumococcal disease; recommended risk groups may differ between those countries and coverage is likely to vary substantially. As most data were only collected for the period up to five years following the introduction of childhood PCV10/13 immunisation, this may not have been sufficient to allow for the development of herd protection.
Based on the findings of the systematic review and meta-analysis we make the following recommendations. First, data on the contribution of pneumococcal serotypes to non-invasive CAP in LMICs are sorely needed to better guide pneumococcal vaccine policy in these regions and tailored vaccine development. The incidence of pneumococcal infection in LMICs is high and pneumococcal vaccines, particularly PCVs, are expensive compared to other vaccines, yet it is not certain that the serotypes included in the current pneumococcal vaccines match the prevailing serotypes causing pneumococcal pneumonia in LMICs. Second, more studies are required to strengthen the evidence on the sero-epidemiology of CAP in older people, as the majority of pneumococcal infections in adults aged ≥50 years are non-invasive yet most of the data come from IPD which is much less common and may be caused by serotypes which do not reflect the pattern of serotypes causing non-invasive pneumonia. Integral to this is the need to develop better serotype-specific diagnostic methods capable of detecting a wider range of serotypes and not restricted to VTs in the existing vaccines so that trends in non-vaccine serotypes can also be detected. This will also be important as new pneumococcal vaccines emerge which cover more serotypes, such as PCVs which are 21-valent. New diagnostics should not rely on respiratory samples, such as sputum PCR, as 30 to 40% of adults with pneumonia do not produce sputum and such specimens are prone to contamination or may only reflect carriage.52,78, 79, 80 Third, we highlight caution when interpreting studies heavily reliant on ss-UADs that target only VT serotypes as they may bias towards over-estimates of the proportions of potentially vaccine-preventable disease.
In conclusion, this review of pneumococcal CAP in adults aged 50 years and over highlights variations in the types of studies and methodologies used to determine the burden of pneumococcal vaccine serotypes. Nonetheless, it demonstrates that there remains a considerable burden of pneumococcal CAP, including potentially vaccine preventable disease, in older people.
Declaration of interests
WSL reports unrestricted investigator-initiated research funding from Pfizer from 2016 to present for an unrelated multi-centre study in pneumonia in which he is the CI, and research funding for unrelated clinical trials in the fields of COVID-19, tuberculosis and community-acquired pneumonia. WSL also declares unpaid roles as the Joint Committee on Vaccination and Immunisation (JCVI) UK Chair of COVID-19 Immunisation, and National Lead of the British Thoracic Society community acquired pneumonia audit programme. LL's salary is funded by the National Institute for Health Research (NIHR) Nottingham Biomedical Research Centre, UK. HL declares voluntary, unpaid membership of the National Confidential Enquiry into Patient Outcome and Death specialist advisory group for Community Acquired Pneumonia. TM and BL declare no competing interests.
Acknowledgments
Acknowledgements
We thank Dr Christos Chalitsios at the University of Nottingham for his technical support.
Contributors
LL and WSL conceived and designed the study. LL, BL, HL, TM and WSL contributed to the acquisition and extraction of data, quality assessment, and data analysis. LL, WSL and TM interpreted the data. LL wrote the first draft of the manuscript. All authors contributed to critical revision of the manuscript, had full access to all the data in the study, and accept responsibility for the decision to submit for publication.
Data sharing statement
The study dataset can be requested from Louise.Lansbury@nottingham.ac.uk. The analysis codes are available at https://github.com/LouiseLansbury/Pneumo_serotypes.git.
Funding
The National Institute for Health Research (NIHR) Nottingham Biomedical Research Centre, UK, provided salary support for this study.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.eclinm.2022.101271.
Appendix. Supplementary materials
References
- 1.Quan T.P., Fawcett N.J., Wrightson J.M., et al. Increasing burden of community-acquired pneumonia leading to hospitalisation, 1998-2014. Thorax. 2016;71(6):535–542. doi: 10.1136/thoraxjnl-2015-207688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Welte T., Torres A., Nathwani D. Clinical and economic burden of community-acquired pneumonia among adults in Europe. Thorax. 2012;67(1):71–79. doi: 10.1136/thx.2009.129502. [DOI] [PubMed] [Google Scholar]
- 3.Cillóniz C., Ewig S., Polverino E., et al. Microbial aetiology of community-acquired pneumonia and its relation to severity. Thorax. 2011;66(4):340–346. doi: 10.1136/thx.2010.143982. [DOI] [PubMed] [Google Scholar]
- 4.Jain S., Self W.H., Wunderink R.G., et al. Community-acquired pneumonia requiring hospitalization among U.S. adults. N Engl J Med. 2015;373(5):415–427. doi: 10.1056/NEJMoa1500245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Almirall J., Boixeda R., Bolíbar I., et al. Differences in the etiology of community-acquired pneumonia according to site of care: a population-based study. Respir Med. 2007;101(10):2168–2175. doi: 10.1016/j.rmed.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 6.Cillóniz C., Ewig S., Polverino E., et al. Community-acquired pneumonia in outpatients: aetiology and outcomes. Eur Respir J. 2012;40(4):931–938. doi: 10.1183/09031936.00168811. [DOI] [PubMed] [Google Scholar]
- 7.Rice L.B. Antimicrobial resistance in gram-positive bacteria. Am J Infect Control. 2006;34(5 Suppl 1) doi: 10.1016/j.ajic.2006.05.220. S11-9; discussion S64-73. [DOI] [PubMed] [Google Scholar]
- 8.Bogaert D., De Groot R., Hermans P.W. Streptococcus pneumoniae colonisation: the key to pneumococcal disease. Lancet Infect Dis. 2004;4(3):144–154. doi: 10.1016/S1473-3099(04)00938-7. [DOI] [PubMed] [Google Scholar]
- 9.GBD 2016 lower respiratory infections collaborators Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory infections in 195 countries, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect Dis. 2018;18(11):1191–1210. doi: 10.1016/S1473-3099(18)30310-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schuchat A., Hilger T., Zell E., et al. Active bacterial core surveillance of the emerging infections program network. Emerg Infect Dis. 2001;7(1):92–99. doi: 10.3201/eid0701.010114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanquet G., Krizova P., Valentiner-Branth P., et al. Effect of childhood pneumococcal conjugate vaccination on invasive disease in older adults of 10 European countries: implications for adult vaccination. Thorax. 2019;74(5):473–482. doi: 10.1136/thoraxjnl-2018-211767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harboe Z.B., Dalby T., Weinberger D.M., et al. Impact of 13-valent pneumococcal conjugate vaccination in invasive pneumococcal disease incidence and mortality. Clin Infect Dis. 2014;59(8):1066–1073. doi: 10.1093/cid/ciu524. [DOI] [PubMed] [Google Scholar]
- 13.Ladhani S.N., Collins S., Djennad A., et al. Rapid increase in non-vaccine serotypes causing invasive pneumococcal disease in England and Wales, 2000-17: a prospective national observational cohort study. Lancet Infect Dis. 2018;18(4):441–451. doi: 10.1016/S1473-3099(18)30052-5. [DOI] [PubMed] [Google Scholar]
- 14.Waight P.A., Andrews N.J., Ladhani S.N., Sheppard C.L., Slack M.P., Miller E. Effect of the 13-valent pneumococcal conjugate vaccine on invasive pneumococcal disease in England and Wales 4 years after its introduction: an observational cohort study. Lancet Infect Dis. 2015;15(5):535–543. doi: 10.1016/S1473-3099(15)70044-7. [DOI] [PubMed] [Google Scholar]
- 15.Rodrigo C., Bewick T., Sheppard C., et al. Impact of infant 13-valent pneumococcal conjugate vaccine on serotypes in adult pneumonia. Eur Respirat J. 2015;45(6):1632–1641. doi: 10.1183/09031936.00183614. [DOI] [PubMed] [Google Scholar]
- 16.Frank O., De Oliveira Bernardo C., González-Chica D.A., Macartney K., Menzies R., Stocks N. Pneumococcal vaccination uptake among patients aged 65 years or over in Australian general practice. Hum Vaccin Immunother. 2020;16(4):965–971. doi: 10.1080/21645515.2019.1682844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gatwood J., Shuvo S., Hohmeier K.C., et al. Pneumococcal vaccination in older adults: an initial analysis of social determinants of health and vaccine uptake. Vaccine. 2020;38(35):5607–5617. doi: 10.1016/j.vaccine.2020.06.077. [DOI] [PubMed] [Google Scholar]
- 18.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lansbury L., Lawrence H., Lim B., McKeever T., Lim W.-.S. The burden of pneumococcal pneumonia due to vaccine-type Streptococcus pneumoniae in adults aged 50 years and over. PROSPERO. 2020 https://www.crd.york.ac.uk/prospero/display_ record.php?ID=CRD42020192002 [Google Scholar]
- 20.Wells G, Shea B, O'Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed 12 May 2021.
- 21.Nyaga V.N., Arbyn M., Aerts M. Metaprop: a Stata command to perform meta-analysis of binomial data. Arch Public Health. 2014;72(1):39. doi: 10.1186/2049-3258-72-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leblanc J., Elsherif M., Ye L., et al. Age-stratified burden of pneumococcal community acquired pneumonia in hospitalised Canadian adults from 2010 to 2015. BMJ Open Resp Res. 2020;7 doi: 10.1136/bmjresp-2019-000550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morimoto K., Suzuki M., Ishifuji T., et al. The burden and etiology of community-onset pneumonia in the aging Japanese population: a multicenter prospective study. PLoS ONE. 2015;10(3) doi: 10.1371/journal.pone.0122247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Menéndez R., España P.P., Pérez-Trallero E., et al. The burden of PCV13 serotypes in hospitalized pneumococcal pneumonia in Spain using a novel urinary antigen detection test. CAPA study. Vaccine. 2017;35(39):5264–5270. doi: 10.1016/j.vaccine.2017.08.007. [DOI] [PubMed] [Google Scholar]
- 25.Vestjens S.M.T., Wagenvoort G.H.J., Grutters J.C., et al. Changes in pathogens and pneumococcal serotypes causing community-acquired pneumonia in the Netherlands. Vaccine. 2017;35(33):4112–4118. doi: 10.1016/j.vaccine.2017.06.049. [DOI] [PubMed] [Google Scholar]
- 26.Kim S., Chung D., Song J., et al. Changes in serotype distribution and antimicrobial resistance of Streptococcus pneumoniae isolates from adult patients in Asia: emergence of drug-resistant non-vaccine serotypes. Vaccine. 2019 doi: 10.1016/j.vaccine.2019.09.065. S0264-410X(19):31293–31299. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 27.Choi M.J., Song J.Y., Cheong H.J., et al. Clinical usefulness of pneumococcal urinary antigen test, stratified by disease severity and serotypes. J Infect Chemother. 2015;21(9):672–679. doi: 10.1016/j.jiac.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Horácio A.N., Silva-Costa C., Lopes E., Ramirez M., M-C J. Conjugate vaccine serotypes persist as major causes of non-invasive pneumococcal pneumonia in Portugal despite declines in serotypes 3 and 19A (2012-2015) PLoS ONE. 2018;13(11) doi: 10.1371/journal.pone.0206912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Noguchi S., Yatera K., Akata K., et al. Distribution and annual changes in the proportion of Streptococcus pneumoniae serotypes in Japanese adults with pneumococcal pneumonia from 2011 to 2017. J Infect Chemother. 2019;25(11):925–929. doi: 10.1016/j.jiac.2019.07.007. [DOI] [PubMed] [Google Scholar]
- 30.Regev-Yochay G.C., Chazan B., Gonzalez E., et al. Distribution of 13-Valent pneumococcal conjugate vaccine serotype streptococcus pneumoniae in adults 50 Years and older presenting with community-acquired pneumonia in Israel. Hum Vaccin Immunother. 2018;14(10):2527–2532. doi: 10.1080/21645515.2018.1475811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sherwin R.L., Gray S., Alexander R., et al. Distribution of 13-valent pneumococcal conjugate vaccine Streptococcus pneumoniae serotypes in US adults aged ≥50 years with community-acquired pneumonia. J Infect Dis. 2013;208(11):1813–1820. doi: 10.1093/infdis/jit506. [DOI] [PubMed] [Google Scholar]
- 32.Oishi K., Yoshimine H., Watanabe H., et al. Drug-resistant genes and serotypes of pneumococcal strains of community-acquired pneumonia among adults in Japan. Respirology. 2006;11(4):429–436. doi: 10.1111/j.1440-1843.2006.00867.x. [DOI] [PubMed] [Google Scholar]
- 33.Prato R., Fortunato F., Cappelli M.G., Chironna M., Martinelli D. Effectiveness of the 13-valent pneumococcal conjugate vaccine against adult pneumonia in Italy: a case-control study in a 2-year prospective cohort. BMJ Open. 2018;8(3) doi: 10.1136/bmjopen-2017-019034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pletz M.W., Ewig S., Rohde G., et al. Impact of pneumococcal vaccination in children on serotype distribution in adult community-acquired pneumonia using the serotype-specific multiplex urinary antigen detection assay. Vaccine. 2016;34(20):2342–2348. doi: 10.1016/j.vaccine.2016.03.052. [DOI] [PubMed] [Google Scholar]
- 35.Sando E., Suzuki M., Furumoto A., et al. Impact of the pediatric 13-valent pneumococcal conjugate vaccine on serotype distribution and clinical characteristics of pneumococcal pneumonia in adults: the Japan Pneumococcal Vaccine Effectiveness Study (J-PAVE) Vaccine. 2019;(37):2687–2693. doi: 10.1016/j.vaccine.2019.04.009. [DOI] [PubMed] [Google Scholar]
- 36.Horácio A.N., Lopes J.P., Ramirez M., Melo-Cristino J. Non-invasive pneumococcal pneumonia in Portugal–serotype distribution and antimicrobial resistance. PLoS ONE. 2014;9(7) doi: 10.1371/journal.pone.0103092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wunderink R.G., Self W.H., Anderson E.J., et al. Pneumococcal community-acquired pneumonia detected by serotype-specific urinary antigen detection assays. Clin Infect Dis. 2018;66(10):1504–1510. doi: 10.1093/cid/cix1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van Werkhoven C.H., Hollingsworth R.C., Huijts S.M., et al. Pneumococcal conjugate vaccine herd effects on non-invasive pneumococcal pneumonia in elderly. Vaccine. 2016;34(28):3275–3282. doi: 10.1016/j.vaccine.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 39.Isturiz R.E., Ramirez J., Self W.H., et al. Pneumococcal epidemiology among us adults hospitalized for community-acquired pneumonia. Vaccine. 2019;37(25):3352–3361. doi: 10.1016/j.vaccine.2019.04.087. [DOI] [PubMed] [Google Scholar]
- 40.Vila-Corcoles A., Ansa X., Ochoa-Gondar O., Satue E., de Diego C., Rodriguez-Blanco T. Pneumococcal pneumonia in adults 60 years or older: incidence, mortality and prevention. Med Clin. 2016;146(5):199–202. doi: 10.1016/j.medcli.2015.09.015. [DOI] [PubMed] [Google Scholar]
- 41.Pick H., Daniel P., Rdrigo C., et al. Pneumococcal serotype trends, surveillance and risk factors in UK adult pneumonia, 2013-18. Thorax. 2020;75:38–49. doi: 10.1136/thoraxjnl-2019-213725. [DOI] [PubMed] [Google Scholar]
- 42.Harat R.A., Gray S., Gutterman E.M., et al. Prospective, population-based surveillance of the burden of Streptococcus pneumoniae in community-acquired pneumonia in older adults, Chrzanów County, Poland, 2010 to 2012. Pneumonol Alergol Pol. 2016;84(2):95–103. doi: 10.5603/PiAP.2016.0007. [DOI] [PubMed] [Google Scholar]
- 43.Benfield T., Skovgaard M., Schønheyder H.C., et al. Serotype distribution in non-bacteremic pneumococcal pneumonia: association with disease severity and implications for pneumococcal conjugate vaccines. PLoS ONE. 2013;8(8):e72743. doi: 10.1371/journal.pone.0072743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Di Pasquale M., Aliberti S., Azzari C., et al. Serotypes and antibiotic susceptibility of Streptococcus pneumoniae isolated from hospitalized patients with community-acquired pneumonia in Italy. SAGE Open Med. 2017;5:1–4. doi: 10.1177/2050312117720058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Domenech A., Ardanuy C., Calatayud L., et al. Serotypes and genotypes of Streptococcus pneumoniae causing pneumonia and acute exacerbations in patients with chronic obstructive pulmonary disease. J Antimicrob Chemother. 2011;66(3):487–493. doi: 10.1093/jac/dkq480. [DOI] [PubMed] [Google Scholar]
- 46.Suzuki M., Dhoubhadel B.G., Ishifuji T., et al. Serotype-specific effectiveness of 23-valent pneumococcal polysaccharide vaccine against pneumococcal pneumonia in adults aged 65 years or older: a multicentre, prospective, test-negative design study. Lancet Infect Dis. 2017;17(3):313–321. doi: 10.1016/S1473-3099(17)30049-X. [DOI] [PubMed] [Google Scholar]
- 47.LeBlanc J., ElSherif M., Ye L., et al. Streptococcus pneumoniae serotype 3 is masking PCV13-mediated herd immunity in Canadian adults hospitalized with community acquired pneumonia: a study from the Serious Outcomes Surveillance (SOS) Network of the Canadian immunization research Network (CIRN) Vaccine. 2019;37:5466–5473. doi: 10.1016/j.vaccine.2019.05.003. [DOI] [PubMed] [Google Scholar]
- 48.Quirk S.J., Haraldsson G., Hjálmarsdóttir M.Á., et al. Vaccination of Icelandic children with the 10-valent pneumococcal vaccine leads to a significant herd effect among adults in Iceland. J Clin Microbiol. 2019;57(4) doi: 10.1128/JCM.01766-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Eletu S.D., Sheppard C.L., Rose S., et al. Re-validation and update of an extended-specificity multiplex assay for detection of Streptococcus pneumoniae capsular serotype/serogroup-specific antigen and cell-wall polysaccharide in urine specimens. Access Microbiol. 2020;2(3) doi: 10.1099/acmi.0.000094. acmi000094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Eletu S.D., Sheppard C.L., Thomas E., et al. Development of an extended-specificity multiplex immunoassay for detection of Streptococcus pneumoniae serotype-specific antigen in urine by use of human monoclonal antibodies. Clin Vaccine Immunol. 2017;24(12) doi: 10.1128/CVI.00262-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sheppard C.L., Harrison T.G., Smith M.D., George R.C. Development of a sensitive, multiplexed immunoassay using xMAP beads for detection of serotype-specific streptococcus pneumoniae antigen in urine samples. J Med Microbiol. 2011;60(Pt 1):49–55. doi: 10.1099/jmm.0.023150-0. [DOI] [PubMed] [Google Scholar]
- 52.Song J.Y., Eun B.W., Nahm M.H. Diagnosis of pneumococcal pneumonia: current pitfalls and the way forward. Infect Chemother. 2013;45(4):351–366. doi: 10.3947/ic.2013.45.4.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Boulware D.R., Daley C.L., Merrifield C., Hopewell P.C., Janoff E.N. Rapid diagnosis of pneumococcal pneumonia among HIV-infected adults with urine antigen detection. J Infect. 2007;55(4):300–309. doi: 10.1016/j.jinf.2007.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Said M.A., Johnson H.L., Nonyane B.A., et al. Estimating the burden of pneumococcal pneumonia among adults: a systematic review and meta-analysis of diagnostic techniques. PLoS ONE. 2013;8(4):e60273. doi: 10.1371/journal.pone.0060273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Horita N., Miyazawa N., Kojima R., et al. Sensitivity and specificity of the Streptococcus pneumoniae urinary antigen test for unconcentrated urine from adult patients with pneumonia: a meta-analysis. Respirology. 2013;18(8):1177–1183. doi: 10.1111/resp.12163. [DOI] [PubMed] [Google Scholar]
- 56.Sinclair A., Xie X., Teltscher M., Dendukuri N. Systematic review and meta-analysis of a urine-based pneumococcal antigen test for diagnosis of community-acquired pneumonia caused by Streptococcus pneumoniae. J Clin Microbiol. 2013;51(7):2303–2310. doi: 10.1128/JCM.00137-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Domínguez J., Galí N., Blanco S., et al. Detection of Streptococcus pneumoniae antigen by a rapid immunochromatographic assay in urine samples. Chest. 2001;119(1):243–249. doi: 10.1378/chest.119.1.243. [DOI] [PubMed] [Google Scholar]
- 58.Gutiérrez F., Masiá M., Rodríguez J.C., et al. Evaluation of the immunochromatographic Binax NOW assay for detection of Streptococcus pneumoniae urinary antigen in a prospective study of community-acquired pneumonia in Spain. Clin Infect Dis. 2003;36(3):286–292. doi: 10.1086/345852. [DOI] [PubMed] [Google Scholar]
- 59.Rosón B., Fernández-Sabé N., Carratalà J., et al. Contribution of a urinary antigen assay (Binax NOW) to the early diagnosis of pneumococcal pneumonia. Clin Infect Dis. 2004;38(2):222–226. doi: 10.1086/380639. [DOI] [PubMed] [Google Scholar]
- 60.Smith M.D., Derrington P., Evans R., et al. Rapid diagnosis of bacteremic pneumococcal infections in adults by using the Binax NOW Streptococcus pneumoniae urinary antigen test: a prospective, controlled clinical evaluation. J Clin Microbiol. 2003;41(7):2810–2813. doi: 10.1128/JCM.41.7.2810-2813.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shoji H., Domenech A., Simonetti A.F., et al. The Alere BinaxNOW pneumococcal urinary antigen test: diagnostic sensitivity for adult pneumococcal pneumonia and relationship to specific serotypes. J Clin Microbiol. 2018;56(2) doi: 10.1128/JCM.00787-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Musher D.M., Abers M.S., Bartlett J.G. Evolving understanding of the causes of pneumonia in adults, with special attention to the role of pneumococcus. Clin Infect Dis. 2017;65(10):1736–1744. doi: 10.1093/cid/cix549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shoar S., Musher D.M. Etiology of community-acquired pneumonia in adults: a systematic review. Pneumonia. 2020;12:11. doi: 10.1186/s41479-020-00074-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Torres A., Blasi F., Dartois N., Akova M. Which individuals are at increased risk of pneumococcal disease and why? Impact of COPD, asthma, smoking, diabetes, and/or chronic heart disease on community-acquired pneumonia and invasive pneumococcal disease. Thorax. 2015;70(10):984–989. doi: 10.1136/thoraxjnl-2015-206780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jamal A., Phillips E., Gentzke A.S., et al. Current Cigarette Smoking Among Adults - United States, 2016. MMWR Morb Mortal Wkly Rep. 2018;67(2):53–59. doi: 10.15585/mmwr.mm6702a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.WHO Regional Office for Europe. Health Topics Tobacco https://www.euro.who.int/en/health-topics/disease-prevention/tobacco. Accessed 12 May 2021.
- 67.Norris T., Vahratian A., Cohen R.A. Vaccination coverage among adults aged 65 and over: united States. NCHS Data Brief. 2015;2017(281):1–8. [PubMed] [Google Scholar]
- 68.Aston S.J., Ho A., Jary H., et al. Etiology and risk factors for mortality in an adult community-acquired pneumonia cohort in Malawi. Am J Respir Crit Care Med. 2019;200(3):359–369. doi: 10.1164/rccm.201807-1333OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Albrich W.C., Pride M.W., Madhi S.A., et al. Multiplex urinary antigen detection for 13 Streptococcus pneumoniae serotypes improves diagnosis of pneumococcal pneumonia in South African HIV-infected adults. J Clin Microbiol. 2017;55(1):302–312. doi: 10.1128/JCM.01573-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Azarian T., Mitchell P.K., Georgieva M., et al. Global emergence and population dynamics of divergent serotype 3 CC180 pneumococci. PLoS Pathog. 2018;14(11) doi: 10.1371/journal.ppat.1007438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Horacio A., Silva-Costa C., Lopez J.P., Ramirez M., Melo-Cristino J. Serotype 3 remains the leading cause of invasive pneumococcal disease in adults in Portugal (2012-2014) despite continued reductions in other 13-valent conjugate vaccine serotypes. Front Microbiol. 2016;7(1616) doi: 10.3389/fmicb.2016.01616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.van der Linden M., Falkenhorst G., Perniciaro S., Imöhl M. Effects of infant pneumococcal conjugate vaccination on serotype distribution in invasive pneumococcal disease among children and adults in Germany. PLoS ONE. 2015;10(7) doi: 10.1371/journal.pone.0131494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Steens A., Bergsaker M.A., Aaberge I.S., Rønning K., Vestrheim D.F. Prompt effect of replacing the 7-valent pneumococcal conjugate vaccine with the 13-valent vaccine on the epidemiology of invasive pneumococcal disease in Norway. Vaccine. 2013;31(52):6232–6238. doi: 10.1016/j.vaccine.2013.10.032. [DOI] [PubMed] [Google Scholar]
- 74.Galanis I., Lindstrand A., Darenberg J., et al. Effects of PCV7 and PCV13 on invasive pneumococcal disease and carriage in Stockholm, Sweden. Eur Respir J. 2016;47(4):1208–1218. doi: 10.1183/13993003.01451-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Moore M.R., Link-Gelles R., Schaffner W., et al. Effect of use of 13-valent pneumococcal conjugate vaccine in children on invasive pneumococcal disease in children and adults in the USA: analysis of multisite, population-based surveillance. Lancet Infect Dis. 2015;15(3):301–309. doi: 10.1016/S1473-3099(14)71081-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dagan R., Patterson S., Juergens C., et al. Comparative immunogenicity and efficacy of 13-valent and 7-valent pneumococcal conjugate vaccines in reducing nasopharyngeal colonization: a randomized double-blind trial. Clin Infect Dis. 2013;57(7):952–962. doi: 10.1093/cid/cit428. [DOI] [PubMed] [Google Scholar]
- 77.Woodhead M.A., Macfarlane J.T., McCracken J.S., Rose D.H., Finch R.G. Prospective study of the aetiology and outcome of pneumonia in the community. Lancet. 1987;1(8534):671–674. doi: 10.1016/s0140-6736(87)90430-2. [DOI] [PubMed] [Google Scholar]
- 78.García-Vázquez E., Marcos M.A., Mensa J., et al. Assessment of the usefulness of sputum culture for diagnosis of community-acquired pneumonia using the PORT predictive scoring system. Arch Intern Med. 2004;164(16):1807–1811. doi: 10.1001/archinte.164.16.1807. [DOI] [PubMed] [Google Scholar]
- 79.Musher D.M., Montoya R., Wanahita A. Diagnostic value of microscopic examination of Gram-stained sputum and sputum cultures in patients with bacteremic pneumococcal pneumonia. Clin Infect Dis. 2004;39(2):165–169. doi: 10.1086/421497. [DOI] [PubMed] [Google Scholar]
- 80.van der Eerden M.M., Vlaspolder F., de Graaff C.S., et al. Comparison between pathogen directed antibiotic treatment and empirical broad spectrum antibiotic treatment in patients with community acquired pneumonia: a prospective randomised study. Thorax. 2005;60(8):672–678. doi: 10.1136/thx.2004.030411. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.