Abstract
Escape behavior is essential for animals to avoid attacks by predators. In some species, multiple escape responses could be employed. However, it remains unknown what aspects of threat stimuli affect the choice of an escape response. We focused on two distinct escape responses (running and jumping) to short airflow in crickets and examined the effects of multiple stimulus aspects including the angle, velocity, and duration on the choice between these responses. The faster and longer the airflow, the more frequently the crickets jumped. This meant that the choice of an escape response depends on both the velocity and duration of the stimulus and suggests that the neural basis for choosing an escape response includes the integration process of multiple stimulus parameters. In addition, the moving speed and distance changed depending on the stimulus velocity and duration for running but not for jumping. Running away would be more adaptive escape behavior.
Keywords: Decision-making, Directionality, Motor performance, Oriented behavior, Insect
Highlights
-
•
Crickets chose their escape behaviors depending on stimulus intensity and duration.
-
•
Crickets changed running speed depending on stimulus parameters.
-
•
Running away was likely more adaptive escape behavior than jumping.
-
•
Crickets controlled escape direction regardless of stimulus velocity or duration.
-
•
Choosing escape action would be based on integration of multiple stimulus aspects.
Decision-making, Directionality, Motor performance, Oriented behavior, Insect.
1. Introduction
Selecting a behavioral response appropriate to a situation is crucial for animals to survive events such as predator attacks. Vertebrates and invertebrates universally exhibit escape behavior to survive such attacks (Domenici et al., 2011a, b; LeDoux and Daw, 2018). Usually, animals quickly move away from a predator as a typical escape response. This increases their survival rate. The more emergent the situation, the more often this escape response is chosen compared to other defensive behaviors such as freezing (Baba and Shimozawa, 1997; Fadok et al., 2017; Evans et al., 2018; Turner et al., 2016). For instance, mice exhibit either flight or freeze in response to the movement of a predator (De Franceschi et al., 2016). They choose a flight response when raptors approach them but exhibit a freezing response when raptors just cruise past. This is because when the predator is unaware of the prey, freezing is a more efficient response to avoid being targeted. In contrast, once the predator is aware of the prey and begins to approach it, flight is an appropriate response to escape the attack (Eilam, 2005). Prey animals are subjected to attacks from various species of predators (Bulbert et al., 2015; Evans et al., 2019), and how to approach by the predator varies in every attack trials (Dangles et al., 2006; Walker et al., 2005). Thus, it is critical for prey animals to know how a predator will approach them. This helps them make an appropriate behavioral choice against an attack.
Animals detect the predator's approach through various parameters of sensory stimuli such as speed, duration, and orientation (Domenici, 2010). The prey regulates its escape response based on the characteristics of the stimuli. The stimulus velocity and orientation are related to the escape distance and direction, respectively (Bhattacharyya et al., 2017; Card, 2012; Domenici et al., 2011a, b; Dunn et al., 2016; Stewart et al., 2013). The size of the looming stimulus indicates the distance of the approaching predator. This has been known to be a sensory signal to determine the initiation of an escape response in certain species (Bhattacharyya et al., 2017; Dunn et al., 2016; Fotowat and Gabbiani, 2007; Preuss et al., 2006). Several neuroscience studies have revealed that some neurons involved in the visually evoked escape behavior respond to the size-to-speed ratio (l/v), which is a key parameter in looming stimuli (Peek and Card, 2016). Various animals employ multiple types of escape behaviors which include distinct movements (Briggman et al., 2005; Liu and Hale, 2017; Takagi et al., 2017). This results in various locomotor performances. Each escape response is considered to have a specific advantage such as speed or controllability. The success or failure of avoiding the predator's attack depends on what advantages the chosen escape behavior has (Card and Dickinson, 2008; Sato et al., 2019). Recent studies suggest that the approaching speed of the predator indicated by the stimulus velocity has an impact on the selection between multiple escape responses. For example, the short or long modes of flies taking-off (Card and Dickinson, 2008; von Reyn et al., 2014, 2017). However, it is unknown what other parameters, including the direction or duration of the stimulus, are involved in the escape action selection process.
We addressed this issue using the wind-elicited escape behavior of the field cricket Gryllus bimaculatus as the experimental model. Crickets exhibit either running or jumping in response to a short air current that is detected as predator approaches (Dupuy et al., 2011; Sato et al., 2017, 2019). The running and jumping movements are different from each other. The cricket either runs on the ground or jumps by strongly kicking the ground. Our previous study revealed a “trade-off” between speed and behavioral flexibility. If the cricket chooses to run rather than jump, it moves more slowly. However, it can respond more flexibly to further attacks received during the response (Sato et al., 2019). Interestingly, crickets can also control their movement direction accurately in response to a stimulus from any direction, not only for running but also for jumping (Sato et al., 2019). This is in contrast to jumping escape responses by locusts (Santer et al., 2005; Simmons et al., 2010). In addition, the longer the stimulus, the longer the distance the crickets run (Oe and Ogawa, 2013). These facts suggest that crickets may be able to take multiple stimulus parameters into account when choosing to either run or jump.
In this study, we manipulated the three parameters of an air-current stimulus (angle, velocity, and duration) and examined their effects on the response probabilities to run or jump that were elicited by the stimulus. Furthermore, to clarify the impact of these parameters on the escape response performance, we examined the relationship between the locomotor parameters including movement speed, travel distance, reaction time, directionality, and other stimulus parameters. Our results demonstrated that crickets choose the escape response and regulate their locomotion based on multiple stimulus parameters.
2. Materials and methods
2.1. Animals
We used the wild-type strain of crickets (Gryllus bimaculatus, Hokudai WT; Watanabe et al., 2018) that were bred in our laboratory. Thirty adult male crickets, less than 14 days after adult molting, were used throughout the experiments. They were reared under 12/12 h light/dark conditions at a constant temperature of 27 °C. All experiments were conducted under white light during the dark phase at 26-28 °C.
2.2. Behavioral experiment
The experimental apparatus used in our previous study (Sato et al., 2019) was used throughout the experiments. We monitored the wind-elicited escape responses using a high-speed digital camera (CH130EX, Shodensha, Osaka, Japan) installed above a circular arena (ø = 260 mm). The inside of the arena wall (height = 155 mm) was painted black with no visual cues so that the crickets could not receive any information regarding their orientation. After anesthetization by cooling with ice (0 °C) for 10 min, crickets were marked with two white spots on the dorsal surface of the head and thorax. The size was large enough to detect the movement of crickets from images. All experiments were performed after 30 min to ensure complete recovery from anesthesia. The cricket was placed in the center of the arena inside an inverted beaker (ø = 50 mm) covered with aluminum foil. After the beaker was carefully lifted, an air-current stimulus was immediately applied to the cricket that was standing still. The response was recorded using a camera (Figure S1A). Thus, when the cricket received the stimulus, its cerci were located within a small circle (ø = 20 mm) at the center of the arena. Based on the video images (shutter speed, 1 ms; sampling rate, 120 frames/s; total recording duration, 1660 ms), the two markers on the animal were automatically traced, and locomotor parameters mentioned in a later section were measured using motion analysis software (Move-tr/2D, Library, Tokyo, Japan). To monitor the entire trajectory during movement, we adopted a 285.7 × 285.7 mm frame size with 1024 × 1024 pixels resolution, which covered the entire arena.
2.3. Stimulation and experimental procedure
The air-current stimulus used throughout the experiments was a short puff of nitrogen gas from a plastic nozzle (ø = 15 mm) connected to a pneumatic picopump (PV820, World Precision Instruments, Sarasota, FL, USA). One air-current nozzle was installed on the inside wall of the arena to be positioned on the same horizontal plane as the animal. Since the crickets were oriented randomly within a beaker, the stimulus angles were measured as the angles of the crickets’ orientation against the stimulus source (left in Figure 1A), which were varied across the trials.
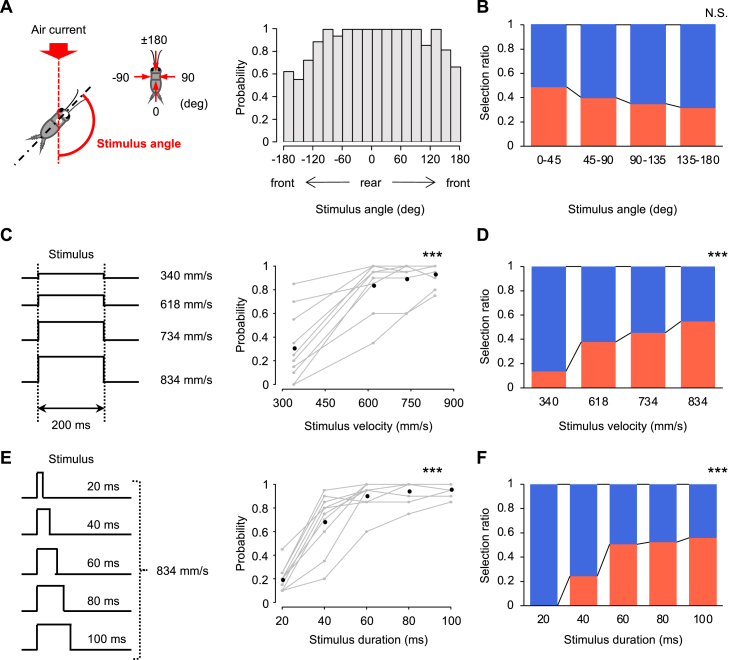
Figure 1.
Effects of three stimulus parameters on the action selection of running and jumping. (A) Distribution of the response probability against the stimulus angle in the angle test. Left diagram shows the definition of the stimulus angle. Right histograms represent the ratio of the number of responses to the number of trials for a range of every 20 degrees of the stimulus angles. The air current with a velocity of 834 mm/s and a duration of 200 ms was used (n = 10 animals). (B) The selection ratio of running (blue) to jumping (red) in the angle test. The data is shown in four divisions based on the absolute value of the stimulus angle. (C) Changes in the response probability over the stimuli with different velocities. Left diagram indicates four types of stimuli used in the velocity test, in which the velocity was varied and the duration was fixed to 200 ms (n = 10 animals). (D) The selection ratio of running (blue) to jumping (red) in the velocity test. (E) Changes in the response probability over the stimuli with various durations. Left diagram indicates five types of stimuli used in the duration test, in which the duration was varied and the velocity was fixed to 834 mm/s (n = 10 animals). (F) The selection ratio of running (blue) to jumping (red) in the duration test. In (C) and (E), gray open circles connected with gray lines represent the probability for each individual and black filled circles represent the mean of the individuals' probability, for each velocity or duration of stimuli. ∗∗∗p < 0.001. N.S. not significant, coefficients for each of the stimulus parameters in logistic regression analysis.
The data of three experiments with various stimulations were analyzed to test the effect of the three stimulus parameters on escape responses: “angle test,” “velocity test,” and “duration test.” A section of the data obtained in our previous study (Sato et al., 2019) was also analyzed for the angle test. The effect of stimulus angles was examined in the escape responses to constant stimulus duration of 200 ms and a velocity of 834 mm/s. The travel time of the air current to the center of the arena was ms. In the previous study using the same experimental apparatus as the present study (Sato et al., 2019), this value has been obtained as the delay in stimulus-evoked ascending spikes of projection neurons that were extracellularly recorded (n = 2 animals, 10 trials in total). In the velocity test, we examined the effect of stimulus velocities on the escape response to the stimuli of 200 ms duration with four different velocities (340, 618, 734, and 834 mm/s). These velocities of the airflows were measured at the center of the arena (left in Figure 1C). The travel times of these stimuli were , , and ms for 340, 618, 734 and 834 mm/s of air currents, respectively. These values were also calculated based on the spike delays to the stimulus recorded extracellularly (n = 2 animals, 10 trials in total for each stimulus velocity). In the duration test, we examined the effect of stimulus duration on the escape response to the stimuli of 834 mm s−1 for four different durations (20, 40, 60, 80, and 100 ms) (left in Figure 1E). The velocity and duration of the air current were regulated by air pressure and the interval between the opening and closing of the valve of the picopump.
In the angle test, 20 trials were repeatedly performed for each individual at inter-trial intervals of 60–90 s. In both the velocity and duration tests, stimuli with various velocities or durations were applied to the same individuals at increasing (n = 5 animals) or decreasing order (n = 5 animals). Twenty trials were performed in one session for each type of stimulus. Therefore in total, 80 or 100 trials were performed for each individual in the tests. The crickets were rested between the sessions for approximately 10 min within a plastic container (138 mm × 220 mm × 135 mm) and had free access to food and water. Ten crickets were tested for each experiment.
2.4. Data analysis
The wind-elicited responses were analyzed as described in our previous study (Sato et al., 2017, 2019). Responses were defined based on the translational velocity of the cricket's movement (Figure S1B). If the velocity exceeded 10 mm/s within 250 ms after the stimulus onset and was greater in its maximum value by more than 50 mm/s, the cricket was considered to respond to the stimulus. If the cricket did not begin to move within 250 ms of the response definition period, the trial was considered as “no response,” including the freezing response. For the angle test, the data in all trials were pooled, and then the response probability was calculated from the number of responding trials per total trials for the range of every 20° of stimulus angles (right in Figure 1A). In the velocity and duration tests, the response probability was calculated from the number of responding trials per 20 trials for each type of stimulus (right in Figure 1C,E).
As in our previous study (Sato et al., 2019), the wind-elicited responses were categorized into “running” or “jumping” according to the movement of legs during the locomotion, which was confirmed visually for all responding trials by frame check of the video. If all six legs were off the ground simultaneously, the response was defined as “jumping,” if any one of the six legs touched the ground during movement, that response was defined as “running” (Figure S1C). We rarely observed complex behaviors combined with running and jumping such as jumping after running and vice versa. The selection ratio of running or jumping was calculated as the proportion of responses for all the responding trials. In the angle test, all trials were pooled and divided into four groups based on the range of absolute stimulus angles, 0°–45°, 45°–90°, 90°–135°, and 135°–180°. The selection ratio was calculated for each angular range (Figure 1B). In the velocity and duration tests, the selection ratio was calculated for each type of stimulus, regardless of the stimulus angle (Figure 1D,F).
We focused on the “initial response” in the responding trial and analyzed the cricket's movement during the initial response as defined in our previous studies (Fukutomi et al., 2015; Oe and Ogawa, 2013; Sato et al., 2017, 2019). The initial response was the first continuous movement (bout) in which the translational velocity was greater in its maximum value than 50 mm/s and never less than 10 mm/s after the stimulus onset. Therefore, the response start was measured as the time when the translational velocity exceeded 10 mm/s after stimulus onset. The response finish was measured as the time when the velocity was less than 10 mm/s. Movement distance, maximum translational velocity, and reaction time were measured as the metric locomotor parameters that characterized the response movements. The movement distance was measured as the entire path length of the moving trajectory. The reaction time was calculated by subtracting the mean travel time of the air current, as mentioned above, from the time from the opening of the picopump to the start of the response. Angular parameters, including movement direction and turn angle, were calculated based on the cricket's body axis, as a vector connecting the thoracic and head markers (Figure 5A and S2A). The movement direction was measured as the angle between the body axis at the starting point of the response and a line connecting the thoracic markers at the start and finish points. Thus, if the cricket moved in the direction opposite to the stimulus source, the movement direction would be equal to the stimulus angle. Since it has been confirmed in a previous study that crickets move in the opposite direction to the stimulus in both running and jumping (Sato et al., 2019), the accuracy in the directional control was assessed by the absolute value of the difference between the movement direction and stimulus angle. The turn angle was measured as the angle between the body axes at the start and finish points. If the cricket was oriented to the exact opposite side of the stimulus source at the finish points, the turn angle would be equal to the stimulus angle.
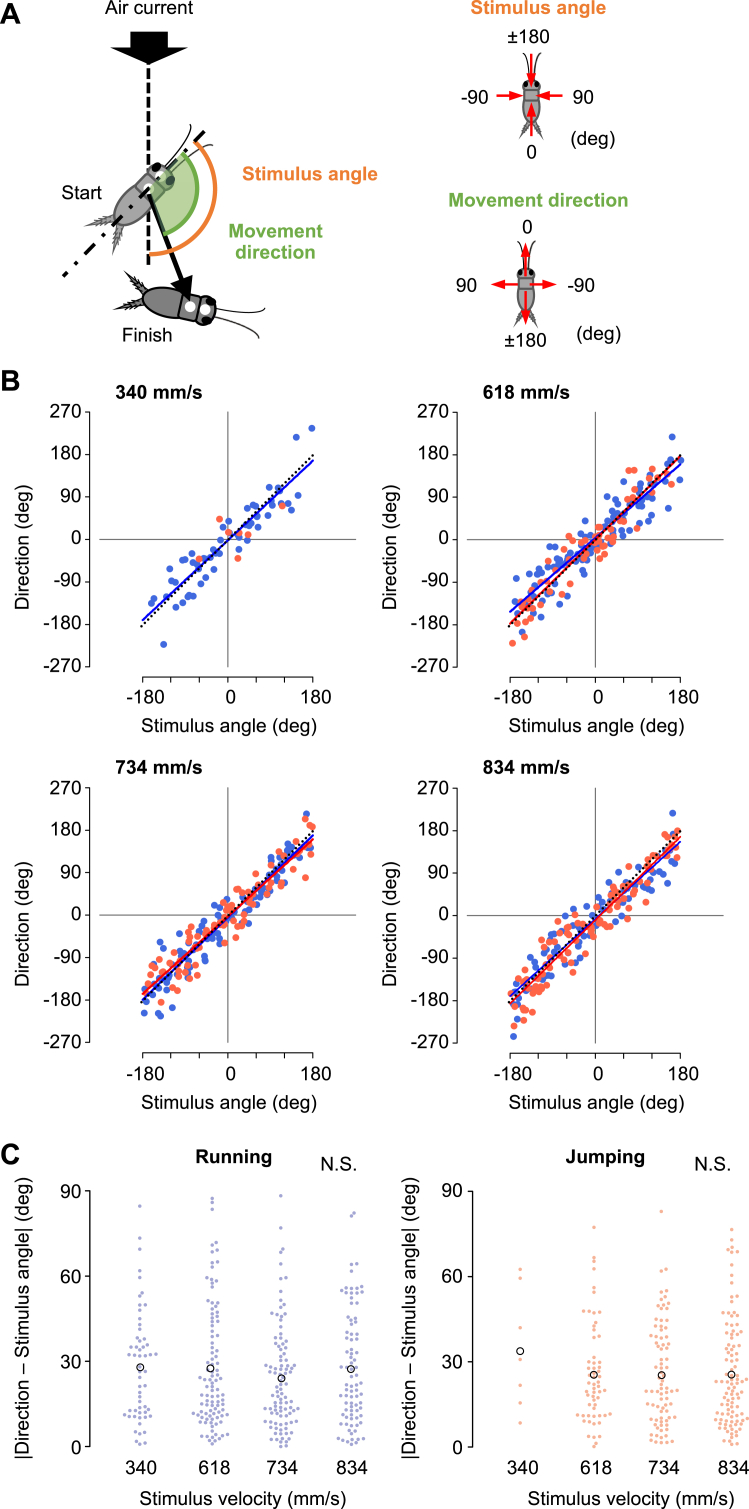
Figure 5.
Effects of stimulus velocity on directional control. (A) Diagram showing the definition of movement direction (green) and stimulus angle (orange). According to this definition, if the movement direction coincides with the stimulus angle, the cricket will move to the opposite direction of the stimulus. (B) Relationships between the movement direction and stimulus angle in running (blue) and jumping (red), for the stimuli of various velocities, 340, 618, 734, and 834 mm/s. Colored lines represent linear regression lines with a significant slope for the data of running (blue) and jumping (red), respectively. Black dotted lines indicate the line of . (C) Absolute values of the angular difference between the movement direction and stimulus angle in running (left) and jumping (right). Colored filled circles represent the data for each trial and black open circles represent the mean of the data in all trials, for each velocity of stimuli. N.S. not significant, likelihood ratio test for LMMs (n = 10 animals).
2.5. Statistical analysis
R programming software (ver. 3.4.4, R Development Core Team) was used for all the statistical analyses. We assessed the effect of the stimulus parameters on the response probability and selection ratio. Both of these parameters were calculated for each individual, using multiple logistic regression analysis in each of the angle, velocity, and duration tests. In addition, trial order was considered as an explanatory variable to test the learning effects by repeatedly receiving stimuli in multiple trials. Thus, the logistic regression considering the two explanatory factors was as follows:
| (1) |
| (2) |
where and are the estimated coefficients, and is the intercept. Then, the significance of the coefficients for stimulus parameters as explanatory variables was checked to assess their effect on the response probability or selection ratio (Table 1).
Table 1.
Results of multiple logistic regression analysis to test the effect of stimulus parameters and trial order on the response probability and selected ratio.
| Experiment | Parameter | Explanatory variable | Result of logistic regression |
||
|---|---|---|---|---|---|
| Coefficient | z value | p value | |||
| Angle test | Selection ratio (Figure 1B) | Stimulus angle | -0.269 | -1.841 | 0.066 |
| Trial order | -0.002 | -0.083 | 0.934 | ||
| Velocity test | All probability (Figure 1C) | Stimulus velocity | 0.008 | 13.81 | <0.001 |
| Trial order | -0.015 | -0.882 | 0.378 | ||
| Selection ratio (Figure 1D) | Stimulus velocity | 0.004 | 5.896 | <0.001 | |
| Trial order | 0.003 | 0.185 | 0.853 | ||
| Duration test | All probability (Figure 1E) | Stimulus duration | 0.072 | 14.57 | <0.001 |
| Trial order | -0.014 | -0.865 | 0.387 | ||
| Selection ratio (Figure 1F) | Stimulus duration | 0.023 | 6.722 | <0.001 | |
| Trial order | 0.006 | 0.473 | 0.636 | ||
To analyze the relationships between the metric locomotor parameters such as movement distance, maximum velocity, or reaction time, and the stimulus angle, which was indicated as an absolute value in degrees from 0 (front) to 90 (lateral) and 180 (behind) we used linear regression analysis as follows:
| (3) |
where is the estimated coefficient and is the intercept. The significance of the coefficients was examined to assess the effect of the stimulus angle on the metric parameters (Table 2).
Table 2.
Results of linear regression analysis to test the relationships between the metric locomotor parameters and stimulus angle.
| Parameter | Response | Result of linear regression |
||
|---|---|---|---|---|
| Estimated slope | t value | p value | ||
| Movement distance (Figure 2A) | Running | -0.104 | -3.346 | 0.001 |
| Jumping | -0.073 | -1.213 | 0.229 | |
| Max. velocity (Figure 2B) | Running | -0.360 | -2.160 | 0.033 |
| Jumping | 0.652 | 2.105 | 0.039 | |
| Reaction time (Figure 2C) | Running | 0.140 | 3.076 | 0.003 |
| Jumping | 0.021 | 0.545 | 0.587 | |
Since the response probability and selection ratio were strongly affected by stimulus velocity and duration, the sample size of data for the locomotor parameters varied among the sessions using various types of stimuli. In particular, slow (340 mm/s of velocity) or short (20 ms of duration) air current rarely or never elicited jumping. Therefore, we could not calculate the mean value of the locomotor parameters for each tested individual in the session using such stimuli. Considering the small and varied sample sizes, a linear mixed-effects model (LMM, the package ‘lme4’ ver. 1.1–23 in R) was used to assess the effects of stimulus velocity and duration on locomotor parameters. We assumed the LMMs with stimulus parameters as explanatory variables, as follows:
| (4) |
where is the intercept, is the estimated coefficient, and is the random effect that the animal IDs were considered. The significance of the effect of stimulus parameters was tested by comparing the LMMs with and without the explanatory variable of stimulus velocity or duration using the likelihood ratio test (Table 3).
Table 3.
Results of likelihood ratio test for LMMs to test the effects of stimulus velocity and duration on the locomotor parameters.
| Experiment | Parameter | Response | Result of likelihood ratio test |
|
|---|---|---|---|---|
| Chi-squared | p value | |||
| Velocity test | Movement distance (Figure 3A) | Running | 9.287 | 0.002 |
| Jumping | 0.254 | 0.614 | ||
| Max. velocity (Figure 3B) | Running | 22.10 | <0.001 | |
| Jumping | 0.035 | 0.851 | ||
| Reaction time (Figure 3C) | Running | 254.7 | <0.001 | |
| Jumping | 149.6 | <0.001 | ||
| Directional accuracy (Figure 5B) | Running | 0.318 | 0.573 | |
| Jumping | 0.530 | 0.467 | ||
| Duration test | Movement distance (Figure 4A) | Running | 17.24 | <0.001 |
| Jumping | 2.284 | 0.131 | ||
| Max. velocity (Figure 4B) | Running | 74.93 | <0.001 | |
| Jumping | 0.785 | 0.376 | ||
| Reaction time (Figure 4C) | Running | 36.20 | <0.001 | |
| Jumping | 3.431 | 0.064 | ||
| Directional accuracy (Figure 6B) | Running | 7.681 | 0.006 | |
| Jumping | 0.787 | 0.375 | ||
Relationships between angular parameters and stimulus angle were analyzed by the regression analyses, which were used in our previous study (Sato et al., 2019). The movement direction was considered as non-circular data and linear regression analysis was used as follows:
| (5) |
where is the estimated coefficient and is the intercept (Table 5). In contrast, the turn angle was considered as circular data. A circular regression analysis was performed as follows:
| (6) |
where and are the intercepts, and are the estimated coefficients of cosine, and and are the estimated coefficients of sine in the numerator and denominator of the model, respectively.
Table 5.
Result of linear regression analysis to test the relationships between the movement direction and stimulus angle.
| Experiment | Stimulus parameter |
Response | Significance of coefficient |
|||
|---|---|---|---|---|---|---|
| Velocity | Duration | Estimated slope | t value | p value | ||
| Velocity test (Figure 5A) | 340 mm/s | 200 ms | Running | 0.938 | 17.65 | <0.001 |
| Jumping | 0.479 | 1.864 | 0.121 | |||
| 618 mm/s | 200 ms | Running | 0.868 | 24.42 | <0.001 | |
| Jumping | 0.986 | 21.61 | <0.001 | |||
| 734 mm/s | 200 ms | Running | 0.968 | 31.46 | <0.001 | |
| Jumping | 0.910 | 26.45 | <0.001 | |||
| 834 mm/s | 200 ms | Running | 0.909 | 26.44 | <0.001 | |
| Jumping | 0.981 | 32.84 | <0.001 | |||
| Duration test (Figure 6A) | 834 mm/s | 20 ms | Running | 0.946 | 9.727 | <0.001 |
| Jumping | - | - | - | |||
| 834 mm/s | 40 ms | Running | 0.934 | 23.49 | <0.001 | |
| Jumping | 1.015 | 14.66 | <0.001 | |||
| 834 mm/s | 60 ms | Running | 0.930 | 23.84 | <0.001 | |
| Jumping | 0.938 | 25.05 | <0.001 | |||
| 834 mm/s | 80 ms | Running | 0.987 | 23.48 | <0.001 | |
| Jumping | 0.908 | 24.81 | <0.001 | |||
| 834 mm/s | 100 ms | Running | 1.002 | 25.63 | <0.001 | |
| Jumping | 0.965 | 32.33 | <0.001 | |||
3. Results
3.1. Faster and longer stimulus elicited the jumping response more frequently
First, we examined the parameters of the stimulus that affected the selection of either running or jumping (Figure S1). We tested the effects of the angle, velocity, and duration of the stimulus. We found that the crickets chose to jump more frequently in response to faster and longer stimuli (Figure 1 and Table 1). In the angle test (Figure 1A,B), the effect of the stimulus angle on the selection ratio of running or jumping was not statistically significant (Table 1), although the jumping tended to be chosen more frequently for a stimulus from behind (approximately 0°). In contrast, the velocity and duration tests demonstrated that the velocity and duration of the stimulus apparently affected both probabilities of the escape response to the stimulus as well as the selection ratio of running or jumping while escaping (Figure 1C–F). The response probability and the ratio of jumping choice to running choice significantly increased as the velocity or duration of the stimulus increased (Table 1). These results indicated that the cricket's choice of an escape response was affected by the two stimulus parameters, which were velocity and duration of the air current. Although we repeated trials multiple times for each individual, the trial orders did not affect the response probabilities or selection ratios in all three experiments (Figure 1 and Table 1). This indicated that there was no effect of repeated stimulation on the behavioral choice during the experiment.
3.2. Crickets changed running speed depending on stimulus parameters
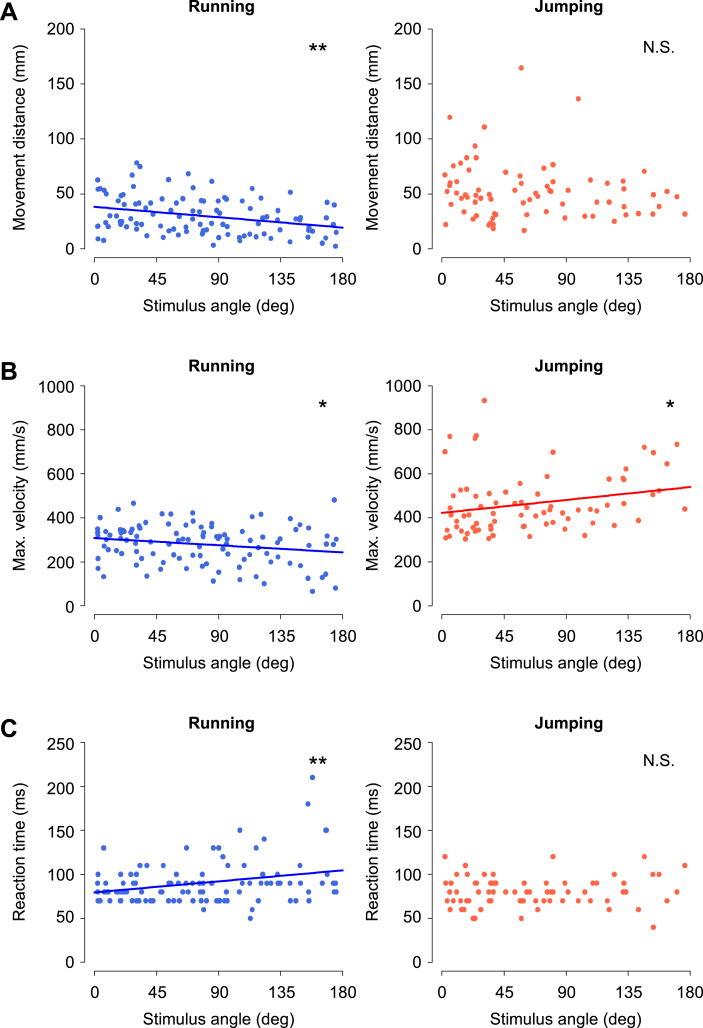
Based on the data from the angle, velocity, and duration tests, we investigated the effects of stimulus parameters on locomotion performance. The performance of the escape behavior was evaluated by metric locomotor parameters such as movement distance, maximum velocity, and reaction time during running or jumping. The relationships between these parameters and the stimulus angle indicated that the crickets changed their locomotion performance according to the stimulus angle for running rather than for jumping (Figure 2). The stimulus angle had a significant impact on all three metric locomotor parameters for running, but only on the maximum velocity for jumping (lines in Figure 2 and Table 2). The closer to behind of the cricket the stimulus was applied from, the quicker the crickets began to respond and the farther and faster they ran (left in Figure 2A–C). In contrast, the closer stimulus angle was to the front of the cricket, the faster the crickets jumped. However, the movement distance and reaction time of jumping were not significantly affected by the stimulus angle (right in Figure 2A–C).
Figure 2.
Effects of stimulus angle on metric locomotor parameters. (A–C) Relationships between the stimulus angle and the movement distance (A), maximum translational velocity (B), or reaction time (C), in running (left) and jumping (right) in the angle test. The velocity and duration of the air current were fixed to 834 mm/s and 200 ms, respectively. The total number of responses used for the analysis were 106 and 76 for running and jumping, respectively. Lines indicate the regression lines with a significant slope. ∗p < 0.05, ∗∗p < 0.01. N.S. not significant, linear regression analysis (n = 10 animals).
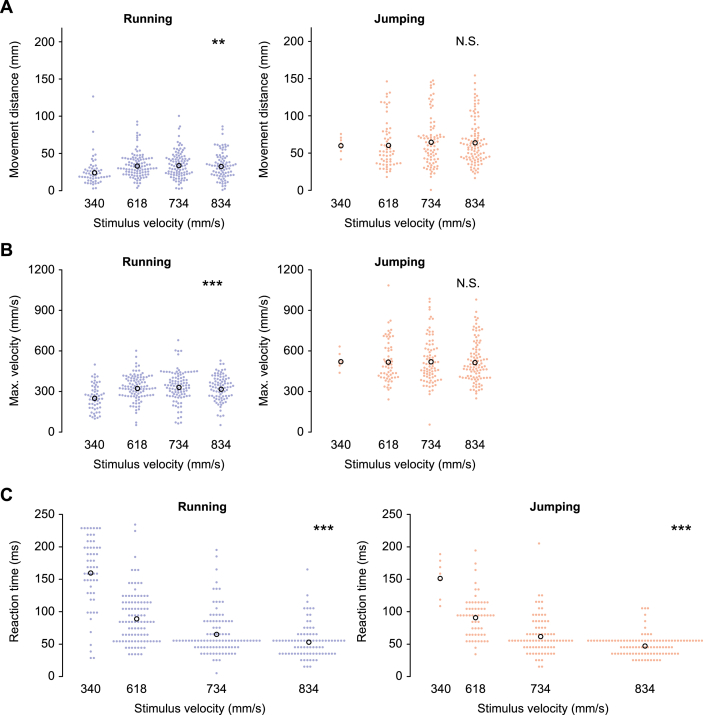
In the velocity and duration tests, we examined the effects of velocity or duration of the stimulus, not only on the metric locomotor parameters (Figures 3 and 4) but also on the movement direction (Figures 5 and 6) of running and jumping. Stimulus velocity and duration influenced the metric locomotor parameters of running rather than jumping. The velocity test indicated that crickets increased distance and speed for running, but not for jumping, as the stimulus velocity increased (Figure 3A,B, and Table 3). The reaction time became shorter as the stimulus velocity increased in both running and jumping. This means that the faster the air current, the quicker the crickets initiated the escape regardless of the response type (Figure 3C and Table 3). When we analyzed the data without responses to the slowest stimulus (340 mm/s), the effect of stimulus velocity on the reaction time was still significant whereas significance of the effects on the distance and speed was lost (Table 4).
Figure 3.
Effects of stimulus velocity on metric locomotor parameters. (A–C) The movement distance (A), maximum translational velocity (B), and reaction time (C), in running (left) and jumping (right). The total number of responses used for the analysis were 56, 101, 98 and 84 for running, and 7, 51, 81 and 100 for jumping, for 340, 618, 734 and 834 mm/s for stimulus velocities, respectively. Colored filled circles represent the data for each trial and black open circles represent the mean of the data in all trials for each velocity of stimuli. ∗∗p < 0.01, ∗∗∗p < 0.001. N.S. not significant, likelihood ratio test for LMMs (n = 10 animals).
Figure 4.
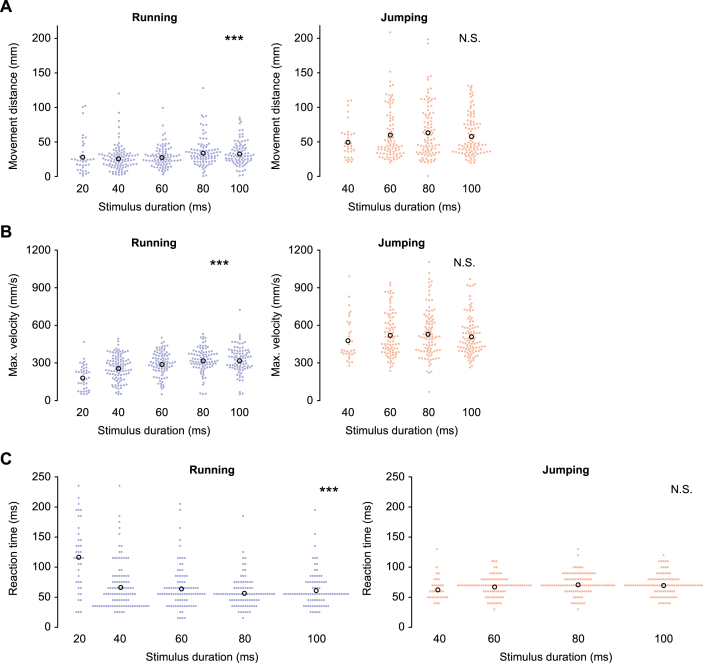
Effects of stimulus duration on metric locomotor parameters. (A–C) The movement distance (A), maximum translational velocity (B), and reaction time (C), in running (left) and jumping (right). The total number of responses used for the analysis were 39, 101, 88, 90 and 85 for running, and 0, 33, 87, 97 and 98 for jumping, for 20, 40, 60, 80 and 100 ms of stimulus durations, respectively. Colored filled circles represent the data for each trial and black open circles represent the mean of the data in all trials for each duration of stimuli. No jumping was elicited by the stimulus of 20-ms duration. ∗∗∗p < 0.001. N.S. not significant, likelihood ratio test for LMMs (n = 10 animals).
Figure 6.
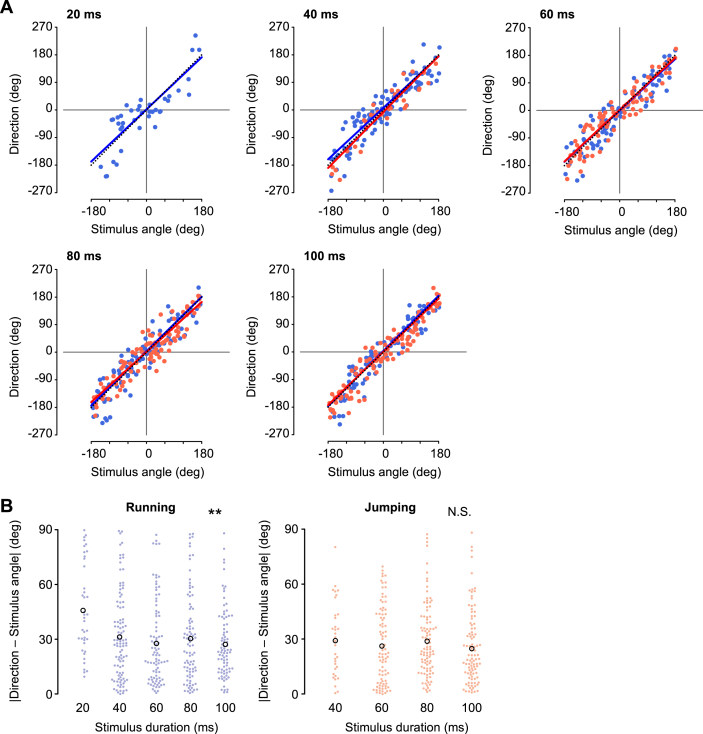
Effects of stimulus duration on directional control. (A) Relationships between the movement direction and stimulus angle in running (blue) and jumping (red), for the stimuli of various durations, 20, 40, 60, 80, and 100 ms. No jumping was elicited by the 20 ms duration stimulus. Colored lines represent linear regression lines with a significant slope for the data of running (blue) and jumping (red), respectively. Black dotted lines indicate the line of . (B) Absolute values of the angular difference between movement direction and stimulus angle in running (left) and jumping (right). Colored filled circles represent the data for each trial and black open circles represent the mean of the data in all trials, for each duration of stimuli. ∗∗p < 0.01, N.S. not significant, likelihood ratio test for LMMs (n = 10 animals).
Table 4.
Results of likelihood ratio test for LMMs to test the effects of stimulus velocity and duration on the locomotor parameters (not including the responses to slowest or shortest stimuli).
| Experiment | Parameter | Response | Result of likelihood ratio test |
|
|---|---|---|---|---|
| Chi-squared | p value | |||
| Velocity test | Movement distance (Figure 3A) | Running | 0.115 | 0.735 |
| Jumping | 0.351 | 0.554 | ||
| Max. velocity (Figure 3B) | Running | 0.170 | 0.680 | |
| Jumping | 0.011 | 0.915 | ||
| Reaction time (Figure 3C) | Running | 66.36 | <0.001 | |
| Jumping | 97.17 | <0.001 | ||
| Directional accuracy (Figure 5B) | Running | 0.070 | 0.792 | |
| Jumping | 0.0004 | 0.983 | ||
| Duration test | Movement distance (Figure 4A) | Running | 20.33 | <0.001 |
| Jumping | 2.284 | 0.131 | ||
| Max. velocity (Figure 4B) | Running | 35.30 | <0.001 | |
| Jumping | 0.785 | 0.376 | ||
| Reaction time (Figure 4C) | Running | 2.925 | 0.087 | |
| Jumping | 3.431 | 0.064 | ||
| Directional accuracy (Figure 6B) | Running | 0.367 | 0.545 | |
| Jumping | 0.787 | 0.375 | ||
In the duration test, the longer the stimulus, the farther and faster the crickets ran. However, they did not change their jumping locomotion depending on the stimulus duration (Figure 4). The effects of the stimulus duration were significant on all of the metric locomotor parameters for running, whereas none of the parameters for jumping (Table 3). Interestingly, the longer the stimulus, the earlier the cricket initiated the running escape response. However, it did not change the start of the jumping response. This was likely because the shortest stimulus (20 ms) elicited running responses with a long latency of over 100 ms after the stimulus was terminated (left in Figure 4C). If the stimulus was too short, it possibly took longer for the crickets to notice it, or such a stimulus would be judged not to be dangerous. When the data of the responses to the shortest stimulus were removed, the effect of the stimulus duration on the reaction time was no longer significant. In contrast, unlike the velocity test, the effects on the distance and speed were still significant (Table 4). Taken together with the low probability of response to the shortest stimulus, this suggests that very short airflows were not perceived as dangerous signal to escape by the crickets.
3.3. Movement direction was controlled regardless of stimulus velocity and duration
Our previous studies have shown that crickets precisely control the direction of their movements against the stimulus angle during both running and jumping (Sato et al., 2019). We then examined whether the velocity and duration of the stimulus affected the directional control of the cricket's escape response (Figures 5 and 6). The crickets could control their movement direction precisely against the stimulus angle, independent of the velocity or duration of the stimulus. Even in response to stimuli of different velocities or durations, plots of the movement direction against stimulus angle were distributed close to the line for running and jumping (Figures 5A, 6A and Table 5). Here, the precise movement of the crickets in the direction opposite to the stimulus was assessed by the absolute value of the angular difference between the moving direction and the stimulus angle. Independent of the stimulus velocity, the absolute angular differences between the movement direction and stimulus angle were approximately 30° when both running and jumping (Figure 5B and Table 3). This indicated that the air current velocity had no effect on the directional control. In addition, in the duration test, the duration of the stimulus had little impact on the directional control of the escape responses (Figure 6). The absolute angular difference between the movement direction and stimulus angle was mostly constant for the stimuli of 40–100 ms duration in both running and jumping (Figure 6B). The significant effect of the stimulus duration was observed in running, however not in jumping (Table 3). This may be because the running directions in response to the shortest (20 ms) stimulus were less correlated with the stimulus angle. In contrast, no jumping was elicited by the shortest stimuli, resulting in no significant difference in the absolute difference value of the stimulus duration for jumping. We also examined the impact of the velocity and duration of the stimulus on the relationship between the turn angle and stimulus angle (Figures S2 and S3). However, there was no apparent difference in the distributions of the plot among the responses either in the velocity or the duration test. Thus, the angular parameters of escape responses, including the movement direction and turn angle, were not significantly affected by the velocity and duration of the stimulus. This was true except for the extremely short stimuli that were close to the threshold to induce a jump.
To conclude, regarding the impact of stimulus parameters on locomotor parameters, crickets altered their locomotion performance such as the moving distance, velocity, and reaction time, according to the angle, velocity, and duration of the stimulus for running but very little for jumping. In contrast, the escape direction was controlled precisely, independent of the velocity and duration of the stimulus in both the running and jumping escape responses. These results suggest that movement performance in speed and distance is modulated for the running response but not for jumping, depending on the stimulus characteristics. The directionality of the escape movements was dictated by the stimulus angle only but was unaffected by the stimulus intensity and duration.
4. Discussion
4.1. The neural system to choose escape responses based on multiple stimulus parameters
The prey animal must perceive how the predator approaches through the multiple aspects of the stimulus in order to perform the most successful escape response. Previously, the choice of escape response has been reported to depend on stimulus velocity (Bhattacharyya et al., 2017; von Reyn et al., 2014, 2017). Our results revealed that both velocity and duration of the stimulus affect the action selection of either running or jumping in wind-elicited escape behavior. In conclusion, crickets likely made a decision regarding their escape response based on multiple aspects of the air-current stimuli that indicate the predator's approach. This finding strongly suggests that the central nervous system of crickets includes an integration process of multiple channels of distinct stimulus information to select an escape action.
While complex neural mechanisms across multiple brain regions are involved in mammalian escape behaviors (Evans et al., 2018; Gross and Canteras, 2012; Shang et al., 2015; Wang et al., 2015), relatively simple neural circuits mediate the escape behavior of fish and invertebrates (Card, 2012; Eaton et al., 2001). In such simple neural systems that mediate escape behaviors, certain neurons have been identified that trigger the escape responses to a specific sensory stimulus indicating the predator's approach. In flies, a key set of neurons called giant fibers directly induces quick taking-off as an escape response (Allen et al., 2006). To choose from two types of escape take-off behaviors depending on the stimulus velocity, the presence of neural circuits in which specific projection neurons provide information on stimulus velocity to the giant fibers have been proposed in the fly (von Reyn et al., 2014, 2017). In context, our results show that crickets choose escape responses based on multiple stimulus parameters for the first time, which would promote an understanding of the mechanism of choosing appropriate behavior according to various situations in the natural field.
The neural basis of the cercal sensory system that processes airflow information, which in turn mediates wind-elicited escape behavior, has been well studied (Jacobs et al., 2008; Baba and Ogawa, 2017). Air currents are detected as air-particle displacement by filiform hairs on the cerci (Miller et al., 2011; Shimozawa and Kanou, 1984). Several wind-sensitive interneurons including giant interneurons (GIs) identified as ascending projection neurons receive excitatory synaptic inputs from the sensory afferents of the mechanoreceptor neurons of the hair sensilla. The GIs encode various characteristics of the surrounding air currents such as velocity, direction, and frequency in their firing activity (Aldworth et al., 2011; Miller et al., 1991; Theunissen and Miller, 1991). These ascending neurons project their long axons through the ventral nerve cord to higher centers including the thoracic ganglia and the brain (Hirota et al., 1993). Because the GIs differ from each other in their sensitivities to the direction and intensity of air currents (Jacobs et al., 2008; Miller et al., 1991; Ogawa et al., 2008; Theunissen and Miller, 1991; Theunissen et al., 1996), the GIs likely provide distinct sensory information to their postsynaptic partners. The GIs also respond to naturalistic airflow stimuli in the field (Dupuy et al., 2012). These facts suggest that the GIs play crucial roles in wind-elicited escape behavior. However, the thresholds for stimulus velocity in the neural response of GIs were much lower than those in the behavioral response reported in this study (Miller et al., 1991). This implies that the central neural circuits receiving ascending signals in the brain may play a decisive role in the behavioral choice and motor control of escape behaviors. Thus, further investigation of the postsynaptic circuit of GIs would clarify the neural mechanism underlying action selection depending on multiple stimulus parameters.
4.2. Stimulus characteristics that were likely to elicit jumping
The faster and longer the air currents, the more frequently the jumping response were elicited. Although it was likely that the air-current stimulus we used differed in detail of characteristics from the real air currents caused by the predator approaching, the velocity and duration of the air-current stimulus are considered to be correlated with the size of the predator and the approaching speed (Casas and Dangles, 2010). Thus, crickets would choose to jump, allowing them to escape farther and faster in response to a more threatening stimulus (Sato et al., 2019).
It should be noted, however, that the jumping probability was saturated at approximately 50% even for the fastest and longest stimulus, which was the most inducible for jumping (Figure 1). Our previous study has reported that cricket’s can more flexibly change their movements during running rather than jumping, responding to additional stimulus (Sato et al., 2019). Therefore, it is likely that crickets exhibit running response more frequently to weaker stimuli suggesting an attack of predator approaching from a greater distance, in order to respond to conceivable additional attack. Another possibility is that additional sensory inputs may be required to further elevate the likelihood of a jumping selection. Animals use a variety of sensory modalities to detect predators. For example, auditory stimuli combined with air currents alter the contents of escape responses in crickets (Fukutomi and Ogawa, 2017; Fukutomi et al., 2015). Even if they do not directly elicit escape responses, other sensory inputs that inform external contexts or internal conditions of the animals affect escape responses (Domenici et al., 2008; Matsuura et al., 2002). Therefore, crickets may jump more frequently than 50% in various contexts or situations.
In contrast, the stimulus angle did not significantly affect the selection of running or jumping. This result is consistent with a previous report where crickets have the same accuracy of directional controllability for running and jumping, regardless of the stimulus angle (Sato et al., 2019). In this study, a strong correlation, which could be approximated by , between the movement direction and stimulus angle, was also observed. This correlation was observed both in the velocity test (Figure 5) and the duration test (Figure 6). These results indicated that the escape direction could be controlled against the stimulus angle in either type of escape response, independent of the stimulus velocity and duration. It is supposed that the sensory information of the stimulus angle would be conveyed by neural channels different from those for stimulus velocity and duration.
4.3. Stimulus dependency of the locomotion performance
The crickets changed their moving speed and travel distance according to the stimulus parameters for running, however not for jumping. This locomotion controllability is the behavioral advantage of running compared to jumping. The dependency of the travel distance and moving speed on the stimulus velocity were not observed if the data for the response to the slowest stimulus was removed. This suggests that the dynamic range for movement regulation to the airflow velocity is narrow and relatively low. In contrast, the invariance of the jumping speed and distance to the stimulus parameters illustrated that jumping was a more inflexible response. This means that jumping response might be more predictable for predators. These results are consistent with our previous reports that running is a more flexible response during which crickets respond to the additional stimulus, compared to jumping (Sato et al., 2019).
Interestingly, the reaction time decreased as the stimulus velocity increased for both running and jumping (Figure 3C). Previous studies have demonstrated that escape responses to visual looming stimuli is initiated at a specific angular size of the stimulus. The size of the stimulus is an important clue for animals to know the distance to the approaching predator. This suggests that the distance to the predators, rather than the speed of the predator's approach, is a more crucial sensory signal for the prey to decide upon an escape response (Bhattacharyya et al., 2017; Dunn et al., 2016; Fotowat and Gabbiani, 2007). In contrast, our results revealed a strong relationship between stimulus velocity and escape latency. This means that the crickets may decide to start escaping based on the stimulus velocity, which indicates the approaching speed of the predator. The faster a predator approaches, the less time it takes to reach its prey. Thus, the reaction time is important for the prey to increase the success rate of its escape behavior (von Reyn et al., 2014; Walker et al., 2005). In particular, when animals of prey cannot visually detect a predator's approach, as in the dark, they will make decisions regarding the initiation of the escape based on the stimulus velocity. This decision making system is likely effective for the survival of nocturnal animals.
Unlike the metric locomotor parameters, the movement direction was accurately controlled almost independently of the velocity or duration of the stimulus. This result was consistent with a previous study reporting that the direction of the escape when running on a treadmill is similarly controlled to stimuli of various durations (Oe and Ogawa, 2013). The accuracy of directional control decreased only in the running response to the shortest stimulus (left in Figure 6B). There is a possibility that a 20 ms stimulus is too brief for the crickets to perceive their direction. This is also supported by the result that the reaction time for a running response to the shortest stimulus was longer than that for stimuli with longer durations (left in Figure 4C). Air currents longer than a certain duration are likely to be necessary for the cricket to perceive stimulus orientation for directional control of the escape response.
Declarations
Author contribution statement
Nodoka Sato: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Hisashi Shidara, Hiroto Ogawa: Conceived and designed the experiments; Wrote the paper.
Funding statement
Hiroto Ogawa was supported by JSPS KAKENHI (16H06544).
Nodoka Sato was supported by JSPS Grant-in Aid for JSPS Research Fellow (19J10862).
Hisashi Shidara was supported by JSPS KAKENHI (19K16283).
Data availability statement
Data will be made available on request.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
We thank Dr. Masayo Soma for helpful advice regarding statistical analysis.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- Aldworth Z.N., Dimitrov A.G., Cummins G.I., Gedeon T., Miller J.P. Temporal encoding in a nervous system. PLoS Comput. Biol. 2011;7 doi: 10.1371/journal.pcbi.1002041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen M.J., Godenschwege T.A., Tanouye M.A., Phelan P. Making an escape: development and function of the Drosophila giant fibre system. Semin. Cell Dev. Biol. 2006;17:31–41. doi: 10.1016/j.semcdb.2005.11.011. [DOI] [PubMed] [Google Scholar]
- Baba Y., Ogawa H. In: The Cricket as a Model Organism. Horch H.W., Mito T., Popadic A., Ohuchi H., Noji S., editors. Springer; 2017. Cercal system-mediated antipredator behaviors; pp. 211–228. [Google Scholar]
- Baba Y., Shimozawa T. Diversity of motor responses initiated by a wind stimulus in the freely moving cricket, Gryllus bimaculatus. Zool. Sci. 1997;14:587–594. [Google Scholar]
- Bhattacharyya K., McLeen D.L., Maclver M.A. Visual threat assessment and reticulospinal encoding of calibrated responses in larval zebrafish. Curr. Biol. 2017;27:2751–2762. doi: 10.1016/j.cub.2017.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggman K.L., Abarbanel H.D., Kristan W.B. Optical imaging of neuronal populations during decision-making. Science. 2005;307:896–901. doi: 10.1126/science.1103736. [DOI] [PubMed] [Google Scholar]
- Bulbert M.W., Page R.A., Bernal X.E. Danger comes from all fronts: predator-dependent escape tactics of túngara frogs. PLoS One. 2015;10 doi: 10.1371/journal.pone.0120546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Card G.M. Escape behaviors in insects. Curr. Opin. Neurobiol. 2012;22:180–186. doi: 10.1016/j.conb.2011.12.009. [DOI] [PubMed] [Google Scholar]
- Card G., Dickinson M. Performance trade-offs in the flight initiation of Drosophila. J. Exp. Biol. 2008;211:341–353. doi: 10.1242/jeb.012682. [DOI] [PubMed] [Google Scholar]
- Casas J., Dangles O. Physical ecology of fluid flow sensing in arthropods. Annu. Rev. Entomol. 2010;55:505–520. doi: 10.1146/annurev-ento-112408-085342. [DOI] [PubMed] [Google Scholar]
- Dangles O., Ory N., Steinmann T., Christides J.-P., Casas J. Spider’s attack versus cricket’s escape: velocity modes determine success. Anim. Behav. 2006;72:603–610. [Google Scholar]
- De Franceschi G., Vivattanasam T., Saleem A.B., Solomon S.G. Vision guides selection of freeze or flight defense strategies in mice. Curr. Biol. 2016;26:2150–2154. doi: 10.1016/j.cub.2016.06.006. [DOI] [PubMed] [Google Scholar]
- Domenici P. Context-dependent variability in the components of fish escape response: integrating locomotor performance and behavior. J. Exp. Zool. 2010;313A:59–79. doi: 10.1002/jez.580. [DOI] [PubMed] [Google Scholar]
- Domenici P., Blagburn J.H., Bacon J.P. Animal escapology I: theoretical issues and emerging trends in escape trajectories. J. Exp. Biol. 2011;214:2463–2473. doi: 10.1242/jeb.029652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domenici P., Blagburn J.M., Bacon J.P. Animal escapology II: escape trajectory case studies. J. Exp. Biol. 2011;214:2474–2494. doi: 10.1242/jeb.053801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domenici P., Booth D., Blagburn J.M., Bacon J.P. Cockroaches keep predators guessing by using preferred escape trajectories. Curr. Biol. 2008;18:1792–1796. doi: 10.1016/j.cub.2008.09.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn T.W., Gebhardt C., Nauman E.A., Riegler C., Ahrens M.B., Engert F., Del Bene F. Neural circuits underlying visually evoked escapes in larval zebrafish. Neuron. 2016;89:613–628. doi: 10.1016/j.neuron.2015.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupuy F., Casas J., Body M., Lazzari C.R. Danger detection and escape behaviour in wood crickets. J. Insect Physiol. 2011;57:865–871. doi: 10.1016/j.jinsphys.2011.03.020. [DOI] [PubMed] [Google Scholar]
- Dupuy F., Steinmann T., Pierre D., Christidès J.P., Cummins G., Lazzari C., John M., Casas J. Responses of cricket cercal interneurons to realistic naturalistic stimuli in the field. J. Exp. Biol. 2012;215:2382–2389. doi: 10.1242/jeb.067405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton R.C., Lee R.K.K., Foreman M.B. The Mauthner cell and other identified neurons of the brainstem escape network of fish. Prog. Neurobiol. 2001;63:476–485. doi: 10.1016/s0301-0082(00)00047-2. [DOI] [PubMed] [Google Scholar]
- Eilam D. Die hard: a blend of freezing and fleeing as a dynamic defense—implications for the control of defensive behavior. Neurosci. Biobehav. Rev. 2005;29:1181–1191. doi: 10.1016/j.neubiorev.2005.03.027. [DOI] [PubMed] [Google Scholar]
- Evans D.A., Stempel A.V., Vale R., Branco T. Cognitive control of escape behaviour. Trends Cognit. Sci. 2019;23:334–348. doi: 10.1016/j.tics.2019.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D.A., Stempel A.V., Vale R., Ruehle S., Lefler Y., Branco R. A synaptic threshold mechanism for computing escape decisions. Nature. 2018;558:590–594. doi: 10.1038/s41586-018-0244-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadok J.P., Krabbe S., Markovic M., Courtin J., Xu C., Massi L., Botta P., Bylund K., Müller C., Kovacevic A., et al. A competitive inhibitory circuit for selection of active and passive fear responses. Nature. 2017;542:96–100. doi: 10.1038/nature21047. [DOI] [PubMed] [Google Scholar]
- Fotowat H., Gabbiani F. Relationship between the phases of sensory and motor activity during a looming-evoked multistage escape behavior. J. Neurosci. 2007;27:10047–10059. doi: 10.1523/JNEUROSCI.1515-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukutomi M., Ogawa H. Crickets alter wind-elicited escape strategies depending on acoustic context. Sci. Rep. 2017;7:15158. doi: 10.1038/s41598-017-15276-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukutomi M., Someya M., Ogawa H. Auditory modulation of wind-elicited walking behavior in the cricket Gryllus bimaculatus. J. Exp. Biol. 2015;218:3968–3977. doi: 10.1242/jeb.128751. [DOI] [PubMed] [Google Scholar]
- Gross C.T., Canteras N.S. The many path to fear. Nat. Rev. Neurosci. 2012;13:651–658. doi: 10.1038/nrn3301. [DOI] [PubMed] [Google Scholar]
- Hirota K., Sonoda Y., Baba Y., Yamaguchi T. Distinction in morphology and behavioral role between dorsal and ventral groups of cricket giant interneurons. Zool. Sci. 1993;10:705–709. [Google Scholar]
- Jacobs G.A., Miller J.P., Aldworth Z. Computational mechanisms of mechanosensory processing in the cricket. J. Exp. Biol. 2008;205:2005–2016. doi: 10.1242/jeb.016402. [DOI] [PubMed] [Google Scholar]
- LeDoux J., Daw N.D. Surviving threats: neural circuit and computational implications of a new taxonomy of defensive behavior. Nat. Rev. Neurosci. 2018;19:269–282. doi: 10.1038/nrn.2018.22. [DOI] [PubMed] [Google Scholar]
- Liu Y.C., Hale M.E. Local spinal cord circuits and bilateral Mauthner cell activity function together to drive alternative startle behaviors. Curr. Biol. 2017;27:697–704. doi: 10.1016/j.cub.2017.01.019. [DOI] [PubMed] [Google Scholar]
- Matsuura T., Kanou M., Yamaguchi T. Motor program initiation and selection in crickets, with special reference to swimming and flying behavior. J. Comp. Physiol. A. 2002;187:987–995. doi: 10.1007/s00359-001-0269-3. [DOI] [PubMed] [Google Scholar]
- Miller J.P., Jacobs G.A., Theunissen F.E. Representation of sensory information in the cricket cercal sensory system. I. Response properties of the primary interneurons. J. Neurophysiol. 1991;66:1680–1689. doi: 10.1152/jn.1991.66.5.1680. [DOI] [PubMed] [Google Scholar]
- Miller J.P., Krueger S., Heys J.J., Gedeon T. Quantitative characterization of the filiform mechanosensory hair array on the cricket cercus. PLoS One. 2011;6 doi: 10.1371/journal.pone.0027873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oe M., Ogawa H. Neural basis of stimulus-angle-dependent motor control of wind-elicited walking behavior in the cricket Gryllus bimaculatus. PLoS One. 2013;8 doi: 10.1371/journal.pone.0080184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa H., Cummins G.I., Jacobs G.A., Oka K. Dendritic design implements algorithm for synaptic extraction of sensory information. J. Neurosci. 2008;28:4592–4603. doi: 10.1523/JNEUROSCI.5354-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preuss T., Osei-Bonsu P.E., Weiss S.A., Wang C., Fabor D.S. Neural representation of object approach in a decision-making motor circuit. J. Neurosci. 2006;26:3454–3464. doi: 10.1523/JNEUROSCI.5259-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peek M.Y., Card G.M. Comparative approaches to escape. Curr. Opin. Neurobiol. 2016;41:167–173. doi: 10.1016/j.conb.2016.09.012. [DOI] [PubMed] [Google Scholar]
- Santer R.D., Yamazaki Y., Rind F.C., Simmons P.J. Motor activity and trajectory control during escape jumping in the locust Locusta migratoria. J. Comp. Physiol. A. 2005;191:965–975. doi: 10.1007/s00359-005-0023-3. [DOI] [PubMed] [Google Scholar]
- Sato N., Shidara H., Ogawa H. Post-molting development of wind-elicited escape behavior in the cricket. J. Insect Physiol. 2017;103:36–46. doi: 10.1016/j.jinsphys.2017.10.003. [DOI] [PubMed] [Google Scholar]
- Sato N., Shidara H., Ogawa H. Trade-off between motor performance and behavioural flexibility in the action selection of cricket escape behaviour. Sci. Rep. 2019;9:18112. doi: 10.1038/s41598-019-54555-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang C., Liu Z., Chen Z., Shi Y., Wang Q., Liu S., Li D., Cao P. A parvalbumin-positive excitatory visual pathway to trigger fear responses in mice. Science. 2015;348:1472–1477. doi: 10.1126/science.aaa8694. [DOI] [PubMed] [Google Scholar]
- Shimozawa T., Kanou M. Varieties of filiform hairs: range fractionation by sensory afferents and cercal interneurons of a cricket. J. Comp. Physiol. A. 1984;155:485–493. [Google Scholar]
- Simmons P.J., Rind F.C., Santer R.D. Escapes with and without preparation: the neuroethology of visual startle in locusts. J. Insect Physiol. 2010;56:876–883. doi: 10.1016/j.jinsphys.2010.04.015. [DOI] [PubMed] [Google Scholar]
- Stewart W.J., Cardenas G.S., McHenry M.J. Zebrafish larvae evade predators by sensing water flow. J. Exp. Biol. 2013;216:388–398. doi: 10.1242/jeb.072751. [DOI] [PubMed] [Google Scholar]
- Takagi S., Cocanougher B.T., Niki S., Miyamoto D., Kohsaka H., Kazama H., Fetter R.D., Truman J.W., Zlatic M., Cardona A., et al. Divergent connectivity of homologous command-like neurons mediates segment-specific touch responses in Drosophila. Neuron. 2017;96:1373–1387. doi: 10.1016/j.neuron.2017.10.030. [DOI] [PubMed] [Google Scholar]
- Theunissen F.E., Miller J.P. Representation of sensory information in the cricket cercal sensory system. II Information theoretic calculation of system accuracy and optimal tuning curve width of four primary interneurons. J. Neurophysiol. 1991;66:1690–1703. doi: 10.1152/jn.1991.66.5.1690. [DOI] [PubMed] [Google Scholar]
- Theunissen F., Roddey J.C., Stufflebeam S., Clague H., Miller J.P. Information theoretic analysis of dynamical encoding by four identified primary sensory interneurons in the cricket cercal system. J. Neurophysiol. 1996;75:1345–1364. doi: 10.1152/jn.1996.75.4.1345. [DOI] [PubMed] [Google Scholar]
- Turner H.N., Amengol K., Patel A.A., Himmel N.J., Sullivan L., Iyer S.C., Bhattacharyya S., Iyer E.P.R., Landry C., Galko M.J., et al. The TRP channels Pkd2, NompC, and Trpm act in cold-sensing neurons to mediate unique aversive behaviors to noxious cold in Drosophila. Curr. Biol. 2016;26:3116–3128. doi: 10.1016/j.cub.2016.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Reyn C.R., Breads P., Peek M.Y., Zheng G.Z., Williamson W.R., Yee A.L., Leonardo A., Card G.M. A spike-timing mechanism for action selection. Nat. Neurosci. 2014;17:962–970. doi: 10.1038/nn.3741. [DOI] [PubMed] [Google Scholar]
- von Reyn C.R., Nern A., Williamson W.R., Breads P., Wu M., Namiki S., Card G.M. Feature integration drives probabilistic behavior in the drosophila escape response. Neuron. 2017;94:1190–1204. doi: 10.1016/j.neuron.2017.05.036. [DOI] [PubMed] [Google Scholar]
- Walker J.A., Ghalambor C.K., Griset O.L., McKenny D., Reznik D.N. Do faster starts increase the probability of evading predators? Funct. Ecol. 2005;19:808–815. [Google Scholar]
- Wang L., Chen I.Z., Lin D. Collateral pathways from the ventromedial hypothalamus mediate defensive behaviors. Neuron. 2015;85:1344–1358. doi: 10.1016/j.neuron.2014.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T., Ugajin A., Aonuma H. Immediate-early promoter-driven transgenic reporter system for neuroethological research in a hemimetabolous insect. eNeuro. 2018;5 doi: 10.1523/ENEURO.0061-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.