Abstract
Liver cancer is the second most occurring cancer worldwide and is one of the leading causes of cancer-related deaths. Hepatocellular carcinoma (HCC) is the most common (80%-90%) type among malignant liver cancers. Sarcopenia occurs very early in HCC and can predict and provide an opportunity to improve muscle health before engaging in the treatment options such as loco-regional, systemic, and transplant management. Multiple prognostic stating systems have been developed in HCC, such as Barcelona Clinic Liver Cancer, Child-Pugh score and Albumin-Bilirubin grade. However, the evaluation of patients’ performance status is a major limitation of these scoring systems. In this review, we aim to summarize the current knowledge and recent advances about the role of sarcopenia in cirrhosis in general, while focusing specifically on HCC. Additionally, the role of sarcopenia in predicting clinical outcomes and prognostication in HCC patients undergoing loco-regional therapies, liver resection, liver transplantation and systematic therapy has been discussed. A literature review was performed using databases PubMed/MEDLINE, EMBASE, Cochrane, Web of Science, and CINAHL on April 1, 2021, to identify published reports on sarcopenia in HCC. Sarcopenia can independently predict HCC-related mortality especially in patients undergoing treatments such as loco-regional, surgical liver transplantation and systemic therapies. Basic research is focused on evaluating a balance of anabolic and catabolic pathways responsible for muscle health. Early clinical studies have shown promising results in methods to improve sarcopenia in HCC which can potentially increase prognosis in these patients. As sarcopenia occurs very early in HCC, it can predict and provide an opportunity to improve muscle health before engaging in the treatment options such as loco-regional, systemic, and transplant management. Further, sarcopenia measurement can obviate the confounding caused by the abdominal ascites in these patients. The use of sarcopenia can add to the existing scoring systems to better prognosticate the HCC.
Keywords: Sarcopenia, Skeletal muscle, Hepatocellular carcinoma, Cirrhosis, Outcomes, Liver cancer
Core Tip: Sarcopenia is a condition defined by the loss of skeletal muscle mass, quality and strength. It is commonly seen as a part of normal aging but can also be noted in multiple conditions such as chronic inflammation, cancers and use of drugs. Sarcopenia is common in liver cirrhosis and is associated with overall poor outcomes (disease-free survival). Recently, the adverse effects of sarcopenia in hepatocellular carcinoma (HCC) has been an area of intense interest. Altered bio-impedence and rapid muscle loss in liver diseases could alter skeletal muscle strength in these patients. Additionally, development of tumor-related cytokines can accelerate the sarcopenia progression which could provide insights into disease progression and response to various therapeutic options. While multiple scoring systems are available to evaluate the HCC progression, sarcopenia provides an additional functional status tool to further refine these systems. In this article, we summarize the role of sarcopenia in HCC progression and changes during locoregional and systemic treatments.
INTRODUCTION
Primary liver cancers include hepatocellular carcinoma (HCC) and other non-HCC tumors. Primary liver cancers are the second most lethal cancer worldwide, fourth leading cause of cancer mortality and sixth frequently diagnosed cancer per year[1]. HCC is the most common cancers among the primary liver cancers, which constitutes 90% of cases. HCC usually develops within a liver cirrhosis (cirrhotic-HCC, 80% of cases), and rarely with no appreciable cirrhosis or advance fibrosis (non-cirrhotic-HCC, 20% of cases)[2]. Due to aggressive nature of HCC, prognosis is poor. This is compounded by delay in the treatment, limiting life expectancy and management options. Early identification of high-risk features for appropriate stratification, and prognostication in HCC is paramount to alter the disease course and improve survival. Several prognostic staging systems and biomarkers have been developed to identify the patients at risk of poor prognosis[3]. Some of these include Cancer of the Liver Italian Program, Barcelona Clinic Liver Cancer (BCLC), Child-Pugh score, Chinese University Prognostic Index score, the Hong-Kong Liver Cancer stating system and Japan Integrated Staging. Further, biomarkers such as alpha-fetoprotein (AFP), des-γ-carboxyprothrombin AFP-L3, vascular endothelial growth factor, and angiopoietin 2 were used as independent prognostic factors in advanced tumors[4]. However, current available staging and prognosticative systems lack parameters that consider nutritional, functional and performance status[5]. Although long-term prognosis is dependent on the liver reserve and staging of the cancer, poor performance can significantly affect clinical outcomes in HCC patients. The use of the Eastern Cooperative Oncology Group classification with BCLC could provide an assessment of patients functional status.
Rosenberg[6] introduced the term “Sarcopenia,” which was coined from the Greek word “sarx,” or “flesh,” and “penia,” or “loss.” It can be defined as loss of skeletal muscle mass, quality, strength with a reduction in the motor unit number, atrophy of type muscle fibers[7], and can contribute to frailty, functional impairment, and disability[8-11]. Three most commonly used diagnostic criteria used for sarcopenia include “muscle mass” (height-adjusted), “muscle strength,” and/or “physical performance”[12]. A focus on muscle function has shown to be a powerful predictor of clinically relevant outcomes rather than muscle mass alone[13]. Recently, body mass index (BMI)-adjusted mass is found to be a better predictor of adverse outcomes than height-adjusted muscle mass[14,15]. Further, multiple muscles or groups of muscles could be utilized to assess sarcopenia. Some of the most commonly used muscles include the paraspinal muscle area (psoas muscle, quadratus lumborum, transverse spinal muscle, erector spinae muscles) and triceps muscles (mid-arm circumference). Loss of skeletal muscle mass can affect static, dynamic and isokinetic strength[16]. It can also be associated with a decline in the maximum oxygen consumption (at a rate of 3%-8% per decade of life starting from 30 years) which ultimately leads to a decrease in overall functioning[17]. Dynamic changes in skeletal muscle mass and function can occur with changes in hormones (daily insulin, glucagon), anabolic steroids, corticosteroids, thyroid (month-to-month), and immune mediators [interleukin (IL)-1, tumor necrosis factor, and IL-2]. Primary sarcopenia is noted to be due to physiological states such as aging and secondary causes (acute or chronic illness)[18]. Individuals with cancer may deplete up to 80% of their muscle mass. Further, sarcopenia can be noted in as high as 80% and 60% of patients with upper gastrointestinal and lung cancers, respectively[19]. Pre-therapeutic sarcopenia is noted with highest prevalence in esophageal and small-cell lung cancers and could have severe consequences in terms of post-operative complications, chemotherapy-related toxicity, and poor overall survival (OS)[20].
Cross-sectional imaging is commonly performed in HCC patients for diagnosis, surveillance, and treatment response[19]. It is logical to use this cross-sectional imaging to evaluate skeletal muscle mass simultaneously for valuable information to assess the prognosis and treatment outcomes. Additionally, patients with cirrhosis and HCC commonly develop ascites spuriously increasing the abdominal girth and weight. Despite this increase, significant proportion of these patients have decreased muscle mass leading to “sarcopenic obesity[21].” Use of an objective tool (which is measurable and reproducible) to assess the survival of HCC patients with ascites remains a challenge. Furthermore, methods to assess the prognosis of HCC patients during/after loco-regional (radiofrequency ablation, radioembolization, chemoembolization), liver transplantation, and systemic therapy (chemotherapy, immunotherapy) could have a long-lasting impact on these individuals. One such objective method is to use sarcopenia to assess the patient response and overall could assist in OS in HCC patients[14,22-34]. Therefore, this manuscript aims to describe the role of sarcopenia in the management and prognosis in HCC. Furthermore, we aim to describe and summarize the methods to improve sarcopenia to enhance the survival of patients undergoing treatment for HCC.
LITERATURE SEARCH
An electronic search was performed using databases PubMed/MEDLINE, EMBASE, Cochrane, Web of Science, and CINAHL on April 1, 2021, to identify published reports on sarcopenia in HCC. We used the following search terms- “carcinoma, hepatocellular” or “cancer, hepatocellular” and ”sarcopenia” or “sarcopenias”. A total of 4762 articles were published on sarcopenia and 167571 on hepatocellular cancers. Both basic science and clinical studies were included. A combined search revealed 2289 articles over the last 12 mo. The authors AP and HG reviewed the articles independently. Clinical reviews, case reports, and case series were excluded. A manual search was performed by evaluating the references from included studies and related articles in multiple databases. If any discrepancies, these articles were re-reviewed by the author RT. After removing non-relevant/duplicates/non-English language articles, including a manual search, 80 full length published articles were finally reviewed.
HCC AND SARCOPENIA
Secondary sarcopenia is a common finding in patients with cirrhosis. Reduction in protein synthesis can lead to a decrease in lean body mass seen in cirrhotics[26]. Protein catabolism seen in disease processes such as neoplasms can lead to significant loss of muscle mass and it can be seen up to 40% of patients with liver cirrhosis[35]. Sarcopenia can be associated with an increased risk of encephalopathy, post-transplant mortality, infections, treatment effectiveness, and quality of life[36-38]. Patients with cirrhosis who were diagnosis with HCC showed accelerated sarcopenia up to 30-40% at the time of diagnosis[39,40]. Sarcopenia in these patients can independently predict HCC-related mortality along with decompensated cirrhosis, performance status, TNM staging, and BCLC class[41,42]. However, each of these have limitations with biggest being lack of prognostication, inability to provide comprehensive tool to assess complex interactions between cirrhosis, HCC and functional capacity[43]. Further, factors responsible for survival differ significantly among patients with compensated and decompensated cirrhosis[44].
As HCC occurs over time in patients with underlying chronic liver disease, assessment of skeletal muscle mass and change overtime can provide important details about deterioration of the disease. A number of tumor-related factors (cytokines and myokines) can change the skeletal muscle mass which can assist to further refine these scoring systems. Furthermore, cirrhotic have ascites, disproportionate loss of muscle compared to fat (altering BMI) leading to difficulty in interpreting bioimpedance, anthropometric measurements. Hence use of tools to integrate degree of sarcopenia-related measurements by CT-based techniques can offer ways to predict change in these patients[45,46].
BIOLOGICAL BASIS OF SARCOPENIA IN HCC
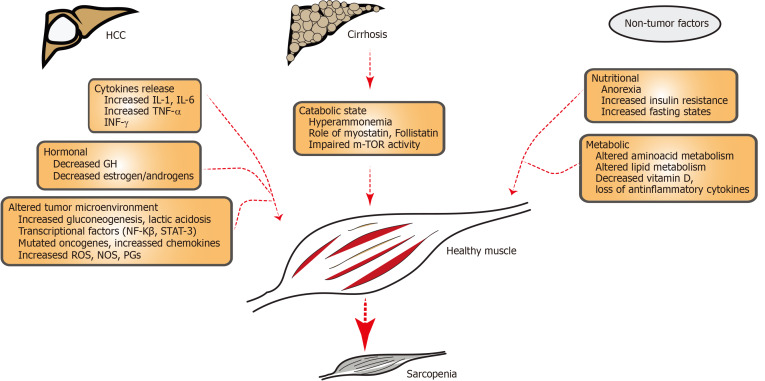
Sarcopenia is the condition characterized by loss of muscle strength, mass, and functional ability. The pathophysiology of this muscle loss can be multifactorial (hormonal, inflammatory, age-related, chronic liver and non-liver states, drug induced). Loss of muscle anabolic activity with nutritional deficiency can further worsen sarcopenia. Loss of skeletal muscle homeostasis especially between hypertrophy and regeneration can lead to sarcopenia. Most of the changes related to sarcopenia originates with normal aging process. A balance of muscle protein anabolic and catabolic pathways are responsible for muscle health. During sarcopenia, multiple cellular changes occur such as the reduction in myofibres (size and number), myosteatosis (development of intramuscular and intermuscular fat infiltration)[47], decreased number of type II fibre satellite cells. Further, loss of mitochondrial integrity, molecular signaling [IGF-1, mammalian target of rapamycin complex 1 (mTOR)], neurological (plaque formation, motor neuron loss), epigenetic change (modulated via microRNAs), endocrine factors (myostatin , osteocalcin and abnormal communication among them) and reactive oxygen species (ROS) imbalance[48] combined with reduced physical activity can all contribute to the muscle loss. Some of the frequent causes of sarcopenia are elucidated in Figure 1. Hyperammonemia, increased autophagy, decreased protein synthesis, abnormal mitochondrial activity, increased proteasomal activity, and low testosterone levels are also responsible for sarcopenia cirrhosis[49,50]. It is compounded by decreased metabolic substrates (especially branched-chain amino acids)[51], extrahepatic gluconeogenesis, and increased insulin resistance/pro-inflammatory cytokines (NFκB signaling, mTOR inhibition, enhanced apoptosis, eukaryotic initiation factor-2[52]. Portal hypertension-related complications and alcohol intake further worsen sarcopenia in cirrhosis[53,54].
Figure 1.
Schematic illustration showing factors contributing to sarcopenia in hepatocellular carcinoma and cirrhosis. Patients with hepatocellular carcinoma have increased release of cytokines, hormonal substances (GH, anabolic steroids) and altered tumor microenvironment (with hypercatabolic state, mutagenesis included by altered DNA, increased reactive oxygen species. Patients with HCC have underlying cirrhosis with hyperammonemia, decreased m-TOR activity which can contribute to sarcopenia. Non-tumor factors include poor nutrition and altered amino acid or lipid metabolism. HCC: Hepatocellular carcinoma; IL-1: Interleukin-1; IL-6: Interleukin-6; TNF-α: Tumor necrosis factor alfa; INF-γ: Interferon gamma; GH: Growth hormone; NF: Nuclear factor kappa B; STAT-3; Signal transducer and activator of transcription 3; ROS: Reactive oxygen species; NOS: Nitric oxide species; PGs: Prostaglandins; mTOR: Mechanistic target of rapamycin.
Early sarcopenia can be seen in HCC individuals[24,55]. Myokines (myostatin, IL-6, follistatin) are cytokines produced and secreted by muscle fibers and can exert paracrine/autocrine effects[33]. Myokines can exert immunological and anti-inflammatory effects and facilitate proinflammatory state of liver fibrosis, cirrhosis, and HCC development. Although myostatin levels in HCC have been a matter of debate, high IL-6 and follistatin levels had a significantly lower 5-year OS rate in HCC and were related to tumor progression by BCLC/TNM staging in HCC[33]. Follistatin is a glycoprotein and inhibitor of the TGF-β superfamily (such as myostatin, activin), and it can be related to tumor stage, size and can play an oncogenic role in hepatocarcinogenesis. These details provide important insights into potential agents such as myostatin inhibitors, mitochondrial protective agents, and antioxidants, which can be utilized for liver cirrhosis or HCC[55]. Such anti-sarcopenic treatments could be used to prolong or further reverse molecular, and metabolic changes noted in HCC patients.
CHANGES IN SARCOPENIA WITH HCC TREATMENTS
Sarcopenia in HCC patients undergoing various treatments (locoregional and systemic) has been shown to impact outcomes and survival. Multiple studies have reported outcomes among these patients. It has been showed that a baseline sarcopenia is associated with lack of response to HCC treatments, further decompensation episodes, and increased mortality[56]. In the following sections, we elaborate on studies evaluating the role of sarcopenia in HCC patients with various treatments such as loco-regional, surgery, transplant, and chemo/immunotherapy.
LOCO-REGIONAL THERAPY
Patients with HCC can be candidates for multiple loco-regional treatment (LRT) options such as radiotherapy, chemoembolization, radioembolization. Data on sarcopenic predicting response to LRT is sparse (Table 1)[14]. Iritani et al[15] reported 217 HCC patients on LRT and evaluated the role of sarcopenia. In this study, L3 skeletal muscle index (SMI) was used to define sarcopenia. Patients with low L3 SMI showed a significantly lower OS compared to those without sarcopenia (P = 0.004). Further, obese sarcopenic patients died earlier (P = 0.013)[15]. In 2015, Fujiwara et al[57] showed a higher risk of HCC recurrence in sarcopenic patients in 515 patients with BCLC stage 0/A who underwent percutaneous radiofrequency ablation (RFA). In 2017, a retrospective study of 182 patients with HCC undergoing percutaneous RFA therapy with curative intent was analyzed[58]. Patient with sarcopenia decreased pretreatment psoas muscle index (PMI) survival (overall cumulative survival) was 51.5% compared to 86.5% without sarcopenia (P < 0.0001. In addition to sarcopenia, total bilirubin ≥ 1.2 mg/dL, des-γ-carboxy prothrombin ≥ 34 mAU/mL (P = 0.009) were found to be adverse predictors of OS [58]. These findings were irrespective of CTP score or achievement of SVR in HCV-related HCC. Furthermore, above findings indicate the usefulness of sarcopenia to assess outcomes of HCC patients undergoing RFA.
Table 1.
Outcomes of hepatocellular carcinoma patients undergoing loco-regional therapy with sarcopenia
|
Ref.
|
Technique
|
n
|
Methods and outcomes
|
| RFA | |||
| Iritani et al[15] (2012-2014, Japan) | RFA | 217 | L3-SMI. B36.0 cm2/m2 for men and B29.0 cm2/m2 for women. Sarcopenia patients had lower OS than those without |
| Fujiwara et al[57] (2015, Japan) | RFA | 515 | L3-SMI used. B36.2 cm2/m2 for men and B29.6 cm2/m2 for women. Sarcopenia was associated with a higher risk of recurrence in very early/early-stage HCC who underwent treatment with RFA. |
| Yuri et al[58] (2017, Japan) | RFA | 182 | PMI used. 6.36 cm2/m2 for men and 3.92 cm2/m2 for women. Sarcopenia was associated with overall reduced HCC survival with no effect on recurrence. |
| TACE | |||
| Dodson et al[38] (2013, United States) | TACE drug eluding TACE | 216 | TPA was used to assess sarcopenia. TPA of < 477 mm/m2 for men and < 338 mm/m2 for woman. Sarcopenia was independently associated with increased risk of death (lowest vs highest TPA quartile, HR = 1.84; P = 0.04) |
| Kobayashi et al[60] (2018, Japan) | TACE | 102 | L3-SMI used. 42 cm2/m2 for men and 38 cm2/m2 for women. Change in L3-SMI was an independent prognostic factor in patients with HCC treated with TACE. |
| Loosen et al[61] (2019, Germany) | TACE | 56 | Mean PMI was 11.81 mm/m2. Low PMI (13.39 mm/m2) had significantly lower median overall survival (491 d) compared to high PMI (1291 d) |
| Fujita et al[59] (2019, Japan) | TACE | 179 | PMI used. < 6.0 cm2/m2 for men and < 3.4 cm2/m2 for women. No difference was normal with low PMI and normal PMI for HCC outcomes. However, changes in PMI were significant after TACE with significant loss of liver function reserves post treatment. |
| TARE | |||
| Faron et al[32] (2020, Europe) | TARE | 58 | MRI derived FFMA were used to predict sarcopenia. FFMA < 3582 mm2 for men and < 2301 mm2 for men. Low FFMA was associated with significantly reduced OS (197 vs 294, P = 0.02). |
Studies depicting various loco-regional treatments utilized in hepatocellular carcinoma in relation to sarcopenia. RFA: Radiofrequency Ablation; TACE: Transarterial chemoembolization; TARE: Transarterial radiofrequency embolization; L3-SMI: Third lumbar vertebrae-skeletal muscle index; OS: Overall survival; HCC: Hepatocellular carcinoma; TPA: Total psoas area; FFMA: Fat-free muscle area; PMI: Psoas muscle index.
Trans-arterial interventions for the HCC can be chemoembolization (TACE) or radioembolization (TARE) and are increasingly being utilized for large or multifocal disease with metastasis or macrovascular invasion[27]. The available data are conflicting about the role of sarcopenia as a predictor of survival in HCC who underwent TACE (Table 1). Fujita et al[59] and Kobayashi et al[60] showed no significant association between muscle volume at baseline and clinical outcomes. On the contrary, Loosen et al[61] and Dodson et al[38] showed that pre-interventional sarcopenia was associated with poor outcomes. Significant heterogeneity was noted in the methods to evaluate sarcopenia in these studies. The total psoas area (TPA), PMI, and L3-SMI were used to evaluate the presence of sarcopenia. If sarcopenia directed these effects (on the TACE efficacy) beyond the patients’ general clinical condition or if this is mere an association, needs further evaluation in a prospective fashion. Data on the effects of sarcopenia on HCC patients with TARE is even more limited. Recently, Faron et al[32] reported 58 HCC patients using MRI-derived fat-free muscle area (FFMA) to predict sarcopenia. The FFMA < 3582 mm2 for men and < 2301 mm2 for women were used. In this study low FFMA was associated with significantly reduced OS (197 vs 294, P = 0.02)[32].
SURGICAL TREATMENTS
Liver resection
The role of sarcopenia in HCC patients undergoing liver resection is increasingly become topic of interest. Since, HCC patients often have poor nutritional status, methods to reduce the catabolic state and improve protein synthesis, regeneration, Fan et al[62] investigated 124 patients to evaluate the role of nutrition in HCC resection. Nutrition therapy given prior to the liver resection with branched chain amino acids (BCAA), lipids, and dextrose have shown to decrease the worsening of liver function, sepsis-related complications, need for treatment for ascites, and overall decreased mortality. There was a reduction in the overall post-operative morbidity in the nutrition group compared to the control group (34% vs 55%; relative risk, 0.66; 95%CI: 0.45-0.96)[62]. In 2013, Harimoto and colleagues[63] studied 186 HCC patients with sarcopenia using L3-SMI (< 43.75 for men, < 41.10 for women), and a significant correlation was noted between sarcopenia and liver dysfunction (indicated by low albumin levels and indocyanine green retention). In patients with and without sarcopenia, the 5-year OS rate was 71% and 83·7%, and the 5-year recurrence-free survival rate was 13% and 33·2%, respectively[63]. Additionally, studies evaluated the relationship between total functional liver volume (TFLV) and sarcopenia (L3-SMI) and found that median TFLV was significantly lower in the sarcopenic group than the normal group (1296 mL vs 1840 mL; P < 0.05)[64].
Sarcopenic obesity characterized by increased fat volume compared to skeletal muscle mass. As obesity and loss of muscle share common pathophysiological mechanisms, combined insult could display a poor outcome. Studies evaluated the effect of sarcopenic obesity in HCC and found that patients with sarcopenic obesity had worse median survival (84.7 mo vs 39.1 mo, P = 0.002) and worse median recurrence-free survival (21.4 mo vs 8.4 mo, P = 0.003)[21]. Additionally, it was identified as an independent risk factor for death and HCC recurrence[21]. Effect of sarcopenia on immediate and short-term clinical outcomes after hepatic resection was examined by Otsuji et al[65] Sarcopenic patients had a higher postoperative length of stay, higher rates of liver failure, major complications, and intra-abdominal abscess formation (Table 2). Multiple other studies have provided similar results with different modalities to evaluate sarcopenia, such as L3-SMI, TPA, and visceral-to-subcutaneous adipose tissue ratio (Table 2). Furthermore, these studies used differing SMI cut-off points to define sarcopenia. The majority of the studies point to poor outcomes in patients with sarcopenia, which might be due to the underlying liver dysfunction and HCC severity. Nevertheless, prospective data with uniform cut-off points to assess SMI to define sarcopenia in these studies to provide concrete evidence of the relationship between sarcopenia and liver resection.
Table 2.
Outcomes of hepatocellular carcinoma patients undergoing liver resection (hepatectomy) with sarcopenia over last 5 years
|
Ref.
|
Technique
|
n
|
Methods and outcomes
|
| Otsuji et al[65] (2015, Japan) | Major hepatectomy and extrahepatic bile resection | 256 | Total psoas area (TPA) was used to assess sarcopenia. TPA of < 567 mm/m2 for men and < 395 mm/m2 for woman. Length of postoperative hospital stay were longer (39 d vs 30 d, P < 0.001, high rate of liver failure (33% vs 16%), major complications (54% vs 37%), intra-abdominal abscess (29% vs 18% compared to those without sarcopenia (P < 0.05)[69]. |
| Voron et al[110] (2015, Japan) | Hepatectomy | 198 | L3-SMI used 52.4 cm2/m2 for men and 38.9 cm2/m2 for women. Sarcopenia was associated with shorter median OS (52.3 mo vs 70.3 mo; P = 0.01 and it was an independent predictor of OS and DFS. |
| Yabusaki et al[111] (2016, Japan) | Primary hepatectomy | 195 | SMI used 43.75 cm2/m2 for men and 41.10 cm2/m2 for women. Sarcopenia was associated with poor cumulative recurrence rate (P = 0.13). |
| Takagi et al[113] (2016, Japan) | Curative hepatectomy | 254 | L3-SMI used 46.4 cm2/m2 for men and 37.6 cm2/m2 for women. The sarcopenic group had a significantly lower 5-yr OS rate than the non-sarcopenic group (58.2% vs 82.4%, P = 0.0002). Further it was an independent predictor of poor survival (HR =2.28, P = 0.002) and poor ASA status (HR = 3.17, P = 0.001). |
| Kobayashi et al[21] (2019, Japan) | Hepatectomy | 465 | L3-SMI used. 40.31 cm2/m2 for men and 30.88 cm2/m2 for women. Sarcopenic obesity as a significant risk factor for mortality (HR = 2.504, P = 0.005) and recurrence of HCC (HR = 2.031, P = 0.006) after hepatectomy for HCC. |
| Hamaguchi et al[112] (2019, Japan) | Hepatectomy | 606 | L3-SMI was used to assess the sarcopenia. SMI of < 40.31 for men and 30.88 for women were used. A high visceral-to-subcutaneous adipose tissue ratio, low SMI, and high IMAC contributed to an increased risk of death (P < 0.001) and HCC recurrence (P < 0.001) in an additive manner. |
| Xu et al[22] (2020, China) | Hepatectomy | 1420 | Authors performed a meta-analysis of six studies and preoperative sarcopenia was significantly associated with poor OS (HR =1.58, 95%CI: 1.34-1.84, P = 0) and shorter DFS (HR =1.54, 95%CI: 1.17-2.02, P = 0.002) in patients with HCC undergoing hepatectomy[24]. |
Studies, techniques and outcomes to evaluate the success of liver resection in patients with sarcopenia and hepatocellular carcinoma. L3-SMI: Third lumbar vertebrae- skeletal muscle Index; OS: Overall survival; SMI: Skeletal muscle index; HR: Hazards ratio; DFS: Disease free survival. HCC: Hepatocellular carcinoma; TPA: Total psoas area; IMAC: Intramuscular adipose tissue content; PMI: Psoas muscle index.
Liver transplantation
Sarcopenia in patients awaiting liver transplantation (LT), perioperative and postoperative outcomes have been studied recently[66-71]. Multiple methods to assess sarcopenia were used (Table 3). For example, L3-SMI, psoas muscle thickness, MELD-sarcopenia score, skeletal muscle mass-to-visceral fat area ratio (SVR), TPA, PMA, and height normalized psoas muscle thickness were used. Among these, L3-SMI is the most commonly used objective way of assessing sarcopenia. Further, studies evaluated the wait times and survival related to sarcopenia (Table 3).
Table 3.
Outcomes of hepatocellular carcinoma patients undergoing liver transplant with sarcopenia over last 5 years
|
Ref.
|
Technique
|
n
|
Methods and outcomes
|
| Itoh et al[114] (2016, Japan) | Living-donor LT | 153 | Based on SVR, patients with low SVR were had poor prognosis than without low SVR for OS (P = 0.03) and recurrence-free survival (P = 0.01). |
| Carey et al[68] (2016, United States) | Awaiting LT | 396 | L3-SMI used. 50 cm2/m2 for men and 39 cm2/m2 for women. Patients who died had lower SMI compared to those who survived (45.6 cm2/m2 vs 48.5 cm2/m2; P < 0.001), and SMI was associated with wait-list mortality (HR, 0.95; P < 0.001)[72]. |
| Wada et al[67] (2017, Japan) | LDLT | 32 | TPA was used. TPA of 791.6 mm2/m2 for men and 488.8 mm2/m2 for women. TPV was used to compare to TPA. Preoperative TPV is a better predictor compared to TPA in assessing post-operative risks in LDLT recipients[71]. |
| Golse et al[70] (2017, Europe) | LT | 256 | PMA, L3-SMI was used. 1561 mm2 for men and 1464 mm2 for women. One and 5-yr OS rates were significantly poorer in the sarcopenic group than in the nonsarcopenic group at 59% vs 94% and 54% vs 80%, respectively (P < 0.001). Authors concluded that pre-LT PMA might be predict 1-yr survival post-LT[74]. |
| Van Vugt et al[69] (2017, Europe) | Listed for LT | 585 | L3-SMI used. 43 to 53 cm2/m2 for men based on the BMI and 41 cm2/m2 for women. Sarcopenia was associated with waiting list mortality in liver transplant candidates with cirrhosis, particularly in patients with lower MELD scores (P < 0.001) [73]. |
| Kim et al[71] (2018, Japan) | LDLT | 92 | Height normalized psoas muscle thickness (< 15.5 mm/m) at L3. HCC recurrence risk was greater in sarcopenic patients in univariable analysis [HR = 8.06 (1.06–16.70), P = 0.044) and in multivariable analysis [HR = 9.49 (1.18–76.32), P = 0.034][75]. |
| Chae et al[66] (2018, South Korea) | LDLT | 408 | This study investigated the association between a perioperative decrease in the PMI and patient mortality after LT. A PMI decrease ≤-11.7% between the day before surgery and POD-7 was an independent predictor of patient mortality after LT[70]. |
Techniques, methods and outcomes to evaluate the success of liver transplantation in patients with sarcopenia and hepatocellular carcinoma. LT: Liver transplant; LDLT: Living-donor LT; SVR: Skeletal muscle mass-to-Visceral fat area ratio; TPV: Total psoas volume; PMA: Psoas muscle area; BMI: Body mass index; L3-SMI: Third lumbar vertebrae- skeletal muscle index; OS: Overall survival; SMI: Skeletal muscle index; HR: Hazards ratio; DFS: Disease free survival; HCC: Hepatocellular carcinoma; TPA: Total psoas area; PMI: Psoas muscle index.
Studies performed on outcomes in LT patients evaluated the preoperative status of the patients (listed and waiting for the transplant), procedural outcomes and post-procedure long-term survival. Carey et al[68] in 2016 used L3-SMI with 50 cm2/m2 for men and 39 cm2/m2 for women and noted that individuals who died had lower SMI compared to those who survived (45.6 cm2/m2 vs 48.5 cm2/m2; P < 0.001), and SMI was associated with wait-list mortality (HR, 0.95; P < 0.001). Wada et al[67] in 2017 considered sarcopenia for TPA of 791.6 mm2/m2 for men and 488.8 mm2/m2 for women. The authors compared TPV to TPA. The preoperative total psoas volume (TPV) was found to be a better predictor than TPA in assessing post-operative risks in living-donor LT recipients[67]. Multiple studies evaluated the LT outcomes and complications such as infections, length of stay, failure to rescue, and surgery-related events[72,73]. The rate of infections was assessed and compared to individuals with sarcopenia. Patients with sarcopenia had a higher prevalence of sepsis, bacterial pneumonia, longer ICU stays, and mortality[2,69]. Postoperative survival was studied by Van Vugt et al[69] and Kaido et al[72] who noted that sarcopenia was inversely associated with clinical outcomes after LT. Few studies noted sarcopenia developing after the LT, which is probably due to underestimation of muscle mass/strength estimation before LT. In addition to underlying cirrhosis, increased catabolism, tumor-related morbidity noted in these patients, the role of immunosuppressant use cannot be underestimated. The use of mTOR and calcineurin inhibitors can potentially lead to sarcopenia[74]. Further, renal dysfunction caused by calcineurin inhibitors can compound these effects. The results of these studies provide an opportunity for improving the nutritional status in sarcopenia LT patients with dietary and exercise measures during pre, peri and post-operative period.
Systemic therapies
The use of chemotherapy and immunotherapy has become the mainstay of treatment for HCC lesions that are not amenable to LRT or LT. Sorafenib is the most studied and prescribed chemotherapeutic agent in HCC[75]. Although it can prolong survival, its use is limited by its adverse effects such as nausea, excessive fatigue, and diarrhea noted in most patients. These studies evaluated multiple outcomes such as OS, progression-free survival, mortality were evaluated in different studies in HCC patients receiving Sorafenib therapy[76-82]. While the ways to assess the sarcopenia differed in these studies, most commonly used method is L3-SMI. Further various cut-off values were utilized in these studies.
Nishikawa et al[78] studied 232 patients to evaluate for OS using L3-SMI. The authors noted that the patients with sarcopenia had significantly low median OS of 174 d compared to 454 d in the non-sarcopenic group (P < 0.0001). Multivariate analysis showed that sarcopenia was an independent predictor of OS. Similarly, Takada et al[81] studied 214 patients in which OS in pre-sarcopenia patients were worse than without pre-sarcopenia (median 252 d vs 284 d, respectively; P = 0.16). Saeki et al[82] reported 100 advanced HCC patients using use of L3-SMI showing individuals without muscle depletion had longer survival was noted (HR = 0.50, P = 0.006). This combined with low tumor number (< 7) and lack of extrahepatic spread offered better survival in these patients[82]. Dynamic assessment of sarcopenia has assisted to compare outcomes before and after starting sorafenib. Few studies noted that sarcopenia worsened after the initiation of sorafenib. If this is due to the progression of HCC or angiogenic (or Carnitine inhibitory) properties of sorafenib needs further evaluation[83]. Further, Cheng et al[34] reported that pre-sarcopenia could independently predict the outcomes in sorafenib-failed HCC.
Use of other modalities such as fat mass indices (visceral, subcutaneous) in combination with L3-SMI and their relative changes (over a period of time) can assist in assessing sarcopenia and can predict outcomes in HCC patients receiving sorafenib[82]. However, more studies are needed to confirm these findings. Recently newer agents for HCC are increasingly utilized such as Regorafenib, Lenvatinib, Nivolumab, the combination of gemcitabine and oxaliplatin (GEMOX regimen)[30,84-86]. Studies showing the effect of sarcopenia on HCC patients' survival using these agent are sparse. Lenvatinib induces minimal muscle loss after 2 years of treatment correlates with its low toxicity[23,30,87]. Combined effects of sarcopenia and inflammation (by high neutrophil-to-lymphocyte ratio and absolute lymphocyte count) have been studied in patients receiving nivolumab in HCC patients[28]. If inflammatory markers are more important than sarcopenia in patients received immunotherapy needs further validation[25,28]. Overall, sarcopenia can predict survival in advanced HCC patients receiving chemotherapeutics such as sorafenib before initiation of the treatment and during and after the treatment. Strategies to improve the muscle mass, nutrition can add to the survival in these patients. Further studies are needed to evaluate the role of sarcopenia for new chemotherapy and for immunotherapy.
METHODS TO IMPROVE SARCOPENIA
As sarcopenia can adversely affect the outcomes of HCC patients undergoing treatments, methods to improve could impact the survival of these patients. As HCC happens with a background of cirrhosis in up to 80%-90% of patients, improving sarcopenia in cirrhotics could assist in improving survival. Reversing pathophysiology by improving myofibres size, number, reversing myosteatosis, inhibiting mitochondrial integrity loss, mTOR signaling, and decreasing ROS accumulation can improve sarcopenia in both HCC and cirrhotics. Two major strategies exist to improve sarcopenia in these patients- nutritional support and physical exercise. Use of L-carnitine, BCAA, leucine have been used in the studies to increase the nutritional component[88,89]. Improvement of skeletal mass (PMI) was noted after the supplementation of these agents in these studies. Physical exercise can recruit more myofibres and at least inhibit sarcopenia. It is unclear if it can reverse the sarcopenia completely. Both isometric (lifting hand weights 2-3 times per week) and isotonic (30-40 min walking 3-4 times per week) have been used to improve muscle strength in these patients[90-94]. Studies have shown an increased muscle cross-sectional area (quadriceps) with exercise in cirrhotics of at least 10%[95]. Although, testosterone supplementation have been reported to improve the sarcopenia, few reports of alpha-alkylated formulation could theoretically increase the risk of HCC formation[96].
The role of non-steroidal Selective Androgen Receptor Modulators (SARMs) is increasingly being recognized in the treatment of sarcopenia[97-99]. SARMs inhibit protein degradation and thereby could decrease the rate of sarcopenia. Multiple animal models have been used to evaluate mechanisms of SARMs to reverse muscle atrophy in degonadized mice. For instance, SARM treatment in ovariectomized rat model can increase muscle mass by enhanced mitochondrial biogenesis, actin and myosin[98]. SARMs can target androgen receptors and decrease sarcopenia via paracrine growth factor signaling on vimentin positive muscle fibroblasts[97]. Further, upregulation of mTOR, glycogen synthase kinase[99]. SARMs also exhibit anabolic effects, increasing the bone and muscle mass which are affected in patients with HCC. A combination approach of nutritional supplementation with physical exercise with a multidisciplinary approach has been tried in cirrhotics and HCC patients[31]. Significant changes in muscle volume was noted after the intervention[95]. Similarly, a combined approach has been tried in a few studies in HCC patients undergoing TACE[100,101]. This approach has been studied in patients waiting or LT, with good response[102,103]. In conclusion, a combined multidisciplinary approach is useful and logical to improve the sarcopenia in cirrhotics and HCC which might eventually improve outcomes of these patients undergoing local, surgical and systemic therapies.
FUTURE DIRECTIONS
Although sarcopenia can offer significant details about the functional status, it can be further enhanced by the use of frailty (using clinical frailty scale, liver frailty index, Karnofsky performance status) and amount of malnutrition (by assessment of BMI, nutritional intake). These can be incorporated into composite scoring to better evaluate the functional status of HCC patients. Recently use of changes in bone resorption via upregulation of inflammatory cytokines opened the concept of sarcopenic osteoporosis[104]. A crosstalk between skeletal muscle, bone homeostatic changes with underlying cirrhosis and HCC can provide pathways for treatments in the future. Myostatin, irisin, osteocalcin, activation of Wnt/β-catenin pathways have been implicated in sarcopenic osteoporosis. Furthermore, biomarkers such as imbalance of plasma free amino acids (BCAA) have been implicated in progression of HCC[105]. If this could be a reliable way to improve the sarcopenia in HCC patients remains to be studied.
Precision medicine tools such as use of radiomics and radiogenomics are emerging for assessing host and tumor-related risk factors in HCC[106,107]. Radiomics uses medical imaging data to develop reproducible quantitative data from qualitative images. This has been utilized for lung cancer assessment of tumor and non-tumor tissue[108]. Development of methods to quantify the amount of normal non-tumor liver tissue in HCC patients is essential for surgeons to evaluate resection strategies. Seror et al[109] noted that use of non-invasive cross-sectional imaging to assess the liver surface nodularity and lean body mass can act as surrogate markers for liver cirrhosis and sarcopenia. Patients with higher liver surface nodularity (OR 7.05, 95%CI: 2.13-23.25) and sarcopenia (OR 6.51, 95%CI: 2.08-20.39) were associated with high risk of complications[109]. A step further in this direction, use of genomics (cellular and molecular changes) to existing radiomics can provide radiogenomic information which can be used to develop molecular signatures for development for actionable clinical targets[107]. Finally use of artificial intelligence and deep learning can lead to next generation biostatistical and informatic data to develop algorithms and pathways to identify optimal clinical patterns[106].
CONCLUSION
Sarcopenia is increasingly recognized as a predictive marker for assessing outcomes in HCC patients. There is increasing evidence to evaluate its role in loco-regional, surgical, transplant, and systemic treatment options in HCC patients. Early recognition to identify sarcopenia, methods to improve the muscle volume, strength, and mass could impact the patient outcome and OS. The use of appropriate nutritional support, physical activity or both could potentially improve muscle volume in these patients. However, it is unclear about the degree of improvement of the sarcopenia with all of these measurement combined. Further, prospective studies aimed at interventions that could potentially reverse sarcopenia to improve HCC patients' outcomes are needed in the future.
Footnotes
Conflict-of-interest statement: None of the authors have no conflicts of interest.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: American College of Gastroenterology; American Society of Gastrointestinal Endoscopy; and American Gastroenterological Association.
Peer-review started: April 19, 2021
First decision: June 23, 2021
Article in press: January 11, 2022
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ogundipe OA S-Editor: Gong ZM L-Editor: A P-Editor: Gong ZM
Contributor Information
Abhilash Perisetti, Department of Internal Medicine, Gastroenterology and Hepatology Division, University of Arkansas for Medical Sciences, Little Rock, AR 72205, United States; Department of Interventional Oncology and Surgical Endoscopy, Parkview Health, Fort Wayne, IN 46825, United States. abhilash.perisetti@gmail.com.
Hemant Goyal, Department of Internal Medicine, The Wright Center for Graduate Medical Education, The Wright Center for Graduate Medical Education, Scranton, PA 18501, United States.
Rachana Yendala, Department of Hematology and Oncology, Conway Regional Medical Center, Conway, AR 72034, United States.
Saurabh Chandan, Department of Internal Medicine, Gastroenterology and Hepatology Division, CHI Creighton University Medical Center, Omaha, NE 68107, United States.
Benjamin Tharian, Department of Internal Medicine, Gastroenterology and Hepatology Division, University of Arkansas for Medical Sciences, Little Rock, AR 72205, United States.
Ragesh Babu Thandassery, Department of Medicine, Central Arkansas Veterans Healthcare System, Little Rock, AR 72205, United States.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Lee DH, Lee JM. Primary malignant tumours in the non-cirrhotic liver. Eur J Radiol. 2017;95:349–361. doi: 10.1016/j.ejrad.2017.08.030. [DOI] [PubMed] [Google Scholar]
- 3.Cabibbo G, Enea M, Attanasio M, Bruix J, Craxì A, Cammà C. A meta-analysis of survival rates of untreated patients in randomized clinical trials of hepatocellular carcinoma. Hepatology. 2010;51:1274–1283. doi: 10.1002/hep.23485. [DOI] [PubMed] [Google Scholar]
- 4.Llovet JM, Peña CE, Lathia CD, Shan M, Meinhardt G, Bruix J SHARP Investigators Study Group. Plasma biomarkers as predictors of outcome in patients with advanced hepatocellular carcinoma. Clin Cancer Res. 2012;18:2290–2300. doi: 10.1158/1078-0432.CCR-11-2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vitale A, Farinati F, Noaro G, Burra P, Pawlik TM, Bucci L, Giannini EG, Faggiano C, Ciccarese F, Rapaccini GL, Di Marco M, Caturelli E, Zoli M, Borzio F, Sacco R, Cabibbo G, Virdone R, Marra F, Felder M, Morisco F, Benvegnù L, Gasbarrini A, Svegliati-Baroni G, Foschi FG, Olivani A, Masotto A, Nardone G, Colecchia A, Fornari F, Marignani M, Vicari S, Bortolini E, Cozzolongo R, Grasso A, Aliberti C, Bernardi M, Frigo AC, Borzio M, Trevisani F, Cillo U Italian Liver Cancer (ITA. LI.CA) group. Restaging Patients With Hepatocellular Carcinoma Before Additional Treatment Decisions: A Multicenter Cohort Study. Hepatology. 2018;68:1232–1244. doi: 10.1002/hep.30185. [DOI] [PubMed] [Google Scholar]
- 6.Rosenberg IH. Summary comments. Am J Clin Nutr . 1989;50:1231–1233. [Google Scholar]
- 7.Brown M, Hasser EM. Complexity of age-related change in skeletal muscle. J Gerontol A Biol Sci Med Sci. 1996;51:B117–B123. doi: 10.1093/gerona/51a.2.b117. [DOI] [PubMed] [Google Scholar]
- 8.Thomas DR. Loss of skeletal muscle mass in aging: examining the relationship of starvation, sarcopenia and cachexia. Clin Nutr. 2007;26:389–399. doi: 10.1016/j.clnu.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 9.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel JP, Rolland Y, Schneider SM, Topinková E, Vandewoude M, Zamboni M European Working Group on Sarcopenia in Older People. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen LK, Liu LK, Woo J, Assantachai P, Auyeung TW, Bahyah KS, Chou MY, Chen LY, Hsu PS, Krairit O, Lee JS, Lee WJ, Lee Y, Liang CK, Limpawattana P, Lin CS, Peng LN, Satake S, Suzuki T, Won CW, Wu CH, Wu SN, Zhang T, Zeng P, Akishita M, Arai H. Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J Am Med Dir Assoc. 2014;15:95–101. doi: 10.1016/j.jamda.2013.11.025. [DOI] [PubMed] [Google Scholar]
- 11.Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, Cooper C, Landi F, Rolland Y, Sayer AA, Schneider SM, Sieber CC, Topinkova E, Vandewoude M, Visser M, Zamboni M Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2), and the Extended Group for EWGSOP2. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48:601. doi: 10.1093/ageing/afz046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen LK, Woo J, Assantachai P, Auyeung TW, Chou MY, Iijima K, Jang HC, Kang L, Kim M, Kim S, Kojima T, Kuzuya M, Lee JSW, Lee SY, Lee WJ, Lee Y, Liang CK, Lim JY, Lim WS, Peng LN, Sugimoto K, Tanaka T, Won CW, Yamada M, Zhang T, Akishita M, Arai H. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J Am Med Dir Assoc. 2020;21:300–307.e2. doi: 10.1016/j.jamda.2019.12.012. [DOI] [PubMed] [Google Scholar]
- 13.Studenski SA, Peters KW, Alley DE, Cawthon PM, McLean RR, Harris TB, Ferrucci L, Guralnik JM, Fragala MS, Kenny AM, Kiel DP, Kritchevsky SB, Shardell MD, Dam TT, Vassileva MT. The FNIH sarcopenia project: rationale, study description, conference recommendations, and final estimates. J Gerontol A Biol Sci Med Sci. 2014;69:547–558. doi: 10.1093/gerona/glu010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yeh WS, Chiang PL, Kee KM, Chang CD, Lu SN, Chen CH, Wang JH. Pre-sarcopenia is the prognostic factor of overall survival in early-stage hepatoma patients undergoing radiofrequency ablation. Medicine (Baltimore) 2020;99:e20455. doi: 10.1097/MD.0000000000020455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iritani S, Imai K, Takai K, Hanai T, Ideta T, Miyazaki T, Suetsugu A, Shiraki M, Shimizu M, Moriwaki H. Skeletal muscle depletion is an independent prognostic factor for hepatocellular carcinoma. J Gastroenterol. 2015;50:323–332. doi: 10.1007/s00535-014-0964-9. [DOI] [PubMed] [Google Scholar]
- 16.Aniansson A, Grimby G, Rundgren A. Isometric and isokinetic quadriceps muscle strength in 70-year-old men and women. Scand J Rehabil Med. 1980;12:161–168. [PubMed] [Google Scholar]
- 17.Astrand I, Astrand PO, Hallbäck I, Kilbom A. Reduction in maximal oxygen uptake with age. J Appl Physiol. 1973;35:649–654. doi: 10.1152/jappl.1973.35.5.649. [DOI] [PubMed] [Google Scholar]
- 18.Shachar SS, Williams GR, Muss HB, Nishijima TF. Prognostic value of sarcopenia in adults with solid tumours: A meta-analysis and systematic review. Eur J Cancer. 2016;57:58–67. doi: 10.1016/j.ejca.2015.12.030. [DOI] [PubMed] [Google Scholar]
- 19.Baracos VE. Management of muscle wasting in cancer-associated cachexia: understanding gained from experimental studies. Cancer. 2001;92:1669–1677. doi: 10.1002/1097-0142(20010915)92:6+<1669::aid-cncr1495>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 20.Pamoukdjian F, Bouillet T, Lévy V, Soussan M, Zelek L, Paillaud E. Prevalence and predictive value of pre-therapeutic sarcopenia in cancer patients: A systematic review. Clin Nutr. 2018;37:1101–1113. doi: 10.1016/j.clnu.2017.07.010. [DOI] [PubMed] [Google Scholar]
- 21.Kobayashi A, Kaido T, Hamaguchi Y, Okumura S, Shirai H, Yao S, Kamo N, Yagi S, Taura K, Okajima H, Uemoto S. Impact of Sarcopenic Obesity on Outcomes in Patients Undergoing Hepatectomy for Hepatocellular Carcinoma. Ann Surg. 2019;269:924–931. doi: 10.1097/SLA.0000000000002555. [DOI] [PubMed] [Google Scholar]
- 22.Xu L, Jing Y, Zhao C, Zhang Q, Zhao X, Yang J, Wu L, Yang Y. Preoperative computed tomography-assessed skeletal muscle index is a novel prognostic factor in patients with hepatocellular carcinoma following hepatectomy: a meta-analysis. J Gastrointest Oncol. 2020;11:1040–1053. doi: 10.21037/jgo-20-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Uojima H, Chuma M, Tanaka Y, Hidaka H, Nakazawa T, Iwabuchi S, Kobayashi S, Hattori N, Ogushi K, Morimoto M, Kagawa T, Tanaka K, Kako M, Koizumi W. Skeletal Muscle Mass Influences Tolerability and Prognosis in Hepatocellular Carcinoma Patients Treated with Lenvatinib. Liver Cancer. 2020;9:193–206. doi: 10.1159/000504604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takada H, Amemiya F, Yasumura T, Yoda H, Okuwaki T, Imagawa N, Shimamura N, Tanaka K, Kadokura M, Maekawa S, Enomoto N. Relationship between presarcopenia and event occurrence in patients with primary hepatocellular carcinoma. Sci Rep. 2020;10:10186. doi: 10.1038/s41598-020-67147-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qayyum A, Bhosale P, Aslam R, Avritscher R, Ma J, Pagel MD, Sun J, Mohamed Y, Rashid A, Beretta L, Kaseb AO. Effect of sarcopenia on systemic targeted therapy response in patients with advanced hepatocellular carcinoma. Abdom Radiol (NY) 2021;46:1008–1015. doi: 10.1007/s00261-020-02751-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marasco G, Serenari M, Renzulli M, Alemanni LV, Rossini B, Pettinari I, Dajti E, Ravaioli F, Golfieri R, Cescon M, Festi D, Colecchia A. Clinical impact of sarcopenia assessment in patients with hepatocellular carcinoma undergoing treatments. J Gastroenterol. 2020;55:927–943. doi: 10.1007/s00535-020-01711-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lanza E, Masetti C, Messana G, Muglia R, Pugliese N, Ceriani R, Lleo de Nalda A, Rimassa L, Torzilli G, Poretti D, D'Antuono F, Politi LS, Pedicini V, Aghemo A Humanitas HCC Multidisciplinary Group. Sarcopenia as a predictor of survival in patients undergoing bland transarterial embolization for unresectable hepatocellular carcinoma. PLoS One. 2020;15:e0232371. doi: 10.1371/journal.pone.0232371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim N, Yu JI, Park HC, Yoo GS, Choi C, Hong JY, Lim HY, Lee J, Choi MS, Lee JE, Kim K. Incorporating sarcopenia and inflammation with radiation therapy in patients with hepatocellular carcinoma treated with nivolumab. Cancer Immunol Immunother. 2021;70:1593–1603. doi: 10.1007/s00262-020-02794-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim JH, Kang SH, Lee M, Youn GS, Kim TS, Jun BG, Kim MY, Kim YD, Cheon GJ, Kim DJ, Baik SK, Choi DH, Suk KT. Serum Myostatin Predicts the Risk of Hepatocellular Carcinoma in Patients with Alcoholic Cirrhosis: A Multicenter Study. Cancers (Basel) 2020;12 doi: 10.3390/cancers12113347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hiraoka A, Kumada T, Kariyama K, Tada T, Tani J, Fukunishi S, Atsukawa M, Hirooka M, Tsuji K, Ishikawa T, Takaguchi K, Itobayashi E, Tajiri K, Shimada N, Shibata H, Ochi H, Kawata K, Yasuda S, Toyoda H, Ohama H, Nouso K, Tsutsui A, Nagano T, Itokawa N, Hayama K, Arai T, Imai M, Koizumi Y, Nakamura S, Joko K, Michitaka K, Hiasa Y, Kudo M Real-life Practice Experts for HCC (RELPEC) Study Group and HCC 48 Group (hepatocellular carcinoma experts from 48 clinics in Japan) Clinical importance of muscle volume in lenvatinib treatment for hepatocellular carcinoma: Analysis adjusted with inverse probability weighting. J Gastroenterol Hepatol. 2021;36:1812–1819. doi: 10.1111/jgh.15336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hashida R, Kawaguchi T, Koya S, Hirota K, Goshima N, Yoshiyama T, Otsuka T, Bekki M, Iwanaga S, Nakano D, Niizeki T, Matsuse H, Kawaguchi A, Shiba N, Torimura T. Impact of cancer rehabilitation on the prognosis of patients with hepatocellular carcinoma. Oncol Lett. 2020;19:2355–2367. doi: 10.3892/ol.2020.11345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Faron A, Sprinkart AM, Pieper CC, Kuetting DLR, Fimmers R, Block W, Meyer C, Thomas D, Attenberger U, Luetkens JA. Yttrium-90 radioembolization for hepatocellular carcinoma: Outcome prediction with MRI derived fat-free muscle area. Eur J Radiol. 2020;125:108889. doi: 10.1016/j.ejrad.2020.108889. [DOI] [PubMed] [Google Scholar]
- 33.Choi K, Jang HY, Ahn JM, Hwang SH, Chung JW, Choi YS, Kim JW, Jang ES, Choi GH, Jeong SH. The association of the serum levels of myostatin, follistatin, and interleukin-6 with sarcopenia, and their impacts on survival in patients with hepatocellular carcinoma. Clin Mol Hepatol. 2020;26:492–505. doi: 10.3350/cmh.2020.0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cheng TY, Lee PC, Chen YT, Chao Y, Hou MC, Huang YH. Pre-sarcopenia determines post-progression outcomes in advanced hepatocellular carcinoma after sorafenib failure. Sci Rep. 2020;10:18375. doi: 10.1038/s41598-020-75198-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dasarathy S. Consilience in sarcopenia of cirrhosis. J Cachexia Sarcopenia Muscle. 2012;3:225–237. doi: 10.1007/s13539-012-0069-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Merli M, Giusto M, Lucidi C, Giannelli V, Pentassuglio I, Di Gregorio V, Lattanzi B, Riggio O. Muscle depletion increases the risk of overt and minimal hepatic encephalopathy: results of a prospective study. Metab Brain Dis. 2013;28:281–284. doi: 10.1007/s11011-012-9365-z. [DOI] [PubMed] [Google Scholar]
- 37.Krell RW, Kaul DR, Martin AR, Englesbe MJ, Sonnenday CJ, Cai S, Malani PN. Association between sarcopenia and the risk of serious infection among adults undergoing liver transplantation. Liver Transpl. 2013;19:1396–1402. doi: 10.1002/lt.23752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dodson RM, Firoozmand A, Hyder O, Tacher V, Cosgrove DP, Bhagat N, Herman JM, Wolfgang CL, Geschwind JF, Kamel IR, Pawlik TM. Impact of sarcopenia on outcomes following intra-arterial therapy of hepatic malignancies. J Gastrointest Surg. 2013;17:2123–2132. doi: 10.1007/s11605-013-2348-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Begini P, Gigante E, Antonelli G, Carbonetti F, Iannicelli E, Anania G, Imperatrice B, Pellicelli AM, Fave GD, Marignani M. Sarcopenia predicts reduced survival in patients with hepatocellular carcinoma at first diagnosis. Ann Hepatol. 2017;16:107–114. doi: 10.5604/16652681.1226821. [DOI] [PubMed] [Google Scholar]
- 40.Meza-Junco J, Montano-Loza AJ, Baracos VE, Prado CM, Bain VG, Beaumont C, Esfandiari N, Lieffers JR, Sawyer MB. Sarcopenia as a prognostic index of nutritional status in concurrent cirrhosis and hepatocellular carcinoma. J Clin Gastroenterol. 2013;47:861–870. doi: 10.1097/MCG.0b013e318293a825. [DOI] [PubMed] [Google Scholar]
- 41.Borzio M, Fornari F, De Sio I, Andriulli A, Terracciano F, Parisi G, Francica G, Salvagnini M, Marignani M, Salmi A, Farinati F, Carella A, Pedicino C, Dionigi E, Fanigliulo L, Cazzaniga M, Ginanni B, Sacco R EpaHCC Group. Adherence to American Association for the Study of Liver Diseases guidelines for the management of hepatocellular carcinoma: results of an Italian field practice multicenter study. Future Oncol. 2013;9:283–294. doi: 10.2217/fon.12.183. [DOI] [PubMed] [Google Scholar]
- 42.Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis . 1999;19:329–338. doi: 10.1055/s-2007-1007122. [DOI] [PubMed] [Google Scholar]
- 43.Cammà C, Cabibbo G. Prognostic scores for hepatocellular carcinoma: none is the winner. Liver Int. 2009;29:478–480. doi: 10.1111/j.1478-3231.2009.01994.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tandon P, Garcia-Tsao G. Prognostic indicators in hepatocellular carcinoma: a systematic review of 72 studies. Liver Int. 2009;29:502–510. doi: 10.1111/j.1478-3231.2008.01957.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Martin L, Birdsell L, Macdonald N, Reiman T, Clandinin MT, McCargar LJ, Murphy R, Ghosh S, Sawyer MB, Baracos VE. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol. 2013;31:1539–1547. doi: 10.1200/JCO.2012.45.2722. [DOI] [PubMed] [Google Scholar]
- 46.Shen W, Punyanitya M, Wang Z, Gallagher D, St-Onge MP, Albu J, Heymsfield SB, Heshka S. Total body skeletal muscle and adipose tissue volumes: estimation from a single abdominal cross-sectional image. J Appl Physiol (1985) 2004;97:2333–2338. doi: 10.1152/japplphysiol.00744.2004. [DOI] [PubMed] [Google Scholar]
- 47.Hamaguchi Y, Kaido T, Okumura S, Kobayashi A, Fujimoto Y, Ogawa K, Mori A, Hammad A, Hatano E, Uemoto S. Muscle Steatosis is an Independent Predictor of Postoperative Complications in Patients with Hepatocellular Carcinoma. World J Surg. 2016;40:1959–1968. doi: 10.1007/s00268-016-3504-3. [DOI] [PubMed] [Google Scholar]
- 48.Ábrigo J, Elorza AA, Riedel CA, Vilos C, Simon F, Cabrera D, Estrada L, Cabello-Verrugio C. Role of Oxidative Stress as Key Regulator of Muscle Wasting during Cachexia. Oxid Med Cell Longev. 2018;2018:2063179. doi: 10.1155/2018/2063179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dasarathy S, Hatzoglou M. Hyperammonemia and proteostasis in cirrhosis. Curr Opin Clin Nutr Metab Care. 2018;21:30–36. doi: 10.1097/MCO.0000000000000426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Davuluri G, Allawy A, Thapaliya S, Rennison JH, Singh D, Kumar A, Sandlers Y, Van Wagoner DR, Flask CA, Hoppel C, Kasumov T, Dasarathy S. Hyperammonaemia-induced skeletal muscle mitochondrial dysfunction results in cataplerosis and oxidative stress. J Physiol. 2016;594:7341–7360. doi: 10.1113/JP272796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Holecek M, Kandar R, Sispera L, Kovarik M. Acute hyperammonemia activates branched-chain amino acid catabolism and decreases their extracellular concentrations: different sensitivity of red and white muscle. Amino Acids. 2011;40:575–584. doi: 10.1007/s00726-010-0679-z. [DOI] [PubMed] [Google Scholar]
- 52.Peth A, Uchiki T, Goldberg AL. ATP-dependent steps in the binding of ubiquitin conjugates to the 26S proteasome that commit to degradation. Mol Cell. 2010;40:671–681. doi: 10.1016/j.molcel.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thapaliya S, Runkana A, McMullen MR, Nagy LE, McDonald C, Naga Prasad SV, Dasarathy S. Alcohol-induced autophagy contributes to loss in skeletal muscle mass. Autophagy. 2014;10:677–690. doi: 10.4161/auto.27918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dasarathy J, Alkhouri N, Dasarathy S. Changes in body composition after transjugular intrahepatic portosystemic stent in cirrhosis: a critical review of literature. Liver Int. 2011;31:1250–1258. doi: 10.1111/j.1478-3231.2011.02498.x. [DOI] [PubMed] [Google Scholar]
- 55.Ha Y, Kim D, Han S, Chon YE, Lee YB, Kim MN, Lee JH, Park H, Rim KS, Hwang SG. Sarcopenia Predicts Prognosis in Patients with Newly Diagnosed Hepatocellular Carcinoma, Independent of Tumor Stage and Liver Function. Cancer Res Treat. 2018;50:843–851. doi: 10.4143/crt.2017.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Badran H, Elsabaawy MM, Ragab A, Aly RA, Alsebaey A, Sabry A. Baseline Sarcopenia is Associated with Lack of Response to Therapy, Liver Decompensation and High Mortality in Hepatocellular Carcinoma Patients. Asian Pac J Cancer Prev. 2020;21:3285–3290. doi: 10.31557/APJCP.2020.21.11.3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fujiwara N, Nakagawa H, Kudo Y, Tateishi R, Taguri M, Watadani T, Nakagomi R, Kondo M, Nakatsuka T, Minami T, Sato M, Uchino K, Enooku K, Kondo Y, Asaoka Y, Tanaka Y, Ohtomo K, Shiina S, Koike K. Sarcopenia, intramuscular fat deposition, and visceral adiposity independently predict the outcomes of hepatocellular carcinoma. J Hepatol. 2015;63:131–140. doi: 10.1016/j.jhep.2015.02.031. [DOI] [PubMed] [Google Scholar]
- 58.Yuri Y, Nishikawa H, Enomoto H, Ishii A, Iwata Y, Miyamoto Y, Ishii N, Hasegawa K, Nakano C, Nishimura T, Yoh K, Aizawa N, Sakai Y, Ikeda N, Takashima T, Takata R, Iijima H, Nishiguchi S. Implication of Psoas Muscle Index on Survival for Hepatocellular Carcinoma Undergoing Radiofrequency Ablation Therapy. J Cancer. 2017;8:1507–1516. doi: 10.7150/jca.19175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fujita M, Takahashi A, Hayashi M, Okai K, Abe K, Ohira H. Skeletal muscle volume loss during transarterial chemoembolization predicts poor prognosis in patients with hepatocellular carcinoma. Hepatol Res. 2019;49:778–786. doi: 10.1111/hepr.13331. [DOI] [PubMed] [Google Scholar]
- 60.Kobayashi T, Kawai H, Nakano O, Abe S, Kamimura H, Sakamaki A, Kamimura K, Tsuchiya A, Takamura M, Yamagiwa S, Terai S. Rapidly declining skeletal muscle mass predicts poor prognosis of hepatocellular carcinoma treated with transcatheter intra-arterial therapies. BMC Cancer. 2018;18:756. doi: 10.1186/s12885-018-4673-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Loosen SH, Schulze-Hagen M, Bruners P, Tacke F, Trautwein C, Kuhl C, Luedde T, Roderburg C. Sarcopenia Is a Negative Prognostic Factor in Patients Undergoing Transarterial Chemoembolization (TACE) for Hepatic Malignancies. Cancers (Basel) 2019;11 doi: 10.3390/cancers11101503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fan ST, Lo CM, Lai EC, Chu KM, Liu CL, Wong J. Perioperative nutritional support in patients undergoing hepatectomy for hepatocellular carcinoma. N Engl J Med. 1994;331:1547–1552. doi: 10.1056/NEJM199412083312303. [DOI] [PubMed] [Google Scholar]
- 63.Harimoto N, Shirabe K, Yamashita YI, Ikegami T, Yoshizumi T, Soejima Y, Ikeda T, Maehara Y, Nishie A, Yamanaka T. Sarcopenia as a predictor of prognosis in patients following hepatectomy for hepatocellular carcinoma. Br J Surg. 2013;100:1523–1530. doi: 10.1002/bjs.9258. [DOI] [PubMed] [Google Scholar]
- 64.Dello SA, Lodewick TM, van Dam RM, Reisinger KW, van den Broek MA, von Meyenfeldt MF, Bemelmans MH, Olde Damink SW, Dejong CH. Sarcopenia negatively affects preoperative total functional liver volume in patients undergoing liver resection. HPB (Oxford) 2013;15:165–169. doi: 10.1111/j.1477-2574.2012.00517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Otsuji H, Yokoyama Y, Ebata T, Igami T, Sugawara G, Mizuno T, Nagino M. Preoperative sarcopenia negatively impacts postoperative outcomes following major hepatectomy with extrahepatic bile duct resection. World J Surg. 2015;39:1494–1500. doi: 10.1007/s00268-015-2988-6. [DOI] [PubMed] [Google Scholar]
- 66.Chae MS, Moon KU, Jung JY, Choi HJ, Chung HS, Park CS, Lee J, Choi JH, Hong SH. Perioperative loss of psoas muscle is associated with patient survival in living donor liver transplantation. Liver Transpl. 2018;24:623–633. doi: 10.1002/lt.25022. [DOI] [PubMed] [Google Scholar]
- 67.Wada Y, Kamishima T, Shimamura T, Kawamura N, Yamashita K, Sutherland K, Takeda H. Pre-operative volume rather than area of skeletal muscle is a better predictor for post-operative risks for respiratory complications in living-donor liver transplantation. Br J Radiol. 2017;90:20160938. doi: 10.1259/bjr.20160938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Carey EJ, Lai JC, Wang CW, Dasarathy S, Lobach I, Montano-Loza AJ, Dunn MA Fitness, Life Enhancement, and Exercise in Liver Transplantation Consortium. A multicenter study to define sarcopenia in patients with end-stage liver disease. Liver Transpl. 2017;23:625–633. doi: 10.1002/lt.24750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.van Vugt JLA, Alferink LJM, Buettner S, Gaspersz MP, Bot D, Darwish Murad S, Feshtali S, van Ooijen PMA, Polak WG, Porte RJ, van Hoek B, van den Berg AP, Metselaar HJ, IJzermans JNM. A model including sarcopenia surpasses the MELD score in predicting waiting list mortality in cirrhotic liver transplant candidates: A competing risk analysis in a national cohort. J Hepatol. 2018;68:707–714. doi: 10.1016/j.jhep.2017.11.030. [DOI] [PubMed] [Google Scholar]
- 70.Golse N, Bucur PO, Ciacio O, Pittau G, Sa Cunha A, Adam R, Castaing D, Antonini T, Coilly A, Samuel D, Cherqui D, Vibert E. A new definition of sarcopenia in patients with cirrhosis undergoing liver transplantation. Liver Transpl. 2017;23:143–154. doi: 10.1002/lt.24671. [DOI] [PubMed] [Google Scholar]
- 71.Kim YR, Park S, Han S, Ahn JH, Kim S, Sinn DH, Jeong WK, Ko JS, Gwak MS, Kim GS. Sarcopenia as a predictor of post-transplant tumor recurrence after living donor liver transplantation for hepatocellular carcinoma beyond the Milan criteria. Sci Rep. 2018;8:7157. doi: 10.1038/s41598-018-25628-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kaido T, Ogawa K, Fujimoto Y, Ogura Y, Hata K, Ito T, Tomiyama K, Yagi S, Mori A, Uemoto S. Impact of sarcopenia on survival in patients undergoing living donor liver transplantation. Am J Transplant. 2013;13:1549–1556. doi: 10.1111/ajt.12221. [DOI] [PubMed] [Google Scholar]
- 73.Underwood PW, Cron DC, Terjimanian MN, Wang SC, Englesbe MJ, Waits SA. Sarcopenia and failure to rescue following liver transplantation. Clin Transplant. 2015;29:1076–1080. doi: 10.1111/ctr.12629. [DOI] [PubMed] [Google Scholar]
- 74.Semsarian C, Wu MJ, Ju YK, Marciniec T, Yeoh T, Allen DG, Harvey RP, Graham RM. Skeletal muscle hypertrophy is mediated by a Ca2+-dependent calcineurin signalling pathway. Nature. 1999;400:576–581. doi: 10.1038/23054. [DOI] [PubMed] [Google Scholar]
- 75.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, Schwartz M, Porta C, Zeuzem S, Bolondi L, Greten TF, Galle PR, Seitz JF, Borbath I, Häussinger D, Giannaris T, Shan M, Moscovici M, Voliotis D, Bruix J SHARP Investigators Study Group. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 76.Mir O, Coriat R, Blanchet B, Durand JP, Boudou-Rouquette P, Michels J, Ropert S, Vidal M, Pol S, Chaussade S, Goldwasser F. Sarcopenia predicts early dose-limiting toxicities and pharmacokinetics of sorafenib in patients with hepatocellular carcinoma. PLoS One. 2012;7:e37563. doi: 10.1371/journal.pone.0037563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Imai K, Takai K, Hanai T, Ideta T, Miyazaki T, Kochi T, Suetsugu A, Shiraki M, Shimizu M. Skeletal muscle depletion predicts the prognosis of patients with hepatocellular carcinoma treated with sorafenib. Int J Mol Sci. 2015;16:9612–9624. doi: 10.3390/ijms16059612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nishikawa H, Nishijima N, Enomoto H, Sakamoto A, Nasu A, Komekado H, Nishimura T, Kita R, Kimura T, Iijima H, Nishiguchi S, Osaki Y. Prognostic significance of sarcopenia in patients with hepatocellular carcinoma undergoing sorafenib therapy. Oncol Lett. 2017;14:1637–1647. doi: 10.3892/ol.2017.6287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hiraoka A, Hirooka M, Koizumi Y, Izumoto H, Ueki H, Kaneto M, Kitahata S, Aibiki T, Tomida H, Miyamoto Y, Yamago H, Suga Y, Iwasaki R, Mori K, Miyata H, Tsubouchi E, Kishida M, Ninomiya T, Abe M, Matsuura B, Kawasaki H, Hiasa Y, Michitaka K. Muscle volume loss as a prognostic marker in hepatocellular carcinoma patients treated with sorafenib. Hepatol Res. 2017;47:558–565. doi: 10.1111/hepr.12780. [DOI] [PubMed] [Google Scholar]
- 80.Yamashima M, Miyaaki H, Honda T, Shibata H, Miuma S, Taura N, Nakao K. Significance of psoas muscle thickness as an indicator of muscle atrophy in patients with hepatocellular carcinoma treated with sorafenib. Mol Clin Oncol. 2017;7:449–453. doi: 10.3892/mco.2017.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Takada H, Kurosaki M, Nakanishi H, Takahashi Y, Itakura J, Tsuchiya K, Yasui Y, Tamaki N, Takaura K, Komiyama Y, Higuchi M, Kubota Y, Wang W, Okada M, Enomoto N, Izumi N. Impact of pre-sarcopenia in sorafenib treatment for advanced hepatocellular carcinoma. PLoS One. 2018;13:e0198812. doi: 10.1371/journal.pone.0198812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Saeki I, Yamasaki T, Maeda M, Kawano R, Hisanaga T, Iwamoto T, Matsumoto T, Hidaka I, Ishikawa T, Takami T, Sakaida I. No Muscle Depletion with High Visceral Fat as a Novel Beneficial Biomarker of Sorafenib for Hepatocellular Carcinoma. Liver Cancer. 2018;7:359–371. doi: 10.1159/000487858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Amanuma M, Nagai H, Igarashi Y. Sorafenib Might Induce Sarcopenia in Patients With Hepatocellular Carcinoma by Inhibiting Carnitine Absorption. Anticancer Res. 2020;40:4173–4182. doi: 10.21873/anticanres.14417. [DOI] [PubMed] [Google Scholar]
- 84.Pinter M, Peck-Radosavljevic M. Review article: systemic treatment of hepatocellular carcinoma. Aliment Pharmacol Ther. 2018;48:598–609. doi: 10.1111/apt.14913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mir O, Coriat R, Boudou-Rouquette P, Ropert S, Durand JP, Cessot A, Mallet V, Sogni P, Chaussade S, Pol S, Goldwasser F. Gemcitabine and oxaliplatin as second-line treatment in patients with hepatocellular carcinoma pre-treated with sorafenib. Med Oncol. 2012;29:2793–2799. doi: 10.1007/s12032-012-0208-x. [DOI] [PubMed] [Google Scholar]
- 86.Dhooge M, Coriat R, Mir O, Perkins G, Brezault C, Boudou-Rouquette P, Goldwasser F, Chaussade S. Feasibility of gemcitabine plus oxaliplatin in advanced hepatocellular carcinoma patients with Child-Pugh B cirrhosis. Oncology. 2013;84:32–38. doi: 10.1159/000342763. [DOI] [PubMed] [Google Scholar]
- 87.Rinninella E, Cintoni M, Raoul P, Mele MC, De Gaetano AM, Marini MG, Mora V, Gasbarrini A. Minimal impact of lenvatinib (Lenvima®) on muscle mass in advanced hepatocellular carcinoma and implications for treatment duration. Two cases from the REFLECT study. Eur Rev Med Pharmacol Sci. 2019;23:10132–10138. doi: 10.26355/eurrev_201911_19583. [DOI] [PubMed] [Google Scholar]
- 88.Ohara M, Ogawa K, Suda G, Kimura M, Maehara O, Shimazaki T, Suzuki K, Nakamura A, Umemura M, Izumi T, Kawagishi N, Nakai M, Sho T, Natsuizaka M, Morikawa K, Ohnishi S, Sakamoto N. L-Carnitine Suppresses Loss of Skeletal Muscle Mass in Patients With Liver Cirrhosis. Hepatol Commun. 2018;2:906–918. doi: 10.1002/hep4.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tsien C, Davuluri G, Singh D, Allawy A, Ten Have GA, Thapaliya S, Schulze JM, Barnes D, McCullough AJ, Engelen MP, Deutz NE, Dasarathy S. Metabolic and molecular responses to leucine-enriched branched chain amino acid supplementation in the skeletal muscle of alcoholic cirrhosis. Hepatology. 2015;61:2018–2029. doi: 10.1002/hep.27717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kruger C, McNeely ML, Bailey RJ, Yavari M, Abraldes JG, Carbonneau M, Newnham K, DenHeyer V, Ma M, Thompson R, Paterson I, Haykowsky MJ, Tandon P. Home Exercise Training Improves Exercise Capacity in Cirrhosis Patients: Role of Exercise Adherence. Sci Rep. 2018;8:99. doi: 10.1038/s41598-017-18320-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Debette-Gratien M, Tabouret T, Antonini MT, Dalmay F, Carrier P, Legros R, Jacques J, Vincent F, Sautereau D, Samuel D, Loustaud-Ratti V. Personalized adapted physical activity before liver transplantation: acceptability and results. Transplantation. 2015;99:145–150. doi: 10.1097/TP.0000000000000245. [DOI] [PubMed] [Google Scholar]
- 92.Zenith L, Meena N, Ramadi A, Yavari M, Harvey A, Carbonneau M, Ma M, Abraldes JG, Paterson I, Haykowsky MJ, Tandon P. Eight weeks of exercise training increases aerobic capacity and muscle mass and reduces fatigue in patients with cirrhosis. Clin Gastroenterol Hepatol. 2014;12:1920–6.e2. doi: 10.1016/j.cgh.2014.04.016. [DOI] [PubMed] [Google Scholar]
- 93.Román E, García-Galcerán C, Torrades T, Herrera S, Marín A, Doñate M, Alvarado-Tapias E, Malouf J, Nácher L, Serra-Grima R, Guarner C, Cordoba J, Soriano G. Effects of an Exercise Programme on Functional Capacity, Body Composition and Risk of Falls in Patients with Cirrhosis: A Randomized Clinical Trial. PLoS One. 2016;11:e0151652. doi: 10.1371/journal.pone.0151652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sinclair M, Gow PJ, Grossmann M, Angus PW. Review article: sarcopenia in cirrhosis--aetiology, implications and potential therapeutic interventions. Aliment Pharmacol Ther. 2016;43:765–777. doi: 10.1111/apt.13549. [DOI] [PubMed] [Google Scholar]
- 95.Aamann L, Dam G, Borre M, Drljevic-Nielsen A, Overgaard K, Andersen H, Vilstrup H, Aagaard NK. Resistance Training Increases Muscle Strength and Muscle Size in Patients With Liver Cirrhosis. Clin Gastroenterol Hepatol. 2020;18:1179–1187.e6. doi: 10.1016/j.cgh.2019.07.058. [DOI] [PubMed] [Google Scholar]
- 96.Sinclair M, Grossmann M, Gow PJ, Angus PW. Testosterone in men with advanced liver disease: abnormalities and implications. J Gastroenterol Hepatol. 2015;30:244–251. doi: 10.1111/jgh.12695. [DOI] [PubMed] [Google Scholar]
- 97.Dubois V, Simitsidellis I, Laurent MR, Jardi F, Saunders PT, Vanderschueren D, Claessens F. Enobosarm (GTx-024) Modulates Adult Skeletal Muscle Mass Independently of the Androgen Receptor in the Satellite Cell Lineage. Endocrinology. 2015;156:4522–4533. doi: 10.1210/en.2015-1479. [DOI] [PubMed] [Google Scholar]
- 98.Shankaran M, Shearer TW, Stimpson SA, Turner SM, King C, Wong PY, Shen Y, Turnbull PS, Kramer F, Clifton L, Russell A, Hellerstein MK, Evans WJ. Proteome-wide muscle protein fractional synthesis rates predict muscle mass gain in response to a selective androgen receptor modulator in rats. Am J Physiol Endocrinol Metab. 2016;310:E405–E417. doi: 10.1152/ajpendo.00257.2015. [DOI] [PubMed] [Google Scholar]
- 99.Jones A, Hwang DJ, Narayanan R, Miller DD, Dalton JT. Effects of a novel selective androgen receptor modulator on dexamethasone-induced and hypogonadism-induced muscle atrophy. Endocrinology. 2010;151:3706–3719. doi: 10.1210/en.2010-0150. [DOI] [PubMed] [Google Scholar]
- 100.Koya S, Kawaguchi T, Hashida R, Goto E, Matsuse H, Saito H, Hirota K, Taira R, Matsushita Y, Imanaga M, Nagamatsu A, Shirono T, Shimose S, Iwamoto H, Niizeki T, Kuromatsu R, Miura H, Shiba N, Torimura T. Effects of in-hospital exercise on liver function, physical ability, and muscle mass during treatment of hepatoma in patients with chronic liver disease. Hepatol Res. 2017;47:E22–E34. doi: 10.1111/hepr.12718. [DOI] [PubMed] [Google Scholar]
- 101.Koya S, Kawaguchi T, Hashida R, Hirota K, Bekki M, Goto E, Yamada M, Sugimoto M, Hayashi S, Goshima N, Yoshiyama T, Otsuka T, Nozoe R, Nagamatsu A, Nakano D, Shirono T, Shimose S, Iwamoto H, Niizeki T, Matsuse H, Koga H, Miura H, Shiba N, Torimura T. Effects of in-hospital exercise on sarcopenia in hepatoma patients who underwent transcatheter arterial chemoembolization. J Gastroenterol Hepatol. 2019;34:580–588. doi: 10.1111/jgh.14538. [DOI] [PubMed] [Google Scholar]
- 102.Le Cornu KA, McKiernan FJ, Kapadia SA, Neuberger JM. A prospective randomized study of preoperative nutritional supplementation in patients awaiting elective orthotopic liver transplantation. Transplantation. 2000;69:1364–1369. doi: 10.1097/00007890-200004150-00026. [DOI] [PubMed] [Google Scholar]
- 103.Brustia R, Savier E, Scatton O. Physical exercise in cirrhotic patients: Towards prehabilitation on waiting list for liver transplantation. A systematic review and meta-analysis. Clin Res Hepatol Gastroenterol. 2018;42:205–215. doi: 10.1016/j.clinre.2017.09.005. [DOI] [PubMed] [Google Scholar]
- 104.Yang YJ, Kim DJ. An Overview of the Molecular Mechanisms Contributing to Musculoskeletal Disorders in Chronic Liver Disease: Osteoporosis, Sarcopenia, and Osteoporotic Sarcopenia. Int J Mol Sci. 2021;22 doi: 10.3390/ijms22052604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sano A, Tsuge S, Kakazu E, Iwata T, Ninomiya M, Tsuruoka M, Inoue J, Masamune A. Plasma free amino acids are associated with sarcopenia in the course of hepatocellular carcinoma recurrence. Nutrition. 2021;84:111007. doi: 10.1016/j.nut.2020.111007. [DOI] [PubMed] [Google Scholar]
- 106.Su TH, Wu CH, Kao JH. Artificial intelligence in precision medicine in hepatology. J Gastroenterol Hepatol. 2021;36:569–580. doi: 10.1111/jgh.15415. [DOI] [PubMed] [Google Scholar]
- 107.Saini A, Breen I, Pershad Y, Naidu S, Knuttinen MG, Alzubaidi S, Sheth R, Albadawi H, Kuo M, Oklu R. Radiogenomics and Radiomics in Liver Cancers. Diagnostics (Basel) 2018;9 doi: 10.3390/diagnostics9010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Coroller TP, Grossmann P, Hou Y, Rios Velazquez E, Leijenaar RT, Hermann G, Lambin P, Haibe-Kains B, Mak RH, Aerts HJ. CT-based radiomic signature predicts distant metastasis in lung adenocarcinoma. Radiother Oncol. 2015;114:345–350. doi: 10.1016/j.radonc.2015.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Seror M, Sartoris R, Hobeika C, Bouattour M, Paradis V, Rautou PE, Soubrane O, Vilgrain V, Cauchy F, Ronot M. Computed Tomography-Derived Liver Surface Nodularity and Sarcopenia as Prognostic Factors in Patients with Resectable Metabolic Syndrome-Related Hepatocellular Carcinoma. Ann Surg Oncol. 2021;28:405–416. doi: 10.1245/s10434-020-09143-9. [DOI] [PubMed] [Google Scholar]
- 110.Voron T, Tselikas L, Pietrasz D, Pigneur F, Laurent A, Compagnon P, Salloum C, Luciani A, Azoulay D. Sarcopenia Impacts on Short- and Long-term Results of Hepatectomy for Hepatocellular Carcinoma. Ann Surg. 2015;261:1173–1183. doi: 10.1097/SLA.0000000000000743. [DOI] [PubMed] [Google Scholar]
- 111.Yabusaki N, Fujii T, Yamada S, Suzuki K, Sugimoto H, Kanda M, Nakayama G, Koike M, Fujiwara M, Kodera Y. Adverse impact of low skeletal muscle index on the prognosis of hepatocellular carcinoma after hepatic resection. Int J Surg. 2016;30:136–142. doi: 10.1016/j.ijsu.2016.04.049. [DOI] [PubMed] [Google Scholar]
- 112.Hamaguchi Y, Kaido T, Okumura S, Kobayashi A, Shirai H, Yao S, Yagi S, Kamo N, Seo S, Taura K, Okajima H, Uemoto S. Preoperative Visceral Adiposity and Muscularity Predict Poor Outcomes after Hepatectomy for Hepatocellular Carcinoma. Liver Cancer. 2019;8:92–109. doi: 10.1159/000488779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Takagi K, Yagi T, Yoshida R, Shinoura S, Umeda Y, Nobuoka D, Kuise T, Watanabe N, Fujiwara T. Sarcopenia and American Society of Anesthesiologists Physical Status in the Assessment of Outcomes of Hepatocellular Carcinoma Patients Undergoing Hepatectomy. Acta Med Okayama. 2016;70:363–370. doi: 10.18926/AMO/54594. [DOI] [PubMed] [Google Scholar]
- 114.Itoh S, Yoshizumi T, Kimura K, Okabe H, Harimoto N, Ikegami T, Uchiyama H, Shirabe K, Nishie A, Maehara Y. Effect of Sarcopenic Obesity on Outcomes of Living-Donor Liver Transplantation for Hepatocellular Carcinoma. Anticancer Res. 2016;36:3029–3034. [PubMed] [Google Scholar]