Summary
Background
Sarcolipin and uncoupling protein 3 (UCP3) mediate muscle-based non-shivering thermogenesis (NST) to improve metabolic homeostasis. The impacts of maternal obesity (MO) and maternal exercise (ME) on NST in offspring muscle remain unexamined.
Methods
Female mice were fed with a control diet or high fat diet to induce obesity. Then, obese mice were further separated into two groups: obesity only (OB) and OB plus daily exercise (OB/Ex). Fetal muscle was collected at embryonic day 18.5 and offspring mice at 3-month-old. Apelin administration during pregnancy and apelin receptor (APJ) knockout mouse were further used for investigating the mediatory role of APJ on muscle-based thermogenesis. To explore the direct effects of exercise on AMP-activated protein kinase (AMPK) downstream targets, AMPK knockout mouse was used.
Findings
MO inhibited while ME activated AMPK and peroxisome proliferator-activated receptor γ coactivator-1α (PGC-1α) in fetal muscle. AMPK activation increased sarcolipin expression, which inhibited the uptake of calcium ions into sarcoplasmic reticulum, thereby activating CaMKK2. Consistently, the expression of UCP3 and sarcolipin was suppressed due to MO but activated in ME fetal muscle. Importantly, changes of UCP3 and sarcolipin maintained in offspring muscle, showing the transgenerational effects. Furthermore, apelin administration during pregnancy mimicked the effects of ME on AMPK and CaMKK2 activation, and UCP3 and sarcolipin expression, underscoring the mediatory roles of apelin-AMPK signaling in improving fetal muscle development.
Interpretation
ME, via activation of apelin signaling-AMPK axis, enhances NST gene expression in fetal and offspring muscle impaired due to MO, which intergenerationally protects offspring from diet-induced obesity and metabolic disorders.
Funding
This work was supported by National Institutes of Health Grant R01-HD067449.
Keywords: Maternal obesity, Sarcolipin, UCP3, PGC-1α, Calcium activity
Research in context.
Evidence before this study
Accumulating evidence points to the importance of muscle-based thermogenesis in maintaining metabolic health. We previously found that maternal exercise (ME) improves mitochondrial biogenesis in the fetal muscle, which generates long-term benefits on metabolic health of offspring. On the other hand, maternal obesity (MO) impairs fetal muscle development and offspring muscle functions. However, the effects of ME on skeletal muscle thermogenesis in fetal/offspring muscle impaired due to MO remain undefined.
Added value of this study
Our findings delineate the important intergenerational role of ME in improving muscle-based thermogenesis and thus improving metabolic homeostasis of the fetal and offspring muscle impaired due to MO. In addition, apelin administration generates similar effects of ME in activating AMP-activated protein kinase (AMPK) and calcium/calmodulin-dependent protein kinase 2 (CaMKK2) signaling, thereby enhancing the expression of muscle-based thermogenic genes, sarcolipin (SLN) and uncoupling protein 3 (UCP3) in MO fetal muscle. These discoveries underscore the mediatory roles of apelin receptor signaling in linking ME and its intergenerational effect on muscle-based thermogenesis.
Implications of all the available evidence
As one of the major underlying mechanisms in regulating muscle-based thermogenic gene expression during fetal muscle development, apelin-AMPK axis represents novel therapeutic targets to improve fetal muscle development impaired due to lack of physical activity and obesity during pregnancy. Considering the commonness of maternal obesity and sedentary lifestyle in western societies and the high accessibility of exercise, our findings have important clinical applications in improving metabolic health of offspring born to obese mothers.
Alt-text: Unlabelled box
Introduction
Overweight and obesity in pregnant women are increasing worldwide,1,2 which predispose their children to obesity and associated metabolic diseases.3 Non-shivering thermogenesis (NST) including thermogenesis of brown adipose tissue (BAT) and skeletal muscle, utilizes excessive energy for heat generation, and their activation protects against metabolic disorders including obesity, type 2 diabetes mellitus and cardiovascular diseases.4,5 We previously found that maternal exercise (ME) enhances BAT NST of their offspring.6 However, despite of its abundance in neonates, BAT amounts decline rapidly as age increases, questioning its importance in maintaining metabolic health of adults.4,7 Skeletal muscle, accounting approximately 30–40% of body mass in adult humans, is mainly responsible for basal metabolism8,9; activation of NST in adult skeletal muscle prevents metabolic impairment due to obesity.10, 11, 12 Supportively, ME intergenerationally remodels mitochondrial oxidative function in the offspring skeletal muscle.13 Up to now, however, the effects of ME and/or maternal obesity (MO) on muscle-based thermogenesis in offspring remain unexamined.
As a basic mechanism of NST in skeletal muscle, sarcoendoplasmic reticulum Ca2+ ATPase (SERCA) pumps Ca2+ ions back into the sarcoplasmic reticulum (SR), critical for muscle relaxation.14,15 SERCA is a central regulator of muscle performance, and its dysfunction is associated in cardiovascular and skeletal muscle diseases.14,15 Sarcolipin (SLN) uncouples Ca2+ pumping from ATP hydrolysis by SERCA, triggering futile energy consumption and muscle-based thermogenesis.16 This futile energy consumption in the skeletal muscle improves the metabolic homeostasis of muscle and the whole body.17,18 On the other hand, its thermogenic function is impaired due to diet-induced obesity.12,19 In addition, uncoupling protein 3 (UCP3), a mitochondrial inner membrane protein is also associated with NST in skeletal muscle.20 Its expression is stimulated by peroxisome proliferator-activated receptor γ coactivator-1α (PGC-1α).21 The thermogenic function of both SLN and UCP3 is up-regulated by elevation of intracellular Ca2+ level,16,22 which is stimulated by exercise training.23 Nevertheless, the mechanisms linking ME to NST via SLN and UCP3 activation remain unexamined.
Apelin, as a small peptide, is an endogenous ligand for a G protein-coupled receptor (APLNR, also known as APJ).24 It was initially identified as a hormone secreted from adipose tissue,25 but also secreted by skeletal muscle26 and other tissues.27,28 Apelin activates Gαq and stimulates phospholipase C, which triggers inositol triphosphate (IP3) release and elevates intracellular Ca2+ levels.29 Enhanced intracellular Ca2+ levels is known to enhance muscle-based thermogenesis.10 We previously found that ME elevates the apelin level in fetal circulation.6 Considering the importance of the fetal stage for skeletal muscle development, in this study, we hypothesized that MO impairs while ME enhances NST of skeletal muscle in offspring via stimulation of apelin/APJ signaling during fetal muscle development. We found that ME enhances mitochondrial activity and SLN/UCP3 expression in fetal muscle impaired due to MO, which enhances muscle-based NST and prevents diet-induced obesity in offspring. In addition, apelin administration during pregnancy mirrors the benefits of ME via the activation of APJ-AMP-activated protein kinase (AMPK) axis, suggesting it is a novel therapeutic target to prevent intergenerational obesity due to MO, which now accounts for approximately 35% of pregnancies in the United States.30
Methods
Animals
Eight-week-old wild-type female C57BL/6 J mice were fed either a control diet (CD; 10% energy from fat, D12450J, Research Diets, New Brunswick, NJ) or high fat diet (HFD, 60% energy from fat; D12492, Research Diets) ad libitum for 8 weeks to induce obesity, defined as gaining more than 20% body weight compared to the CD group.31, 32, 33 Then, mice were randomized into three groups: control (CON, n = 6), obese (OB, n = 6), and obese with exercise (OB/Ex, n = 6). For ME, female mice were acclimated to treadmill running for one week, when 60% HFD was changed to 45% HFD (D12451; Research Diets) to protect neonatal death.31 Then, female mice were mated with age-matched male mice fed a conventional chow diet. Determination of mating was confirmed by the presence of vaginal smears. During pregnancy, mice continued to be fed CON or 45% HFD and exercised daily. Maternal treadmill exercise protocol was conducted as previously described.34 Briefly, maternal mice were subjected to flat degree treadmill exercise training during pregnancy from E1.5 to E16.5 for fetal muscle sampling at E18.5, or from E1.5 to E20.5 for delivering offspring. We did not conduct exercise training 2 days before tissue collection to avoid the acute effects of exercise. The exercise intensities were follows: E1.5 to E7.5 (40%), E8.5 to E14.5 (65%) and E15.5 to E16.5 (for fetal muscle sampling) or to E20.5 (for delivering offspring) (50%) of the maximal oxygen consumption rates (VO2max).31,34
At embryonic day 18.5 (E18.5), maternal gastrocnemius and fetal muscle were collected after anesthetization by carbon dioxide inhalation and cervical dislocation.31 One female and one male fetuses randomly selected from each litter for further analyses. Fetal sex determination was conducted using a PCR method.35
To examine the long-term effects of exercise and/or obesity during pregnancy, the same number of maternal mice were prepared for delivering offspring. After birth and weaning (4-week-old), one female and one male offspring were randomly selected from one litter and weaned onto CD (10% energy from fat, D12450J, Research Diets, New Brunswick, NJ) or HFD (60% energy from fat; D12492, Research Diets) to mimic the common post-weaning obesogenic diet, which resulted in three treatment groups: maternal control with offspring CD (M-Ctrl_CD), M-Ctrl with offspring HFD (M-Ctrl_HFD), and maternal exercise (M-Ex) with offspring HFD (M-Ex_HFD) for 8 weeks. Then, these offspring mice (12-week-old) were anesthetized for further analyses after 5 h fasting (Supplementary Fig. 1a).
In the apelin administration study, 8 weeks of female mice fed a HFD (60%) for 8 weeks were randomized into two groups injected with either PBS or apelin (OB/PBS, n = 6; OB/APN, n = 6). One weeks before mating, HFD was changed to the 45% HFD so that mice were adapted to this diet and also to protect neonatal death. [Pyr1]apelin-13 (AAPPTec, Louisville, KY) was injected daily at 0.5 μmol/kg per day intraperitoneally (i.p.) from E1.5 to E16.5 during pregnancy, as previously described.6,26 In addition, age-matched female mice fed a CON diet were injected (i.p.) with PBS daily (CD/PBS, n = 6), which served as a reference for the improvement of OB fetal development due to apelin injection.
Ethics
All animal studies were conducted in AAALAC-approved facilities in accordance with the Animals in Research: Reporting In Vitro Experiments (ARRIVE) guidelines36 and approved by the Institute of Animal Care and Use Committees (IACUC) at the Washington State University (ASAF# 6704).
Primary myogenic cells
To explore the mediatory roles of APJ signaling pathway, Apjflox/flox mice27 were cross bred with Tmem163Tg(ACTB-cre)2Mrt (β-actinCre, #019099, Jackson Lab) transgenic mice. Primary myogenic cells were isolated from the gastrocnemius muscle of β-actinCre/Apj cKO or Apjflox/flox (as a control group) weanling offspring mice. Separately, in the AMPK deficient model, Gt(ROSA)26Sortm1(cre/ERT)Nat/J mice (R26CreER, #004847, Jackson Lab) were cross-bred with Prkaa1flox/flox (#014141, Jackson Lab) to obtain ROSACre/Prkaa1 mice. Then, myogenic cells were isolated from ROSACre/Prkaa1 mice, treated with 4‑hydroxy-tamoxifen and then induced myogenic differentiation with/without 100 nM [Pyr1]apelin-13 treatment as previously described.37
Indirect calorimetry
Indirect calorimetric (open-circuit) measurements were conducted using Comprehensive Lab Animal Monitoring System (Columbus Instruments) in accordance with the manufacturer's instructions. Mice were fed ad libitum with the diet and water provided.6
Thermal imaging
An E6 Thermal Imaging Infrared Camera (FLIR Systems, Wilsonville, OR, USA) were used to measure surface temperatures, and average surface temperatures of whole body were analyzed using FLIR-Tools-Software (FLIR System), as preciously described.6
Quantification of mtDNA copy number
Extracted total DNA was utilized for measuring mitochondrial DNA copy number, which was quantified by using mitochondrial DNA, NADH dehydrogenase subunit 1 (Nd1), and nuclear DNA, lipoprotein lipase (Lpl) genes based on a PCR method.38 The primers were mtDNA Nd1 forward, 5′-CACTATTCGGAGCTTTACG-3′; reverse, 5′-TGTTTCTGCTAGGGTTGA-3′; Lpl forward, 5′-GAAAGGGCTCTGCCTGAGTT-3′; and reverse, 5′-TAGGGCATCTGAGAGCGAGT-3′.
RNA sequencing
Alternative polyadenylation with whole transcriptome termini site sequencing (WTTS seq) was conducted based on a previous report.39 After trimming, alignment was performed and mapped to the reference genome (Mus_musculus.GRCm38.fa). The gene ontology (GO) and differential expressed gene (DEG) analyses were performed.
Real time PCR
Total RNA was extracted using TRIzol reagent (Invitrogen, Grand Island, NY) and cDNA was synthesized using 500 ng of total RNA with an iScript™ cDNA Synthesis Kit (Bio-Rad, Hercules, CA), as previously described.31 RT-PCR gene expression analysis was performed using custom-designed primers (Supplementary Table 1) with SsoAdvanced™ Universal SYBR® Green Supermix (Bio-Rad). 18S rRNA and β-Actin were used for normalization, and relative mRNA was calculated based on a 2−ΔΔCt method described in the previous study.6
Western blotting
Protein expression levels were determined by western blot analyses as previously described.40 Fetal and offspring muscle tissues were homogenized, and proteins were extracted using lysis buffer (100 mM Tris–HCl, pH 6.8, 2.0% SDS, 20% glycerol, 0.02% bromophenol blue, 5% 2-mercaptoethanol, 100 mM NaF and 1 mM Na3VO4). Electrophoresis and transfer processes were followed based on a previous study.6 The following primary antibodies were used: SLN polyclonal antibody (#18395–1-AP; RRID: AB_2286622), UCP3 polyclonal antibody (#10750–1-AP; RRID: AB_2272729), apelin polyclonal antibody (#11497–1-AP; RRID: AB_2877771) and APJ polyclonal antibody (#20341–1-AP; RRID: AB_2878676) were purchased from Proteintech (Rosemont, IL, USA). AMPKα (#2793; RRID: AB_915794), phospho-AMPKα (#2535; RRID: AB_331250), CaMKK2 (#16810; RRID: AB_2798771) and phospho-CaMKK2 (#16737; RRID: AB_2798769) were further obtained from Cell Signaling Technology (Danvers, MA, USA). As secondary antibodies, IRDye 680 goat anti-mouse secondary (dilution 1:10,000; RIDD: AB_621840) and IRDye 800CW goat anti-rabbit secondary antibody (dilution 1:10,000; RRID: AB_621843) were used (LI-COR Biosciences, Lincoln, NE). Membranes were scanned using an Odyssey Infrared Imaging System (LI-COR Biosciences), and target proteins were detected and analyzed as previously described.6
Statistical analysis
Three biological replicates were used for animal and cell culture studies. Statistical analyses were conducted using statistical analysis software (SAS Institute Inc., Cary, NC), which was visualized by GraphPad Prism 7 for Windows (GraphPad Software, San Diego, CA). Data were presented as mean ± SEM, and statistical significance was defined as P < 0.05. One-way repeated analysis of variance (ANOVA) followed by post-hoc (Tukey's test) analysis or independent Student's t-test was used for comparison. The number of samples for each measurement and statistical significances are presented in the figure legends. R statistics and SPSS Statistics ver.21 (IBM Corp., Armonk, NY, USA) were used for transcriptome analysis.
Role of the funding source
The funding sources have not been involved in study design, data collection, data analyses, interpretation, and/or writing of the report.
Results
Exercise during pregnancy induces muscle-based thermogenic gene expression in both mothers and fetuses
In our previous study, we found that obesity reduced the surface temperature of dams during pregnancy, which was recovered by exercise.31 Consistently, MO down-regulated the expression of thermogenic markers in maternal skeletal muscle, which was alleviated due to ME (Supplementary Fig. 1e). ME was correlated with increased muscle weights and activation of calcium signaling (Supplementary Fig. 1b,e), and the expression of SLN and UCP3 was elevated, consistent with a previous report.41 We further analyzed the effects of ME on NST gene expression in fetal muscle. Notably, the surface temperature of neonates at postnatal day 1 (P1) was lower in the M-OB group compared to M-Ctrl, which was prevented by ME (M-OB/Ex neonates) (Figure 1a), consistent with our previous study showing that ME enhances thermogenic function as indicated by higher surface temperature during 2 days cold exposure.6 On the other hand, M-OB increased neonatal body weight at P1, which was prevented by ME (Supplementary Fig. 1d). Similarly, ME enhanced the expression of NST marker genes in fetal muscle at embryonic day 18.5 (E18.5) (Figure 1b,c,e-g). Consistently, the expression of calcium ion channel-related genes and phosphorylation of calcium/calmodulin-dependent protein kinase kinase 2 (CaMKK2), a key mediator of calcium signaling, were elevated in the M-OB/Ex fetal muscle compared to M-OB, showing that ME enhanced calcium signaling in fetal muscle impaired due to MO (Figure 1d,h). Of note, markers of mitochondrial biogenesis in female and male fetal muscles were similarly recovered in M-OB/Ex group (Figure 1i,j), in agreement with a previous report showing that SLN and UCP3 expression correlates with mitochondrial biogenesis.38 Taken together, exercise during pregnancy stimulates muscle-based thermogenesis in both mothers and their fetuses, as shown by elevated expression of SLN, UCP3 and mitochondrial biogenesis.
Figure 1.
Maternal exercise enhances muscle-based non-shivering thermogenesis in fetal muscle. (a) Representative thermographic images (left) and calculated averages (right) of surface temperature of M-Ctrl, M-OB and M-OB/Ex neonates at postnatal day 1 (P1) (n = 6). (b-d) mRNA levels of Sln (b), Ucp3 (c) and calcium ion channel related genes (d) in the muscle of M-Ctrl, M-OB and M-OB/Ex fetuses (n = 6). (e-h) Cropped western blots of SLN, UCP3 and p-CaMKK2 in fetal muscle (β-tubulin was used for normalization; n = 6). (i) mitochondrial DNA (mtDNA) copy number in the muscle of M-Ctrl, M-OB and M-OB/Ex fetuses (n = 6). (j) mRNA expression of mitochondrial biogenesis-related genes in the muscle of M-Ctrl, M-OB and M-OB/Ex fetuses (n = 6). Data are mean ± SEM, and each dot represents one litter; P values by one way ANOVA followed by post hoc (Tukey's test) analysis (a–j).
Maternal exercise improves metabolic-related molecular expression in the offspring challenged with HFD
Our previous study showed the long-lasting effects of ME, which reduced body weight gain and subcutaneous fat weight in the offspring mice fed a HFD.6 To determine the long-term effects of ME on offspring metabolic health, we analyzed SLN and UCP3 expression in adult offspring muscle challenged with either a CD or a HFD (Supplementary Fig. 1a) and found that ME persistently increased SLN and UCP3 expression in offspring muscle, in agreement with increase in CaMKK2 phosphorylation (Figure 2a–c). The gene expression associated with fatty acid oxidation was reduced due to HFD challenge, but preferentially up-regulated by ME (Figure 2d). ME further improved lipid metabolism in both female and male adult offspring challenged with HFD (Figure 2e). Altogether, ME has long-term effects in enhancing muscle-based NST, and protects offspring from diet-induced obesity, which relates to enhanced fatty acid β-oxidation.
Figure 2.
Maternal exercise long-termly activates muscle thermogenic gene expression impaired due to HFD feeding. One female and one male offspring per dam were weaned onto either a control diet (CD) or high fat diet (HFD) for 8 weeks (M-Ctrl_CD, M-Ctrl_HFD and M-Ex_HFD). (a-b) mRNA levels of Sln (a) and Ucp3 (b) genes in female and male offspring challenged with CD or HFD (n = 6). (c) Cropped western blots of SLN, UCP3 and p-CaMKK2 in female and male offspring challenged with CD or HFD (β-tubulin was used for normalization; n = 6). (d-e) mRNA expression of mitochondrial fatty acid oxidation (d) or lipid metabolism (e)-related genes (n = 6). Data are mean ± SEM, and each dot represents one litter; P values by one way ANOVA followed by post hoc (Tukey's test) analysis (a-e).
Apelin mirrors benefits of exercise on gene expression in fetal muscle
Given that exercise during pregnancy increased circulating apelin levels in maternal and fetal serum, and in fetal muscle from obese mothers (Supplementary Figs. 1c and 3a,b), we conducted another animal study to explore whether exogenous apelin administration exerts the beneficial effects of ME on NST in fetal muscle (Supplementary Fig. 2a). Consistent with the ME study, apelin administration elevated fetal muscle weight (Supplementary Fig. 2b), and apelin treatment mirrored the beneficial effects of ME in preventing the deterioration of thermogenic gene expression in MO fetal muscle (Figure 3c–g). Notably, apelin administration elevated CaMKK2 phosphorylation and mitochondrial biogenesis impaired due to MO (Figure 3h–j). These changes were further confirmed by RNA-seq using fetal muscle in response to maternal apelin administration. To test weather apelin and ME share mirroring common mechanism to mediate muscle thermogenesis, we also measured mitochondrial fatty acid oxidation in the fetal muscle of maternal apelin administration, showing highly active gene expression related to fatty acid oxidation (Figure 3k). In addition, in gene ontology (GO) analyses, we found that mitochondrial respiratory capacity and calcium ion activity-related pathways were down-regulated in fetal muscle of OB/PBS group compared to CD/PBS, whereas pathways related to mitochondrial biogenesis and function were up-regulated in response to apelin administration (OB/APN) (Figure 4a,b). Of note, MO (vs. control) or MO with apelin administration (vs. MO) generated similar effects in improving gene expression based on differentially expressed genes (DEGs) and Veen diagram analyses (Figure 4c–e). Consistent with RNA-seq data, respiratory exchange ratios in dams were down-regulated in OB/PBS, but up-regulated in response to apelin administration (Supplementary Fig. 2c–e). Taken together, apelin administration during pregnancy mirrors beneficial effects of ME on gene expression in fetal muscle.
Figure 3.
Apelin induces muscle-based thermogenesis in fetal muscle. (a) Circulating apelin levels in the fetal serum at E18.5 (n = 6). (b) Cropped western blots of apelin in female and male fetuses from maternal exercise (ME) study (β-tubulin was used for normalization; n = 6). (c-d) mRNA expression of Sln and Ucp3 in fetal muscle of CD/PBS, OB/PBS, and OB/APN (n = 6). (e–h) Cropped western blots of SLN (F), UCP3 (g) and CaMKK2 phosphorylation (h) in fetal muscle of apelin supplementation study (β-tubulin was used for normalization; n = 6). (i,j) mRNA expression of mitochondrial biogenic genes in fetal muscle of CD/PBS, OB/PBS and OB/APN (n = 6). (k) Gene expression related to mitochondrial fatty acid oxidation by RNA-seq data in fetal muscle of CD/PBS, OB/PBS, and OB/APN (n = 4). Data are mean ± SEM, and each dot represents one litter; P values by one way ANOVA followed by post hoc (Tukey's test) analysis (a-j).
Figure 4.
Transcriptome analysis (RNA-seq) of fetal muscle from the apelin supplementation study. (a) Principal-component analysis of fetal muscle of CD/PBS, OB/PBS and OB/APN (n = 4). (b) Expression profile and gene ontology (GO) analysis of up-/down-regulated genes related to muscle development, mitochondrial biogenesis and calcium mediated signaling pathways (n = 4). (c,d) Volcano plots of DEGs in fetal muscle of OB/PBS vs. CD/PBS (c) and OB/PBS vs. OB/APN (d). (e) Venn diagram of differentially expressed genes (DEGs) in fetal muscle of CD/PBS, OB/PBS and OB/APN (n = 4).
APJ is required for muscle-based non-shivering thermogenesis
To test the mediatory roles of apelin/APJ in muscle-based NST gene expression during fetal development, we isolated primary myogenic cells from Apj deficient mice (Figure 5a,d), as previously described.40 The average surface temperature in both Apj deficient female and male weanling offspring were lower than wild-type mice, but the body weight did not differ (Figure 5b,c). Positive correlations between thermogenic markers and average surface temperature were observed (Figure 5g). In cultured myogenic cells, the muscle-based thermogenic gene expression and protein levels were reduced due to Apj deficiency (Figure 5e,f). Together, APJ signaling stimulates NST gene expression, which correlates with muscle-based thermogenesis.
Figure 5.
Apj is required for muscle-based non-shivering thermogenesis. (a) Generation of Apj deficient mice. (b) Body weight in both female and male Floxed or Knockdown (KD) mice (n = 3). (c) Representative heat images (left) and means (right) of female and male Floxed or KD mice (n = 3). (d) Myogenic cell isolation from β-actinCre/ApjFlox/+ mice. (e) mRNA levels of Sln (left) and Ucp3 (right) in myogenic cells isolated from female and male Floxed or KD (n = 3). (f) Cropped western blots of SLN and UCP3 in myogenic cells isolated from female and male Floxed or KD (β-tubulin was used for normalization; n = 3). (g) Pearson correlation between surface temperature and SLN or UCP3 protein levels in Apj deficient model (Flox; n = 3, KD; n = 3). Data are mean ± SEM, and each dot represents one litter; two-sided P values by unpaired Student's t-test (b–f) and Pearson correlation (g).
Acute exercise stimulates muscle-based thermogenic gene expression by AMPKα1 activation
We found that ME or apelin treatment robustly stimulated AMPK activation, while MO inhibited AMPK in both female and male fetal muscle (Figure 6a,b). To investigate direct effects of exercise on respiratory capacity, mitochondrial biogenesis, and muscle-based thermogenic gene expression in AMPKα1 deficient mice, we generated an inducible Prkaa1 knockout (AMPKα1 KO) mouse (Rosa-cre;Prkaa1flox/flox) for in vivo study of exercise intervention (Figure 6c,d). As we expected, muscular gene expression including thermogenesis (Sln and Ucp3) and mitochondrial biogenesis (Ppargc1a and Tfam) was down-regulated in AMPK deficient mice, whereas no difference in PGC-1α protein level between with and without AMPK was found (Figure 6e,f). However, PGC-1α protein level was up-regulated in response to single bout of exercise in control mice, while its elevation in AMPK deficient mice was not found (Figure 6f). Consistently, oxygen consumption rates (OCRs), cardon dioxide production, respiratory exchange ratios (RERs), and carbohydrate (CHO) oxidation were reduced in AMPK KO mice (Figure 6g and Supplementary Fig. 3a–h). Then, we measured maximal capacity of oxygen consumption (VO2max) during exercise to test direct effects of endurance exercise via single bout of exhaustive exercise. Expectedly, VO2max in AMPK KO mice was reduced compared to control mice (Prkaa1flox/flox). Consistent with respiratory capacity, muscular endurance and maximal strengths were impaired due to AMPK deficiency without changes in the body weight (Supplementary Fig. 4a–e). Taken together, AMPK activation is required for exercise-dependent mitochondrial and muscle-based thermogenic gene expression.
Figure 6.
Exercise directly activates mitochondrial biogenesis via AMPK activation. (a,b) Cropped western blots of AMPK phosphorylation in fetal muscle of maternal exercise (ME; a) or apelin supplementation (b) studies (β-tubulin was used for normalization; n = 6). (c,d) Generation of Prkaa1 knockout mice. (e) Mitochondrial biogenesis-related gene expression in the Gastrocnemius muscle of female and male Prkaa1 deficient mice. (f) Cropped western blots of PGC-1α in Prkaa1 deficient muscle before and after single bout of exercise (β-tubulin was used for normalization; n = 5 for pre-Exercise; n = 6 for post-Exercise). (g) Time-resolved oxygen consumption and analysis of female and male Flox or Prkaa1 deficient mice (n = 6). Data are mean ± SEM, and each dot represents one litter; P values by one way ANOVA followed by post hoc (Tukey's test) analysis (a,b); two-sided P values by unpaired Student's t-test (e–g).
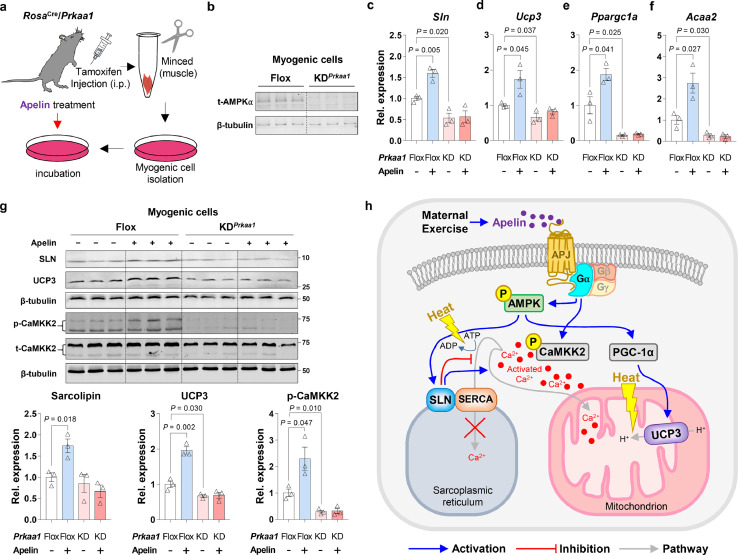
ME stimulates muscle-based thermogenesis by stimulating apelin-dependent AMPKα1 activation
Since AMPKα1 deficiency is impactful in regulating apelin signaling (Supplementary Fig. 4f), we hypothesized that AMPK is a downstream target of apelin signaling in mediating muscle-based thermogenic gene expression. To determine whether AMPK activation is required for stimulating muscle-based NST due to apelin administration, we utilized an inducible Prkaa1 knockout (AMPKα1 KO) mice and isolated primary myogenic cells (Figure 7a,b). Expectedly, apelin treatment induced muscle thermogenesis-related gene expression, the expression of mitochondrial biogenesis-related gene, Ppragc1a, and fatty acid oxidation-related gene, Acss2; these effects of apelin were abolished due to AMPKα1 deficiency (Figure 7c–f). Consistently, we found that apelin up-regulated SLN and UCP3 protein levels in isolated myogenic cells, which were absent in AMPKα1-deficient myogenic cells (Figure 7g). Furthermore, apelin elevated calcium mediated signaling, but absent due to AMPKα1 deficiency (Figure 7g). Together, apelin stimulates the expression of SLN and UCP3 through AMPKα1 activation (Figure 7h).
Figure 7.
Muscle-based non-shivering thermogenesis is activated by apelin-AMPK axis. (a) Myogenic cell isolation from male β-actinCre/Apjflox/+ weanling mice and treated with apelin. (b) Cropped western blots to show Prkaa1 deficiency in the primary myogenic cells (β-tubulin was used for normalization; n = 3). (c–f) mRNA expression of Sln, Ucp3, Ppargc1a, and Acaa2 in the primary myogenic cells with/without apelin treatment (n = 3). (g) Cropped western blots of SLN, UCP3 and p-CaMKK2 in the primary myogenic cells with/without apelin treatment (β-tubulin was used for normalization; n = 3). (h) Working model: ME-induced apelin activates AMPK and CaMKK2 phosphorylation, thereby leading to SLN and UCP3-dependent non-shivering thermogenesis. Data are mean ± SEM, and each dot represents one litter; P values by two-way ANOVA followed by post hoc (Tukey's test) analysis (c–g).
Discussion
The rate of pediatric obesity is growing worldwide, which necessitates discovery of underlying mechanisms and developing molecular and physiological therapies to combat obesity during childhood and adolescence.42 AMPK is an essential regulator of metabolic gene expression and calcium ion activity.43,44 As a G protein-coupled receptor, APJ is activated by apelin, which induces AMPK phosphorylation and mitochondrial biogenesis.40 Exercise stimulates apelin-AMPK axis and increases cytosolic Ca2+ level.28,45, 46, 47 Whereas the effects of ME on thermogenic gene expression in fetal muscle and its intergenerational effects of exercise during pregnancy on offspring metabolism have not been examined. We found that ME stimulates apelin production, AMPK activation, and the expression of muscle-based thermogenic genes in fetal muscle. Furthermore, the up-regulation of thermogenic genes persisted in offspring, which accelerates energy consumption through NST, thereby improving metabolic homeostasis of both female and male offspring mice.6,40 Our findings are clinically important, because past studies of NST focused on brown fat, but the amount of brown fat gradually reduces when age increases, and metabolic diseases are common in aged populations.4 Thus, muscle-based NST is important to improve metabolism and combat obesity.10 Our data are consistent with previous meta-analyses where children outcomes are improved due to ME.48,49
Calcium ion is a second messenger required for diverse biological processes, including muscle contraction and neural transmission.50 In skeletal muscle, SERCA pump and Ryanodine receptor (RYR) regulate intracellular calcium ion concentration.47,51 Sarcolipin-dependent SERCA inactivation elevates cytoplasmic calcium ion levels, which stimulates muscular NST,12,16 and protects against metabolic impairment of muscle due to obesity.12 Furthermore, elevated cytosolic calcium ions stimulate CaMKK2 phosphorylation and induces muscular UCP3 expression, enhancing fatty acid oxidation.19,20 While obesity suppresses oxidative metabolism in skeletal muscle,52 activation of sarcolipin signaling pathways promotes mitochondrial biogenesis and oxidative phosphorylation,38 preventing obesity-related metabolic dysfunction.10 Similarly, chronic cold acclimation stimulates futile calcium ion cycling in muscle, enhancing NST.53 In the current study, we found that ME activates thermogenic gene expression in fetal muscle; importantly, ME intergenerationally increased the expression of sarcolipin and UCP3 in adult offspring, which might be due to persistent elevation of PGC-1α and sensitization of apelin/AMPK axis, enhancing NST of muscle. Remarkably, ME offspring were protected against diet-induced obesity,6,40 consistent with earlier reports.12,16
Apelin binds to the C-terminal of APJ and activates G protein subunits, including Gαq and Gαi proteins, leading to Gαq-mediated AMPK activation.29 Besides, AMPK activates CaMKK2 and its associated calcium signaling pathways.44 AMPK phosphorylation further activates PGC-1α and stimulates SLN expression.54 SLN blocks SERCA-mediated calcium ion takeback into the sarcoplasmic reticulum (SR), which elevates cytosolic Ca2+ concentration55 and oxidative metabolism.19 In addition to stimulation of SLN-dependent calcium signaling, PGC-1α mediates UCP3 expression in skeletal muscle through partnering with the peroxisome proliferator-activated receptor β (PPARβ).21 Besides, UCP3 mediates protons (H+) transfer from inner to outer mitochondrial membrane, dissipating energy for heat generation.11,56 In addition to apelin-induced Gαq activation, apelin also regulates Gαi and we previously showed that the phosphorylation of protein kinase A (PKA) as a down-stream target of Gαi was inhibited in the fetal tissue by apelin supplementation during pregnancy,6 consistent with the previous report showing that apelin-activated APJ (Gαi) reduces intracellular cyclic adenosine monophosphate (cAMP) level.29 In our previous study, we found that ME stimulates placental apelin secretion, which elevates apelin level in the fetal circulation.6 In the current study, we found that apelin activates AMPK to enhance mitochondrial biogenesis and the expression of thermogenic genes in muscle cells, which are highly stimulated by exercise. Supportively, AMPK activation due to regular exercise enhances glucose and fatty acid uptake by muscle, improves mitochondrial homeostasis and metabolic capacity of muscle, and ameliorates the hyperglycemic state and metabolic dysfunction due to obesity and type 2 diabetes.43 Consistently, we previously reported that ME induces apelin activation and prevents metabolic dysfunction in offspring due to MO as indicated by reduced blood glucose and insulin levels, and alleviated insulin resistance.31 Apelin has key roles in maintenance of insulin sensitivity57 and stimulating glucose utilization58 in the skeletal muscle, which might be due to the specific activation of Gαq and AMPK in skeletal muscle, which improves glucose uptake and insulin sensitivity.59
Although we showed that apelin supplementation during pregnancy activated AMPK and stimulated CaMKK2 phosphorylation, we did not measure changes in calcium ion levels upon apelin-APJ activation, which is a limitation in the current study. Furthermore, our conclusion of the muscle-based NST in offspring could be further strengthened by measuring changes in ME-dependent or apelin-dependent mitochondrial respiratory activity. We plan to address these limitations in the future studies.
In summary, we have demonstrated that ME stimulates calcium signaling and mitochondrial biogenesis in fetal muscle impaired due to maternal obesity. Furthermore, we found the long-term effects of ME on sarcolipin and UCP3 activation in offspring muscle, which protects offspring against diet-induced obesity and metabolic disorders. We identified apelin-APJ-AMPK axis as one of the major underlying mechanisms in regulating the expression of muscle-based thermogenic gene expression during fetal muscle development. Considering the commonness of maternal obesity and the high accessibility of exercise, our findings have important clinical applications in improving fetal muscle development and long-term metabolic health of offspring born to obese mothers.
Contributors
J.S.S. and S.A.C. designed and performed experiments, and wrote the manuscript. L.Z. and J.M.D. contributed to caring animals and conducted experiments. H.W. and Z.J contributed the analysis of RNA-seq data. M-J.Z. analyzed the data and provided oversight for nutrient experiments. M.D. and J.S.S. designed experiments, analyzed the data, and edited the manuscript. J.S.S., S.A.C. and M.D. are the guarantors of this work and had full access to all the data in the current study.
Declaration of interests
The authors declare no competing interests.
Acknowledgments
Acknowledgments
This work was supported by National Institutes of Health Grant R01-HD067449.
Data Sharing
All data needed to evaluate the conclusions in the paper are present in the paper or the Supplementary Materials. RNA-seq transcriptome data have been deposited to the NCBI Sequence Read Archive (SRA) repository: https://www.ncbi.nlm.nih.gov/bioproject/PRJNA674423/.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.ebiom.2022.103842.
Contributor Information
Jun Seok Son, Email: junseok.son@som.umaryland.edu.
Min Du, Email: min.du@wsu.edu.
Appendix. Supplementary materials
References
- 1.Overweight, obesity, and health risk. Arch Intern Med. 2000;160(7):898–904. doi: 10.1001/archinte.160.7.898. [DOI] [PubMed] [Google Scholar]
- 2.Heslehurst N., Rankin J., Wilkinson J.R., Summerbell C.D. A nationally representative study of maternal obesity in England, UK: trends in incidence and demographic inequalities in 619 323 births, 1989–2007. Int J Obes. 2010;34(3):420–428. doi: 10.1038/ijo.2009.250. (2005) [DOI] [PubMed] [Google Scholar]
- 3.Catalano P.M., Shankar K. Obesity and pregnancy: mechanisms of short term and long term adverse consequences for mother and child. BMJ Clin Res Ed. 2017;356:j1. doi: 10.1136/bmj.j1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cypess A.M., Lehman S., Williams G., et al. Identification and importance of brown adipose tissue in adult humans. N Engl J Med. 2009;360(15):1509–1517. doi: 10.1056/NEJMoa0810780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saito M., Okamatsu-Ogura Y., Matsushita M., et al. High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes. 2009;58(7):1526–1531. doi: 10.2337/db09-0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Son J.S., Zhao L., Chen Y., et al. Maternal exercise via exerkine apelin enhances brown adipogenesis and prevents metabolic dysfunction in offspring mice. Sci Adv. 2020;6(16):eaaz0359. doi: 10.1126/sciadv.aaz0359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pfannenberg C., Werner M.K., Ripkens S., et al. Impact of age on the relationships of brown adipose tissue with sex and adiposity in humans. Diabetes. 2010;59(7):1789–1793. doi: 10.2337/db10-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DeFronzo R.A., Jacot E., Jequier E., Maeder E., Wahren J., Felber J.P. The effect of insulin on the disposal of intravenous glucose. Results from indirect calorimetry and hepatic and femoral venous catheterization. Diabetes. 1981;30(12):1000–1007. doi: 10.2337/diab.30.12.1000. [DOI] [PubMed] [Google Scholar]
- 9.Thiebaud D., Jacot E., DeFronzo R.A., Maeder E., Jequier E., Felber J.P. The effect of graded doses of insulin on total glucose uptake, glucose oxidation, and glucose storage in man. Diabetes. 1982;31(11):957–963. doi: 10.2337/diacare.31.11.957. [DOI] [PubMed] [Google Scholar]
- 10.Betz M.J., Enerback S. Targeting thermogenesis in brown fat and muscle to treat obesity and metabolic disease. Nat Rev Endocrinol. 2018;14(2):77–87. doi: 10.1038/nrendo.2017.132. [DOI] [PubMed] [Google Scholar]
- 11.Li H., Wang C., Li L., Li L. Skeletal muscle non-shivering thermogenesis as an attractive strategy to combat obesity. Life Sci. 2021;269 doi: 10.1016/j.lfs.2021.119024. [DOI] [PubMed] [Google Scholar]
- 12.Maurya S.K., Periasamy M. Sarcolipin is a novel regulator of muscle metabolism and obesity. Pharmacol Res. 2015;102:270–275. doi: 10.1016/j.phrs.2015.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Siti F., Dubouchaud H., Hininger I., et al. Maternal exercise before and during gestation modifies liver and muscle mitochondria in rat offspring. J Exp Biol. 2019;222(10):jeb194969. doi: 10.1242/jeb.194969. [DOI] [PubMed] [Google Scholar]
- 14.Goonasekera S.A., Lam C.K., Millay D.P., et al. Mitigation of muscular dystrophy in mice by SERCA overexpression in skeletal muscle. J Clin Invest. 2011;121(3):1044–1052. doi: 10.1172/JCI43844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Odermatt A., Taschner P.E., Khanna V.K., et al. Mutations in the gene-encoding SERCA1, the fast-twitch skeletal muscle sarcoplasmic reticulum Ca2+ ATPase, are associated with brody disease. Nat Genet. 1996;14(2):191–194. doi: 10.1038/ng1096-191. [DOI] [PubMed] [Google Scholar]
- 16.Bal N.C., Maurya S.K., Sopariwala D.H., et al. Sarcolipin is a newly identified regulator of muscle-based thermogenesis in mammals. Nat Med. 2012;18(10):1575–1579. doi: 10.1038/nm.2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brownstein A.J., Veliova M., Acin-Perez R., Liesa M., Shirihai O.S. ATP-consuming futile cycles as energy dissipating mechanisms to counteract obesity. Rev Endocr Metab Disord. 2021 doi: 10.1007/s11154-021-09690-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Periasamy M., Herrera J.L., Reis F.C.G. Skeletal muscle thermogenesis and its role in whole body energy metabolism. Diabetes Metab J. 2017;41(5):327–336. doi: 10.4093/dmj.2017.41.5.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maurya S.K., Bal N.C., Sopariwala D.H., et al. Sarcolipin is a key determinant of the basal metabolic rate, and its overexpression enhances energy expenditure and resistance against diet-induced obesity. J Biol Chem. 2015;290(17):10840–10849. doi: 10.1074/jbc.M115.636878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Costford S.R., Seifert E.L., Bézaire V., et al. The energetic implications of uncoupling protein-3 in skeletal muscle. Appl Physiol Nutr Metab. 2007;32(5):884–894. doi: 10.1139/H07-063. Physiologie appliquee, nutrition et metabolisme. [DOI] [PubMed] [Google Scholar]
- 21.Lima T.I., Guimarães D., Sponton C.H., et al. Essential role of the PGC-1α/PPARβ axis in Ucp3 gene induction. J Physiol. 2019;597(16):4277–4291. doi: 10.1113/JP278006. [DOI] [PubMed] [Google Scholar]
- 22.Waldeck-Weiermair M., Malli R., Naghdi S., Trenker M., Kahn M.J., Graier W.F. The contribution of UCP2 and UCP3 to mitochondrial Ca(2+) uptake is differentially determined by the source of supplied Ca(2+) Cell Calcium. 2010;47(5):433–440. doi: 10.1016/j.ceca.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 23.Convertino V.A., Morey E.R., Greenleaf J.E. Reduction in plasma calcium during exercise in man. Nature. 1982;299(5884):658. doi: 10.1038/299658a0. [DOI] [PubMed] [Google Scholar]
- 24.Tatemoto K., Hosoya M., Habata Y., et al. Isolation and characterization of a novel endogenous peptide ligand for the human APJ receptor. Biochem Biophys Res Commun. 1998;251(2):471–476. doi: 10.1006/bbrc.1998.9489. [DOI] [PubMed] [Google Scholar]
- 25.Akcılar R., Turgut S., Caner V., et al. The effects of apelin treatment on a rat model of type 2 diabetes. Adv Med Sci. 2015;60(1):94–100. doi: 10.1016/j.advms.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 26.Vinel C., Lukjanenko L., Batut A., et al. The exerkine apelin reverses age-associated sarcopenia. Nat Med. 2018;24(9):1360–1371. doi: 10.1038/s41591-018-0131-6. [DOI] [PubMed] [Google Scholar]
- 27.Hwangbo C., Wu J., Papangeli I., et al. Endothelial APLNR regulates tissue fatty acid uptake and is essential for apelin’s glucose-lowering effects. Sci Transl Med. 2017;9(407):eaad4000. doi: 10.1126/scitranslmed.aad4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang G., Anini Y., Wei W., et al. Apelin, a new enteric peptide: localization in the gastrointestinal tract, ontogeny, and stimulation of gastric cell proliferation and of cholecystokinin secretion. Endocrinology. 2004;145(3):1342–1348. doi: 10.1210/en.2003-1116. [DOI] [PubMed] [Google Scholar]
- 29.Yue P., Jin H., Xu S., et al. Apelin decreases lipolysis via G(q), G(i), and AMPK-dependent mechanisms. Endocrinology. 2011;152(1):59–68. doi: 10.1210/en.2010-0576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Flegal K.M., Carroll M.D., Kit B.K., Ogden C.L. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA. 2012;307(5):491–497. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 31.Son J.S., Liu X., Tian Q., et al. Exercise prevents the adverse effects of maternal obesity on placental vascularization and fetal growth. J Physiol. 2019;597(13):3333–3347. doi: 10.1113/JP277698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aye I.L., Rosario F.J., Powell T.L., Jansson T. Adiponectin supplementation in pregnant mice prevents the adverse effects of maternal obesity on placental function and fetal growth. Proc Natl Acad Sci U S A. 2015;112(41):12858–12863. doi: 10.1073/pnas.1515484112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang Q., Liang X., Sun X., et al. AMPK/alpha-ketoglutarate axis dynamically mediates DNA demethylation in the Prdm16 promoter and brown adipogenesis. Cell Metab. 2016;24(4):542–554. doi: 10.1016/j.cmet.2016.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chae S.A., Son J.S., Zhu M.J., de Avila J.M., Du M. Treadmill running of mouse as a model for studying influence of maternal exercise on offspring. Bio Protoc. 2020;10(23):e3838. doi: 10.21769/BioProtoc.3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tunster S.J. Genetic sex determination of mice by simplex PCR. Biol Sex Differ. 2017;8(1):31. doi: 10.1186/s13293-017-0154-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kilkenny C., Browne W.J., Cuthill I.C., Emerson M., Altman D.G. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. Osteoarthr Cartil. 2012;20(4):256–260. doi: 10.1016/j.joca.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 37.Than A., He H.L., Chua S.H., et al. Apelin enhances brown adipogenesis and browning of white adipocytes. J Biol Chem. 2015;290(23):14679–14691. doi: 10.1074/jbc.M115.643817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maurya S.K., Herrera J.L., Sahoo S.K., et al. Sarcolipin signaling promotes mitochondrial biogenesis and oxidative metabolism in skeletal muscle. Cell Rep. 2018;24(11):2919–2931. doi: 10.1016/j.celrep.2018.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou X., Li R., Michal J.J., et al. Accurate profiling of gene expression and alternative polyadenylation with whole transcriptome termini site sequencing (WTTS-Seq) Genetics. 2016;203(2):683–697. doi: 10.1534/genetics.116.188508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Son J.S., Chae S.A., Wang H., et al. Maternal inactivity programs skeletal muscle dysfunction in offspring mice by attenuating apelin signaling and mitochondrial biogenesis. Cell Rep. 2020;33(9) doi: 10.1016/j.celrep.2020.108461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jones T.E., Baar K., Ojuka E., Chen M., Holloszy J.O. Exercise induces an increase in muscle UCP3 as a component of the increase in mitochondrial biogenesis. Am J Physiol Endocrinol Metab. 2003;284(1):E96–101. doi: 10.1152/ajpendo.00316.2002. [DOI] [PubMed] [Google Scholar]
- 42.Cardel M.I., Atkinson M.A., Taveras E.M., Holm J.C., Kelly A.S. Obesity treatment among adolescents: a review of current evidence and future directions. JAMA Pediatr. 2020;174(6):609–617. doi: 10.1001/jamapediatrics.2020.0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Herzig S., Shaw R.J. AMPK: guardian of metabolism and mitochondrial homeostasis. Nat Rev Mol Cell Biol. 2018;19(2):121–135. doi: 10.1038/nrm.2017.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Takabatake S., Ohtsuka S., Sugawara T., et al. Regulation of Ca(2+)/calmodulin-dependent protein kinase kinase β by cAMP signaling. Biochim Biophys Acta Gen Subj. 2019;1863(4):672–680. doi: 10.1016/j.bbagen.2018.12.012. [DOI] [PubMed] [Google Scholar]
- 45.Hu H., He L., Li L., Chen L. Apelin/APJ system as a therapeutic target in diabetes and its complications. Mol Genet Metab. 2016;119(1–2):20–27. doi: 10.1016/j.ymgme.2016.07.012. [DOI] [PubMed] [Google Scholar]
- 46.Niederberger E., King T.S., Russe O.Q., Geisslinger G. Activation of AMPK and its impact on exercise capacity. Sports Med. 2015;45(11):1497–1509. doi: 10.1007/s40279-015-0366-z. (Auckland, NZ) [DOI] [PubMed] [Google Scholar]
- 47.Berchtold M.W., Brinkmeier H., Müntener M. Calcium ion in skeletal muscle: its crucial role for muscle function, plasticity, and disease. Physiol Rev. 2000;80(3):1215–1265. doi: 10.1152/physrev.2000.80.3.1215. [DOI] [PubMed] [Google Scholar]
- 48.Davenport M.H., Meah V.L., Ruchat S.M., et al. Impact of prenatal exercise on neonatal and childhood outcomes: a systematic review and meta-analysis. Br J Sports Med. 2018;52(21):1386–1396. doi: 10.1136/bjsports-2018-099836. [DOI] [PubMed] [Google Scholar]
- 49.Chen Y., Ma G., Hu Y., et al. Effects of maternal exercise during pregnancy on perinatal growth and childhood obesity outcomes: a meta-analysis and meta-regression. Sports Med. 2021;51(11):2329–2347. doi: 10.1007/s40279-021-01499-6. (Auckland, NZ) [DOI] [PubMed] [Google Scholar]
- 50.Gorski P.A., Ceholski D.K., Young H.S. Structure-function relationship of the SERCA pump and its regulation by phospholamban and sarcolipin. Adv Exp Med Biol. 2017;981:77–119. doi: 10.1007/978-3-319-55858-5_5. [DOI] [PubMed] [Google Scholar]
- 51.Santulli G., Nakashima R., Yuan Q., Marks A.R. Intracellular calcium release channels: an update. J Physiol. 2017;595(10):3041–3051. doi: 10.1113/JP272781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Paran C.W., Verkerke A.R., Heden T.D., et al. Reduced efficiency of sarcolipin-dependent respiration in myocytes from humans with severe obesity. Obesity. 2015;23(7):1440–1449. doi: 10.1002/oby.21123. (Silver Spring, Md) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Teulier L., Rouanet J.L., Rey B., Roussel D. Ontogeny of non-shivering thermogenesis in muscovy ducklings (Cairina moschata) Comp Biochem Physiol Part A Mol Integr Physiol. 2014;175:82–89. doi: 10.1016/j.cbpa.2014.05.012. [DOI] [PubMed] [Google Scholar]
- 54.Sepa-Kishi D.M., Sotoudeh-Nia Y., Iqbal A., Bikopoulos G., Ceddia R.B. Cold acclimation causes fiber type-specific responses in glucose and fat metabolism in rat skeletal muscles. Sci Rep. 2017;7(1):15430. doi: 10.1038/s41598-017-15842-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.MacLennan D.H., Asahi M., Tupling A.R. The regulation of SERCA-type pumps by phospholamban and sarcolipin. Ann N Y Acad Sci. 2003;986:472–480. doi: 10.1111/j.1749-6632.2003.tb07231.x. [DOI] [PubMed] [Google Scholar]
- 56.Bézaire V., Seifert E.L., Harper M.E. Uncoupling protein-3: clues in an ongoing mitochondrial mystery. FASEB J. 2007;21(2):312–324. doi: 10.1096/fj.06-6966rev. [DOI] [PubMed] [Google Scholar]
- 57.Yue P., Jin H., Aillaud M., et al. Apelin is necessary for the maintenance of insulin sensitivity. Am J Physiol Endocrinol Metab. 2010;298(1):E59–E67. doi: 10.1152/ajpendo.00385.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dray C., Knauf C., Daviaud D., et al. Apelin stimulates glucose utilization in normal and obese insulin-resistant mice. Cell Metab. 2008;8(5):437–445. doi: 10.1016/j.cmet.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 59.Bone D.B.J., Meister J., Knudsen J.R., et al. Skeletal muscle-specific activation of G(q) signaling maintains glucose homeostasis. Diabetes. 2019;68(6):1341–1352. doi: 10.2337/db18-0796. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.