Highlights
-
•
Thioredoxin reductase (TrxR), a seleno enzyme, regulates cellular redox.
-
•
Several human cancers are known to overexpress TrxR.
-
•
Inhibitors of TrxR have enhanced radiation induced cytotoxicity in multiple cancers.
-
•
TrxR could be a potential target during radiotherapy of cancer patients.
Keywords: Thioredoxin reductase, Thioredoxin, Redox homeostasis, Radiation
Abbreviations: AIF, apoptosis inducing factor; APE1, apurinic/apyrimidinic endonuclease 1; ARE, antioxidant response element; ASK1, apoptosis signaling kinase; BSO, buthionine sulfoximine; Con A, concanavalin A; CypD, cyclophilin D; FAD, flavine adenine dinucleotide; G.R., glutathione reductase; Grx2, glutaredoxin 2; GNP, gold nanoparticle; Hif1α, hypoxia inducible factor 1 α; HNSCC, head & neck squamous cell carcinoma; IQ9, indolequinone 9; IR, ionizing radiation; LLC, Lewis lung carcinoma; LPS, lipopolysaccharide; MSR, methionine sulfoxide reductase; MnSOD, manganese superoxide dismutase; NADPH, nicotinamide adenine dinucleotide phosphate; N.F.-κB, nuclear factor kappa B; Nrf2, nuclear factor erythroid 2 related factor 2; NSCLC, non-small cell lung cancer; PDI, protein disulfide isomerase; PRX, peroxiredoxin; PTEN, Phosphatase and Tensin Homolog; PMA, phorbol-12-myristic acid; PEG, polyethylene glycol; ROS, reactive oxygen species; Ref-1, redox factor 1; RNR, ribonucleotide reductase; SAPK, stress activated protein kinase; Sec, selenocysteine; SLNB, solid lipid nanoparticles of baicalein; Trx, thioredoxin; TrxR, thioredoxin reductase; TNF, tumor necrosis factor
Abstract
Novel agents are required to increase the radiosensitivity of cancer and improve the outcome of radiotherapy. Thioredoxin (Trx) and thioredoxin reductase (TrxR) reduce the oxidized cysteine thiols in several proteins, which regulate cellular redox, survival, proliferation, DNA synthesis, transcription factor activity and apoptosis. TrxR is essential for maintaining a conducive redox state for tumor growth, survival and resistance to therapy. Therefore, it is an appealing pharmacological target for the radiosensitization of tumors. Ionizing radiation (IR) is known to cause cytotoxicity through ROS, oxidative stress and DNA damage. Inhibition of thioredoxin system augments IR induced oxidative stress and potentiates cytotoxic effects. However, TrxR also regulates several critical cellular processes in normal cells. Here, we highlight the pre-clinical research and pharmacological studies to surmise possible utility of different TrxR inhibitors for radiosensitization. This review provides a succinct perspective on the role of TrxR inhibitors during the radiotherapy of cancer.
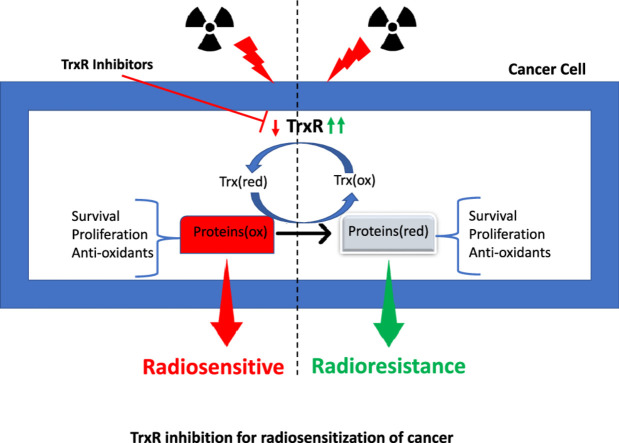
Graphical abstract
Introduction
Radiotherapy (RT) remains the mainstay of cancer treatment as a palliative or curative therapy. However, intrinsic and acquired tumor radioresistance often reduces the desired therapeutic outcome. This leads to tumor recurrence and metastasis and impacts cancer patients' progression-free survival and quality of life [1]. There is unmet clinical requirement of agents that can enhance the sensitivity of cancer cells to radiotherapy. Safe and efficacious radiosensitizers will improve the therapeutic ratio of RT [2]. IR-induced cellular damage is primarily manifested during conventional RT through ROS-mediated infliction of critical cellular targets [3]. Radiosensitizer accentuates IR mediated tissue injury to achieve better tumor control. Cells have an inherent tendency to establish redox homeostasis after perturbations caused by oxidative stress-inducing agents [4]. Their ability to achieve steady-state redox equilibrium depends on the inherent pool of antioxidants and reducing equivalents. Cancer cells are equipped to overcome excess ROS burden by upregulating cellular antioxidant defense system that functions as rheostats for oxidative stress. Many cancers inherently over-express genes encoding cellular redox regulatory proteins [5].
Thioredoxin reductase is a seleno-enzyme from the flavoprotein family of oxido-reductases. It is the only pyridine nucleotide disulfide containing enzyme that reduces oxidized thioredoxin [6]. Human thioredoxin is a peptide enzyme with 105 amino acid residues (∼12 kDa) with disulfide oxido-reductase activity. It regulates the reduction of a wide variety of biological proteins such as ribonucleotide reductase, peroxiredoxin, phosphatase and tensin homolog (PTEN), redox factor-1, methionine sulfoxide reductase, transcription factors (viz. Nrf2, p53, NF-κB) and apoptosis signalling kinase [6]. TrxR is overexpressed in several cancers, given its crucial role in numerous physiological processes [7]. Elevated TrxR levels help cancer cells to cope with excess oxidative burden induced by ionizing radiation exposure. Cancer cells exploit TrxR for selective advantage over neighbouring normal cells during radiotherapy and exhibit resilience to conventional radiotherapy. Inhibition of TrxR makes cells more susceptible to IR induced damage, thereby amplifying the debilitating effects of the radiation-induced injury.
Since cells do not have an alternative arm to compensate for different functions of TrxR, they succumb to IR induced damage to critical biomolecules. Hence TrxR is an attractive druggable target to enhance the radiosensitivity of cancer cells [8]. Various TrxR inhibitors are under pre-clinical and clinical testing as anticancer and chemotherapeutic agents [7]. However, researchers are exploring TrxR as one of the exploitable targets for radiosensitization to develop a specific and selective radiosensitizer with a better therapeutic index. Investigators have reported natural, semi-synthetic and synthetic TrxR inhibitors as radiosensitizing agents in various types of cancers, including non-small cell lung cancer (NSCLC) [9], head and neck squamous cell carcinoma (HNSCC) [10], glioma [11], cervical cancer [12], prostate cancer [13], breast cancer [14] and colorectal cancer [15]. Several prospective and informative reviews describe the anticancer efficacy of different TrxR inhibitors. However, the utility of TrxR inhibitors as radiosensitizers has not been systematically reviewed. Here, we present a succinct overview of the studies on the use of TrxR inhibitors as radiosensitizers and provide a novel perspective on additional cellular targets involved in specific radiosensitization of cancer cells. TrxR is an attractive radiobiological target that warrants further investigation for its clinical development as a radiosensitizer.
Thioredoxin system
Thioredoxin
Thioredoxin was first reported in E.coli by Laurent in 1964 [16], and subsequently, Moore reported its presence in mammalian cells in 1967 [6,17]. Trx enzymes from different species show remarkable structural similarity with conserved cysteine-selenocysteine residues, N-terminal β-sheet, followed by α-helix and three β-sheets and C-terminal α-helix [6]. It is a small disulfide oxido-reductase protein that regulates the reduction of a wide variety of biological proteins such as ribonucleotide reductase, peroxiredoxin, phosphatase and tensin homolog (PTEN), redox factor-1, methionine sulfoxide reductase, transcription factors (viz. Nrf2, p53, NF-κB) and apoptosis signalling kinase [18]. Depending on the intracellular location of thioredoxin, it is named as (cytosolic) Trx1 and (mitochondrial) Trx2 [4]. The active site of Trx is conserved throughout all the organisms with CXXC motif with active site cysteines at positions 32 and 35 in humans [4]. Mammalian Trx1 contains additional cysteine residues at positions 62, 69 and 73 away from the active site. These residues are not found in Trx2 [6,7] and participate in post-translational regulation of signal transduction through glutathionylation, s-nitrosylation and thiol oxidation [18]. Proteomic studies have shown that these residues also affect the enzymatic activity of Trx [4,6,7].
The Trx fold contains a βαβ motif at the N-terminal and a ββα motif at the C-terminal. It is also found in other proteins that interact with disulfide or dithiols, such as glutaredoxin, glutathione S-transferase and protein disulfide isomerase [4,6]. N-terminal cysteine residue of Trx forms an intermolecular mixed disulfide with target proteins following nucleophilic attack [4,6,18]. Subsequently, C-terminal cysteine thiolate of Trx attacks this mixed disulfide producing reduced target protein and oxidized Trx [4,6,18]. Interestingly, oxidized (Trxox) and reduced (Trxred) forms of Trx constitute a conjugate redox pair, and their redox potential depends upon the ratio of these two forms present inside cells.
The gene encoding Trx is present on human chromosome 9, spans a 13 kb region (band 9q31.3) and contains five exons. The promoter region has a consensus sequence with a regulatory motif for constitutive/inducible expression, and antioxidant response element (ARE for binding of Nrf2). A variety of stimuli can activate Trx gene expression, including irradiation (X-ray and UV), oxidative stress (ROS), mitogenic stimuli (LPS, Concanavalin A), hormones (estradiol, testosterone), chemicals (viz. PMA), hypoxia and TNF [6].
The Human Trx2 gene is present on chromosome 22, which codes for mitochondrial Trx (Trx2) protein. Trx2 is essential during murine embryo development as a homozygous knockout is lethal and results in death on day 10 [6] [18]. Trx2 has only two cysteine residues, unlike Trx1, which contain additional three cysteine residues for post-translational regulation [6,18]. Trx2 maintains Bcl-XL protein level in mitochondria and prevents membrane permeabilization and membrane potential loss. Further, Trx2 can inhibit mitochondrial located ASK1 activity and inhibit the Src homology/collagen (Shc) adaptor protein p66Shc, an important regulator of mitochondrial redox and metabolism. Similar to Trx1, the redox status of Trx2 is very critical in determining cell viability and it can also be similarly detected by performing redox Western blotting [4,6,18,19].
Thioredoxin reductase
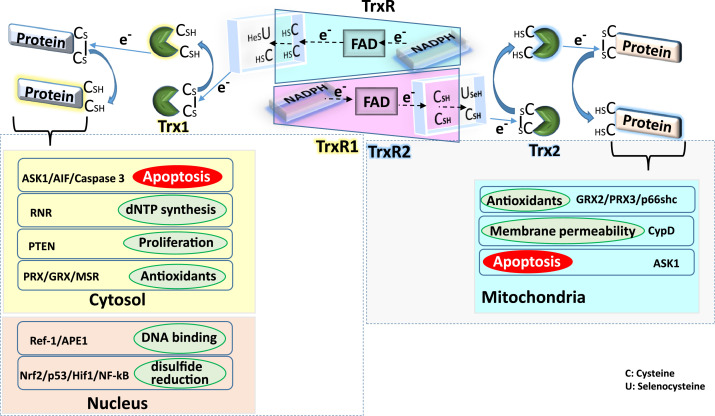
It is a seleno-enzyme belonging to the flavoprotein family of pyridine nucleotide disulfide containing oxido-reductase [6]. It is the lone oxido-reductase enzyme that can catalyze the reduction of disulfides in thioredoxin substrate [4]. The peculiarity of TrxR is that it contains selenocysteine (Sec, U) in the C-terminus active site consensus sequence Gly-Cys-SeCys-Gly and has broad substrate specificity other than Trx, which include glutaredoxin, protein disulfide isomerase, selenite, cytochrome C, lipoic acid and ubiquinone [6]. Cells contain three isoforms of TrxR in the cytosol (TrxR1), mitochondria (TrxR2) and testes (TrxR3), also known as thioredoxin glutathione reductase [6]. In humans, these three isoforms are encoded by three different genes, namely TXNRD1, TXNRD2 & TXNRD3 [6]. Human TrxR and glutathione reductase (GR) have a similar structure [18]. Incorporation of Sec into polypeptide chain by a stop codon (UGA) requires Sec insertion sequence element (SECIS), tRNA[Sec] and SECIS binding protein 2 (SBP2) [18]. Selenium is critical for TrxR activity, and its addition to culture medium increases cellular TrxR activity. Dietary intake of selenium modulates TrxR activity in vivo by regulating the protein levels of TrxR [20]. Fig. 1 shows different functions of thioredoxin system in cytosol, nucleus and mitochondria.
Fig. 1.
General functions of the thioredoxin system in cellular compartments
Trx1 reduces the disulfide bond in the proteins present in cytosol and nucleus and gets oxidized in the process. Active site Cys/SeC residues of TrxR1 reduce the oxidized Trx1 protein by transferring electron from NADPH through FAD. In cytosol, Trx1 is involved in the disulfide reduction of proteins involved in antioxidant defense (PRX/GRX/MSR), proliferation (PTEN), dNTP synthesis (RNR) and apoptosis (ASK1/AIF/Caspase 3). Whereas in the nucleus, Trx1 reduces disulfide bonds in transcription factors (NF-κB, Nrf-2, Hif1α, p53) and proteins regulating DNA binding (Ref-1, APE1).
In mitochondria, Trx2 reduces disulfide bonds in the proteins regulating mitochondrial redox (p66shc, GRX2 and PRX3), membrane permeability (CypD) and apoptosis (ASK1).
Inhibitors of thioredoxin reductase
The complex structure and catalysis of TrxR make it challenging to design specific TrxR inhibitors. TrxR inhibitors are divided into four major classes i) metal complexes ii) Michael acceptors, iii) natural molecules iv) Sulphur/Se/Te containing compounds. They can be further subdivided based on hard and soft acid & base, reversible & irreversible inhibitors, suicide substrates, cross-linking reactants, enzyme modifiers and so on [7,8,18]. The specificity of TrxR inhibition depends upon the biochemical differences between cysteine and selenocysteine. At physiological pH, thiols are converted into thiolates, and selenols predominantly exist as selenolates [21,22]. Therefore, it is challenging to design and develop a highly selective and specific inhibitor of TrxR. Further, considering the pivotal role of the thioredoxin system in normal cell survival, its inhibition invariably raises a possible threat of shutting down thioredoxin functions in non-malignant cells [21]. Thus, selective targeting of TrxR in cancer cells and its specific inhibition are the two important challenges in this field. However, a few potential molecules have been identified as TrxR inhibitors [21].
Current status of radiosensitizers
A radiosensitizer is an agent/molecule that can enhance the cytotoxic effects of radiation towards cancer cells and improve the outcome of radiotherapy [2]. According to G. E. Adams, radiosensitization can be classically achieved using one of the following five approaches: (i) inhibition of endogenous radioprotective substances (ii) conversion of radiosensitizer into the cytotoxic product due to radiolysis (iii) inhibition of repair (iv) thymine analogues for DNA and (v) oxygen mimics [2,23,24]. Recent technological advances have invigorated the field of radiosensitizer development. The current status of radiosensitizer research is systematically reviewed elsewhere, which advocates a multi-target approach for superior efficacy [2,24]. TrxR is a suitable candidate in this category as it is involved in critical redox pathways, which cannot be performed by any surrogate macromolecule [5].
TrxR inhibitors as radiosensitizers
NSCLC
One of the first reports regarding radiosensitization of NSCLC used a novel organoselenium TrxR inhibitor ethaselen, also known as BBSKE [9]. BBSKE (5µM) inhibited IR induced increase in TrxR activity in A549 and H1299 cells. The clonogenic assay showed that BBSKE significantly increased radiosensitivity of TrxR addicted A549 and H1299 cells but not H1666 cells expressing low levels of TrxR. In vivo studies using lung tumor xenograft model of Lewis Lung Carcinoma (LLC) cells in C57BL6 mice revealed significant synergism as evinced by the enhancement factor of 1.5 for the combination of IR (8Gy) and BBSKE (36 mg/kg b.w. for 14 days). Further, mean survival time of mice increased from 18±1 days in tumor implanted group to more than 50 days in BBSKE+IR group as against 33±5 and 27±4 days for BBSKE and IR alone groups respectively [9]. The mechanism of radiosensitization appears to be via inhibition of IR induced NF-κB transcriptional activity. NF-κB is a transcription factor that regulates the expression of antioxidant and anti-apoptotic genes and therefore reduces cell death in response to radiation. NF-κB over-expression is implicated in intrinsic and acquired tumor radioresistance [25]. Hence, downregulating NF-κB transcriptional activity by BBSKE helps to alleviate tumor radioresistance.
Our previous study using a metabolically stable analogue of curcumin revealed that dimethoxycurcumin (DiMC) interacts with side chains E & F of TrxR and inhibits its activity in cell-free system. DiMC significantly increased the radiosensitivity of A549 cells via suppression of TrxR activity. Direct involvement of TrxR inhibition was evinced from the studies showing that TrxR overexpression significantly ameliorated the radiosensitizing effect of DiMC in human lung cancer cells [26]. These findings also indicated a possible involvement of TrxR in determining the intrinsic radiosensitivity of human lung cancer cells.
Radiation resistant subline of HCC827 cells were obtained by sub-culturing the survivors after exposure to multiple doses of 2Gy radiation over a few weeks. miRNA profiling showed that downregulation of miR-124 correlates with radioresistance in HCC827 cells. HCC827 cells exhibit elevated levels of TrxR after IR exposure. Overexpression of miR-124 decreased TrxR1 expression, whereas inhibition of miR-124 showed the opposite effect. miR-124 interacts with 3’-UTR of TrxR mRNA and inhibits transcription and translation of TrxR. siRNA mediated knockdown of TrxR sensitized HCC827 cells to IR induced killing [27].
The 10 nm amine-PEG functionalized gold nanoparticles (GNPs) exhibited synergistic killing of A549 cells when combined with 225 kV X-rays or 25 keV/µm proton beam irradiation. High Z gold nanometal exhibits enhanced dose delivery and damage to DNA inducing cell death. GNP (50µg/ml gold, 8.22 nM concentration of 10 nm size GNPs) caused 71% inhibition of TrxR activity. The combination of GNP with 2Gy IR killed 25% more cells as compared with IR alone, whereas a combination with 5Gy IR killed about 76% of lung cancer cells. Following uptake of GNPs after receptor-mediated endocytosis, lysosomal acidic pH triggers the release of gold ions which interact with selenol moiety of TrxR, leading to cytotoxicity. Further, TrxR siRNA treated A549 cells showed higher radiosensitivity [28].
A well-characterized phosphine gold Au(I) compound [Au(SCN)(PEt3)] has also been shown to sensitize radioresistant U810 cells. U810 cells were treated with [Au(SCN)(PEt3)] for 24 h before γ-radiation and 24 h post-irradiation followed by continuous culture in the presence of 0.5 µM drug. U810 cells exhibited survival fractions of 0.71 and 0.51 at 2Gy & 5Gy respectively after treatment with TrxR inhibitor as opposed to survival fractions of 0.94 and 0.65 at similar doses without TrxR inhibition [29]. Microarray analysis revealed that genes related to DNA repair, replication and chromatin remodeling were significantly affected due to combination treatment.
Baicalein is a flavone, and it is naturally found in the roots of Scutellaria baicalensis. It has been shown to inhibit TrxR activity and Trx nuclear localization by directly binding to the active site of TrxR. Encapsulation of baicalein into in situ solid lipid nanoparticles (SLN) increased in vitro radiosensitivity of A549 cells as evinced by clonogenic and MTT assays. Treatment of A549 cells with SLNB (25 µM) in combination with IR 4Gy exhibited synergistic killing. Treatment of A549 cells with SLNB inhibited TrxR activity and increased ROS levels in combination with IR [30].
Breast cancer
A classical TrxR inhibitor auranofin (AF 3-10 µM) exhibited potent radiosensitization of murine mammary carcinoma cells 4T1 and EMT6 under both normoxic and hypoxic culture conditions in vitro. AF mediated TrxR inhibition lead to mitochondrial dysfunction, ROS overproduction, DNA damage and cell death [31]. In another study, AF caused 63 and 27% decrease in TrxR activity in SUM159 and MDA-MB-231 cells in vitro at 50 and 250nM concentrations, respectively. Treatment with 500nM AF sensitized breast cancer stem cells to IR induced cell death in MDA-MB-231 and SUM159 cells [32].
A potent TrxR inhibitor indolequinone 9 (IQ9) exhibited a sensitization enhancement ratio of 1.2, 1.3 and 1.43 in triple-negative breast cancer cell lines MDA-MB-231, MDA-MB-468 and MDA-MB-436, respectively. However, no significant enhancement was observed in luminal breast cancer cell lines MCF-7 and T47D. IQ9 exhibited IC50 of 0.272 to 0.44 nM for TrxR inhibition in TNBC cells as against 0.64 and 0.62 nM in luminal cells [14].
Curcumin inhibited TrxR activity more prominently in 2D cell culture than in 3D spheroid model of MCF-7 cells when combined with radiation. However, cell viability was significantly reduced under both the culture settings [33].
Cervical cancer
Selenadizole derivative SeD-3 inhibited TrxR in HeLa cells with EC50 value of 18.7 µM. SeD-3 (4 µM) increased the radiosensitivity of HeLa cells exposed to X-rays . Sequential treatment with SeD-3 (4 µM) and 2Gy radiation inhibited TrxR activity by 93.6%, leading to ROS over-production, increased γ-H2Ax foci formation, impaired repair of DNA damage and cell death [34].
Prostate cancer
miR-17-3p has been shown to simultaneously inhibit mitochondrial MnSOD, glutathione peroxidase 2 and thioredoxin reductase 2. Upregulation of miR-17-3p in PC3 cells enhanced radiation-induced cytotoxic effects through increased ROS production and decreased mitochondrial respiration. Transfection with miR-17-3p inhibitor or ectopic expression of mitochondrial antioxidant enzymes reversed the radiosensitivity of PC3 cells [13].
We have shown that DU145 cells exhibit higher basal radioresistance than PC3 cells due to inherent higher expression of Nrf2, GSH and thioredoxin reductase [35]. These results indicate probable involvement of TrxR in determining the cell type-specific radioresistance of tumor cells from a common tissue origin.
Head & neck cancer
Radioresistant human papilloma virus-negative (HPV-) (HNSCC) FaDu, JHU-022 and SQ20B cells have been shown to overexpress TrxR. However, HPV+ HNSCC UPCI-SCC090 cells do not exhibit TrxR overexpression and also show radiosensitivity. Curcumin enhances the radiosensitivity of HPV- HNSCC cells but not HPV+ HNSCC cells. Pretreatment of HPV- HNSCC cells with 10 µM curcumin increased radiation-induced killing with sensitization enhancement ratio (SER) of 1.41, 1.39 and 1.21 for FaDu, SQ20B, JHU-022 cells, respectively. Stable transfection with shRNA of TrxR in FaDu cells abolished the radiosensitization exhibited by curcumin [10].
Glioma
Silencing TP53-induced glycolysis and apoptosis regulator (TIGAR) increases the radiosensitivity of glioma cells by aggravating IR induced oxidative stress. Genetic ablation of TIGAR reduces radiation-induced migration of Trx1 to the nucleus in A172 and T98G cells. Overexpression of Trx1 in A172 glioma cells increased nuclear migration of Trx1 in TIGAR silenced cells. Trx1 overexpression reduced the radiosensitivity of TIGAR silenced glioma cells [11,36].
Colorectal cancer
Piperlongumine (PL) increased radiation-induced killing of CT26 and DLD-1 cells at 15 µM and 10 µM with enhancement ratios of 1.58 and 1.74 respectively under normoxic conditions and 1.62 and 1.78 under hypoxic conditions. PL significantly decreased TrxR activity in these cells via ROS overproduction which was reversed by antioxidant N-acetyl cysteine [15].
Other cancers
1,2,5-selenadiazole, a TrxR inhibitor, significantly reduced the IC50 from 12–28 µg/ml to 2–9 µg/ml when combined with 8Gy radiation in A375 melanoma cells. Selenadizaole exhibited enhanced TrxR inhibition combined with radiation followed by ROS generation, mitochondrial dysfunction and apoptosis induction. Similar radiosensitizing effects of selenadiazoles were also observed in HeLa cells through synergistic inhibition of TrxR [37,38]. We observed that human T cell lymphoma Jurkat cells are radioresistant as compared to normal human PBMNCs. Further studies revealed that Jurkat cells upregulate Nrf2 dependent antioxidant defense after exposure to radiation, which includes Trx and TrxR proteins. Interestingly, shRNA mediated knockdown of Trx1 or Trx2 significantly enhanced the radiosensitivity of human T cell lymphoma Jurkat cells indicating a probable role of Trx system in determining the intrinsic radioresistance of Jurkat cells [39].
Simultaneous inhibition of TrxR enzyme activity and glutathione production can shut down the redox metabolism in the cells and effectively increase their radiosensitivity. AF and BSO have been shown to delay the tumor growth by 13 days and 9 days in 4T1 and EMT tumor models via suppression of TrxR activity and production of GSH, respectively [32]. 2-AAPA has been reported to inhibit GR and TrxR activity in MDA-MB-231 and SUM159 cells and enhanced radiation (2Gy) induced cell death in MDA-MB-231 cells but not in SUM159 cells. Simultaneous inhibition of GSH and Trx metabolism exhibited additive killing in MDA-MB-231, SUM159 and PANC-1 cells when combined with 2Gy irradiation [32]. Simultaneous inhibition of glycolysis, glutathione and thioredoxin has been reported to increase the radiosensitivity of different types of cervical cancer cells. Treatment of SiHa cells with 2-deoxyglucose, AF and BSO significantly increased radiation-induced cell death in vitro. Combination of these inhibitors along with 4Gy dose of radiation most effectively limited the tumor growth in SiHa xenograft bearing mice [12]. Curcumin induced radiosensitization in squamous cell carcinoma HeLa and FaDu in TrxR1 dependent manner and knockdown of TrxR1 abolished radiosensitizing effects of curcumin [40]. Table 1 shows an updated compilation of TrxR inhibitors reported as radiosensitizers in various cancer types. Graphical abstract depicts the use of TrxR inhibitors for radiosensitization of cancer (Scheme 1).
Table 1.
TrxR inhibitors as radiosensitizers in different cancers.
| Sr. No. | Compound(s) | Mechanism | In vitro (effective concentration) | Cancer Type /Cell line | Ref. |
|---|---|---|---|---|---|
| In vivo (effective dose) | |||||
| 1. | Auranofin | Interaction of gold (soft acid) with catalytic SeC (soft base) of TrxR | 3–10 μM; 0.05 and 0.25 μM | Murine mammary carcinoma (4T1, EMT6); human breast cancer (MDA-MB-231 and SUM159) | [31] |
| Balb/c mice 3mg/kg/day subcutaneous for 10 days | 4T1 / EMT6 tumor bearing mice | ||||
| Gold nanoparticles | Interaction of gold (soft acid) with catalytic SeC (soft base) of TrxR | 8.22 nM | NSCLC (A549) | [28] | |
| 2. | [Au(SCN)(PEt3)] | i. Isothiocyanate mediated reversible TrxR inhibition | 2.5 μM | NSCLC (U1810) | [29] |
| ii. Au(I)-selenol interaction mediated TrxR inhibition | |||||
| 3. | Selenium containing i. BBSKE | Reversible inhibition of TrxR | 5 μM | NSCLC (A549, H1299) | [9] |
| C57 mice (36 mg/kg/day oral for 14 days) | Lewis Lung Carcinoma (LLC) tumor bearing mice | ||||
| ii. Se-D3 | Reversible inhibition of TrxR | 4–10 μg/ml | Melanoma (A375) | [37] | |
| 4. | i. Curcumin | Michael addition to TrxR and conversion into ROS generating enzyme | 30–100 µM | Breast (MCF7 2D monolayer and 3D spheroid) | [33] |
| 10 µM | Squamous carcinoma (HeLa & FaDu) | [40] | |||
| 10 µM | HNSCC (FaDu, JHU022, SQ20B) | [10] | |||
| Diet enriched with 1% w/v curcumin beginning on day 7 after tumor transplant (2Gy x 3) | FaDu-CMV-Luciferase cells in to athymic nude mice | ||||
| ii. dimethoxy-curcumin | 3.1 µM | NSCLC (A549) | [26] | ||
| 5. | Piperlongumine | Michael addition to Cys498 and hydrogen bond formation with Cys-497, Gln-494 and Trp-407 | 15 μM | Colorectal cancer (CT26) | [15] |
| 10 μM | Colorectal cancer DLD-1 | ||||
| 6. | Indolequinone (IQ9) | Alkylation of SeC498 of TrxR | 565.0 ± 29.5 nM | Brest cancer (MDA-MB-231) | [14] |
| 501.6 ± 170.7 nM | Breast cancer (MDA-MB-468) | ||||
| 194.6 ± 4.5 nM | Breast cancer (MDA-MB-436) |
Discussion and future directions
Despite recent advents in the delivery of radiation, the prognosis of patients is poor with several treatment-related morbidities. Radiotherapy as a non-invasive effort has a limited range of success for the local management of cancer as a monotherapy. However, it needs to be combined with adjuvants for achieving therapy goals effectively [41]. Combining chemo-radiotherapy for therapy-resistant cancers to salvage the patients has also yielded limited success. Elegant progression in clinical research on non-invasive efforts to improve tumor control is also associated with risk of failure and metastatic progression of the disease [41]. There have been several efforts to integrate surgical extirpation of radioresistant tumors with chemo-radiotherapy for improved overall survival. There is a pressing need for an alternative effective drug strategy for cancers that are resistant to standard-of-care therapy. A safe, well-tolerated and efficacious multi-target radiosensitizer may provide more viable treatment options.
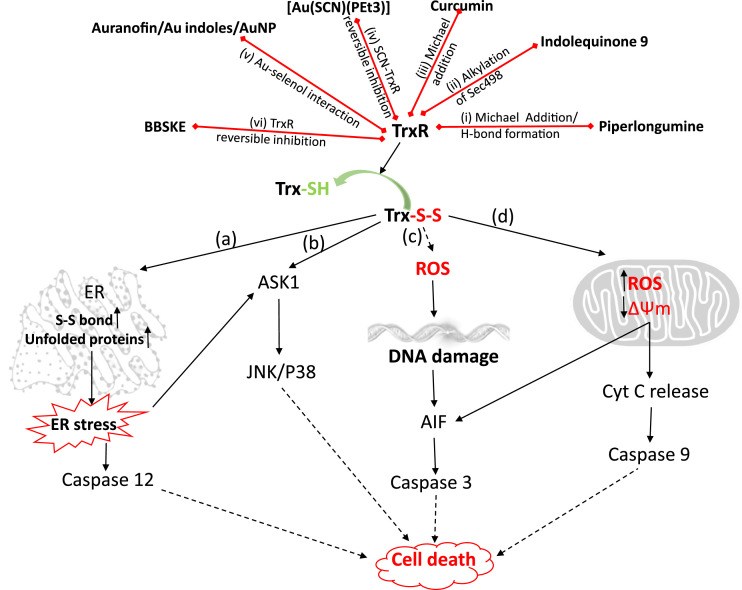
TrxR is an operator of several cellular processes in cells, and tumor cells elevate levels of TrxR, which helps in tumorigenesis and angiogenesis in the early stages. It also promotes metastasis and radioresistance during late stages of cancer [4,[6], [7], [8]]. Tumor cells harbour excess ROS levels than normal cells, which make them more vulnerable to TrxR inhibition. TrxR inhibitors per se exhibit strong cytotoxicity against cancer cells. Fig. 2 shows multiple modes of cell death induced by TrxR inhibitors.
Fig. 2.
Mechanisms of cell death after TrxR inhibition
Compounds depicted in the figure inhibits (red arrows) TrxR via mechanisms described in (i) to (vi). Inhibition of TrxR leads to exhaustion of the pool of reduced Trx (Trx-SH) and accumulation of oxidized Trx (Trx-S-S). Depending upon the location, oxidized Trx leads to the initiation of a cascade of events leading to cell death. (a) In the endoplasmic reticulum, lack of reduction of disulfide bonds in the protein disulfide isomerase (PDI) leads to accumulation of non-native disulfide bonds in the proteins initiating unfolded protein response. This leads to ER stress associated activation of caspase-12 and ASK1. (b) Oxidation of Trx1 dissociates ASK1 signalosome initiating downstream apoptotic signaling through SAPK (JNK/P38) in the cytosol. (c) Lack of functional antioxidant enzymes due to oxidized Trx1 leads to accumulation of ROS, leading to DNA damage initiated apoptotic signaling. (d) Oxidized Trx2 leads to disruption of mitochondrial redox, loss of mitochondrial membrane potential and release of several pro-apoptotic factors into the cytosol, including cytochrome c resulting in activation of apoptotic signaling.
Auranofin, an anti-rheumatic drug, is one of the most extensively used TrxR inhibitors, a gold phosphine with oxidation state Au(I). It exhibits strong anticancer activity in vitro and in vivo [37]. Auranofin can inhibit cytosolic and mitochondrial TrxR via interaction with Sec moiety in the catalytic site of TrxR [21,42]. It can increase the radiosensitivity of human breast cancer stem cells (MDA-MB-231), murine mammary carcinoma cells and different cervical cancer cells. It increases ROS production, mitochondrial dysfunction and DNA damage leading to cell death. Clinical studies have reported around 3 µM concentration of auranofin in human plasma, and hence it is exploitable for radiosensitization. To improve the selectivity towards tumor cells, phosphine gold (I) containing compounds of AF with diverse ligands are being tested [43]. In an attempt to achieve strong TrxR inhibition, thiocyanate containing [Au(SCN)(PEt3)] compound was tested for its tumor cytotoxicity. Gold-containing indoles have also been reported to potentiate cytotoxic effects of ionizing radiation in MCF-7, HeLa, HCT116 and CEM cells [44].
Advances in nanotechnology have resulted in sophisticated synthesis methods, ease of fabrication, functionalization, enhanced biocompatibility, high stability, versatile delivery, low cytotoxicity, favourable kinetics and higher tumor tissue accumulation of nanomaterials, making them promising candidates as radiosensitizers [2,24]. Gold nanoparticles (GNPs) have been shown to enhance X-ray and proton beam induced cell death in NSCLC. Gold has a high atomic number which also interacts with photons of IR thereby generating secondary electrons due to the photoelectric and Compton effect leading to local dose enhancement and better tumor regression [2]. Overall gold-containing compounds and nanomaterials have shown great promise as radiosensitizers.
The α,β unsaturated carbon bond present in the curcumin molecule function as Michael acceptor. It is a potent electrophile that reacts with selenocyteine residue of TrxR [45]. Curcumin exhibited TrxR dependent radiosensitization of HNSCC. A metabolically stable curcumin analogue exhibited radiosensitization in NSCLC. However, curcumin has poor bioavailability, low stability and low efficacy. Hence, other curcumin variants and curcumin-based nanoparticles with improved pharmacokinetics and bioavailability are being investigated.
Selenium-based ethaselen and selenadiazole derivatives along with a novel TrxR inhibitor indolequinone 9 have shown promising radiosensitizing potential, which warrants further investigation regarding safety, tolerability, pharmacokinetics and pharmacodynamics in pre-clinical and clinical models.
Strong addiction of certain cancers to TrxR offers a clinically actionable target and may serve as a predictive/prognostic marker for targeted molecular therapy. Use of selective small-molecule inhibitors of TrxR may offer a survival benefit in patients with TrxR over-expressing tumors. A recent report proposes TrxR to be associated with chemoresistance and poor prognosis in NSCLC patients. High TrxR protein levels correlated with shorter overall and disease-free survival in a cohort of patients that underwent surgery and/or platinum-based adjuvant chemotherapy [46]. Trx is essential in the metabolic reprogramming of T cells during immune activation. Deletion of TrxR1 impairs the expansion of activated T cells, and it is required for thymocyte and peripheral T cell proliferation, making it an attractive target for T-cell leukaemia [47].
Cancer cells not exhibiting elevated TrxR levels were not prone to radiosensitization by TrxR inhibition [40]. This points to the important fact that the normal cells which do not harbor elevated TrxR levels may be spared by the use of TrxR inhibitor during radiotherapy. However, it is primitive to comment on this aspect as more studies need to be conducted to experimentally establish the validity of this assumption.
Despite several decades of research on radiosensitizer development, practice-changing clinical treatment options are not available for patient care. The existing gap between pre-clinical studies and clinical translation of effective molecules needs to be filled by collated efforts of the team of professionals with multilateral expertise in drug development. To improve the outcome of cancer radiotherapy, continuous and exhaustive efforts are needed worldwide to design better treatment regimen. Research on exploiting TrxR inhibitors as radiosensitizer is attracting the attention of researchers and more efforts are required in order to verify the clinical relevance of novel TrxR inhibitors.
CRediT authorship contribution statement
Raghavendra S. Patwardhan: Conceptualization, Writing – original draft, Data curation, Formal analysis. Deepak Sharma: Validation, Writing – review & editing. Santosh K. Sandur: Conceptualization, Project administration, Resources, Supervision, Writing – review & editing.
Declaration of Competing Interest
The authors declare that there is no conflict of interest regarding the publication of this article.
Funding
The research is funded by the Department of Atomic Energy, Government of India.
Contributor Information
Deepak Sharma, Email: dsharma@barc.gov.in.
Santosh K. Sandur, Email: sskumar@barc.gov.in.
References
- 1.Barker H.E., Paget J.T., Khan A.A., Harrington K.J. The tumour microenvironment after radiotherapy: mechanisms of resistance and recurrence. Nat. Rev. Cancer. 2015;15:409–425. doi: 10.1038/nrc3958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang H., Mu X., He H., Zhang X.D. Cancer Radiosensitizers. Trends Pharmacol. Sci. 2018;39:24–48. doi: 10.1016/j.tips.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 3.Huang G., Pan S.T. ROS-mediated therapeutic strategy in chemo-/radiotherapy of head and neck cancer. Oxid. Med. Cell. Longev. 2020;2020 doi: 10.1155/2020/5047987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hanschmann E.M., Godoy J.R., Berndt C., Hudemann C., Lillig C.H. Thioredoxins, glutaredoxins, and peroxiredoxins–molecular mechanisms and health significance: from cofactors to antioxidants to redox signaling. Antioxid. Redox. Signal. 2013;19:1539–1605. doi: 10.1089/ars.2012.4599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dagnell M., Schmidt E.E., Arner E.S.J. The A to Z of modulated cell patterning by mammalian thioredoxin reductases. Free Radic. Biol. Med. 2018;115:484–496. doi: 10.1016/j.freeradbiomed.2017.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mahmood D.F., Abderrazak A., El Hadri K., Simmet T., Rouis M. The thioredoxin system as a therapeutic target in human health and disease. Antioxid. Redox. Signal. 2013;19:1266–1303. doi: 10.1089/ars.2012.4757. [DOI] [PubMed] [Google Scholar]
- 7.Jia J.J., Geng W.S., Wang Z.Q., Chen L., Zeng X.S. The role of thioredoxin system in cancer: strategy for cancer therapy. Cancer Chemother. Pharmacol. 2019;84:453–470. doi: 10.1007/s00280-019-03869-4. [DOI] [PubMed] [Google Scholar]
- 8.Biaglow J.E., Miller R.A. The thioredoxin reductase/thioredoxin system: novel redox targets for cancer therapy. Cancer Biol. Ther. 2005;4:6–13. doi: 10.4161/cbt.4.1.1434. [DOI] [PubMed] [Google Scholar]
- 9.Wang L., Fu J.N., Wang J.Y., Jin C.J., Ren X.Y., Tan Q., Li J., Yin H.W., Xiong K., Wang T.Y., Liu X.M., Zeng H.H. Selenium-containing thioredoxin reductase inhibitor ethaselen sensitizes non-small cell lung cancer to radiotherapy. Anticancer. Drugs. 2011;22:732–740. doi: 10.1097/CAD.0b013e32834618bc. [DOI] [PubMed] [Google Scholar]
- 10.Tuttle S., Hertan L., Daurio N., Porter S., Kaushick C., Li D., Myamoto S., Lin A., O'Malley B.W., Koumenis C. The chemopreventive and clinically used agent curcumin sensitizes HPV (-) but not HPV (+) HNSCC to ionizing radiation, in vitro and in a mouse orthotopic model. Cancer Biol. Ther. 2012;13:575–584. doi: 10.4161/cbt.19772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang H., Gu C., Yu J., Wang Z., Yuan X., Yang L., Wang J., Jia Y., Liu J., Liu F. Radiosensitization of glioma cells by TP53-induced glycolysis and apoptosis regulator knockdown is dependent on thioredoxin-1 nuclear translocation. Free Radic. Biol. Med. 2014;69:239–248. doi: 10.1016/j.freeradbiomed.2014.01.034. [DOI] [PubMed] [Google Scholar]
- 12.Rashmi R., Huang X., Floberg J.M., Elhammali A.E., McCormick M.L., Patti G.J., Spitz D.R., Schwarz J.K. Radioresistant cervical cancers are sensitive to inhibition of glycolysis and redox metabolism. Cancer Res. 2018;78:1392–1403. doi: 10.1158/0008-5472.CAN-17-2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu Z., Zhang Y., Ding J., Hu W., Tan C., Wang M., Tang J., Xu Y. miR-17-3p downregulates mitochondrial antioxidant enzymes and enhances the radiosensitivity of prostate cancer cells. Mol. Ther. Nucleic Acids. 2018;13:64–77. doi: 10.1016/j.omtn.2018.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abdullah N.A., Inman M., Moody C.J., Storr S.J., Martin S.G. Cytotoxic and radiosensitizing effects of a novel thioredoxin reductase inhibitor in breast cancer. Invest. New Drugs. 2021;39(5):1232–1241. doi: 10.1007/s10637-021-01106-5. In this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang H., Jiang H., Corbet C., de Mey S., Law K., Gevaert T., Feron O., De Ridder M. Piperlongumine increases sensitivity of colorectal cancer cells to radiation: Involvement of ROS production via dual inhibition of glutathione and thioredoxin systems. Cancer Lett. 2019;450:42–52. doi: 10.1016/j.canlet.2019.02.034. [DOI] [PubMed] [Google Scholar]
- 16.Laurent T.C., Moore E.C., Reichard P. Enzymatic synthesis of deoxyribonucleotides: IV. Isolation and characterization of thioredoxin, the hydrogen donor from Escherichia coli B. J. Biol. Chem. 1964;239:3436–3444. [PubMed] [Google Scholar]
- 17.Moore E.C. A thioredoxin-thioredoxin reductase system from rat tumor. Biochem. Biophys. Res. Commun. 1967;29:264–268. doi: 10.1016/0006-291x(67)90446-9. [DOI] [PubMed] [Google Scholar]
- 18.Lu J., Holmgren A. Thioredoxin system in cell death progression. Antioxid. Redox. Signal. 2012;17:1738–1747. doi: 10.1089/ars.2012.4650. [DOI] [PubMed] [Google Scholar]
- 19.Chen Y., Cai J., Jones D.P. Mitochondrial thioredoxin in regulation of oxidant-induced cell death. FEBS Lett. 2006;580:6596–6602. doi: 10.1016/j.febslet.2006.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Venardos K., Harrison G., Headrick J., Perkins A. Effects of dietary selenium on glutathione peroxidase and thioredoxin reductase activity and recovery from cardiac ischemia-reperfusion. J. Trace Elem. Med. Biol. 2004;18:81–88. doi: 10.1016/j.jtemb.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 21.Zhang B., Zhang J., Peng S., Liu R., Li X., Hou Y., Han X., Fang J. Thioredoxin reductase inhibitors: a patent review. Expert Opin. Ther. Pat. 2017;27:547–556. doi: 10.1080/13543776.2017.1272576. [DOI] [PubMed] [Google Scholar]
- 22.Saccoccia F., Angelucci F., Boumis G., Carotti D., Desiato G., Miele A.E., Bellelli A. Thioredoxin reductase and its inhibitors. Curr. Protein Pept. Sci. 2014;15:621–646. doi: 10.2174/1389203715666140530091910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Adams G. Chemical radiosensitization of hypoxic cells. Br. Med. Bull. 1973;29(1):48–53. doi: 10.1093/oxfordjournals.bmb.a070956. Jan 1973. [DOI] [PubMed] [Google Scholar]
- 24.Gong L., Zhang Y., Liu C., Zhang M., Han S. Application of radiosensitizers in cancer radiotherapy. Int. J. Nanomed. 2021;16:1083–1102. doi: 10.2147/IJN.S290438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bai M., Ma X., Li X., Wang X., Mei Q., Li X., Wu Z., Han W. The accomplices of NF-κB lead to radioresistance. Curr. Protein Pept. Sci. 2015;16:279–294. doi: 10.2174/138920371604150429152328. [DOI] [PubMed] [Google Scholar]
- 26.Jayakumar S., Patwardhan R.S., Pal D., Sharma D., Sandur S.K. Dimethoxycurcumin, a metabolically stable analogue of curcumin enhances the radiosensitivity of cancer cells: Possible involvement of ROS and thioredoxin reductase. Biochem. Biophys. Res. Commun. 2016;478:446–454. doi: 10.1016/j.bbrc.2016.06.144. [DOI] [PubMed] [Google Scholar]
- 27.Hao C., Xu X., Ma J., Xia J., Dai B., Liu L., Ma Y. MicroRNA-124 regulates the radiosensitivity of non-small cell lung cancer cells by targeting TXNRD1. Oncol. Lett. 2017;13:2071–2078. doi: 10.3892/ol.2017.5701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Penninckx S., Heuskin A.C., Michiels C., Lucas S. The role of thioredoxin reductase in gold nanoparticle radiosensitization effects. Nanomedicine. 2018;13:2917–2937. doi: 10.2217/nnm-2018-0171. Lond. [DOI] [PubMed] [Google Scholar]
- 29.Selenius M., Hedman M., Brodin D., Gandin V., Rigobello M.P., Flygare J., Marzano C., Bindoli A., Brodin O., Bjornstedt M., Fernandes A.P. Effects of redox modulation by inhibition of thioredoxin reductase on radiosensitivity and gene expression. J. Cell. Mol. Med. 2012;16:1593–1605. doi: 10.1111/j.1582-4934.2011.01469.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Joshi H.A., Patwardhan R.S., Sharma D., Sandur S.K., Devarajan P.V. Pre-clinical evaluation of an innovative oral nano-formulation of baicalein for modulation of radiation responses. Int. J. Pharm. 2021;595 doi: 10.1016/j.ijpharm.2020.120181. [DOI] [PubMed] [Google Scholar]
- 31.Wang H., Bouzakoura S., de Mey S., Jiang H., Law K., Dufait I., Corbet C., Verovski V., Gevaert T., Feron O., Van den Berge D., Storme G., De Ridder M. Auranofin radiosensitizes tumor cells through targeting thioredoxin reductase and resulting overproduction of reactive oxygen species. Oncotarget. 2017;8:35728–35742. doi: 10.18632/oncotarget.16113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rodman S.N., Spence J.M., Ronnfeldt T.J., Zhu Y., Solst S.R., O'Neill R.A., Allen B.G., Guan X., Spitz D.R., Fath M.A. Enhancement of radiation response in breast cancer stem cells by inhibition of thioredoxin- and glutathione-dependent metabolism. Radiat. Res. 2016;186:385–395. doi: 10.1667/RR14463.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.El Feky S.E., Ghany Megahed M.A., Abd El Moneim N.A., Zaher E.R., Khamis S.A., Ali L.M.A. Cytotoxic, chemosensitizing and radiosensitizing effects of curcumin based on thioredoxin system inhibition in breast cancer cells: 2D vs. 3D cell culture system. Exp. Ther. Med. 2021;21:506. doi: 10.3892/etm.2021.9937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.He L., Ji S., Lai H., Chen T. Selenadiazole derivatives as theranostic agents for simultaneous cancer chemo-/radiotherapy by targeting thioredoxin reductase. J. Mater. Chem. B. 2015;3:8383–8393. doi: 10.1039/c5tb01501d. [DOI] [PubMed] [Google Scholar]
- 35.Jayakumar S., Kunwar A., Sandur S.K., Pandey B.N., Chaubey R.C. Differential response of DU145 and PC3 prostate cancer cells to ionizing radiation: role of reactive oxygen species, GSH and Nrf2 in radiosensitivity. Biochim. Biophys. Acta. 2014;1840:485–494. doi: 10.1016/j.bbagen.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 36.Zhang Y., Chen F., Tai G., Wang J., Shang J., Zhang B., Wang P., Huang B., Du J., Yu J., Zhang H., Liu F. TIGAR knockdown radiosensitizes TrxR1-overexpressing glioma in vitro and in vivo via inhibiting Trx1 nuclear transport. Sci. Rep. 2017;7:42928. doi: 10.1038/srep42928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liang Y.W., Zheng J., Li X., Zheng W., Chen T. Selenadiazole derivatives as potent thioredoxin reductase inhibitors that enhance the radiosensitivity of cancer cells. Eur. J. Med. Chem. 2014;84:335–342. doi: 10.1016/j.ejmech.2014.07.032. [DOI] [PubMed] [Google Scholar]
- 38.Xie Q., Zhou Y., Lan G., Yang L., Zheng W., Liang Y., Chen T. Sensitization of cancer cells to radiation by selenadiazole derivatives by regulation of ROS-mediated DNA damage and ERK and AKT pathways. Biochem. Biophys. Res. Commun. 2014;449:88–93. doi: 10.1016/j.bbrc.2014.04.151. [DOI] [PubMed] [Google Scholar]
- 39.Patwardhan R.S., Sharma D., Checker R., Thoh M., Sandur S.K. Spatio-temporal changes in glutathione and thioredoxin redox couples during ionizing radiation-induced oxidative stress regulate tumor radio-resistance. Free Radic. Res. 2015;49:1218–1232. doi: 10.3109/10715762.2015.1056180. [DOI] [PubMed] [Google Scholar]
- 40.Javvadi P., Hertan L., Kosoff R., Datta T., Kolev J., Mick R., Tuttle S.W., Koumenis C. Thioredoxin reductase-1 mediates curcumin-induced radiosensitization of squamous carcinoma cells. Cancer Res. 2010;70:1941–1950. doi: 10.1158/0008-5472.CAN-09-3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sher D.J. Neoadjuvant chemoradiotherapy for stage III non-small cell lung cancer. Front. Oncol. 2017;7:281. doi: 10.3389/fonc.2017.00281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Onodera T., Momose I., Kawada M. Potential Anticancer activity of auranofin. Chem. Pharm. Bull. 2019;67:186–191. doi: 10.1248/cpb.c18-00767. Tokyo. [DOI] [PubMed] [Google Scholar]
- 43.Gandin V., Fernandes A.P., Rigobello M.P., Dani B., Sorrentino F., Tisato F., Bjornstedt M., Bindoli A., Sturaro A., Rella R., Marzano C. Cancer cell death induced by phosphine gold(I) compounds targeting thioredoxin reductase. Biochem. Pharmacol. 2010;79:90–101. doi: 10.1016/j.bcp.2009.07.023. [DOI] [PubMed] [Google Scholar]
- 44.Craig S., Gao L., Lee I., Gray T., Berdis A.J. Gold-containing indoles as anticancer agents that potentiate the cytotoxic effects of ionizing radiation. J. Med. Chem. 2012;55:2437–2451. doi: 10.1021/jm2005942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu Z., Du Z.Y., Huang Z.S., Lee K.S., Gu L.Q. Inhibition of thioredoxin reductase by curcumin analogs. Biosci. Biotechnol. Biochem. 2008;72:2214–2218. doi: 10.1271/bbb.80229. [DOI] [PubMed] [Google Scholar]
- 46.Delgobo M., Gonçalves R.M., Delazeri M.A., Falchetti M., Zandoná A., das Neves R.N., Almeida K., Fagundes A.C., Gelain D.P., Fracasso J.I. Thioredoxin reductase-1 levels are associated with NRF2 pathway activation and tumor recurrence in non-small cell lung cancer. Free Radic. Biol. Med. 2021;177:58–71. doi: 10.1016/j.freeradbiomed.2021.10.020. [DOI] [PubMed] [Google Scholar]
- 47.Muri J., Heer S., Matsushita M., Pohlmeier L., Tortola L., Fuhrer T., Conrad M., Zamboni N., Kisielow J., Kopf M. The thioredoxin-1 system is essential for fueling DNA synthesis during T-cell metabolic reprogramming and proliferation. Nat. Commun. 2018;9:1–16. doi: 10.1038/s41467-018-04274-w. [DOI] [PMC free article] [PubMed] [Google Scholar]