Abstract
Sigmar1 is a widely expressed molecular chaperone protein in mammalian cell systems. Accumulating research demonstrated the cardioprotective roles of pharmacologic Sigmar1 activation by ligands in preclinical rodent models of cardiac injury. Extensive biochemical and immuno-electron microscopic research demonstrated Sigmar1’s sub-cellular localization largely depends on cell and organ types. Despite comprehensive studies, Sigmar1’s direct molecular role in cardiomyocytes remains elusive. In the present study, we determined Sigmar1’s subcellular localization, transmembrane topology, and function using complementary microscopy, biochemical, and functional assays in cardiomyocytes. Quantum dots in transmission electron microscopy showed Sigmar1 labeled quantum dots on the mitochondrial membranes, lysosomes, and sarcoplasmic reticulum-mitochondrial interface. Subcellular fractionation of heart cell lysates confirmed Sigmar1’s localization in purified mitochondria fraction and lysosome fraction. Immunocytochemistry confirmed Sigmar1 colocalization with mitochondrial proteins in isolated adult mouse cardiomyocytes. Sigmar1’s mitochondrial localization was further confirmed by Sigmar1 colocalization with Mito-Tracker in isolated mouse heart mitochondria. A series of biochemical experiments, including alkaline extraction and proteinase K treatment of purified heart mitochondria, demonstrated Sigmar1 as an integral mitochondrial membrane protein. Sigmar1’s structural requirement for mitochondrial localization was determined by expressing FLAG-tagged Sigmar1 fragments in cells. Full-length Sigmar1 and Sigmar1’s C terminal-deletion fragments were able to localize to the mitochondrial membrane, whereas N- terminal deletion fragment was unable to incorporate into the mitochondria. Finally, functional assays using extracellular flux analyzer and high-resolution respirometry showed Sigmar1 siRNA knockdown significantly altered mitochondrial respiration in cardiomyocytes. Overall, we found that Sigmar1 localizes to mitochondrial membranes and is indispensable for maintaining mitochondrial respiratory homeostasis in cardiomyocytes.
Keywords: Sigma-1 receptor, subcellular localization, mitochondria
Graphical Abstract

Introduction
Sigmar1 is a multifunctional, ubiquitously expressed chaperone protein encoded by the SIGMAR1 gene (Aishwarya et al., 2021). The purification and cloning of Sigmar1-binding site from guinea pig liver using Sigmar1 specific probes initiated the molecular characterization of Sigmar1 (Hanner et al., 1996a). Since the discovery, extensive studies have used pharmacologic approach to define Sigmar1 ligands binding stoichiometry, and determining their physiological and pharmacological profiles (Culp et al., 1992; de Costa et al., 1990; Leonardo et al., 2010; Matsuno et al., 1996; Rao et al., 1990). These studies revealed the potential roles of Sigmar1 agonists and antagonists in the diverse central nervous system (CNS)-associated disorders utilizing in vitro neuronal cell lines and in vivo rodent models. Comprehensive studies using these ligands showed Sigmar1’s biological roles in neuroinflammation, cognitive dysfunction, memory deficits, psychiatric disorders, motor neuron diseases, drug addiction, and stroke (Hayashi and Su, 2008; Penke et al., 2018; Ryskamp et al., 2019). However, most of these Sigmar1 ligands also demonstrated a binding affinity for other receptors, including serotonin receptors, serotonin reuptake transporters, and metabotropic receptors in the CNS (Fisher et al., 2016; Fontanilla et al., 2009; Hayashi and Su, 2008; Narita et al., 1996; Penke et al., 2018; Rao et al., 1990; Smith et al., 1998; Zou et al., 1998). Moreover, most of these studies were limited to dissecting the pharmacological effects of Sigmar1 ligands and lacked validation of the Sigmar1’s direct involvement by using genetic loss-of-function studies utilizing Sigmar1 null cell lines or rodent models. Thus, the precise molecular role of Sigmar1 in eliciting diverse cellular and physiological functions requires further investigation.
Despite extensive reports, the subcellular localization of Sigmar1 remains elusive. An early study demonstrated widespread expression of Sigmar1 in the CNS in rats (Alonso et al., 2000). Subsequent studies reported a diverse subcellular distribution of Sigmar1, including the plasma membrane, plasma membrane subsurface cisternae of endoplasmic reticulum (ER), ER, nuclear envelope, nucleoplasmic reticulum, mitochondria-associated ER membranes (MAM), and mitochondria (Hayashi and Su, 2003a, b, 2004, 2007; Mavlyutov et al., 2017b). Sigmar1 was also reported to localize at the ER and nuclear envelope in human immune cells (Dussossoy et al., 1999). Subsequently, immunoelectron microscopy (immune-EM) studies confirmed several of these reported subcellular distributions of Sigmar1 (Mavlyutov et al., 2017a; Mavlyutov et al., 2016a; Mavlyutov et al., 2015a; Mavlyutov et al., 2010; Mavlyutov et al., 2012; Mavlyutov et al., 2013; Mavlyutov and Guo, 2017; Mavlyutov et al., 2015b; Mavlyutov et al., 2011; Mavlyutov and Ruoho, 2007; Mavlyutov et al., 2017b; Yang et al., 2017). However, immuno-EM examinations were unable to detect Sigmar1 at the plasma membrane (Mavlyutov et al., 2017a; Mavlyutov et al., 2016a; Mavlyutov et al., 2015a; Mavlyutov et al., 2010; Mavlyutov et al., 2012; Mavlyutov et al., 2013; Mavlyutov and Guo, 2017; Mavlyutov et al., 2015b; Mavlyutov et al., 2011; Mavlyutov and Ruoho, 2007; Mavlyutov et al., 2017b; Yang et al., 2017). Sigmar1 was also detected on mitochondria isolated from rat liver (depicted as Sigmar1-like receptor) using ligand-based studies and immunostaining (Klouz et al., 2002). (+)-Pentazocine ligand binding and enzyme binding assays (using monoamine oxidases and cytochrome c oxidases) showed Sigmar1 in the outer mitochondrial membrane in the isolated mitochondrial fraction and liver mitochondria have a Sigmar1 ligand binding site compared to that of brain mitochondria (Klouz et al., 2002). All these studies together suggest organ and tissue-specific Sigmar1 localization and function in the body. However, the subcellular distributions and molecular function of Sigmar1 in cardiomyocytes remain unknown.
Extensive studies from our group and others reported ubiquitous expression of Sigmar1 in the heart. Subsequent studies utilizing Sigmar1 ligands (i.e., dehydroepiandrosterone and fluvoxamine) showed activation of Sigmar1 ameliorates pressure-overload induced hypertrophy and cardiac dysfunctions in ovariectomized rats (Bhuiyan and Fukunaga, 2009; Bhuiyan et al., 2011) and transverse aortic stenosis mice (Bhuiyan et al., 2010; Tagashira et al., 2014). In line with these findings, chronic activation of Sigmar1 with fluvoxamine attenuated ventricular remodeling and arrhythmias after myocardial infarction in rats (Fo et al., 2020). We have recently reported the development of systolic cardiac dysfunction with coinciding abnormal mitochondrial ultrastructure, altered mitochondrial dynamic regulatory protein expression, phosphorylation states, and compromised mitochondrial respiration in global Sigmar1 null (Sigmar1−/−) mice hearts with ageing (Abdullah et al., 2018b). However, despite these encouraging studies, there has been no study to determine subcellular localization and organelle-specific molecular functions of Sigmar1 in cardiomyocytes to pave rational drug design and discovery targeting Sigmar1. In the current study, we performed complementary biochemical, immunohistochemistry, immunocytochemistry, and ultrastructural immunostaining at the whole heart level, isolated cardiomyocytes, and isolated mitochondria to delineate Sigmar1’s distribution in cardiomyocytes. Further, we extended these findings to determine whether Sigmar1 plays any essential functions in maintaining cardiomyocyte bioenergetics and mitochondrial respirations by conducting extracellular flux analysis and high-resolution respirometry studies.
Results
Sigmar1 localizes to subcellular organelles in cardiomyocytes
Earlier studies reported activation of Sigmar1 using an agonist, fluvoxamine (which is also a selective serotonin reuptake inhibitor), elicits cardioprotective effects in pressure overload-induced myocardial hypertrophy and dysfunction (Bhuiyan and Fukunaga, 2009; Bhuiyan et al., 2011; Bhuiyan et al., 2010; Tagashira et al., 2014). On the contrary, pharmacologic Sigmar1 inhibition by an antagonist, haloperidol (an antipsychotic drug), aggravates cardiac pathology in pressure overload-induced heart failure in mice (Shinoda et al., 2016). Moreover, Sigmar1 ligands were tested in human clinical trials, including cutamesine (SA4503) for ischemic stroke (Phase 2) (Urfer et al., 2014), and sertraline (SADHART-CHF) for depression in patients with heart failure (Jiang et al., 2008; Serebruany et al., 2005; Xiong et al., 2015). However, all these preclinical studies are limited to assessing Sigmar1 activation and inhibition using non-specific chemical ligands having affinity to other receptors. Despite all these comprehensive studies, subcellular localization and molecular functions of Sigmar1 in the heart remain largely underexplored. In the present study, we performed fluorescence anti-Sigmar1 (green) immunostaining in human and mouse heart sections to visualize the endogenous Sigmar1’s expression pattern (Fig. 1). Sigmar1 appeared as distinct green puncta in both human and mouse hearts counterstained for cardiomyocytes (red) with cardiac Troponin I (TnI) (Fig. 1). To visualize Sigmar1’s presence and localization to subcellular compartments, we performed anti-Sigmar1 targeted quantum dot nanocrystals (Q-dots) labeling on ultra-thin adult mouse heart sections followed by transmission electron microscopy (TEM) imaging. The Q-dots TEM showed the ultrastructural localization and enrichment of endogenous Sigmar1 in the heart. Electron-dense anti-Sigmar1 labeled Q-dots (pseudocolored red dots) were observed on mitochondrial (light blue-colored) outer- (OMM) and inner-membrane (IMM) (brown arrows), lysosomes (yellow-colored), and on the sarcoplasmic reticulum/endoplasmic reticulum (SR/ER) membranes (light green-colored)-mitochondrial interface (Fig. 2A, 2B). TEM image of heart sections labeled with rabbit IgG with Q-dots was used as an Isotype control for anti-Sigmar1 rabbit primary antibody (Fig. 2A, 2B). Therefore, Q-dots TEM showed the endogenous Sigmar1 localization mostly on the mitochondrial membranes and lysosomes.
Fig. 1. Sigmar1 expression in human and mouse ventricular heart tissues.
Representative confocal microscopy images of human (top panel) and mouse (bottom panel) heart sections co immunostained with anti-Sigmar1 (green) and cardiac anti-Troponin I (cTnI, red) antibodies. Representative images showing a longitudinal view of the left ventricular sections of the human (upper panel) and mouse (lower panel) heart. Sigmar1 (green) immunostaining appeared as punctate dots in confocal fluorescence microscope images, and cTnI (red) counterstaining delineated cardiomyocytes in the heart. Representative confocal fluorescence microscope images are selected from n=3 stained human and mouse heart tissue sections. Scale bars: 20 μm.
Fig. 2. Sigmar1 immuno- labeled quantum dot (Q-dots) TEM and subcellular fractionation showing subcellular localization in adult mice heart myocytes.
(A) Representative transmission electron microscopy (TEM) image demonstrates electron-dense anti-Sigmar1 labeled Q-dots (pseudocolored in red) localization on distinct subcellular organelles in mouse heart ventricular tissues. TEM images were post-processed, and organelles were pseudocolored using GIMP 2.10 software. Mitochondria are delineated in light blue, lysosomes are in yellow, and SR/ER are in light green. TEM image of heart sections labeled with rabbit IgG and Q-dots was used as isotype control. L lysosome, M mitochondria, SR/ER sarcoplasmic reticulum/endoplasmic reticulum. Scale bar: 200 nm. (B) Representative magnified images demonstrating anti-Sigmar1 positive Q-dots localization on mitochondrial outer- and inner-membrane cristae (brown arrows). Sigmar1 positive labeled Q-dots were also detected in close proximity of mitochondria on sarcoplasmic reticulum (SR)/endoplasmic reticulum (ER) membranes (blue arrows) and lysosomes (L). TEM image of heart sections labeled with rabbit IgG and Q-dots was used as isotype control. Representative images are from n=4 adult mouse hearts in three independent experiments in duplicates for each heart section. M mitochondria; SR/ER sarcoplasmic reticulum/endoplasmic reticulum, L lysosomes. Scale bar: 200 nm. (C) Western blot images of adult mouse heart tissue subcellular fractions consisting of whole-cell (WC) lysates, cytosolic fractions (C), lysosomal fractions (L), and mitochondrial fraction (M). Isolated subcellular fractions were subjected to Western blot analysis to assess Sigmar1’s presence and partitioning in subcellular compartments. Cytosolic protein GAPDH is used to verify isolated subcellular fractions purity. Lysosomal membrane protein LAMP2a, outer mitochondrial membrane protein MFN2, and inner mitochondrial integral membrane protein Tim23 were used to assess the successful enrichment of lysosomes and mitochondria fractions, respectively. Representative Western blots are from n>5 subcellular fractionation-based experiments using adult mouse hearts.
To further confirm the Q-dots TEM data, we performed subcellular fractionation to purify isolated mitochondria and lysosomes from mouse heart lysates. Western blot analysis with anti-Sigmar1 antibody confirmed Sigmar1 enrichment in the purified mitochondria and lysosome fraction but not in cytosol fractions (Fig. 2C). We used cytosolic protein GAPDH to verify the purity of isolated subcellular fractions. Lysosomal membrane protein LAMP2a, outer mitochondrial membrane protein MFN2, and inner mitochondrial integral membrane protein Tim23 was used as a control to determine the successful enrichment of lysosomes and mitochondria, respectively (Fig. 2C). In summary, Q-dots TEM and subcellular fractionation data suggested abundant Sigmar1 localization in the mitochondria in mouse heart.
Sigmar1's presence and localization to mitochondria
The heart is the most metabolically active organ in the body and possesses the highest content of mitochondria of any tissue. Therefore, we focused on Sigmar1’s role in mitochondria in subsequent studies. To corroborate our observations of endogenous Sigmar1 localization to mitochondria enriched fractions and anti-Sigmar1 labeled Q-dots appeared on mitochondrial membranes, we utilized co-immunofluorescence-based studies for anti-Sigmar1 mitochondria specific labeling in isolated primary adult mouse left ventricular cardiomyocytes (Fig. 3). We immunostained isolated primary adult mouse cardiomyocytes with anti-Sigmar1 antibody (in green) followed by co-immunostaining with mitochondrial electron transport chain supercomplexes subunit-specific cocktail antibody OXPHOS (in red) (Fig. 3A, 3B) and mitochondrial outer membrane protein Tom20 (in red) (Fig. 3C, 3D), respectively. We observed distinct yellow pixels in merged green and red channel images suggesting colocalization of anti-Sigmar1 stained green fluorescence pixels to anti-OXPHOS and anti-Tom20 stained red fluorescence pixels. Image quantifications corroborate the notable colocalization of Sigmar1-to-OXPHOS and Sigmar1-to-Tom20 stained mitochondria as evident in Mander’s colocalization coefficient and the number of colocalized voxels between green-to-red fluorescence pixels in adult mouse cardiomyocytes (Fig. 3B, 3D).
Fig. 3. Sigmar1 localizes to mitochondria in cardiomyocytes.
(A) Confocal immunofluorescence micrographs of anti-Sigmar1 (green) and anti-OXPHOS (red) co-immunostaining in isolated cardiomyocytes from adult mouse heart ventricles. Confocal microscope imaging revealed Sigmar1 puncta (green) colocalize to OXPHOS (red) stained mitochondria as evident in yellow pixels in overlay image of green and red fluorescence channel images of cardiomyocytes. Representative confocal fluorescent micrographs from two independent experiments. Scale bars: 20 μm. (B) Box plots represent Mander’s colocalization coefficient showing the extent of colocalization between Sigmar1 stained green pixels-to-OXPHOS stained red pixels and number of colocalized voxels (volume data set containing volume elements consisting of the smallest unit within an image with precise information of measured fluorescence intensity) per cardiomyocytes. Image analysis was done on sequential z-stack confocal images of N>20 adult mouse cardiomyocytes from two independent experiments. Boxes represent interquartile ranges, lines represent medians, and whiskers represent ranges. (C) Confocal immunofluorescence micrographs of anti-Sigmar1 (green) and mitochondrial outer membrane protein anti-Tom20 (red) co-immunostaining in isolated cardiomyocytes from adult mouse heart ventricles. Anti-Sigmar1 stained puncta (green) colocalize to Tom20 (red) stained mitochondria as evident in yellow pixels in overlay images of green and red fluorescence channel images of cardiomyocytes. Representative confocal fluorescent micrographs are from three independent experiments. Scale bars: 20 μm. (D) Box plots represent Mander’s colocalization coefficient showing the extent of colocalization between Sigmar1 stained green pixels-to-Tom20 stained red pixels and number of colocalized voxels (volume data set containing volume elements consisting of the smallest unit within an image with precise information of measured fluorescence intensity) per cardiomyocytes. Image analysis was done on sequential z-stack confocal images of N>40 adult mouse cardiomyocytes from three independent experiments. Boxes represent interquartile ranges, lines represent medians, and whiskers represent ranges.
To substantiate our observations of the presence of Sigmar1 in mitochondria in adult cardiomyocytes, we isolated mitochondria from adult mouse hearts. We labeled the active mitochondria with mitochondrial membrane potential sensitive MitoTracker Red dye (Fig. 4). Anti-Sigmar1 immunofluorescence staining reveals notable colocalization to Mitotracker red labeled mitochondria (Fig. 4A) as reflected in Mander’s colocalization coefficient values (Fig. 4C) and Pearson’s correlation coefficient (Fig. 4D) of colocalized pixels between Sigmar1-to-Mitotracker red in isolated cardiac mitochondria. Mitochondria isolated from Sigmar1 knockout mouse heart was used as a negative control and did not exhibit any distinguishable anti-Sigmar1 staining confirming the specificity of anti-Sigmar1 primary antibody staining (Fig. 4A). We used mitochondrial outer membrane protein anti-Tom20 and mitochondrial respiratory chain super complexes subunit-specific cocktail anti-OXPHOS immunofluorescence staining to confirm the isolated mitochondrial purity and entirety (Fig. 4B). Anti-OXPHOS staining showed significantly higher Mander’s colocalization coefficient to Mitotracker red-stained mitochondria than anti-Sigmar1-to-Mitotracker red (Fig. 4C). On the other hand, anti-Sigmar1 staining exhibited significantly higher Pearson’s correlation coefficient to Mitotracker red staining than anti-Tom20 and anti-OXPHOS staining, indicating the presence of similar intensity and closely existed anti-Sigmar1 stained pixels to Mitotracker red-stained pixels in isolated mitochondria (Fig. 4D). We also determined whether commercially available anti-Sigmar1 antibodies staining coincides with Mitotracker Red labeled cardiac mitochondria. We utilized a panel of anti-Sigmar1 antibodies raised against N-terminal and C-terminal amino acid sequences of Sigmar1 protein (Supplementary Fig. 1A). We used rabbit IgG as isotype control to assess the specificity of utilized primary antibodies staining (Supplementary Fig. 1B). Notably, we observed a comparatively similar degree of colocalization of all anti-Sigmar1 antibodies staining signals to Mitotracker Red labeled isolated cardiac mitochondria as evidenced in comparative Mander’s colocalization coefficient and Pearson’s correlation coefficient values except a C-terminal directed anti-Sigmar1 antibody speculatively due to antibody access to the targeted epitope of Sigmar1 against which the antibody has been developed and Sigmar1’s membrane topology (Supplementary Fig. 1C and Supplementary Fig. 1D). Altogether, our confocal immunofluorescence microscopic studies recapitulated Sigmar1’s localization to mitochondria in adult cardiomyocytes.
Fig. 4. Sigmar1 localization was confirmed in isolated cardiac mitochondria.
(A) Representative confocal images of anti- Sigmar1 antibody (green) and MitoTracker (red) loaded isolated mitochondria from adult mouse heart ventricles. Anti-Sigmar1 staining (green) showed high colocalization with MitoTracker red (red) staining appeared as yellow pixels in overlay images. Sigmar1 knockout mice heart ventricular tissue-derived mitochondria were used as a negative control to determine the primary antibody staining specificity. (B) Mitochondrial outer membrane integral protein anti-Tom20 and electron transport system respiratory supercomplexes subunit-specific cocktail anti-OXPHOS antibody staining are used to verify MitoTracker red labeled mitochondrial purity and entirety. Both anti-Tom20 (green) and anti-OXPHOS (green) staining show high colocalization to MitoTracker red, appearing in yellow pixels in overlay images. Rabbit IgG is used as an isotype control for anti-Sigmar1 rabbit primary antibodies. (C) Box plots representing Mander’s colocalization coefficient values illustrating fractions of anti-Sigmar1, anti-Tom20, and anti-OXPHOS (green) fluorescence colocalized towards corresponding MitoTracker Red fluorescence. (D) Box plots representing Pearson’s correlation coefficient values illustrating linearity of colocalized pixels intensity of anti-Sigmar1, anti-Tom20, and anti-OXPHOS (green) fluorescence pixels towards corresponding MitoTracker Red fluorescence. Representative images are from three independent experiments for each antibody tested. Scale bars: 20 μm. Boxes represent interquartile ranges, lines represent medians, and whiskers represent ranges. P values were determined by One-way ANOVA followed by Tukey’s multiple comparisons test.
Sigmar1 is an integral mitochondrial membrane protein
Proteins residing in the mitochondria can be either integral membrane proteins, peripheral proteins loosely bound to mitochondrial membranes, or amphitropic proteins localizing to mitochondria in response to stimuli. As anti-Sigmar1 labeled Q-dots appeared in the outer- and inner mitochondrial membranes, we further dissected the type of binding of Sigmar1 to mitochondrial membranes by alkaline extraction and dose-dependent Proteinase K enzyme digestion of isolated mitochondria. To confirm whether Sigmar1 resides as an integral mitochondrial membrane protein, we subjected the isolated cardiac mitochondria with Na2CO3(pH 11.5) to extract soluble and loosely bound peripheral proteins (Fujiki et al., 1982; Zhao et al., 2009). Sigmar1 remained in the mitochondrial pellet, whereas cytochrome c was released into the supernatant, suggesting Sigmar1 is an integral mitochondrial membrane protein (Fig. 5A). In control experiments, mitochondria treated with Triton-X 100 extracted all membrane-bound proteins to the supernatant fraction (Fig 5A). To further dissect Sigmar1’s mitochondrial membrane localization, mitochondria were treated with increasing amounts of proteinase K (0.25 μg/ml to 2 μg/ml), which digests mitochondrial membrane proteins (Murakawa et al., 2015). Our immunoblot analysis revealed that Sigmar1 showed a dose-dependent digestion pattern similar to mitochondrial membrane proteins. We used OMM resident MAM proteins (MFN2 and DRP1), SR/ER-resident proteins (IRE1α, VCP, PDI), and cytosolic protein (Actin and GAPDH) as a control to demonstrate the purity of the mitochondrial fraction. Whole-cell lysate (60μg protein) was used as a positive control (Fig 5B). A high amount of proteinase K (25 μg/ml) treatment to isolated mitochondria completely digested Sigmar1 similar to mitochondrial membrane protein Tom20 (Fig. 5C). We also confirmed the enriched presence of Sigmar1 in human and rat ventricular heart tissue-derived purified mitochondrial fractions (Fig. 5D). Altogether, our complementary alkaline, detergent, and proteinase K enzyme-based digestion assays conceded that Sigmar1 is an integral mitochondrial membrane protein.
Fig. 5. Sigmar1 is an integral mitochondrial membrane resident protein.
(A) Representative Western blot images of anti-Sigmar1 and anti- cytochrome C (Cyt C) antibodies probed membranes following alkaline (Na2CO3, pH11.5) and detergent-based (10% v/v Triton X-100) extraction of mitochondrial proteins from isolated mitochondria of adult mouse hearts. S, supernatant fractions, P, mitochondrial pellet fractions. Western blot images are representative of three independent experiments using n=3 individual adult mouse hearts. (B) Representative Western blot images of mitochondria pellet (P) and supernatant fractions (S) probed with labeled antibodies following dose-dependent Proteinase K enzyme-based digestion of isolated mitochondria from adult mouse hearts. We used OMM resident MAM proteins (MFN2 and DRP1), IMM proteins (OPA1 and Tim23), SR/ER-resident proteins (IRE1α, VCP, PDI), and cytosolic protein (Actin and GAPDH) as a control to demonstrate the purity of the mitochondrial fraction. The whole cell lysate was used as a positive control. (C) Representative western blot images of adult mouse heart-derived mitochondria pellet (P) and supernatant fractions (S) following buffer and Proteinase K (25 μg/mL) digestion. (D) Human and rat heart tissue-derived whole cell lysates and isolated mitochondria fractions immunoblotted with anti-Sigmar1 antibody. Tom20 was used to confirm the enrichment of mitochondrial fractions. M mitochondria, WC whole cell lysates. All western blot images represent n>3 subcellular fractionation-based experiments using the biologically independent adult mouse, human, and rat hearts.
Sigmar1 N-terminal is required for incorporation to mitochondrial membranes
The crystal structure of Sigmar1 protein revealed a single transmembrane domain residing at amino-terminal (N-terminal) with a flat membrane-associated carboxy-terminal (C-terminal) (Schmidt et al., 2016). To dissect the location of amino acid residues sequence required for mitochondrial membrane localization of Sigmar1, we conducted in silico and in vitro molecular studies (Fig. 6). Our in silico Kyte-Doolittle hydrophobicity plot showed the presence of hydrophobic amino acid (aa) residues that appeared as a prominent hydrophobic peak near the N-terminal of full-length Sigmar1 protein (Fig. 6A). Next, we exogenously expressed FLAG-tagged full length, N- and C-terminal truncation mutant Sigmar1 fragments in HEK293T cells. We confirmed the mutant construcťs expression by anti-FLAG immunoblotting in HEK293T cell lysates (Fig. 6B and 6C). Subsequently, exogenously expressed Sigmar1 fragments were detected with anti-FLAG (green) immunofluorescence staining (Fig. 6D and 6E). We found strong colocalization of full-length Sigmar1 with gradual attrition with C-terminal truncation fragments of Sigmar1 (aa1–169 and aa1–101) to Mitotracker red-stained mitochondria (Fig. 6D). Interestingly, N-terminal truncated fragments of Sigmar1 aa102–223 appeared as diffuse puncta and failed to exhibit discernible colocalization to Mitotracker red-stained mitochondria (Fig. 6D). We also observed a similar trend of colocalization between anti-FLAG stained Sigmar1 fragments-to-ER tracker staining with lesser colocalization with N-terminal truncated Sigmar1 fragment (Fig. 6E). These observations were reflected in Mander’s colocalization coefficient values of anti-FLAG stained Sigmar1 constructs to Mitotracker red and ER tracker blue fluorescence signals (Fig. 6F and 6G). Altogether, our data indicate Sigmar1’s N-terminal domain is required for mitochondrial membrane incorporation and stability.
Fig. 6. Sigmar1’s N-terminal possesses amino acid residues required for mitochondrial membrane localization.
(A) Kyte and Doolittle hydrophobicity plot of full-length mouse Sigmar1 protein’s amino acid sequence (graphed using ProtScale, Expasy). The hydrophobicity plot shows a distinct hydrophobic peak residing at Sigmar1’s N-terminal (indicated with red arrow) indicative of a putative transmembrane domain. (B) Schematic diagram showing full-length and Sigmar1 fragments. Full-length Sigmar1 amino acids (aa) 1–223, C-terminal truncation fragment of Sigmar1 (aa1–169 and aa1–101) constructs containing putative transmembrane domain (amino acid residues 11–30) at Sigmar1’s N-terminal sequence. N-terminal deletion fragment of Sigmar1 aa102–223 construct lacking N-terminal residing putative transmembrane region. (C) Representative anti-FLAG antibody labeled Western blot images demonstrating expression of FLAG-tagged full length and Sigmar1 fragment plasmid constructs in HEK293T cells. Western blot images are from n=5 independent transfection experiments in HEK293T cells. (D) Representative confocal fluorescence images of MitoTracker red (red) and anti-FLAG antibody (green) stained HEK293T cells. Sigmar1 fragments possessing putative transmembrane domain at N-terminal (Sigmar1 aa1–223, Sigmar1 aa1– 169, Sigmar1 aa1–101) expression (anti-FLAG, green) showed significant colocalization with MitoTracker Red stained mitochondria (yellow pixels in represented merged green and red fluorescent channel images). In contrast, N-terminal lacking Sigmar1 aa102–223 construct expression showed diffused puncta (green) with lesser colocalization with MitoTracker red-stained mitochondria than full length and C-terminal truncation mutant Sigmar1 constructs. (E) Representative confocal fluorescence microscope images of ER-Tracker blue (blue) and anti-FLAG antibody (green) stained HEK293T cells. FLAG-tagged full length and Sigmar1 fragment coding plasmids showed little co-localization in anti-FLAG (green)-to-ER-Tracker (blue) overlay images. Representative images are from three independent experiments. Scale bars: 5 μm. (F) – (G) Box plots represent Mander’s colocalization coefficient illustrating fractions of anti-Sigmar1 (green) fluorescence colocalized towards corresponding MitoTracker Red and ER Tracker Blue fluorescence. Image analysis results are from N>100 cells for each group of cells. Boxes represent interquartile ranges, lines represent medians, and whiskers represent ranges. P values were determined by One-way ANOVA followed by Tukey’s multiple comparisons test. NS not significant.
Sigmar1 is required for physiological mitochondrial respiration
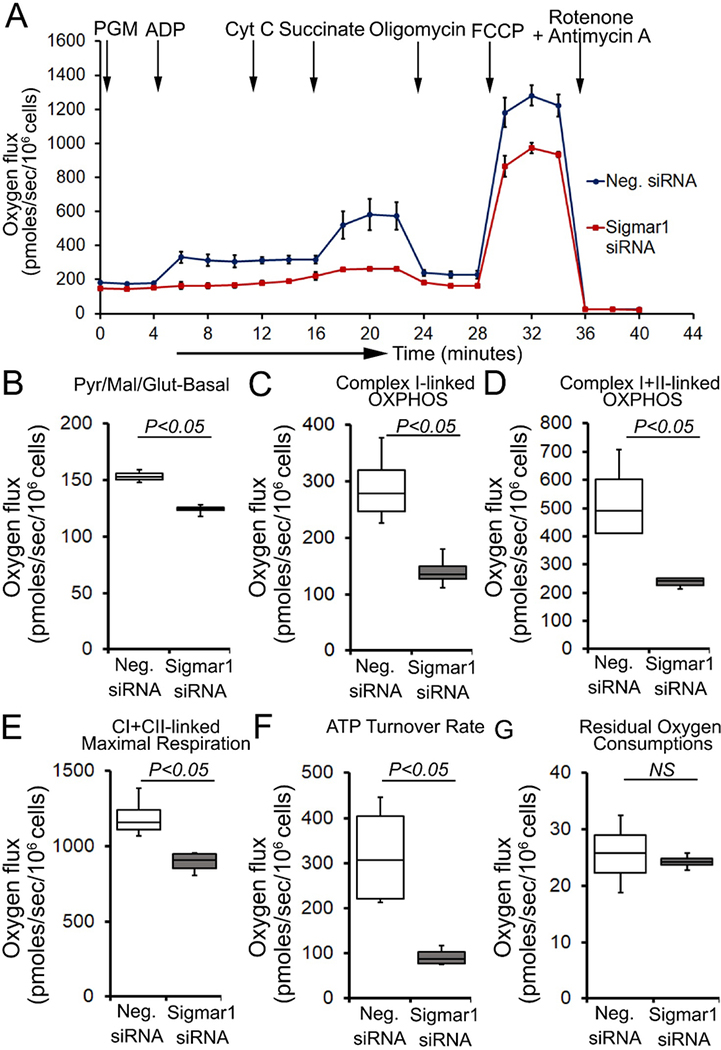
To determine whether Sigmar1 plays any functional role in mitochondria, we utilized neonatal rat ventricular cardiomyocytes (NRCs), knocked down Sigmar1 by siRNA transfection, and performed mitochondrial respiration on intact cardiomyocytes. We confirmed a nearly complete ablation of Sigmar1 protein in NRCs by siRNA transfection at 5 days after siRNA transfection (Supplementary Fig. 2). Mitochondrial oxygen consumption rates (OCRs) were measured in control siRNA (negative siRNA) and Sigmar1 siRNA knockdown cardiomyocytes using the Seahorse extracellular flux analyzer (Fig. 7A) (Alam et al., 2017). Real-time OCRs in intact cardiomyocytes showed that basal respiration, representing the sum of all physiological mitochondrial oxygen consumption, decreased in the Sigmar1 knockdown cardiomyocytes, indicating lower respiratory function compared with control siRNA groups (Fig. 7B). The injection of oligomycin leads to a decrease in basal respiration that is reflective of oxygen consumption used to generate ATP and was not different in between the two groups (Fig. 7C). The addition of FCCP uncouples respiration from oxidative phosphorylation and allows the measurement of maximal OCR, which was lower in Sigmar1 knockdown cardiomyocytes, indicating lower overall mitochondrial activity (Fig. 7D). The extent of nonmitochondrial oxygen-consuming processes measured by inhibiting the respiratory chain with rotenone and antimycin A, was significantly greater in Sigmar1 siRNA knockdown cardiomyocytes (Fig. 7E). Further, mitochondrial respiratory reserve capacity (Fig. 7F) as calculated by subtracting basal OCR from FCCP-stimulated OCR and ATP turnover (Fig. 7G) measured by nonmitochondrial respiration subtracted from the ATP-linked OCR was significantly attenuated in Sigmar1 knockdown cardiomyocytes. The maximal respiration yielded by nonmitochondrial respiration subtracted from FCCP-stimulated OCR was also significantly reduced in Sigmar1 knockdown cardiomyocytes (Fig. 7H). The proton leak through the inner mitochondrial membrane, represented by nonmitochondrial respiration subtracted from the post-oligomycin OCR, was unchanged across the treatments (Fig. 7I). In summary, we found that the absence of Sigmar1 compromised mitochondrial bioenergetics profiles in cardiomyocytes.
Fig. 7. Genetic ablation of Sigmar1 suppresses mitochondrial respiration in cardiomyocytes.
(A) Line graphs represent mitochondrial oxygen consumption rates (OCRs) in intact neonatal rat cardiomyocytes treated with control siRNA (Neg. siRNA) and Sigmar1 directed siRNA. Black arrows indicate sequential addition of Oligomycin (1 μM), carbonyl cyanide 4-(trifluoromethoxy) phenylhydrazone (FCCP) (4 μM), and Rotenone (0.5 μM) plus Antimycin A (0.5 μM). OCR values are expressed as picomoles O2 per minute per μg protein. Each point represents average OCR values from five wells per treatment in two independent experiments. Box plots represent OCRs at (B) baseline as well as with the addition of (C) oligomycin, (D) FCCP, and (E) rotenone plus antimycin A addition. Key mitochondrial respiratory parameters, including (F) reserve capacity, (G) ATP turnover, and (H) maximal respiration was significantly attenuated in Sigmar1 siRNA treated cardiomyocytes compared to control siRNA treated cardiomyocytes. (I) Proton leak was unchanged between the two groups of cardiomyocytes. Boxes depict interquartile ranges, lines represent medians, and whiskers represent ranges. P values were determined by the nonparametric Kolmogorov-Smirnov test. Neg. negative, siRNA small interfering RNA, NS not significant.
Next, we determined whether defects in physiological fuel substrates supported mitochondrial respiration were linked to the compromised bioenergetics profiles observed in Sigmar1 knocked down cardiomyocytes. We subjected Sigmar1 siRNA knockdown cardiomyocytes to high-resolution respirometry using Oroboros O2k-chamber. Control siRNA-treated cardiomyocytes served as an experimental control. To achieve convergent electron flow through complex I and complex II, NADH (N)-generating substrates (donates an electron to complex I, CI), i.e., pyruvate, malate, glutamate, and succinate (S) (activates complex II, CII), were sequentially added under saturating ADP concentration to achieve oxidative phosphorylation (OXPHOS) state (Fig. 8A). Next, Oligomycin, an ATP synthase (Complex V) inhibitor, is injected to measure complex I+II-linked leak respiration in the presence of fuel substrates and excess adenylates (Fig. 8A and 8E). FCCP is injected to uncouple mitochondrial respiration from OXPHOS, allowing measurement of maximal respiration. Mitochondrial respiratory complex I inhibitor (rotenone) and complex III inhibitor (antimycin A) were injected at the end to measure residual oxygen consumptions exclusive of mitochondrial respiration (Fig. 8A). Sigmar1 knockdown in cardiomyocytes ensues in reduced oxygen flux when supplied with NADH-linked substrates pyruvate, malate, and glutamate under both basal and OXPHOS state (in the presence of excess ADP) (Fig. 8B and 8C). The suppression of oxygen flux persisted when cardiomyocytes were supplied with succinate to activate convergent electron flow in the mitochondrial electron transfer system through NS-pathway (Fig. 8D). FCCP-stimulated uncoupled maximal respiration linked to CI+CII-linked oxygen flux, and ATP turnover rate was significantly attenuated in Sigmar1 siRNA-treated cardiomyocytes compared to control cardiomyocytes (Fig. 8E and 8F). We have not found any significant difference in non-mitochondrial residual oxygen flux between treatment groups (Fig. 8G). Overall, our data suggest the essential role of Sigmar1 in maintaining physiological mitochondrial fuel substrates-supported OXPHOS-linked respirations.
Fig. 8. Sigmar1 knockdown attenuates fuel substrates supported OXPHOS in cardiomyocytes.
(A) Representative oxygraph constituting temporal real-time oxygen flux in Saponin (15 μg/ml) permeabilized neonatal rat cardiomyocytes treated with control siRNA and Sigmar1 targeted siRNA. NADH (N)-linked substrates pyruvate (P) (4 mM), glutamate (G) (10 mM), and malate (M) (4 mM) were injected to induce electron flow at complex I of electron transfer system (ETS). ADP (2 mM) was injected to attain complex I (CI) supported oxidative phosphorylation (OXPHOS) state. Cytochrome C (10 μM) was injected to ensure outer mitochondrial membrane integrity. Subsequently, succinate (S) (20 mM) was injected to induce convergent electron flow through the NS pathway and to measure CI+CII-linked OXPHOS. Mitochondrial proton motive force uncoupler FCCP (4 μM) was injected following oligomycin (1 μM) to measure CI+CII-linked maximal respiration. rotenone (1 μM) and antimycin A (2 μM) were co-injected to measure non-mitochondrial oxygen fluxes. Black arrows indicate the site of substrates and inhibitors injection. Each point represents average oxygen fluxes from four independent experiments per treatment group. Oxygen fluxes are expressed as picomoles (pmoles) O2/[sec*106 cells]. Data points are expressed as mean ± SEM. (B) – (G) Box plots represent oxygen fluxes following supplementation of (B) pyruvate, malate, and glutamate (PGM) at baseline, (C) complex I-linked OXPHOS, (D) complex I+II-linked OXPHOS, (E) FCCP stimulated CI+CII-linked maximal respiration, (F) ATP turnover rate constituting oxygen flux after subtracting post-Oligomycin oxygen flux from CI+CII-linked OXPHOS state oxygen flux under available fuel substrates and excess ADP, and (G) residual oxygen consumptions (ROX) following Rotenone and Antimycin A injection. The mitochondrial respiratory parameters were calculated by subtracting ROX values in the respective experiment to attain mitochondrial respiration-specific oxygen fluxes in cardiomyocytes. Boxes represent interquartile ranges, lines represent medians, and whiskers represent ranges. P values were determined by the nonparametric Kolmogorov-Smirnov test. Neg. negative, siRNA small interfering RNA, Pyr pyruvate, Mal malate, Glut Glutamate, CI complex I, CII complex II, NS not significant.
Discussion
Earlier studies to characterize Sigmar1 subcellular localization are mostly limited to brain, retina, and spinal cord sections, primary neuronal cultures, and neuroblastoma cell lines. Unlike other cell systems, the heart is a special organ composed of cardiomyocytes requiring a constant energy supply for their proper functioning. Cardiac myocytes contain nearly 30% to 40% of their volume with highly organized mitochondria residing at subsarcolemmal spaces, interfibrillar spaces, and perinuclear areas (Hollander et al., 2014). In contrast, cardiomyocytes contains approximately 3.5% of sarcoplasmic reticulum (SR) (Katz, 2011). The striated muscle SR is a specialized ER subcompartment in which general ER markers (e.g. Bip and calnexin) coexist with the major SR proteins specifically responsible for Ca2+ uptake, storage, and release (Katz, 2011; Volpe et al., 1992). The SR surrounds the myofilaments and functions in collaboration with deep invaginations of the sarcolemma (transverse (t)-tubules) to activate myocyte contraction by releasing calcium from SR. Despite the well documented presence of SR in cardiomyocytes, functional relationship between the ER and SR in cardiomyocytes has remained confusing. As the literature supports Sigmar1’s localization in the ER and ER-associated mitochondrial membranes in cells containing ER-network, the lack of similar structures in cardiomyocytes provided a compelling rationale to study Sigmar1 function in the heart. Nonetheless, to our best knowledge, Sigmar1’s subcellular distribution and organelle-specific functions in cardiac myocytes have been largely overlooked in the literature.
In the present study, we demonstrated Sigmar1’s localization in the mitochondrial membranes, lysosomes and SR/ER to mitochondrial interface using Q-dots TEM and subcellular fractionation of heart cell lysates. As mitochondria comprise the majority of the cell volume of cardiomyocytes and play a crucial role for the normal functioning of the heart, we focused on elucidating the molecular role of Sigmar1 in mitochondria in the cardiomyocytes. We confirmed the Sigmar1 mitochondrial localization by quantifying the extent of endogenous Sigmar1 partitioning to mitochondria by co-immunostaining isolated adult cardiac myocytes with anti-OXPHOS, anti-Tom20, and anti-Sigmar1 antibodies followed by confocal immunofluorescent Z-stack imaging. Mander’s colocalization coefficient and the number of colocalized voxels confirmed Sigmar1 colocalizes to OXPHOS and Tom20 stained mitochondria in adult cardiac myocytes. This was further confirmed by Mitotracker red labeled isolated cardiac mitochondria to anti-Sigmar1 staining. The accuracy of the Sigmar1’s immunostaining on isolated mitochondria was validated using commercially available N-terminal and C-terminal targeted Sigmar1 antibodies. Both N-terminal and C-terminal targeted Sigmar1 antibody stained endogenous Sigmar1 exhibited comparable values of colocalization parameters (Mander’s and Pearson’s coefficients) to Mitochondria with relatively smaller values when stained with C-terminal targeted antibodies. These observations indicate plausible differential membrane tropisms of Sigmar1 N-terminal and C-terminal amino acid residues. Along with these observations, series of biochemical experiments using alkaline extraction and proteinase K treatment of purified mitochondria demonstrated Sigmar1 as an integral mitochondrial membrane protein.
Earlier studies reported Sigmar1 localization in mitochondrial membranes, vesicles, or elongated cisternae of ER in the hypothalamus and hippocampus in rats (Alonso et al., 2000). In contrast, exogenously expressed GFP-tagged Sigmar1 showed plasma membrane localization in Xenopus Oocytes and colocalized to Kv1.4 potassium channel (Aydar et al., 2002). Sigmar1 was also detected in perinuclear areas, plasmalemma regions of cell-cell contact, growth cones of neurites in mouse neuroblastoma NG-108–15 cells, focal adhesion contacts in Chinese hamster ovary (CHO)-K1 cells (Mavlyutov and Ruoho, 2007). Interestingly, Sigmar1 was detected in ER-associated detergent-insoluble lipid droplets in NG-108 cells and rat hippocampal differentiated oligodendrocytes (Hayashi and Su, 2003a, b). Notably, Sigmar1 consistently appeared in isolated mitochondria fractions and colocalized to mitochondria along with mitochondria-associated ER membranes (MAM) in CHO cells (Hayashi and Su, 2007; Mori et al., 2013). Intriguingly, Sigmar1 was found on ER and nuclear envelope (NE) and showed regulating chromatin remodeling complex on NE in NG-108 and Neuro-2a cells (Tsai et al., 2015). Mouse retinal photoreceptor cells exhibited predominant Sigmar1 expression of NE and sparse distribution at plasma membrane subsurface ER cisternae but not on plasma membrane itself (Mavlyutov et al., 2015a). These observations were further extended in NSC34 neuronal cells and human retinal pigment epithelial ARPE-19 cells where Sigmar1 localization was found on nucleoplasmic reticulum and ER (Mavlyutov et al., 2017b). On the other hand, Sigmar1 localization was detected on the plasma membrane, ER, and NE in mouse and rat dorsal ganglion cells (Mavlyutov et al., 2016b). Subsequent studies from the same author groups reported the presence of Sigmar1 in ER, MAM, and plasma membrane subsurface ER cisternae but not at the plasma membranes in primary rat DRG neurons utilizing C-terminal tagged EGFP-APEX2-Sigmar1 fusion construct adenoviral-mediated gene delivery to rat (Mavylutov et al., 2018). Altogether, these studies indicate cell-specific diverse subcellular localization of Sigmar1 and suggest organelle-specific molecular functions of Sigmar1 under physiological and pathophysiological condition.
Accumulating studies corroborate Sigmar1 as an integral membrane protein. However, its membrane topology remains elusive due to divergent observations in literary reports. Initial studies characterized Sigmar1 with only one transmembrane domain at 80–100 amino acid (aa) sequence that reverberates in later hydrophobicity analysis, confirming a single-pass transmembrane topology of Sigmar1 (Hanner et al., 1996b; Kekuda et al., 1996; Seth et al., 1997). However, another bioinformatics TMbase platform yielded two TM domains at 10–30 aa and 80–100 aa sequence of Sigmar1 (Aydar et al., 2002). In the same study, authors reported that N- and C-terminal GFP-tagged Sigmar1 fusion protein immunofluorescence signal could only be recovered following 0.5% acetone permeabilization in Xenopus oocytes (Aydar et al., 2002). These observations indicate Sigmar1 N-and C-termini are inaccessible from outside the cells, and both termini reside at the cytoplasm. In contrast, based on endogenous Sigmar1 immunostaining using N- and C-terminal targeted antibodies in CHO cells, studies also showed that both N- and C-terminal of Sigmar1 might reside in ER lumen as immunofluorescence signals can only be observed following CHAPS or Triton X-100 mediated cell permeabilization (Hayashi and Su, 2007). These apparent discrepancies in the Sigmar1 tropism might be due to altered membrane insertion of GFP-fused Sigmar1 protein and/or cell type-specific differences in Sigmar1 localization. To this point, C-terminal EYFP-tagged Sigmar1, not N-terminal EYFP-tagged Sigmar1, exhibited a distribution pattern as seen for endogenous Sigmar1 immunostaining (Hayashi and Su, 2007). Moreover, recently reported crystal structure of purified full-length human Sigmar1 protein reconstituted into lipidic cubic phase consisting of lipid: protein at a ratio of 1.5:1.0. The crystal structure of Sigmar1 in this artificially reconstituted membrane exhibited a short single transmembrane domain residing at N-terminal facing towards ER lumen. In contrast, the C-terminal domain is oriented to the cytosolic side (Schmidt et al., 2016). The notion of a single transmembrane domain in Sigmar1 further supported based on GFP-ascorbate peroxidase 2 (APEX2)-tagged Sigmar1 fusion protein immuno-electron microscopy pattern in ND9/27 cells suggesting transmembrane domain lies between amino acids 1–80 (Mavylutov et al., 2018). However, utilizing N-terminal and C-terminal GFP-APEX2 tagged Sigmar1 fusion constructs overexpression studies in ND7/23 cells, studies also reported that electron-dense precipitates observed in the cytosol in case of N-terminal tagged Sigmar1 constructs while C-terminal tagged constructs give rise to electron-dense precipitates in the ER lumen, MAMs and plasma membrane subsurface ER cisternae in immune-electron micrographs (Mavylutov et al., 2018). These observations suggested reversed topology of Sigmar1 in contrast to its reported crystal structure model with cytosol facing N-terminal and ER lumen facing C-terminal.
To dissect out amino acid residues required for Sigmar1 mitochondrial localization, we expressed flag-tagged full-length Sigmar1, C-terminal, and N-terminal deletion mutant Sigmar1 constructs in HEK293T cells. In consort to our and earlier published in silico observations of a hydrophobic, N-terminal transmembrane domain in Sigmar1, N-terminal deletion mutant (102–223 aa) exhibited diffused dots in transfected HEK cells with significantly smaller values for Mander’s colocalization coefficient compared to C-terminal deletion mutants. These findings underscore the presence of N-terminal residing membrane anchoring transmembrane domain in 1–101 amino acid residues consistent with recent reports. We observed a similar pattern when ER was stained with ER Tracker blue in HEK293T cells, although Mander’s colocalization coefficient values were lower than Mitotracker red. While preparing the current manuscript, a recent study also reported exogenously expressed GFP-tagged Sigmar1 fusion protein colocalization to mCherry-tagged Sec61β protein in HEK293T cells, where protein transport protein Sec61 subunit beta (Sec61β) is used as ER marker protein (Zhemkov et al., 2021). Although authors used Tom20 as mitochondria marker, Mander’s colocalization coefficient of Sigmar1-to-Tom 20 stained fluorescent signals was overlooked. The differing Mander’s colocalization values in our studied Flag-tagged Sigmar1-to-ER Tracker Blue and reported GFP-tagged-Sigmar1-to-mCherrySec61β fluorescent signals might arise from multiple reasons. First, due to the utilized labeling procedure, we used antibody and protein tagging free ER specific dye to label endogenous ER compartment compared to mCherry-tagged exogenously expressed Sec61β protein as ER. Second, physiological interactions between Sigmar1 and Sec61β protein as their endogenous interactions have not been considered. Third, intrinsic limitations of fluorescence microscopes resolution yielding slightly greater overlap coefficient values due to a large molecular mass of GFP (about 27 kDa) and mCherry (28 kDa) tagging themselves, resulting in greater spreading of fluorescence signals in digital micrographs. Nonetheless, we were able to demonstrate that Sigmar1 N-terminal possesses the transmembrane domain required for membrane localization.
Cardiac myocytes' physiological and functional homeostasis depends on mitochondrial oxidative phosphorylation (OXPHOS) (Zhou and Tian, 2018). To determine whether Sigmar1 imparts any functional significance in mitochondrial OXPHOS, we conducted extracellular flux analysis and high-resolution respirometry analysis in neonatal rat cardiac myocytes following Sigmar1 knockdown. Mitochondrial stress test assay in intact isolated cardiac myocytes utilizing cell-permeable inhibitors and uncouplers revealed significantly reduced bioenergetic profiles in Sigmar1 knockout cardiac myocytes than the control group of cardiac myocytes. These data indicate the absence of Sigmar1 in cardiac myocytes negatively affects mitochondrial electron transport system (ETS) linked respiration. To determine specific fuel substrate linked ETS supported oxygen flux, next, we conducted high-resolution respirometry in permeabilized cardiac myocytes supplemented with Complex I and Complex II linked substrates in the presence of saturating ADP concentrations to achieve OXPHOS state. Notably, Sigmar1 knockout resulted in significant attenuation of mitochondrial Complex I and Complex II-linked OXPHOS in the presence of NADH-linked substrates, i.e., pyruvate, malate, glutamate, and succinate under saturating ADP concentrations. This compromised oxygen flux ensued in reduced maximal respirations following FCCP-induced uncoupling of mitochondrial respiration from intermembrane proton motive force in Sigmar1 knockout cardiac myocytes. These data underscore the necessity of Sigmar1 in maintaining physiological mitochondrial respirations in cardiac myocytes.
Overall, our current study brings to light uncharted and novel subcellular localization and organelle-specific functions of Sigmar1 in cardiac myocytes. We discovered that Sigmar1 is an integral mitochondrial membrane protein in the mammalian heart through complementary biochemical, immunohistochemistry, immunocytochemistry, and immune-electron microscopic study. Through a genetic loss-of-function approach to knock down Sigmar1 in primary cardiac myocytes, we determined the essential functions of Sigmar1 in maintaining physiological mitochondrial fuel substrates-linked respirations. Hence, our findings corroborate novel Sigmar1-dependent mitochondrial electron transfer system (ETS)-linked respirations. Altogether, our results laid the foundation of novel molecular functions of Sigmar1 in mitochondrial physiological functions. In future studies, we aim to employ biochemical and proteomics approaches to discover novel associate protein partners of Sigmar1 in regulating physiological mitochondrial structure and functions to pave rational drug design targeting Sigmar1 in cardiovascular diseases.
Methods
Animals
We have used 8- to 10-week-old male and female C57BL/6 mice (Jackson Laboratory, Bar Harbor, ME) for heart tissue immunohistochemistry, isolation of mitochondria, subcellular fractionation, and isolation of adult cardiac myocytes in the current study. All mice were housed in cages supplied with ad libitum water and food with a 12-hour light-dark cycle. We obtained timed pregnant Sprague-Dawley rats from Charles River Laboratories (Portage, MI) to isolate primary neonatal rat cardiomyocytes from 1–2 day old newborn rat pups. All animals were handled and cared for according to the Guide for the Care and Use of Laboratory Animals (Eighth Edition, National Institutes of Health, Bethesda, MD). The Institutional Animal Care and Use Committee approved all animal experiment procedures at LSU Health Sciences Center-Shreveport.
Human Heart Tissues
We obtained human heart specimens from autopsies of patients who died due to accidental events in collaboration with the Department of Pathology and Translational Pathobiology at LSU Health Sciences Center-Shreveport. The LV walls were either snap-frozen in liquid nitrogen for subsequent cellular fractionation studies or fixed in 10% v/v formalin jars. Fixed LV tissues were dehydrated in serial alcohol and xylene wash, followed by paraffin infiltration and embedding. Embedded heart tissues were then sectioned at serial 5μm thin sections on a microtome for subsequent immunohistochemistry studies (Abdullah et al., 2020b; Alam et al., 2020). These experiments using frozen and formalin-fixed human heart tissue were deemed non-human research by the Institutional IRB due to the exclusive use of postmortem samples.
Immunofluorescence staining on heart tissue sections
Formalin-fixed, paraffin-embedded 5 μm thin human and mouse heart sections were first deparaffinized in serial xylene, and alcohol wash. Rehydrated in 1X phosphate-buffered saline (PBS, pH 7.4). Antigen retrieval was carried out by boiling the slides in 0.1M sodium citrate buffer containing 10mM EDTA (pH 6.2) for 30 minutes. The slides were cooled down to room temperature and washed in 1X PBS. Heart sections were then blocked in Blocking buffer containing 1% w/v Bovine Serum Albumin, 0.1% v/v cold water fish skin gelatin, 0.1% v/v Tween20, and 0.05% w/v sodium azide in 1X PBS at room temperature (RT) for 1 hour. Subsequently, the heart sections were incubated with rabbit anti-Sigmar1 (1:100) (OAAB01426, Aviva Systems Biology) (for human heart sections) and rabbit anti-Sigmar1 (1:100) (42–3300, Invitrogen) (for mouse heart sections) primary antibodies overnight at 4 °C. The next morning, heart sections were washed in 1X PBS and incubated with Alexa Fluor 488 donkey anti-rabbit (A21206, Invitrogen) secondary antibody at 1:100 dilution in Blocking buffer for 1 hour at RT. The slides were washed in 1X PBS and incubated with mouse anti-Troponin I (1:1000) antibody (MAB1691, Sigma Aldrich) for 1 hour at RT. The slides were subsequently washed in 1X PBS and incubated with Alexa Fluor™ 568 donkey anti-mouse (A10037, Invitrogen) secondary antibody at 1:100 dilution in Blocking buffer for 1 hour at RT. The slides were then washed in 1X PBS. All washes were carried out 3 times 5 minutes each at RT in 1X PBS. Nuclei were counterstained with 4’,6-diamidino-2-phenylindole (DAPI) (D1306, Invitrogen) and mounted with Vectashield Hardset anti-fade mounting media (Vector Laboratories) to reduce photobleaching. Stained heart sections were subsequently observed on a Nikon high-speed, high-resolution A1R confocal microscope (Nikon Instruments Inc., Melville, NY). Fluorophores were excited using 405nm, 488nm, and 561nm lasers, and fluorescence was detected in standard (for 405 lasers) and GaAsP PMT detectors (488 and 561 lasers) in a sequential pattern. Digital fluorescent micrographs were captured with Nikon NIS elements C software (v4.13.04) with a ×60 oil objective lens (NA = 1.4).
Cardiac mitochondria isolation
Cardiac mitochondria were isolated from freshly dissected hearts of anesthetized mice and rats. In the case of human heart tissue, snap-frozen autopsy heart LV tissues kept at −80 °C were used. First, finely chopped heart ventricles in 1 mm3 piece were washed and homogenized in mannitol-sucrose-ethylene glycol tetraacetic acid (MS-EGTA) buffer constituting 225 mM mannitol, 75 mM sucrose, 5 mM HEPES, and 1 mM EGTA (pH 7.4) using a glass/Teflon Potter Elvehjem homogenizer (Abdullah et al., 2020a; Abdullah et al., 2018b; Alam et al., 2018; Alam et al., 2020). Homogenized heart lysates were first centrifuged at 600g for 5 minutes twice at 4 °C to pelleted out unbroken cells and nuclei. The supernatant containing mitochondria was subjected to further centrifugation at 10,000g for 10 minutes 4 °C. The pelleted mitochondria were rewashed in MS-EGTA buffer and re-suspended in MS-EGTA buffer.
Subcellular fractionation
Subcellular fractionation to isolate the purified mitochondria, lysosomes, and cytosol fractions were prepared from mouse heart as described previously (Han et al., 2021; Wieckowski et al., 2009). Mice heart ventricles were cut into 1 mm3 piece and homogenized using glass/Teflon Potter Elvehjem homogenizer. A fraction of the initial heart lysates were kept to use as whole cell lysates. Crude mitochondria from the heart were isolated using the MS-EGTA buffer constituting 225 mM mannitol, 75 mM sucrose, 5 mM HEPES, and 1 mM EGTA (pH 7.4). The crude mitochondrial fraction was pelleted by centrifugation at 10,000 g for 10 min, and the supernatants were centrifuged at 20,000 g for 30 min at 4°C to collect lysosomal fractions as a pellet. The supernatant was subjected to 100,000 × g centrifugation for 1 hour at 4°C to isolate cytosol (Wieckowski et al., 2009). Further, the lysosomal fraction was re-suspended in gradient dilution buffer (equal volume of lysosome enrichment reagents A and B) and layered on a discontinuous OptiPrep Gradients according to manufacturer’s instructions (Lysosome Enrichment Kit for Tissue and Cultured Cells, Catalogue Number: 89839, Thermo Scientific). The prepared gradient is then subjected to 145,000 × g ultracentrifugation for 2 hours at 4 °C. The lysosomal fractions were collected subsequently from the top of the gradient following ultracentrifugation and washed with 1X PBS to obtain enriched lysosomal fractions according to the manufacturer’s instructions (Thermo Scientific). The crude mitochondria fraction was washed and resuspended in mitochondrial reconstitution buffer (MRB) [250 mM mannitol, 5 mM HEPES (pH 7.4), and 0.5 mM EGTA], and layered on top of a 30% Percoll gradient buffer [225 mM mannitol, 25 mM HEPES (pH 7.4), 1 mM EGTA, and 30% Percoll](Wieckowski et al., 2009). After centrifugation at 95,000 g for 30 min, a dense band containing the pure mitochondria was recovered at the bottom of the gradient. All final organelle pellets were resuspended and lysed in RIPA buffer supplemented with protease inhibitor cocktail; the protein concentrations were determined and subsequently subjected to immune blotting experiments. Equal amounts of protein (5 μg) were loaded for each fraction per lane.
Immunofluorescence staining on adult mouse cardiomyocytes
We have isolated primary adult mouse cardiomyocytes according to the protocol reported earlier (Abdullah et al., 2020b; Ackers-Johnson et al., 2016; Alam et al., 2018; Alam et al., 2017). Briefly, isoflurane euthanized mice heart first perfused with EDTA buffer (pH 7.8) containing 5 mM EDTA through the right ventricle. Aorta was clamped with Lahey forceps, and the heart was dissected out by cutting the aorta behind the clamp. Hearts were perfused more through the left ventricle (LV) with EDTA buffer following perfusion buffer wash containing no EDTA. Clamped hearts were then transferred to Petri dishes containing perfusion buffer supplemented with collagenase II (0.5 mg/ml), collagenase IV (0.5 mg/ml) and Protease XIV (0.05 mg/ml). Clamped hearts were then slowly perfused through LV with the above digestion buffer (30 ml to 50 ml) until heart ventricles were visibly pale and flaccid. LVs were then separated, gently pulled into 1 mm3 pieces using fine tip forceps. Cardiomyocytes dissociation was further completed through gentle trituration using wide-bore pipette tips, and enzymes activity was stopped by adding 5 mL of stop buffer (perfusion buffer containing 5% v/v Fetal Bovine Serum). Cell suspensions were then filtered through a 100-μm filter and subjected to 4 consecutive rounds of gravity settling using 3 intermediate calcium reintroduction buffers to restore calcium concentration to physiological levels in cardiomyocytes gradually. The cell pellet in each round of gravity settling was enriched with cardiac myocytes and ultimately constituted a highly pure rod-shaped myocytes fraction. Isolated cardiac myocytes were then plated into laminin (5 μg/ml) coated Nunc Lab Tek 2-well glass chamber slides at 1 × 105 cells per well. Following 2 hours of plating, cardiomyocytes mitochondria were stained by incubating with 200 nM MitoTracker Red CMXRos (M7512, Molecular Probes, Invitrogen) diluted in culture medium for 40 minutes at 37 °C. A set of wells were kept un-stained for dual immunostaining with mitochondrial outer membrane protein Tom20 and Sigmar1. Cardiomyocytes were then immediately fixed and permeabilized in 4% v/v paraformaldehyde with 0.5% v/v Triton X-100 in PBS for 10 minutes. Cardiomyocytes were washed twice 5 minutes each, and formaldehyde was quenched by incubating the myocytes in 0.1M glycine (pH 3.5) for 30 minutes at room temperature (RT). The myocytes were then blocked with 1% w/v bovine serum albumin, 0.1% v/v cold water fish skin gelatin, 0.1% v/v Tween 20, and 0.05% w/v sodium azide in 1X PBS for 1 hour at RT. The myocytes were then incubated with rabbit anti-Sigmar1 (1:100) (42–3300, Invitrogen) and anti-mouse Tom20 (1:100) (ab56783, Abcam) primary antibodies overnight at 4 °C. The next morning, myocytes were washed twice for 5 minutes each with 1X PBS at RT. The cells were then incubated with Alexa Fluor 488 donkey anti-rabbit (A21206, Invitrogen) and Alexa Fluor 555 donkey anti-mouse (A-31570, Invitrogen) secondary antibodies at 1:100 dilution in Blocking buffer for 1 hour at RT. The chamber wells were washed twice with PBS for 5 minutes each. Nuclei were stained with DAPI (Invitrogen) and washed with PBS. The stained myocytes were mounted with Vectashield Hardset anti-fade mounting media (Vector Laboratories) to reduce photobleaching. Adult cardiomyocytes were subsequently examined on Nikon A1R confocal microscope (Nikon Instruments Inc., Melville, NY). Fluorophores were excited using 405nm, 488nm, and 561nm lasers, and fluorescence was recorded in standard (for 405 lasers) and GaAsP PMT detectors (488 and 561 lasers) in a sequential pattern to avoid overlaps between fluorophores signals. Digital fluorescent micrographs were captured with Nikon NIS elements C software (v4.13.04) with ×60 oil objective lens (NA = 1.4). The extent of colocalization between anti-Sigmar1 stained green fluorescence signals-to-MitoTracker Red stained red fluorescence signals were carried out on confocal z-stack images (3–5 μm optical volume) using ‘Colocalization’ module of Imaris 9.2 image analysis software (Bitplane AG, OXFORD Instruments) based on intensity thresholding (Marvizon et al., 2007). Colocalization coefficient values and colocalized voxels were derived from a 2D pixel intensity map of green and red fluorescent pixels of respective images. Crosshairs on 2D pixel intensity map were manually set to quantify high-intensity green-to-red pixels colocalization (Aaron et al., 2018; Dunn et al., 2011; Manders et al., 1993; Marvizon et al., 2007). Following the thresholds were set, the program provided numerical values of colocalization coefficients and numbers of colocalized voxels.
Quantum dot nanocrystals coupled transmission electron microscopy
We have utilized quantum dot nanocrystals (Q-dots) to delineate the subcellular localization of Sigmar1 at the ultrastructural level as previously described (Killingsworth and Bobryshev, 2016). Briefly, mice were anesthetized with isoflurane, and lack of toe pinch reflex was examined to ensure surgical anesthesia induction. The hearts were immediately excised, atria and right ventricle were dissected out. The left ventricles (LV) were bifurcated and put in 3% glutaraldehyde in 0.1M sodium Cacodylate buffer. The LV tissues were cut into about 1 mm3 piece. The dissected heart tissues were fixed in 3% glutaraldehyde (in 0.1 M sodium cacodylate buffer pH 7.4) for 24 hours at 4 °C in a glass sample tube. The fixed tissues were then processed for Q-dots labeling following consecutive washes with 0.1M sodium Cacodylate buffer 20 minutes twice, osmium tetraoxide (2% OsO4, pH 7.4) for 4 hours, 2% sodium acetate for 10 minutes, and 2% uranyl acetate for 1 hour at room temperature. The stained tissues were then subjected to sequential dehydration in 50% ethanol, 70% ethanol, 95% ethanol, 100% ethanol, and 100% acetone. The LV tissues were then impregnated in low viscosity epoxy resin (preparation as described in Killingsworth, M. C., & Bobryshev, Y. V, 2016). The impregnated tissues were then put in resin molds and cured at 70 °C overnight. The embedded tissue sections were cut into thin sections of 90 nm slices and put on the dull side of the nickel mesh grid. Antigen unmasking was carried out by incubating the tissue sections with sodium metaperiodate (5% in distilled water) for 30 minutes, followed by a wash with distilled water for 60 seconds. The residual aldehydes were quenched using 0.05M Glycine and blocked for 20 minutes in blocking buffer constituting 1% normal goat serum (PCN5000, Invitrogen) and 1% modified BSA-c (25557, Electron Microscopy Sciences) in 1X PBS. The blocked grids were then incubated with antibody diluent (S3022, Dako) for 10 minutes followed by incubation with rabbit anti-Sigmar1 (diluted at 1:10 ratio in antibody diluent) (61994, Cell Signaling) primary antibody at room temperature (RT) for 1 hour 30 minutes. The grids were then washed with antibody diluent twice 5 minutes each and incubated with biotinylated goat anti-rabbit (1:10) secondary antibody for 1 hour at RT. Next, the grids were washed twice with antibody diluent 5 minutes each and incubated with Qdot 655 streptavidin conjugates (1:10) (Q10121MP, Invitrogen) (fluorescently labeled quantum dot nanocrystals with covalently attached streptavidin) for 1 hour at RT. The grids were then washed with antibody diluent twice 5 minutes each, followed by a wash in distilled water for 2 minutes. Tissue grids that received only Q-dots incubation served as a negative control of the staining process. The stained grids were blot dried and imaged with JEOL JEM-1400 transmission electron microscope (JEOL, Peabody, MA) with Advanced Microscopy Techniques imaging software (AMT, Woburn, MA). TEM images were post-processed, and organelles were pseudocolored using GIMP 2.10 software to delineate particular organelles.
Isolated cardiac mitochondria staining
Isolated cardiac mitochondria staining was conducted according to the reported methods elsewhere (Martin et al., 2012; Ponnalagu et al., 2016; Singh et al., 2013; Singh et al., 2012). Briefly, freshly isolated cardiac mitochondria were incubated with 200 nM MitoTracker Red CMXRos (M7512, Molecular Probes, Invitrogen) for 45 minutes in 1 mL MS-EGTA buffer at 37 °C. MitoTracker labeled mitochondria then diluted 5 times in MS-EGTA buffer and seeded dropwise on 0.1% w/v Poly-L-lysine (P8920, Sigma Aldrich) coated glass coverslips in 24-well cell culture plates and incubated for at 37 °C in a cell culture incubator for 1 hour. Seeded mitochondria were then immediately fixed and permeabilized in 4% v/v paraformaldehyde containing 0.5% v/v Triton X-100 in PBS for 10 minutes at room temperature (RT). Formalin was quenched by adding 0.1M glycine (pH 3.5) into the wells for 30 minutes at RT. Mitochondria were then blocked in a blocking buffer containing 1% w/v bovine serum albumin, 0.1% v/v cold water fish skin gelatin, 0.1% v/v Tween 20, and 0.05% w/v sodium azide in PBS at RT for 1 hour. Following blocking, mitochondria were incubated with rabbit anti-Sigmar1 (1:100) (OAAB01426, Aviva Systems Biology) (anti-Sigmar1 N-terminal directed antibody), rabbit anti-Sigmar1 (1:100) (AP12379PU-N, Acris, OriGene) (anti-Sigmar1 N-terminal directed antibody), rabbit anti-Sigmar1 (1:100) (42–3300, Invitrogen) (anti-Sigmar1 C-terminal directed antibody), rabbit anti-Sigmar1 (1:100) (PA5–12327, Invitrogen) (anti-Sigmar1 C-terminal directed antibody), rabbit anti-tom20 (1:100) (sc-11415, Santa Cruz) and mouse anti-OXPHOS (1:100) (ab110413, Abcam) primary antibodies overnight at 4 °C in respective wells. Isotype control mitochondria wells were incubated with normal rabbit IgG (1:100) (12–370, EMD Millipore). The next morning, mitochondria wells were washed twice for 5 minutes each with 1X PBS at RT. The mitochondria were then incubated with respective Alexa Fluor 488 donkey anti-rabbit (A21206, Invitrogen) and Alexa Fluor 488 donkey anti-mouse (A10037, Invitrogen) secondary antibodies at a dilution of 1:100 in Blocking buffer at RT for 1 hour. The wells were subsequently washed twice with PBS. The stained mitochondria coverslips were mounted with Vectashield Hardset anti-fade mounting media (Vector Laboratories) and kept in the dark at 4 °C until confocal fluorescence imaging. Mitochondria were subjected to high-resolution microscopy using Nikon A1R confocal microscope (Nikon Instruments Inc., Melville, NY) and observed with ×60 oil objective lens (NA = 1.4). Antibody-stained fluorochromes were excited using 405nm, 488nm, and 561nm lasers coupled to standard (for 405 lasers) and GaAsP PMT detectors (for 488 and 561 lasers). Digital fluorescent micrographs were captured with Nikon NIS elements C software (v4.13.04). The extent of colocalization between anti-Sigmar1, anti-Tom20, anti-OXPHOS, and anti-rabbit IgG stained green fluorescence signals-to-MitoTracker Red stained red fluorescence signals were analyzed using the ‘Colocalization’ module in Imaris 9.2 (Bitplane AG, OXFORD Instruments). Mander’s colocalization coefficient and Pearson’s correlation coefficient between the respective fluorescence pixels were calculated in a 2D pixel intensity map of green-to-red fluorescent pixels. Crosshairs on 2D pixel intensity map were manually set to quantify high-intensity green-to-red pixels colocalization (Aaron et al., 2018; Manders et al., 1993; Marvizon et al., 2007). After thresholding, the program generated numerical values of colocalization coefficients, and numbers of colocalized voxels between the respective fluorescent signals were recorded.
Biochemical experiments on isolated mitochondria
Freshly isolated mitochondria from adult mouse heart ventricles were subjected to alkaline, detergent, and Proteinase K-based mitochondrial protein extraction experiments to biochemically determine the association of Sigmar1 to mitochondria (Arasaki et al., 2015; de Brito and Scorrano, 2008; Filadi et al., 2018; Kim et al., 2015). 100 μg of mitochondria were incubated with 1M Na2CO3 and 10% v/v Triton X-100 for 30 minutes on ice in MS-EGTA buffer (pH 7.4). The mitochondrial suspensions were then centrifuged at 15,000 rpm for 10 minutes at 4 °C. Protein samples for western blot analysis were prepared using 6x Laemmli’s buffer containing 30% v/v β-mercaptoethanol to mitochondrial pellet containing integral membrane proteins and supernatants containing soluble and loosely bound proteins. In separate experiments, an equal amount of isolated cardiac mitochondria (100 μg) were exposed to Proteinase K-based digestion with 0.25 μg/ml to 25 μg/ml doses and incubated for 30 minutes on ice. After digestion, mitochondrial suspensions were centrifuged at 15,000 rpm for 10 minutes at 4 °C. Mitochondrial pellet and supernatants were separated, and protein samples were prepared using 6× Laemmli’s buffer containing 30% v/v β-mercaptoethanol. In all experiments, mitochondrial fractions subjected to the same treatment except the presence of alkali, Triton X-100, and Proteinase K, respectively, served as experimental controls.
Western blot analyses
Human, rat, and mouse heart subcellular fractions were prepared by homogenizing flash-frozen hearts in ice-cold Tris-HCl buffer (pH 7.5) containing 0.5% Triton-X 100, 4 mM EGTA, 10 mM ethylenediaminetetraacetic acid (EDTA), 150 mM sodium chloride, 1 mM sodium orthovanadate, 30 mM sodium pyrophosphate, 50 mM sodium fluoride, and a complete mixture of protease inhibitor cocktail (Roche) (Abdullah et al., 2019; Abdullah et al., 2018b; Alam et al., 2018). HEK293T cells were lysed with Cell Lytic M lysis buffer (Sigma-Aldrich) containing a protease inhibitor cocktail (Roche)(Abdullah et al., 2020b). The lysed cells were sonicated and centrifuged at 14,000 × g for 15 minutes to sediment insoluble cell debris. The lysed subcellular fractions and HEK cells protein concentrations were measured through a modified Bradford assay (Bio-Rad) relative to bovine serum albumin (BSA) standard curve(Abdullah et al., 2020b; Abdullah et al., 2019; Abdullah et al., 2018a). Protein samples were prepared with 6× Laemmli’s buffer containing 30% v/v β-mercaptoethanol. HEK293T cell lysates, mouse heart subcellular fractions, alkali, Triton X-100, and Proteinase K treated resultant pellet, and supernatant fractions were resolved through sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) using 7.5–12% gels and transferred to polyvinylidene difluoride (PVDF) membranes (Bio-Rad). PVDF membranes were blocked with 5% non-fat dried milk in 1X TBST buffer for 1 hour and incubated with primary antibodies overnight on slow rocking at 4°C. The following primary antibodies were used in the current study for immunoblotting: Sigmar1 (1:1000, 61994, Cell Signaling), Flag (1:500, F3165, Sigma Aldrich), GAPDH (1:1000, MAB374, EMD Millipore), LAMP2a (1:1000, 512200, Invitrogen), Mfn2 (1:1000, 9482, Cell Signaling), Cytochrome C (1:1000, SC7159, Santa Cruz), Tom20 (1:1000, sc-11415, Santa Cruz), Fis1 (1:500, sc-98900, Santa Cruz), OPA1 (1:1000, 80471, Cell Signaling), Tim23 (1:500, sc-514463, Santa Cruz), Drp1 (1:1000, 14647, Cell Signaling), IRE1α (1:1000, NB100–2323, Novus Biologicals), VCP (1:1000, 2648, Cell Signaling), PDI (1:1000, 3501, Cell Signaling) and β-actin (1:1000, 47778, Santa Cruz). Membranes were subsequently washed with 1X TBST buffer and incubated with alkaline-phosphatase-conjugated secondary antibodies (Jackson ImmunoResearch Laboratories, Inc.). Membranes were exposed to ECF substrate (Amersham) and imaged on a ChemiDoc Touch Imaging System (Bio-Rad).
Flag-tagged full-length Sigmar1 and deletion mutant Sigmar1 expression plasmids
We prepared FLAG-tagged full-length Sigmar1 and a series of FLAG-tagged Sigmar1 fragments (Sigmar1 aa1–223, Sigmar1 aa1– 169, Sigmar1 aa1–101, and Sigmar1 aa102–223) based on the possible TM domains predicted by Kyte-Doolittle analysis and the UniProt database (http://www.uniprot.org) using a mouse cDNA library by standard PCR methods(Bhuiyan et al., 2012). PCR-amplified DNAs were cloned in pCMV3 vector to express in HEK293T cells.
Plasmids transfection and immunostaining in HEK293T cells
HEK293T cells (CRL-11268, ATCC) were seeded on glass coverslips in 24-well plates at 5 × 104 cells per well in DMEM-GlutaMAX (Gibco) culture media supplemented with 10% FBS (Gibco) and 1% antibiotic–antimycotic (Gibco). N-terminal Flag-tagged Sigmar1 expression plasmid in pCMV3 vector was transfected in seeded HEK293T cells using Lipofectamine 2000 (11668019, Invitrogen) according to the manufacturer’s instructions (Abdullah et al., 2020b). Mock wells received only pCMV3 vector plasmid. Following 48 hours of transfection, cells were labeled with 200 nM MitoTracker Red CMXRos (M7512, Molecular Probes, Invitrogen) and with 200 nM ER-Tracker Blue-White DPX (E12353, Molecular Probes, Invitrogen) diluted in culture medium for 40 minutes at 37 °C. Cells were subsequently fixed and permeabilized with 4% paraformaldehyde and 0.5% v/v Triton-× 100 in PBS (pH 7.4) for 20 minutes at room temperature (RT). Next, formalin was neutralized with 0.1 M glycine (pH 3.5). Cells were blocked with blocking buffer containing 1% bovine serum albumin, 0.1% cold water fish skin gelatin, 0.1% Tween 20, and 0.05% sodium azide in PBS (pH 7.4) for 1 hour at RT. Cells were then immunolabeled with mouse monoclonal anti-Flag (1:100) (F3165, Sigma Aldrich) primary antibody to specifically stain exogenously expressed Sigmar1 protein followed by incubation with Alexa Fluor 488 donkey anti-mouse (A10037, Invitrogen) secondary antibody. Cells were subsequently washed with PBS and mounted with Vectashield Hardset (Vector Laboratories) mounting media. Immunofluorescently labeled HEK293T cells were examined on a Leica TCS SP5 spectral confocal microscope using a ×63 oil objective (NA=1.4), and images were acquired with Leica LAS (AF 2.6.3) software.
siRNA knockdown of Sigmar1
Sigmar1 siRNA knockdown in cardiomyocytes was achieved by methods as described previously (Alam et al., 2017). Briefly, 24 hours after plating, cardiomyocytes were transfected with Sigmar1 siRNA(GGAUCACCCUGUUUCUGACUAUUGU and ACAAUAGUCAGAAACAGGGUGAUCC) (Invitrogen) at 25nM concentrations with Lipofectamine 2000 (Initrogen) in OptiMEM (Gibco) media for 16 hours. A non-specific siRNA was used as a negative control in all the silencing experiments.
Mitochondrial bioenergetics
The mitochondrial bioenergetics stress test was conducted to measure mitochondrial oxygen consumption rates (OCRs) using a Seahorse XFe24 Extracellular Flux Analyzer (Agilent, Santa Clara, CA) (Abdullah et al., 2019; Aishwarya et al., 2020; Alam et al., 2018). Neonatal rat ventricular cardiomyocytes (NRCs) were plated at a density of 8 × 10 4 cells per well into 0.1% w/v gelatin-coated Seahorse XFe24 plates with α-MEM culture media containing 10% FBS with 1% antibiotic-antimycotic mixture (Gibco). After 24 hours of plating, cardiomyocytes were transfected with Sigmar1 siRNA and control siRNA for 16 hours. NRCs were subsequently cultured in DMEM-Glutamax™ culture media containing 2% FBS with 1% antibiotic-antimycotic mixture (Gibco) for 5 days. On day 5, NRCs were incubated with DMEM (containing no glucose and no pyruvate; Gibco) containing 10 mM glucose and 2 mM sodium pyruvate in a CO2 free incubator at 37 °C for 1 hour before loading the plate in the XFe24 analyzer. The OCRs were recorded over 90 minutes following consecutive injections of oligomycin (1 μM), FCCP (4 μM), and rotenone (0.5 μM) plus antimycin A (0.5 μM) to each well at the given time points. Following completion of the test protocol, total protein concentrations were measured in individual wells to normalize the OCR values as expressed as pmoles/min/μg protein.
High-resolution respirometry
Isolated primary neonatal rat ventricular cardiomyocytes (NRCs) were plated at a density of 2 × 106 cells per 10 cm2 tissue culture plates in α-MEM culture media containing 10% FBS with 1% antibiotic–antimycotic mixture (Gibco). After 24 hours of plating, cardiomyocytes were transfected with Sigmar1 siRNA and control siRNA for 16 hours. NRCs were subsequently cultured in DMEM-Glutamax culture media containing 2% FBS with 1% antibiotic–antimycotic mixture (Gibco) for 5 days. On day 5, NRCs were gently scraped from the plates following incubation with 0.05% Trypsin-EDTA (Gibco) for 5 minutes and permeabilized with saponin (15 μg/ml) for 8 minutes in mitochondrial respiration medium, MiR05 (60101–01, Oroboros). Permeabilized NRCs were counted and subjected to respirometry studies at 1.5 to 2 × 106 cells/ml into each respiration chambers containing MiR05 respiration medium at 37 °C (O2k; Oroboros Oxygraph-2k, Innsbruck, Austria) (Djafarzadeh and Jakob, 2017; Hughey et al., 2011; Lemieux et al., 2017; Long et al., 2019; Mattox et al., 2014). Instrumental and chemical oxygen background fluxes were calibrated as a function of oxygen concentration and subtracted from the total volume-specific oxygen flux according to the manufacturer’s instructions (Datlab software, Oroboros Instruments). Stable oxygen fluxes were recorded over 3 minutes following subsequent injections of NADH (N)-linked substrates pyruvate (P) (4 mM), glutamate (G) (10 mM), and malate (M) (4 mM). ADP (2 mM) was injected to attain a complex I (CI) supported oxidative phosphorylation (OXPHOS) state. Cytochrome c (10 μM) was injected to ensure outer mitochondrial membrane integrity. Cytochrome c control factors (change of respiration divided by cytochrome c stimulated respiration) were in the range of 0.02 to 0.15 in both negative and Sigmar1 siRNA treated NRCs. Next, succinate (S) (20 mM) was injected to induce convergent electron flow through the NS pathway and measure CI+CII-linked OXPHOS. Mitochondrial proton motive force uncoupler FCCP (4 μM) was injected following Oligomycin (1 μM) to measure CI+CII-linked maximal respiration. Rotenone (1 μM) and Antimycin A (2 μM) were co-injected to measure non-mitochondrial oxygen fluxes. Mitochondria-specific oxygen fluxes are determined by subtracting non-mitochondrial oxygen fluxes and expressed as pmoles O2/[sec*106 cells].
Statistical analyses and scientific rigor
All cell culture experiments were repeated two to three times. For all imaging studies, slides and acquired microscopic images were alphanumerically labeled. After completing the study, individual groups ID and image ID numbers were cross-referenced with treatment to permit analysis. Statistical analyses were conducted in GraphPad Prism (v8.2.1, La Jolla, CA). Data were presented in graphs showing median and interquartile ranges. P values less than 0.05 (95% confidence interval) were considered significant.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health grants: R00HL122354, R01HL145753, R01HL145753–01S1, and R01HL145753–03S1; LSUHSC-S CCDS Finish Line Award, COVID-19 Research Award, and LARC Research Award to MSB; P20GM121307 and R01 HL149264 to C.G.K.; NIH R01 HL098435, HL133497, and HL141155 to A.W.O; HL141998 to S.M.; AA025744 and AA026708 to M.P.; LSUHSC-S Malcolm Feist Cardiovascular and AHA Postdoctoral Fellowship to CSA (20POST35210789); and LSUHSC-S Malcolm Feist Pre-doctoral Fellowship to RA.
Footnotes
Data availability
All original data underlying selected data shown in the figures and supplementary figures are available from the corresponding author upon reasonable request. A reporting summary for this article is available as a Supplementary Information file.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aaron JS, Taylor AB, Chew TL, 2018. Image co-localization - co-occurrence versus correlation. J Cell Sci 131. [DOI] [PubMed] [Google Scholar]
- Abdullah CS, Aishwarya R, Alam S, Morshed M, Remex NS, Nitu S, Kolluru GK, Traylor J, Miriyala S, Panchatcharam M, Hartman B, King J, Bhuiyan MAN, Chandran S, Woolard MD, Yu X, Goeders NE, Dominic P, Arnold CL, Stokes K, Kevil CG, Orr AW, Bhuiyan MS, 2020a. Methamphetamine induces cardiomyopathy by Sigmar1 inhibition-dependent impairment of mitochondrial dynamics and function. Commun Biol 3, 682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdullah CS, Aishwarya R, Alam S, Morshed M, Remex NS, Nitu S, Kolluru GK, Traylor J, Miriyala S, Panchatcharam M, Hartman B, King J, Bhuiyan MAN, Chandran S, Woolard MD, Yu X, Goeders NE, Dominic P, Arnold CL, Stokes K, Kevil CG, Orr AW, Bhuiyan MS, 2020b. Methamphetamine induces cardiomyopathy by Sigmar1 inhibition-dependent impairment of mitochondrial dynamics and function. Communications Biology 3, 682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdullah CS, Alam S, Aishwarya R, Miriyala S, Bhuiyan MAN, Panchatcharam M, Pattillo CB, Orr AW, Sadoshima J, Hill JA, Bhuiyan MS, 2019. Doxorubicin-induced cardiomyopathy associated with inhibition of autophagic degradation process and defects in mitochondrial respiration. Sci Rep 9, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdullah CS, Alam S, Aishwarya R, Miriyala S, Panchatcharam M, Bhuiyan MAN, Peretik JM, Orr AW, James J, Osinska H, Robbins J, Lorenz JN, Bhuiyan MS, 2018a. Cardiac Dysfunction in the Sigma 1 Receptor Knockout Mouse Associated With Impaired Mitochondrial Dynamics and Bioenergetics. J Am Heart Assoc 7, e009775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdullah CS, Alam S, Aishwarya R, Miriyala S, Panchatcharam M, Bhuiyan MAN, Peretik JM, Orr AW, James J, Osinska H, Robbins J, Lorenz JN, Bhuiyan MS, 2018b. Cardiac Dysfunction in the Sigma 1 Receptor Knockout Mouse Associated With Impaired Mitochondrial Dynamics and Bioenergetics. Journal of the American Heart Association 7, e009775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ackers-Johnson M, Li PY, Holmes AP, O'Brien SM, Pavlovic D, Foo RS, 2016. A Simplified, Langendorff-Free Method for Concomitant Isolation of Viable Cardiac Myocytes and Nonmyocytes From the Adult Mouse Heart. Circ Res 119, 909–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aishwarya R, Abdullah CS, Morshed M, Remex NS, Bhuiyan MS, 2021. Sigmar1's Molecular, Cellular, and Biological Functions in Regulating Cellular Pathophysiology. Front Physiol 12, 705575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aishwarya R, Alam S, Abdullah CS, Morshed M, Nitu SS, Panchatcharam M, Miriyala S, Kevil CG, Bhuiyan MS, 2020. Pleiotropic effects of mdivi-1 in altering mitochondrial dynamics, respiration, and autophagy in cardiomyocytes. Redox Biol 36, 101660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam S, Abdullah CS, Aishwarya R, Miriyala S, Panchatcharam M, Peretik JM, Orr AW, James J, Robbins J, Bhuiyan MS, 2018. Aberrant Mitochondrial Fission Is Maladaptive in Desmin Mutation–Induced Cardiac Proteotoxicity. Journal of the American Heart Association 7, e009289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam S, Abdullah CS, Aishwarya R, Morshed M, Nitu SS, Miriyala S, Panchatcharam M, Kevil CG, Orr AW, Bhuiyan MS, 2020. Dysfunctional Mitochondrial Dynamic and Oxidative Phosphorylation Precedes Cardiac Dysfunction in R120G-αB-Crystallin-Induced Desmin-Related Cardiomyopathy. Journal of the American Heart Association 9, e017195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam S, Abdullah CS, Aishwarya R, Orr AW, Traylor J, Miriyala S, Panchatcharam M, Pattillo CB, Bhuiyan MS, 2017. Sigmar1 regulates endoplasmic reticulum stress-induced C/EBP-homologous protein expression in cardiomyocytes. Biosci Rep 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso G, Phan V, Guillemain I, Saunier M, Legrand A, Anoal M, Maurice T, 2000. Immunocytochemical localization of the sigma(1) receptor in the adult rat central nervous system. Neuroscience 97, 155–170. [DOI] [PubMed] [Google Scholar]
- Arasaki K, Shimizu H, Mogari H, Nishida N, Hirota N, Furuno A, Kudo Y, Baba M, Baba N, Cheng JL, Fujimoto T, Ishihara N, Ortiz-Sandoval C, Barlow LD, Raturi A, Dohmae N, Wakana Y, Inoue H, Tani K, Dacks JB, Simmen T, Tagaya M, 2015. A Role for the Ancient SNARE Syntaxin 17 in Regulating Mitochondrial Division. Dev Cell 32, 304–317. [DOI] [PubMed] [Google Scholar]
- Aydar E, Palmer CP, Klyachko VA, Jackson MB, 2002. The sigma receptor as a ligand-regulated auxiliary potassium channel subunit. Neuron 34, 399–410. [DOI] [PubMed] [Google Scholar]
- Bhuiyan MS, Fukunaga K, 2009. Stimulation of Sigma-1 receptor signaling by dehydroepiandrosterone ameliorates pressure overload-induced hypertrophy and dysfunctions in ovariectomized rats. Expert Opinion on Therapeutic Targets 13, 1253–1265. [DOI] [PubMed] [Google Scholar]
- Bhuiyan MS, Gulick J, Osinska H, Gupta M, Robbins J, 2012. Determination of the critical residues responsible for cardiac myosin binding protein C's interactions. J Mol Cell Cardiol 53, 838–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhuiyan MS, Tagashira H, Fukunaga K, 2011. Dehydroepiandrosterone-Mediated Stimulation of Sigma-1 Receptor Activates Akt-eNOS Signaling in the Thoracic Aorta of Ovariectomized Rats with Abdominal Aortic Banding. Cardiovascular Therapeutics 29, 219–230. [DOI] [PubMed] [Google Scholar]
- Bhuiyan MS, Tagashira H, Shioda N, Fukunaga K, 2010. Targeting sigma-1 receptor with fluvoxamine ameliorates pressure-overload-induced hypertrophy and dysfunctions. Expert Opin Ther Targets 14, 1009–1022. [DOI] [PubMed] [Google Scholar]
- Culp SG, Rominger D, Tam SW, De Souza EB, 1992. [3H]DuP 734 [1-(cyclopropylmethyl)-4-(2'-(4''-fluorophenyl)-2'- oxoethyl)-piperidine HBr]: a receptor binding profile of a high-affinity novel sigma receptor ligand in guinea pig brain. J Pharmacol Exp Ther 263, 1175–1187. [PubMed] [Google Scholar]
- de Brito OM, Scorrano L, 2008. Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature 456, 605–U647. [DOI] [PubMed] [Google Scholar]
- de Costa BR, Rice KC, Bowen WD, Thurkauf A, Rothman RB, Band L, Jacobson AE, Radesca L, Contreras PC, Gray NM, et al. , 1990. Synthesis and evaluation of N-substituted cis-N-methyl-2-(1-pyrrolidinyl)cyclohexylamines as high affinity sigma receptor ligands. Identification of a new class of highly potent and selective sigma receptor probes. J Med Chem 33, 3100–3110. [DOI] [PubMed] [Google Scholar]
- Djafarzadeh S, Jakob SM, 2017. High-resolution Respirometry to Assess Mitochondrial Function in Permeabilized and Intact Cells. J Vis Exp [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn KW, Kamocka MM, McDonald JH, 2011. A practical guide to evaluating colocalization in biological microscopy. Am J Physiol Cell Physiol 300, C723–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dussossoy D, Carayon P, Belugou S, Feraut D, Bord A, Goubet C, Roque C, Vidal H, Combes T, Loison G, Casellas P, 1999. Colocalization of sterol isomerase and sigma(1) receptor at endoplasmic reticulum and nuclear envelope level. Eur J Biochem 263, 377–386. [DOI] [PubMed] [Google Scholar]
- Filadi R, Leal NS, Schreiner B, Rossi A, Dentoni G, Pinho CM, Wiehager B, Cieri D, Cali T, Pizzo P, Ankarcrona M, 2018. TOM70 Sustains Cell Bioenergetics by Promoting IP3R3-Mediated ER to Mitochondria Ca(2+) Transfer. Curr Biol 28, 369–382 e366. [DOI] [PubMed] [Google Scholar]
- Fisher A, Bezprozvanny I, Wu L, Ryskamp DA, Bar-Ner N, Natan N, Brandeis R, Elkon H, Nahum V, Gershonov E, LaFerla FM, Medeiros R, 2016. AF710B, a Novel M1/sigma1 Agonist with Therapeutic Efficacy in Animal Models of Alzheimer's Disease. Neurodegener Dis 16, 95–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fo Y, Zhang C, Chen X, Liu X, Ye T, Guo Y, Qu C, Shi S, Yang B, 2020. Chronic sigma-1 receptor activation ameliorates ventricular remodeling and decreases susceptibility to ventricular arrhythmias after myocardial infarction in rats. Eur J Pharmacol 889, 173614. [DOI] [PubMed] [Google Scholar]
- Fontanilla D, Johannessen M, Hajipour AR, Cozzi NV, Jackson MB, Ruoho AE, 2009. The hallucinogen N,N-dimethyltryptamine (DMT) is an endogenous sigma-1 receptor regulator. Science 323, 934–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiki Y, Hubbard AL, Fowler S, Lazarow PB, 1982. Isolation of intracellular membranes by means of sodium carbonate treatment: application to endoplasmic reticulum. The Journal of cell biology 93, 97–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S, Zhao F, Hsia J, Ma X, Liu Y, Torres S, Fujioka H, Zhu X, 2021. The role of Mfn2 in the structure and function of endoplasmic reticulum-mitochondrial tethering in vivo. J Cell Sci 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanner M, Moebius FF, Flandorfer A, Knaus HG, Striessnig J, Kempner E, Glossmann H, 1996a. Purification, molecular cloning, and expression of the mammalian sigma1-binding site. Proceedings of the National Academy of Sciences 93, 8072–8077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanner M, Moebius FF, Flandorfer A, Knaus HG, Striessnig J, Kempner E, Glossmann H, 1996b. Purification, molecular cloning, and expression of the mammalian sigma1-binding site. Proc Natl Acad Sci U S A 93, 8072–8077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Su TP, 2003a. Intracellular dynamics of sigma-1 receptors (sigma(1) binding sites) in NG108–15 cells. J Pharmacol Exp Ther 306, 726–733. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Su TP, 2003b. Sigma-1 receptors (sigma(1) binding sites) form raft-like microdomains and target lipid droplets on the endoplasmic reticulum: roles in endoplasmic reticulum lipid compartmentalization and export. J Pharmacol Exp Ther 306, 718–725. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Su TP, 2004. Sigma-1 receptors at galactosylceramide-enriched lipid microdomains regulate oligodendrocyte differentiation. Proc Natl Acad Sci U S A 101, 14949–14954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Su TP, 2007. Sigma-1 receptor chaperones at the ER-mitochondrion interface regulate Ca(2+) signaling and cell survival. Cell 131, 596–610. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Su TP, 2008. An update on the development of drugs for neuropsychiatric disorders: focusing on the sigma 1 receptor ligand. Expert Opin Ther Targets 12, 45–58. [DOI] [PubMed] [Google Scholar]
- Hollander JM, Thapa D, Shepherd DL, 2014. Physiological and structural differences in spatially distinct subpopulations of cardiac mitochondria: influence of cardiac pathologies. Am J Physiol Heart Circ Physiol 307, H1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughey CC, Hittel DS, Johnsen VL, Shearer J, 2011. Respirometric oxidative phosphorylation assessment in saponin-permeabilized cardiac fibers. J Vis Exp [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang W, O'Connor C, Silva SG, Kuchibhatla M, Cuffe MS, Callwood DD, Zakhary B, Henke E, Arias RM, Krishnan R, Investigators S-C, 2008. Safety and efficacy of sertraline for depression in patients with CHF (SADHART-CHF): a randomized, double-blind, placebo-controlled trial of sertraline for major depression with congestive heart failure. Am Heart J 156, 437–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz AM, 2011. Physiology of the heart, 5th ed Lippincott, Williams &Wilkins; pp. 16–25. [Google Scholar]
- Kekuda R, Prasad PD, Fei Y-J, Leibach FH, Ganapathy V, 1996. Cloning and Functional Expression of the Human Type 1 Sigma Receptor (hSigmaR1). Biochemical and Biophysical Research Communications 229, 553–558. [DOI] [PubMed] [Google Scholar]
- Killingsworth MC, Bobryshev YV, 2016. Correlative Light- and Electron Microscopy Using Quantum Dot Nanoparticles. J Vis Exp [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HY, Botelho SC, Park KJ, Kim H, 2015. Use of carbonate extraction in analyzing moderately hydrophobic transmembrane proteins in the mitochondrial inner membrane. Protein Sci 24, 2063–2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klouz A, Sapena R, Liu J, Maurice T, Tillement J-P, Papadopoulos V, Morin D, 2002. Evidence for sigma-1-like receptors in isolated rat liver mitochondrial membranes. British Journal of Pharmacology 135, 1607–1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemieux H, Blier PU, Gnaiger E, 2017. Remodeling pathway control of mitochondrial respiratory capacity by temperature in mouse heart: electron flow through the Q-junction in permeabilized fibers. Sci Rep 7, 2840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonardo CC, Hall AA, Collier LA, Green SM, Willing AE, Pennypacker KR, 2010. Administration of a Sigma Receptor Agonist Delays MCAO-Induced Neurodegeneration and White Matter Injury. Transl Stroke Res 1, 135–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long Q, Huang L, Huang K, Yang Q, 2019. Assessing Mitochondrial Bioenergetics in Isolated Mitochondria from Mouse Heart Tissues Using Oroboros 2k-Oxygraph. Methods Mol Biol 1966, 237–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manders EMM, Verbeek FJ, Aten JA, 1993. Measurement of co-localization of objects in dual-colour confocal images. J Microsc 169, 375–382. [DOI] [PubMed] [Google Scholar]
- Martin LJ, Lau E, Singh H, Vergnes L, Tarling EJ, Mehrabian M, Mungrue I, Xiao S, Shih D, Castellani L, Ping P, Reue K, Stefani E, Drake TA, Bostrom K, Lusis AJ, 2012. ABCC6 localizes to the mitochondria-associated membrane. Circ Res 111, 516–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marvizon JC, Perez OA, Song B, Chen W, Bunnett NW, Grady EF, Todd AJ, 2007. Calcitonin receptor-like receptor and receptor activity modifying protein 1 in the rat dorsal horn: localization in glutamatergic presynaptic terminals containing opioids and adrenergic alpha2C receptors. Neuroscience 148, 250–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuno K, Nakazawa M, Okamoto K, Kawashima Y, Mita S, 1996. Binding properties of SA4503, a novel and selective sigma 1 receptor agonist. Eur J Pharmacol 306, 271–279. [DOI] [PubMed] [Google Scholar]
- Mattox TA, Young ME, Rubel CE, Spaniel C, Rodriguez JE, Grevengoed TJ, Gautel M, Xu Z, Anderson EJ, Willis MS, 2014. MuRF1 activity is present in cardiac mitochondria and regulates reactive oxygen species production in vivo. J Bioenerg Biomembr 46, 173–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavlyutov TA, Baker EM, Losenegger TM, Kim JR, Torres B, Epstein ML, Ruoho AE, 2017a. The Sigma-1 Receptor-A Therapeutic Target for the Treatment of ALS? Adv Exp Med Biol 964, 255–265. [DOI] [PubMed] [Google Scholar]
- Mavlyutov TA, Duellman T, Kim HT, Epstein ML, Leese C, Davletov BA, Yang J, 2016a. Sigma-1 receptor expression in the dorsal root ganglion: Reexamination using a highly specific antibody. Neuroscience 331, 148–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavlyutov TA, Duellman T, Kim HT, Epstein ML, Leese C, Davletov BA, Yang J, 2016b. Sigma-1 Receptor Expression in the Dorsal Root Ganglion: Reexamination Using a Highly Specific Antibody. Neuroscience 331, 148–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavlyutov TA, Epstein M, Guo LW, 2015a. Subcellular localization of the sigma-1 receptor in retinal neurons - an electron microscopy study. Sci Rep 5, 10689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavlyutov TA, Epstein ML, Andersen KA, Ziskind-Conhaim L, Ruoho AE, 2010. The sigma-1 receptor is enriched in postsynaptic sites of C-terminals in mouse motoneurons. An anatomical and behavioral study. Neuroscience 167, 247–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavlyutov TA, Epstein ML, Liu P, Verbny YI, Ziskind-Conhaim L, Ruoho AE, 2012. Development of the sigma-1 receptor in C-terminals of motoneurons and colocalization with the N,N'-dimethyltryptamine forming enzyme, indole-N-methyl transferase. Neuroscience 206, 60–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavlyutov TA, Epstein ML, Verbny YI, Huerta MS, Zaitoun I, Ziskind-Conhaim L, Ruoho AE, 2013. Lack of sigma-1 receptor exacerbates ALS progression in mice. Neuroscience 240, 129–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavlyutov TA, Guo LW, 2017. Peeking into Sigma-1 Receptor Functions Through the Retina. Adv Exp Med Biol 964, 285–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavlyutov TA, Guo LW, Epstein ML, Ruoho AE, 2015b. Role of the Sigma-1 receptor in Amyotrophic Lateral Sclerosis (ALS). J Pharmacol Sci 127, 10–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavlyutov TA, Nickells RW, Guo LW, 2011. Accelerated retinal ganglion cell death in mice deficient in the Sigma-1 receptor. Mol Vis 17, 1034–1043. [PMC free article] [PubMed] [Google Scholar]
- Mavlyutov TA, Ruoho AE, 2007. Ligand-dependent localization and intracellular stability of sigma-1 receptors in CHO-K1 cells. J Mol Signal 2, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavlyutov TA, Yang H, Epstein ML, Ruoho AE, Yang J, Guo LW, 2017b. APEX2-enhanced electron microscopy distinguishes sigma-1 receptor localization in the nucleoplasmic reticulum. Oncotarget 8, 51317–51330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavylutov T, Chen X, Guo LW, Yang J, 2018. APEX2-tagging of Sigma 1-receptor indicates subcellular protein topology with cytosolic N-terminus and ER luminal C-terminus. Protein Cell 9, 733–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori T, Hayashi T, Hayashi E, Su TP, 2013. Sigma-1 receptor chaperone at the ER-mitochondrion interface mediates the mitochondrion-ER-nucleus signaling for cellular survival. PLoS One 8, e76941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakawa T, Yamaguchi O, Hashimoto A, Hikoso S, Takeda T, Oka T, Yasui H, Ueda H, Akazawa Y, Nakayama H, Taneike M, Misaka T, Omiya S, Shah AM, Yamamoto A, Nishida K, Ohsumi Y, Okamoto K, Sakata Y, Otsu K, 2015. Bcl-2-like protein 13 is a mammalian Atg32 homologue that mediates mitophagy and mitochondrial fragmentation. Nat Commun 6, 7527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita N, Hashimoto K, Tomitaka S, Minabe Y, 1996. Interactions of selective serotonin reuptake inhibitors with subtypes of sigma receptors in rat brain. Eur J Pharmacol 307, 117–119. [DOI] [PubMed] [Google Scholar]
- Penke B, Fulop L, Szucs M, Frecska E, 2018. The Role of Sigma-1 Receptor, an Intracellular Chaperone in Neurodegenerative Diseases. Curr Neuropharmacol 16, 97–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponnalagu D, Gururaja Rao S, Farber J, Xin W, Hussain AT, Shah K, Tanda S, Berryman M, Edwards JC, Singh H, 2016. Molecular identity of cardiac mitochondrial chloride intracellular channel proteins. Mitochondrion 27, 6–14. [DOI] [PubMed] [Google Scholar]
- Rao TS, Cler JA, Mick SJ, Ragan DM, Lanthorn TH, Contreras PC, Iyengar S, Wood PL, 1990. Opipramol, a potent sigma ligand, is an anti-ischemic agent: neurochemical evidence for an interaction with the N-methyl-D-aspartate receptor complex in vivo by cerebellar cGMP measurements. Neuropharmacology 29, 1199–1204. [DOI] [PubMed] [Google Scholar]
- Ryskamp DA, Korban S, Zhemkov V, Kraskovskaya N, Bezprozvanny I, 2019. Neuronal Sigma-1 Receptors: Signaling Functions and Protective Roles in Neurodegenerative Diseases. Front Neurosci 13, 862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt HR, Zheng S, Gurpinar E, Koehl A, Manglik A, Kruse AC, 2016. Crystal structure of the human sigma1 receptor. Nature 532, 527–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serebruany VL, Suckow RF, Cooper TB, O'Connor CM, Malinin AI, Krishnan KR, van Zyl LT, Lekht V, Glassman AH, Sertraline Antidepressant Heart Attack Randomized, T., 2005. Relationship between release of platelet/endothelial biomarkers and plasma levels of sertraline and N-desmethylsertraline in acute coronary syndrome patients receiving SSRI treatment for depression. Am J Psychiatry 162, 1165–1170. [DOI] [PubMed] [Google Scholar]
- Seth P, Leibach FH, Ganapathy V, 1997. Cloning and structural analysis of the cDNA and the gene encoding the murine type 1 sigma receptor. Biochem Biophys Res Commun 241, 535–540. [DOI] [PubMed] [Google Scholar]
- Shinoda Y, Tagashira H, Bhuiyan MS, Hasegawa H, Kanai H, Fukunaga K, 2016. Haloperidol aggravates transverse aortic constriction-induced heart failure via mitochondrial dysfunction. J Pharmacol Sci 131, 172–183. [DOI] [PubMed] [Google Scholar]
- Singh H, Lu R, Bopassa JC, Meredith AL, Stefani E, Toro L, 2013. MitoBK(Ca) is encoded by the Kcnma1 gene, and a splicing sequence defines its mitochondrial location. Proc Natl Acad Sci U S A 110, 10836–10841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh H, Lu R, Rodriguez PF, Wu Y, Bopassa JC, Stefani E, Toro L, 2012. Visualization and quantification of cardiac mitochondrial protein clusters with STED microscopy. Mitochondrion 12, 230–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RL, Canton H, Barrett RJ, Sanders-Bush E, 1998. Agonist properties of N,N-dimethyltryptamine at serotonin 5-HT2A and 5-HT2C receptors. Pharmacol Biochem Behav 61, 323–330. [DOI] [PubMed] [Google Scholar]
- Tagashira H, Bhuiyan MS, Shioda N, Fukunaga K, 2014. Fluvoxamine rescues mitochondrial Ca2+ transport and ATP production through sigma(1)-receptor in hypertrophic cardiomyocytes. Life Sci 95, 89–100. [DOI] [PubMed] [Google Scholar]
- Tsai SY, Chuang JY, Tsai MS, Wang XF, Xi ZX, Hung JJ, Chang WC, Bonci A, Su TP, 2015. Sigma-1 receptor mediates cocaine-induced transcriptional regulation by recruiting chromatin-remodeling factors at the nuclear envelope. Proc Natl Acad Sci U S A 112, E6562–6570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urfer R, Moebius HJ, Skoloudik D, Santamarina E, Sato W, Mita S, Muir KW, Cutamesine Stroke Recovery Study, G., 2014. Phase II trial of the Sigma-1 receptor agonist cutamesine (SA4503) for recovery enhancement after acute ischemic stroke. Stroke 45, 3304–3310. [DOI] [PubMed] [Google Scholar]
- Volpe P, Villa A, Podini P, Martini A, Nori A, Panzeri MC, Meldolesi J, 1992. The endoplasmic reticulum-sarcoplasmic reticulum connection: distribution of endoplasmic reticulum markers in the sarcoplasmic reticulum of skeletal muscle fibers. Proc Natl Acad Sci U S A 89, 6142–6146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieckowski MR, Giorgi C, Lebiedzinska M, Duszynski J, Pinton P, 2009. Isolation of mitochondria-associated membranes and mitochondria from animal tissues and cells. Nat Protoc 4, 1582–1590. [DOI] [PubMed] [Google Scholar]
- Xiong GL, Prybol K, Boyle SH, Hall R, Streilein RD, Steffens DC, Krishnan R, Rogers JG, O'Connor CM, Jiang W, Investigators S-C, 2015. Inflammation Markers and Major Depressive Disorder in Patients With Chronic Heart Failure: Results From the Sertraline Against Depression and Heart Disease in Chronic Heart Failure Study. Psychosom Med 77, 808–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Fu Y, Liu X, Shahi PK, Mavlyutov TA, Li J, Yao A, Guo SZ, Pattnaik BR, Guo LW, 2017. Role of the sigma-1 receptor chaperone in rod and cone photoreceptor degenerations in a mouse model of retinitis pigmentosa. Mol Neurodegener 12, 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Liu T, Jin SB, Tomilin N, Castro J, Shupliakov O, Lendahl U, Nister M, 2009. The novel conserved mitochondrial inner-membrane protein MTGM regulates mitochondrial morphology and cell proliferation. J Cell Sci 122, 2252–2262. [DOI] [PubMed] [Google Scholar]
- Zhemkov V, Ditlev JA, Lee WR, Wilson M, Liou J, Rosen MK, Bezprozvanny I, 2021. The role of sigma 1 receptor in organization of endoplasmic reticulum signaling microdomains. Elife 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B, Tian R, 2018. Mitochondrial dysfunction in pathophysiology of heart failure. J Clin Invest 128, 3716–3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou LB, Yamada K, Nabeshima T, 1998. Sigma receptor ligands (+)-SKF10,047 and SA4503 improve dizocilpine-induced spatial memory deficits in rats. Eur J Pharmacol 355, 1–10. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.