Abstract
Hepatitis C virus (HCV) was transmitted from a chronic carrier to his female partner during unprotected anal and vaginal intercourse. Based on HVR1 and phylogenetic tree analysis, the couple had closely related isolates. These findings confirm sexual transmission of HCV without other risk factors.
CASE REPORT
A 32-year-old heterosexual woman, the index patient, presented in March 1997 with jaundice and severe fatigue; her alanine aminotransferase level was 732 IU/liter (normal level, <31). An enzyme immunosorbent assay for hepatitis C virus (HCV) (Ortho Diagnostics, Raritan, N.J.) was positive, and viremia was detected by PCR (Amplicor HCV; Roche Molecular Systems, Neuilly, France). Other causes of acute viral hepatitis were excluded, the autoantibody screening result was negative, and the patient tested negative for human immunodeficiency virus (HIV) infection.
In November 1997, a liver biopsy showed chronic active hepatitis, and her alanine aminotransferase level was 130 IU/liter. Over the 6 months before her illness, she had only one active physical relationship, which included oral sex and vaginal and anal intercourse with her partner. She was negative for HCV infection during this period. Her partner, a 35-year-old man, had had an episode of jaundice with an increase in transaminase activity in 1983 after a blood transfusion. In 1994, he was positive for HCV and his liver biopsy showed, chronic active hepatitis. The temporal relationship between HCV positivity and sexual intercourse led us to suspect a sexually transmitted infection.
In this study, we investigated by HVR1 sequence analysis a case of transmission of an HCV isolate from a chronic carrier to his female partner during unprotected anal and vaginal intercourse.
HCV is transmitted mainly through direct percutaneous exposure to infected blood. Among subjects infected by HCV, approximately 40% have no history of blood transfusion or intravenous drug abuse (18). Perinatal transmission is possible, although the risk is low. The role of sexual transmission in the spread of HCV infection is still debated, but many studies have demonstrated it (12, 18). Such transmission is rare; it probably results from common risk factors or sharing toilet instruments and follows a parenteral route rather than occurring via sexual intercourse, which plays a minor role except in sexually transmitted disease with genital lesions (1, 3, 5, 10, 12). Transmission of HCV infection to sexual partners is favored by a high concentration of circulating HCV or by concomitant HIV infection (3).
HCV, like other RNA viruses, exhibits enormous genomic diversity. HCV isolates show four levels of genetic variability: types, subtypes, isolates, and quasispecies (QS). This heterogeneity is a consequence of high error rates in RNA replication. HCV circulates as a heterogeneous population of genetically different but closely related genomes, the QS (14). The HVR1 region of the genome is highly variable among and within patients and can be used to identify individual HCV isolates, which is of particular interest for epidemiological studies (9).
Microbiological findings.
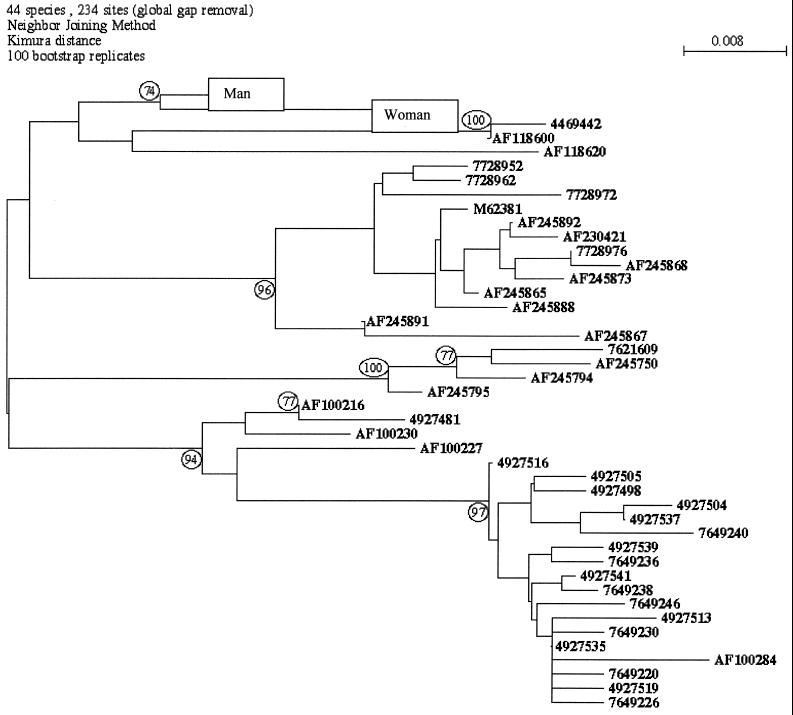
In order to find out if the virus had been transmitted between the two patients, we performed sequence analysis of part of the HVR1 region of the genome of virus isolated from these two patients. The HVR1 fragment (nucleotide positions 1156 to 1234) was chosen for sequence analysis because this domain exhibits a sufficiently high degree of variability to distinguish between HCV isolates of the same subtype. The two HVR1 fragments isolated from the index patient and her partner were aligned with the SEAVIEW program (8). Representative HVR1 sequences of each subtype of genotype 3a were used as control sequences for the HVR1 fragments. A set of control sequences was built with a BLASTn search of the nonredundant databases of the National Center for Biotechnology Information (NCBI), with the consensus sequence of the family as a query.
The trees were inferred through the neighbor-joining (NJ) method, with Kimura's two-parameter distance. The NJ tree was generated from the distance matrix on the basis of all pairwise comparisons of sequences. The trees obtained were unrooted. In order to assess the confidence placed in tree topology, we used the bootstrap method. We used the tools in the PHYLIP package, version 3.52, to estimate the tree.
The viral sequences isolated from the index patient and her partner were genotype 3a. Nucleotide sequencing of the HVR1 region showed that the two patients were infected by the same isolate because there were 97% homologies between the sequences from the source and the index patient.
The tree obtained with the NJ method is shown in Fig. 1. In this tree, the representative 42 nonredundant sequences of genotype 3a and the two HVR1 fragments of the patient and her partner are compared. The two patients are in the same branch and distinct from the other branches of the tree. The HVR1 patient subtree was supported by bootstrap values of 74% (NJ). Such a low level of confidence was expected because the patient sequences are characterized by only four substitutions relative to the genotype 3a control sequences.
FIG. 1.
Phylogenetic analysis of HCV isolates from a couple (man and woman) with HCV viremia based on the nucleotide sequence of part of the HVR1 region from the index patient. The phylogenetic tree was constructed by the NJ method program in the PHYLIP package (version 3.5). Numbers at the forks show the number of occurrences of the repetitive groups to the right out of 100 bootstrap samples.
Discussion.
The timing of the events and the molecular characterization of the two HCV isolates provide strong evidence that HCV was transmitted during sexual intercourse. The similarity between the sequences in the HVR1 regions of the isolates demonstrates that a cluster of strains exists in both patients. It should be noted that this region showed sufficient variability to distinguish isolates and is the most adequate to document person-to-person transmission from samples taken close together in time. The results from molecular biological investigations and epidemiological evaluation are complementary pieces of evidence in inquiries on possible intraspousal transmission of HCV (17). Sexual intercourse seems to be the only important risk factor. No parenteral or other risk factors for transmission of HCV, such as drug addiction, hospitalization, a history of acupuncture, ear piercing, tattooing, or sharing used razors, were identified for the couple. Altogether, these data suggest that this HCV isolate was transmitted from the chronically infected male to his female partner, probably by sexual intercourse. It has been suggested that the low risk of sexual transmission of HCV may be due to infected blood passed during intercourse through abrasions of mucosa rather than through HCV-infected semen (4). However, anal rather than vaginal intercourse constitutes a major cause of abrasions of mucosa. The possibility of anal transmission of HCV has been suggested by Balasekaran et al. (2). In this study, in a cohort of non-drug-addicted homosexual men, the prevalence of HCV antibodies was related to the number of acts of anal intercourse. In contrast to HIV and HBV, the risk of HCV transmission through anal intercourse remains controversial, since several studies failed to find a correlation between HCV infection and anal intercourse among male homosexuals (16). However, it has been reported that women engaging in very high risk sexual behavior were 14.2 times more likely to have HCV than other women (7). In case-control studies, HCV infection is associated with sexual promiscuity and sex with a partner who has a past history of hepatitis (13, 19).
The last consensus conferences of the European Association for the Study of the Liver and the National Institutes of Health (6, 15) do not recommend barrier precautions for stable monogamous sexual partners, including the use of latex condoms. Our study points out the possibility of transmission of HCV during anal sexual intercourse without safe-sex precautions. Barrier contraception with a condom should be suggested for serodiscordant couples with risky sexual practices, such as sexual anal intercourse. This recommendation could help reduce the possibility, as reported for HIV with antiretroviral therapy (11), of transmission of interferon-selected QS mutants.
REFERENCES
- 1.Ackerman Z, Ackerman E, Paltiel O. Intrafamilial transmission of hepatitis C virus: a systematic review. J Viral Hepat. 2000;7:93–103. doi: 10.1046/j.1365-2893.2000.00203.x. [DOI] [PubMed] [Google Scholar]
- 2.Balasekaran R, Bulterys M, Jamal M M, Quinn P G, Johnston D E, Skipper B, Chaturvedi S. A case-control study of risk factors for sporadic hepatitis C virus infection in the southwestern United States. Am J Gastroenterol. 1999;94:1341–1346. doi: 10.1111/j.1572-0241.1999.01084.x. [DOI] [PubMed] [Google Scholar]
- 3.Chayama K, Kobayashi M, Tsubota A, Koida I, Arase Y, Saitoh S, Ikeda K, Kumada H. Molecular analysis of intraspousal transmission of hepatitis C virus. J Hepatol. 1995;22:431–439. doi: 10.1016/0168-8278(95)80106-5. [DOI] [PubMed] [Google Scholar]
- 4.Debono E, Halfon P, Bourliere M, Gerolami V, Gastaldi M, Castellani P, Gauthier A P. Absence of hepatitis C genome in semen of infected men by polymerase chain reaction, branched DNA and in situ hybridization. Liver. 2000;20:257–261. doi: 10.1034/j.1600-0676.2000.020003257.x. [DOI] [PubMed] [Google Scholar]
- 5.Dienstag J L. Sexual and perinatal transmission of hepatitis C. Hepatology. 1997;26(Suppl. 1):66S–70S. doi: 10.1002/hep.510260712. [DOI] [PubMed] [Google Scholar]
- 6.European Association for the Study of the Liver. EASL International Consensus Conference on Hepatitis C, Paris, 26–28 February 1999. Consensus statement. J Hepatol. 1999;30:956–961. [PubMed] [Google Scholar]
- 7.Feldman J G, Minkoff H, Landesman S, Dehovitz J. Heterosexual transmission of hepatitis C, hepatitis B, and HIV-1 in a sample of inner city women. Sex Transm Dis. 2000;6:338–342. doi: 10.1097/00007435-200007000-00007. [DOI] [PubMed] [Google Scholar]
- 8.Galtier N, Gouy M, Gautier C. Seaview and Phylo-Win: two graphic tools for sequence alignment and molecular phylogeny. Comput Appl Biosci. 1996;6:543–548. doi: 10.1093/bioinformatics/12.6.543. [DOI] [PubMed] [Google Scholar]
- 9.Halfon P, Quentin Y, Roquelaure B, Sarles J, Halimi G, Gérolami V, Khiri H, Bourliere M, Cartouzou G. Mother-to-infant transmission of hepatitis C virus: molecular evidence of superinfection by homologous virus in children. J Hepatol. 1999;30:970–978. doi: 10.1016/s0168-8278(99)80248-7. [DOI] [PubMed] [Google Scholar]
- 10.Healey C J, Smith D B, Walker J L, Holmes B C, Fleming K A, Chapman R W G, Simmonds P. Acute hepatitis C infection after sexual exposure. Gut. 1995;36:148–150. doi: 10.1136/gut.36.1.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hecht F M, Grant R M, Petropoulos C J, Dillon B, Chesney M A, Tian H, Hellmann N S. Sexual transmission of an HIV-1 variant resistant to multiple reverse-transcriptase and protease inhibitors. N Engl J Med. 1998;339:307–311. doi: 10.1056/NEJM199807303390504. [DOI] [PubMed] [Google Scholar]
- 12.Leruez-Ville M, Kuntsmann J M, De Almeida M, Rouzioux C, Chaix M L. Detection of hepatitis C virus in semen of infected men. Lancet. 2000;356:42. doi: 10.1016/S0140-6736(00)02435-1. [DOI] [PubMed] [Google Scholar]
- 13.Lima M P, Pedro R J, Rocha M D. Prevalence and risk factors for hepatitis C virus (HCV) infection among pregnant Brazilian women. Int J Gynaecol Obstet. 2000;70:319–326. doi: 10.1016/s0020-7292(00)00209-5. [DOI] [PubMed] [Google Scholar]
- 14.Martell M, Esteban J L, Quer J, Genesca J, Weiner A, Esteban R, Guardia J, Gomez J. Hepatitis C virus (HCV) circulates as a population of different but closely related genomes: quasispecies nature of HCV genome distribution. J Virol. 1992;66:3225–3226. doi: 10.1128/jvi.66.5.3225-3229.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National Institutes of Health. Consensus Development Conference Panel statement: management of hepatitis C. Hepatology. 1997;26(Suppl. 1):2S–10S. doi: 10.1002/hep.510260701. [DOI] [PubMed] [Google Scholar]
- 16.Osella A R, Massa M A, Joekes S, Blanch N, Yacci M R, Centonze S, Sileoni S. Hepatitis B and C virus sexual transmission among homosexual men. Am J Gastroenterol. 1998;93:49–52. doi: 10.1111/j.1572-0241.1998.049_c.x. [DOI] [PubMed] [Google Scholar]
- 17.Ross R S, Viazov S, Varenholz C, Roggendorf M. Inquiries on intraspousal transmission of hepatitis C virus: benefits and limitations of genome sequencing and phylogenetic analysis. Forensic Sci Int. 1999;100:69–76. doi: 10.1016/s0379-0738(98)00200-x. [DOI] [PubMed] [Google Scholar]
- 18.Serfaty L. Nontransfusional and non-intravenous-drug-addiction-related transmission of hepatitis C. Presse Med. 1999;28:1135–1140. [PubMed] [Google Scholar]
- 19.Wejstal R. Sexual transmission of hepatitis C virus. J Hepatol. 1999;31(Suppl. 1):92–95. doi: 10.1016/s0168-8278(99)80382-1. [DOI] [PubMed] [Google Scholar]