Abstract
Breast cancer is the most frequent cancer among women and causes the greatest number of cancer-related death among women all over the world. It approximately accounts for 15% of all cancer death. The human microbiota is the term applied to the aggregate of microbes that live in different habitats of living organisms 'bodies, including the gut, skin, vagina, and mouth, as well as nose, conjunctiva, pharynx, and urethra, among others. Increasing evidence is pointing to the role of the microbiome in the occurrence and development of a variety of cancers. Intestinal microbiome imbalance is related to the occurrence of gastrointestinal tumors, such as esophageal, gastric, colorectal, and gallbladder cancer. The present study aimed to identify the role of microbiota in the development of breast cancer. The women with breast cancer (n=130) in this study were in the age range of 25-75 years. The study was conducted in Kirkuk city of Iraq from September 10, 2019, to March 15, 2020. The control group included 20 women diagnosed with benign breast lesions in the age range 25-75 years, who matched the women in the patient group. Blood samples and breast tissue samples were taken from patients with breast cancer and benign breast lesions. Blood samples were examined through immunological methods, enzyme-linked immunosorbent assay (ELISA) was adopted for the detection of interleukin-19 (IL-19). Breast tissue samples were taken from breast cancer and benign breast lesions patients to isolate and identify bacteria. Based on the obtained results, only 6 out of 30 (20%) cultured breast tissue samples from women with breast cancer showed bacterial growth. In total, 4 (67%) and 2(33%) of these 6 positive cultures were Escherichia coli was and Staphylococcus aureus, respectively, and this relation was statistically significant. However, no bacterial growth was observed on the cultured breast tissue samples taken from women with benign breast lesions. Moreover, the difference between women with a positive and negative result of bacterial culture and stages of breast cancer was statistically non-significant. It is worth mentioning that 50 % of women with breast cancer and bacterial growth were within the age range of 40-49 year. The present study revealed that the difference between women with breast cancer and those with benign breast lesions was statistically highly significant according to the place of residence. In addition, the mean level of IL-19 among women with breast cancer was lower than that in women with benign breast lesions, and this relation was statistically highly significant.
Keywords: Breast Cancer; IL-19, Microbiota
1. Introduction
Breast cancer is the most prevalent cancer in women and the leading cause of cancer-related death in women worldwide. It is responsible for about 15% of all cancer deaths ( 1 ). Following the discovery of an elevated risk of breast cancer in women with a positive family history of breast cancer, the cases were divided into hereditary (familial) and sporadic instances. Obesity and other risk factors are linked to the development of breast cancer ( 2 ).
The term “human microbiota” refers to the aggregate of bacteria that reside in various habitats throughout the human body (e.g., the gut, skin, vagina, mouth, nose, conjunctiva, pharynx, and urethra). Each organ’s microbiota is unique, and microbiomes exhibit significant and functionally meaningful inter-individual variability, which makes them a potential predictor of illness development, including cancer. The microbiota plays a role in a variety of diseases, including metabolic disorders, inflammatory and autoimmune diseases, allergies, and even conditions in which microbiome involvement seems to be implausible ( 3 - 5 ). A number of studies have shown that the microbiome has a role in the occurrence and progression of a range of malignancies. The results of one of these studies revealed that gastrointestinal malignancies, such as esophageal, gastric, colorectal, and gallbladder cancers are linked to gut microbiota imbalance ( 6 ). Furthermore, malignancies in other regions of the body, including hepatocellular carcinoma, breast cancer, pancreatic cancer, prostate cancer, and ovarian cancer are caused and proliferated by disruptions in the intestinal microbiome ( 7 ).
IL-19 is a cytokine belonging to the IL-10 family that contains IL-10, IL-19, IL-20, IL-22, IL-24, and IL-26 as well. This cytokine is produced by Monocytes, macrophages, B cells, endothelium, and epithelial cells, as well as immune and non-immune cells ( 8 ). IL-19 was shown to induce the production of Tumor Necrosis Factor (TNF)-ɑ and IL-6 by mouse monocytes, while it increased IL-10 and decreased TNF-ɑ in humans ( 9 , 10 ). IL-19 has a role to play in post-cardiopulmonary bypass immunosuppression and can act as an anti-inflammatory agent in inflammatory bowel disease ( 11 - 13 ).
2. Material and Methods
This cross-sectional study was carried out in Kirkuk city of Iraq from September 10, 2019, to March 15, 2020. The women with breast cancer (n=130) in this study were in the age range of 25-75 years. An interview was carried out with these patients using a questionnaire form designed by the investigator. The control group who were matched to the breast cancer patients included 20 women diagnosed with benign breast lesions in the age range of 25-75 years. Women in groups of patients and control were presented to Kirkuk Oncology Center and Azadi Teaching Hospital in Kirkuk, Iraq.
Blood samples and breast tissue samples were taken from patients with breast cancer and those with benign breast lesions. Blood samples were examined using immunological methods , and enzyme-linked immunosorbent assay (ELISA) for detection of IL-19. For this purpose, 5ml of blood samples in plain tubes were left for 30 min at 37 °C and then centrifuged at 3000 rounds per minute (rpm) for 15 min. afterward, the clot was removed and the residuals were re- centrifuged at 3000 rpm for 10 min. Subsequently, the obtained sera were aspirated using an automatic micropipette and transferred into two clean test tubes for serological tests. Labels were fixed on each test tube which was then stored and freezed at -20°C for late serological testing to determine the level of IL-19 using ELISA technique.
In total, 50, 30, and 20 fresh breast tissue samples were collected from the same women undergoing breast surgery at Azadi Teaching General Hospital, breast cancer patients, and those with benign breast lesions, respectively. Afterward, excised tissue samples were placed into tubes containing physiological saline solution for bacterial isolation.
The isolated samples of breast tissue were cultured for about 2 h after taking the sample and incubated aerobically and anaerobically at 37°C. Subsequently, the growing microbial cultures were observed in the culture media and approved through microscopical, cultural, and biochemical diagnostic methods. Detection of IL-19 was performed using ELISA kits.
3. Results
In the present study, the result of bacterial culture was positive in only 6 (20%) out of 30 women with breast cancer, which was statistically significant. However, no bacterial growth was observed on the cultured breast tissue samples taken from women with benign breast lesions (Table1).
Table 1.
Status of bacterial growth on culture in study groups
| Culture result | Breast cancer | Benign breast lesion | ||
|---|---|---|---|---|
| No. | % | No. | % | |
| Positive | 6 | 20 | 0 | 0.0 |
| Negative | 24 | 80 | 20 | 100.0 |
| Total | 30 | 100 | 20 | 100.0 |
| X2 | 10.8 | |||
| P-value | 0.001 | |||
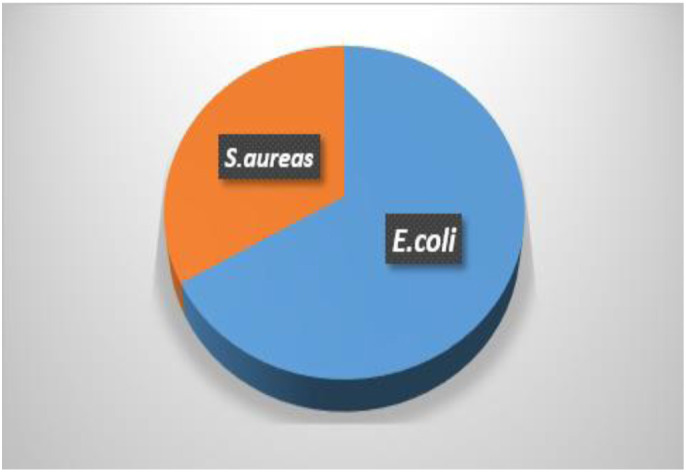
About 4 out of 6 (67%) positive cultures of samples from women with breast cancer were Escherichia coli, while 2(33%) were Staphylococcus aureus (Figure 1).
Figure 1.
Types of bacteria grown on culture media
The present studies revealed that 50% of women with breast cancer and with bacterial growth on their cultured breast tissue samples were in the age range of 40-49 years, while the other half were in the age range of 50-59 years. This ratio was not statistically significant (Table 2).
Table 2.
The age distribution of women with breast cancer and bacterial growth
| Age(year) | ||||
|---|---|---|---|---|
| Breast cancer group | ||||
| Positive culture | Negative culture | |||
| No. | % | No. | % | |
| <39 | 0 | 0 | 1 | 4.2 |
| 40-49 | 3 | 50 | 8 | 33.3 |
| 50-59 | 3 | 50 | 7 | 29.2 |
| 60-69 | 0 | 0 | 8 | 33.3 |
| Total | 6 | 100 | 24 | 100 |
| X2 | 0.000 | 5.66 | ||
| P-value | 1 | 1.29 | ||
The present study revealed that majority of women with breast cancer was from rural areas, while the majority of women with benign breast lesion were from urban areas. The difference between the two groups of study in terms of the place of residence was statistically significant (Table 3).
Table 3.
Distribution of the study groups according to the place of residence
| Residence | Breast cancer | Benign breast lesion | ||
|---|---|---|---|---|
| No. | % | No. | % | |
| Rural | 71 | 54.60 | 3 | 15 |
| Urban | 59 | 45.40 | 17 | 85 |
| Total | 130 | 100 | 20 | 100 |
| X2 | 1.108 | 9.8 | ||
| P-value | 0.29 | 0.002* | ||
| X2 | 10.883 | |||
| P-value | 0.001* | |||
The results of this study revealed that the majority of women with a positive result of bacterial culture (50%) were in stage II of cancer, followed by those in stage III (33.30%) and stage I (16.7%,).The majority of women with a negative result of bacterial culture (54.2%) were in stage II of cancer, followed by those in stage III (29.2%) and stage I (16.7%). The difference between women with a positive and negative result of bacterial culture was statistically non-significant (Table 4).
Table 4.
Relation between stage of breast cancer and results of bacterial culture
| Culture | ||||
|---|---|---|---|---|
| Stage of breast cancer | Positive | Negative | ||
| No. | % | No. | % | |
| Stage I | 1 | 16.70 | 4 | 16.70 |
| Stage II | 3 | 50 | 13 | 54.20 |
| Stage III | 2 | 33.30 | 7 | 29.20 |
| Total | 6 | 100 | 24 | 100 |
| X2 | 1 | 5.25 | ||
| P-value | 0.6 | 0.07 | ||
| X2 | 0.04 | |||
| P-value | 0.97 | |||
The study showed that the mean±SD level of IL-19 among women with breast cancer was determined at 40.3±23.3, which was lower than in the group of women with benign breast lesions (63.6±19.1). This relation was statistically significant (Table 5).
Table 5.
Relation between breast cancer and IL-19
| Study Group | No. | Mean± SD | P-value | |
|---|---|---|---|---|
| IL-19 (pg/ml) | Breast Cancer | 130 | 40.3±23.3 | <0.01 |
| Benign breast lesion | 20 | 63.6±19.1 |
4. Discussion
Breast cancer is the most prevalent cancer in women and the leading cause of cancer-related death in women worldwide. It is responsible for about 15% of all cancer deaths ( 1 ). In this study, the result of bacterial culture was positive in only 6 (20%) out of 30 women with breast cancer, which was statistically significant. In total, four out of six positive cultures among breast cancer cases were proved to be Escherichia coli, while the other two were Staphylococcus aureus. Although some studies found no evidence of bacterial abundance in breast cancer ( 14 ), others found that diversity ( 15 ) and abundance ( 16 ) of related taxa were reduced with breast cancer. The relative abundance of Enterobacteriaceae, Bacillus, and Staphylococcus spp. had increased in individuals with breast cancer, according to one 16S rRNA study ( 16 ).
In breast cancer cells, Escherichia coli (Enterobacteriaceae) and Staphylococcus epidermides both cause double-stranded DNA breaks ( 17 ). The breast microbiota of women with malignant cancer differed significantly from that of women with benign disease, according to Hieken, Chen ( 18 ). Prevotella, Lactococcus, Streptococcus, Corynebacterium, and Micrococcus were found in significantly higher relative abundances in healthy patients, compared to breast cancer patients. Moreover, Bacillus, Staphylococcus, Enterobacteriaceae, Comamondaceae, and Bacteroidetes were found to have significantly higher relative abundances in patients with breast cancer. In vitro, these bacteria were capable of causing DNA damage.
There was also a decrease in some lactic acid bacteria, which are renowned for their health benefits, such as anti-carcinogenic capabilities ( 19 ). Proteobacteria were the most prevalent phylum in breast tissue; however, members of this phylum account for a minor proportion of the overall bacterial community in the vagina, oral cavity, skin, and gastrointestinal system ( 20 - 22 ).
In this study, 50% of women with breast cancer with positive bacterial culture were within the age range of40-49 years, while the other half was within the age range of 50-59 year, and this ratio was statistically not significant. The results of a study conducted by Urbaniak, Gloor ( 17 ) showed that the mean age differed between the two groups. In their study, the cancer cohort had a mean and median age of 62 years, while the healthy cohort had a mean age of 49 years and a median age of 53 years ( 17 ). Moreover, the mean and median ages of the benign group were 38 years and 36 years, respectively, and the microbial profiles did not differ between the benign and cancer groups. Due to the fact that human microbiota is dynamic and evolves with age and exposure to environmental stimuli, microbiome changes are another important aspect of the association between cancer and aging ( 23 , 24 ). Exponential growth in invasive breast cancer and ductal carcinoma in situ has been documented in women over the age of 50, up until menopause, which accounts for 80% of all breast cancers. At age 40, the 10-year risk of invasive breast cancer is 1.5 % which rises to 3 % and 4 % by age 50 and 70, respectively. In total, the prevalence of breast cancer among women is 13.2 % or one in eight women ( 25 ).
The present study revealed that the majority (54.60%) of women with breast cancer were from rural areas, which was in line with the results obtained byAbdullahAbbas and Saadoon ( 26 ). Breast cancer was shown to be the leading cause of morbidity and mortality among Indian women in a study performed on breast cancer epidemiology. In India, the place of residence (urban/rural) and environmental factors were key risk factors for an increase in cancer incidence. Breast cancer development was also affected by activities among urban and rural women ( 27 ).
Based on the obtained results in this study, the majority of women with a positive result of bacterial culture (50%) were in stage II of cancer, followed by those in stage III (33.30%) and stage I (16.7%,). The result was statistically non-significant. The results of a study conducted by Xuan, Shamonki ( 14 ) indicated that there was an inverse relationship between breast cancer stage and bacterial load in tumor tissue, but not in paired normal tissue ( 14 ). Tumors from Stage 1 patients had the highest copy number of bacterial DNA followed by Stage 2 patients, and the lowest copy number of bacterial DNA was observed in Stage 3 patients. These findings imply that the number of germs was not significantly different between matched normal tissues from patients with breast cancer and healthy breast tissue from healthy individuals. In another study in which patients were stratified into grade I (n=7), grade II (n=36), grade III (n=13), although there was no significant difference among grade I, II, and III, on the other hand the grade III tissue showed higher of number of germs compared with grade I and II ( 28 ).
The involvement of the immune response in the development or control of breast cancer is difficult to assess. Nonetheless, there is strong evidence indicating that the immune response in this disease is not a host defensive response and may lead to cancer development. The creation of direct or indirect modulators of breast cancer cell development, such as cytokines, by inflammatory cell infiltrates, could be one mechanism for these effects ( 29 ). It was reported that interleukins stimulate cancer cell growth and contribute to locoregional relapse as well as metastasis ( 30 ).
Increased levels of proinflammatory cytokines have been associated with distressing symptoms in people with breast cancer, in addition to having a negative impact on disease development ( 31 ). Cytokines may be involved in the pathogenesis of neuropsychiatric disorders of immunological and neuroendocrine systems. Proinflammatory cytokines have been linked to depression in cancer patients undergoing treatment as well as fatigue in breast cancer survivors ( 32 ).
The present study showed that the mean±SD level of IL-19 among breast cancer women (40.3±23.3pg/ml) was lower than that in women with benign breast lesions (63.6±19.1pg/ml), and this relation was statistically highly significant. Mehdipour, Malekzadeh ( 33 ) Showed a significantly lower median serum IL-19 level in breast cancer patients (median: 27.3 pg/ml; range: 10.5-2443.6 pg/ml), compared to the healthy control group. In the study conducted by Hsing, Cheng ( 34 ) no difference was observed in the serum level of IL-19 between patients with low and high IL-19 expression in tumors. One explanation for this result is that probablyIL-19 acted primarily as a local mediator in the breast cancer cells’ microenvironment.
The IL-19 is a member of the IL-10 family, expressed in different types of tumor cells. In breast cancer, it has been expressed in the late stages of the tumor. It has also been linked to an increase in mitosis and a considerable increase in metastasis, both of which lead to a poor prognosis ( 35 ). The IL-19 can upregulate TGF-β, IL-1β, and IL-6, and all factors that induce the migration of breast cancer cells. It is also involved in breast tumor metastasis. Santulli, Borghese ( 36 ) found a lower level of IL-19 and IL-22 in the sera of patients with ovarian endometriosis, compared to control women without endometriosis. They also showed a significant correlation between the levels of serum IL-19 and IL-2 and clinical features of disease severity ( 36 ). Low levels of IL-19 in the blood have been linked to severe dyspareunia. Following the detection ofIL-19, determination of its amount can determine the disease’s activity to some extent. This is the reason why the negative findings were ignored when the attempt was made to link interleukin levels to disease activity ( 37 ).
The majority of women with breast cancer were within the age range of40-49years, while the highest rate of women with benign breast lesions was within the age range of50-59 years, and no significant relation was observed between these groups. However, the relation between the place of residence in women with breast cancer and those with benign breast lesions was highly significant. The majority of women with breast cancer were within stage II of breast cancer. The result of bacterial culture was positive in only 6 out of 30 women with breast cancer, which was statistically significant. Eventually, the mean level of IL-19 in breast cancer women was lower than that in women with benign breast lesions, and this relation was statistically highly significant.
Authors' Contribution
Study concept and design: A. S. H.
Acquisition of data: N. I.
Analysis and interpretation of data: I. H. S.
Drafting of the manuscript: A. S. H.
Critical revision of the manuscript for important intellectual content: N. I.
Statistical analysis: I. H. S.
Administrative, technical, and material support: A. S. H.
Ethics
All procedures performed in the study involving human participants were in accordance with the ethical standards of the Kirkuk University, Kirkuk, Iraq under the project number of 2021-4587924.
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- 1.Breast cancer [Internet] WHO. 2018. Available from: http://www.who.int/cancer/prevention/diagnosis-screening/breast-cancer/en/
- 2.S B. Molecular Pathology of Breast Cancer. 1st ed. New York: Springer-Verlag; 2016. [Google Scholar]
- 3.Caputi V, Giron MC. Microbiome-gut-brain axis and toll-like receptors in Parkinson’s disease. Int J Mol Sci. 2018;19(6):1689. doi: 10.3390/ijms19061689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rea D, Coppola G, Palma G, Barbieri A, Luciano A, Del Prete P, et al. Microbiota effects on cancer: from risks to therapies. Oncotarget. 2018;9(25):17915. doi: 10.18632/oncotarget.24681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Selber-Hnatiw S, Rukundo B, Ahmadi M, Akoubi H, Al-Bizri H, Aliu AF, et al. Human gut microbiota: toward an ecology of disease. Front Microbiol. 2017;8:1265. doi: 10.3389/fmicb.2017.01265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rajagopala SV, Vashee S, Oldfield LM, Suzuki Y, Venter JC, Telenti A, et al. The human microbiome and cancer. Cancer Prev Res. 2017;10(4):226–34. doi: 10.1158/1940-6207.CAPR-16-0249. [DOI] [PubMed] [Google Scholar]
- 7.Chase D, Goulder A, Zenhausern F, Monk B, Herbst-Kralovetz M. The vaginal and gastrointestinal microbiomes in gynecologic cancers: a review of applications in etiology, symptoms and treatment. Gynecol Oncol. 2015;138(1):190–200. doi: 10.1016/j.ygyno.2015.04.036. [DOI] [PubMed] [Google Scholar]
- 8.Sabat R. IL-10 family of cytokines. Cytokine & growth factor reviews. 2010;21(5):315–24. doi: 10.1016/j.cytogfr.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 9.Jordan WJ, Eskdale J, Boniotto M, Lennon GP, Peat J, Campbell JD, et al. Human IL‐19 regulates immunity through auto‐induction of IL‐19 and production of IL‐10. Eur J Immunol. 2005;35(5):1576–82. doi: 10.1002/eji.200425317. [DOI] [PubMed] [Google Scholar]
- 10.Liao Y-C, Liang W-G, Chen F-W, Hsu J-H, Yang J-J, Chang M-S. IL-19 induces production of IL-6 and TNF-α and results in cell apoptosis through TNF-α. J Immunol. 2002;169(8):4288–97. doi: 10.4049/jimmunol.169.8.4288. [DOI] [PubMed] [Google Scholar]
- 11.Azuma Y-T, Matsuo Y, Nakajima H, Yancopoulos GD, Valenzuela DM, Murphy AJ, et al. Interleukin-19 is a negative regulator of innate immunity and critical for colonic protection. J Pharmacol Sci. 2011;115(2):105–11. doi: 10.1254/jphs.10r02cr. [DOI] [PubMed] [Google Scholar]
- 12.Cantó E, Garcia Planella E, Zamora-Atenza C, Nieto JC, Gordillo J, Ortiz MA, et al. Interleukin-19 impairment in active Crohn’s disease patients. PLoS One. 2014;9(4):e93910. doi: 10.1371/journal.pone.0093910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yeh C-H, Cheng B-C, Hsu C-C, Chen H-W, Wang J-J, Chang M-S, et al. Induced interleukin-19 contributes to cell-mediated immunosuppression in patients undergoing coronary artery bypass grafting with cardiopulmonary bypass. Ann Thorac Surg. 2011;92(4):1252–9. doi: 10.1016/j.athoracsur.2011.04.061. [DOI] [PubMed] [Google Scholar]
- 14.Xuan C, Shamonki JM, Chung A, DiNome ML, Chung M, Sieling PA, et al. Microbial dysbiosis is associated with human breast cancer. PloS one. 2014;9(1):e83744. doi: 10.1371/journal.pone.0083744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goedert JJ, Jones G, Hua X, Xu X, Yu G, Flores R, et al. Investigation of the association between the fecal microbiota and breast cancer in postmenopausal women: a population-based case-control pilot study. J Natl Cancer Inst. 2015;107(8) doi: 10.1093/jnci/djv147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luu TH, Michel C, Bard J-M, Dravet F, Nazih H, Bobin-Dubigeon C. Intestinal proportion of Blautia sp. is associated with clinical stage and histoprognostic grade in patients with early-stage breast cancer. Nutr Cancer. 2017;69(2):267–75. doi: 10.1080/01635581.2017.1263750. [DOI] [PubMed] [Google Scholar]
- 17.Urbaniak C, Gloor GB, Brackstone M, Scott L, Tangney M, Reid G. The microbiota of breast tissue and its association with breast cancer. Appl Environ Microbiol. 2016;82(16):5039–48. doi: 10.1128/AEM.01235-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hieken TJ, Chen J, Hoskin TL, Walther-Antonio M, Johnson S, Ramaker S, et al. The microbiome of aseptically collected human breast tissue in benign and malignant disease. Sci Rep. 2016;6(1):1–10. doi: 10.1038/srep30751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koller VJ, Marian B, Stidl R, Nersesyan A, Winter H, Simić T, et al. Impact of lactic acid bacteria on oxidative DNA damage in human derived colon cells. Food Chem Toxicol. 2008;46(4):1221–9. doi: 10.1016/j.fct.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 20.Hummelen R, Fernandes AD, Macklaim JM, Dickson RJ, Changalucha J, Gloor GB, et al. Deep sequencing of the vaginal microbiota of women with HIV. PloS one. 2010;5(8):e12078. doi: 10.1371/journal.pone.0012078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wade WG. The oral microbiome in health and disease. Pharmacol Res. 2013;69(1):137–43. doi: 10.1016/j.phrs.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 22.Wolfe AJ, Toh E, Shibata N, Rong R, Kenton K, FitzGerald M, et al. Evidence of uncultivated bacteria in the adult female bladder. J Clin Microbiol. 2012;50(4):1376–83. doi: 10.1128/JCM.05852-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goldsmith F, O’Sullivan A, Smilowitz JT, Freeman SL. Lactation and intestinal microbiota: how early diet shapes the infant gut. J Mammary Gland Biol Neoplasia. 2015;20(3):149–58. doi: 10.1007/s10911-015-9335-2. [DOI] [PubMed] [Google Scholar]
- 24.Thongaram T, Hoeflinger JL, Chow J, Miller MJ. Human milk oligosaccharide consumption by probiotic and human-associated bifidobacteria and lactobacilli. J Dairy Sci. 2017;100(10):7825–33. doi: 10.3168/jds.2017-12753. [DOI] [PubMed] [Google Scholar]
- 25.Quong J, Eppenberger-Castori S, Moore D, Scott GK, Birrer MJ, Kueng W, et al. Age-dependent changes in breast cancer hormone receptors and oxidant stress markers. Breast Cancer Res Treat. 2002;76(3):221–36. doi: 10.1023/a:1020886801674. [DOI] [PubMed] [Google Scholar]
- 26.AbdullahAbbas A, Saadoon IH. Relation of Epstein Barr virus with Interleukin-6 Level among Women with Breast Cancer in Ramadi City. Med-Leg Update. 2020;20(1):1393–8. [Google Scholar]
- 27.Malvia S, Bagadi SA, Dubey US, Saxena S. Epidemiology of breast cancer in Indian women. Asia Pac J Clin Oncol. 2017;13(4):289–95. doi: 10.1111/ajco.12661. [DOI] [PubMed] [Google Scholar]
- 28.Meng S, Chen B, Yang J, Wang J, Zhu D, Meng Q, et al. Study of microbiomes in aseptically collected samples of human breast tissue using needle biopsy and the potential role of in situ tissue microbiomes for promoting malignancy. Front Oncol. 2018;8:318. doi: 10.3389/fonc.2018.00318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stewart T, Heppner G. Immunological enhancement of breast cancer. Parasitology. 1997;115(7):141–53. doi: 10.1017/s0031182097001832. [DOI] [PubMed] [Google Scholar]
- 30.S SP. Serum cytokines levels of interleukin -8 and tumor necrosis factor in breast cancer patients treated with tamoxifen and supplemented with co-enzyme Q10, riboflavin and niacin. Basic Clin Pharmacol Toxicol. 2007;100:387–91. doi: 10.1111/j.1742-7843.2007.00065.x. [DOI] [PubMed] [Google Scholar]
- 31.Musselman DL, Miller AH, Porter MR, Manatunga A, Gao F, Penna S, et al. Higher than normal plasma interleukin-6 concentrations in cancer patients with depression: preliminary findings. Am J Psychiatry. 2001;158(8):1252–7. doi: 10.1176/appi.ajp.158.8.1252. [DOI] [PubMed] [Google Scholar]
- 32.Lyon DE, McCain NL, Walter J, Schubert C. Cytokine comparisons between women with breast cancer and women with a negative breast biopsy. Nurs Res. 2008;57(1):51. doi: 10.1097/01.NNR.0000280655.58266.6c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mehdipour F, Malekzadeh M, Talei A, Ghaderi A. Decreased Serum Level of Interleukin-19 in Iranian Patients with Breast Cancer. Middle East J Cancer. 2015;6(4):203–9. [Google Scholar]
- 34.Hsing C-H, Cheng H-C, Hsu Y-H, Chan C-H, Yeh C-H, Li C-F, et al. Upregulated IL-19 in breast cancer promotes tumor progression and affects clinical outcome. Clin Cancer Res. 2012;18(3):713–25. doi: 10.1158/1078-0432.CCR-11-1532. [DOI] [PubMed] [Google Scholar]
- 35.Chen Y-Y, Li C-F, Yeh C-H, Chang M-S, Hsing C-H. Interleukin-19 in breast cancer. Clin Dev Immunol. 2013;2013 doi: 10.1155/2013/294320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Santulli P, Borghese B, Chouzenoux S, Streuli I, Borderie D, de Ziegler D, et al. Interleukin-19 and interleukin-22 serum levels are decreased in patients with ovarian endometrioma. Fertil Steril. 2013;99(1):219–26. doi: 10.1016/j.fertnstert.2012.08.055. [DOI] [PubMed] [Google Scholar]
- 37.Santulli P, Borghese B, Chouzenoux S, Vaiman D, Borderie D, Streuli I, et al. Serum and peritoneal interleukin-33 levels are elevated in deeply infiltrating endometriosis. Hum Reprod. 2012;27(7):2001–9. doi: 10.1093/humrep/des154. [DOI] [PubMed] [Google Scholar]


